Introduction
Recurrent spontaneous abortion (RSA) is defined as
≥3 pregnancy losses within the first 20 weeks of gestation, whereas
an increasing number of researchers hold the opinion that ≥2
sequential pregnancy losses are sufficient for defining RSA.
Approximately 5% of females of childbearing age suffer from RSA and
the cause is unknown in over half of the cases, which makes it
difficult to perform any evidence-based diagnosis and treatment
(1). The etiology of RSA involves
multiple factors, including immune disorders, chromosomal
abnormalities, endocrine disorders and uterine abnormalities, among
which immune disorders are a key factor, and efforts have been made
in recent years to control RSA through agents targeting the immune
system (2,3). Currently available treatments for
RSA, including aspirin, anticoagulants and hormonal support, have
been indicated to exert immunomodulatory effects, suggesting that
targeting immunological disorders may be of value in the treatment
of RSA (4).
Tumor necrosis factor (TNF)-α is a pro-inflammatory
T helper (Th)1 cell cytokine, which regulates the inflammatory
mechanism in various pathologies, including RSA (5–7).
Regarding the immunological background of RSA, the imbalance
between Th1 cytokines, particularly TNF-α, and Th2 cytokines, such
as IL-10, is profound; therefore, TNF inhibitors, which are
commonly used treatments for inflammatory and immune diseases, have
been applied for the treatment of females with RSA (8–16).
The focus of the present review was to outline the mechanistic
involvement of TNF-α in RSA immune disorder and discuss clinical
studies that have attempted to improve pregnancy outcomes in
patients with RSA by using TNF inhibitors.
Immunological background in RSA
The immunological mechanism is largely altered
during pregnancy in response to the development of the fetomaternal
relationship. Embryos are considered as allografts to the mother,
as the antigens expressed by the embryos at the fetomaternal
interface are paternal and foreign, and these antigens may
subsequently induce alloimmune responses in the mother (3,17);
this means that the maternal immune tolerance may be broken down by
the implantation of the embryo and, thus, pro-inflammatory
cytokines (such as the TNF-α) may be released (Fig. 1). In addition, certain autoimmune
disorders, such as the antiphospholipid syndrome (APS) and
positivity for other antibodies such as anti-citrulline protein
antibody, are also characterized by the overexpression of
pro-inflammatory cytokines in peripheral blood mononuclear cells
(PBMCs), and the overproduced TNF-α may be released into the local
circulation of the implantation site, further contributing to
embryonic/fetal morbidity (Fig.
1) (18,19). Approximately 5–15% of females with
RSA present with APS, which refers to the presence of
antiphospholipid antibodies (aPLs) and their association with
venous/arterial thrombosis and hypercoagulation. The presence of
aPLs has been indicated to coexist with activated CD4+ T
cells and disrupted Th1/Th2 cytokine homeostasis (18). The dysregulated immune responses
damage the placental villi and embryonic tissues, interrupting the
implantation of the fertilized oocyte and embryonic development,
resulting in pregnancy failure. For instance, the Th17 cell and
regulatory T cell (Treg) imbalance (specifically, increased numbers
of Th17 cells and decreased numbers of Tregs) may be an important
immune factor contributing to the occurrence of RSA (20). In addition, in the case of RSA, it
has been hypothesized that the adaptive immune system recognizes
the alloantigen from the father, so that any further attempts at
fertilization with the same partner would result in failure
(3).
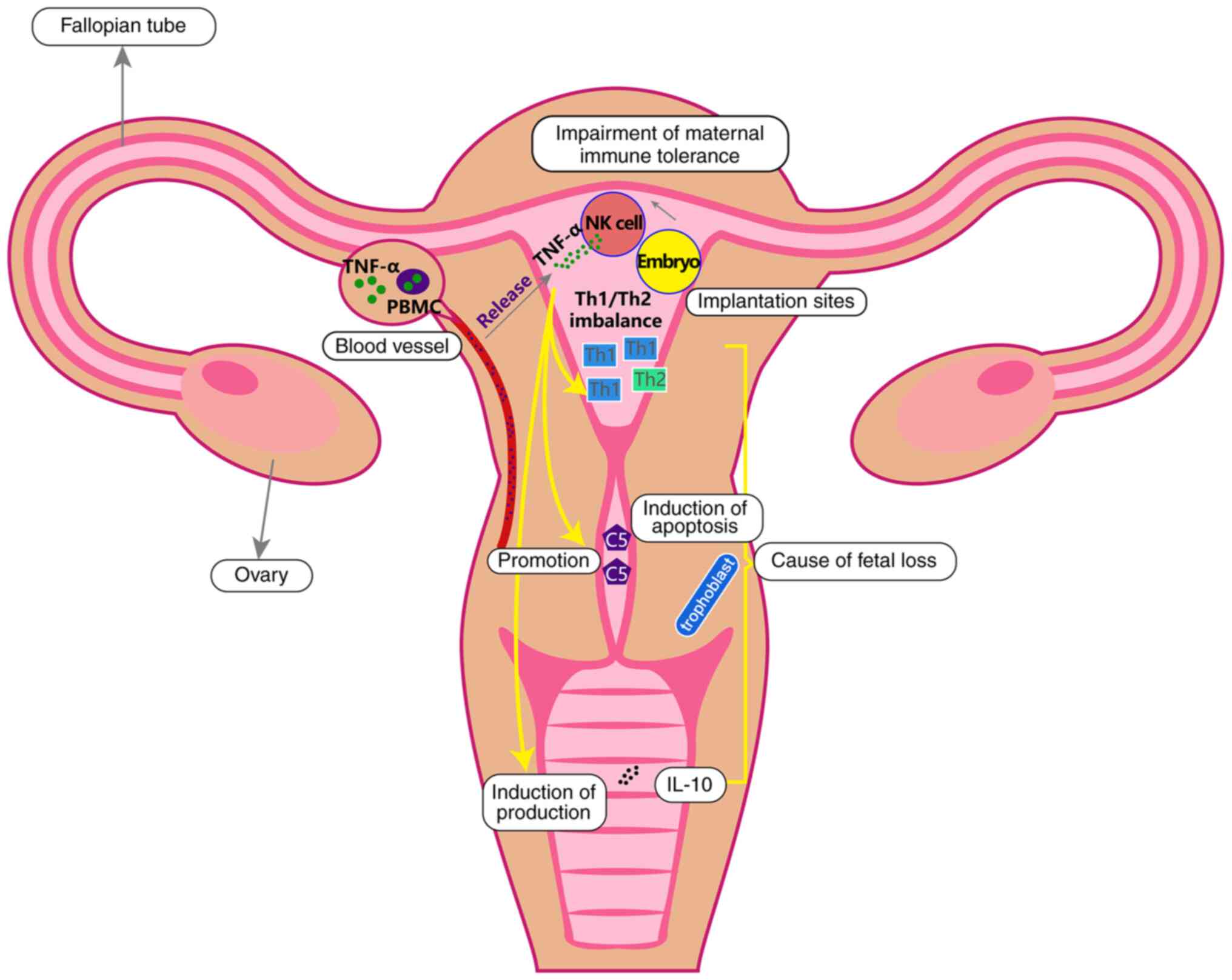 | Figure 1.TNF-α origin and potential mechanism
underlying its effects on RSA. After the implantation of the
embryo, the maternal immune tolerance is broken down and TNF-α is
then released by NK cells. In addition, in patients with autoimmune
disorders, TNF-α is also released from PBMCs. TNF-α may cause RSA
though disrupting the Th1/Th2 cell balance, inducing apoptosis of
trophoblast cells via promoting the secretion of C5 and coagulation
and promoting the production of IL-10. TNF, tumor necrosis factor;
RSA, recurrent spontaneous abortion; C5, complement component 5;
