Introduction
Pancreatic cancer, of which the most common
histological subtype is pancreatic ductal adenocarcinoma (PDAC),
has worldwide incidence and mortality rates of eight cases per
100,000 person/year and seven deaths per 100,000 person/year,
respectively (1). By 2017,
pancreatic cancer had become the third-leading cause of
cancer-associated mortality in the United States. The incidence and
mortality of pancreatic cancer are continuing to increase, and it
will be the second largest cause of cancer-related mortality
worldwide by 2030, based on predicted data (2). Pancreatic cancer is an aggressive and
fatal disease, and most patients with pancreatic cancer succumb to
the disease within 5 years (3). The
5-year overall survival rate is 8%, but in patients with advanced
disease, this decreases to 3% (4).
The causes of the poor prognosis of patients with pancreatic cancer
include a lack of early symptoms, no clinically validated screening
methods in the early stage and early invasion of nearby vessels,
amongst other factors (3,5). For pancreatic cancer, the only current
treatment option is surgery. However, only 10–15% of newly
diagnosed patients qualify for surgery, as pancreatic cancer is
often in the late stage when diagnosed (3,5,6).
Currently, therapies for pancreatic cancer are
seriously lacking. Combination chemotherapies, which have been
previously used for the treatment of patients with pancreatic
cancer, such as Folfirinox and gemcitabine/nab-paclitaxel, have
only extended the patients' survival by several months and cause
evident toxic side effects (7,8). In
addition, immunotherapy has not been shown to be effective against
PDAC. It has also been revealed that whole-cell therapeutic
vaccines show no effect in patients with advanced-stage pancreatic
cancer (9,10). Therefore, more effective and less
toxic drugs are urgently required for patients with pancreatic
cancer.
An increasing number of studies have revealed that
the activation of the mevalonate pathway or cholesterol intake via
low density lipoprotein receptor (LDLR) is associated with the
development of pancreatic cancer (10–12).
High expression levels of 3-hydroxy-3-methylglutaryl-coenzyme A
(HMG-CoA) reductase and LDLR were observed in a PDAC mouse model
(10). These findings suggested
that cholesterol level could influence the development of PDAC.
Cholesterol is a basic and important functional and structural
element of cell membranes, and it served a key role in cell
differentiation, proliferation and apoptosis (2,6,13). In
the process of synthesizing cholesterol from cytoplasmic
acetyl-CoA, HMG-CoA reductase catalyzes the conversion of HMG-CoA
to mevalonate, and this is the rate-limiting step of the pathway
(14). Moreover, inhibitors of
HMG-CoA reductase can inhibit the proliferation of some cancer
cells, such as breast cancer cells and ovarian cancer cells, by
blocking cholesterol biosynthesis (15–17).
The conversion of 2, 3-monoepoxysqualene to
lanosterol, which is catalyzed by a key enzyme 2, 3-oxidosqualene
cyclase (OSC), is a critical step downstream of HMG-CoA reductase
in the process of cholesterol biosynthesis (14). OSC has become a feasible new target
for inhibiting cholesterol biosynthesis (18). RO 48-8071 (RO) is an inhibitor of
OSC (19). It has been reported
that RO has an inhibitory effect on the proliferation of prostate
and breast cancer cells (20,21).
Inhibitors of cholesterol synthesis can regulate apoptosis,
angiogenesis and metastasis via the ERK and JNK signaling pathway
in breast and pancreatic cancer (22,23).
The present study conducted a set of experiments to investigate the
effects of RO on pancreatic cancer and to determine the possible
molecular mechanisms.
Materials and methods
Reagents
RO was purchased from MedChemExpress (cat. no.
HY-18630A) and dissolved in DMSO with a storage concentration of 10
mM. DMEM was purchased from Gibco (Thermo Fisher Scientific, Inc.),
while FBS was obtained from Clark Bioscience. Specific antibodies
against β-actin, p27, phosphorylated (p)ERK1/2, ERK1/2, pJNK, JNK,
cyclin B1 and cyclin E were purchased from Santa Cruz
Biotechnology, Inc. A specific antibody for Ki67
immunohistochemical staining was obtained from OriGene
Technologies, Inc. HRP-conjugated secondary antibodies were
obtained from MilliporeSigma, and used for western blotting.
HRP-conjugated secondary antibodies and a DAB HRP Color Development
kit for immunohistochemistry were purchased from OriGene
Technologies, Inc.
Cell culture
The human pancreatic tumor cell line PANC-1 was
purchased from the American Type Culture Collection. Cells were
maintained routinely in complete DMEM (DMEM with 10% FBS) with 1%
penicillin-streptomycin in a 5% CO2 humidified
atmosphere at 37°C.
Animals
A total of 12 male BALB/c nude mice aged 4 weeks
(weight, 16.86±9.3 g) for the in vivo study were provided by
Beijing Vital River Laboratory Animal Technology Co., Ltd., and
reared according to the Animal Research Committee's guidelines of
Anhui Medical University (Hefei, China). The mice were raised in
the specific-pathogen-free laboratory animal room under humane
conditions at 22±2°C with 55±5% humidity under a 12-h light/dark
cycle with food and water provided ad libitum. The animal
protocols were approved by the Committee for Ethics of Animal
Experimentation of Anhui Medical University (approval no.
20150136).
Cell viability assay
An MTS assay (Promega Corporation) was performed to
assess cell viability. Following trypsinization, ~2,000 PANC-1
cells in the logarithmic growth phase were prepared as a
unicellular suspension to seed in a 96-well cell culture plate with
100 µl volume in triplicate. Cells were treated with RO at
different concentrations (1, 3, 10, 30 and 100 µM) for 72 h when
they reached 60–70% confluence. Then, the MTS reagent was added to
the 96-well cell culture plate (20 µl per well), which was followed
by incubation at 37°C for 2 h. The absorbance value (A value) at
the wavelength of 490 nm was measured on a universal microplate
reader (ELx800; BioTek Instruments, Inc.), with six samples
measured in each group. Using this method, an appropriate
concentration of RO for the treatment of PANC-1 cells was
determined and used in the subsequent experiments. RO at the
selected concentration was used to treat PANC-1 cells as
aforementioned for 0, 24, 48 and 72 h to detect the time-dependent
effects of RO on PANC-1 cell viability.
Cell cycle analysis via flow cytometry
(FCM)
PANC-1 cells at a density of 1×105 cells
per well were inoculated into 6-well cell culture plates and
cultured in a 5% CO2 humidified atmosphere at 37°C.
