Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common type of non-Hodgkin lymphoma globally, accounting for 30–40%
of all cases in different geographic regions. DLBCL is an
aggressive disease with heterogeneous genes, phenotypes and
clinical characteristics (1). The
patients most often present with a rapidly growing tumor mass in a
single or multiple lymph nodes, and even outside the lymph nodes.
Combining rituximab and cyclophosphamide, Adriamycin, vincristine
and prednisone (CHOP regimen) can significantly improve the
prognosis of patients with DLBCL, and the 3-year recurrence-free
survival period can reach 65–80%. However, 30–40% of patients with
DLBCL will relapse or develop refractory disease, which is still
the main cause of patient-related deaths. Therefore, molecular
discovery may be the best way forward for designing new therapies
in precision medicine. In addition to traditional diagnostic
functions, it is also necessary to explore prognostic markers and
potential therapeutic targets (2).
MicroRNA (miRNA/miR) is a type of endogenous
non-coding RNA, 18–25 nucleotides in length, which is ubiquitously
expressed both intra- and extracellularly. Its biological function
can lead to mRNA degradation or translation inhibition, thereby
playing an important regulatory role in animal and plant cells
(3). This post-transcriptional
regulation can directly affect the function of target genes and
participate in the physiological and pathological processes of
multiple systems of the body, including the digestive system. At
present, a large number of clinical and basic studies have revealed
that miRNA can act as an oncogene or tumor suppressor and has key
functions in tumorigenesis and malignant progression (4,5).
Several studies have suggested that Homo
sapiens (hsa)-miR-429 acts as a tumor suppressor in a variety
of malignant tumors. hsa-miR-429 can inhibit the Raf/MEK/ERK
pathway by targeting CRK like proto-oncogene adaptor protein to
reduce the migration of liver cancer cells and reverse
epithelial-mesenchymal transition (6,7).
Moreover, it can inhibit hepatocyte proliferation and liver
regeneration by targeting the JUN/MYC/BCL2/cyclin D1 axis (8). hsa-miR-429 exerts a
tumor-suppressive effect by targeting fascin actin-bundling protein
1 in gastric cancer cells (9). In
addition, the expression of hsa-miR-429 in patients with bladder
cancer surviving for 5 years was revealed to be significantly
higher compared with that in deceased patients, and it was a
potential prognostic indicator of bladder cancer (10). hsa-miR-429 can also promote the
proliferation of bladder cancer cells by inhibiting
cyclin-dependent kinase inhibitor 2B (11). hsa-miR-429 can inhibit the
progression and metastasis of osteosarcoma by targeting zinc finger
E-box binding homeobox 1 (12).
hsa-miR-429 also regulates the proliferation and apoptosis of
Wilms' tumor cells by targeting c-Myc, reduces the perineural
invasion of pancreatic cancer by inhibiting neurotrophic factor-3,
and inhibits the migration and invasion of esophageal squamous cell
carcinoma cells by targeting Slug (13–15). Previous studies have suggested
that long non-coding RNA (lncRNA) small nucleolar RNA host gene 6
(SNHG6) is a regulatory factor of hsa-miR-429. When the expression
levels of SNHG6 increase, the concentration of free hsa-miR-429 in
the cell is significantly reduced, which promotes the progression
of Wilms' tumor (16).
The role of hsa-miR-429 in breast cancer is
controversial. A previous study has revealed that hsa-miR-429
inhibits the proliferation and invasion of breast cancer cells by
inhibiting the Wnt/β-catenin signaling pathway (17). Moreover, in erb-b2 receptor
tyrosine kinase 2 positive (HER2+) breast cancer,
hsa-miR-429 could regulate the hypoxia-inducible factor 1 subunit α
(HIF1α) pathway by directly targeting von Hippel-Lindau tumor
suppressor mRNA, an important molecule that degrades HIF1α.
Overexpression of hsa-miR-429 in HER2+ breast cancer was
revealed to lead to an increase in breast cancer cell proliferation
and migration, whereas silencing of hsa-miR-429 could delay tumor
growth. This suggests that hsa-miR-429 may play a dual role in
different tumors (18). However,
to the best of our knowledge, the molecular function and mechanism
of action of hsa-miR-429 in DLBCL have never been evaluated.
Materials and methods
Cell culture
The DLBCL cell lines, SUDHL-4 and DB, were provided
by The Chinese Academy of Sciences Stem Cell Bank. Both are
suspension cells, cultured with 90% RPMI-1640 + 10% FBS (both from
HyClone; Cytiva) at 37°C and 5% CO2.
Clinical sample collection
A total of 10 newly diagnosed patients with DLBCL
admitted to the Department of Hematology, Jingzhou Central Hospital
from January 2019 to June 2019 were included in the present study.
There were 7 males and 3 females with a median age of 65 years
(50–76 years). Inclusion criteria were as follows: lymph node
enlargement was the first manifestation of all patients. The lymph
nodes with suspected lesions were completely resected and further
analyzed by immunohistochemistry after paraffin embedding. The
immunohistochemical analysis included CD20, CD3, CD5, CD10, CD45,
CD30, Bcl-2, Bcl-6, Ki-67, Mum-1, and MYC. The pathological
diagnosis was in line with the World Health Organization (WHO)
diagnostic criteria for hematopoietic and lymphoid tissue tumors in
2008. Exclusion criteria were as follows: previous hematopoietic
and lymphoid tumor. Normal tissue was obtained from patients
hospitalized at the Department of Hematology at the same period,
with lymphadenopathy as the main manifestation, and lymphadenitis
confirmed by immunohistochemical analysis after complete excision
of disaffected lymph nodes. The samples used in this study were
collected from the discarded samples after lymph node biopsy of
patients without affecting the disease diagnosis and treatment of
patients, with the consent of patients and their family members.
