Introduction
Endometrial cancer (EC) is the sixth most commonly
diagnosed cancer worldwide (1). It
has been estimated that the incidence of EC in China will increase
twofold by 2030 to reach >120,000 cases (2).
Treatments for EC include radiotherapy,
chemotherapy, surgery, hormone and molecular targeted therapy and
immune-checkpoint inhibitors (3).
The prognosis of EC is poor, so developing safe and efficacious
treatments is key to improve patient outcomes.
Paclitaxel (PTX) is an anti-cancer drug isolated and
extracted from yew trees that is used in the treatment of breast
and ovarian cancer, EC and other types of disease (4,5). PTX
kills dividing cancer cells by stabilizing microtubules of mitotic
spindles (6) but PTX is also highly
toxic to healthy cells in the human body (7). Improving the anti-tumor effects of
drugs and decreasing their systemic side effects is important.
Human epidermal growth factor receptor-2, also known
as C-erbB-2, is a transmembrane tyrosine kinase receptor
(8). C-erbB-2 is expressed
at low levels in the epithelial cells of most organs in healthy
human tissue and at slightly higher levels in fetal tissue
(9,10). Studies have shown that
C-erbB-2 is overexpressed in various types of tumor, such as
EC, breast cancer, ovarian cancer, stomach and lung cancer
(11–14) and its overexpression is associated
with proliferation of tumor cells. Zhou et al (15) note that high expression of
C-erbB-2 is closely related to the prognosis of ovarian
cancer; Li et al (16)
suggest that C-erbB-2 is a potential therapeutic target for
lung cancer and Erickson et al (17) state that C-erbB-2 is also an
effective potential therapeutic target in endometrial cancer.
Therefore, directly knocking out C-erbB-2 to explore the
effect on the endometrium is a feasible method
Gene-level editing is based on clustered regularly
interspaced short palindromic repeats (CRISPR)/CRISPR-associated
protein 9 (Cas9) technology. CRISPR technology was first used in
Escherichia coli (18,19).
CRISPR enables correction of errors in the genome and gene
regulation in cells and organisms to be performed rapidly, cheaply
and with relative ease (20). Guide
(g)RNA matches a desired target gene and Cas9 (an endonuclease)
causes a double-stranded DNA break, thereby allowing modifications
to the genome (21). The
CRISPR/Cas9 system is the most powerful gene-editing method
worldwide (22) but its safe and
efficient use in the human body is a major challenge (23).
Ultrasound non-invasively controls the release of
drugs and carriers wrapped in or around gas-filled microbubbles
(MBs) (24). Ultrasound also
induces cavitation effects, which can produce transient pores in
cell membranes. This increases cell permeability and enhances the
efficiency of drug delivery (25,26).
The surface of cationic (C) gas-filled MBs has a positive charge,
allowing effective combination with negatively charged plasmid DNA
to increase the loading rate of plasmids (27). Hence, CMBs are used to deliver drugs
or genes.
In the present study, the EC cell line HEC-1A was
cultured in vitro. CMB were used to carry PTX and the
CRISPR/Cas9 gene-targeted editing system under ultrasonic
irradiation. The present study aimed to explore the interference
effect of C-erbB-2 knockout by CRISPR/Cas9-PTX-CMB on
endometrial cancer cells.
Materials and methods
Cell culture
Wuhan Procell Life Technology (Wuhan, China)
provided the human EC cell line (HEC-1A). The HEC-1A cell line was
supplemented with 10% fetal bovine serum (Biosharp Life Sciences)
and 5% penicillin-streptomycin (Biosharp Life Sciences). Cells were
cultured (37°C; 5% CO2) in complete medium [DMEM (Gibco;
Thermo Fisher Scientific, Inc.) + 10% fetal bovine serum (Biosharp
Life Sciences) + 5% penicillin-streptomycin (Biosharp Life
Sciences)] lacking bacteria, yeasts, fungi or
mycoplasma.
Construction of primers and
plasmids
The sequences of three candidate gRNAs targeting
C-erbB-2 were selected. For gRNA1, target 1 was
5′-TCATCGCTCACAACCAAGTG-3′ and target 2 was
5′-CAGGGGTGGTATTGTTCAGC-3′. For gRNA2, target 1 was
5′-TCATCGCTCACAACCAAGTG-3′ and target 2 was
5′-CGGGTCTCCATTGTCTAGCA-3′. For gRNA3, target 1 was
5′-CGCTCACAACCAAGTGAGGC-3′ and target 2 was
5′-ACAGGGGTGGTATTGTTCAG-3′.
These sequences were cloned into the pGE-5 plasmid
encoding Cas9 and the gRNA scaffold. The empty pGE-5 plasmid
(Shanghai GenePharma Co., Ltd.) was designed as a negative control.
Synthesis was performed by Shanghai GenePharma Co., Ltd. The pGE-5
plasmid contained enhanced green fluorescent protein, ampicillin,
puromycin-resistance genes and other markers, to aid selection of
the best gRNA sequence and screen out successfully transfected
cells.
Screening for the best targeting
C-erbB-2-knockout gRNA
HEC-1A cells were inoculated in a 6-well plate
(4×105/well) and large petri dish
(1×106/dish). Then, 2.5 ml complete medium [DMEM (Gibco;
Thermo Fisher Scientific, Inc.) + 10% fetal bovine serum (Biosharp
Life Sciences) + 5% penicillin-streptomycin (Biosharp Life
Sciences)] was added to each well of the 6-well plate and 10 ml
complete medium was added to the large dish in a 37°C incubator.
Culture was performed for 24 h until cells adhered to the wall and
confluence reached 70%. Cells were washed once with
phosphate-buffered saline (PBS) before transfection. A total of 2.5
ml Opti-MEM Reduced-Serum Medium (Gibco; Thermo Fisher Scientific,
Inc.) was added each well of the 6-well plate and 5 ml Opti-MEM
Reduced-Serum Medium (Gibco; Thermo Fisher Scientific, Inc.) was
added to the large dish, followed by incubation at 37°C for 2 h.
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) was
employed to transfect different CRISPR/Cas9 plasmids into HEC-1A
cells according to the manufacturer's instructions.
Lipofectamine® 3000 6 µl or 18 µl (Thermo Fisher
Scientific, Inc.) and gRNA plasmid 2 µg or 9 µg and P3000 4 µl or
18 µl (Thermo Fisher Scientific, Inc.) were mixed gently and added
to a 6-well plate (Lipofectamine® 3000 6 µl+ gRNA
plasmid 2 µg + P3000 4 µl) or a large dish
(Lipofectamine® 3000 18 µl + gRNA plasmid 9 µg + P3000
18 µl) for cell growth after standing at room temperature for 15
minutes. The cells were placed in a 37°C incubator with 5%
CO2 for transfection for 6 h and then the medium in the
6-well plate and the large dish was changed to complete medium, the
cells continued to be cultured in an incubator at 37°C with 5%
CO2, Images were captured using a fluorescence
microscope (cat. no. BX51; Olympus Corporation) after 24 h and 48
h. After capturing the 48 h image, RNA from cells in the 6-well
plate was collected for reverse transcription-quantitative
(RT-q)PCR. The total protein from cells in the large dish was
collected for western blotting to measure the expression of
C-erbB-2 protein and verify the knockout efficiency of each
group of plasmids.
CMB construction
Stearoyl phosphatidylcholine (Avanti Polar Lipids,
Inc.), (2,3-dioleoyl-propyl) trimethylammonium chloride (Avanti
Polar Lipids) and polyoxyethylene stearate (Shanghai Trustin
Chemical Co., Ltd.) were weighed accurately and used at a molar
ratio of 70:16:14. Reagents were dissolved in chloroform and the
organic solvent was removed by rotary evaporation under reduced
pressure following uniform mixing. The mixture was placed in an
ultrasonic water bath at 60°C for 15 min to obtain a lipid
suspension. The suspension was divided into vials, filled with
sulfur hexafluoride (SF6) gas and shaken mechanically
for 1 min to obtain CMB.
