Introduction: Identification of the COVID-19
pandemic
In December 2019, the novel coronavirus (2019-nCoV)
emerged, eventually causing a global pandemic of severe acute
respiratory syndrome (SARS). Soon after the outbreak, the virus was
identified in January of 2020 and was temporally named 2019-nCoV,
and then labelled SARS-CoV-2 by the World Health Organization
(WHO). Later on, the disease was re-named as coronavirus disease
2019 (COVID-19) to differentiate it from other coronavirus
infections of SARS or Middle Eastern respiratory syndrome (MERS).
The acute clinical symptoms of patients with COVID-19 include
fever, dry cough and dyspnea, which are similar to those of SARS
and MERS. By now, more than several million confirmed COVID-19
cases have been reported in >200 countries, with an estimated
mortality risk of 2–6% worldwide (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen).
The entire genome of novel SARS-CoV-2 (MN908947.3)
was soon sequenced and determined to have 29,903 nucleotides. It
was revealed to have 79.5% nucleotide similarity with SARS-CoV,
which appeared in Guangdong Province in China in 2002, >50%
similarity with MERS-CoV, which emerged in Middle Eastern countries
in 2012, and 96% similarity to the bat coronavirus bat-CoV-RaTG13
(1). The coronavirus genome
structures of SARS-CoV, MERS-CoV and SARS-CoV-2 are similar in that
they contain a single strand of RNA, which is organized by the
5′-leader-UTR-RNA-dependent RNA polymerase (RdRp)-spike
(S)-envelope (E)-Membrane (M)-nucleocapsid (N)-3′UTR poly(A) tail,
with accessory non-structural genes interspersed throughout the
structural genes, as presented in Fig. 1A-D (2).
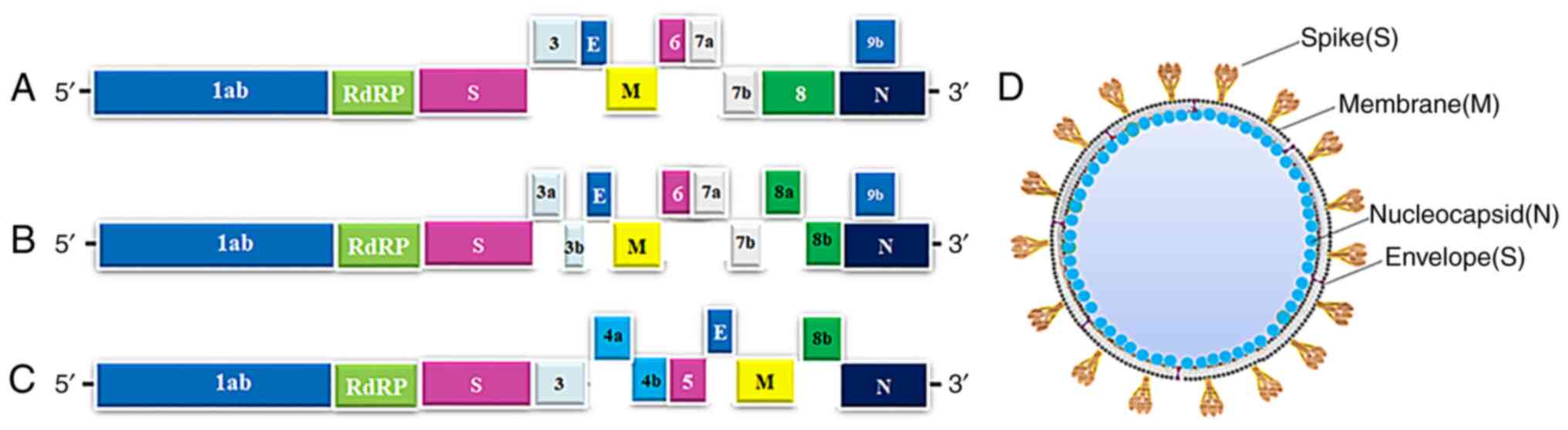 | Figure 1.Genomic structure and potential drug
targets for SARS-Cov-2, SARS-CoV and MERS-CoV. (A-C) Genomic
organization of (A) SARS-Cov-2 (GenBank ID, MN908947.3), (B)
SARS-CoV and (C) MERS-CoV, indicating the coding regions for
proteins that are potential drug targets. (D) The main structural
proteins of SARS-Cov-2 were indicated on viral particles. SARS-CoV,
severe acute respiratory syndrome coronavirus; MERS-CoV, Middle
Eastern respiratory syndrome coronavirus; RdRp, RNA-dependent RNA
polymerase; S, spike; M, membrane; N, nucleocapsid; E,
envelope. |
In the history of human coronavirus-caused
infections, at the international level, there have been six types
of coronavirus disease: 229E, NL63, HKU1, OC43, MERS and SARS. The
first human coronavirus was identified in the 1960s and SARS-CoV
and MERS-CoV caused epidemics in 2002 and 2012, respectively.
SARS-CoV2 is the seventh coronavirus, which triggered the most
severe global spread of COVID-19 in humans ever recorded. The virus
particle is composed of a single-stranded, positive-sense RNA
enveloped by glycoproteins and has a typical coronavirus appearance
under an electron microscope. As that of SARS-CoV or MERS-CoV, the
genomic RNA of SARS-CoV2 encodes for non-structural proteins, such
as RdRps, other viral enzymes and at least four structural
proteins: The S protein, the E protein, the M protein and the N
protein, as visualized in Fig.
1D. The N protein of SARS-CoV2 has nearly 90% similarity with
the amino acid sequence of SARS-CoV (1).
Along with SARS-CoV and MERS-CoV, SARS-CoV2 belongs
to the subgenus Sarbecovirus of the genus β-coronavirus, which is
closely related to bat-SL-CoVZC45 or bat-SL-CoVZXC21. Although
several animals are potential carriers of SARS-CoV2, none has been
confirmed to be an intermediate host for SARS-CoV2. Further studies
have indicated that SARS-CoV2 uses its S protein to bind to the
angiotensin-converting enzyme 2 (ACE2) receptors in human cells,
similar to how SARS-CoV used to infect humans. Other studies have
demonstrated that both SARS-CoV and SARS-CoV-2 use S proteins to
bind to the ACE2 receptors of host cells for viral infection
(2–6).
The pathogenesis of SARS-CoV-2 infection is composed
of four stages including at least two consecutive stages: The viral
replication phase and the immunological response phase (7–9).
The viral phase is the initial one during which ~80% of patients
with COVID-19 may be asymptomatic or have mild symptoms. Whereas,
~20% of patients enter the second immunological phase in which
their condition becomes more severe and their immune system becomes
hyper-responsive. Certain studies have reported that the
circulating natural killer (NK) cells of patients with COVID-19
have been reduced, whereas the expression of inhibitory receptor
NKG2A in NK cells is increased to lower the cytolytic activity of
NK cells. Furthermore, dendritic cells, activated neutrophils and
inflammatory monocytes increase in patients with COVID-19, with
higher percentages of patients experiencing severe damage to their
respiratory tract and lungs (10,11). At times, a third phase of
hypercoagulability among patients is followed again by the second
immunological phase. Finally, in the fourth stage, organ injury and
failure occur in the most severe COVID-19 cases.
Although no drugs have been approved to treat
coronavirus infections, certain existing antiviral drugs for SARS,
MERS or other viral infections have been used as emerging
treatments for COVID-19 and demonstrated beneficial effects in
patients. In order to provide solid evidence to select therapeutics
to immediately treat COVID-19, the PubMed database was searched for
the present review using the key words ‘therapeutics’ and
‘COVID-19’ to identify articles published since December 2019.
Recent advances in the use of chloroquine (CQ; 4-aminoquinoline),
remdesivir, ribavirin, favipiravir, galidesivir, monoclonal
antibodies (mAbs), patient-derived plasma, natural products and
Chinese herbal medicine (CHM) for the treatment of SARS or MERS and
COVID-19 were reviewed in order to outline treatment options for
health professionals and medical researchers (12–14).
Chloroquine and hydroxychloroquine
In vitro and in vivo studies suggested
that CQ and hydroxychloroquine (HCQ) are effective against SARS and
MERS. CQ was initially approved as an immuno-modulating drug to
treat malaria (15). Later on,
the less toxic HCQ was synthesized and approved by the US Food and
Drug Administration (FDA) to treat malaria (16). Both CQ and HCQ appear to generate
antiviral actions either by inhibiting the virus from binding to
the ACE2 receptors on human cells or through their
anti-inflammatory effect. In cultured primate cells treated with CQ
either prior to or after SARS-CoV infection, CQ had strong
antiviral effects, indicating that CQ may be used to treat SARS-CoV
infections, including SARS-CoV-2 (17). By detecting viral RNA copy numbers
and the N protein expression of SARS-CoV-2 in the supernatant of
treated Vero E6 cells, it was revealed that CQ strongly inhibited
the RNA replication of SARS-CoV-2 (18).
In several clinical trials in China, CQ and HCQ were
tested as emerging treatments for COVID-19 and improved patients'
recovery. CQ was effective against SARS-CoV2 infections in >100
patients by shortening their hospital stay and alleviating their
clinical symptoms (19). In a
clinical study involving 22 patients with COVID-19, the percentage
of patients who became SARS-CoV2-negative in the CQ group was
slightly higher than that in the lopinavir/ritonavir group at days
7, 10 and 14 of treatment with CQ (20). Another study reported that HCQ had
stronger antiviral activity against COVID-19 than CQ. A similar
therapeutic effect was indicated in the use of HCQ sulfate for
patients with COVID-19 compared to the use of CQ phosphate
(21). In addition, a group of
French patients with COVID-19 were treated with a combination of
HCQ and azithromycin, which significantly reduced the viral load in
the patients after 6 days of treatment. This aforementioned study
suggests that azithromycin has a synergistic, antiviral effect with
HCQ. To validate this outcome, the SARS-CoV-2 IHUMI-3 strain from
the same group of patients was used to infect the cultured Vero E6
cells in order to observe the combined antiviral effects of HCQ and
azithromycin. The viral replication copy numbers from treated cell
lysates decreased significantly through a combined treatment
involving HCQ and azithromycin (22,23). A multi-center retrospective study
was performed to include patients with a COVID-19-related admission
in six hospitals in Detroit (USA) from March 10 to May 2, 2020. In
total, 2,541 patients were enrolled and followed up for 28.5 days;
the patients had a median hospitalization time of 6 days. HCQ
reduced the mortality of infected patients by 66% and HCQ combined
with azithromycin decreased the mortality of infected patients by
71% compared to that of patients who did not receive any HCQ or
azithromycin. This clinical study implied that treatment with HCQ
alone or in combination with azithromycin is associated with
significantly reduced mortality among patients with COVID-19
(24).