Ethical approval was obtained from The Ethics Committee of Jingzhou
Central Hospital (Jingzhou, China). Written informed consent was
obtained from the patients for their anonymized information to be
published in this article. All patients consented to participate in
the study by having their samples used in clinical research.
Quantitative PCR (qPCR)
Total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.) from DLBCL tissue.
Bulge-Loop hsa-miR-429 Primer Set (cat. no. MQPS0001297-1-100) was
used to quantify hsa-miR-429 by qPCR with SYBR-Green (FP411-02;
TIANGEN). The thermocycling conditions were as follows: Initial
denaturation, 95°C, 15 min; followed by a 45-cycle, 94°C, 20 sec,
60°C, 34 sec). The primer sequences of U6 (internal reference) were
as follows: forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′. The aforementioned kit was purchased
from Guangzhou RiboBio Co., Ltd. It was used according to the
manufacturer's instructions. The relative expression levels were
calculated using the 2−ΔΔCq method (19).
Transfection
hsa-miR-429 mimic (cat. no. miR10001536-1-5;
sequence: 5′-UAAUACUGUCUGGUAAAACCGU-3′), mimic negative control
(cat. no. miR1N0000001-1-5; sequence:
5′-UUUGUACUACACAAAAGUACUG-3′), hsa-miR-429 inhibitor (cat. no.
miR20001536-1-5; sequence: 5′-ACGGUUUUACCAGACAGUAUUA-3′) and
inhibitor negative control (cat. no. miR2N0000001-1-5; sequence:
5′-CAGUACUUUUGUGUAGUACAAA-3′) were purchased from Guangzhou RiboBio
Co., Ltd. miRNA mimic and inhibitor were transfected into cell
culture plates with Ribofect™ CP Reagent (cat. no. C10511-05;
Guangzhou RiboBio Co., Ltd.), and cultured at 37°C for 48 h. The
transfection concentrations were 50 nM (miRNA mimic) and 100 nM
(miRNA inhibitor). A total of 20 µM miRNA mimic or inhibitor was
diluted with 1X Ribofect™ CP Buffer as recommended and gently
mixed. The Ribofect™ CP Reagent was added, mixed gently, and
incubated at room temperature for 10–15 min. The Ribofect™ CP
mixture was added to the complete medium and mixed gently.
Subsequently, the culture plate was placed in a CO2
incubator at 37°C for 48 h, and the related cellular and molecular
experiments were carried out.
Cell proliferation experiment
Cell proliferation was evaluated using a Cell
Counting Kit-8 (CCK-8; cat. no. C0037; Beyotime Institute of
Biotechnology) assay. A total of 1×103 cells in 100 µl
medium were plated into a 96-well plate. After 48 h incubation at
37°C, 10 µl of CCK8 was added to each well and incubated at 37°C in
dark for 1 h. The absorbance was measured at 450 nm.
Cell colony formation assay
The cells were seeded into a 6-well plate at a
concentration of 1×103 cells in 2 ml complete medium and
incubated at 37°C, 5% CO2 for 14 days. The medium was
changed every 3 days. After the formation of visible colonies, the
cells were fixed with 4% formaldehyde for 20 min at room
temperature and stained with 0.1% crystal violet for 10–15 min at
room temperature. After washing away the background color,
photomicrographs were obtained and the number of colonies was
counted by eye.
Apoptosis analysis
The cells were stained with Annexin V-FITC Apoptosis
Detection kit (cat. no. C1062L; Beyotime Institute of
Biotechnology) at room temperature in dark for 10 min. The kit was
used according to the manufacturer's instructions. The apoptotic
rate was detected by flow cytometry (BD LSRFortessa and
FlowJo7.6.5; BD Biosciences).
Cell cycle analysis
The cells at 70% confluent were collected by
centrifugation at 1,000 × g for 5 min at 22°C and fixed with
ice-cold 70% ethanol at 4°C for 30 min or longer. The cells were
stained with Cell Cycle and Apoptosis Analysis kit (cat. no. C1052;
Beyotime Institute of Biotechnology) at 37°C in dark for 30 min.
The kit was used according to the manufacturer's instructions. The
cell cycle distribution was detected by flow cytometry (BD
LSRFortessa and FlowJo7.6.5; BD Biosciences).
Target gene prediction
TargetScan Human 7.2 (version 7.2; targetscan.org/vert_72/) was used to predict the
target gene of hsa-miR-429.
Dual luciferase reporter gene
assay
hsa-miR-429 mimic (cat. no. miR10001536-1-5;
Sequence: 5′-UAAUACUGUCUGGUAAAACCGU-3′), mimic negative control
(cat. no. miR1N0000001-1-5; sequence:
5′-UUUGUACUACACAAAAGUACUG-3′), hsa-miR-429 inhibitor (cat. no.
miR20001536-1-5; sequence: 5′-ACGGUUUUACCAGACAGUAUUA-3′) and
inhibitor negative control (cat. no. miR2N0000001-1-5; sequence:
5′-CAGUACUUUUGUGUAGUACAAA-3′) were purchased from Guangzhou RiboBio
Co., Ltd. A dual luciferase reporter assay was used to verify the
binding of hsa-miR-429 to the 3′ untranslated region (UTR) of CBX8.
The 3′-UTR wild-type (WT) sequence and 3′-UTR mutant (MUT) sequence
of CBX8 were cloned into the pGL6-miR plasmid (cat. no. D2106;
Beyotime Institute of Biotechnology). The recombinant plasmid (2
µg) and hsa-miR-429 mimic (50 nM) or mimic negative control (50 nM)
were co-transfected into 293T cells at 40% confluent (supplied by
the Chinese Academy of Sciences Stem Cell Bank) seeded in a 6-well
plate. The transfection reagent was Lipofectamine 3000 (cat. no.