Construction of PTX-loaded CMB
Stearoyl phosphatidylcholine, trimethyl ammonium
chloride and polyoxyethylene stearate were weighed accurately and
used at a molar ratio of 70:16:14. Reagents were dissolved in
chloroform. PTX dissolved in methanol (1 mg/ml) was added.
Following uniform mixing, the organic solvent was removed by rotary
evaporation under reduced pressure. The lipid film containing PTX
was added to a buffer solution. A PTX-containing lipid suspension
was obtained following placement in an ultrasonic bath at 60°C for
15 min. After dispensing in vials, the suspension was filled with
SF6 gas and shaken mechanically for 1 min to obtain
membrane shells containing PTX (i.e., PTX-CMB).
Physical measurement of CMB and
PTX-CMB
CMB were observed under an optical microscope to
detect their morphology and distribution. The particle size and
surface potential were detected by a laser particle size and
surface potential detector (Malvern Instruments).
Combination and identification of
CRISPR/Cas9 with CMB
CMB and PTX-CMB (both 160 ml) were collected and 20
ml 3,3′-dioctadecyloxacarbocyanine perchlorate fluorescent dye
(Dio; 7.561 mM) was added. After mixing and incubation at room
temperature for 15 min, 40 mg plasmid was added and a Cy3
nucleic-acid labeling kit (Label IT® Tracker™
Intracellular Nucleic Acid Localization kit; Mirus Bio, LLC) was
used for labeling. CMB, PTX-CMB and plasmids were mixed thoroughly
and incubated at 4°C for 20 min. The binding of MB and plasmids was
observed under a confocal microscope (magnification, ×600).
MB transfection
To prepare MB-plasmid mixture, 10 µg plasmid and
1.14×109 MB was added to a sterile centrifuge tube,
followed by gentle blowing with a pipette tip to aid mixing. The
mixture was allowed to stand for 20 min at 4°C. For cell
preparation, 2×105 cells were added to a 6-well plate
with a sterile 22×22 mm cover glass at the bottom and then placed
in a 37°C incubator for 24 h. After 24 h, the original medium [DMEM
(Gibco; Thermo Fisher Scientific, Inc.) + 10% fetal bovine serum
(Biosharp Life Sciences) + 5% penicillin-streptomycin (Biosharp
Life Sciences)] was aspirated and washed with PBS. Tweezers were
used to place the coverslip on the cover of the 6-well plate with
the cell surface facing the liquid surface. Complete medium (as
aforementioned) was added to each well of the 6-well plate until
the liquid overflowed. The MB-plasmid mixture was added to the
upper layer and placed on the 6-hole plate cover. A small
ultrasonic instrument was used to sonicate MBs and cells at the
bottom of the 6-well plate. The sonication conditions were 1 MHz at
0.75 W/cm2 for 30 sec. The CMB and PTX-CMB group were
sonicated under identical conditions. For the group carrying PTX
and CRISPR/Cas9 transfection (CRISPR/Cas9-PTX-CMB), the cells were
cultured at 37°C for 24 h, and a Puro drug sieve was used for 7-day
screening. The expanded culture was used for subsequent
experiments.
RT-qPCR
An RNA extraction kit (Qiagen GmbH) was used to
extract RNA from HEC-1A cells in the CMB, PTX, PTX-CMB,
CRISPR/Cas9-CMB and CRISPR/Cas9-PTX-CMB groups. RT-qPCR was
performed using a RevertAid First Strand cDNA Synthesis kit (Thermo
Fisher Scientific, Inc.; 42°C for 60 min, then 25°C for 5 min, then
42°C for 60 min and 70°C for 5 min.) and PowerUp SYBR Green Master
Mix (Applied Biosystems) to determine cellular mRNA levels of
C-erbB-2, mechanistic target of rapamycin (mTOR),
Bcl-2 associated death promoter (Bad), P27 and
P21 in each group. The primer sequence of each gene is as
follows: C-erbB-2: F 5′-ACCCAGCTCTTTGAGGACAA-3′ R
5′-ATCGTGTCCTGGTAGCAGAG-3′; mTOR: F
5′-CCTGCCTTTGTCATGCCTTT-3′ R 5′-CTGGGTTTGGATCAGGGTCT-3′;
Bad: F 5′-GAAGACTCCAGCTCTGCAGA-3′ R
5′-CATCCCTTCGTCGTCCTCC-3′; P27: F 5′-AGGAACTCGACTCAGACGTG-3′
R 5′-TATTTGGAGGCACAGCAGGA-3′; P21: F
5′-GCCCAGTGGACAGCGAGCAG-3′ R 5′-GCCGGCGTTTGGAGTGGTAGA-3′;
β-actin (Reference gene): F 5′-AAGGATTCCTATGTGGGCGAC-3′ R
5′-CGTACAGGGATAGCACAGCC-3′. Thermal cycling conditions were: 50°C
for 2 min, 95°C for 2 min, then 40 cycles of 95°C for 15 sec, 60°C
for 15 sec, and 72°C for 1 min. The method of quantification was
the 2−ΔΔCq method (28).
Western blotting
RIPA (Beyotime Institute of Biotechnology) lysis
solution [200 µl; containing 2 µl PMSF (Beyotime Institute of
Biotechnology) + 2 µl Cocktail (Cell Signaling Technology, Inc.)]
was added to the cell pellets of each group (CMB, PTX, PTX-CMB,
CRISPR/Cas9-CMB and CRISPR/Cas9-PTX-CMB groups) to lyse the cells.
The cells were dispersed and mixed well and then placed on ice for
30 min lysis. Following centrifugation at 12,000 × g for 10 min at
4°C, the supernatant was aspirated. The protein concentration was
determined by a bicinchoninic acid kit (Beyotime Institute of
Biotechnology). Total protein (30 µg/well) was separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis using 8% gels
and transferred to polyvinylidene fluoride (PVDF) membranes. After
fixing with anhydrous methanol at room temperature for 10 sec, PVDF
membranes were washed with TBST(0.05% Tween-20) and blocked with
TBST (0.05% Tween-20) containing 3% bovine serum albumin(Beyotime
Institute of Biotechnology) at room temperature for 1 h. PVDF
membranes were incubated with anti-C-erbB-2 monoclonal
antibody (Abcam; cat. no. ab16901, 1:1,000) and
anti-β-actin-monoclonal antibody (Cell Signaling Technology,
Inc.; cat. no. 4970s, 1:1,000) overnight at 4°C, washed three times
with TBST, and incubated with horseradish peroxidase-labeled
secondary antibody (Abcam; cat. no. ab205719, 1:10,000) and
β-actin secondary antibody(Cell Signaling Technology, Inc.;
cat. no. 7074P2, 1:2,000) for 1 h at room temperature on a shaker.
at room temperature for 1 h on a shaker. After washing three times
with TBST, bands were visualized using chemiluminescent reagents (A
and B solution; Immobilon Western; Sigma-Aldrich; Merck KGaA) in a
dark room and analyzed using ImageJ (National Institutes of Health;
v1.50i).