NK, natural killer; PBMC, peripheral blood mononuclear cell; Th, T
helper. |
NK cells
As a critical part of the innate immune system,
natural killer (NK) cells are considered as a strong risk factor
for RSA and they may be classified as peripheral and uterine NK
cells (1). Uterine NK cells were
initially referred to as large granular lymphocytes due to the
presence of granules in their cytoplasm and shared properties
(CD56+) with the peripheral NK cells. However, uterine
NK cells (CD16−/CD3−) are different from
peripheral NK cells (CD16+/CD3+) regarding
the expression of the CD16 and CD3 antigens (21). Peripheral NK cells are antiviral
and antineoplastic due to their cytotoxic nature, while uterine NK
cells are less cytotoxic and they produce receptors responsible for
the recognition of antigens on the extravillous trophoblast surface
and the secretion of cytokines (21).
Uterine NK cells are specifically reported to be
associated with various reproductive disorders, including RSA,
uterine fibroids and fetal growth restriction (22). Several studies using
immunohistochemistry as the detection tool have reported that
uterine NK cell numbers are increased in the endometrium of females
with RSA at the mid-secretory phase. By contrast, studies using
flow cytometry have indicated no change in uterine NK cell numbers
in females with RSA (23–25). Due to the discrepancies in uterine
NK cell numbers among different patients, it remains to be
determined whether uterine NK cells are functional in RSA. It was
previously suggested that mRNA expression is altered in endometrial
uterine NK cells in early pregnancy compared with non-pregnancy NK
cells (26); however, other
studies indicated that endometrial uterine NK cells are
non-functional in females with RSA (27,28). In addition, the adverse effects of
uterine NK cells on trophoblasts, such as invasion of the
trophoblast and development of trophoblast abnormalities, still
require confirmation (29–31).
It has been indicated that uterine NK cells are capable of
promoting angiogenesis, since they also produce important
pro-angiogenic factors, including vascular endothelial growth
factors, placental growth factors and angiopoietin 2, and uterine
NK cell deficiency caused poor spiral artery development in a mouse
model (21). However, whether the
involvement of uterine NK cells in angiogenesis contributes to the
pathogenesis of RSA remains to be fully elucidated.
T cells
In immune responses, a Th1/Th2 cell ratio <10.3
is considered a safe range for successful pregnancy (32). The Th1 cells, along with the
subsequent immune activation, increase the secretion of IFN-γ, IL-2
and TNF-α, which induce toxicity against the trophoblast and
trigger placental injury via activating immune cells, such as NK
cells. With regard to Th2 cells, they produce cytokines such as
IL-4, IL-13 and IL-10, which promote trophoblast growth and favor
pregnancy maintenance. The Th2 cells protect the embryo by
inhibiting immune rejection by the maternal immune system (33). The evaluation of Th1/Th2 cytokines
suggests that, in the first trimester of pregnancy, the
concentrations of Th1 cytokines were higher in PBMCs of females
with RSA compared with those with normal pregnancies (34). In addition, females who are prone
to RSA but have a successful pregnancy tend to express lower Th2
cytokine levels compared with those who are not prone to RSA and
suffer a pregnancy loss (35).
Detailed flow cytometry data also revealed that females with RSA
have significantly lower IL-10-producing
CD3+/CD8+ T-cell counts but higher
TNF-α-producing CD3+/CD4+ T-cell counts
compared with non-pregnant fertile controls (36). The aforementioned evidence
suggests the implication of Th1/Th2 imbalance in the pathogenesis
of RSA (Fig. 1).
Tregs suppress the alloimmune response towards the
fetus (37). In cases of
allogeneic organ grafts, Tregs prevent autoimmunity, whereas in
RSA, the number of available studies is limited (38). In a mouse model of spontaneous
abortion, Tregs (CD4+/CD25+) from normal
pregnant/non-pregnant mice inhibited lymphocyte proliferation and
IFN-γ secretion in vitro, whereas in vivo, the
adoptive transfer of Tregs from normal pregnant mice to maternal
mice with abortion prevented fetal rejection, indicating the
function of Tregs in promoting maternal tolerance of the fetus
(39).