However, certain studies have indicated that CQ or
HCQ does not improve the clinical outcomes of patients with
COVID-19. A systematic review using PubMed, EMBASE, the Cochrane
Library, MedRxiv and International Clinical Trials Registry
Platform suggested that five of seven completed clinical trials
indicated positive results using CQ or HCQ to treat COVID-19,
whereas two of seven trials revealed no improvement in patients
with COVID-19 treated with CQ or HCQ. To further learn about the
potential benefits and harms of CQ or HCQ for treating COVID-19,
four randomized controlled trials (RCTs), 10 cohort studies and 9
case series published between December 2019 and May 2020 were
collected and analyzed in order to determine possible treatment
effects of CQ or HCQ. The efficacy of CQ or HCQ treatment for
COVID-19 is relatively low and some results are positive, whereas
some results are negative (25).
To systematically review the available data on the efficacy and
safety of CQ and HCQ for treating COVID-19, 12 observational and 3
RCTs to include 10,659 patients were reviewed. The efficacy of
CQ/HCQ for COVID-19 was analyzed for 5,713 patients who received
CQ/HCQ in comparison with 4,966 patients who only received standard
care. Meta-analysis indicated no significant reduction in the
mortality of COVID-19 patients by treatment with HCQ. Hence, CQ or
HCQ may not improve clinical outcomes for COVID-19 (26,27). A recent study also searched
PubMed, Embase and Web of Science for RCTs and assessed the
efficacy and safety of HCQ/CQ therapy alone for SARS-CoV-2
infection. HCQ did not reduce the requirement for hospitalization
among outpatients, but led to a significantly higher rate of any
adverse event compared to the control group (28).
Overall, the antiviral activity of CQ and HCQ is
evident in both experimental and clinical studies, even though
certain research has reported negative outcomes of CQ and HCQ for
treating COVID-19 in clinical trials. This discrepancy may be due
to several reasons. First, CQ and HCQ may have been used in
patients with different time phases of the disease in different
clinical trials. Since CQ and HCQ inhibit the entry and replication
of the virus into lung cells, the therapeutic effect may be
decreased if patients are in the late stage of the disease
progression. Furthermore, the severity of a patient may impact the
therapeutic effects. The therapeutic effects of CQ and HCQ may be
weaker or have no significant impact on patients with a severe
status. Finally, the therapeutic effects of CQ and HCQ are
influenced by treatment time and dosage. It usually takes 5–7
continuous days to apply CQ or HCQ to patients with COVID-19 to
achieve clinical improvements.
Remdesivir and other nucleoside
analogues
As non-structural proteins, such as RdRp and
helicase, have a critical role in the replication, transcription
and translation processes of the coronavirus, nucleoside analogues
(NAs), which have similar structures to adenosine or guanine, are
able to target RdRp and be integrated into RNA synthesis to inhibit
viral infection (12). However,
it is far more difficult to develop NAs against coronaviruses such
as SARS-CoV, MERS-CoV and SARS-CoV-2 due to the unique proofreading
of 3′-5′ exoribonuclease in coronaviruses (29). The NAs and remdesivir (GS-5734),
which was originally approved as a reverse transcriptase inhibitor
to treat HIV, produced potential antiviral actions against
coronaviruses. In 2016, Warren et al (30) reported on the antiviral activity
of remdesivir against Ebola virus (EBOV) by inhibiting viral
replication, decreasing pathological changes and reducing clinical
symptoms in non-human primate models with EBOV infections. Later,
in 2017, remdesivir strongly inhibited viral replication in primary
human airway cultures infected with coronavirus and effectively
decreased the viral load in a mouse model infected with SARS-CoV.
In 2018, remdesivir was reported to significantly inhibit SARS-CoV
and MERS-CoV infections in in vitro and in vivo
models. Other studies also suggested that remdesivir has
significant antiviral activity against several CoVs, including
SARS-CoV, MERS-CoV, the highly pathogenic SARS-CoV-2 and emergent
Bat CoVs in cultured cells through in vitro experiments and
mouse models in vivo (31,32). Recently, a combination of
remdesivir and GC376, an inhibitor of the 3C-like protease (3CLpro)
for treating feline infectious peritonitis (coronavirus infection),
had a synergistic antiviral effect against SARS-CoV-2 in Vero
cells, implying that these compounds inhibit the replication of
SARS-CoV-2 through different drug targets (33).
Several clinical trials have been performed to
evaluate the efficacy and safety of intravenous remdesivir for
patients with SARS-CoV-2 infection (NCT04292899, NCT04292730,
NCT04257656, NCT04252664 and NCT04280705) (34). In 2020, a clinical trial to use
remdesivir to treat patients with severe COVID-19 suggested that 36
out of 53 patients (68%) exhibited significant improvement in
clinical symptoms. The 53 patients were given remdesivir for a
period of 10 days at a dose of 200 mg on day 1, followed by 100 mg
daily for the remaining 9 days of treatment (35). An RCT involving 596 patients with
SARS-CoV-2 infection at 105 hospitals in the US, Europe and Asia
was performed from March 15 through to April 18, 2020. Patients
were randomized into three groups with a 10-day treatment with
remdesivir (n=197), a 5-day treatment with remdesivir (n=199) or
standard care (n=200). Patients in the 5-day remdesivir group were
significantly improved regarding their clinical symptoms compared
to those who received standard care (36). Another large-scale,
double-blinded, randomized, placebo-controlled trial enrolled 1,062
patients; 541 were treated with remdesivir and 521 were treated
with placebo. The patients with COVID-19 treated with remdesivir
recovered more rapidly than the patients treated with placebo.
Compared to the placebo, remdesivir shortened the recovery time by
5 days (37). Recently, a
retrospective comparative study was performed in 5 hospitals in the
US. Out of 2,483 patients with confirmed COVID-19, 342 were treated
with remdesivir. 184 patients received remdesivir and
corticosteroids, while 158 patients were given remdesivir alone.
Remdesivir-treated patients recovered more rapidly than the matched
control patients who did not receive remdesivir (38). In 2020, the FDA approved
remdesivir for the emerging treatment of hospitalized patients with
COVID-19 and it was recommended for use in adult and pediatric
patients requiring hospitalization aged >12 years with a
bodyweight of at least 40 kg (39).
In addition to remdesivir, other NAs are able to
target RdRp and inhibit the RNA replication of SARS-CoV-2. A
synthetic RdRp model with 801 amino acid residues was generated to
have >97% sequence identity to the RdRp of SARS-CoV-2 and the
FDA-approved drugs of ribavirin, galidesivir and remdesivir were
tested in this model. These compounds use different hydrogen bonds
to interact with the residues of RdRp of SARS-CoV-2 (40). Ribavirin was originally approved
to treat the hepatitis C virus (HCV), respiratory syncytial virus
and EBOV infection. As a guanine derivative, ribavirin targets RdRp
and interferes with RNA synthesis and the mRNA capping of viruses.
Recent studies have also indicated that ribavirin has antiviral
activity against SARS-COV-2 (3,40).
Importantly, ribavirin, combined with interferon, significantly
increased antiviral activity against SARS-CoV and MERS-CoV via
in vitro and in vivo studies, including in primate
models (41,42).
Favipiravir also effectively inhibited the RdRp of
RNA viruses, such as EBOV and HCV (42,43). In a recent study, favipiravir
inhibited the replication of SARS-COV-2 in cultured Vero E6 cells
(44). In clinical studies, the
symptoms of patients with COVID-19 improved significantly after
they were treated with favipiravir for 2.5 days, compared to 4.2
days in control patients at hospitals in Wuhan and Shenzhen (China)
(45). Galidesivir (BCX4430) is
an adenosine analogue that was developed to treat RNA viral
infections, including HCV. Galidesivir significantly decreased the
viral load and improved the survival of animal models with MERS-CoV
and SARS-CoV infections (46). In
the recently validated RdRp model, created to examine the antiviral
activity of drugs against COVID-19, ribavirin, remdesivir and
galidesivir effectively inhibited the replication of SARS-COV-2,
further suggesting their potential use in clinical practice
(40).
Antibody-based therapy for COVID-19
Antibodies (Abs) have long been used to neutralize
viral antigens to treat viral diseases. One adaptive immune
response in patients with SARS-CoV-2 infection is the production of
specific Abs. The Abs from patients with COVID-19 are able to bind
to the S protein or RBD of SARS-CoV-2 to block it from interacting
with ACE2 receptors in order to prevent viral replication. A subset
of the Abs may be able to inhibit authentic SARS-CoV-2 infection.
Although the titers of neutralizing Abs against SARS-CoV-2 in human
plasma eventually decrease over time, these Abs may remain in the
human body for at least three months (47,48). mAbs are able to specifically bind
to epitope proteins on virions to inhibit virus packaging. The
neutralizing mAbs are generated to target the S protein of SARS-CoV
and MERS-CoV to inhibit them from entering the human body (49,50). CR3022 is a neutralizing mAb used
to combat SARS-CoV; it binds to the receptor-binding domain (RBD)
of the S protein to inhibit the virus from entering. Thus, it may
be used to treat COVID-19 (51).
