Introduction
Osteoarthritis (OA) is the most common bone and
joint disease in the elderly population. Its main characteristics
include synovitis, degeneration and destruction of cartilage, and
sclerosis of subchondral bone (1). Epidemiological statistics show that
the incidence rate of OA among patients >55 years of age is
44–70% (1). Patients with OA have
a poorer quality of life, present with numerous clinical symptoms
and the efficacy of treatment is low, which poses a heavy burden
for patients' relatives and society. There is currently no
effective treatment to prevent the occurrence and delay the
progression of OA (2,3). During OA, biochemical pathways of
chondrocytes are altered, leading to an increase in inflammatory
factors and the degradation of the extracellular matrix (ECM)
(4). Abnormal gene expression in
chondrocytes has been reported to be associated with cartilage
erosion (5). Therefore, it is
necessary to explore the regulatory mechanisms of genes affecting
chondrocyte inflammation and ECM degradation.
Fatty acid binding protein (FABP) is a fat-binding
protein present inside cells and is a member of the intracellular
lipid-binding protein family (6),
which consists of intracellular proteins with low molecular weight,
126–134 amino acid sequences and a strong affinity for long-chain
fatty acids (7). FABP4 is a
member of the FABP family, and is mainly expressed in mature
adipocytes and macrophages, and is secreted by adipocytes in
response to cAMP, thus playing a role in cellular lipid fluxes,
metabolism and signaling (8). In
addition to regulating lipid metabolism, FABP4 has been found to be
involved in multiple biological processes, including inflammation
(9). FABP4 has been found to
contribute to the pathogenesis of inflammatory-mediated diseases,
and downregulation of it can suppress inflammation, apoptosis and
oxidative stress in these diseases (10–13). As an inflammatory arthritis, OA
has been reported to be associated with the abnormal expression of
FABP4. A previous study suggested that FABP4 expression is
increased in plasma and synovial fluid in patients with OA
(14). Another study validated
that FABP4 could serve as a biomarker of OA via bioinformatics
analysis (15). Recently, FABP4
levels were found to be negatively associated with cartilage
thickness in end-stage knee OA (16). The aforementioned reports provide
evidence that FABP4 is involved in the occurrence and progression
OA. However, the specific effect of FABP4 in the regulation of OA
has not been investigated thus far, to the best of our knowledge.
Therefore, the present study aimed to clarify the effect of FABP4
on IL-1β-induced chondrocyte inflammation and the potential
mechanisms in vitro.
Materials and methods
Cell culture
ATDC5 cells were purchased from BeNa Culture
Collection (Beijing Beina Chunglian Institute of Biotechnology).
The cells were cultured in DMEM/Ham's F12 (Thermo Fisher
Scientific, Inc.) supplemented with 5% FBS (Thermo Fisher
Scientific, Inc.) and 100 U/ml penicillin/streptomycin in a
humidified incubator at 37°C with 5% CO2. Cells were
then treated with 10 ng/ml IL-1β (MilliporeSigma), with or without
10 µM GW9662 [peroxisome proliferator-activated receptor (PPAR)γ
inhibitor; Sigma-Aldrich; Merck KGaA] co-treatment at 37°C for 24
h.
Cell transfection
Small interfering RNAs (siRNAs) against FABP4
(si-FABP4-1 and si-FABP4-2) and siRNA negative control (NC; si-NC)
were purchased from Shanghai GenePharma Co., Ltd. ATDC5 cells
(1×106 per well) were seeded into 6-well plates and
cultured with complete medium without antibiotics for ≥24 h prior
to transfection. Next, cells were transiently transfected with 2
µg/ml siRNAs using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) and incubated at 37°C for 6 h. The
cells were harvested 48 h for analysis post-transfection. The
sequences were as follows: si-FABP4-1, 5′-GACGUUGACCUGGACUGAAd
TdT-3′ and 3′-UUCAGUCCAGGUCAACGUCdTdT-5′; si-FABP4-2,
5′-GUGGGAUAUAUUGUUCAAAdTdT-3′ and 3′-UUUGAACAAUAU-AUCCCACdTdT-5′;
and si-NC, 5′-UUAUGCCGAUCGCGUCACATT-3′ and
3′-TTAAUACGGCUAGCGCAG-UGU-5′.
Bioinformatics analysis
To verify whether PPARγ was a target of FABP4, the
database Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING; http://string-db.org/) was searched.
Cell Counting Kit-8 (CCK-8) assay
ATDC5 cells were plated into four 96-well plates
(2×104 cells per well). Upon culture overnight for
adherence, cells were treated with IL-1β or GW9662 co-treatment for
24 h, then CCK-8 solution (Dojindo Laboratories, Inc.) was diluted
with culture medium and added to the 96-well plates, followed by
incubation for 1 h at 37°C. Finally, the absorbance at 450 nm was
detected with a spectrophotometer (Thermo Fisher Scientific,
Inc.).
TUNEL assay
TUNEL staining was performed by using an In Situ
Apoptosis Detection Kit (MilliporeSigma) according to the
manufacturer's protocol. Briefly, ATDC5 cells (2×104)
cultured in 96-well plates were washed with PBS and fixed with 4%
paraformaldehyde for 30 min at room temperature. Cells were then
incubated for 90 min at 37°C with terminal deoxynucleotidyl
transferase (TdT) incubation buffer. NC cells were incubated
without TdT enzyme. The reaction was terminated by washing the
cells with PBS. The nuclei were stained with 5 µg/ml DAPI at room
temperature for 5 min, followed by observation under a fluorescence
microscope in three fields of view (Nikon Eclipse 80i; Nikon
Corporation; magnification, ×200).
ELISA
The levels of TNF-α (cat. no. PT512) and IL-6 (cat.
no. PI326) in ATDC5 cell culture supernatant were measured with
commercially available standard sandwich ELISA kits (Beyotime
Institute of Biotechnology) in accordance with the manufacturer's
instructions. Each sample was measured in triplicate. The level of
prostaglandin E2 (PGE2) was determined using a
Prostaglandin E2 ELISA kit (cat. no. ab133021; Abcam). Reactive
oxygen species (ROS) content in cells was detected by using a
ROS/Superoxide Detection Assay kit (Cell-based) (cat. no. ab139476;
Abcam). The expression of superoxide dismutase (SOD) was detected
using a SOD kit (cat. no. ab277415; Abcam). The expression of
glycosaminoglycan (GAG) was detected by spectrophotometry using
1,9-dimethylmethylene blue (DMMB; Sigma-Aldrich; Merck KGaA), which
can be used to monitor the level of sulfated GAG, as previously
reported (17).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from ATDC5 cells was extracted with
TRIzol® (Thermo Fisher Scientific, Inc.) according to
the supplier's protocol. Next, RT kits (Takara Bio, Inc.) were used
to reverse transcribe RNA into cDNA according to the manufacturer's
protocol. Subsequently, 50 ng cDNA was used for qPCR using TB
Green® Fast qPCR Mix (Takara Bio, Inc.) and an Applied
Biosystems 7500 Real-Time PCR System (Thermo Fisher Scientific,
Inc.). The following thermocycling conditions were used for qPCR:
95°C for 2 min; followed by 40 cycles of 95°C for 20 sec and 65°C
for 40 sec. The results were analyzed using the 2−ΔΔCq
method (18) with Gapdh as the
internal reference gene. The primers were as follows: FABP4
forward, 5′-TTCCTTCAAACTGGGCGTGG-3′ and reverse,
5′-GCCTTTCATAACACATTCCACC-3′; matrix metalloproteinase (Mmp)3
forward, 5′-TCCCACATCACCTACAGGATTG-3′ and reverse,
5′-CAGGCCCATCAAAAGGGACA-3′; Mmp9 forward,
5′-CAGCCGACTTTTGTGGTCTTC-3′ and reverse,
5′-CGGTACAAGTATGCCTCTGCCA-3′; Mmp13 forward,
5′-GGAGCCCTGATGTTTCCCAT-3′ and reverse,
5′-GTCTTCATCGCCTGGACCATA-3′; ADAM metalloproteinase with
thrombospondin type 1 motif 4 (Adamts-4) forward,
5′-CAAGCATCCGAAACCCTGTC-3′ and reverse, 5′-ACACAGGTCCTGCCGGG-3′;
and Gapdh forward, 5′-GGGTCCCAGCTTAGGTTCATC-3′ and reverse,
5′-CCAATACGGCCAAATCCGTTC-3′.