Cell scratching and healing
Each cell group (CMB, PTX, PTX-CMB, CRISPR/Cas9-CMB
and CRISPR/Cas9-PTX-CMB groups) was cultured in a Medium petri dish
containing complete culture medium as aforementioned for 24 h at
37°C and 5% CO2. The cells were trypsinized and
centrifuged at 80 × g for 3 min at room temperature. After
aspirating the supernatant, 1 ml serum-free medium [DMEM (Gibco;
Thermo Fisher Scientific, Inc.) + 5% penicillin-streptomycin
(Biosharp Life Sciences)] was used to resuspend the cells. Then,
190 µl serum-free medium was added to 10 µl resuspended cells,
followed by thorough mixing. Then, 10 µl cell suspension was
removed for counting. The density of HEC-1A cells was maintained at
7×105/ml. Next, 70 µl cell suspension was placed in the
wound-healing inserts (ibidi GmbH) and cultured in a 37°C incubator
until the cells in the chambers on both sides of the wound-healing
inserts covered the field of view. The scratch cell was pulled out
vertically and the cells washed gently with PBS. Then, 1 ml
serum-free medium was added and culturing allowed to continue.
Images were recorded under a light microscope (magnification, ×100)
every 4 h and stopped when the cells began to fuse. ImageJ
(National Institutes of Health, v1.50i) was used to measure the
healing area of each group of cells.
Clone formation
From each group (CMB, PTX, PTX-CMB, CRISPR/Cas9-CMB
and CRISPR/Cas9-PTX-CMB groups) cells in the logarithmic growth
phase were collected and inoculated in a 6-well plate at
1×103 cells/well. Then, 2 ml complete medium was added
and changed every 3 days. Cells were cultured for 7 days in an
incubator at 37°C. The culture was stopped when a clonal population
of cells was visible to the naked eye. The medium was aspirated,
washed with PBS and cells were fixed at room temperature with 4%
paraformaldehyde for 20 min. After aspirating the 4%
paraformaldehyde, 1 ml 10% Giemsa stain was added to each well at
room temperature for 5 min and images were captured. In each group,
the number of clones was manually counted in four randomly selected
fields of view under a light microscope (magnification, ×40);
>50 cells was considered as one colony.
Cell invasion
Matrigel was spread at 37°C on a 24-well plate
chamber (membrane pore size: 8 µm) and aspirated after 1 h.
Opti-MEM Reduced-Serum Medium (Gibco; Thermo Fisher Scientific,
Inc.) was used to resuspend 1.5×105 cells in each group
(CMB, PTX, PTX-CMB, CRISPR/Cas9-CMB and CRISPR/Cas9-PTX-CMB groups)
into the upper chamber and complete medium was added to the lower
chamber. Following 36 h culture at 37°C, the medium in the upper
chamber was removed and washed three times with PBS. After removing
the medium in the lower chamber, cells were fixed at room
temperature with 4% paraformaldehyde for 30 min. Giemsa solution
(10%; 600 µl) was added for staining at room temperature for 10
min. A total of 4 randomly selected fields of view were observed
under a light microscope(×200) and images were captured.
Cell Counting Kit (CCK)-8 assay
CCK-8 (Beyotime Institute of Biotechnology) was used
to measure the viability and proliferation of cells. A total of
2×103 cells/well of each group (CMB, PTX, PTX-CMB,
CRISPR/Cas9-CMB and CRISPR/Cas9-PTX-CMB groups) were inoculated
into 96-well plates. Three replicate wells were set up for each
group and each time point. There were 75 wells in total, 15 holes
in each group and 3 holes were used per group per day). After the
cells were cultured at 37°C for 24 h, 3-wells were selected for
each group. The cells were washed once with PBS, then 10 µl of
CCK-8 reagent was added and the cells were cultured at 37°C for 2
h. Then, a microplate reader (Gene Company Limited) was used to
measure the absorbance of the cells at 450 nm this was recorded as
Day 1. After the cells were cultured at 37°C for 48 h, 3-wells were
selected for each group again, rinsed with PBS, CCK-8 reagent added
and cultured at 37°C for 2 h. Then, a microplate reader (Gene
Company Limited) was used to measure the absorbance of the cells at
450 nm and recorded as Day 2. These steps were repeated until the
fifth day of measurement.
Statistical analysis
The cell invasion and clone formation were repeated
four times, the other experiments were repeated three times. Data
are presented as the mean ± standard deviation. Statistical
analysis were performed using SPSS 24.0 (IBM Corp.). Differences
between multiple groups were assessed using independent samples
Kruskal-Wallis test followed by Dunn's post hoc test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
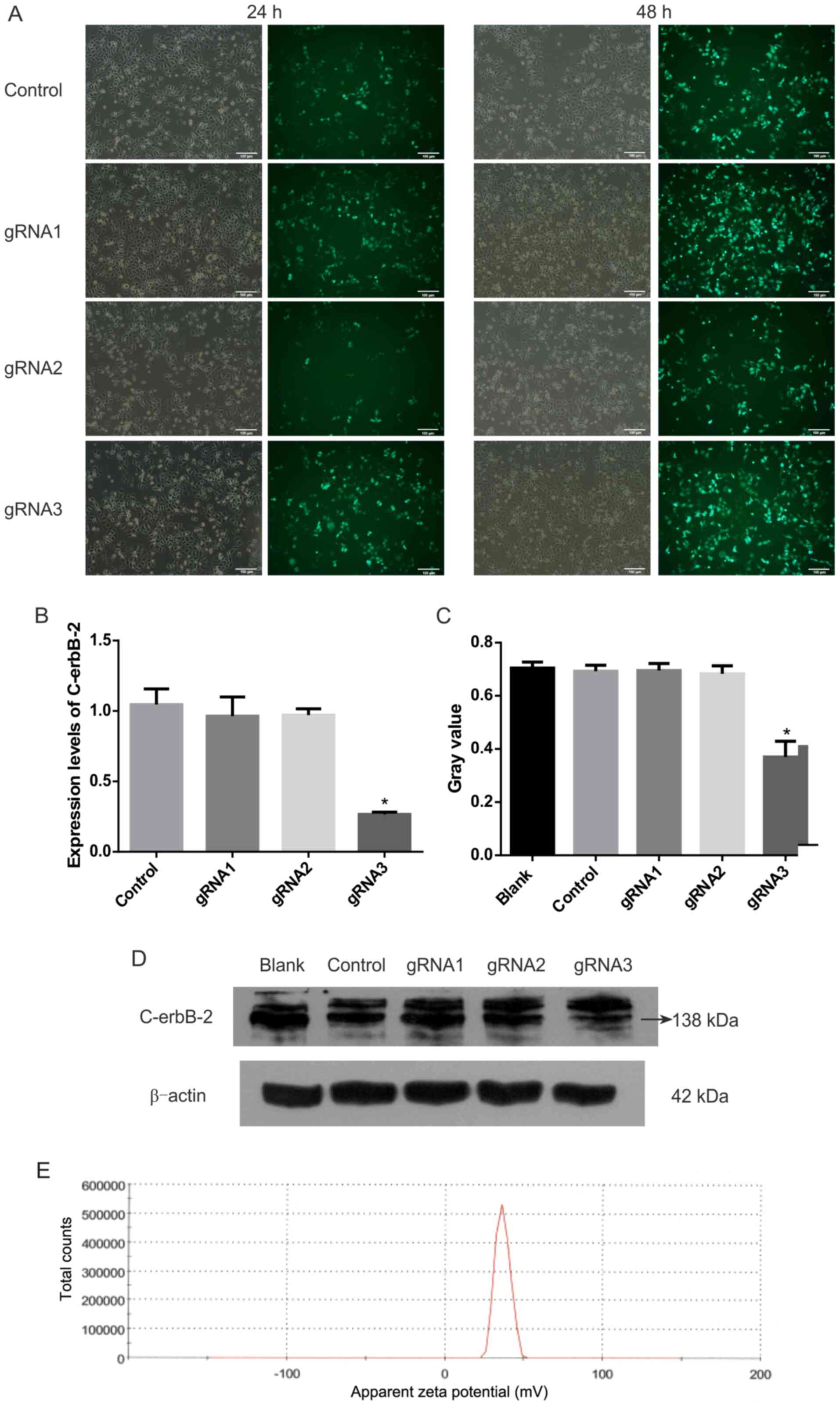
gRNA3 sequence has the highest
efficiency in knocking out C-erbB-2
After three gRNA sequences were transfected into
HEC-1A cells, the expression levels of C-erbB-2 and protein
significantly decreased (Fig. 1).
gRNA3 was the best sequence to knock out C-erbB-2 among the
three gRNA sequences tested.