Since the viruses tend to develop mutations in the
targeted epitopes to escape Ab-mediated neutralization, a cocktail
of mAbs that targets different epitopes in the viruses to
neutralize mutant viruses is required in experimental and clinical
therapy. Previously, a more effective mAb against SARS-CoV was
obtained by combing several mAbs to target conserved epitopes of
the coronavirus (52). In January
2020, Distributed Bio developed a panel of ultra-high affinity mAbs
to recognize and neutralize a panel of isotopes on SARS-CoV-2 to
block COVID-19 infection. Certain mAbs against COVID-19 have been
developed and proceeded to clinical trials (53). An RCT against EBOV indicated that
single or triple mAbs significantly reduced the mortality of
patients with Ebola virus disease, providing clinical evidence that
mAbs have potential utility for the treatment of COVID-19 (54).
To date, >30 Abs have demonstrated a potentially
neutralizing effect against SARS-CoV-2 infection. Most Abs have
been generated from B cells or fresh peripheral blood mononuclear
cells, depending on the neutralizing targets on SARS-CoV-2. The
antiviral efficacy of most of these Abs has been demonstrated in
preclinical studies and certain Abs, including CT-P59, ADG20,
CB6LALA, AZD8895, AZD1061 and DXP-593, are in phase 2 or 3 clinical
trials against COVID-19 (55,56). Most recently, a clinical trial
included 452 COVID-19 patients to test the efficacy of a
neutralizing Ab, LY-CoV555, based on three doses (700, 2,800 or
7,000 mg) or a placebo; the clinical outcomes were evaluated at the
time of the interim analysis of the phase 2 trial. From days 2 to
6, patients who had received LY-CoV555 had slightly lower severity
of symptoms than those who had received the placebo. The LY-CoV555
appeared to accelerate the natural decline of viral load over time
(ClinicalTrials.gov; no. NCT04427501)
(57). Since the immune response
factors are usually upregulated in the pathogenesis of SARS-CoV-2
infection, a human mAb of canakinumab, targeting interleukin
(IL)-1β, was used to treat 17 patients with mild or severe
non-intensive care COVID-19. Another 17 patients with similar
symptoms were treated with standard HCQ plus lopinavir/ritonavir.
Canakinumab therapy caused rapid, long-lasting improvement in
oxygenation levels in 60.3% of the patients with COVID-19, which is
better than that of standard therapy (58).
To improve patient survival, another Ab-based
treatment that has been developed involves using convalescent
plasma (CP) or immunoglobulins isolated from patients who have
recovered from SARS, MERS or COVID-19. Over the past two decades,
CP immunotherapy has been indicated to be effective and safe to
treat patients with SARS, MERS and H1N1. Clinical studies have
reported that patients with SARS treated with CP had lower
mortality and shorter hospital stays than those not treated with CP
(59–61). Since the patients' viral peak
usually appears in the first week of infection in most viral
diseases and a primary immune response of the host normally
develops 10–14 days after infection (62), the CP should be collected earlier
during the second or third week of the SARS-CoV-2 infection. During
the recent outbreak of COVID-19, one study used CP transfusion to
treat 5 critical patients with laboratory-confirmed COVID-19 in
Shenzhen (China). The donor patients had been previously diagnosed
with confirmed COVID-19 and were subsequently screened to exclude
patients who were positive for SARS-CoV-2, hepatitis B virus, HCV,
HIV and other respiratory viruses at the time of blood donation.
Prior to and after CP transfusion, the patients were evaluated and
had an increased pressure of arterial oxygen/fraction of inspired
oxygen ratio, from 172–276 to 206–290 for 4 out of 5 patients
within 7 days after transfusion, indicating therapeutic efficacy of
CP for COVID-19 (63). A Korean
group reported that two patients with severe and acute pneumonia
caused by SARS-CoV-2 improved after the infusion of CP from donors
(64). Hence, the clinical
symptoms of transfused patients were improved and the patients had
a significant increase in oxyhemoglobin saturation and lymphocyte
counts. The average time from disease onset to transfusion was 16.5
days among these patients and the neutralizing Ab was maintained at
a high level (above 1:640) after transfusion. In addition, the
viral particles were undetectable and no severe adverse effects
were observed in patients after transfusion, thus supporting the
efficacy and safety of CP for treating SARS-CoV-2 infection
(65).
Chinese herbal medicine for treating
SARS-COV-2 infection
CHM is a potential treatment option for SARS-CoV-2
infection. Natural compounds from plants or CHM may have antiviral
effects against SARS-CoV in certain aspects. Due to its safety and
lower toxicity, combined with other conventional drugs, CHM has
beneficial effects in terms of decreasing mortality and relieving
the symptoms of patients with SARS-CoV in clinical trials (66,67).
In further studies, the clinical antiviral impact of
CHM on SARS-CoV infection was supported through the identification
of the active components in Chinese herbs. As a major component of
the Chinese herb liquorice root (Glycyrrhiza uralensis),
glycyrrhizin inhibited the replication of clinically isolated
SARS-CoV (68). Wang et al
(69) indicated that a compound
derived from CHM, MOL376, had potential antiviral efficacy against
SARS. Certain Chinese herbal components, such as emodin from the
genus Rheumand polygonum, baicalin from Scutellaria
baicalensis, scutellarin from Erigeron breviscapus,
tetra-O-galloyl-β-D-glucose from Galla chinensis and
luteolin from Veronicalina riifolia markedly inhibited the
interaction of the S-protein of SARS-CoV with ACE2 receptors in
human cells, implying that CHM exerts its antiviral activity by
inhibiting viral entry into human cells. However, the antiviral
activity of these compounds in relation to SARS-CoV-2 requires to
be validated in future studies (70).
During the outbreak of COVID-19, common Chinese herb
formulae have been used to treat SARS-CoV-2 infection. Based on the
pathological mechanism of viral infection, CHM, with a matched
structure to target ACE2 receptors in human cells, holds the
antiviral potential to prevent SARS-CoV-2 infection. A Chinese
group reported that more than 40 active ingredients of seven
Chinese herbs, including Lonicerae Japonicae Flos and Mori Folium,
displayed an antiviral effect against SARS-CoV by screening the CHM
drug library acting on the S-protein-binding site of human ACE2
receptors for SARS-CoV (71).
Lianhuaqingwen capsule is a Traditional Chinese Medicine (TCM)
formula that is commonly used to treat influenza and other viral
diseases. In a recent study, it was demonstrated to inhibit the
replication of SARS-CoV-2 and its related inflammatory activity
in vitro (72). As a
widely used antiviral herb, Scutellariae radix the root of
Scutellaria baicalensis, was studied for its antiviral
action against SARS-CoV2. The ethanolic extract of Scutellaria
baicalensis inhibited the replication of SARS-CoV2 by
interfering with a key protease, 3CLpro enzyme of SARS-CoV2, in
Vero cells. Further investigation identified that the major
antiviral component of Scutellaria baicalensis is baicalein,
which may effectively inhibit the 3CLpro activity of SARS-CoV2 with
an IC50 of 0.39 µM (73). Liquiritin is one of the main
flavonoids in Glycyrrhiza uralensis and has an antiviral
function by mimicking type I interferon. In a transcriptional
analysis to screen for potentially antiviral compounds, liquiritin
significantly inhibited the replication of SARS-CoV2 in Vero E6
cells; hence, liquiritin has therapeutic effects on SARS-CoV2
infection (74). Other studies
have extensively examined >100 herbal formulae in Chinese
government-issued guidelines and Korean guidelines for treating
COVID-19 with traditional medicine and support the idea that the
formula of Qinfei Paidu Tang is recommended in both Chinese and
Korean guidelines. Qinfei Paidu Tang is mainly composed of >20
formula, including Ephedrae herba, Glycyrrhizae radix and Rhizoma
(75–77).
As CHM contains millions of natural compounds and it
is tedious to screen for herbs effective against SARS-CoV-2, a
recently developed computational prediction was employed as a rapid
and efficient method to identify potentially active compounds of
CHM against SARS-CoV-2, based on the interactions between the herb
in question and the protein structures of the S-/N-proteins or the
RdRp of SARS-CoV-2. The publicly available TCMSP database, a common
antiviral and gene database, has provided invaluable as a source to
predict the molecular mechanisms of CHM in relation to the target
proteins of SARS-CoV2 (http://sm.nwsuaf.edu.cn/lsp/tcmsp.php). Certain novel
components of CHM have been discovered to have antiviral activity
against SARS-CoV-2 (78,79). Most recently, a study confirmed
natural compounds against SARS-CoV and MERS-CoV in published
papers, cross-checked these compounds in Chinese herbal databases
to determine the commonly shared compounds and then used
computer-based prediction to identify 13 compounds in CHM with
potential antiviral activity in relation to SARS-CoV-2. In
addition, >125 Chinese herbs contain 2 or more of these 13
compounds (80). These predicted
herbs still require to be validated for their antiviral effects
against COVID-19 through experimental and clinical studies.
Other options for treating COVID-19
In addition to the above-mentioned therapeutics,
certain other drugs have demonstrated antiviral effects on
SARS-CoV-2 and in patients with COVID-19. Tocilizumab is a
recombinant, anti-human mAb of IgG1, which improved a patient's
condition by inhibiting the IL-6 receptors. A clinical trial in
China used tocilizumab to treat 20 patients with acute COVID-19 and
indicated that 95% of patients (19) were cured and discharged from the
hospital after two weeks (81).
Tocilizumab has been approved to treat severe complications related
to SARS-CoV-2 in China. Recent clinical trials have demonstrated
the therapeutic efficacy of tocilizumab in severe COVID-19 cases.