Western blot assay
Total protein was extracted from ATDC5 cells using a
RIPA kit (Beyotime Institute of Biotechnology). Harvested cells
were lysed on ice. Protein concentrations of the cell supernatants
were determined using a BCA Protein Assay kit (Beyotime Institute
of Biotechnology). The samples were mixed with loading buffer, and
heated in boiling water for 5 min. Equal quantities of proteins (40
µg per lane) were separated via 10–12% SDS-PAGE and then
transferred to a polyvinylidene fluoride membrane (MilliporeSigma).
After blocking with 5% non-fat milk in TBS with Tween-20 (TBST) for
2 h at room temperature, the membranes were incubated at 4°C
overnight with specific primary antibodies. Following washing in
TBST, the membranes were incubated with secondary goat anti-rabbit
IgG antibody (Abcam; 1:10,000; cat. no. ab6721) at room temperature
for 2 h and then washed again. Specific protein bands were
visualized by using an ECL Plus kit (cat. no. WBKLS0500;
MilliporeSigma) with a bio-imaging system (Quantity One, version
4.6.2; Bio-Rad Laboratories, Inc.). The densitometry analysis was
performed using ImageJ software (version 1.8.0; National Institutes
of Health). All experiments were performed three times
independently. The rabbit primary antibodies (Abcam) used included:
FABP4 (1:5,000; cat. no. ab92501), Bcl2 (1:1,000; cat. no.
ab32124), Bax (1:5,000; cat. no. ab32503), cleaved caspase3 (1:500;
cat. no. ab32042), caspase3 (1:2,000; cat. no. ab32351), MMP3
(1:10,000; cat. no. ab52915), MMP9 (1:10,000; cat. no. ab76003),
MMP13 (1:1,000; cat. no. ab219620), phosphorylated (p)-NF-κB p65
(1:1,000; cat. no. ab76302), NF-κB p65 (1:5,000; cat. no. ab16502),
PPARγ (1:1,000; cat. no. ab178860), β-actin (1:5,000; cat. no.
ab8227) and GAPDH (1:10,000; cat. no. ab9485).
Statistical analysis
All experiments performed in the present study were
repeated at least three times. The data were analyzed using
GraphPad Prism 7.0 (GraphPad Software, Inc.), and are presented as
the mean ± SD. Data were confirmed to be normally distributed using
the Shapiro-Wilk test. The comparison of two groups was performed
using an unpaired Student's t-test. Comparisons between multiple
groups were analyzed using one-way ANOVA followed by Tukey's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Knockdown of FABP4 suppresses the
activity and inflammatory damage of IL-1β-induced ATDC5 cells
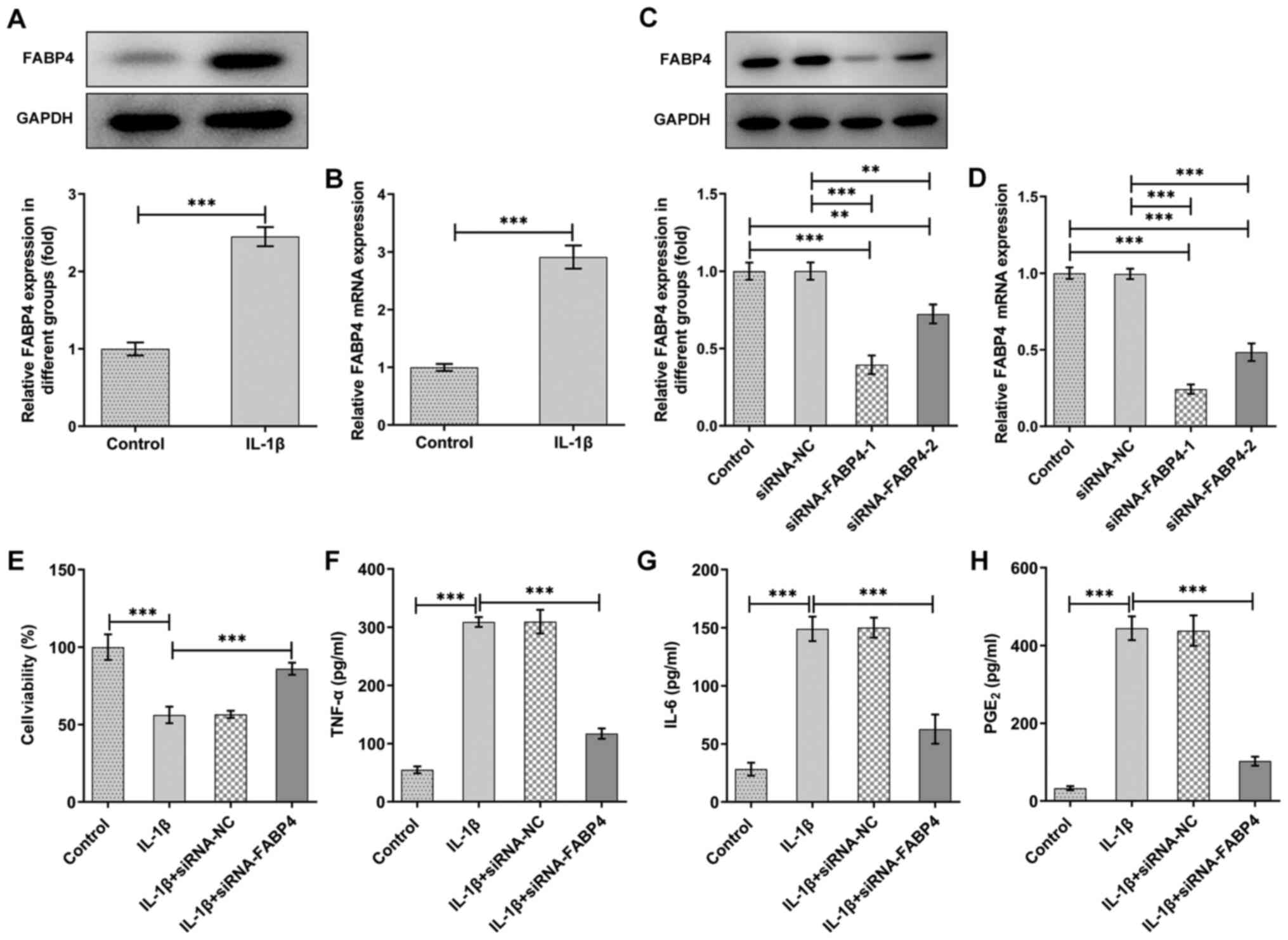
To detect the effect of FABP4 on IL-1β-induced ATDC5
cells, the expression of FABP4 was detected in untreated ATDC5
cells and IL-1β-induced ATDC5 cells by using RT-qPCR and western
blotting. As shown in Fig. 1A and
B, the expression of FABP4 was upregulated in IL-1β-induced
ATDC5 cells compared with that of control ATDC5 cells.