L3000008; Thermo Fisher Scientific). After 48 h at 37 °C, the
relative luciferase activity was detected with Dual-Luciferase
Reporter Assay Systems (Promega Corporation). The results were
normalized with Renilla luciferase activity.
Overexpression and knockdown of
CBX8
The coding sequence of the human CBX8 (gene ID,
57332) was cloned into the pcDNA3.1 (Invitrogen; Thermo Fisher
Scientific, Inc.) vector to generate an overexpression vector.
Small interfering RNA (siRNA) was used to knock down the expression
of the CBX8 gene. CBX8 siRNA was purchased from Guangzhou RiboBio
Co., Ltd. In order to avoid off-target effects, two different pairs
of CBX8 siRNA were selected for the experiment (cat. no.
siG000057332A-1-5 and siG000057332B-1-5; siG000057332A-1-5 target
sequence: 5′-GAGGAAAACATCCTGGATGC-3′; and siG000057332B-1-5 target
sequence: 5′-TGCTCGCAGCCTTTGAGGAA-3′). siN0000002-1-5 (Guangzhou
RiboBio Co., Ltd.; target sequence: 5′-TTCTCCGAACGTGTCACGT-3′) was
used as a negative control. Cells were transfected with the CBX8
expression vector (6 µg DNA per 10-cm dish) and siRNA (100 nM) to
overexpress or knock down the expression of CBX8 by PolyFect
Transfection Reagent (cat. no. 301108; QIAGEN) at room temperature,
respectively. The transfection lasted 6 h. The medium containing
the transfection reagent was removed and replaced with fresh
medium. After culturing in a CO2 incubator at 37°C for
48 h, the subsequent experiments were carried out.
Western blot analysis
The anti-CBX8 (cat. no. 61237), anti-GAPDH
(HRP-conjugated; cat. no. HRP-60004) and goat anti-rabbit IgG
(HRP-conjugated; cat. no. SA00001-2) antibodies were purchased from
ProteinTech Group, Inc. The total protein was extracted by RIPA
lysis buffer (P0013B, Beyotime Biotechnology). The protein was
quantified by BCA method. A total of 10 µg of protein was loaded
per lane of 10% SDS-PAGE gel. PVDF membrane was used. The PVDF
membrane was blocked with 5% skimmed milk at room temperature for
30 min. The primary antibodies were diluted at a ratio of 1:1,000
and the secondary antibodies were diluted at a ratio of 1:5,000.
PVDF membrane was incubated with primary and secondary antibodies
at room temperature for 1 h each. It was washed with TBST with
0.05% Tween-20. Western blotting was performed according to
conventional methods. Finally, ECL chemiluminescence solution
(SuperSignal™ West Pico PLUS, 34577; Thermo Fisher Scientific,
Inc.) was used to develop the image.
Statistical analysis
All experiments were repeated three times. The data
were analyzed using SPSS 17.0 software (SPSS, Inc.) package and are
presented as the mean ± SD. One-way ANOVA was used to analyze the
data and S-N-K method was used as the post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Graphs were generated using GraphPad Prism 5 software (GraphPad
Software, Inc).
Results
Expression of hsa-miR-429 is
significantly downregulated in DLBCL tissue
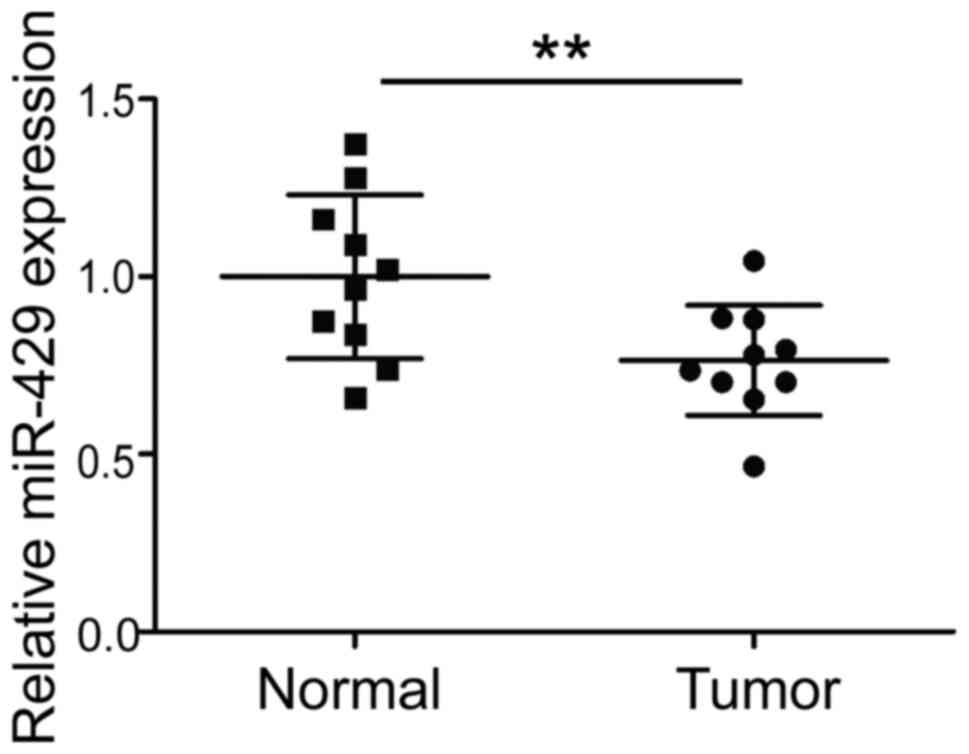
Tumor and normal tissue samples from 10 patients
with DLBCL were collected. The expression of hsa-miR-429 was
detected by qPCR. The results revealed that hsa-miR-429 expression
was significantly reduced in DLBCL tissue (P<0.01; Fig. 1).