TNF-α in RSA
TNF-α is a Th1 pro-inflammatory cytokine that is
located on chromosome 6p21.3 and acts via binding to TNF receptor
(TNFR)I and TNFRII on cells. It was previously reported that TNF-α
is abundant in the body fluids of patients with autoimmune diseases
and that it is closely associated with neurodegenerative diseases
(40). For instance, patients
with RA or systemic lupus erythematosus frequently exhibit high
levels of TNF-α and TNFRI (41).
Elevated TNF-α levels were also reported in patients with multiple
sclerosis (42). In addition, the
levels of TNF-α along with those of other pro-inflammatory
cytokines are increased during the progression of Alzheimer's
disease and may be involved in neurological deterioration through
nitric oxide pathways (43).
TNF-α is produced by multiple cell types, including
immune cells (such as Th1 cells, NK cells, neutrophils, monocytes
and macrophages) and non-immune cells (such as neuronal cells)
(44). These cells are also key
regulators of placentation, the dysregulation of which is a
critical contributor to the pathogenesis of RSA. It has been
reported that TNF-α may cause unsuccessful pregnancy through
several mechanisms, including the following: i) induction of
inflammation and disruption of the Th1/Th2 balance; ii) activation
of the complement, causing trophoblast cell death; and iii)
upregulation of IL-10 levels, leading to fetal loss (Fig. 1). In detail, TNF-α is considered
to be responsible for inducing inflammation during placentation and
implantation, and the balance between Th1 cytokines (predominantly
TNF-α) and Th2 cytokines (predominantly IL-10) is critical for
successful pregnancy (7). TNF-α
may also stimulate the production of IFN-γ though promoting Treg
cell differentiation to Th1 cells, while excessive expression of
TNF-α and IFN-γ may activate the complement, thus leading to
trophoblast cell death (45,46). In addition, TNF-α has been
reported to upregulate programmed cell death-1 levels in monocytes
and induce IL-10 production, which underlies part of the
immunological mechanisms involved in normal pregnancy (47), whereas in RSA, a tendency for a
disequilibrium between TNF-α and IL-10 is present and the
TNF-α/IL-10 ratio appears to be elevated in females with
implantation failure, recurrent fetal loss and other complications,
such as hypertensive syndrome (Fig.
1) (48,49). Initially, studies based on animals
revealed that injecting TNF-α causes embryonic death in pregnant
mice and TNF-α was indicated to be upregulated in a mouse model
with a high incidence of RSA (50). In vivo, TNF-α was observed
to be detrimental to mouse and cattle blastocysts due to its toxic
effect, and it was also demonstrated to be involved in
α-galactosylceramide (ligand expressed by Va14 NK T cells)-induced
embryonic death (51).
In pregnant female patients, TNF-α is primarily
produced by macrophages in the placenta during the first trimester
and a high TNF-α level is a pivotal factor for adverse pregnancy
conditions, including RSA, gestational hypertension and gestational
diabetes mellitus (6). In
addition, TNF-α gene polymorphisms may be associated with increased
RSA risk. In Saudi females, the −308 G/A polymorphism in the TNF-α
gene promoter was reported to be correlated with the occurrence of
unexplained RSA (52). Another
study reported that the −863C/A and −238G/A TNF-α polymorphisms,
which are at the promoter region, are also risk factors for RSA
(53). One study attempted to
recreate the increased maternal TNF-α level by exogenously applying
TNF-α to the culture media of first-trimester villous placental
explants. The inflammatory antibody arrays and ELISA suggested that
granulocyte/macrophage-colony-stimulating factor (CSF), C-C motif
chemokine ligand 5 and IL-10 were upregulated, whereas IL-4 and
macrophage CSF levels were decreased in the presence of TNF-α
(6). Therefore, targeting TNF-α
may be a key strategy in immunological treatments for RSA.
TNF-α inhibitors in RSA
TNF inhibitors suppress TNF-α binding to TNFRI and
TNFRII, thus inhibiting the immune response, dendritic cells and
the differentiation of Tregs, effects that are considered to be
anti-abortive, and are thus applied in RSA (54–59). For instance, TNF inhibitors may
suppress the expression of transcription factors, proteases or
protein kinases (including NF-κB, caspases and MAPK) and inhibit
the release of pro-inflammatory cytokines, chemokines and adhesion
molecules (including IL-1, IL-6, IL-8, MMPs and intercellular
adhesion molecule 1) through blocking TNF-α binding to TNFRI and
TNFRII, and further inhibiting the activation of TNF-α that would
lead to the inflammatory and immune response (54–56). On the other hand, TNF inhibitors
may also restrain the activation of immune cells (including
macrophages, T-cells and B-cells), inhibit the differentiation from
CD4+ T cells to Th1 and Th17 cells and suppress the
maturation of dendritic cells, which further lowers the levels of
pro-inflammatory cytokines (including IL-1, IL-17 or IFN-α), thus
suppressing the inflammatory reaction (57–59). In rats with abnormal maternal
inflammation-induced RSA, administration of IL-10 or TNF inhibitor
(etanercept) prevented pregnancy loss (13). Another study reported that
treatment with etanercept blocked TNF-α activity, reduced fetal
loss and restored the junctional zone ratio, placental invasion and
thinning of spiral arterial walls in a syngeneic mouse model of
placental insufficiency (14). It
was also reported that etanercept achieved a 62% reduction of
inflammation-associated coagulopathies in a rat model, which may
reduce pregnancy-related disorders, including RSA (60).