After treatment with RO (24, 48 and 72 h) when cells reached 60–70%
confluence, a Cell Cycle Analysis kit (Beyotime Institute of
Biotechnology) was used to detect the cell cycle, according to the
manufacturer's instructions. Briefly, the cells were collected and
counted after trypsin digestion. Then, cells were fixed in cold 70%
ethanol for 30 min at 4°C and stained with PI in the dark for ~20
min after washing twice with cold PBS. The cell cycle was then
assessed via FCM (BD FACSVerse™; BD Biosciences). Data analysis was
performed using FlowJo software (FlowJo version 10.5.4; FlowJo
LLC).
Western blotting
PANC-1 cells were treated with RO for 24, 48 and 72
h, and were harvested in RIPA lysis buffer (25 mM Hepes; 1.5%
Triton X-100; 1% sodium deoxycholate; 0.1%SDS; 0.5 M NaCl; 5 mM
EDTA; 50 mM NaF; 0.1 mM sodium vanadate; 1 mM phenylmethylsulphonyl
fluoride; 0.1 g/l leupeptin; pH 7.8) on ice. Cell lysates were
centrifuged at 14,000 × g for 30 min at 4°C to obtain the total
soluble protein by collecting the supernatant. The concentration of
the extracted proteins from each group was determined using a BCA
assay (Beyotime Institute of Biotechnology). Then, 30 µg/lane of
total protein from each treatment group, which had been boiled
together with SDS-PAGE loading buffer, was loaded onto 12% SDS-PAGE
gels and subsequently transferred onto PVDF membranes after
electrophoresis separation. The blots were run on separate gels
simultaneously by loading the same concentrations of the proteins
derived from the same sample into each gel. After non-specific
blocking in 5% skimmed milk for 2 h at room temperature, the PVDF
membranes were washed with PBS containing 0.1% Tween-20 three times
and incubated at 4°C overnight with specific primary antibodies
against β-actin (cat. no. sc-47778; 1:1,000), cyclin B1 (cat. no.
sc-245; 1:500), cyclin E (cat. no. sc-377100; 1:500), p27 (cat. no.
sc-56338; 1:500), pERK (cat. no. sc-81492; 1:500), pJNK (cat. no.
sc-6254; 1:500), ERK1/2 (cat. no. sc-514302; 1:1,000) and JNK (cat.
no. sc-7345; 1:1,000). Subsequently, the membranes were reacted
with the corresponding HRP-conjugated secondary antibody (goat
anti-mouse IgG; cat. no. AP130P; 1:10,000) diluted in TBS solution
for 2 h at room temperature. After another washing step to remove
unbound secondary antibodies, the specific bands were developed
using ECL reagents (SuperSignal™ West Femto kit; Pierce; Thermo
Fisher Scientific, Inc.), and images were captured using a
ChemiScope (Clinx Science Instruments Co., Ltd.). The signals of
the specific bands were identified and detected with the Quantity
One analysis software (version 4.6.6; Bio-Rad Laboratories, Inc.).
Target bands for cyclin B1, cyclin E and p27 were normalized to the
reference gene β-actin, and specific pERK and pJNK bands were
normalized to total ERK1/2 or JNK, respectively.
In vivo experiment
For the in vivo tumorigenic study, 12 male
BALB/c nude mice were inoculated subcutaneously in the right hips
with 1×106 PANC-1 cells suspended in PBS. When the
tumors reached 0.4–0.5 cm, the mice were injected with 50 µl RO at
a concentration of 5 mg/kg/day or DMSO at a relevant concentration
(n=6 for each group) via the vena cava caudalis once a day for the
first 5 days, then once every other day for six more injections
(20). The tumor volumes were
measured every 3 days at day 0, 3, 6, 9, 12, 15, 18 and 21 of RO
injection. The tumor size was calculated as
ab2/2, where a was the longer diameter and
b was the smaller diameter of the two dimensions. The
maximum tumor size obtained in the present study was ~250
mm3. At day 24 after RO injection, the mice were
anesthetized with an intraperitoneal injection of pentobarbital (50
mg/kg) and euthanized via cervical dislocation. Surgery for
subcutaneous tumor dissection was performed to obtain the formed
xenograft tumors.
H&E and tissue immunohistochemical
staining
The excised xenograft tumors were immediately fixed
in 10% formalin for 24 h at room temperature, followed by
dehydration in a gradient series of ethanol and paraffin embedding.
Formalin-fixed and paraffin-embedded tissues were sliced (4-µm
thick) and conventionally stained with hematoxylin for 5 min at
room temperature and eosin for 10 sec at room temperature. The
H&E-stained sections were visualized using a light microscope
(magnification, ×400) after sealing the slides with neutral
balsam.
For immunohistochemical staining, the sections(4-µm
thick) obtained from the formalin-fixed and paraffin-embedded
tissues were blocked with 3% BSA (Beyotime Institute of
Biotechnology) diluted in 0.2% Triton X-100/PBS at room temperature
for 30 min and stained with a Ki67 specific antibody (cat. no.
ZA-0502; OriGene Technologies, Inc.) overnight at 4°C, followed by
incubation with HRP-conjugated secondary antibody (cat. no.
PV-6001) for 2 h at room temperature and development with a DAB
Horseradish Peroxidase Color Development kit (cat. no. ZLI-9018;
OriGene Technologies, Inc.). The images of the stained sections
were captured using a light microscope (magnification, ×400;
Olympus Corporation).