In a clinical trial, tocilizumab was used to treat 100 patients
with COVID-19. After 10 days, 77 (77%) patients had improved in
terms of respiratory conditions. Of the 77 patients, 61 achieved
significant improvement as indicated by chest X-ray. As such, the
therapeutic efficacy of tocilizumab for COVID-19 is rapid,
sustained and associated with significant clinical improvement
(82,83).
Ivermectin is an FDA-approved anti-parasitic drug
that was effective against the SARS-CoV-2 virus in an in
vitro study (84). Cultured
Vero/hSLAM cells were infected with SARS-CoV-2 isolate and 5 µM
ivermectin was then added to treat the cells for 24–72 h. After 24
h, there was a 93% reduction of viral RNA in the supernatant of
samples treated with ivermectin compared to the control, DMSO. By
48 h, a 99.8% reduction in cell-associated viral RNA was observed
with ivermectin treatment. Another study indicated that the
antiviral activity of ivermectin targets and inhibits the host's
importin α/β1 nuclear transport proteins of viral proteins, as also
indicated for other types of RNA (85).
Based on the pathological immune regulation of
COVID-19, blocking the IL signaling pathway may control the
cytokine release from SARS-CoV-2 infection. Thus, a potential
therapeutic option is to use the IL-1 receptor antagonist anakinra.
Since anakinra blocks the activity of the pro-inflammatory
cytokines IL-1α and IL-1β, and is commonly used to treat
rheumatologic ailments (such as systemic-onset juvenile idiopathic
arthritis), anakinra reduced mortality in pediatric and adult
patients with secondary hemophagocytic lymphohistiocytosis
triggered by a virus (86). A
clinical study involving 112 patients with COVID-19 (56 treated
with anakinra and 56 controls) was performed to evaluate the
therapeutic effect of anakinra. The survival rate at day 28 was
obtained for 69 patients (61.6%) and was significantly higher in
anakinra-treated patients than in the controls (75.0 vs. 48.2%,
P=0.007). Thus, anakinra significantly improved overall survival of
COVID-19 patients (87).
Anti-inflammatory treatment is another option for
preventing COVID-19. Several clinical trials have used
anti-inflammatory drugs, including corticosteroids, cytokines and
drugs that interfere with cytokine activities (such as tocilizumab
and sarilumab) to block IL-6 activity, or infliximab and adalimumab
to block TNF-α, and/or baricitinib and ruxolitinib as JAK1/2
signaling pathway inhibitors. The efficacy of corticosteroids was
supported for the treatment of patients with severe COVID-19,
although certain studies suggested that treatment with
corticosteroid may not have an effect in hospitalized patients. A
systematic analysis of several clinical trials that included 1,703
critically ill patients determined that corticosteroid treatment
resulted in lower mortality among COVID-19 cases compared to
standard care or a placebo (the WHO Rapid Evidence Appraisal for
COVID-19 Therapies 2020) (88–90). In a recent clinical trial, 2,014
patients with COVID-19 were treated with dexamethasone; 22.9% of
patients in the dexamethasone group and 25.7% of patients in the
usual care group died within 28 days after treatment, suggesting
antiviral efficacy of dexamethasone. Thus, dexamethasone is
strongly recommended for hospitalized COVID-19 cases who require
oxygen delivery (ClinicalTrials.gov no. NCT04381936; ISRCTN no.
50189673) (91).
The therapeutic efficacy of other drugs, such as
pidotimod and flavonoids, has also been investigated for COVID-19.
One clinical study enrolled 20 patients with COVID-19 and divided
them into two groups: The pidotimod group and the control group.
These patients were treated for 14 days and pidotimod significantly
reduced their symptoms (92).
Flavonoids are abundant in plants, fruits and vegetables and have
antiviral activities that may offer protection against COVID-19.
The flavonoids exhibit potential inhibitory activity against
SARS-CoV-2 by binding to essential viral targets required for viral
entry and/or replication. Flavonoids also exert marked
immune-modulatory and anti-inflammatory effects, including the
inhibition of various inflammatory cytokines. Further studies
suggested that flavonoids are able to reduce the severity of
COVID-19 by promoting lipids metabolism. Two flavonoids, namely
quercetin and luteolin, have demonstrated promising multi-target
activity against SARS-CoV-2 infection. Therefore, flavonoid-rich
plants may be recommended as a supplementary treatment for
SARS-CoV-2 infection (14). The
mechanism of the antiviral activity of quercetin against SARS-CoV-2
including effects on 3CLpro, peptidase, the S glycoprotein, RNA
replicase, RNA binding protein and the papain-like protease of
SARS-CoV-2, to regulate microRNA genes involved in viral
pathogenesis (92). To accelerate
the identification of therapeutic drugs to treat COVID-19
effectively, a recent study used an in silico analysis of
the immune protein network, single-cell RNA sequencing and neural
networks to search for potential therapeutic drug targets against
COVID-19. After screening for 1,584 immune proteins in cells to
co-express the receptors of ACE2 and TMPRSS2, 25 potential
therapeutic targets were determined to be significantly
overexpressed in nasal goblet secretory cells, lung type II
pneumocytes and ileal absorptive enterocytes in patients with
several immunopathologies. Overall, 10,672 drugs with potential
antiviral activity were predicted, which may be used to treat
COVID-19. These drugs require to be further validated in
experiments and approved for clinical trials to treat patients with
COVID-19. (https://github.com/muntisa/immuno-drug-repurposing-COVID-19)
(93,94).
Antiviral mechanisms of therapeutic agents
against SARS-CoV2
As discussed above, certain therapeutic drugs or Abs
are able to inhibit SARS-CoV2 infection by targeting the different
pathological stages of the disease involved in the virus' entry,
RNA replication, synthesis of the polymerases, proteases, the
non-structural proteins and structural proteins of SARS-CoV2, as
well as the adaptive immune response to SARS-CoV2 infection.
Fig. 2 displays the molecular
pathogenesis of the viral infection and antiviral mechanisms by
which therapeutic agents block SARS-CoV2 infection. Based on the
therapeutic targets of SARS-CoV2, the antiviral agents may be
classified into four major categories.
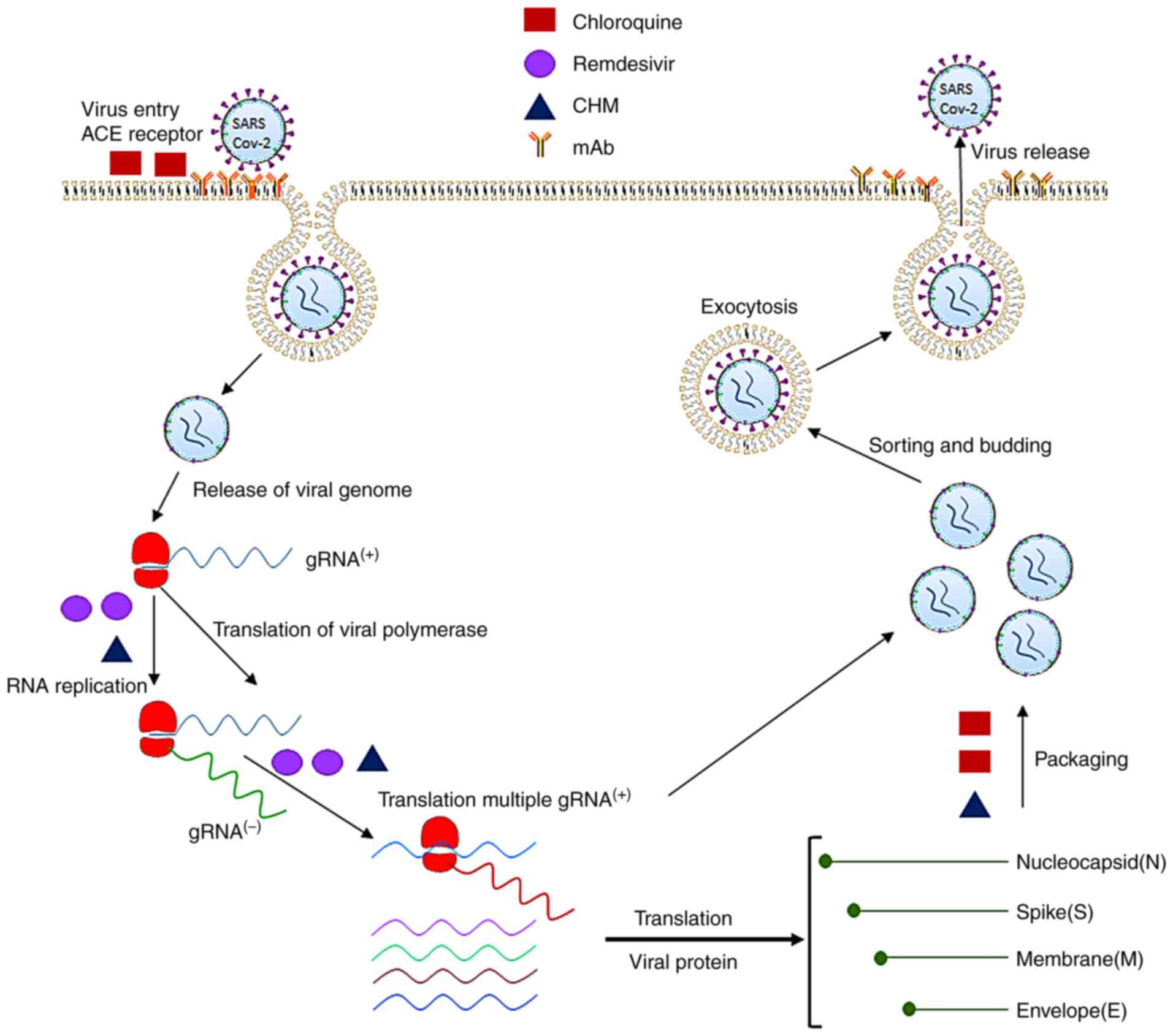 | Figure 2.Pathogenesis of SARS-CoV-2 infection
and molecular targets of antiviral therapeutics. The SARS-CoV-2
virus uses its spike proteins to bind to ACE2 receptors to enter
human cells. Its RNA is released into the cytoplasm and translated
to non-structural proteins such as RNA-dependent RNA polymerase.