The expression of FABP4 was downregulated in the
siRNA-FABP4 groups, and the inhibitory effect of siRNA-FABP4-1 was
higher than that of siRNA-FABP4-2 (Fig. 1C and D). Thus, siRNA-FABP4-1 was
selected for subsequent experiments. Next, a CCK-8 assay was
performed to detect the changes in cell viability of IL-1β-induced
ATDC5 cells. The results shown in Fig. 1E revealed that ATDC5 cell
viability was reduced after induction with IL-1β, and the
inhibitory effect was partly abolished with knockdown of FABP4.
Overproduction of pro-inflammatory cytokines plays a
key role in the pathophysiology of OA (4). In the present study, it was observed
that IL-1β could significantly elevate the levels of the
pro-inflammatory cytokines, TNF-α and IL-6 (Fig. 1F and G), as well as those of the
cell growth and regulatory factor, PGE2 (Fig. 1H). However, knockdown of FABP4
suppressed the production of pro-inflammatory cytokines (Fig. 1F and G) and PGE2 in
IL-1β-induced ATDC5 (Fig.
1H).
Knockdown of FABP4 suppresses
oxidative stress and apoptosis in IL-1β-induced ATDC5 cells
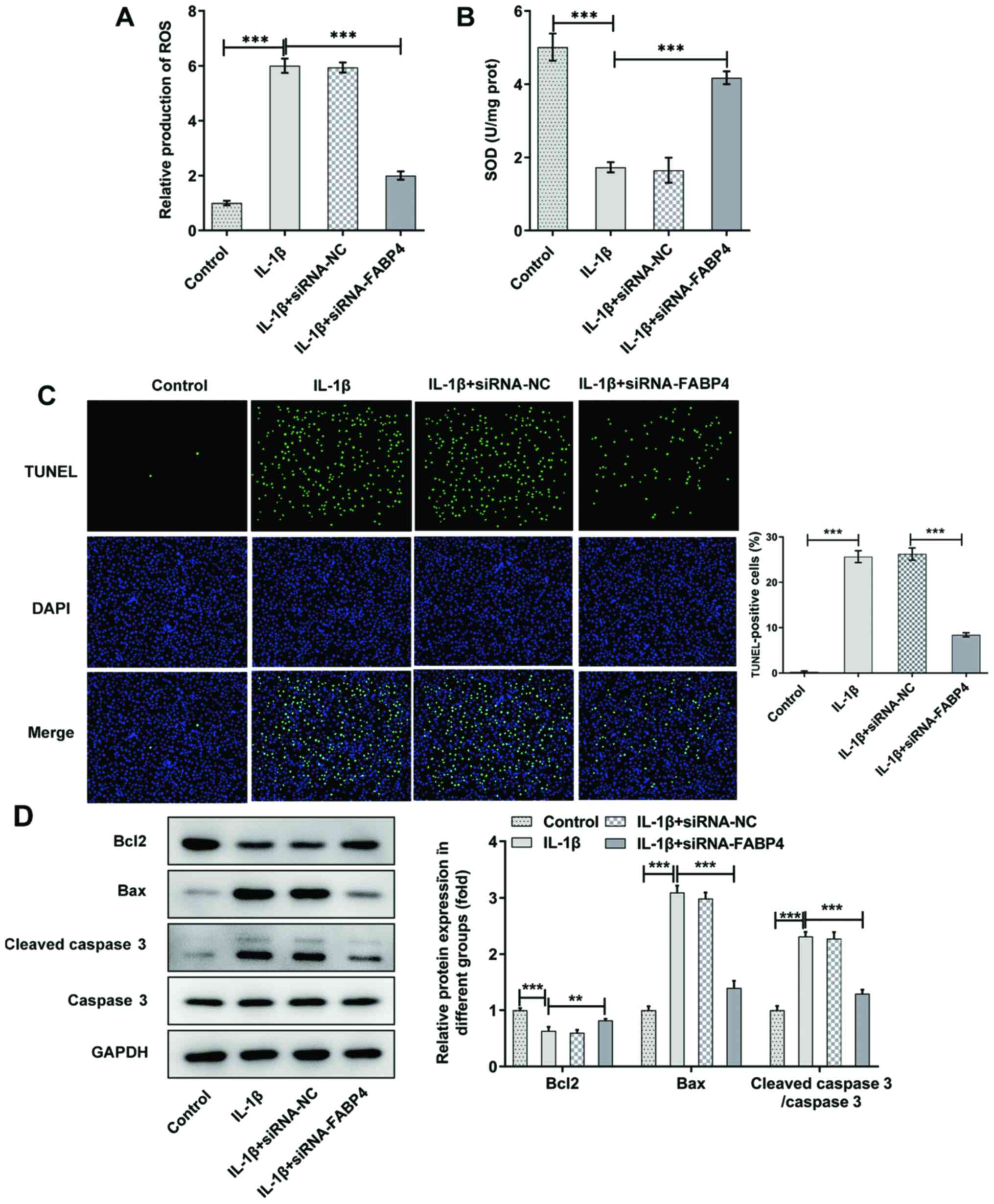
The levels of ROS and SOD were detected in untreated
ATDC5 cells or IL-1β-induced ATDC5 cells. As shown in Fig. 2A, the expression of ROS was
significantly increased (P<0.001) in IL-1β-induced ATDC5 cells
and downregulated after the knockdown of FABP4. The expression
trend of SOD showed the opposite trend to that of ROS (Fig. 2B).
TUNEL staining revealed that IL-1β increased
apoptosis, and knockdown of FABP4 could weaken the pro-apoptotic
effect of IL-1β (Fig. 2C). As
shown in Fig. 2D, compared with
IL-1β-induced ATDC5 cells, it was found that knockdown of FABP4
reduced cell apoptosis through downregulating Bax and cleaved
caspase 3 expression, and upregulating Bcl-2 expression in
IL-1β-induced ATDC5 cells.