hsa-miR-429 inhibits the proliferation
and promotes the apoptosis of SUDHL-4 and DB cells
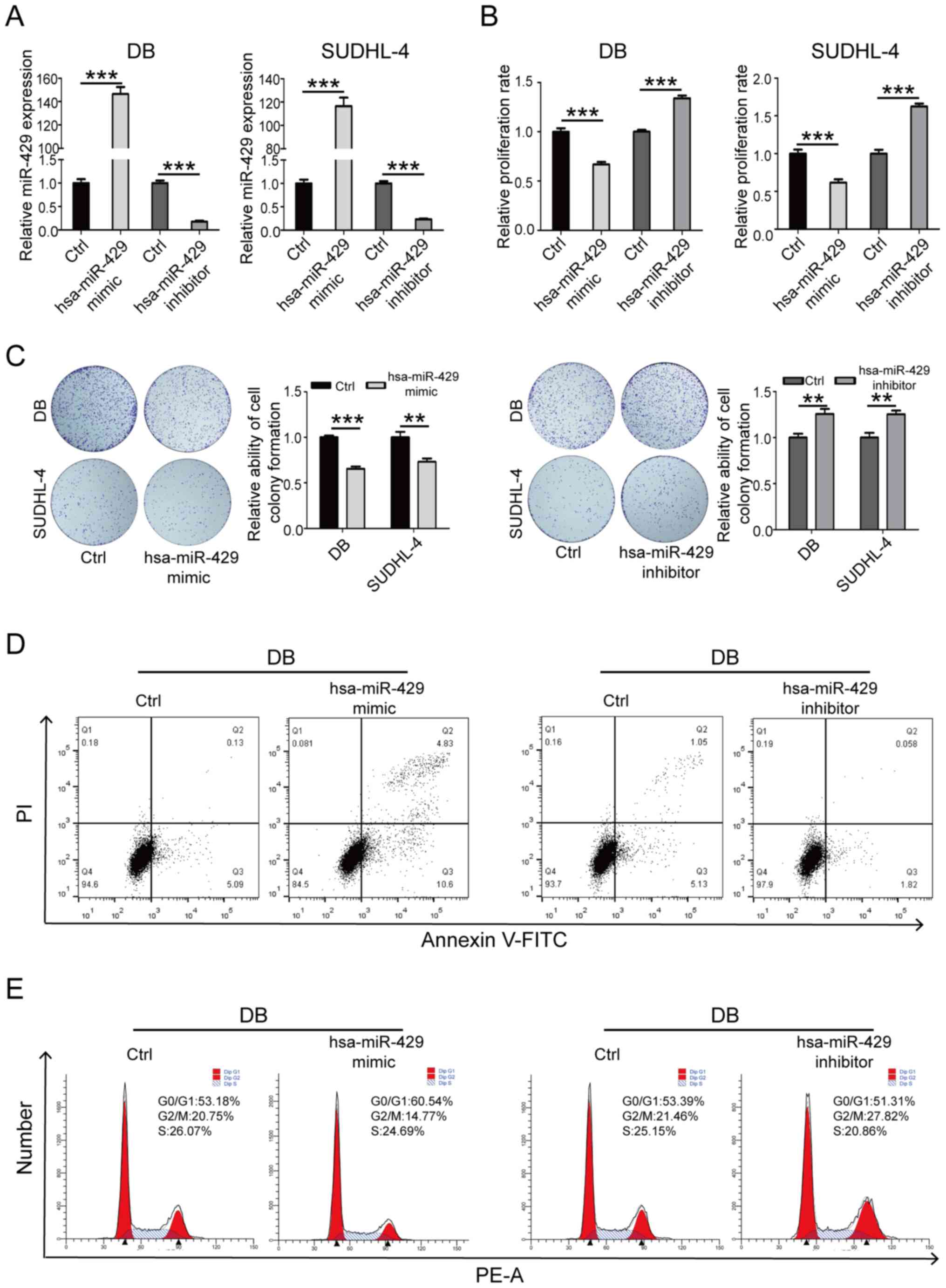
SUDHL-4 and DB cells were transfected with
hsa-miR-429 mimic and inhibitor (Fig.
2A). CCK-8 and colony formation assay results revealed that
transfection with hsa-miR-429 mimic significantly inhibited cell
proliferation, while transfection with hsa-miR-429 inhibitor
significantly promoted cell proliferation (Fig. 2B and C). Moreover, the detection
of the cell apoptotic rate and cell cycle distribution by flow
cytometry indicated that transfection with hsa-miR-429 mimic
resulted in a significant increase in the cell apoptotic rate and
decrease in the proportion of cells in the G2/M-phase of
the cell cycle. The experimental groups transfected with
hsa-miR-429 inhibitor revealed the opposite results (Fig. 2D and E). The aforementioned
results indicated that hsa-miR-429 could inhibit the proliferation
and promote the apoptosis of DLBCL cells.