The positive strand of viral RNA is replicated to produce the
negative strand of viral RNA, which is then transcribed to multiple
positive-strand subgenomic RNA. The viral structural proteins,
including the spike, envelope, membrane and nucleocapsid proteins,
are translated and inserted into the endoplasmic reticulum. The
subgenomic RNAs then combine with structural proteins and are
packaged into the virus, which enters the Golgi vesicle to form the
mature virion. Finally, the virion-containing vesicle fuses with
the membrane of the host's cell to release the virus particles. The
molecular targets of the antiviral therapeutics are denoted in
green (chloroquine), purple (remdesivir), orange (mAbs) and black
(CHM). SARS-CoV-2, severe acute respiratory syndrome coronavirus 2;
mAb, monoclonal antibodies; CHM, Chinese herbal medicine; ACE,
angiotensin-converting enzyme; gRNA, guide RNA. |
Prevention of binding of coronavirus
proteins to human cell receptors and inhibition of viral
self-assembly
SARS-CoV-2 uses human ACE2 receptors for the virus'
S protein to bind to host cells so that the virus is able to invade
the host cells. Thus, the application of drugs that block the S
proteins of SARS-CoV-2 from binding to ACE2 receptors in human
cells is an effective antiviral approach (95–98). ACE2 receptors have a high
similarity of amino acid sequences to the RBD of SARS-CoVs. CQ and
HCQ exert antiviral effects against SARS-CoV-2 by inhibiting the
coronavirus binding to the ACE2 receptors on a cell's surface. Due
to their alkaline nature, CQ and HCQ may also increase the pH
within a cell's cytoplasm to block the virus from entering and
facilitate transport to inhibit SARS-CoV-2 infection both in
vitro and in vivo. In addition, CQ and HCQ may interfere
with the packaging process of viral genomic positive RNAs and
structural proteins to block or delay the formation of viral
particles and their release from infected cells.
Certain Chinese herbs and natural flavonoids (e.g.,
licoflavonol from Glycyrrhiza uralensis) blocked the binding
of the viral S protein to ACE2 receptors to exert an antiviral
effect (80). Pudilan (PDL) is a
four-herb formula that includes Ban Lan Gen (Isatis
indigotica Fort., indigowoad root) and Huang Qin
(Scutellaria baicalensis Georgi., Baikal skullcap). PDL has
been recommended to treat H1N1 infection. An experimental study
revealed that PDL has a therapeutic effect against SARS-CoV-2 by
blocking the virus from binding to the ACE2 receptors in cultured
Vero E6 cells and in hACE2-transgenic mice (99).
Inhibition of the replication of
coronavirus RNA by action on polymerases
Coronaviral non-structural proteins (nsps), such as
RdRp, are involved in the virus' RNA replication, transcription and
protein translation, as well as the modification and processing of
proteins. RdRp (nsp12) is the critical enzyme of the viral
replication and transcriptional complex, which is conserved in
coronaviruses. Therefore, RdRp has been used as an important
antiviral target to develop drugs against SARS, MERS and COVID-19
(44,100). As a small molecule NA,
remdesivir was developed to mimic the structure of adenosine and to
target RdRp for integration into the replication synthesis of RNA
to inhibit coronavirus replication. After remdesivir enters a
cell's cytoplasm, it is metabolized into the active triphosphate
metabolite NTP, which interferes with the RdRp of SARS-CoV-2.
Incorporation of remdesivir at position i causes the termination of
RNA synthesis at position i+3. Of note, the same antiviral effects
are obtained by interfering with the RdRps of SARS-CoV, MERS-CoV
and SARS-CoV-2 after the cells are treated with remdesivir and
other NAs (101). Besides
remdesivir, other NAs, such as ribavirin, favipiravir and
galidesivir have potential antiviral actions against SARS-CoV-2 by
inhibiting the activity of RdRps. One study indicated that
favipiravir exerted an antiviral effect through the lethal
mutagenesis of RdRp of SARS-CoV-2. The SARS-CoV RdRp complex is far
more active than any other viral RdRps. During the replication of
viral RNA, favipiravir was inserted into viral RNA, inducing C-to-U
and G-to-A transitions in the SARS-CoV-2 genome, leading to the
mutated RNA to block viral replication (102). The latter study further
supported the concept of NAs as promising treatments for
COVID-19.
Certain Chinese herbs or natural products have also
demonstrated antiviral activity by binding to RdRp; betulonal (from
Cassine xylocarpa), gnidicin and gniditrin (from Gnidia
lamprantha) bind to and inhibit the RdRp of coronaviruses. The
active theaflavin of CHM markedly inhibited RdRp to block
SARS-CoV-2 replication. Through structural prediction and screening
for a total of 6,842 natural drugs from natural resources in South
Africa, four natural products (diosmetin-7-O-β-d-apiofuranoside,
3-O-α-l-arabinopyranosyl-echinocystic acid, 3′-epi-afroside and
genkwanin-8-C-β-glucopyranoside) were identified to have a similar
binding domain to that of remdesivir, allowing them to block the
RdRp-mediated RNA replication of SARS-CoV-2. Each of these products
had similar antiviral activity to remdesivir based on computational
screening and molecular docking predictions, with docking scores
between −7.1 and −10. 4 kcal/mol (103).
Inhibition of synthesis of viral
protein and the enzymes of the coronavirus
The coronavirus main proteinase 3CLpro, papain-like
proteinase (PLpro) and helicase are potential molecular targets for
antiviral small molecules to inhibit the viral RNA replication of
SARS-CoV-2. 3CLpro is responsible for the processing and maturation
of the viral polyproteins; thus, it is a promising target for
developing drugs against SARS-CoV-2 infection. Recently, treatment
combining GC376 and remdesivir significantly inhibited viral
replication by binding to the catalytically active site of 3CLpro
of SARS-CoV-2 as a major antiviral mechanism (33). The Chinese herb, the root extract
of Isatis indigotica (Ban Lan Gen), dose-dependently
inhibited the cleavage activity of 3CLpro of SARS-coronavirus to
exert its antiviral action (104). The active component of the
natural compound, resveratrol, which is widely present in different
plants, including Vitis vinifera, Polygonum cuspidatum and
Vaccinium macrocarpon, has antiviral effects on MERS-CoV by
targeting the N protein to block the packaging of viral
particles.
Ab-mediated antiviral mechanisms for
blocking SARS-CoV-2 infection
Neutralizing Abs are useful for treating coronavirus
infections such as COVID-19. These Abs may be obtained either by
isolation from the CP of a patient with SARS-CoV-2 or produced by a
biotech company (43). In most
viral infections, the titer of the antiviral serum usually reaches
a higher concentration after the first week of viral infection.
Thus, the early transfusion of CP is probably more effective to
improve the survival rate of critical COVD-19 patients in the early
stage of the disease (52,54).
It should be noted that CP transfusion may not be useful for
patients with end-stage disease, given the severity of the disease.
On the other hand, certain patients with mild disease may
self-recover; hence, CP transfusion may not be required (59).
mAbs may be designed to target the specific viral
antigens in different steps of the pathogenesis of SARS-CoV-2
infection. Usually, mAbs that combat SARS-CoV-2 are able to
neutralize the virus by targeting the virus' structural proteins or
host receptors to inhibit the virus from attaching and entering.
Certain mAbs are able to inhibit the virus' replication and
transcription or increase the immune response of patients with
SARS-CoV-2 infection. Since SARS-CoV-2 enters human cells through
their RBD in the S1 subunit of the S protein to bind to ACE2
receptors, mAbs (targeting S proteins), are the most effective Abs
against SARS-CoV-2 infection (105). In addition to the Abs that
inhibit the virus from entering, other Abs may neutralize the
specific enzymes or proteins of SARS-CoV-2 by preventing its
replication. These Abs are required to cross the cell membrane of
infected cells to neutralize the RdRp or other enzymes to inhibit
viral replication. The critical viral enzymes, such as PLpro,
3CLpro and certain nsps, may be targets to develop Abs to block the
RNA replication cycle of SARS-CoV-2 (106,107).
Anti-inflammatory mechanism of drugs
for inhibiting the pathogenesis of SARS-CoV-2
In addition to inhibiting the entry and replication
of the SARS-CoV-2, certain drugs exert antiviral effects by
decreasing the pathological inflammatory response induced by
SARS-CoV-2. CQ and HCQ may also have an immunomodulatory effect to
inhibit pathogenesis by decreasing cytokine production and
inhibiting cellular autophagy in infected cells. HCQ was reported
to inhibit the release of IL-6, IL-1β and TNF-α (108–110). It was also reported that the
combination of azithromycin with HCQ increased viral clearance in
certain patients with COVID-19. Various anti-inflammatory drugs,
such as corticosteroids, cytokines and drugs that interfere with
cytokine activities (such as tocilizumab and sarilumab) to block
IL-6 activity for decreasing the pathological inflammatory reaction
of SARS-CoV-2, were described in the above sections.
Conclusions and future perspectives
Although experimental and clinical studies indicated
that certain therapeutic agents may be used to treat or prevent
SARS-CoV-2 infection, major challenges remain to develop effective
drugs and vaccines for SARS-CoV-2 infection.
First, the novel NAs and single compounds of Chinese
herbs should be further identified and modified to validate their
antiviral efficacy against COVID-19. Certain NAs such as remdesivir
cannot be administered to the human body directly in the active
triphosphate form. NAs are usually given as a prodrug that requires
to be phosphorylated into the triphosphate form to be recognized by
the RdRp once it enters a cell. To overcome this problem, the
nucleosides of NAs require to be modified to allow active NAs to
cross the cell membrane (40,44). Numerous natural products and
Chinese herbs inhibit the entry and packaging of SARS-CoV-2, but
the molecular targets of the compounds in Chinese herbs require to
be extensively characterized to increase their antiviral activity.