CMBs and PTX-CMBs are positively
charged on the surface
The particle size of CMBs was 1.213 µm and that of
PTX-CMBs was 1.970 µm. The surface potential of CMBs was 36.70 mV
(Fig. 1E) and that of PTX-CMBs was
20.41 mV (Fig. 2A). The
concentration of CMBs and PTX-CMBs was calculated using a cell
counter (CMBs, 28.38×109/ml; PTX-CMBs,
7.84×108/ml). Compared with CMB, the positive surface
potential of PTX-CMBS decreased slightly and the particle size
increased.
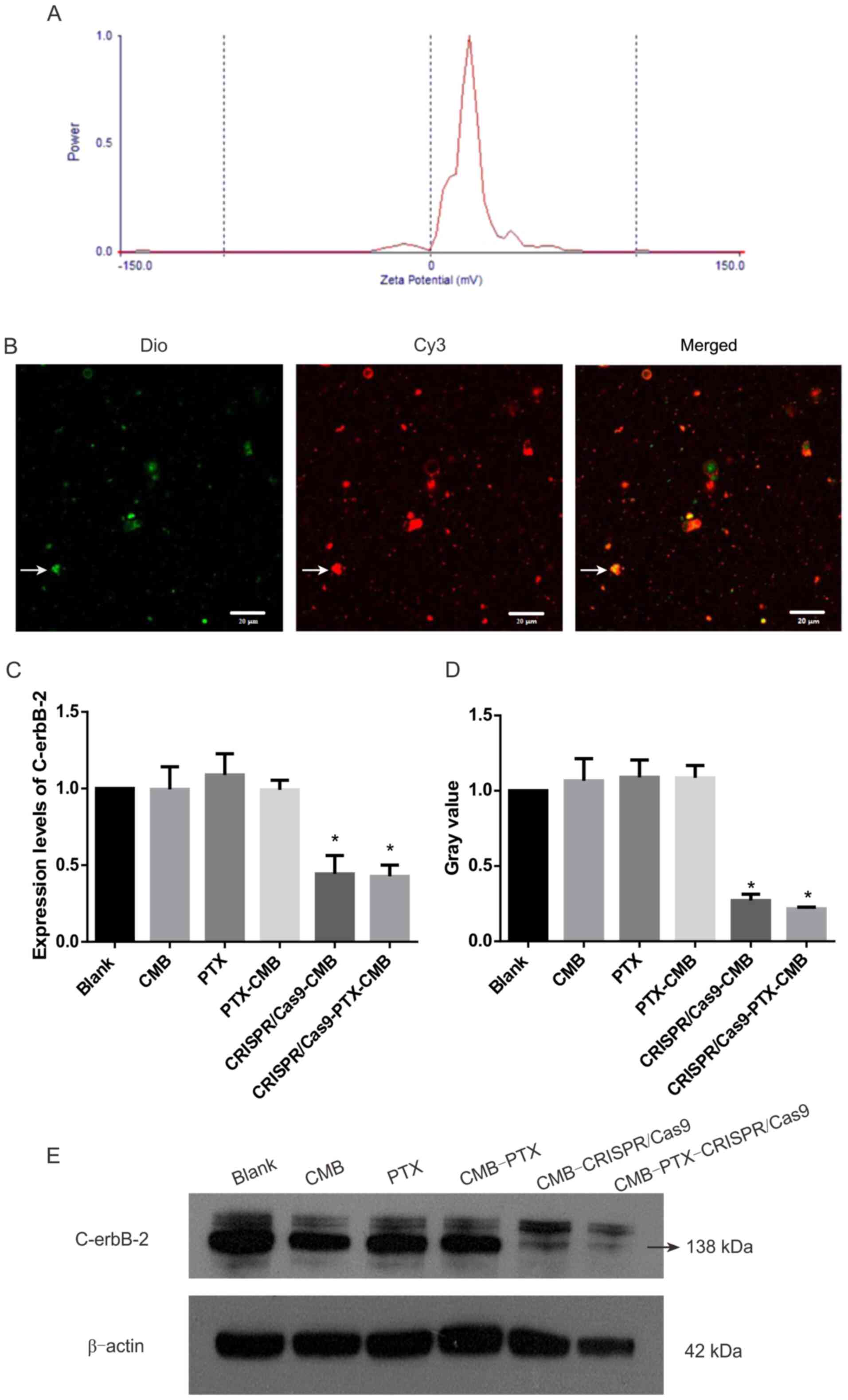
Preparation and in vitro
identification of CRISPR/Cas9-CMB
CMBs (160 µl) and Cy3-labeled Cas9 plasmids (40 µg)
were incubated for 20 min. Under a fluorescence microscope, green
MBs stained with Dio and Cy3-labeled Cas9 plasmids were observed
(Fig. 2B). These data confirmed the
effective combination of CMBs and plasmids.
CRISPR/Cas9-CMB and
CRISPR/Cas9-PTX-CMB groups can effectively knock out C-erbB-2
Expression of C-erbB-2 protein in the
CRISPR/Cas9-CMB and CRISPR/Cas9-PTX-CMB group decreased
significantly compared with that in the other groups (Fig. 2D-E). This confirmed the
effectiveness of transfection and establishment of a
C-erbB-2 knockout line. Compared with the blank group,
RT-qPCR demonstrated significant gene knockout in the
CRISPR/Cas9-CMB and CRISPR/Cas9-PTX-CMB groups (Fig. 2C).