Statistical analysis
All data were analyzed using SPSS version 15.0
software (SPSS, Inc.). All data are presented as the mean ± SEM
(n=3 for the in vitro assays and n=6 mice/group for the
in vivo assays). The significant differences between groups
were determined using one-way ANOVA followed by a Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
RO inhibits the viability of PANC-1
cells
PANC-1 cells in the 96-well culture plate were
treated with RO at a variety of concentrations (1, 3, 10, 30 and
100 µM) for 72 h to determine cell viability using a colorimetric
MTS assay. It was found that RO inhibited PANC-1 cell viability in
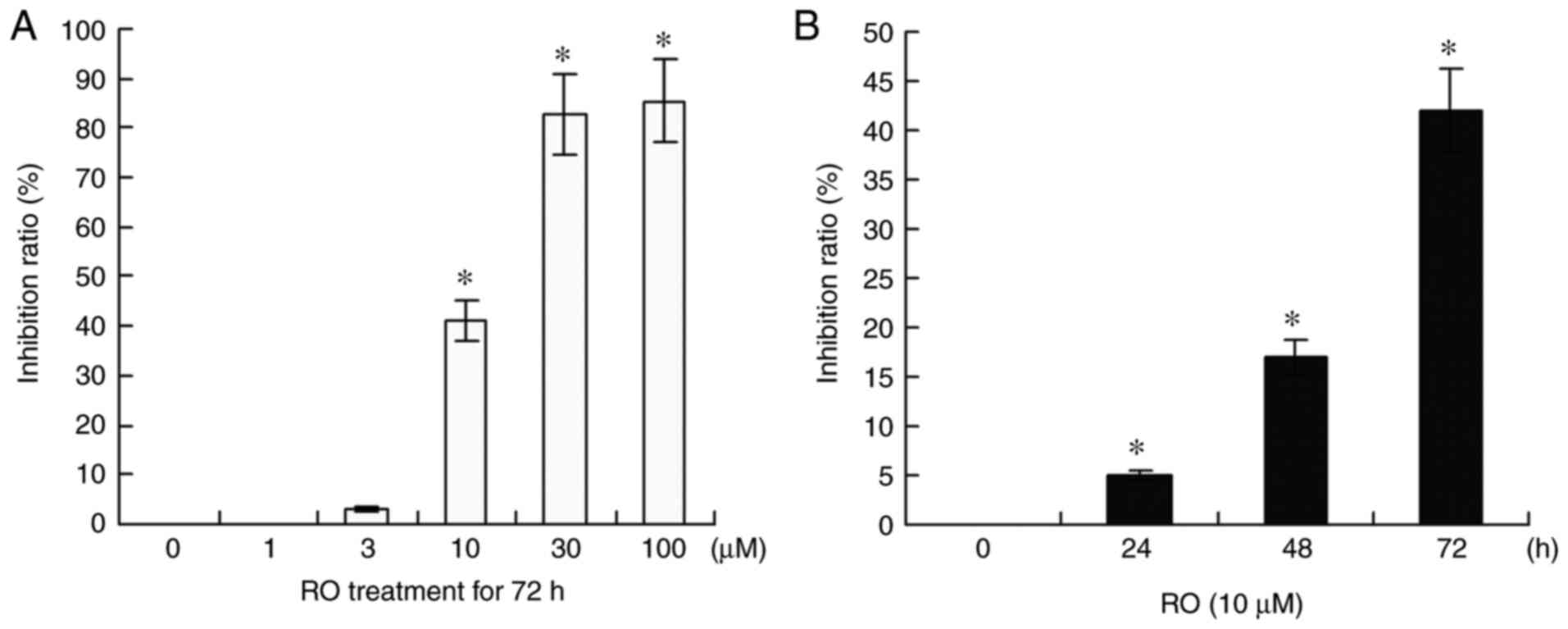
a dose-dependent manner (Fig. 1A).
RO showed a nearly 40% (P<0.05) inhibitory ratio at 10 µM, and
an 80% inhibitory ratio (P<0.05) at 30 and 100 µM after
treatment for 72 h. However, at 1 and 3 µM concentration, RO did
not have an effect on the viability of PANC-1 cells.
It was demonstrated that the effects of RO on PANC-1
cell viability were in a time-dependent manner. The growth
inhibition percentages were 4% (P<0.05), 17% (P<0.05) and 42%
(P<0.05) when PANC-1 cells were treated with 10 µM RO for 24, 48
and 72 h, respectively (Fig. 1B).
In accordance with these results from the MTS assay, RO at a
concentration of 10 µM was selected to treat PANC-1 cells in the
subsequent experiments.
RO inhibits the G1-S-phase
transition of PANC-1 cells
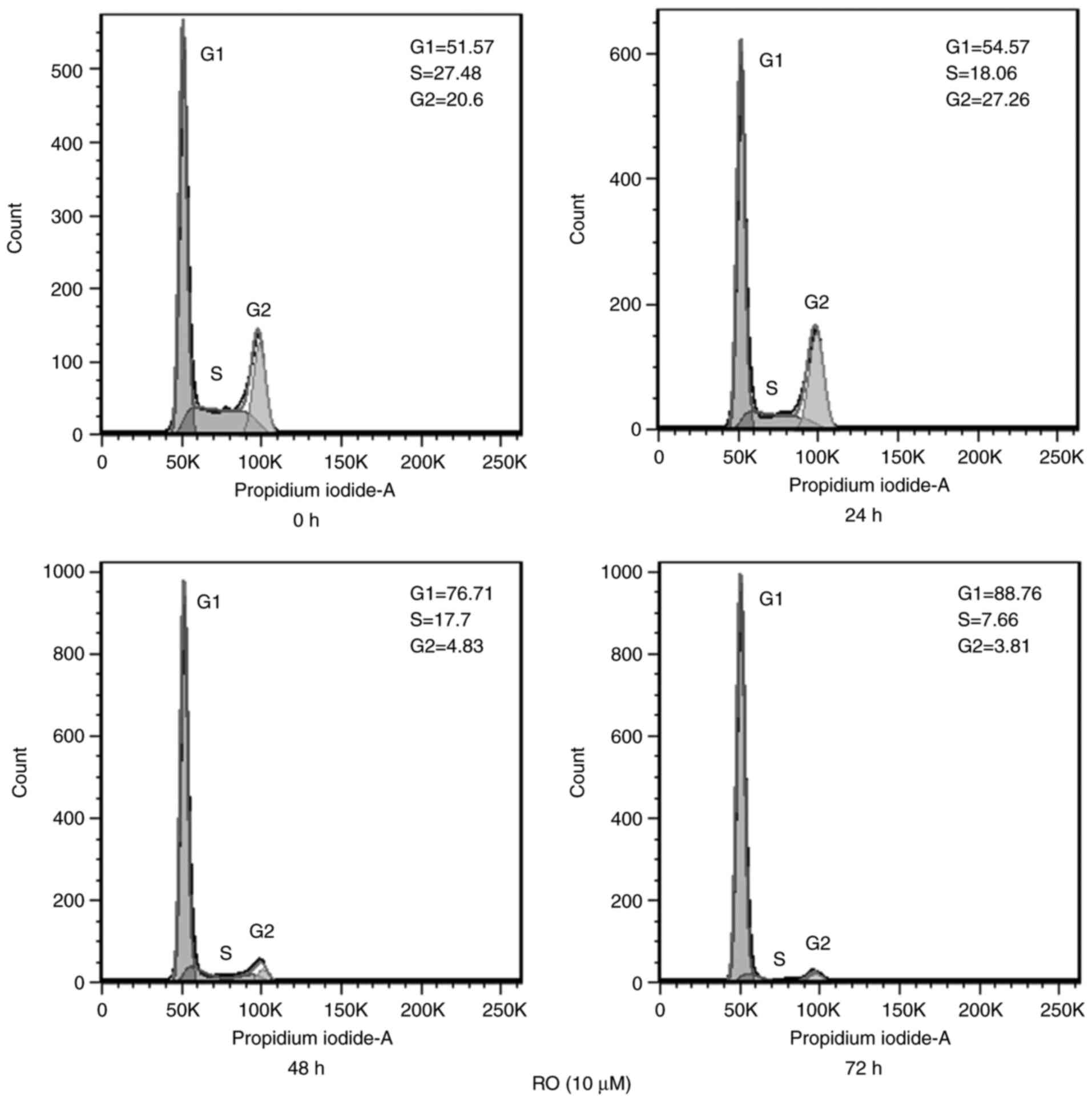
The influence of RO on the cell cycle phase
distribution of PANC-1 cells was determined via FCM after treatment
with 10 µM RO for different durations. It was identified that RO
treatment for 72 h increased G1 phase arrest from 51.57%
(0 h control treatment group) to 88.76%, and reduced the S and
G2 phase percentages from 27.48 and 20.6% to 7.66 and
3.81%, respectively (Fig. 2).