In addition, since common treatments, including
prednisone, heparin, aspirin, cyclosporine A or intravenous
immunoglobulin (Ig), have been indicated to not be efficacious in
reducing TNF-α and the cytotoxicity of NK cells in a number of
patients with RSA, TNF inhibitors, which function in the innate
immune system, are increasingly being investigated for their
efficacy in reducing RSA. As shown in Table I, A randomized controlled trial
enrolling patients with RSA with innate immune disorders reported
that etanercept significantly reduced TNF-α and NK cell activity at
weeks 4-10 of gestation and female patients treated with etanercept
had higher live birth rates compared with those treated with
placebo (9). Among females with
RSA who are treated with anticoagulants, the addition of TNF
inhibitors (etanercept or adalimumab) combined with intravenous
immunoglobulin increased the live birth rate; however, whether the
addition of TNF inhibitors alone is able to improve the live birth
rate remains to be determined (10). In a study of 30 females with a
history of RSA or failure of in vitro fertilization, 4 doses
(25 mg) of etanercept twice weekly prior to conception
significantly reduced NK cell activity, particularly among females
with RSA (8). Other studies
comparing adalimumab + intravenous immunoglobulin with intravenous
immunoglobulin- or adalimumab-alone (16) and evaluating the efficacy of
etanercept-alone or in combination with methotrexate and rofecoxib
in patients with RSA are shown in Table I (8–12,16).
 | Table I.Application of TNF inhibitors for
recurrent spontaneous abortion in clinical studies. |
Table I.
Application of TNF inhibitors for
recurrent spontaneous abortion in clinical studies.
| Author (year) | Sample size, n | Treatment | Outcomes | (Refs.) |
|---|
| Fu et al
(2019) | 188 | Etanercept vs.
placebo | Delivery of healthy
infant: Etanercept group, 89.47% vs. placebo group, 72.04% |
(9) |
| Jerzak et al
(2012) | 30 | Etanercept | NK cell activity
was reduced after etanercept treatment |
(8) |
| Winger et al
(2009) | 75 | Adalimumab plus
intravenous immunoglobulin vs. intravenous immunoglobulin vs.
adalimumab vs. none | Live birth rate:
Adalimumab plus intravenous immunoglobulin group, 73% vs.
intravenous immunoglobulin group, 52% vs. adalimumab group, 50% vs.
no treatment group, 0% | (16) |
| Winger and Reed
(2008) | 75 | TNF inhibitor
(adalimumab or etanercept) vs. anticoagulants | Live birth rate:
TNF inhibitor group 71% vs. anticoagulants group, 19%, | (10) |
| Sills et al
(2009) | 1 | Etanercept | Successful
pregnancy and delivery after etanercept treatment | (11) |
| Sills et al
(2001) | 1 | Etanercept,
methotrexate and rofecoxib | Successful
ovulation induction, conception and normal delivery after
etanercept, methotrexate and rofecoxib treatment | (12) |
For the subsequent analysis of the currently
available data on the application of TNF inhibitors for RSA, the
use of TNF inhibitors, the treatment regimens, complications and
clinical follow-up were compared among the available clinical
studies on RSA (8–12,16) and various noteworthy points became
apparent: i) Among all of the TNF inhibitors, etanercept was the
most frequently used in clinical practice for the treatment of RSA.
In detail, 5 out of the 6 studies used etanercept for the treatment
of RSA, while only two studies used adalimumab (adalimumab and
etanercept were both used in one study) (10)); in addition, the use of etanercept
achieved a higher rate of healthy deliveries compared with
adalimumab; ii) TNF inhibitor monotherapy was more commonly used
than combined therapy, although the results suggested that combined
therapy may achieve a higher live birth rate; iii) in terms of
complications, only a small number of studies performed to date
have evaluated the complications among patients with RSA who
received TNF inhibitor treatment and, thus, no relevant data are
provided in the present review; and iv) regarding the clinical
follow-up, most studies terminated the follow-up at live birth or
the occurrence of abortion, with the exception of one study that
continued the follow-up until 3 months after the delivery. The most
attractive endpoint is the live birth rate, which ranged from 50 to
89.47% in patients with RSA after treatment with TNF
inhibitors.
The safety of TNF inhibitors during pregnancy has
long been studied, since they are widely applied drugs for
rheumatoid arthritis (RA), including patients with RA during
pregnancy. Successful ovulation induction, conception and normal
delivery are reported among females receiving long-term TNF
inhibitors as anti-cytokine treatment for RA (11,12). Regarding pregnant females with
inflammatory bowel disease, infliximab and other TNF inhibitors
have been reported to be safe, as there was no reported increased
incidence of spontaneous abortion or other adverse pregnancy
outcomes (61). However,
according to the official guidelines of the German Society of
Gynecology and Obstetrics, the Austrian Society of Gynecology and
Obstetrics and the Swiss Society of Gynecology and Obstetrics, the
use of TNF inhibitors should be reserved for specific conditions,
such as Crohn's disease and other autoimmune diseases, due to
possible side effects such as skin irritation and drug-induced
lupus (15).
Progress of TNF inhibitor application
As one of the initially approved TNF inhibitors,
etanercept, a fusion protein comprised of the extracellular domain
of human TNFRII receptor and the Fc region of human IgG1, was
approved for RA treatment in the US and Europe in 1998 (62,63). In the same year, infliximab, a
chimeric monoclonal antibody that contains a murine antigen-binding
region joined to the human IgG1 constant region, was approved for
the treatment of Crohn's disease in the US and soon after (in 1999)
in Europe, launching the new era of TNF inhibitors in bowel
diseases (64). Both etanercept
and infliximab were later applied and approved for other
inflammatory and autoimmune diseases, such as ankylosing
spondylitis and ulcerative colitis. Etanercept is administered as a
weekly subcutaneous injection, while infliximab is delivered
intravenously in-hospital (63,64). Following the success of the
aforementioned treatments, the fully human antibodies adalimumab
and golimumab were produced by immunizing genetically engineered
mice with human TNF-α, which solved the problem of the immunogenic
nature of infliximab (a chimeric monoclonal antibody), as well as
its relatively severe adverse effects such as the production of
anti-antibody or the occurrence of allergy (62,63,65–71). The release of TNF inhibitors to
the market has revolutionized the treatment of RA, ankylosing
spondylitis, psoriasis, Chron's disease and ulcerative colitis,
particularly RA and ankylosing spondylitis, for which TNF
inhibitors have become a standard treatment, and they are generally
well-tolerated, with long-term efficacy (72). In addition, the open-label
extensions of clinical trials of TNF inhibitors and observations
post-market release also support their efficacy and safety.