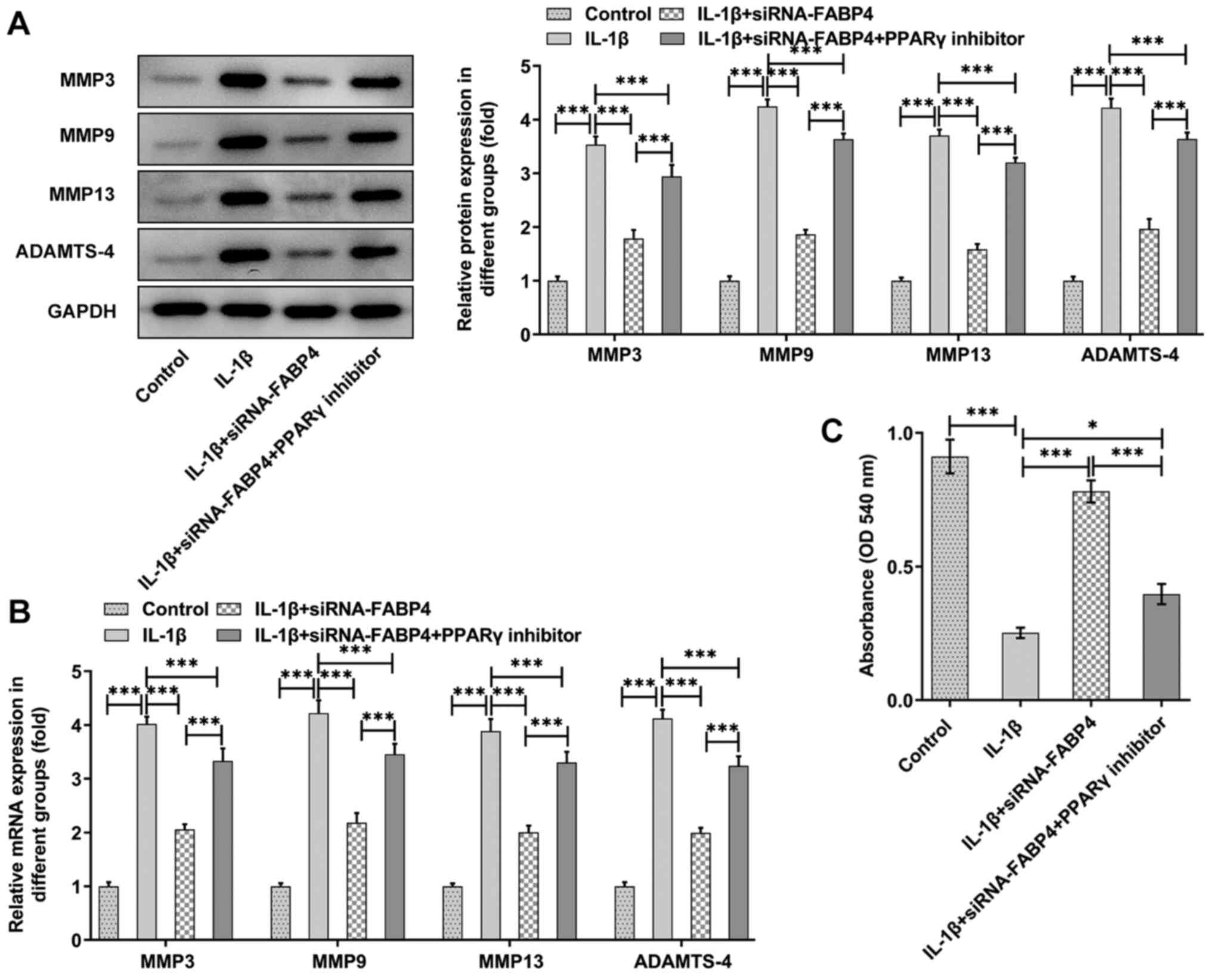
Knockdown of FABP4 suppresses the
expression of MMPs and promotes the expression of GAG
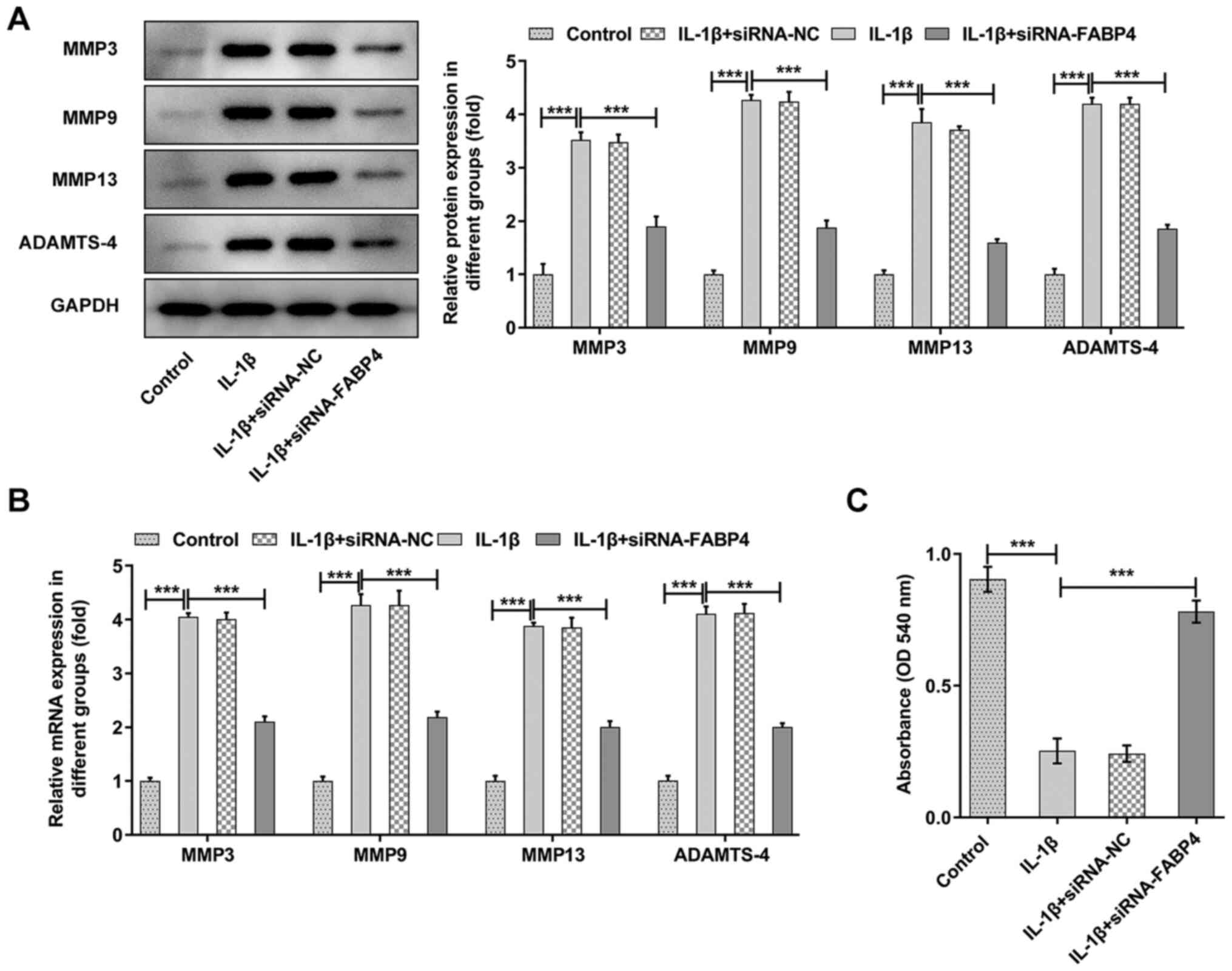
The expression of MMP3, MMP9, MMP13 and ADAMTS-4 was
significantly increased in the IL-1β group (P<0.001), and
knockdown of FABP4 could partly reverse the expression of MMP3,
MMP9, MMP13 and ADAMTS-4 (Fig. 3A and
B). Next, the expression of GAG was detected with a GAG ELISA
kit. As shown in Fig. 3C, it was
observed that IL-1β could significantly (P<0.001) reduce the
levels of GAG, and knockdown of FABP4 could partly reverse this
trend.