RO regulates the expression levels of
cell cycle-related genes and the phosphorylation levels of ERK and
JNK in PANC-1 cells
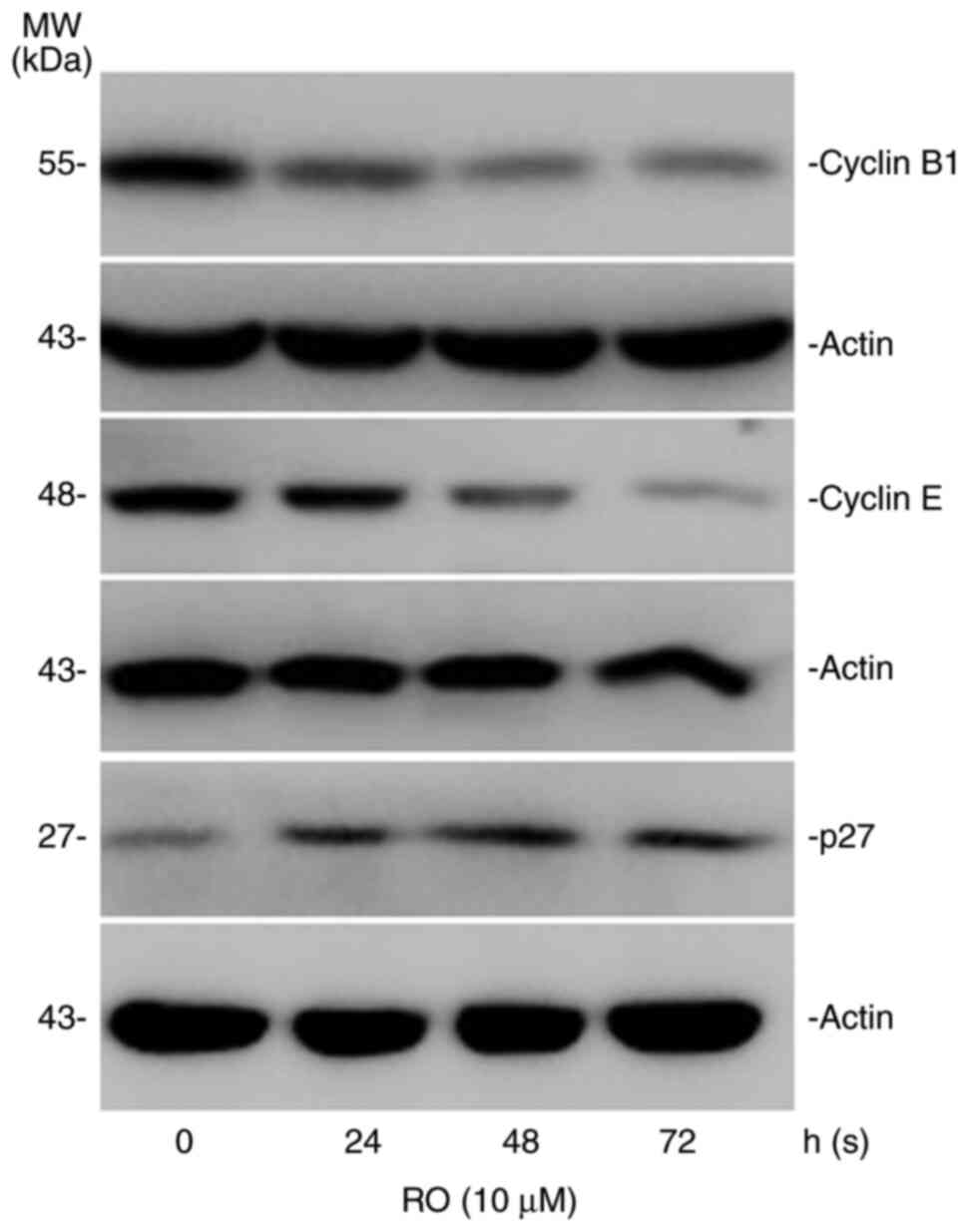
In order to understand how RO could cause cell cycle
arrest at the G1 phase, the expression of cell
cycle-related proteins, cyclin B1, cyclin E and p27 were measured
in RO-treated cells. The results suggested that RO treatment
decreased the expression levels of cyclin B1 and cyclin E (Fig. 3). However, RO increased the
expression levels of p27.