Furthermore, Ab-based therapy appears to be the most validated
treatment for COVID-19. Since mAbs have the advantages of
specifically neutralizing viral antigens and may be produced on a
large scale within a short period, a standard process of
development and production is required to accelerate the approval
of mAbs to treat COVID-19. Due to the diversity of the epitopes on
SARS-CoV-2, a combination of several mAbs or CP should be used to
achieve therapeutic efficacy. In addition, it must be decided how
many injections of the Abs are required to maintain a sufficient
concentration and efficacy of mAbs in treated patients. Secondly,
certain natural plant compounds and TCMs have strong antiviral
properties and no toxicity and are thus recommended as candidate
drugs for immediate treatment of SARS-CoV-2 infection (111,112). Finally, as the genomic RNA,
structural proteins and non-structural proteins of SARS-CoV-2 have
been characterized, the effective DNA or mRNA, or inactivated-virus
vaccines, have been developed for immunization of non-affected
populations to prevent the further spread of SARS-CoV-2. Certain
mRNA vaccines or inactivated virus vaccines for SARS-CoV2 have been
approved and have been used to immunize normal populations in
numerous countries, including the USA, Canada, China, Japan and the
UK. Therefore, with fast advances in drug discovery and vaccine
development, the risk of SARS-CoV2 infection in different
populations around the world will largely decline in the near
future.
Acknowledgements
Not applicable.
Funding
This study was funded by the Start-up Research fund
of Shandong University of Traditional Chinese Medicine (grant no.
2020-220259). Furthermore, the Quancheng Talent Scholar Fund of
Jinan City (grant no. 5150) provided financial support.
Availability of data and materials
Not applicable.
Authors' contributions
FH conceived and designed the review. FH and JW were
major contributors to writing the manuscript. YY, YL, MM, CW and JC
wrote parts of the manuscript and prepared the figures. FH, YL and
JC performed the literature search and selection, and were
responsible for editing the references. All authors read and
approved the final version of the manuscript. All authors are
responsible for all aspects of the work and approve the submission
in its current form. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–733.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H,
Wang W, Song H, Huang B, Zhu N, et al: Genomic characterisation and
epidemiology of 2019 novel coronavirus: Implications for virus
origins and receptor binding. Lancet. 395:565–574. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Q, Zhang Y, Wu L, Niu S, Song C,
Zhang Z, Lu G, Qiao C, Hu Y, Yuen KY, et al: Structural and
functional basis of SARS-CoV-2 entry by using human ACE2. Cell.
181:894–904. e9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara
H, Geng Q, Auerbach A and Li F: Structural basis of receptor
recognition by SARS-CoV-2. Nature. 581:221–224. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuhn JH, Radoshitzky SR, Li W, Wong SK,
Choe H and Farzan M: The SARS Coronavirus receptor ACE 2 A
potential target for antiviral therapy. New Concepts of Antiviral
Therapy. Holzenburg A and Bogner E: Springer US; Boston, MA: pp.
397–418. 2006, View Article : Google Scholar
|
|
6
|
Letko M, Marzi A and Munster V: Functional
assessment of cell entry and receptor usage for SARS-CoV-2 and
other lineage B betacoronaviruses. Nat Microbiol. 5:562–569. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rodriguez Y, Novelli L, Rojas M, De Santis
M, Acosta-Ampudia Y, Monsalve DM, Ramírez-Santana C, Costanzo A,
Ridgway WM, Ansari AA, et al: Autoinflammatory and autoimmune
conditions at the crossroad of COVID-19. J Autoimmun.
114:1025062020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tay MZ, Poh CM, Rénia L, MacAry PA and Ng
LFP: The trinity of COVID-19: Immunity, inflammation and
intervention. Nat Rev Immunol. 20:363–374. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fricke-Galindo I and Falfán-Valencia R:
Genetics insight for COVID-19 susceptibility and severity: A
review. Front Immunol. 12:6221762021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maggi E, Canonica GW and Moretta L:
COVID-19: Unanswered questions on immune response and pathogenesis.
J Allergy Clin Immunol. 146:18–22. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahmed-Hassan H, Sisson B, Shukla RK,
Wijewantha Y, Funderburg NT, Li Z, Hayes D Jr, Demberg T and
Liyanage NPM: Innate immune responses to highly pathogenic
coronaviruses and other significant respiratory viral infections.
Front Immunol. 11:19792020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li G and De Clercq E: Therapeutic options
for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov.
19:149–150. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zumla A, Chan JF, Azhar EI, Hui DS and
Yuen KY: Coronaviruses-drug discovery and therapeutic options. Nat
Rev Drug Discov. 15:327–347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alzaabi MM, Hamdy R, Ashmawy NS, Hamoda
AM, Alkhayat F, Khademi NN, Al Joud SMA, El-Keblawy AA and Soliman
SSM: Flavonoids are promising safe therapy against COVID-19.
Phytochem Rev. May 22–2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Savarino A, Di Trani L, Donatelli I, Cauda
R and Cassone A: New insights into the antiviral effects of
chloroquine. Lancet Infect Dis. 6:67–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Bari MA: Chloroquine analogues in drug
discovery: New directions of uses, mechanisms of actions and toxic
manifestations from malaria to multifarious diseases. J Antimicrob
Chemother. 70:1608–1621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keyaerts E, Vijgen L, Maes P, Neyts J and
Van Ranst M: In vitro inhibition of severe acute respiratory
syndrome coronavirus by chloroquine. Biochem Biophys Res Commun.
323:264–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu
M, Shi Z, Hu Z, Zhong W and Xiao G: Remdesivir and chloroquine
effectively inhibit the recently emerged novel coronavirus
(2019-nCoV) in vitro. Cell Res. 30:269–271. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao J, Tian Z and Yang X: Breakthrough:
Chloroquine phosphate has shown apparent efficacy in treatment of
COVID-19 associated pneumonia in clinical studies. Biosci Trends.
14:72–73. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang M, Tang T, Pang P, Li M, Ma R, Lu J,
Shu J, You Y, Chen B, Liang J, et al: Treating COVID-19 with
chloroquine. J Mol Cell Biol. 12:322–325. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu
P, Liu X, Zhao L, Dong E, Song C, et al: In vitro antiviral
activity and projection of optimized dosing design of
hydroxychloroquine for the treatment of severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 71:732–739.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andreani J, Le Bideau M, Duflot I, Jardot
P, Rolland C, Boxberger M, Wurtz N, Rolain JM, Colson P, La Scola B
and Raoult D: In vitro testing of combined hydroxychloroquine and
azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog.
145:1042282020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gautret P, Lagier JC, Parola P, Hoang VT,
Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE,
et al: Hydroxychloroquine and azithromycin as a treatment of
COVID-19: Results of an open-label non-randomized clinical trial.
Int J Antimicrob Agents. 56:1059492020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arshad S, Kilgore P, Chaudhry ZS, Jacobsen
G, Wang DD, Huitsing K, Brar I, Alangaden GJ, Ramesh MS, McKinnon
JE, et al: Treatment with hydroxychloroquine, azithromycin, and
combination in patients hospitalized with COVID-19. Int J Infect
Dis. 97:396–403. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hernandez AV, Roman YM, Pasupuleti V,
Barboza JJ and White CM: Hydroxychloroquine or chloroquine for
treatment or prophylaxis of COVID-19: A living systematic review.
Ann Intern Med. 173:287–296. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chowdhury MS, Rathod J and Gernsheimer J:
A rapid systematic review of clinical trials utilizing chloroquine
and hydroxychloroquine as a treatment for COVID-19. Acad Emerg Med.
27:493–504. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elavarasi A, Prasad M, Seth T, Sahoo RK,
Madan K, Nischal N, Soneja M, Sharma A, Maulik SK, Shalimar and
Garg P: Chloroquine and hydroxychloroquine for the treatment of
COVID-19: A systematic review and meta-analysis. J Gen Intern Med.
35:3308–3314. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar J, Jain S, Meena J and Yadav A:
Efficacy and safety of hydroxychloroquine/chloroquine against
SARS-CoV-2 infection: A systematic review and meta-analysis. J
Infect Chemother. 27:882–889. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eckerle LD, Becker MM, Halpin RA, Li K,
Venter E, Lu X, Scherbakova S, Graham RL, Baric RS, Stockwell TB,
et al: Infidelity of SARS-CoV Nsp14-exonuclease mutant virus
replication is revealed by complete genome sequencing. PLoS Pathog.
6:e10008962010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Warren TK, Jordan R, Lo MK, Ray AS,
Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, et
al: Therapeutic efficacy of the small molecule GS-5734 against
Ebola virus in rhesus monkeys. Nature. 531:381–385. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong L, Hu S and Gao J: Discovering drugs
to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther.
14:58–60. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Agostini ML, Andres EL, Sims AC, Graham
RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, et al:
Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is
mediated by the viral polymerase and the proofreading
exoribonuclease. mBio. 9:e00221–e00228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu L, Ye F, Feng Y, Yu F, Wang Q, Wu Y,
Zhao C, Sun H, Huang B, Niu P, et al: Both boceprevir and GC376
efficaciously inhibit SARS-CoV-2 by targeting its main protease.
Nat Commun. 11:44172020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Holshue ML, DeBolt C, Lindquist S, Lofy
KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural
A, et al: First case of 2019 Novel Coronavirus in the United
States. N Engl J Med. 382:929–936. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grein J, Ohmagari N, Shin D, Diaz G,
Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, et
al: Compassionate use of remdesivir for patients with severe
Covid-19. N Engl J Med. 382:2327–2336. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Spinner CD, Gottlieb RL, Criner GJ,
Arribas López JR, Cattelan AM, Soriano Viladomiu A, Ogbuagu O,
Malhotra P, Mullane KM, Castagna A, et al: Effect of remdesivir vs
standard care on clinical status at 11 days in patients with
moderate COVID-19: A randomized clinical trial. JAMA.