Knockdown of FABP4 suppresses the cell
viability and levels of inflammatory factors in IL-1β-induced ATDC5
cells by activating PPARγ
Data from the STRING database showed that FABP4 had
the potential to regulate the expression of PPARγ (Fig. 4A). The results of the present
analysis also showed that the expression of p-NF-κB p65 was
increased and that of PPARγ was decreased in IL-1β-induced ATDC5
cells. Knockdown of FABP4 significantly downregulated p-NF-κB p65
expression and upregulated the expression of PPARγ compared with
that of IL-1β-induced ATDC5 cells (Fig. 4B).
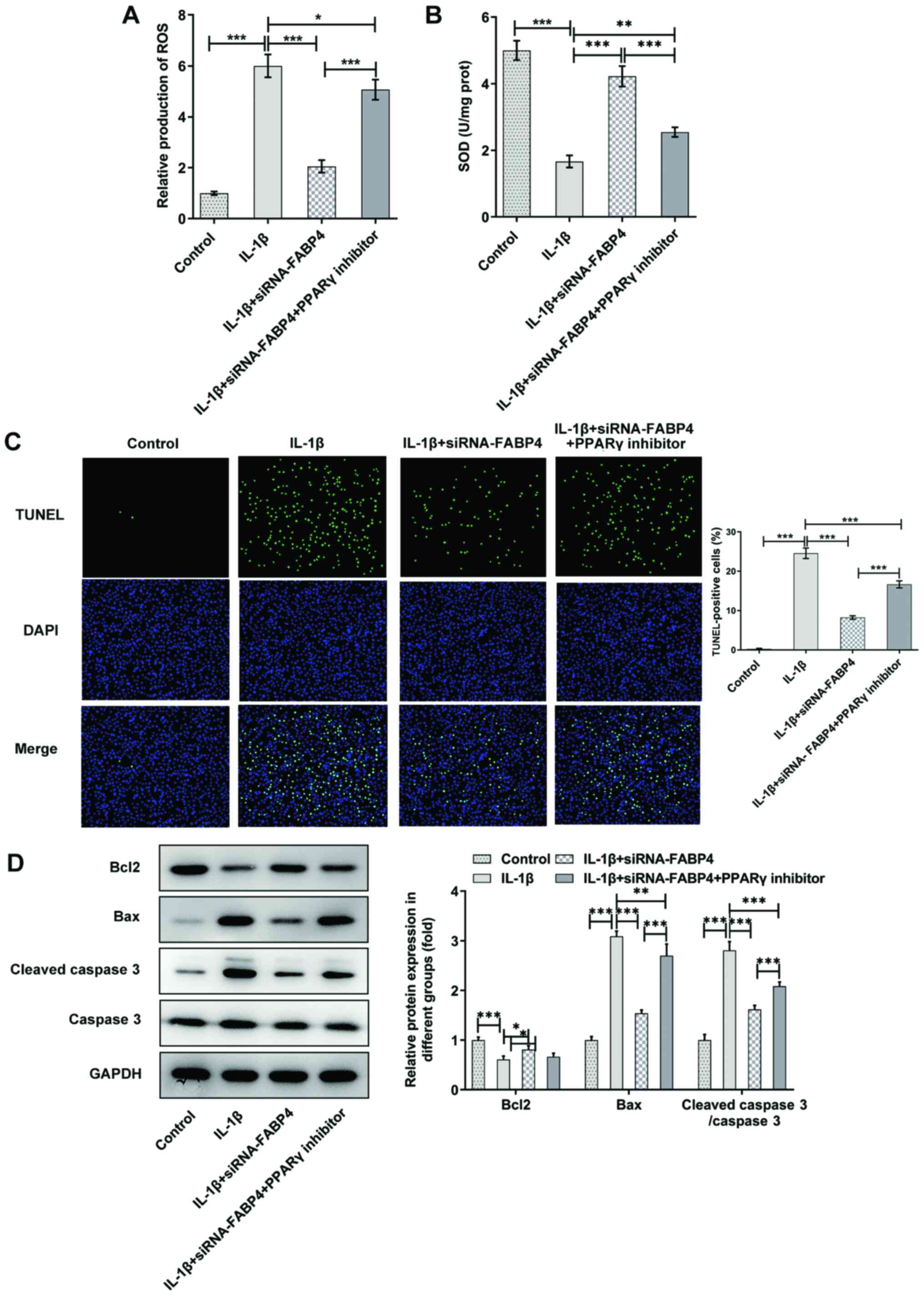
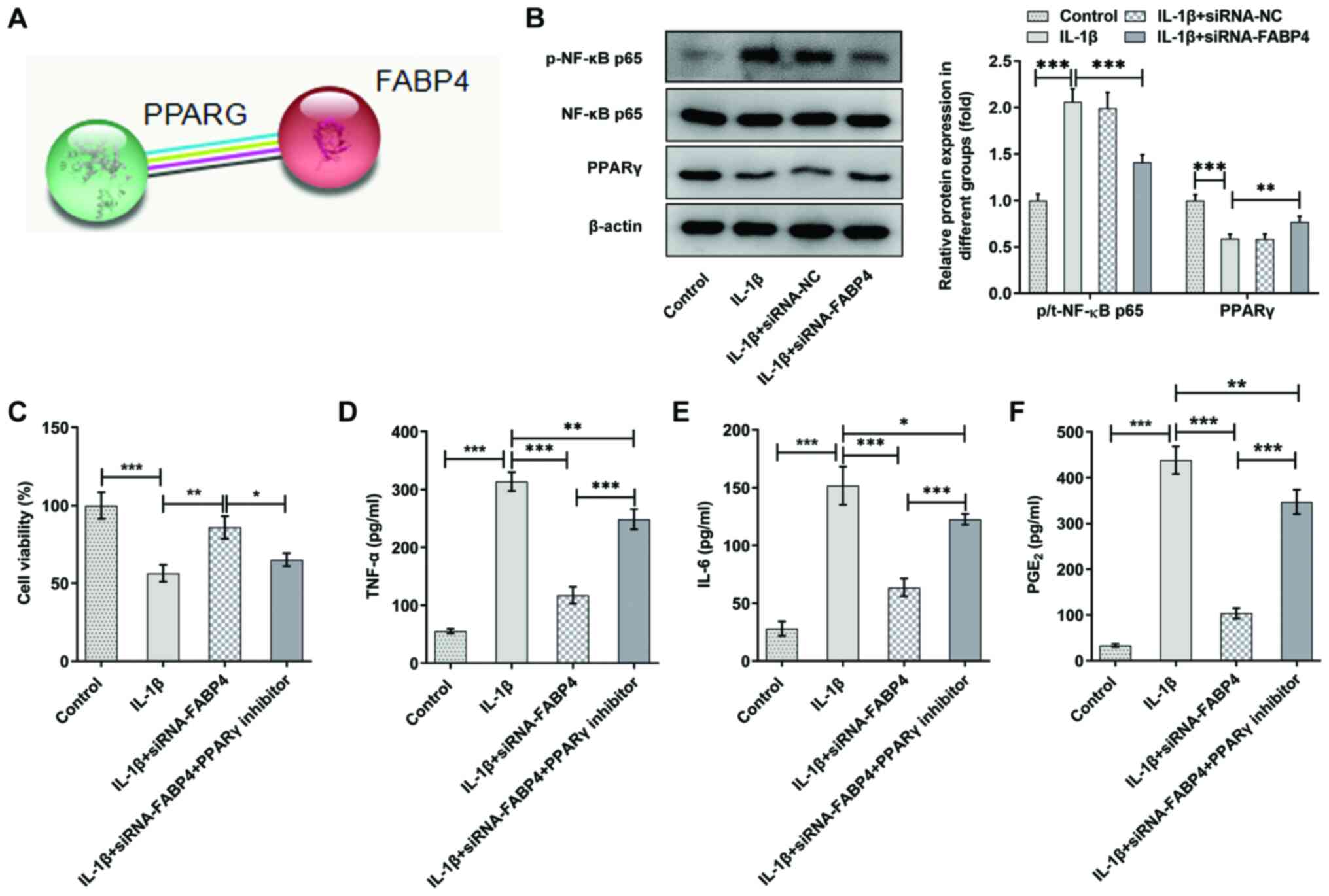 | Figure 4.Knockdown of FABP4 suppresses the
cell viability and inflammatory factors of IL-1β-induced ATDC5
cells by activating PPARγ. (A) Data from the Search Tool for the
Retrieval of Interacting Genes/Proteins database showed that FABP4
had the potential to regulate the expression of PPARγ. (B)
Expression levels of PPARγ and NF-κB p65 signaling proteins were
detected using western blotting (n=3). (C) Cell Counting Kit-8
assay was performed to detect the viability of IL-1β-induced ATDC5
cells with or without PPARγ inhibitor treatment (n=6). The levels
of (D) TNF-α, (E) IL-6 and (F) the cell growth and regulatory
factor PGE2 were measured by using ELISAs (n=4).
*P<0.05, **P<0.01, ***P<0.001. FABP4, fatty acid-binding
protein 4; PPARγ, peroxisome proliferator-activated receptor γ;
PGE2, prostaglandin E2; siRNA, small interfering RNA;
NC, negative control; p-, phosphorylated; t-, total. |
To explore the potential mechanisms of FABP4 in
ATDC5 cells, 10 µM GW9662 (a PPARγ inhibitor) was added to the
cells, which were transfected with siRNA-FABP4 and treated with
IL-1β. As shown in Fig. 4C, it
was found that knockdown of FABP4 could increase the viability of
IL-1β-induced ATDC5 cells by activating PPARγ. The expression
levels of TNF-α (Fig. 4D), IL-6
(Fig. 4E) and PGE2
(Fig. 4F) were upregulated in the
IL-1β + siRNA-FABP4 + PPARγ inhibitor group compared with that in
the IL-1β + siRNA-FABP4 group in IL-1β-induced ATDC5 cells. These
findings demonstrated that knockdown of FABP4 suppressed
inflammatory factors in IL-1β-induced ATDC5 cells by activating
PPARγ.
Knockdown of FABP4 suppresses
oxidative stress and apoptosis in IL-1β-induced ATDC5 cells by
activating PPARγ
The expression of ROS and SOD was detected in the