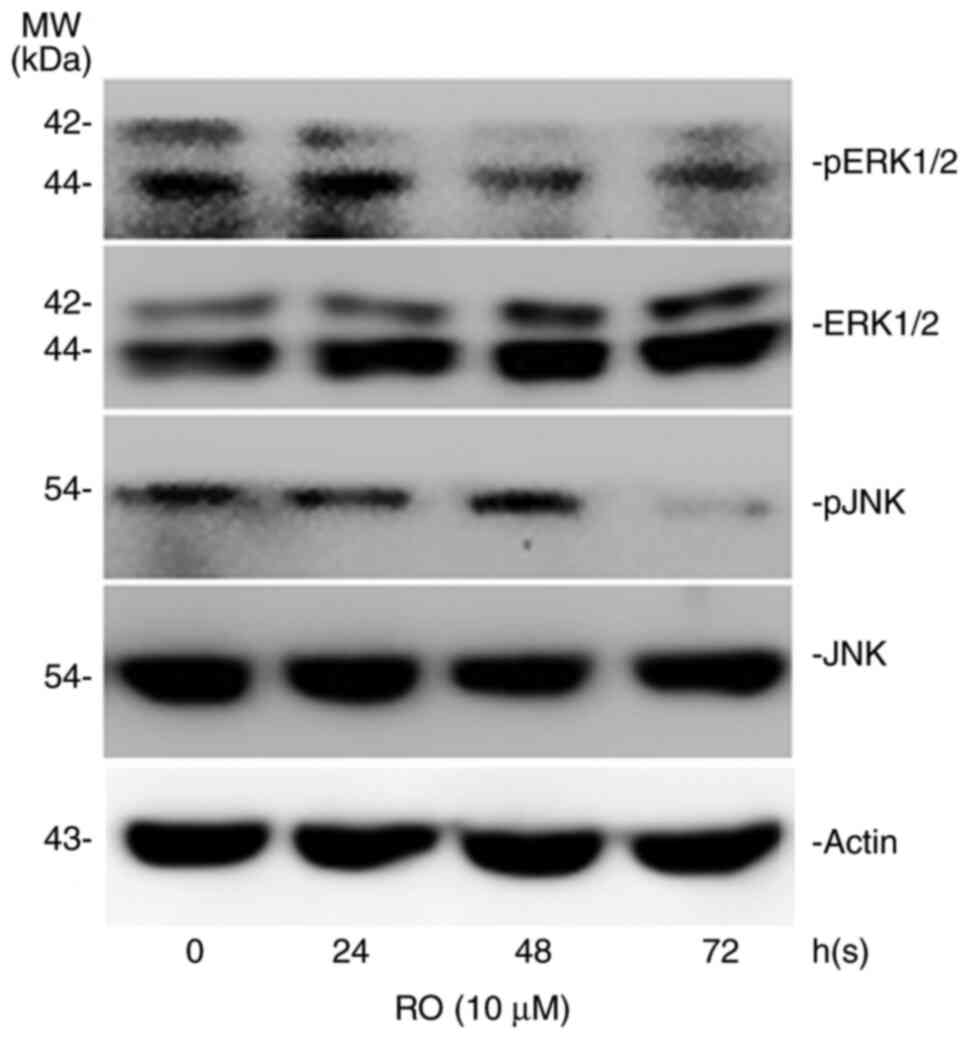
The MAPK signaling pathway serves an important role
in controlling cellular biological behavior, such as cell
proliferation, apoptosis and migratory potential (24). Thus, the phosphorylation levels of
ERK and JNK were determined to observe whether RO exerts its roles
via the ERK and JNK MAPK signaling pathway. It was found that RO
reduced the expression levels of pERK1/2 and pJNK in the PANC-1
cells, which also had a constant level of ERK1/2 and JNK expression
(Fig. 4).
RO inhibits the oncogenicity of PANC-1
cells in vivo
In order to further confirm the inhibitory effects
of RO on pancreatic cancer in vivo, a xenograft model of
subcutaneous tumor growth was established in nude mice. The
experimental mice were sacrificed by cervical dislocation after the
xenografts were treated with RO via intravenous injection for 3
weeks. The tumor volumes were measured every 3 days at day 0, 3, 6,
9, 12, 15, 18, 21 and 24 of RO injection, and the tumor volumes
shown as the ordinate are the average tumor volume of each mouse.
As presented in Fig. 5A-D, the
weights and volume of tumors formed by RO-treated PANC-1 cells were
significantly lower and smaller compared with those of the control
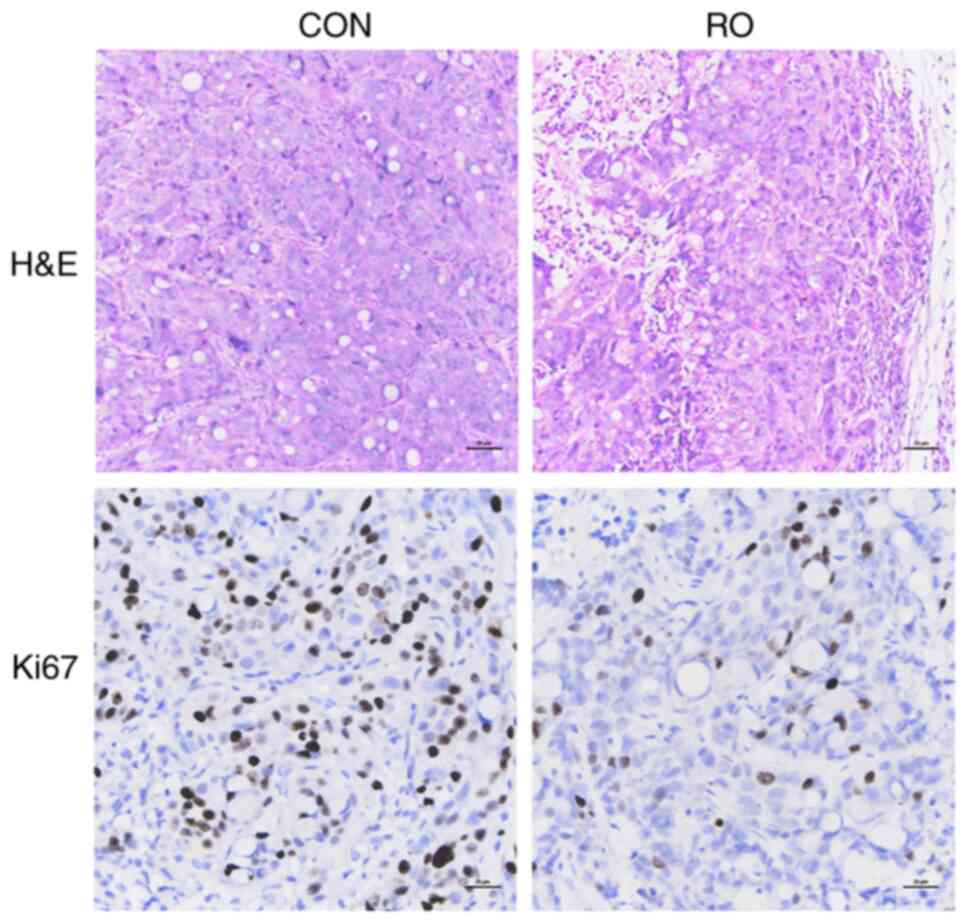
tumors. Moreover, Ki67-specific staining of the tumor tissue
sections from the xenograft animal model revealed that RO treatment
markedly inhibited cell proliferation in vivo, which was
demonstrated by a weaker staining of Ki67 when compared with the
control group (Fig. 6).