TNF inhibitors are among the best-selling drugs
worldwide (73). However, the
cost of these biologicals is high, with an annual cost per patient
of >20,000 USD. Therefore, in addition to the TNF inhibitors
already launched, several other biologicals are available,
including biosimilars and novel drugs under investigation. For
instance, a humanized anti-TNF monoclonal antibody, SSS-07, is
currently under investigation in a clinical trial for RA in China
[National Clinical Trial (NCT) no. NCT02460393] (74). In addition, the approved
biosimilars of infliximab include CT-P13 (66) and SB2 (67); the approved biosimilars of
etanercept include GP2015 (68)
and SB4 (62); and the approved
biosimilars of adalimumab include ABP501, BI695501, GP2017 and SB5
(69). So far, >20 newly
developed anti-TNF biologicals were in the pipeline, as well as new
indications of the already approved drugs (73).
With research efforts focused on drug development,
the dosing of TNF inhibitors may become more practicable and
cost-effective in clinical practice and their application may be
expanded to immunomodulatory conditions other than the commonly
approved indications, e.g., the immune disorder underlying RSA.
Concluding remarks
There are currently numerous studies on the
application of TNF inhibitors for the treatment of autoimmune and
inflammatory diseases. Considering the contribution of
immunological disorders to the mechanism underlying RSA, the
application of TNF inhibitors in patients with RSA aims to target
the immune aspect of this condition. In pre-clinical studies, TNF
inhibitors have been indicated to successfully reduce NK cell
numbers and Th1-related cytokine levels, which are risk factors for
RSA, and clinical attempts have already achieved an improvement in
the live birth rate. However, therapies for RSA using TNF
inhibitors still lack sufficient supportive data. In addition, the
development of TNF inhibitors in the pharmaceutical industry is
still growing, which may be promising for the future clinical
application of TNF inhibitors in RSA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
FM conceived and supervised the study. HW and QY
participated in the acquisition of data and collated the data. YJ
analyzed the data and wrote, edited and revised the manuscript. All
authors participated in revising the manuscript. All authors read
and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RSA
|
recurrent spontaneous abortion
|
|
TNF-α
|
tumor necrosis factor-α
|
|
Th1
|
T helper 1
|
|
IL
|
interleukin
|
|
NK
|
natural killer
|
|
Treg
|
regulatory T cell
|
|
RA
|
rheumatoid arthritis
|
References
|
1
|
Toth B, Vomstein K, Togawa R, Böttcher B,
Hudalla H, Strowitzki T, Daniel V and Kuon RJ: The impact of
previous live births on peripheral and uterine natural killer cells
in patients with recurrent miscarriage. Reprod Biol Endocrinol.
17:722019. View Article : Google Scholar
|
|
2
|
Ozkan ZS, Deveci D, Simsek M, Ilhan F,
Risvanli A and Sapmaz E: What is the impact of SOCS3, IL-35 and
IL17 in immune pathogenesis of recurrent pregnancy loss? J Matern
Fetal Neonatal Med. 28:324–328. 2015. View Article : Google Scholar
|
|
3
|
Pei CZ, Kim YJ and Baek KH: Pathogenetic
factors involved in recurrent pregnancy loss from multiple aspects.
Obstet Gynecol Sci. 62:212–223. 2019. View Article : Google Scholar
|
|
4
|
Ou H and Yu Q: Efficacy of aspirin,
prednisone, and multivitamin triple therapy in treating unexplained
recurrent spontaneous abortion: A cohort study. Int J Gynaecol
Obstet. 148:21–26. 2020. View Article : Google Scholar
|
|
5
|
Alijotas-Reig J, Esteve-Valverde E,
Ferrer-Oliveras R, Llurba E and Gris JM: Tumor Necrosis
Factor-Alpha and pregnancy: Focus on biologics. An updated and
comprehensive review. Clin Rev Allergy Immunol. 53:40–53. 2017.
View Article : Google Scholar
|
|
6
|
Siwetz M, Blaschitz A, El-Heliebi A, Hiden
U, Desoye G, Huppertz B and Gauster M: TNF-alpha alters the
inflammatory secretion profile of human first trimester placenta.
Lab Invest. 96:428–438. 2016. View Article : Google Scholar
|
|
7
|
Saito S, Nakashima A, Shima T and Ito M:
Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J
Reprod Immunol. 63:601–610. 2010. View Article : Google Scholar
|
|
8
|
Jerzak M, Ohams M, Gorski A and Baranowski
W: Etanercept immunotherapy in women with a history of recurrent
reproductive failure. Ginekol Pol. 83:260–264. 2012.
|
|
9
|
Fu J, Li L, Qi L and Zhao L: A randomized
controlled trial of etanercept in the treatment of refractory
recurrent spontaneous abortion with innate immune disorders. Taiwan
J Obstet Gynecol. 58:621–625. 2019. View Article : Google Scholar
|
|
10
|
Winger EE and Reed JL: Treatment with
tumor necrosis factor inhibitors and intravenous immunoglobulin
improves live birth rates in women with recurrent spontaneous
abortion. Am J Reprod Immunol. 60:8–16. 2008. View Article : Google Scholar
|
|
11
|
Sills ES, Walsh DJ, Shkrobot LV, Palermo
GD and Walsh AP: Clinical experience with intravenous
immunoglobulin and tnf-a inhibitor therapies for recurrent
pregnancy loss. Ulster Med J. 78:57–58. 2009.
|
|
12
|
Sills ES, Perloe M, Tucker MJ, Kaplan CR
and Palermo GD: Successful ovulation induction, conception, and
normal delivery after chronic therapy with etanercept: A
recombinant fusion anti-cytokine treatment for rheumatoid
arthritis. Am J Reprod Immunol. 46:366–368. 2001. View Article : Google Scholar
|
|
13
|
Renaud SJ, Cotechini T, Quirt JS,
Macdonald-Goodfellow SK, Othman M and Graham CH: Spontaneous
pregnancy loss mediated by abnormal maternal inflammation in rats
is linked to deficient uteroplacental perfusion. J Immunol.