IL-1β + siRNA-FABP4 groups with or without PPARγ inhibitor
treatment. As shown in Fig. 5A,
the levels of ROS were significantly decreased in the IL-1β +
siRNA-FABP4 group compared with those in the IL-1β group, and were
upregulated after PPARγ inhibitor treatment. The expression trend
of SOD in the IL-1β + siRNA-FABP4 + PPARγ inhibitor group was
opposite to that recorded for ROS (Fig. 5B). TUNEL staining (Fig. 5C) and western blotting (Fig. 5D) showed that the knockdown of
FABP4 suppressed the apoptosis of IL-1β-induced ATDC5 cells by
activating PPARγ.
Knockdown of FABP4 suppresses MMP
expression and promotes the expression of GAG by activating
PPARγ
The expression levels of MMP3, MMP9, MMP13 and
ADAMTS-4 were detected using RT-qPCR and western blot analyses. The
results revealed that the expression levels of MMP3, MMP9, MMP13
and ADAMTS-4 were downregulated in the IL-1β + siRNA-FABP4 group,
which was reversed by the addition of the PPARγ inhibitor (Fig. 6A and B). Similarly, the expression
of GAG was detected by using a GAG ELISA kit, and the PPARγ
inhibitor also attenuated the FABP4 knockdown-induced upregulation
of GAG compared with that of the IL-1β + siRNA-FABP4 group
(Fig. 6C).
Discussion
OA is a complex disease regulated by multiple
factors, including age, sex, genetic factors and physical trauma,
but its pathogenesis remains unclear (2). It is generally accepted that the
pathological features of OA are structural destruction and
functional loss of articular cartilage caused by the imbalance of
articular cartilage ECM synthesis and metabolism (4,5).
Previous studies have suggested that FABP4 expression is increased
in the plasma and synovial fluid of patients with OA (14,15). The present study found that FABP4
knockdown suppressed inflammation, apoptosis and ECM degradation of
IL-1β-induced chondrocytes by activating PPARγ to regulate the
NF-κB signaling pathway.
The release of inflammatory cytokines is one of the
important inducing factors of OA (19). IL-1β serves an important role in
altering the normal structure and function of chondrocytes,
promoting the apoptosis of chondrocytes, degrading chondrocyte ECM,
participating in synovial inflammatory lesions and affecting bone
metabolism (20–22). Therefore, IL-1β was used in the
present study to induce ATDC5 cells in order to establish an
inflammatory environment.