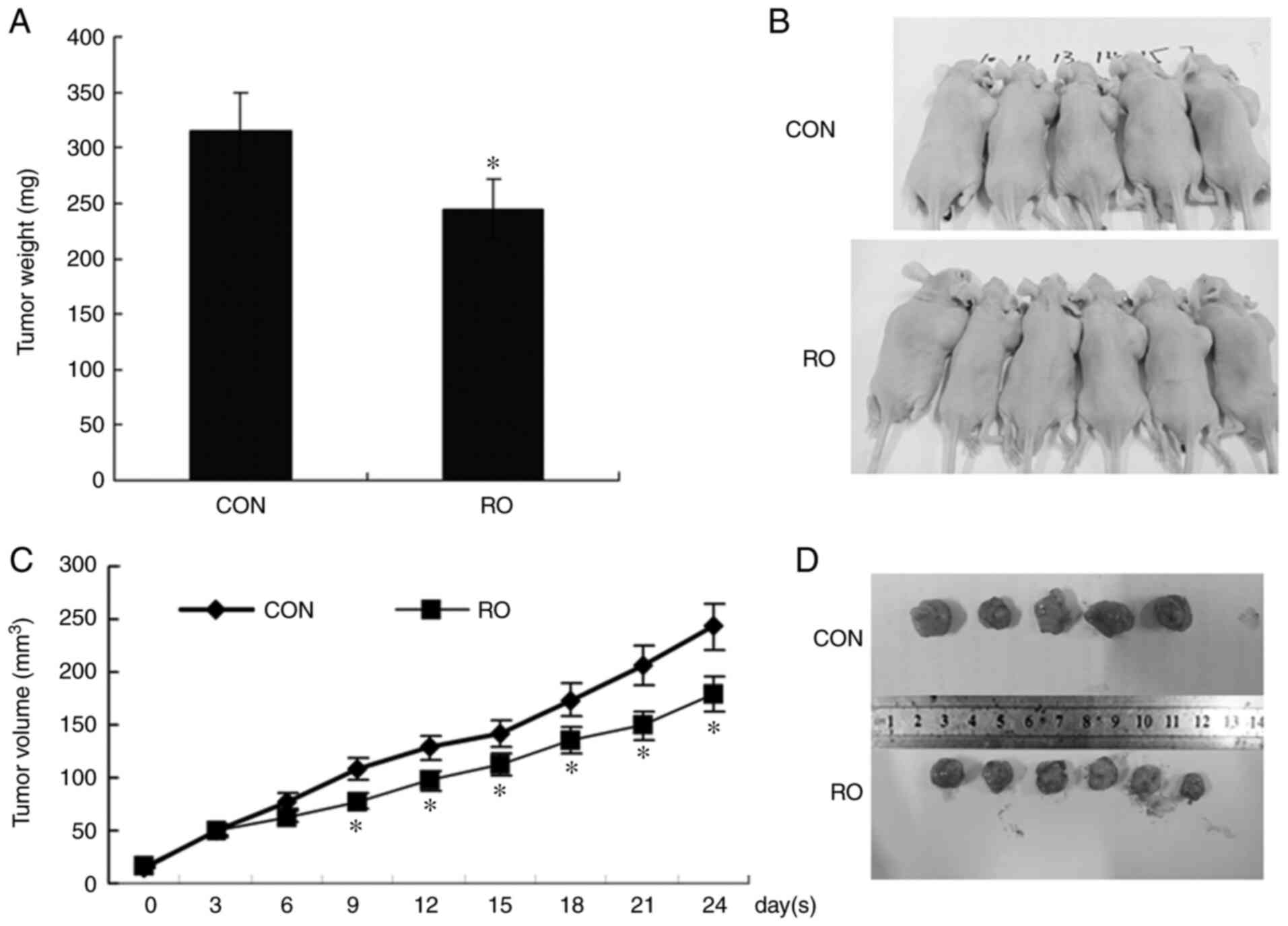 | Figure 5.RO inhibits tumor growth in nude
mice. An in vivo experiment was performed to examine the
inhibitory effect of RO on tumor growth. Male BALB/c nude mice were
inoculated subcutaneously in the right flanks with PANC-1 cells to
form xenograft tumors, followed by intravenous injection with RO or
DMSO (as CON). The tumor volumes were measured every 3 days at days
0, 3, 6, 9, 12, 15, 18, 21 and 24. (A) Tumors weights of the two
groups. (B) Representative images of tumor-bearing mice. (C) Tumor
volume analysis. The tumor volumes shown as the ordinate are the
average tumor volume of each mouse. (D) Resected xenograft tumors
from the two groups. *P<0.05 vs. CON group. CON, control; RO, RO
48-8071. |
Discussion
In total,~85,100 individuals died from pancreatic
cancer in 2016, which has become the third-leading cause of
cancer-associated mortality worldwide, with a 5-year overall
survival rate of 8% (4), mainly due
to a lack of efficacious methods to treat or control pancreatic
cancer. The reasons for the poor prognosis of pancreatic cancer
include uncontrolled and excessive proliferation, and early
invasion (3,5). The main strategy of tumor treatment is
to effectively suppress the malignant behaviors of tumor cells.
High levels of HMG-CoA reductase and LDLR have been
observed in a PDAC mouse model, and that LDLR-mediated activation
of the mevalonate pathway or cholesterol intake is associated with
the development of pancreatic cancer (10–12),
which suggests that cholesterol levels may influence the
development of PDAC. Cholesterol is important in the regulation of
cellular processes, such as cell proliferation, differentiation and
apoptosis (2,6,13). In
the process of synthesizing cholesterol from cytoplasmic
acetyl-CoA, HMG-CoA reductase catalyzes the conversion of HMG-CoA
to mevalonate, which is the rate-limiting step of the pathway
(14). Inhibitors of HMG-CoA
reductase can inhibit the proliferation of some cancer cells by
blocking cholesterol biosynthesis (15–17).