CBX8 is the target gene of
hsa-miR-429
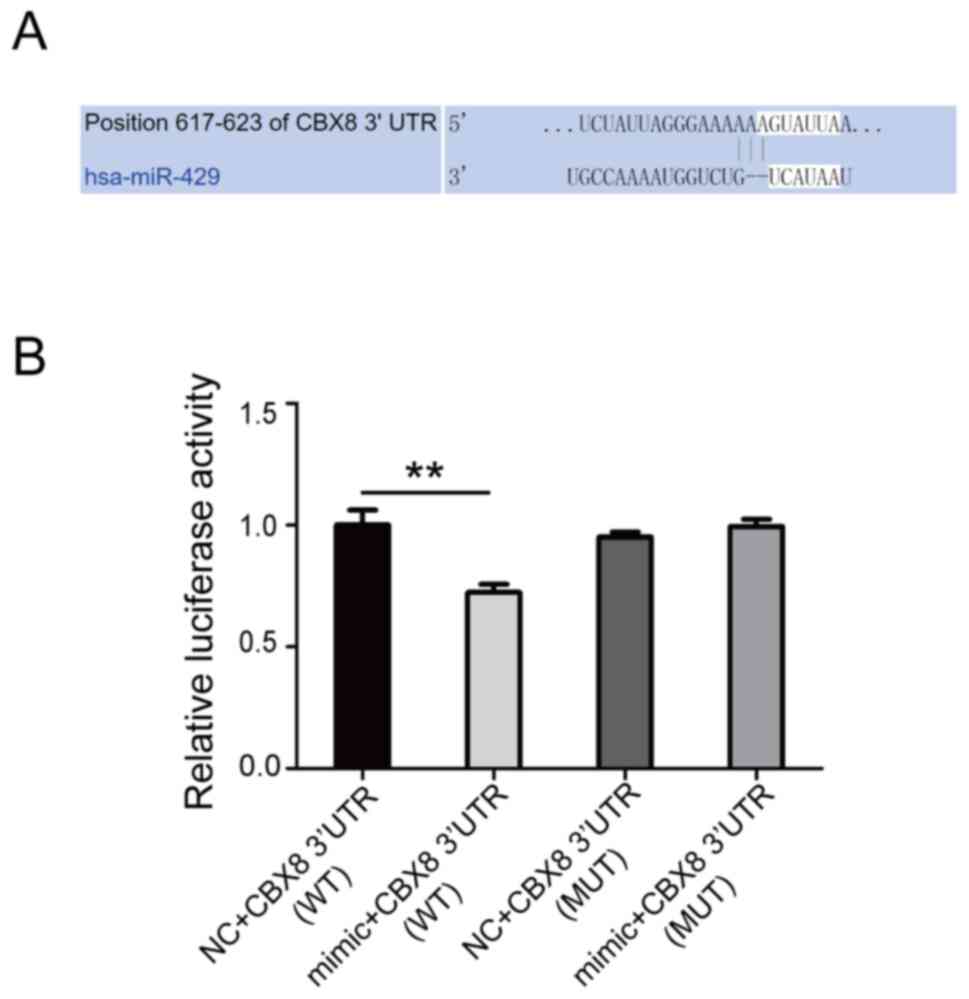
TargetScanHuman 7.2 (http://www.targetscan.org/vert_72/) predicted that
CBX8 may be the target gene of hsa-miR-429 (Fig. 3A). CBX8 exerts cancer-promoting
effects on a variety of malignant tumors, such as liver, colon and
esophageal cancer (20–25). The WT and MUT 3′-UTR (mutated at
positions 617–623) sequences of CBX8 were cloned into the pGL6-miR
vector. miR-429 mimic and mimic negative control were
co-transfected into 293T cells with the pGL6-miR 3′-UTR WT and
pGL6-miR 3′-UTR MUT plasmid. Luciferase activity was then detected
using a dual luciferase detection system. The results demonstrated
that the luciferase activity of the hsa-miR-429 mimic + pGL6-miR
3′-UTR WT group was significantly reduced. After mutating positions
617–623, the luciferase activity was not significantly altered
(Fig. 3B). This result indicated
that hsa-miR-429 regulated its transcription levels by targeting
the 617–623 position of the 3′-UTR of CBX8.
CBX8 promotes the proliferation and