Pro-inflammatory cytokines, such as TNF-α and IL-6,
and ROS are known to be involved in the initiation and progression
of OA (23). IL-6 is mainly
secreted by osteoblasts and stromal cells, and plays a role in
regulating osteoclast formation and bone resorption (24). Activated immature osteoclasts
participate directly in bone resorption, while MMPs degrade bone
ECM and induce bone resorption (25). TNF-α induces the expression of
MMPs and increases their activity, thereby inhibiting the synthesis
of proteoglycan and collagen, and accelerating the decomposition of
the cartilage ECM (25). IL-6 and
TNF-α cause the destruction of the ECM, but also inhibit its repair
(26). The current study found
that knockdown of FABP4 suppressed pro-inflammatory cytokines and
ROS production, but increased SOD concentration in IL-1β-induced
ATDC5 cells. The degeneration of cartilage may be caused by the
reduced number of chondrocytes in the articular cartilage, which
fails to regenerate and remodel the cartilage appropriately.
Increased apoptosis of chondrocyte induced by ROS or
pro-inflammatory cytokines has been documented to reduce the number
of chondrocytes, resulting in OA initiation and progression
(27). In the current study,
IL-1β stimulation markedly increased ATDC5 cell apoptosis, but
FABP4 knockdown effectively reduced the ratio of apoptotic
cells.
OA is regulated by a variety of factors whose common
pathway is the inability of chondrocytes to maintain a balance
between ECM synthesis and degradation (28). The two main structural components
of chondrocyte ECM are proteoglycan and type II collagen (29). OA is characterized by early loss
of proteoglycans, followed by irreversible degradation of collagen,
and the depletion of proteoglycans is mainly due to aggregation of
proteoglycans (30,31). ADAMTS4 belongs to the ADAMTS
family, and is a marker of cartilage degradation in OA (30). In addition, MMPs play an important
role in cartilage degradation in OA, and the loss of GAG is a
fundamental factor in the development of OA (32,33). Since knockdown of FABP4 suppressed
pro-inflammatory cytokines production, the present study next
detected the main components of chondrocyte ECM and the expression
of MMPs. It was found that knockdown of FABP4 suppressed MMPs and
ADAMTS-4 expression, and promoted the expression of GAG.
FABP4 was predicted to regulate PPARγ expression
using the STRING database. Moreover, a previous study reported that
FABP4 downregulated PPARγ expression to regulate adipogenesis
(34). In addition, activation of
PPARγ can inhibit the expression of NF-κB signaling, thereby
suppressing the injury of chondrocytes caused by LPS, and
ultimately alleviating OA (35,36). Upon stimulation, the activated
p-NF-κB p65 can trigger the expression of an array of genes that
induce destruction of the articular joint, leading to OA onset and
progression (37). Therefore, to
further explore the molecular mechanism of FABP4 involved in the
regulation of OA, the present study analyzed the effect of FABP4 on
PPARγ and NF-κB p65 expression levels. It was found that IL-1β
significantly reduced PPARγ expression and increased p-p65
expression levels, but FABP4 knockdown recovered PPARγ and p-p65
expression levels. These results were in accordance with previous
studies, which revealed that activation of PPARγ effectively
suppressed IL-1β-induced inflammation in OA (38,39).
To further validate whether the effect of FABP4
knockdown on ATDC5 cell was dependent on activating PPARγ, PPARγ
inhibitor GW9662 was added. Following the addition of GW9662 to the
cells, it was found that the presence of GW9662 blocked the effects
of FABP4 knockdown on IL-1β-induced ATDC5 cell. However, these
results were based on in vitro experiment, lacking the
validation of in vivo studies. Besides, whether FABP4
exerted its effect on OA via other pathways needs to be elucidated.
Moreover, the chondrogenic potential of ATDC5, such as the
expression of collagen type 2 and aggrecan needs to be investigated
in subsequent experiments.
Overall, the present findings clarified the effect
of FABP4 on IL-1β-induced chondrocytes. The results indicated that
FABP4 knockdown suppressed inflammation, apoptosis, oxidative
stress and ECM degradation of IL-1β-induced chondrocytes by
activating PPARγ to regulate the NF-κB signaling pathway. The
findings of the present study could also provide a novel option for
the clinical treatment of OA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Zhejiang Province (grant no. Y19H060053).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HM and WW conceived and designed the study. HM, BH,
HL and YT performed the experiments to acquire the data. HM and BH
analysed and interpreted the data. HM and WW drafted the manuscript
and revised it for critically important intellectual content. All
authors read and approved the final manuscript. HM and WW confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McAlindon TE and Bannuru RR: OARSI
recommendations for the management of hip and knee osteoarthritis:
The semantics of differences and changes. Osteoarthritis Cartilage.
18:473–475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bian Q, Wang YJ, Liu SF and Li YP:
Osteoarthritis: Genetic factors, animal models, mechanisms, and
therapies. Front Biosci (Elite Ed). 4:74–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moon KH: New view on the initial
development site and radiographic classification system of
osteoarthritis of the knee based on radiographic analysis. Int J
Biomed Sci. 8:233–243. 2012.PubMed/NCBI
|
|
4
|
Troeberg L and Nagase H: Proteases
involved in cartilage matrix degradation in osteoarthritis. Biochim
Biophys Acta. 1824:133–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boehme KA and Rolauffs B: Onset and
Progression of Human Osteoarthritis-Can Growth Factors,
Inflammatory Cytokines, or Differential miRNA Expression
Concomitantly Induce Proliferation, ECM Degradation, and
Inflammation in Articular Cartilage? Int J Mol Sci. 19:22822018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Ding L, Yang J, Liu L and Dong L:
Intestinal fatty acid-binding protein, a biomarker of intestinal
barrier dysfunction, increases with the progression of type 2
diabetes. PeerJ. 9:e108002021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sung CH, Hsu BG, Tasi JP, Wang CH and Kuo
CH: Positive Associations between Adipocyte Fatty Acid-Binding
Protein Level and Central Arterial Stiffness in Peritoneal Dialysis
Patients. Int J Hypertens. 2021:88491152021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lei C, Li M, Zhang M, Wang S, Tian J, Wen
J and Li Y: Cloning, molecular characterization, and nutritional
regulation of fatty acid-binding protein family genes in gold
pompanos (Trachinotus ovatus). Comp Biochem Physiol B
Biochem Mol Biol. 246-247:1104632020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hotamisligil GS and Bernlohr DA: Metabolic
functions of FABPs - mechanisms and therapeutic implications. Nat
Rev Endocrinol. 11:592–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao F, Jiang DD, Guo WH, Guo LS, Gao MM,
Bai Y, Wang X and Zhang LS: FABP4 inhibitor attenuates inflammation
and endoplasmic reticulum stress of islet in leptin receptor
knockout rats. Eur Rev Med Pharmacol Sci. 24:12808–12820.
2020.PubMed/NCBI
|
|
11
|
Ge XN, Bastan I, Dileepan M, Greenberg Y,
Ha SG, Steen KA, Bernlohr DA, Rao SP and Sriramarao P: FABP4
regulates eosinophil recruitment and activation in allergic airway
inflammation. Am J Physiol Lung Cell Mol Physiol. 315:L227–L240.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gong Y, Yu Z, Gao Y, Deng L, Wang M, Chen
Y, Li J and Cheng B: FABP4 inhibitors suppress inflammation and
oxidative stress in murine and cell models of acute lung injury.
Biochem Biophys Res Commun. 496:1115–1121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Furuhashi M: Fatty Acid-Binding Protein 4
in Cardiovascular and Metabolic Diseases. J Atheroscler Thromb.