186:1799–1808. 2011. View Article : Google Scholar
|
|
14
|
Gelber SE, Brent E, Redecha P, Perino G,
Tomlinson S, Davisson RL and Salmon JE: Prevention of defective
placentation and pregnancy loss by blocking innate immune pathways
in a syngeneic model of placental insufficiency. J Immunol.
195:1129–1138. 2015. View Article : Google Scholar
|
|
15
|
Toth B, Wurfel W, Bohlmann M, Zschocke J,
Rudnik-Schöneborn S, Nawroth F, Schleußner E, Rogenhofer N,
Wischmann T, von Wolff M, et al: Recurrent Miscarriage: Diagnostic
and Therapeutic Procedures. Guideline of the DGGG, OEGGG and SGGG
(S2k-Level, AWMF Registry Number 015/050). Geburtshilfe
Frauenheilkd. 78:364–381. 2018. View Article : Google Scholar
|
|
16
|
Winger EE, Reed JL, Ashoush S, Ahuja S,
El-Toukhy T and Taranissi M: Treatment with adalimumab (Humira) and
intravenous immunoglobulin improves pregnancy rates in women
undergoing IVF. Am J Reprod Immunol. 61:113–120. 2009. View Article : Google Scholar
|
|
17
|
Baines MG, Duclos AJ, Antecka E and Haddad
EK: Decidual infiltration and activation of macrophages leads to
early embryo loss. Am J Reprod Immunol. 37:471–477. 1997.
View Article : Google Scholar
|
|
18
|
Garrido-Gimenez C and Alijotas-Reig J:
Recurrent miscarriage: Causes, evaluation and management. Postgrad
Med J. 91:151–162. 2015. View Article : Google Scholar
|
|
19
|
Boomsma CM, Kavelaars A, Eijkemans MJ,
Lentjes EG, Fauser BC, Heijnen CJ and Macklon NS: Endometrial
secretion analysis identifies a cytokine profile predictive of
pregnancy in IVF. Hum Reprod. 24:1427–1435. 2009. View Article : Google Scholar
|
|
20
|
Guo Z, Xu Y, Zheng Q, Liu Y and Liu X:
Analysis of chromosomes and the T helper 17 and regulatory T cell
balance in patients with recurrent spontaneous abortion. Exp Ther
Med. 19:3159–3166. 2020.
|
|
21
|
Quenby S and Farquharson R: Uterine
natural killer cells, implantation failure and recurrent
miscarriage. Reprod Biomed Online. 13:24–28. 2006. View Article : Google Scholar
|
|
22
|
Lash GE and Bulmer JN: Do uterine natural
killer (uNK) cells contribute to female reproductive disorders? J
Reprod Immunol. 88:156–164. 2011. View Article : Google Scholar
|
|
23
|
Tuckerman E, Laird SM, Prakash A and Li
TC: Prognostic value of the measurement of uterine natural killer
cells in the endometrium of women with recurrent miscarriage. Hum
Reprod. 22:2208–2213. 2007. View Article : Google Scholar
|
|
24
|
Tuckerman E, Mariee N, Prakash A, Li TC
and Laird S: Uterine natural killer cells in peri-implantation
endometrium from women with repeated implantation failure after
IVF. J Reprod Immunol. 87:60–66. 2010. View Article : Google Scholar
|
|
25
|
Shimada S, Kato EH, Morikawa M, Iwabuchi
K, Nishida R, Kishi R, Onoé K, Minakami H and Yamada H: No
difference in natural killer or natural killer T-cell population,
but aberrant T-helper cell population in the endometrium of women
with repeated miscarriage. Hum Reprod. 19:1018–1024. 2004.
View Article : Google Scholar
|
|
26
|
Manaster I and Mandelboim O: The unique
properties of human NK cells in the uterine mucosa. Placenta. 29
(Suppl A):S60–S66. 2008. View Article : Google Scholar
|
|
27
|
Manaster I, Mizrahi S, Goldman-Wohl D,
Sela HY, Stern-Ginossar N, Lankry D, Gruda R, Hurwitz A, Bdolah Y,
Haimov-Kochman R, et al: Endometrial NK cells are special immature
cells that await pregnancy. J Immunol. 181:1869–1876. 2008.
View Article : Google Scholar
|
|
28
|
Kopcow HD, Allan DS, Chen X, Rybalov B,
Andzelm MM, Ge B and Strominger JL: Human decidual NK cells form
immature activating synapses and are not cytotoxic. Proc Natl Acad
Sci USA. 102:15563–15568. 2005. View Article : Google Scholar
|
|
29
|
Guimond MJ, Wang B and Croy BA:
Engraftment of bone marrow from severe combined immunodeficient
(SCID) mice reverses the reproductive deficits in natural killer
cell-deficient tg epsilon 26 mice. J Exp Med. 187:217–223. 1998.
View Article : Google Scholar
|
|
30
|
Rai R, Sacks G and Trew G: Natural killer
cells and reproductive failure-theory, practice and prejudice. Hum
Reprod. 20:1123–1126. 2005. View Article : Google Scholar
|
|
31
|
Quenby S, Vince G, Farquharson R and Aplin
J: Recurrent miscarriage: A defect in nature's quality control? Hum
Reprod. 17:1959–1963. 2002. View Article : Google Scholar
|
|
32
|
Nakagawa K, Kwak-Kim J, Ota K, Kuroda K,
Hisano M, Sugiyama R and Yamaguchi K: Immunosuppression with
tacrolimus improved reproductive outcome of women with repeated
implantation failure and elevated peripheral blood TH1/TH2 cell
ratios. Am J Reprod Immunol. 73:353–361. 2015. View Article : Google Scholar
|
|
33
|
Raghupathy R, Makhseed M, Azizieh F,
Hassan N, Al-Azemi M and Al-Shamali E: Maternal Th1- and Th2-type
reactivity to placental antigens in normal human pregnancy and
unexplained recurrent spontaneous abortions. Cell Immunol.