It has been shown that statins, which are inhibitors of HMG-CoA
reductase, can also be used to treat cancer (25). However, statins cause a shortage of
numerous downstream intermediate products, including farnesyl
pyrophosphate and geranylgeranyl diphosphate, which are involved in
the roles of isoprenoids, and membrane structure and function, and
thus produces considerable side effects (26). The conversion of
2,3-monoepoxysqualene to lanosterol by OSC is a key step that is
downstream of HMG-CoA reductase in the process of cholesterol
biosynthesis. Previously, RO, an inhibitor of OSC, has become a
possible novel target to inhibit cholesterol biosynthesis (17,18).
Furthermore, RO may cause fewer side effects than statins, as it
primarily only decreases the level of cholesterol, without
decreasing the levels of other intermediate products of the
mevalonate pathway (21).
It has been reported that RO 48-8071 has an
inhibitory effect on the proliferation of prostate and breast
cancer cells (20,21,27)
and leukemia cells (28). The
present study also demonstrated its inhibitory roles on pancreatic
cancer development and provided insights into the potential
molecular mechanisms of RO. Moreover, the current study found
notable inhibitory effects of RO on pancreatic cancer cells
(PANC-1) in vitro. It was also identified that RO markedly
inhibited cell proliferation in vivo, as shown by a lower
Ki67 expression in xenograft tissues.
Cyclin B and cyclin E, which are commonly known
factors involved in G1/S cell cycle-regulation, interact
with CDKs to stimulate the transition from the G1 phase
to the S phase, while p27 acts as a CDK inhibitor to inhibit the
activities of cyclin/CDK complexes (29). The overexpression of cyclin B and
cyclin E appear to be correlated with high tumor grade in breast
cancer (30). By detecting the cell
cycle and cell viability influenced by RO, the present study found
that RO caused G1 phase arrest and inhibited cell
viability by regulating the expression levels of the cell
cycle-related proteins p27, cyclin B1 and cyclin E. This was
consistent with previous findings concerning the action of RO on
other cancer cells (20,21). It is commonly known that cholesterol
is a component of the cell structure. A noteworthy hypothesis is
that RO causes a shortage of cholesterol, which consequently causes
the inhibition of cell viability (31). The present results revealed that RO
mediated the suppression of G1/S-CDK activity and the
activation of the CDK inhibitor p27, which caused replicative
arrest, and this could be one of the mechanisms of action of the
antitumor roles of RO.
The MAPK signaling pathways are widely known to be
involved in multiple cell behaviors and are associated with tumor
development. ERK is an important mitogen-activated proliferation
factor that can promote cell survival, whereas the JNK/MAPK pathway
serves key roles in apoptotic processes (32). The ERK and JNK/MAPK signaling
pathways are often overactivated in most types of cancer cells and
serve a key role in controlling cell proliferation, apoptosis and
motility (33,34). Cholesterol depletion on the membrane
surface can inhibit PI3K/Akt and ERK pathways in several types of
tumors (35). RO 48-8071 has been
reported to inhibit ERK phosphorylation in both HCT116 and HPAF-II
cells (23). The present
mechanistic studies revealed that RO reduced the expression level
of pERK1/2 and pJNK, which suggested that RO inactivated the ERK
and JNK signaling pathway in PANC-1 cells.
In conclusion, the present results demonstrated that
the anti-pancreatic cancer effects of RO occurred via the
regulation of the cell cycle to inhibit the viability of pancreatic
cancer cells. RO may exert these roles by regulating the expression
levels of cell cycle-related proteins, concomitantly with the
inhibition of the ERK and JNK pathway. A preliminary conclusion
could be drawn from the data obtained in the present study that RO
could be used as an antitumor reagent to limit the development of
pancreatic cancer. Due to its low side effects, RO may facilitate
the treatment of pancreatic cancer. The effects of RO on cell
viability and the cell cycle identified in the present study could
be due to either a RO-induced decreased in cholesterol level or
mechanisms that are independent of the cholesterol levels.
Moreover, RO could be used to exert a direct effect on tumor cells.
The major limitation of the present study is that only one cell
line was used. Further in-depth studies using multiple pancreatic
cancer cell lines are necessary to clarify and provide details on
the specific underlying mechanisms.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
General Program of National Natural Science Foundation of China
(grant no. 81271748) and the University Science Research Project of
Anhui Province (grant nos. KJ2020A0143 and KJ2017A195).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ShZ and YQ designed the study and revised the
manuscript. ZD and YG performed the majority of the experiments. DH
performed the histological examination and interpreted the data. YG
and SuZ analyzed and interpreted the data, and wrote and revised
the manuscript. HZ, TZ and XL analyzed and interpreted the data.
ShZ and YQ confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The animals involved in the present study were
reared according to the Animal Research Committee's guidelines of
Anhui Medical University (Hefei, China). The animal protocols were
approved by the Committee for Ethics of Animal Experimentation of
Anhui Medical University (approval no. 20150136).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xiao AY, Tan MLY, Wu LM, Asrani VM,
Windsor JA, Yadav D and Petrov MS: Global incidence and mortality
of pancreatic diseases: A systematic review, meta-analysis, and
meta-regression of population-based cohort studies. Lancet
Gastroenterol Hepatol. 1:45–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
American Cancer Society, . Cancer Facts
& Figures 2017. American Cancer Society; 2017
|
|
5
|
Makohon-Moore A and Iacobuzio-Donahue CA:
Pancreatic cancer biology and genetics from an evolutionary
perspective. Nat Rev Cancer. 16:553–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kleeff J, Korc M, Apte M, La Vecchia C,
Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH
and Neoptolemos JP: Pancreatic cancer. Nat Rev Dis Primers.
2:160222016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vaccaro V, Sperduti I and Milella M:
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med. 365:768–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morrison AH, Byrne KT and Vonderheide RH:
Immunotherapy and prevention of pancreatic cancer. Trends Cancer.