inhibits the apoptosis of SUDHL-4 and DB cells
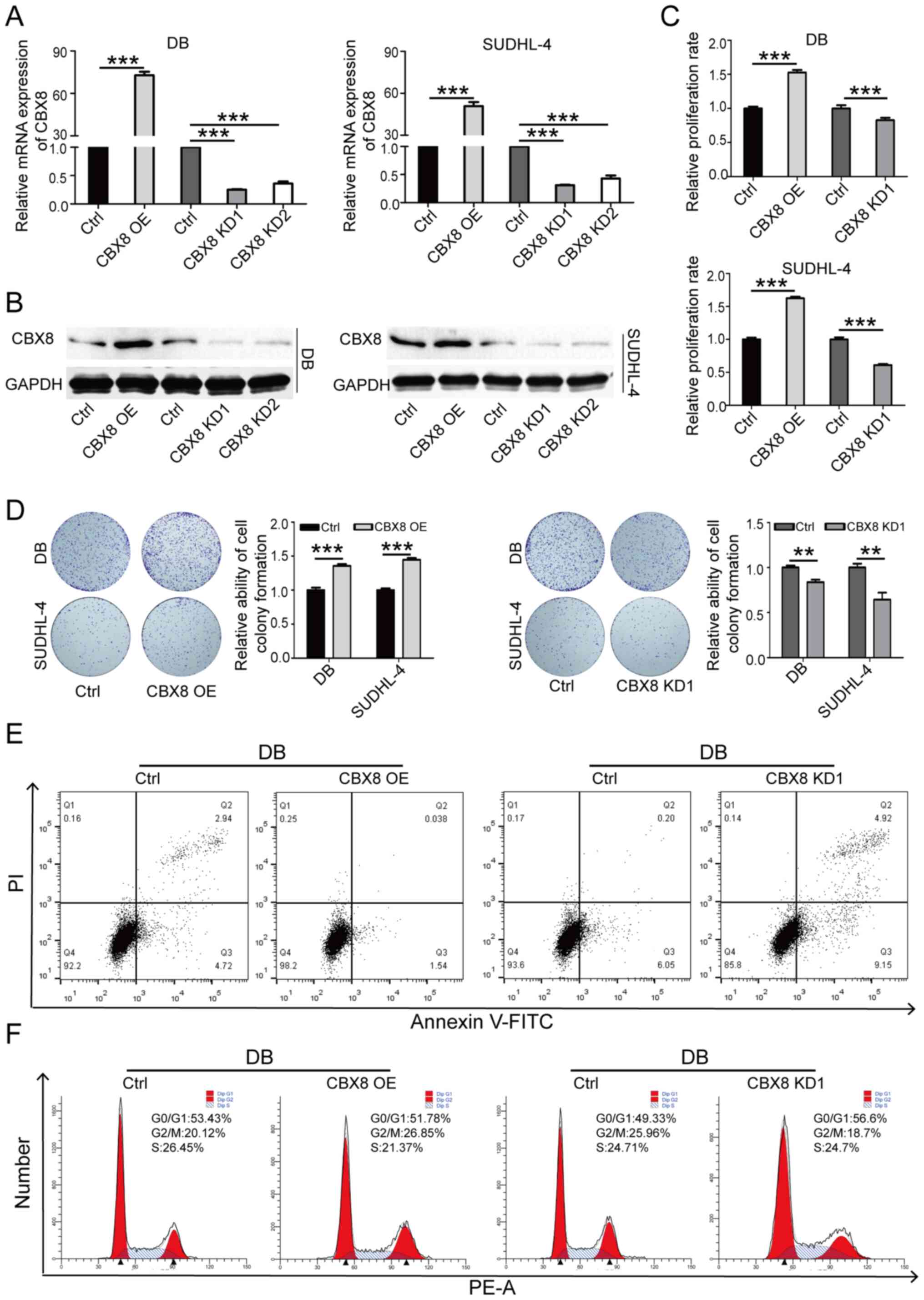
Overexpression of CBX8 in SUDHL-4 and DB cells led
to a significant increase in cell proliferation (Fig. 4A-D), a distinct decrease in the
cell apoptotic rate (Fig. 4E) and
a marked increase in the proportion of cells in the G2/M
phase (Fig. 4F). CBX8 knockdown
had the opposite effects. The aforementioned results indicated that
CBX8 may promote oncogenesis in DLBCL cells.
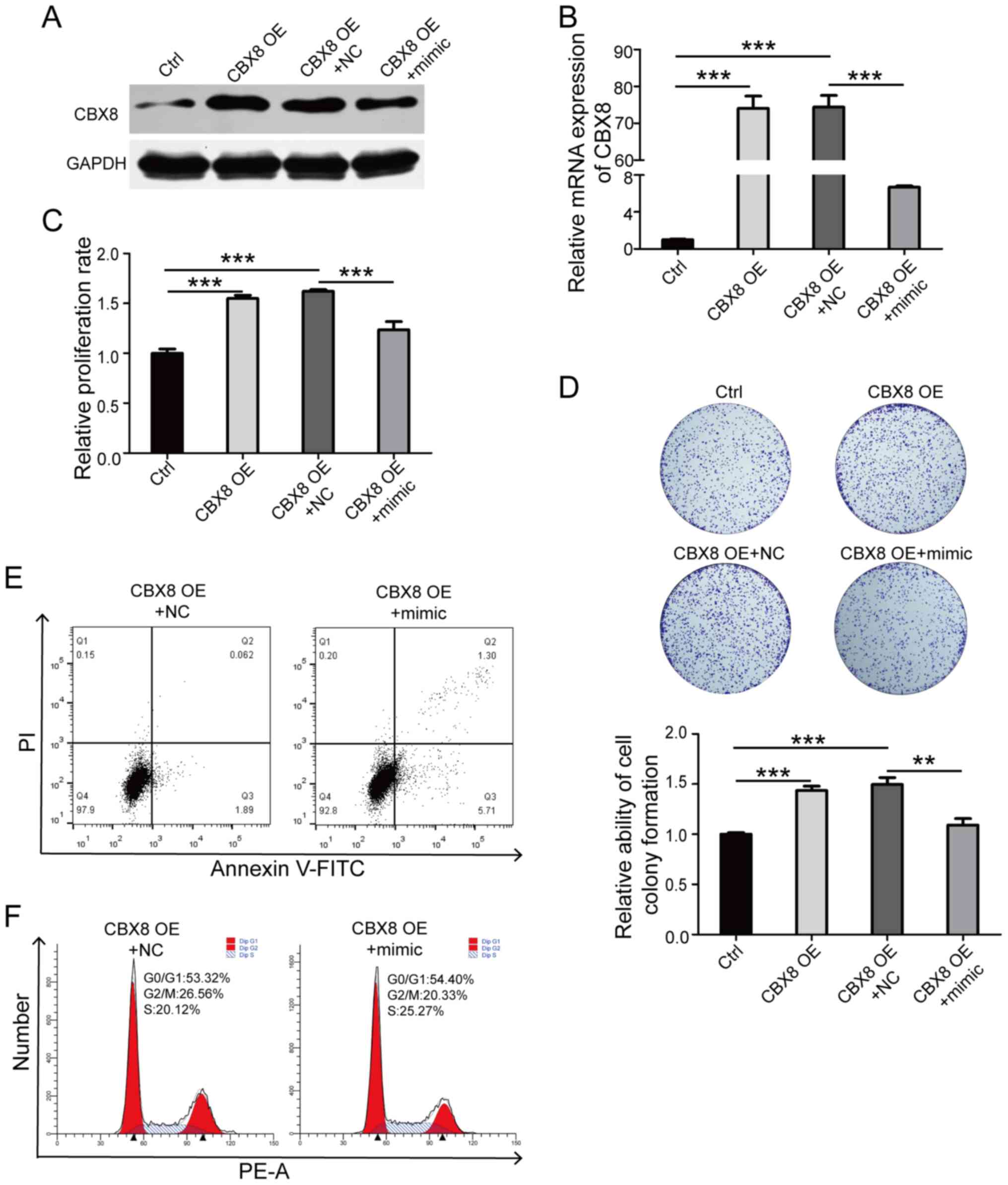
hsa-miR-429 antagonizes the effect of
CBX8 on DB cell proliferation and inhibition of apoptosis
hsa-miR-429 mimic was transfected into DB cells
overexpressing CBX8. Western blot analysis demonstrated that
hsa-miR-429 mimic significantly downregulated the protein and mRNA
expression levels of CBX8 in DB cells (Fig. 5A and B). The results of the cell
proliferation experiments and cell colony formation assays revealed
that hsa-miR-429 mimic antagonized the positive effect of CBX8 on
the proliferation of DB cells (Fig.