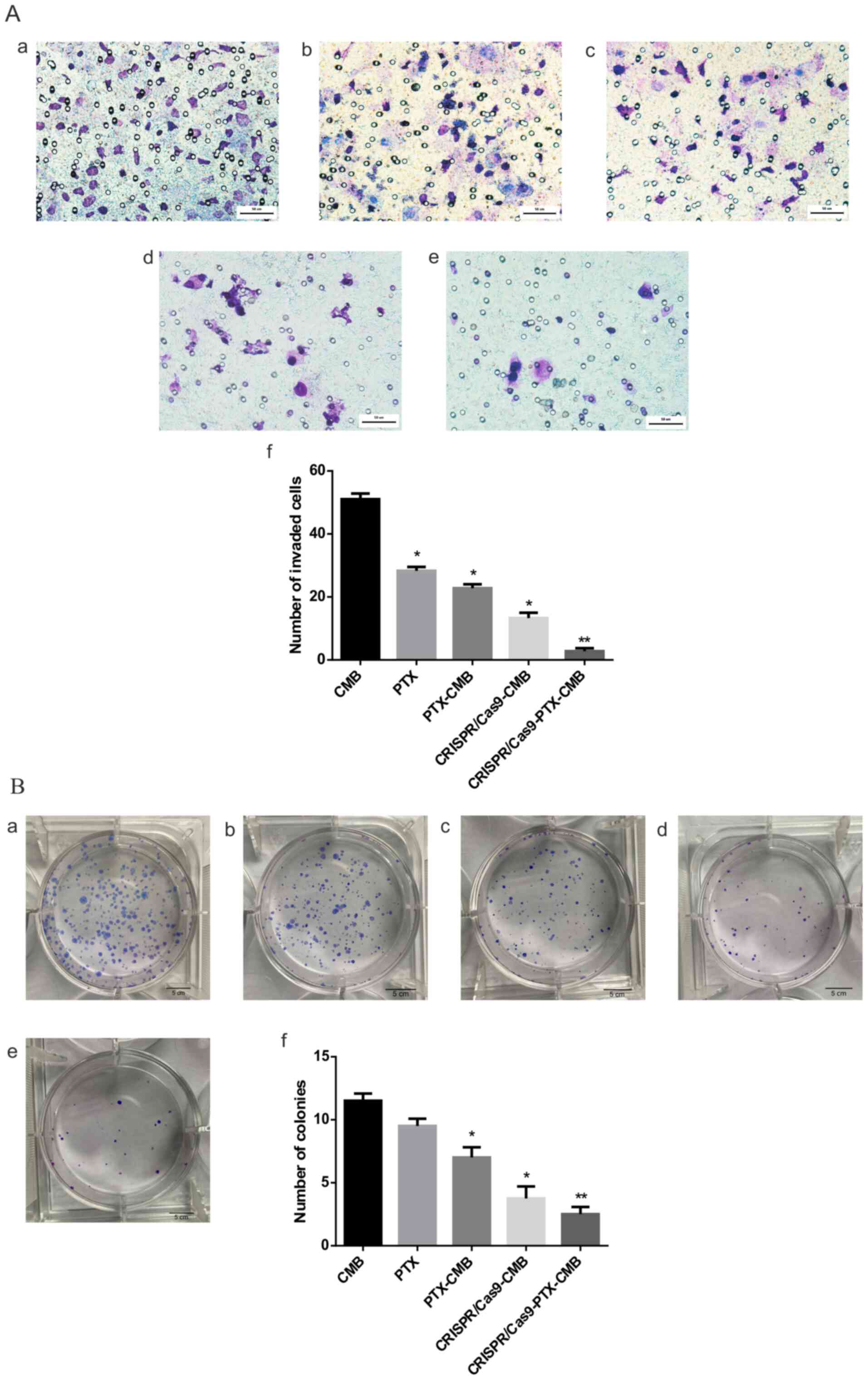
Knockout of C-erbB-2 in vitro can
effectively inhibit cell invasion
Compared with the CMB group, the number of invaded
cells in the PTX, PTX-CMB, CRISPR/Cas9-CMB and CRISPR/Cas9-PTX-CMB
groups significantly decreased (Fig.
3A-a-e). The CRISPR/Cas9-PTX-CMBs group had the lowest number
of invading cells (Fig. 3A-f).
These data confirmed that the invasion ability of cells treated
with PTX or gene knockout was effectively inhibited, and combined
drug/gene therapy exhibited the most notable inhibitory effect.
Knockout of C-erbB-2 in vitro can
effectively inhibit cell clone formation
Compared with the CMB group, CRISPR/Cas9-CMB and
CRISPR/Cas9-PTX-CMB groups had fewer cell clones, with the lowest
number observed in the CRISPR/Cas9-PTX-CMB group (Fig. 3B-a-f). The PTX-CMB group exhibited
fewer cell clones than the PTX group. CRISPR/Cas9-PTX-CMBs
exhibited fewer cell clones than the CRISPR/Cas9-CMB group. The
results of cell clone formation experiments showed that PTX and
CRISPR/Cas9 knockout of C-erbb-2 exhibited an inhibitory effect on
HEC-1A cells, while CRISPR/Cas9-PTX-CMB exhibited the greatest
inhibitory effect.
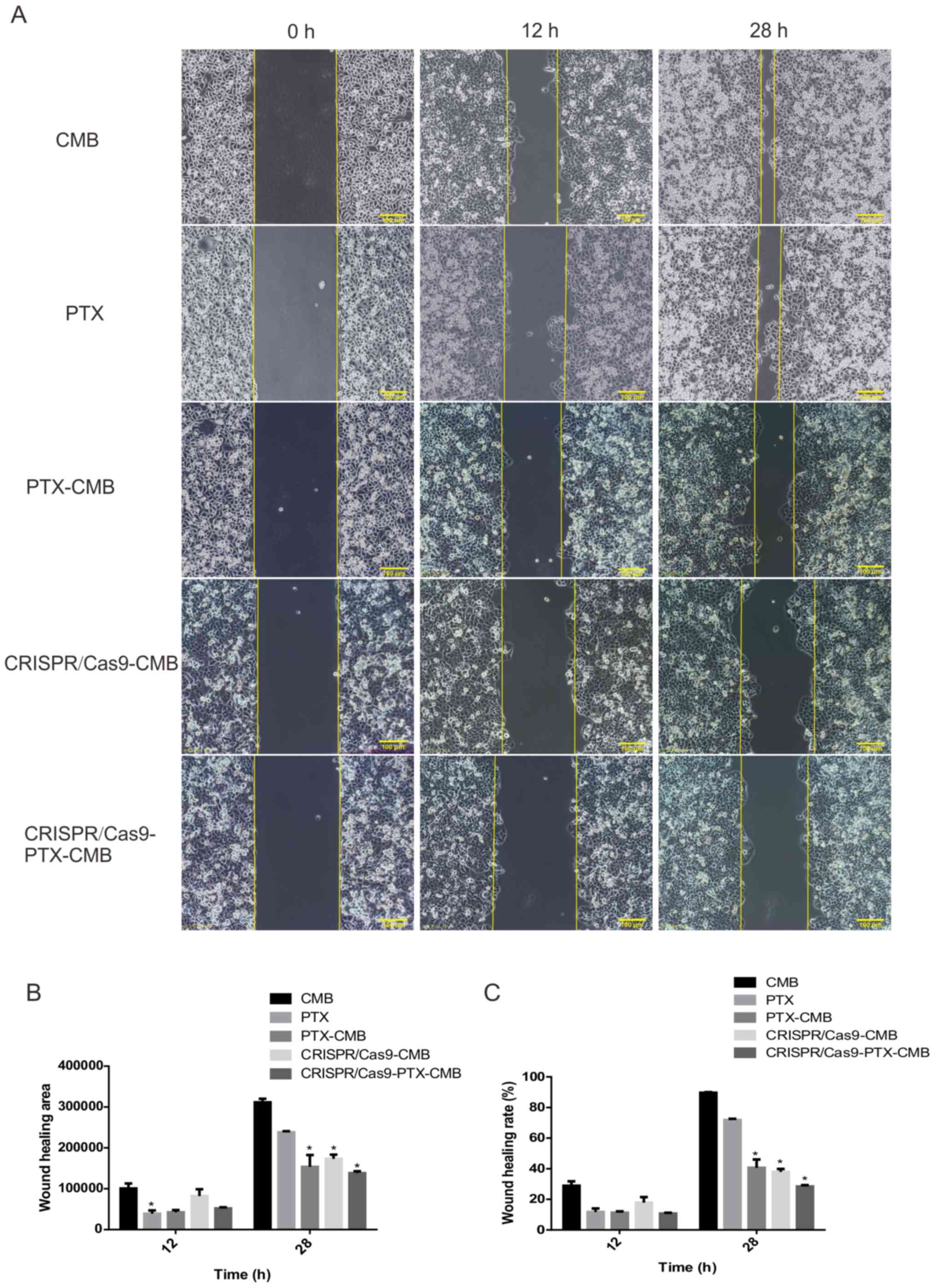
Knockout of C-erbB-2 in vitro can
effectively inhibit cell migration
At 48 h, compared with the CMB group, the wound
healing rate of the CMB-PTX, CRISPR/Cas9-CMB and
CRISPR/Cas9-PTX-CMB groups was inhibited (Fig. 4B and C). The cell scratch experiment
demonstrated that the proliferation of cells in the
CRISPR/Cas9-PTX-CMB was significantly inhibited.