4:418–428. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guillaumond F, Bidaut G, Ouaissi M,
Servais S, Gouirand V, Olivares O, Lac S, Borge L, Roques J, Gayet
O, et al: Cholesterol uptake disruption, in association with
chemotherapy, is a promising combined metabolic therapy for
pancreatic adenocarcinoma. Proc Natl Acad Sci. 112:2473–2478. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Qin S, Wang M, Zhang T and Zhang
S: Association between cholesterol intake and pancreatic cancer
risk: Evidence from a meta-analysis. Sci Rep. 5:82432015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sumi S, Beauchamp RD, Townsend CM Jr,
Uchida T, Murakami M, Rajaraman S, Ishizuka J and Thompson JC:
Inhibition of pancreatic adenocarcinoma cell growth by lovastatin.
Gastroenterology. 103:982–989. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neoptolemos JP, Palmer DH, Ghaneh P,
Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA,
Cunningham D, Wadsley J, et al: European study group for pancreatic
cancer. Comparison of adjuvant gemcitabine and capecitabine with
gemcitabine monotherapy in patients with resected pancreatic cancer
(ESPAC-4): A multicentre, open-label, randomised, phase 3 trial.
Lancet. 389:1011–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ikonen E: Cellular cholesterol trafficking
and compartmentalization. Nat Rev Mol Cell Biol. 9:125–138. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cuccioloni M, Bonfili L, Mozzicafreddo M,
Cecarini V, Scuri S, Cocchioni M, Nabissi M, Santoni G, Eleuteri AM
and Angeletti M: Mangiferin blocks proliferation and induces
apoptosis of breast cancer cells via suppression of the mevalonate
pathway and by proteasome inhibition. Food Funct. 7:4299–4309.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeganeh B, Wiechec E, Ande SR, Sharma P,
Moghadam AR, Post M, Freed DH, Hashemi M, Shojaei S, Zeki AA and
Ghavami S: Targeting the mevalonate cascade as a new therapeutic
approach in heart disease, cancer and pulmonary disease. Pharmacol
Ther. 143:87–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato S, Liberona MF, Cerda-Infante J,
Sánchez M, Henríquez J, Bizama C, Bravo ML, Gonzalez P, Gejman R,
Brañes J, et al: Simvastatin interferes with cancer ‘stem-cell’
plasticity reducing metastasis in ovarian cancer. Endocr Relat
Cancer. 25:821–836. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Charlton-Menys V and Durrington PN:
Squalene synthase inhibitors: Clinical pharmacology and
cholesterol-lowering potential. Drugs. 67:11–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morand OH, Aebi JD, Dehmlow H, Ji YH,
Gains N, Lengsfeld H and Himber J: RO 48-8.071, a new
2,3-oxidosqualene: Lanosterol cyclase inhibitor lowering plasma
cholesterol in hamsters, squirrel monkeys, and minipigs: Comparison
to simvastatin. J Lipid Res. 38:373–390. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang Y, Mafuvadze B, Aebi JD and Hyder
SM: Cholesterol biosynthesis inhibitor RO 48-8071 suppresses growth
of hormone-dependent and castration-resistant prostate cancer
cells. Onco Targets Ther. 9:3223–3232. 2016.PubMed/NCBI
|
|
21
|
Liang Y, Besch-Williford C, Aebi JD,
Mafuvadze B, Cook MT, Zou X and Hyder SM: Cholesterol biosynthesis
inhibitors as potent novel anti-cancer agents suppression of
hormone-dependent breast cancer by the oxidosqualene cyclase
inhibitor RO 48-8071. Breast Cancer Res Treat. 146:51–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gopalan A, Yu W, Sanders BG and Kline K:
Simvastatin inhibition of mevalonate pathway induces apoptosis in
human breast cancer cells via activation of JNK/CHOP/DR5 signaling
pathway. Cancer Lett. 329:9–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maione F, Oliaro-Bosso S, Meda C, Di
Nicolantonio F, Bussolino F, Balliano G, Viola F and Giraudo E: The
cholesterol biosynthesis enzyme oxidosqualene cyclase is a new
target to impair tumour angiogenesis and metastasis dissemination.
Sci Rep. 5:90542015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garcia-Ruiz C, Morales A and
Fernandez-Checa JC: Statins and proteinprenylation in cancer cell
biology and therapy. Anticancer Agents Med Chem. 12:303–315. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McTaggart SJ: Isoprenylated proteins. Cell
Mol Life Sci. 63:255–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grinter SZ, Liang Y, Huang SY, Hyder SM
and Zou X: An inverse docking approach for identifying new
potential anti-cancer targets. J Mol Graph Model. 29:795–799. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vilimanovich U, Bosnjak M, Bogdanovic A,
Markovic I, Isakovic A, Kravic-Stevovic T, Mircic A, Trajkovic V
and Bumbasirevic V: Statin-mediated inhibition of cholesterol
synthesis induces cytoprotective autophagy in human leukemic cells.
Eur J Pharmacol. 765:415–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaldis P and Aleem E: Cell cycle sibling
rivalry: Cdc2 vs. Cdk2. Cell Cycle. 4:1491–1494. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan L, Cao X, Yan H, Wang Q, Tian X, Zhang
L, He X, Borjihan G and Morigen: The synthetic antihyperlipidemic
drug potassium piperate selectively kills breast cancer cells
through inhibiting G1-S-phase transition and inducing apoptosis.
Oncotarget. 8:47250–47268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Sukumaran P, Varma A, Derry S,
Sahmoun AE and Singh BB: Cholesterol-induced activation of TRPM7
regulates cell proliferation, migration, and viability of human
prostate cells. Biochim Biophys Acta. 1843:1839–1850. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen X, Cui X, Cui H, Jin Y, Jin W and Sun
H: Geraniol and lupeol inhibit growth and promote apoptosis in
human hepatocarcinoma cells through the MAPK signaling pathway. J
Cell Biochem. 120:5033–5041. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu X, Yang Q, Yan J, Zhang X and Zheng M:
LncRNA MNX1-AS1 promotes the progression of cervical cancer through
activating MAPK pathway. J Cell Biochem. 120:4268–4277. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fang Z, Tang Y, Fang J, Zhou Z, Xing Z,
Guo Z, Guo X, Wang W, Jiao W, Xu Z and Liu Z: Simvastatin inhibits
renal cancer cell growth and metastasis via AKT/mTOR, ERK and
JAK2/STAT3 pathway. PLoS One. 8:e628232013. View Article : Google Scholar : PubMed/NCBI
|