5C and D). In addition, the apoptotic rate of DB cells
overexpressing CBX8 transfected with hsa-miR-429 mimic was
increased (Fig. 5E), and the
proportion of cells in the G2/M phase was significantly
reduced (Fig. 5F). The
aforementioned results demonstrated the antagonistic effect of
hsa-miR-429 on the oncogenic role of CBX8.
Discussion
miRNA is widely expressed in animal and plant cells.
miRNA can be combined with lipids or lipoproteins and other
components in cells to form microvesicles, which can be
disseminated outside of the cell and enter the peripheral blood
circulation. It can also enter recipient cells through endocytosis,
thereby exerting specific biological effects and regulating target
genes (4,5). Previous studies have revealed that
different types of malignant tumors may aberrantly express specific
tumor-associated miRNA molecules (4,5).
The aberrant expression of miRNA can play a key role in multiple
biological processes, such as tumor cell survival, invasion and
metastasis.
In the present study, hsa-miR-429 played a
tumor-suppressive role in DLBCL. High levels of hsa-miR-429
inhibited cell proliferation, promoted apoptosis and changed the
distribution of cells in the cell cycle. Bioinformatics prediction
revealed that CBX8 was the target of hsa-miR-429. The dual
luciferase experiment demonstrated that hsa-miR-429 regulated its
transcription levels by targeting the 617–623 positions in the
3′-UTR of CBX8. It has been reported that, in hepatocellular
carcinoma, CBX8 exhibits oncogenic activity through AKT/β-catenin
activation (20,21). It has also been demonstrated to
interact with Y-Box binding protein 1 (YBX1) to promote the
proliferation of liver cancer cells (22). CBX8 promoted tumorigenesis and
radiation tolerance of esophageal squamous cell carcinoma cells by
targeting APAF1 (23). It has
also been reported that CBX8 participates in the DNA repair process
and promotes the occurrence of esophageal cancer (24). In addition, CBX8 has been revealed
to promote the progression and drug resistance of colon cancer,
breast cancer, leukemia, bladder cancer and prostate cancer
(25–29). To the best of our knowledge, the
role of CBX8 in DLBCL has not yet been studied. The present study
demonstrated that CBX8 also played a role in promoting DLBCL.
Overexpression of CBX8 in SUDHL-4 and DB cells led to a significant
increase in cell proliferation, a notable decrease in the cell
apoptotic rate and a marked increase in the proportion of cells in
the G2/M phase of the cell cycle. Transfection of
hsa-miR-429 mimic into cells reversed these results. Therefore, in
DLBCL, the tumor-suppressive effect of hsa-miR-429 was achieved by
targeted downregulation of CBX8. This result indicated that
hsa-miR-429 may be used as a diagnostic marker and a potential
nucleic acid drug for DLBCL. CBX8 may also become an effective
therapeutic target for DLBCL.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and ZJY performed the experiments. ML, HML, JZZ,
TX and YYT confirmed the authenticity of all the raw data. YL and
ZJY performed the statistical analysis. ML, HML, JZZ, TX and YYT
performed the bioinformatics analysis. ZPH designed the study and
YL drafted the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jingzhou Central Hospital (Jingzhou, China). All
participants signed informed consent forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sehn LH and Gascoyne RD: Diffuse large
B-cell lymphoma: Optimizing outcome in the context of clinical and
biologic heterogeneity. Blood. 125:22–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sehn LH and Salles G: Diffuse large B-cell
lymphoma. N Engl J Med. 384:842–858. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maltby S, Plank M, Ptaschinski C, Mattes J
and Foster PS: MicroRNA function in mast cell biology: Protocols to
characterize and modulate microRNA expression. Methods Mol Biol.
1220:287–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hata A and Lieberman J: Dysregulation of
microRNA biogenesis and gene silencing in cancer. Sci Signal.
8:re32015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng CJ, Bahal R, Babar IA, Pincus Z,
Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM,
et al: MicroRNA silencing for cancer therapy targeted to the tumour
microenvironment. Nature. 518:107–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xue H and Tian GY: miR-429 regulates the
metastasis and EMT of HCC cells through targeting RAB23. Arch
Biochem Biophys. 637:48–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo C, Zhao D, Zhang Q, Liu S and Sun MZ:
miR-429 suppresses tumor migration and invasion by targeting CRKL
in hepatocellular carcinoma via inhibiting Raf/MEK/ERK pathway and
epithelial-mesenchymal transition. Sci Rep. 8:23752018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang C, Chang C, Gao H, Wang Q, Zhang F
and Xu C: miR-429 regulates rat liver regeneration and hepatocyte
proliferation by targeting JUN/MYC/BCL2/CCND1 signaling pathway.