324:1048–1057. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Beigel JH, Tomashek KM, Dodd LE, Mehta AK,
Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, et
al: Remdesivir for the treatment of Covid-19-final report. N Engl J
Med. 383:1813–1826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Garibaldi BT, Wang K, Robinson ML, Zeger
SL, Bandeen-Roche K, Wang MC, Alexander GC, Gupta A, Bollinger R
and Xu Y: Comparison of time to clinical improvement with vs
without remdesivir treatment in hospitalized patients with
COVID-19. JAMA Netw Open. 4:e2130712021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aleissa MM, Silverman EA, Paredes Acosta
LM, Nutt CT, Richterman A and Marty FM: New perspectives on
antimicrobial agents: Remdesivir treatment for COVID-19. Antimicrob
Agents Chemother. 65:e01814–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Elfiky AA: Ribavirin, remdesivir,
sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA
dependent RNA polymerase (RdRp): A molecular docking study. Life
Sci. 253:1175922020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Falzarano D, de Wit E, Martellaro C,
Callison J, Munster VJ and Feldmann H: Inhibition of novel β
coronavirus replication by a combination of interferon-α2b and
ribavirin. Sci Rep. 3:16862013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Falzarano D, de Wit E, Rasmussen AL,
Feldmann F, Okumura A, Scott DP, Brining D, Bushmaker T, Martellaro
C, Baseler L, et al: Treatment with interferon-α2b and ribavirin
improves outcome in MERS-CoV-infected rhesus macaques. Nat Med.
19:1313–1317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
De Clercq E: New nucleoside analogues for
the treatment of hemorrhagic fever virus infections. Chem Asian J.
14:3962–3968. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Choy KT, Wong AY, Kaewpreedee P, Sia SF,
Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PP, Huang X, et al:
Remdesivir, lopinavir, emetine, and homoharringtonine inhibit
SARS-CoV-2 replication in vitro. Antiviral Res. 178:1047862020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen C, Zhang Y, Huang J, Yin P, Cheng Z,
Wu J, Chen S, Zhang Y, Chen B, Lu M, et al: Favipiravir versus
Arbidol for COVID-19: A randomized clinical trial. medRxiv:
2020.03.17.20037432. 2020. View Article : Google Scholar
|
|
46
|
Taylor R, Kotian P, Warren T, Panchal R,
Bavari S, Julander J, Dobo S, Rose A, El-Kattan Y, Taubenheim B, et
al: BCX4430-A broad-spectrum antiviral adenosine nucleoside analog
under development for the treatment of Ebola virus disease. J
Infect Public Health. 9:220–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shu H, Wang S, Ruan S, Wang Y, Zhang J,
Yuan Y, Liu H, Wu Y, Li R, Pan S, et al: Dynamic changes of
antibodies to SARS-CoV-2 in COVID-19 patients at early stage of
outbreak. Virol Sin. 35:744–751. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Brouwer PJM, Caniels TG, van der Straten
K, Snitselaar JL, Aldon Y, Bangaru S, Torres JL, Okba NMA,
Claireaux M, Kerster G, et al: Potent neutralizing antibodies from
COVID-19 patients define multiple targets of vulnerability.
Science. 369:643–650. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chan KH, Chan JF, Tse H, Chen H, Lau CC,
Cai JP, Tsang AK, Xiao X, To KK, Lau SK, et al: Cross-reactive
antibodies in convalescent SARS patients' sera against the emerging
novel human coronavirus EMC (2012) by both immunofluorescent and
neutralizing antibody tests. J Infect. 67:130–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mair-Jenkins J, Saavedra-Campos M, Baillie
JK, Cleary P, Khaw FM, Lim WS, Makki S, Rooney KD, Nguyen-Van-Tam
JS and Beck CR; Convalescent Plasma Study Group, : The
effectiveness of convalescent plasma and hyperimmune immunoglobulin
for the treatment of severe acute respiratory infections of viral
etiology: A systematic review and exploratory meta-analysis. J
Infect Dis. 211:80–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tian X, Li C, Huang A, Xia S, Lu S, Shi Z,
Lu L, Jiang S, Yang Z, Wu Y and Ying T: Potent binding of 2019
novel coronavirus spike protein by a SARS coronavirus-specific
human monoclonal antibody. Emerg Microbes Infect. 9:382–385. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sui J, Deming M, Rockx B, Liddington RC,
Zhu QK, Baric RS and Marasco WA: Effects of human anti-spike
protein receptor binding domain antibodies on severe acute
respiratory syndrome coronavirus neutralization escape and fitness.
J Virol. 88:13769–13780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiang S, Hillyer C and Du L: Neutralizing
antibodies against SARS-CoV-2 and other human coronaviruses:
(Trends in Immunology 41, 355–359; 2020). Trends Immunol.
41:5452020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mulangu S, Dodd LE, Davey RT Jr, Tshiani
Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D,
Tshomba Oloma A, Ibanda A, et al: A randomized, controlled trial of
Ebola virus disease therapeutics. N Engl J Med. 381:2293–2303.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shi R, Shan C, Duan X, Chen Z, Liu P, Song
J, Song T, Bi X, Han C, Wu L, et al: A human neutralizing antibody
targets the receptor-binding site of SARS-CoV-2. Nature.
584:120–124. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Valdez-Cruz NA, García-Hernández E,
Espitia C, Cobos-Marín L, Altamirano C, Bando-Campos CG,
Cofas-Vargas LF, Coronado-Aceves EW, González-Hernández RA,
Hernández-Peralta P, et al: Integrative overview of antibodies
against SARS-CoV-2 and their possible applications in COVID-19
prophylaxis and treatment. Microb Cell Fact. 20:882021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen P, Nirula A, Heller B, Gottlieb RL,
Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, et al:
SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with
Covid-19. N Engl J Med. 384:229–237. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Katia F, Myriam DP, Ucciferri C, Auricchio
A, Di Nicola M, Marchioni M, Eleonora C, Emanuela S, Cipollone F
and Vecchiet J: Efficacy of canakinumab in mild or severe COVID-19
pneumonia. Immun Inflamm Dis. 9:399–405. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hung IF, To KK, Lee CK, Lee KL, Chan K,
Yan WW, Liu R, Watt CL, Chan WM, Lai KY, et al: Convalescent plasma
treatment reduced mortality in patients with severe pandemic
influenza A (H1N1) 2009 virus infection. Clin Infect Dis.
52:447–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ko JH, Seok H, Cho SY, Ha YE, Baek JY, Kim
SH, Kim YJ, Park JK, Chung CR, Kang ES, et al: Challenges of
convalescent plasma infusion therapy in Middle East respiratory
coronavirus infection: A single centre experience. Antivir Ther.
23:617–622. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Soo YO, Cheng Y, Wong R, Hui DS, Lee CK,
Tsang KK, Ng MH, Chan P, Cheng G and Sung JJ: Retrospective
comparison of convalescent plasma with continuing high-dose
methylprednisolone treatment in SARS patients. Clin Microbiol
Infect. 10:676–678. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Casadevall A, Grossman BJ, Henderson JP,
Joyner MJ, Shoham S, Pirofski LA and Paneth N: The assessment of
convalescent plasma efficacy against COVID-19. Med (N Y). 1:66–77.
2020.PubMed/NCBI
|
|
63
|
Arabi Y, Balkhy H, Hajeer AH, Bouchama A,
Hayden FG, Al-Omari A, Al-Hameed FM, Taha Y, Shindo N, Whitehead J,
et al: Feasibility, safety, clinical, and laboratory effects of
convalescent plasma therapy for patients with Middle East
respiratory syndrome coronavirus infection: A study protocol.
Springerplus. 4:7092015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH,
Baek YJ, Jeong SJ, Kim JH, Ku NS, Yeom JS, et al: Use of
convalescent plasma therapy in two COVID-19 patients with acute
respiratory distress syndrome in Korea. J Korean Med Sci.
35:e1492020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Duan K, Liu B, Li C, Zhang H, Yu T, Qu J,
Zhou M, Chen L, Meng S, Hu Y, et al: Effectiveness of convalescent
plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA.
117:9490–9496. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu S, Shergis J, Chen X, Yu X, Guo X,
Zhang AL, Lu C and Xue CC: Chinese herbal medicine (weijing
decoction) combined with pharmacotherapy for the treatment of acute
exacerbations of chronic obstructive pulmonary disease. Evid Based
Complement Alternat Med. 2014:2570122014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Leung PC: The efficacy of Chinese medicine
for SARS: A review of Chinese publications after the crisis. Am J
Chin Med. 35:575–581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cinatl J, Morgenstern B, Bauer G, Chandra
P, Rabenau H and Doerr HW: Glycyrrhizin, an active component of
liquorice roots, and replication of SARS-associated coronavirus.
Lancet. 361:2045–2046. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang SQ, Du QS, Zhao K, Li AX, Wei DQ and
Chou KC: Virtual screening for finding natural inhibitor against
cathepsin-L for SARS therapy. Amino Acids. 33:129–135. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ho TY, Wu SL, Chen JC, Li CC and Hsiang
CY: Emodin blocks the SARS coronavirus spike protein and
angiotensin-converting enzyme 2 interaction. Antiviral Res.
74:92–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Niu M, Wang RL, Wang ZX, Zhang P, Bai ZF,
Jing J, Guo YM, Zhao X, Zhan XY, Zhang ZT, et al: Rapid
establishment of traditional Chinese medicine prevention and
treatment of 2019-nCoV based on clinical experience and molecular
docking. Zhongguo Zhong Yao Za Zhi. 45:1213–1218. 2020.(In
Chinese). PubMed/NCBI
|
|
72
|
Runfeng L, Yunlong H, Jicheng H, Weiqi P,
Qinhai M, Yongxia S, Chufang L, Jin Z, Zhenhua J, Haiming J, et al:
Lianhuaqingwen exerts anti-viral and anti-inflammatory activity
against novel coronavirus (SARS-CoV-2). Pharmacol Res.