26:216–232. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, Li T, Chiu KY, Wen C, Xu A and
Yan CH: FABP4 as a biomarker for knee osteoarthritis. Biomarkers
Med. 12:107–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu HY, Yang M, Guo J, Zhang C, Lin LL, Liu
Y and Wei RX: Identification of the Biomarkers and Pathological
Process of Osteoarthritis: Weighted Gene Co-expression Network
Analysis. Front Physiol. 10:2752019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schadler P, Lohberger B, Thauerer B,
Faschingbauer M, Kullich W, Stradner MH, Husic R, Leithner A and
Steinecker-Frohnwieser B: Fatty Acid-Binding Protein 4 (FABP4) Is
Associated with Cartilage Thickness in End-Stage Knee
Osteoarthritis. Cartilage. 194760352110115202021.PubMed/NCBI
|
|
17
|
Huang TL, Yang CH, Yanai G, Liao JY, Sumi
S and Yang KC: Synergistic effect of l-ascorbic acid and hyaluronic
acid on the expressions of matrix metalloproteinase-3 and −9 in
human chondrocytes. J Biomed Mater Res B Appl Biomater.
106:1809–1817. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brown KA, Davidson EJ, Johnson AL, Wulster
KB and Ortved K: Inflammatory cytokines in horses with cervical
articular process joint osteoarthritis on standing cone beam
computed tomography. Equine Vet J. 2020.PubMed/NCBI
|
|
20
|
Hwang HS, Park IY, Kim DW, Choi SY, Jung
YO and Kim HA: PEP-1-FK506BP12 inhibits matrix metalloproteinase
expression in human articular chondrocytes and in a mouse
carrageenan-induced arthritis model. BMB Rep. 48:407–412. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhi LQ, Yao SX, Liu HL, Li M, Duan N and
Ma JB: Hydroxytyrosol inhibits the inflammatory response of
osteoarthritis chondrocytes via SIRT6-mediated autophagy. Mol Med
Rep. 17:4035–4042. 2018.PubMed/NCBI
|
|
22
|
Zhang Y, Huang X and Yuan Y:
Anti-inflammatory capacity of Apremilast in human chondrocytes is
dependent on SOX-9. Inflamm Res. 69:1123–1132. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oliviero F, Ramonda R, Scanu A, Galozzi P,
Favero M and Punzi L: Levels of inflammatory cytokines and
metalloproteinases are increased in knee synovial fluid of patients
with concomitant erosive hand osteoarthritis. Clin Exp Rheumatol.
38:8002020.PubMed/NCBI
|
|
24
|
Deng Z, Zhang R, Li M, Wang S, Fu G, Jin
J, Wang Z, Ma Y and Zheng Q: STAT3/IL-6 dependent induction of
inflammatory response in osteoblast and osteoclast formation in
nanoscale wear particle-induced aseptic prosthesis loosening.
Biomater Sci. 9:1291–1300. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang TTCC, Chen CL, Lin WC, Lin YS, Tseng
PC, Hsieh CY, Chen YH, Huang WC, Tsai CC, Wang CY, et al: Glycogen
synthase kinase-3β inactivation is an intracellular marker and
regulator for endotoxemic neutrophilia. J Mol Med (Berl).
91:207–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Solovykh EA, Karaoglanova TB, Kushlinskii
NE and Yanushevich OO: Matrix metalloproteinases and inflammatory
cytokines in the oral fluid of patients with chronic generalized
periodontitis various structural materials restoration of teeth and
dentition. Klin Lab Diagn. 55-58:18–21. 2013.(In English, Russian).
PubMed/NCBI
|
|
27
|
Hwang HS and Kim HA: Chondrocyte Apoptosis
in the Pathogenesis of Osteoarthritis. Int J Mol Sci.
16:26035–26054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding W, Du D and Chen S: LIPUS promotes
synthesis and secretion of extracellular matrix and reduces cell
apoptosis in human osteoarthritis through upregulation of SOX9
expression. Int J Clin Exp Pathol. 13:810–817. 2020.PubMed/NCBI
|
|
29
|
Gepstein A, Arbel G, Blumenfeld I, Peled M
and Livne E: Association of metalloproteinases, tissue inhibitors
of matrix metalloproteinases, and proteoglycans with development,
aging, and osteoarthritis processes in mouse temporomandibular
joint. Histochem Cell Biol. 120:23–32. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wen Y, Qin J, Deng Y, Wang H, Magdalou J
and Chen L: The critical role of UDP-galactose-4-epimerase in
osteoarthritis: Modulating proteoglycans synthesis of the articular
chondrocytes. Biochem Biophys Res Commun. 452:906–911. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Slovacek H, Khanna R, Poredos P, Jezovnik
M, Hoppensteadt D, Fareed J and Hopkinson W: Interrelationship of
Osteopontin, MMP-9 and ADAMTS4 in Patients With Osteoarthritis
Undergoing Total Joint Arthroplasty. Clin Appl Thromb Hemost.
26:10760296209648642020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ojanen SP, Finnilä MAJ, Reunamo AE,
Ronkainen AP, Mikkonen S, Herzog W, Saarakkala S and Korhonen RK:
Site-specific glycosaminoglycan content is better maintained in the
pericellular matrix than the extracellular matrix in early
post-traumatic osteoarthritis. PLoS One. 13:e01962032018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mehana EE, Khafaga AF and El-Blehi SS: The
role of matrix metalloproteinases in osteoarthritis pathogenesis:
An updated review. Life Sci. 234:1167862019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garin-Shkolnik T, Rudich A, Hotamisligil
GS and Rubinstein M: FABP4 attenuates PPARγ and adipogenesis and is
inversely correlated with PPARγ in adipose tissues. Diabetes.
63:900–911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang JS, Xiao WW, Zhong YS, Li XD, Du SX,
Xie P, Zheng GZ and Han JM: Galectin-3 deficiency protects
lipopolysaccharide-induced chondrocytes injury via regulation of
TLR4 and PPAR-γ-mediated NF-κB signaling pathway. J Cell Biochem.
120:10195–10204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kobayashi T, Notoya K, Naito T, Unno S,
Nakamura A, Martel-Pelletier J and Pelletier JP: Pioglitazone, a
peroxisome proliferator-activated receptor gamma agonist, reduces
the progression of experimental osteoarthritis in guinea pigs.
Arthritis Rheum. 52:479–487. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rigoglou S and Papavassiliou AG: The NF-κB
signalling pathway in osteoarthritis. Int J Biochem Cell Biol.
45:2580–2584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jia T, Cai M, Ma X, Li M, Qiao J and Chen
T: Oridonin inhibits IL-1β-induced inflammation in human
osteoarthritis chondrocytes by activating PPAR-γ. Int
Immunopharmacol. 69:382–388. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qu Y, Zhou L and Wang C: Mangiferin
Inhibits IL-1β-Induced Inflammatory Response by Activating PPAR-γ
in Human Osteoarthritis Chondrocytes. Inflammation. 40:52–57. 2017.
View Article : Google Scholar : PubMed/NCBI
|