Knockout of C-erbB-2 in vitro can
effectively inhibit cell proliferation
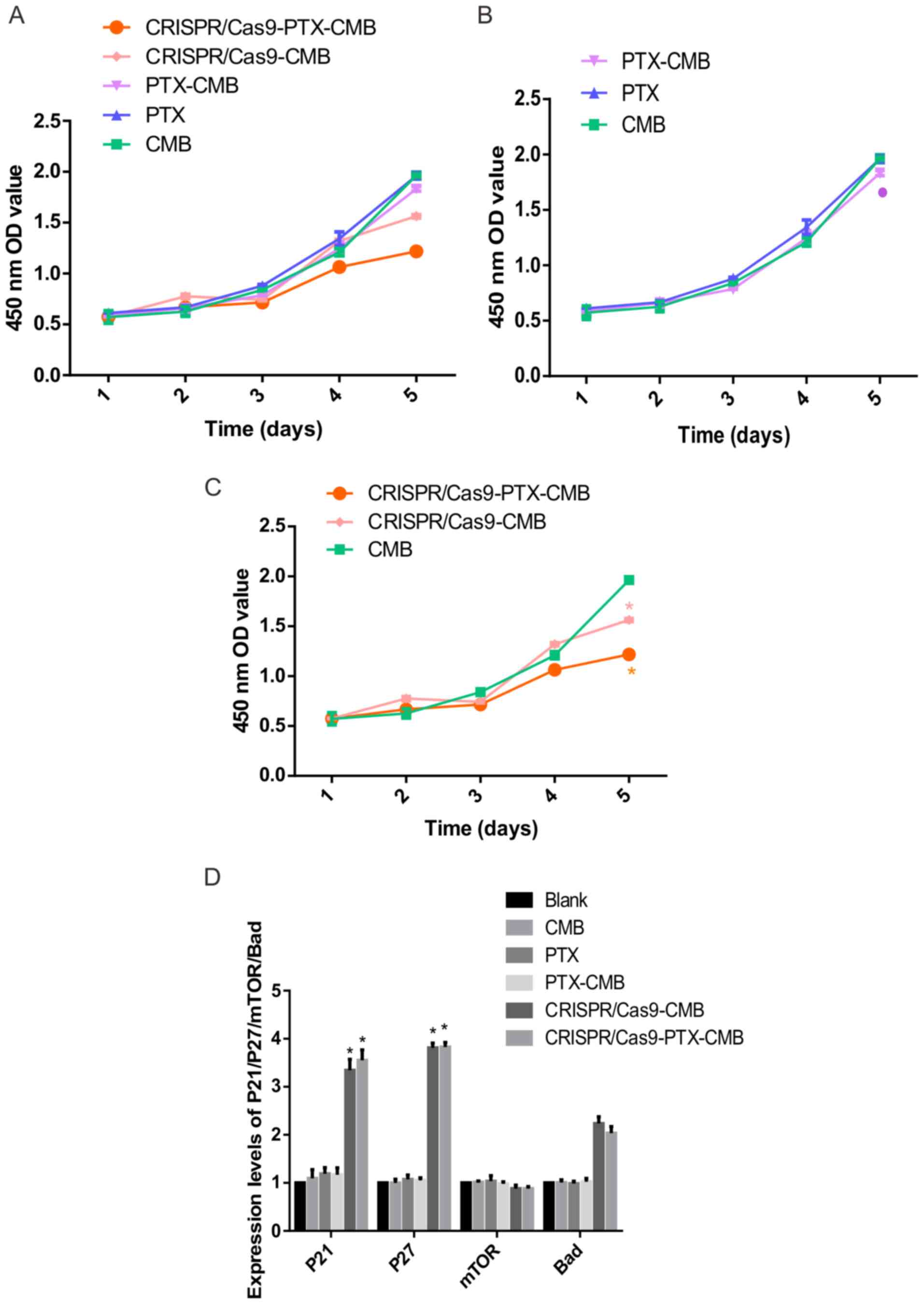
Absorbance values at 450 nm were measured to assess
proliferation (Fig. 5A). On day 5,
compared with the CMB group, the absorbance value of the cells in
the PTX-CMB group decreased (Fig.
5B). At the same time, compared with the CMB group, the
absorbance values of the CRISPR/Cas9-CMB and CRISPR/Cas9-PTX-CMB
groups were significantly decreased (Fig. 5C). These results suggested that
proliferation of cells treated with drug-gene combination was
inhibited.
Knockout of C-erbB-2 gene in vitro can
increase the expression level of P21 and P27 genes
To assess the effect of knockout of C-erbB-2
cell line constructed by CMB burst transfection on the expression
of its downstream genes, RT-qPCR was performed. Compared with the
CMB, PTX, PTX-CMB, CRISPR/Cas9-CMB and CRISPR/Cas9-PTX-CMB groups,
the expression of P21 and P27 was increased
significantly (Fig. 5D). Hence,
C-erbB-2 may participate in the occurrence and progression
of EC by regulating expression of P27 and P21.
Discussion
In recent years, CRISPR/Cas9 systems have been
developed, including use of lentiviruses, lipid nanoparticles,
artificial viruses, and non-viral method (29,30).
All of these systems have drawbacks; adeno-associated virus
(AAV)-mediated Cas9 delivery may cause inadvertent interruption of
the expression of important genes. In addition, the long-term
existence of a virus-mediated drug delivery system will increase
the accumulation of off-target cleavage, therefore, virus-mediated
methods are usually limited in vitro. As to non-viral-mediated
physical methods such as microscopic injection and electroporation;
microscopic injection requires high labor costs and harsh
experimental conditions and electroporation requires specific plans
for different types of cells, which makes the operation
complicated. (31,32). The present study described an
efficient delivery system using CMB and ultrasound. CMB has a
positive charge on its surface and is fat-soluble (33,34),
so it has advantages when loading DNA materials such as plasmids
and carrying PTX drugs (24,35).
CMB not only retains the physical and chemical properties of
ordinary MBs but also significantly improves the ability to carry
plasmid DNA due to its positive charge, which improves the
efficiency of ultrasound targeted transfection, confirming the
efficiency of CMB as a delivery system (36). Ultrasound targeted MBs destruction
(UTMD) is an emerging method for delivering target genes into cells
or living animal organs. It has become a research hotspot due to
its advantages of simplicity, non-invasiveness, targeting and
reproducibility (37–39). Low-frequency ultrasound has little
effect on the biological interaction between healthy cells and
normal tissue and is safe. The low-frequency ultrasound principle
is to combine non-invasive MBs with drugs, DNA or RNA vectors (such
as adenovirus, plasmids or nanoparticles) (37,38).
Low frequency and high mechanical index ultrasound are used to
destroy MBs and release drugs, adenovirus, plasmids or
nanoparticles to specific areas or organs (40). At the same time, it produces a
non-invasive cavitation effect: Multiple reversible pores with
diameters of hundreds of nanometers appear in the cell membrane.
Non-invasive cavitation effect can further improve the transmission
efficiency (41,42).
Cell function experiments showed that HEC-1A cells
treated with CRISPR/Cas9-CMBs or CRISPR/Cas9-PTX-CMBs knockout
C-erbB-2, The rate of proliferation, healing, as well as
cloning, migration and invasion ability of HEC-1A cells were
weakened. These data demonstrated in vitro proliferation of the EC
cell line HEC-1A after knocking out C-erbB-2. The group
CRISPR/Cas9-PTX-CMB simultaneously weakened the proliferation and
invasion ability of HEC-1A cells most robustly. The present study
demonstrated the possibility of delivery of genes or drugs based on
CMB-targeted destruction. At the same drug concentration, compared
with the PTX group, the anti-tumor effect of CMBs following release
of PTX was improved. Combination therapy is expected to overcome
the limitations of traditional treatments that rely on only one
therapy, including the adverse reactions and toxic effects caused
by ineffective increase of drug doses, off-target effects of gene
editing and the effects of drugs or surgery on normal tissue and
organs (43,44). Gene therapy combining
chemotherapeutic agents and genetic material has become a promising
combination therapy strategy due to its synergistic effect and
ability to decrease chemotherapeutic dose without affecting
anti-tumor activity (45).