Cell Signal. 50:80–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang M, Dong BB, Lu M, Zheng MJ, Chen H,
Ding JZ, Xu AM and Xu YH: miR-429 functions as a tumor suppressor
by targeting FSCN1 in gastric cancer cells. OncoTargets Ther.
9:1123–1133. 2016.PubMed/NCBI
|
|
10
|
Wu CL, Ho JY, Hung SH and Yu DS: miR-429
expression in bladder cancer and its correlation with tumor
behavior and clinical outcome. Kaohsiung J Med Sci. 34:335–340.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang J, Liu Y, He A, Liu Y, Wu J, Liao X,
Lv Z, Wang F and Mei H: hsa-miR-429 promotes bladder cancer cell
proliferation via inhibiting CDKN2B. Oncotarget. 8:68721–68729.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng Y, Luan F, Zeng L, Zhang Y and Ma K:
miR-429 suppresses the progression and metastasis of osteosarcoma
by targeting ZEB1. EXCLI J. 16:618–627. 2017.PubMed/NCBI
|
|
13
|
Wang HF, Wang WH, Zhuang HW and Xu M:
miR-429 regulates the proliferation and apoptosis of nephroblastoma
cells through targeting c-myc. Eur Rev Med Pharmacol Sci.
22:5172–5179. 2018.PubMed/NCBI
|
|
14
|
Liu D, Song L, Dai Z, Guan H, Kang H,
Zhang Y, Yan W, Zhao X and Zhang S: miR-429 suppresses
neurotrophin-3 to alleviate perineural invasion of pancreatic
cancer. Biochem Biophys Res Commun. 505:1077–1083. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zong M, Liu Y, Zhang K, J Y and Chen L:
The effects of miR-429 on cell migration and invasion by targeting
Slug in esophageal squamous cell carcinoma. Pathol Res Pract.
215:1525262019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Liu J, Yao Q, Wang Y, Liu Z and
Zhang L: LncRNA SNHG6 promotes Wilms' tumor progression through
regulating miR-429/FRS2 axis. Cancer Biother Radiopharm. Jan
22–2021.(Epub ahead of print). View Article : Google Scholar
|
|
17
|
Zhang L, Liu Q, Mu Q, Zhou D, Li H, Zhang
B and Yin C: miR-429 suppresses proliferation and invasion of
breast cancer via inhibiting the Wnt/β-catenin signaling pathway.
Thorac Cancer. 11:3126–3138. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cava C, Novello C, Martelli C, Lodico A,
Ottobrini L, Piccotti F, Truffi M, Corsi F, Bertoli G and
Castiglioni I: Theranostic application of miR-429 in
HER2+ breast cancer. Theranostics. 10:50–61. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang CZ, Chen SL, Wang CH, He YF, Yang X,
Xie D and Yun JP: CBX8 exhibits oncogenic activity via
AKT/β-catenin activation in hepatocellular carcinoma. Cancer Res.
78:51–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang B, Tian Y, Liao Y, Li Z, Yu S, Su H,
Zhong F, Yuan G, Wang Y, Yu H, et al: CBX8 exhibits oncogenic
properties and serves as a prognostic factor in hepatocellular
carcinoma. Cell Death Dis. 10:522019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao L, Zhou Z, Li W, Peng J, Sun Q, Zhu
H, Song Y, Hou JL, Sun J, Cao HC, et al: Chromobox homolog 8 (CBX8)
Interacts with Y-Box binding protein 1 (YBX1) to promote cellular
proliferation in hepatocellular carcinoma cells. Aging (Albany NY).
11:7123–7149. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Chen H, Zhu H and Sun X: CBX8
promotes tumorigenesis and confers radioresistance in esophageal
squamous cell carcinoma cells through targeting APAF1. Gene.
711:1439492019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao W, Ou C, Qin J, Xing F, Sun Y, Li Z
and Qiu J: CBX8, a novel DNA repair protein, promotes tumorigenesis
in human esophageal carcinoma. Int J Clin Exp Pathol. 7:4817–4826.
2014.PubMed/NCBI
|
|
25
|
Tang J, Wang G, Zhang M, Li FY, Sang Y,
Wang B, Hu K, Wu Y, Luo R, Liao D, et al: Paradoxical role of CBX8
in proliferation and metastasis of colorectal cancer. Oncotarget.
5:10778–10790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SH, Um SJ and Kim EJ: CBX8 suppresses
Sirtinol-induced premature senescence in human breast cancer cells
via cooperation with SIRT1. Cancer Lett. 335:397–403. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SH, Um SJ and Kim EJ: CBX8 antagonizes
the effect of Sirtinol on premature senescence through the
AKT-RB-E2F1 pathway in K562 leukemia cells. Biochem Biophys Res
Commun. 469:884–890. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng F, Luo L, Li D, Guo J and Guo M:
KPNA2 interaction with CBX8 contributes to the development and
progression of bladder cancer by mediating the PRDM1/c-FOS pathway.
J Transl Med. 19:1122021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang S, Tailor K and Kwabi-Addo B:
Androgen-induced epigenetic profiles of polycomb and trithorax
genes in prostate cancer cells. Anticancer Res. 40:2559–2565. 2020.
View Article : Google Scholar : PubMed/NCBI
|