196:122–130. 1999. View Article : Google Scholar
|
|
34
|
Darmochwal-Kolarz D, Rolinski J,
Leszczynska-Goarzelak B and Oleszczuk J: The expressions of
intracellular cytokines in the lymphocytes of preeclamptic
patients. Am J Reprod Immunol. 48:381–386. 2002. View Article : Google Scholar
|
|
35
|
Kwak-Kim JYH, Gilman-Sachs A and Kim CE: T
helper 1 and 2 immune responses in relationship to pregnancy,
nonpregnancy, recurrent spontaneous abortions and infertility of
repeated implantation failures. Chem Immunol Allergy. 88:64–79.
2005.
|
|
36
|
Kwak-Kim JY, Chung-Bang HS, Ng SC,
Ntrivalas EI, Mangubat CP, Beaman KD, Beer AE and Gilman-Sachs A:
Increased T helper 1 cytokine responses by circulating T cells are
present in women with recurrent pregnancy losses and in infertile
women with multiple implantation failures after IVF. Hum Reprod.
18:767–773. 2003. View Article : Google Scholar
|
|
37
|
Wang WJ, Hao CF, Qu QL, Wang X, Qiu LH and
Lin QD: The deregulation of regulatory T cells on
interleukin-17-producing T helper cells in patients with
unexplained early recurrent miscarriage. Hum Reprod. 25:2591–2596.
2010. View Article : Google Scholar
|
|
38
|
Zelenika D, Adams E, Humm S, Lin CY,
Waldmann H and Cobbold SP: The role of CD4+ T-cell subsets in
determining transplantation rejection or tolerance. Immunol Rev.
182:164–179. 2001. View Article : Google Scholar
|
|
39
|
Zenclussen AC, Gerlof K, Zenclussen ML,
Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J and Volk HD:
Abnormal T-cell reactivity against paternal antigens in spontaneous
abortion: Adoptive transfer of pregnancy-induced CD4+CD25+ T
regulatory cells prevents fetal rejection in a murine abortion
model. Am J Pathol. 166:811–822. 2005. View Article : Google Scholar
|
|
40
|
Yan L, Zheng D and Xu RH: Critical role of
tumor necrosis factor signaling in mesenchymal stem cell-based
therapy for autoimmune and inflammatory diseases. Front Immunol.
9:16582018. View Article : Google Scholar
|
|
41
|
Gabay C, Cakir N, Moral F, Roux-Lombard P,
Meyer O, Dayer JM, Vischer T, Yazici H and Guerne PA: Circulating
levels of tumor necrosis factor soluble receptors in systemic lupus
erythematosus are significantly higher than in other rheumatic
diseases and correlate with disease activity. J Rheumatol.
24:303–308. 1997.
|
|
42
|
Sharief MK and Hentges R: Association
between tumor necrosis factor-alpha and disease progression in
patients with multiple sclerosis. N Engl J Med. 325:467–472. 1991.
View Article : Google Scholar
|
|
43
|
Belkhelfa M, Rafa H, Medjeber O,
Arroul-Lammali A, Behairi N, Abada-Bendib M, Makrelouf M, Belarbi
S, Masmoudi AN, Tazir M and Touil-Boukoffa C: IFN-ү and TNF-α are
involved during Alzheimer disease progression and correlate with
nitric oxide production: A study in Algerian patients. J Interferon
Cytokine Res. 34:839–847. 2014. View Article : Google Scholar
|
|
44
|
Idriss HT and Naismith JH: TNF alpha and
the TNF receptor superfamily: Structure-function relationship(s).
Microsc Res Tech. 50:184–195. 2000. View Article : Google Scholar
|
|
45
|
Clark DA: Immunological factors in
pregnancy wastage: Fact or fiction. Am J Reprod Immunol.
59:277–300. 2008. View Article : Google Scholar
|
|
46
|
Clark DA: Anti-TNFalpha therapy in
immune-mediated subfertility: State of the art. J Reprod Immunol.
85:15–24. 2010. View Article : Google Scholar
|
|
47
|
Said EA, Dupuy FP, Trautmann L, Zhang Y,
Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y, et al:
Programmed death-1-induced interleukin-10 production by monocytes
impairs CD4+ T cell activation during HIV infection. Nat Med.
16:452–459. 2010. View Article : Google Scholar
|
|
48
|
Wedekind L and Belkacemi L: Altered
cytokine network in gestational diabetes mellitus affects maternal
insulin and placental-fetal development. J Diabetes Complications.
30:1393–1400. 2016. View Article : Google Scholar
|
|
49
|
Banerjee P, Jana SK, Pasricha P, Ghosh S,
Chakravarty B and Chaudhury K: Proinflammatory cytokines induced
altered expression of cyclooxygenase-2 gene results in unreceptive
endometrium in women with idiopathic recurrent spontaneous
miscarriage. Fertil Steril. 99:179–187 e2. 2013. View Article : Google Scholar
|
|
50
|
Tangri S and Raghupathy R: Expression of
cytokines in placentas of mice undergoing immunologically mediated
spontaneous fetal resorptions. Biol Reprod. 49:850–856. 1993.
View Article : Google Scholar
|
|
51
|
Ito K, Karasawa M, Kawano T, Akasaka T,
Koseki H, Akutsu Y, Kondo E, Sekiya S, Sekikawa K, Harada M, et al:
Involvement of decidual Valpha14 NKT cells in abortion. Proc Natl
Acad Sci USA. 97:740–744. 2000. View Article : Google Scholar
|
|
52
|
Alkhuriji AF, Alhimaidi AR, Babay ZA and
Wary AS: The relationship between cytokine gene polymorphism and
unexplained recurrent spontaneous abortion in Saudi females. Saudi
Med J. 34:484–489. 2013.
|
|
53
|
Aboutorabi R, Behzadi E, Sadegh MJ, Fatehi
SP, Semsarzadeh S, Zarrin Y, Kazemi M, Rafiee L and Mostafavi FS:
The study of association between polymorphism of TNF-α gene's
promoter region and recurrent pregnancy loss. J Reprod Infertil.