The present data suggested that the mechanism of
C-erbB-2 gene regulation of EC may involve regulation of
expression of P27 and P21. P21 and P27
are tumor suppressor genes that effectively inhibit the
proliferation and division of tumor cells and are also known as
negative cell cycle regulators (46). Although the tumor-suppressive
function of P21 is one of the most studied aspects of this
protein in cancer, the role of P21 in phenotypic plasticity
and its carcinogenic/anti-apoptotic function depends on the
subcellular localization of P21. P21 can be an
oncogenic protein or a tumor suppressor, depending on its
localization in the cytoplasm or the nucleus, respectively
(47,48). Huang et al (49) found that nuclear p21 inhibits, but
cytoplasmic p21 promotes, cell migration and invasion abilities.
The present data suggested that the high expression of P21
and P27 in EC primarily serves a role in suppressing tumors,
which also indicated that C-erbB-2 may be downregulated by
P21. The expression of P27 serves a role in promoting
proliferation of tumor cells (50).
Bad is a mitochondrial pro-apoptotic factor, the primary
function of which is to promote cell apoptosis (51). In the present experiment, it remains
unknown whether C-erbB-2 regulates expression of Bad
and affects the occurrence and progress of EC. The mTOR
pathway is a central signaling pathway that controls metabolic
processes, such as protein synthesis, growth and metabolism
(52). It supports proliferation by
controlling cell growth and metabolism. Preclinical studies have
shown that inhibiting mTOR results in anti-tumor activity
(53,54). However, after knocking out
C-erbB-2 gene in the present experiment, Changes in
mTOR gene were not statistically significant. The present
cell function experiments demonstrated that proliferation of the
CRISPR/Cas9-CMB and CRISPR/Cas9-PTX-CMB decreased. Therefore, it
was hypothesized that C-erbB-2 downregulates P21 and
P27 to promote the proliferation of HEC-1A cells. It was
also speculated that the targeted release of PTX may decrease its
systemic toxic and side effects when used against EC. This should
be validated in larger studies involving animal models.
EC (HEC-1A) cells and CMBs were used as carriers for
drug and gene delivery. HEC-1A cells treated with CRISPR/Cas9-CMBs
or CRISPR/Cas-9PTX-CMBs knocked out C-erbB-2. The
proliferation, healing, as well as cloning, migration and invasion
ability of HEC-1A cells were weakened. CMB-assisted transfection
method based on ultrasound may aid development of novel treatment
methods against EC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hainan Key
Program of Research and Development (grant no. ZDYF2019123).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SP and JC performed experiments and data analysis
and wrote the manuscript. SB designed the study, revised the
manuscript and obtained funding. SP and JC confirm the authenticity
of all the raw data, All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu KH and Broaddus RR: Endometrial Cancer.
N Engl J Med. 383:2053–2064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Njoku K, Abiola J, Russell J and Crosbie
EJ: Endometrial cancer prevention in high-risk women. Best Pract
Res Clin Obstet Gynaecol. 65:66–78. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aoki Y, Kanao H, Wang X, Yunokawa M,
Omatsu K, Fusegi A and Takeshima N: Adjuvant treatment of
endometrial cancer today. Jpn J Clin Oncol. 50:753–765. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Habibi Jouybari M, Hosseini S, Mahboobnia
K, Boloursaz LA, Moradi M and Irani M: Simultaneous controlled
release of 5-FU, DOX and PTX from
chitosan/PLA/5-FU/g-C3N4-DOX/g-C3N4-PTX triaxial nanofibers for
breast cancer treatment in vitro. Colloids Surf B Biointerfaces.
179:495–504. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou X, Cao C, Li N and Yuan S: SYL3C
aptamer-anchored microemulsion co-loading β-elemene and PTX
enhances the treatment of colorectal cancer. Drug Deliv.
26:886–897. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ebeid K, Meng X, Thiel KW, Do AV, Geary
SM, Morris AS, Pham EL, Wongrakpanich A, Chhonker YS, Murry DJ, et
al: Synthetically lethal nanoparticles for treatment of endometrial
cancer. Nat Nanotechnol. 13:72–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dinkic C, Kruse A, Zygmunt M, Schuetz F,
Brucker J, Rom J, Sohn C and Fluhr H: Influence of Paclitaxel and
Heparin on Vitality, Proliferation and Cytokine Production of
Endometrial Cancer Cells. Geburtshilfe Frauenheilkd. 77:1104–1110.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Q, Liu F, Wang B, Li Z, Zhou D, Yang
Q, Dong J and Li J: HER-2 expression in biopsy and surgical
specimen on prognosis of osteosarcoma: A systematic review and
meta-analysis of 16 studies. Medicine (Baltimore). 95:e36612016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Press MF, Cordon-Cardo C and Slamon DJ:
Expression of the HER-2/neu proto-oncogene in normal human adult
and fetal tissues. Oncogene. 5:953–962. 1990.PubMed/NCBI
|
|
10
|
Mori S, Akiyama T, Yamada Y, Morishita Y,
Sugawara I, Toyoshima K and Yamamoto T: C-erbB-2 gene product, a
membrane protein commonly expressed on human fetal epithelial
cells. Lab Invest. 61:93–97. 1989.PubMed/NCBI
|
|
11
|
Hua S, Chuanbo F, Zhonglin W, Shuangjiu Z
and Xinwen Z: The expression and prognostic significance of Topo-II
and c-erbB-2 in breast cancer. Minerva Med. Jul 17–2020.(Epub ahead
of print). doi: 10.23736/S0026-4806.20.06637-9. PubMed/NCBI
|
|
12
|
Wang K, Liu J, Duan Y, Wu J, Dongye S,
Wang Y, Liu Z and Han G: C-erbB-2 expression is related with
pathological progression of gastric cancer: Results of a
non-radioactive in situ hybridization. Int J Clin Exp Pathol.
10:9649–9653. 2017.PubMed/NCBI
|
|
13
|
Canoz O, Ozkan M, Arsav V, Er O, Coskun
HS, Soyuer S and Altinbas M: The role of c-erbB-2 expression on the
survival of patients with small-cell lung cancer. Lung.
184:267–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao W, Dong X, Zhao H, Han S, Nie R,
Zhang X and An R: Expression of MIF and c-erbB-2 in endometrial
cancer. Mol Med Rep. 13:3828–3834. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Q, Hou CN, Yang HJ, He Z and Zuo MZ:
Distinct expression and prognostic value of members of the
epidermal growth factor receptor family in ovarian cancer. Cancer
Manag Res. 10:6937–6948. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li BT, Ross DS, Aisner DL, Chaft JE, Hsu
M, Kako SL, Kris MG, Varella-Garcia M and Arcila ME: HER2
Amplification and HER2 Mutation Are Distinct Molecular Targets in
Lung Cancers. J Thorac Oncol. 11:414–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Erickson BK, Zeybek B, Santin AD and Fader
AN: Targeting human epidermal growth factor receptor 2 (HER2) in
gynecologic malignancies. Curr Opin Obstet Gynecol. 32:57–64. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho S, Shin J and Cho BK: Applications of
CRISPR/Cas System to Bacterial Metabolic Engineering. Int J Mol
Sci. 19:10892018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishino Y, Shinagawa H, Makino K, Amemura M
and Nakata A: Nucleotide sequence of the iap gene, responsible for
alkaline phosphatase isozyme conversion in Escherichia coli,
and identification of the gene product. J Bacteriol. 169:5429–5433.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hryhorowicz M, Lipiński D, Zeyland J and
Słomski R: CRISPR/Cas9 Immune System as a Tool for Genome
Engineering. Arch Immunol Ther Exp (Warsz). 65:233–240. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhan T, Rindtorff N, Betge J, Ebert MP and
Boutros M: CRISPR/Cas9 for cancer research and therapy. Semin
Cancer Biol. 55:106–119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta D, Bhattacharjee O, Mandal D, Sen
MK, Dey D, Dasgupta A, Kazi TA, Gupta R, Sinharoy S, Acharya K, et
al: CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life
Sci. 232:1166362019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu C, Zhang L, Liu H and Cheng K:
Delivery strategies of the CRISPR-Cas9 gene-editing system for
therapeutic applications. J Control Release. 266:17–26. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang F, Li Y, Liufu C, Wang Y and Chen Z:
Preparation of Cationic Lipid-coated Ultrasound Contrast Agents and
Noninvasive Gene Transfection Via Ultrasound-targeted Microbubble
Destruction. Curr Pharm Des. 24:3587–3595. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Delalande A, Bastié C, Pigeon L, Manta S,
Lebertre M, Mignet N, Midoux P and Pichon C: Cationic gas-filled
microbubbles for ultrasound-based nucleic acids delivery. Biosci
Rep. 37:BSR201606192017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Q, Deng Q, Hu B, Wang YJ, Chen JL,
Cui JJ, Cao S and Song HN: Ultrasound combined with targeted
cationic microbubble-mediated angiogenesis gene transfection
improves ischemic heart function. Exp Ther Med. 13:2293–2303. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manta S, Renault G, Delalande A, Couture
O, Lagoutte I, Seguin J, Lager F, Houzé P, Midoux P, Bessodes M, et
al: Cationic microbubbles and antibiotic-free miniplasmid for
sustained ultrasound-mediated transgene expression in liver. J
Control Release. 262:170–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu X, Wan T, Xin H, Li D, Pan H, Wu J and
Ping Y: Delivery of CRISPR/Cas9 for therapeutic genome editing. J
Gene Med. 21:e31072019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lino CA, Harper JC, Carney JP and Timlin
JA: Delivering CRISPR: A review of the challenges and approaches.
Drug Deliv. 25:1234–1257. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen M, Mao A, Xu M, Weng Q, Mao J and Ji
J: CRISPR-Cas9 for cancer therapy: Opportunities and challenges.
Cancer Lett. 447:48–55. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yip BH: Recent Advances in CRISPR/Cas9
Delivery Strategies. Biomolecules. 10:8392020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Li X, Liu L, Liu B, Wang F and
Chen C: Tissue Targeting and Ultrasound-Targeted Microbubble
Destruction Delivery of Plasmid DNA and Transfection In Vitro. Cell
Mol Bioeng. 13:99–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kooiman K, Roovers S, Langeveld SAG,
Kleven RT, Dewitte H, O'Reilly MA, Escoffre JM, Bouakaz A, Verweij
MD, Hynynen K, et al: Ultrasound-Responsive Cavitation Nuclei for
Therapy and Drug Delivery. Ultrasound Med Biol. 46:1296–1325. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu X, Guo J, He C, Geng H, Yu G, Li J,
Zheng H, Ji X and Yan F: Ultrasound triggered image-guided drug
delivery to inhibit vascular reconstruction via paclitaxel-loaded
microbubbles. Sci Rep. 6:216832016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang D, Yang L, Tian H, Fu B, Chen W, Liu
K, Jia Z, Jiang S, Han X and Sun L: [Enhancement of gene
transfection efficiency and therapeutic effect of
ultrasound-targeted microbubble destruction in vivo with cationic
microbubble]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 32:228–236.
2018.(In Chinese). PubMed/NCBI
|
|
37
|
Du M, Chen Z, Chen Y and Li Y:
Ultrasound-Targeted Delivery Technology: A Novel Strategy for
Tumor- Targeted Therapy. Curr Drug Targets. 20:220–231. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Leon A, Perera R, Nittayacharn P,
Cooley M, Jung O and Exner AA: Ultrasound Contrast Agents and
Delivery Systems in Cancer Detection and Therapy. Adv Cancer Res.
139:57–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Endo-Takahashi Y and Negishi Y:
Microbubbles and Nanobubbles with Ultrasound for Systemic Gene
Delivery. Pharmaceutics. 12:9642020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qian L, Thapa B, Hong J, Zhang Y, Zhu M,
Chu M, Yao J and Xu D: The present and future role of ultrasound
targeted microbubble destruction in preclinical studies of cardiac
gene therapy. J Thorac Dis. 10:1099–1111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Szablowski JO, Bar-Zion A and Shapiro MG:
Achieving Spatial and Molecular Specificity with
Ultrasound-Targeted Biomolecular Nanotherapeutics. Acc Chem Res.
52:2427–2434. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou LQ, Li P, Cui XW and Dietrich CF:
Ultrasound nanotheranostics in fighting cancer: Advances and
prospects. Cancer Lett. 470:204–219. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu J, Chen J, Feng Y, Zhang S, Lin L, Guo
Z, Sun P, Xu C, Tian H and Chen X: An immune cocktail therapy to
realize multiple boosting of the cancer-immunity cycle by
combination of drug/gene delivery nanoparticles. Sci Adv.
6:eabc78282020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu P, Liu X, Cheng Y, Zhong S, Shi X,
Wang S, Liu M, Ding J and Zhou W: Core-Shell Nanosystems for
Self-Activated Drug-Gene Combinations against Triple-Negative
Breast Cancer. ACS Appl Mater Interfaces. 12:53654–53664. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han H, Kim D, Jang Y, Seo M, Kim K, Lee JB
and Kim H: Focused ultrasound-triggered chemo-gene therapy with
multifunctional nanocomplex for enhancing therapeutic efficacy. J
Control Release. 322:346–356. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou X, Yang Y, Ma P, Wang N, Yang D, Tu
Q, Sun B, Xiang T, Zhao X, Hou Z, et al: TRIM44 is indispensable
for glioma cell proliferation and cell cycle progression through
AKT/p21/p27 signaling pathway. J Neurooncol. 145:211–222. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shamloo B and Usluer S: p21 in Cancer
Research. Cancers (Basel). 11:11782019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rodriguez-Cupello C, Dam M, Serini L, Wang
S, Lindgren D, Englund E, Kjellman P, Axelson H, García-Mariscal A
and Madsen CD: The STRIPAK Complex Regulates Response to
Chemotherapy Through p21 and p27. Front Cell Dev Biol. 8:1462020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang Y, Wang W, Chen Y, Huang Y, Zhang J,
He S, Tan Y, Qiang F, Li A, Røe OD, et al: The opposite prognostic
significance of nuclear and cytoplasmic p21 expression in
resectable gastric cancer patients. J Gastroenterol. 49:1441–1452.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Razavipour SF, Harikumar KB and
Slingerland JM: p27 as a Transcriptional Regulator: New Roles in
Development and Cancer. Cancer Res. 80:3451–3458. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu P, Redd Bowman KE, Brown SM,
Joklik-Mcleod M, Vander Mause ER, Nguyen HTN and Lim CS: p53-Bad: A
Novel Tumor Suppressor/Proapoptotic Factor Hybrid Directed to the
Mitochondria for Ovarian Cancer Gene Therapy. Mol Pharm.
16:3386–3398. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Murugan AK: mTOR: Role in cancer,
metastasis and drug resistance. Semin Cancer Biol. 59:92–111. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hua H, Kong Q, Zhang H, Wang J, Luo T and
Jiang Y: Targeting mTOR for cancer therapy. J Hematol Oncol.
12:712019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Magaway C, Kim E and Jacinto E: Targeting
mTOR and Metabolism in Cancer: Lessons and Innovations. Cells.
8:15842019. View Article : Google Scholar : PubMed/NCBI
|