19:211–218. 2018.
|
|
54
|
Gerriets V, Bansal P, Goyal A and Khaddour
K: Tumor Necrosis Factor Inhibitors. StatPearls [Internet].
StatPearls Publishing; Treasure Island, FL: 2021
|
|
55
|
Wang T and He C: TNF-α and IL-6: The link
between immune and bone system. Curr Drug Targets. 21:213–227.
2020. View Article : Google Scholar
|
|
56
|
Zhang Y, Liu H, Tang W, Qiu Q and Peng J:
Resveratrol prevents TNF-α-induced VCAM-1 and ICAM-1 upregulation
in endothelial progenitor cells via reduction of NF-κB activation.
J Int Med Res. 48:3000605209451312020.
|
|
57
|
Brunner C, Seiderer J, Schlamp A,
Bidlingmaier M, Eigler A, Haimerl W, Lehr HA, Krieg AM, Hartmann G
and Endres S: Enhanced dendritic cell maturation by TNF-alpha or
cytidine-phosphate-guanosine DNA drives T cell activation in vitro
and therapeutic anti-tumor immune responses in vivo. J Immunol.
165:6278–6286. 2000. View Article : Google Scholar
|
|
58
|
Psarras A, Antanaviciute A, Alase A, Carr
I, Wittmann M, Emery P, Tsokos GC and Vital EM: TNF-α regulates
human plasmacytoid dendritic cells by suppressing IFN-α production
and enhancing T cell activation. J Immunol. 206:785–796. 2021.
View Article : Google Scholar
|
|
59
|
Conrad C, Di Domizio J, Mylonas A,
Belkhodja C, Demaria O, Navarini AA, Lapointe AK, French LE, Vernez
M and Gilliet M: TNF blockade induces a dysregulated type I
interferon response without autoimmunity in paradoxical psoriasis.
Nat Commun. 9:252018. View Article : Google Scholar
|
|
60
|
Falcon BJ, Cotechini T,
Macdonald-Goodfellow SK, Othman M and Graham CH: Abnormal
inflammation leads to maternal coagulopathies associated with
placental haemostatic alterations in a rat model of foetal loss.
Thromb Haemost. 107:438–447. 2012. View Article : Google Scholar
|
|
61
|
Dignass AU, Hartmann F, Sturm A and Stein
J: Management of inflammatory bowel diseases during pregnancy. Dig
Dis. 27:341–346. 2009. View Article : Google Scholar
|
|
62
|
Pelechas E and Drosos AA: Etanercept
biosimilar SB-4. Expert Opin Biol Ther. 19:173–179. 2019.
View Article : Google Scholar
|
|
63
|
Spencer-Green G: Etanercept (Enbrel):
Update on therapeutic use. Ann Rheum Dis. 59 (Suppl 1):i46–i49.
2000. View Article : Google Scholar
|
|
64
|
Elliot MJ, Maini RN, Feldmann M, Long-Fox
A, Charles P, Katasikis P, Brennan FM, Bijl H, Ghrayeb J and Woody
JN: Treatment of rheumatoid arthritis with chimeric monoclonal
antibodies to tumor necrosis factor alpha. Arthritis Rheum. 58 (2
Suppl):S92–S101. 2008. View Article : Google Scholar
|
|
65
|
Hanauer SB, Feagan BG, Lichtenstein GR,
Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson
A, Bao W, et al: Maintenance infliximab for Crohn's disease: The
ACCENT I randomised trial. Lancet. 359:1541–1549. 2002. View Article : Google Scholar
|
|
66
|
Yoo DH, Prodanovic N, Jaworski J, Miranda
P, Ramiterre E, Lanzon A, Baranauskaite A, Wiland P, Abud-Mendoza
C, Oparanov B, et al: Efficacy and safety of CT-P13 (biosimilar
infliximab) in patients with rheumatoid arthritis: Comparison
between switching from reference infliximab to CT-P13 and
continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis.
76:355–363. 2017. View Article : Google Scholar
|
|
67
|
Zheng MK, Shih DQ and Chen GC: Insights on
the use of biosimilars in the treatment of inflammatory bowel
disease. World J Gastroenterol. 23:1932–1943. 2017. View Article : Google Scholar
|
|
68
|
Deeks ED: GP2015: An etanercept
biosimilar. BioDrugs. 31:555–558. 2017. View Article : Google Scholar
|
|
69
|
Fiorino G, Gilardi D, Correale C, Furfaro
F, Roda G, Loy L, Argollo M, Allocca M, Peyrin-Biroulet L and
Danese S: Biosimilars of adalimumab: The upcoming challenge in IBD.
Expert Opin Biol Ther. 19:1023–1030. 2019. View Article : Google Scholar
|
|
70
|
Nelson AL, Dhimolea E and Reichert JM:
Development trends for human monoclonal antibody therapeutics. Nat
Rev Drug Discov. 9:767–774. 2010. View Article : Google Scholar
|
|
71
|
Deeks ED: Certolizumab pegol: A review in
inflammatory autoimmune diseases. BioDrugs. 30:607–617. 2016.
View Article : Google Scholar
|
|
72
|
Maxwell LJ, Zochling J, Boonen A, Singh
JA, Veras MM, Tanjong Ghogomu E, Benkhalti Jandu M, Tugwell P and
Wells GA: TNF-alpha inhibitors for ankylosing spondylitis. Cochrane
Database Syst Rev CD005468. 2015. View Article : Google Scholar
|
|
73
|
Steeland S, Libert C and Vandenbroucke RE:
A new venue of TNF targeting. Int J Mol Sci. 19:14422018.
View Article : Google Scholar
|
|
74
|
U.S. National Library of Medicine (NIH), .
A Trial With Humanized TNFα Monoclonal Antibody Injection by Single
Dose and Dose Escalation in Healthy Subjects. ClinicalTrials.gov
Identifier: NCT02460393. NIH; Bethesda, MD: 2015, https://clinicaltrials.gov/ct2/show/NCT02460393June
2–2015
|