156:1047612020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu H, Ye F, Sun Q, Liang H, Li C, Li S,
Lu R, Huang B, Tan W and Lai L: Scutellaria baicalensis
extract and baicalein inhibit replication of SARS-CoV-2 and its
3C-like protease in vitro. bioRxiv: 2020.04.10.035824. 2020.
|
|
74
|
Zhu J, Deng YQ, Wang X, Li XF, Zhang NN,
Liu Z, Zhang B, Qin CF and Xie Z: An artificial intelligence system
reveals liquiritin inhibits SARS-CoV-2 by mimicking type I
interferon. bioRxiv: doi:
https://doi.org/10.1101/2020.05.02.074021. View Article : Google Scholar
|
|
75
|
Chen Z and Nakamura T: Statistical
evidence for the usefulness of Chinese medicine in the treatment of
SARS. Phytother Res. 18:592–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Luo H, Tang QL, Shang YX, Liang SB, Yang
M, Robinson N and Liu JP: Can Chinese medicine be used for
prevention of corona virus disease 2019 (COVID-19)? A review of
historical classics, research evidence and current prevention
programs. Chin J Integr Med. 26:243–250. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ang L, Lee HW, Choi JY, Zhang J and Soo
Lee M: Herbal medicine and pattern identification for treating
COVID-19: A rapid review of guidelines. Integr Med Res.
9:1004072020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yu S, Wang J and Shen H: Network
pharmacology-based analysis of the role of traditional Chinese
herbal medicines in the treatment of COVID-19. Ann Palliat Med.
9:437–446. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wu C, Liu Y, Yang Y, Zhang P, Zhong W,
Wang Y, Wang Q, Xu Y, Li M, Li X, et al: Analysis of therapeutic
targets for SARS-CoV-2 and discovery of potential drugs by
computational methods. Acta Pharm Sin B. 10:766–788. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang DH, Wu KL, Zhang X, Deng SQ and Peng
B: In silico screening of Chinese herbal medicines with the
potential to directly inhibit 2019 novel coronavirus. J Integr Med.
18:152–158. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang Q, Wang Y, Qi C, Shen L and Li J:
Clinical trial analysis of 2019-nCoV therapy registered in China. J
Med Virol. 92:540–545. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kulanthaivel S, Kaliberdenko VB,
Balasundaram K, Shterenshis MV, Scarpellini E and Abenavoli L:
Tocilizumab in SARS-CoV-2 patients with the syndrome of cytokine
storm: A narrative review. Rev Recent Clin Trials. 16:138–145.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Toniati P, Piva S, Cattalini M, Garrafa E,
Regola F, Castelli F, Franceschini F, Airò P, Bazzani C, Beindorf
EA, et al: Tocilizumab for the treatment of severe COVID-19
pneumonia with hyperinflammatory syndrome and acute respiratory
failure: A single center study of 100 patients in Brescia, Italy.
Autoimmun Rev. 19:1025682020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Caly L, Druce JD, Catton MG, Jans DA and
Wagstaff KM: The FDA-approved drug ivermectin inhibits the
replication of SARS-CoV-2 in vitro. Antiviral Res. 178:1047872020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yang SNY, Atkinson SC, Wang C, Lee A,
Bogoyevitch MA, Borg NA and Jans DA: The broad spectrum antiviral
ivermectin targets the host nuclear transport importin α/β1
heterodimer. Antiviral Res. 177:1047602020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Monteagudo LA, Boothby A and Gertner E:
Continuous intravenous anakinra infusion to calm the cytokine storm
in macrophage activation syndrome. ACR Open Rheumatol. 2:276–282.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Franzetti M, Forastieri A, Borsa N,
Pandolfo A, Molteni C, Borghesi L, Pontiggia S, Evasi G, Guiotto L,
Erba M, et al: IL-1 receptor antagonist anakinra in the treatment
of COVID-19 acute respiratory distress syndrome: A retrospective,
observational study. J Immunol. 206:1569–1575. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Romani L, Tomino C, Puccetti P and Garaci
E: Off-label therapy targeting pathogenic inflammation in COVID-19.
Cell Death Discov. 6:492020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Puccetti M, Costantini C, Ricci M and
Giovagnoli S: Tackling immune pathogenesis of COVID-19 through
molecular pharmaceutics. Pharmaceutics. 13:4942021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
D'Ardes D, Pontolillo M, Esposito L,
Masciarelli M, Boccatonda A, Rossi I, Bucci M, Guagnano MT,
Ucciferri C, Santilli F, et al: Duration of COVID-19: Data from an
Italian cohort and potential role for steroids. Microorganisms.
8:13272020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Horby P, White NJ and Landray MJ:
Hydroxychloroquine in hospitalized patients with Covid-19. Reply. N
Engl J Med. 384:8822021.PubMed/NCBI
|
|
92
|
Ucciferri C, Barone M, Vecchiet J and
Falasca K: Pidotimod in paucisymptomatic SARS-CoV2 infected
patients. Mediterr J Hematol Infect Dis. 12:e20200482020.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Saakre M, Mathew D and Ravisankar V:
Perspectives on plant flavonoid quercetin-based drugs for novel
SARS-CoV-2. Beni Suef Univ J Basic Appl Sci. 10:212021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
López-Cortés A, Guevara-Ramírez P,
Kyriakidis NC, Barba-Ostria C, León Cáceres Á, Guerrero S,
Ortiz-Prado E, Munteanu CR, Tejera E, Cevallos-Robalino D, et al:
In silico analyses of immune system protein interactome network,
single-cell rna sequencing of human tissues, and artificial neural
networks reveal potential therapeutic targets for drug repurposing
against COVID-19. Front Pharmacol. 12:5989252021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hong W: Combating COVID-19 with
chloroquine. J Mol Cell Biol. 12:249–250. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S,
Zhang Q, Shi X, Wang Q, Zhang L and Wang X: Structure of the
SARS-CoV-2 spike receptor-binding domain bound to the ACE2
receptor. Nature. 581:215–220. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H,
Li Y, Hu Z, Zhong W and Wang M: Hydroxychloroquine, a less toxic
derivative of chloroquine, is effective in inhibiting SARS-CoV-2
infection in vitro. Cell Discov. 6:162020. View Article : Google Scholar
|
|
98
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kong Q, Wu Y, Gu Y, Lv Q, Qi F, Gong S and
Chen X: Analysis of the molecular mechanism of Pudilan (PDL)
treatment for COVID-19 by network pharmacology tools. Biomed
Pharmacother. 128:1103162020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chu CK, Gadthula S, Chen X, Choo H, Olgen
S, Barnard DL and Sidwell RW: Antiviral activity of nucleoside
analogues against SARS-coronavirus (SARS-coV). Antivir Chem
Chemother. 17:285–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gordon CJ, Tchesnokov EP, Woolner E, Perry
JK, Feng JY, Porter DP and Götte M: Remdesivir is a direct-acting
antiviral that inhibits RNA-dependent RNA polymerase from severe
acute respiratory syndrome coronavirus 2 with high potency. J Biol
Chem. 295:6785–6797. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shannon A, Selisko B, Le NT, Huchting J,
Touret F, Piorkowski G, Fattorini V, Ferron F, Decroly E, Meier C,
et al: Rapid incorporation of Favipiravir by the fast and
permissive viral RNA polymerase complex results in SARS-CoV-2
lethal mutagenesis. Nat Commun. 11:46822020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Khan A, Khan M, Saleem S, Babar Z, Ali A,
Khan AA, Sardar Z, Hamayun F, Ali SS and Wei DQ: Phylogenetic
analysis and structural perspectives of RNA-dependent
RNA-polymerase inhibition from SARs-CoV-2 with natural products.
Interdiscip Sci. 12:335–348. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lin CW, Tsai FJ, Tsai CH, Lai CC, Wan L,
Ho TY, Hsieh CC and Chao PD: Anti-SARS coronavirus 3C-like protease
effects of Isatis indigotica root and plant-derived phenolic
compounds. Antiviral Res. 68:36–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Park T, Lee SY, Kim S, Kim MJ, Kim HG, Jun
S, Kim SI, Kim BT, Park EC and Park D: Spike protein binding
prediction with neutralizing antibodies of SARS-CoV-2. bioRxiv:
doi: 2020.02.22.951178. 2020. View Article : Google Scholar
|
|
106
|
Anand K, Ziebuhr J, Wadhwani P, Mesters JR
and Hilgenfeld R: Coronavirus main proteinase (3CLpro) structure:
Basis for design of anti-SARS drugs. Science. 300:1763–1767. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhou G and Zhao Q: Perspectives on
therapeutic neutralizing antibodies against the novel coronavirus
SARS-CoV-2. Int J Biol Sci. 16:1718–1723. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Quiros Roldan E, Biasiotto G, Magro P and
Zanella I: The possible mechanisms of action of 4-aminoquinolines
(chloroquine/hydroxychloroquine) against Sars-Cov-2 infection
(COVID-19): A role for iron homeostasis? Pharmacol Res.
158:1049042020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhou D, Dai SM and Tong Q: COVID-19: A
recommendation to examine the effect of hydroxychloroquine in
preventing infection and progression. J Antimicrob Chemother.
75:1667–1670. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Devaux CA, Rolain JM, Colson P and Raoult
D: New insights on the antiviral effects of chloroquine against
coronavirus: What to expect for COVID-19? Int J Antimicrob Agents.
55:1059382020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Adhikari B, Marasini BP, Rayamajhee B,
Bhattarai BR, Lamichhane G, Khadayat K, Adhikari A, Khanal S and
Parajuli N: Potential roles of medicinal plants for the treatment
of viral diseases focusing on COVID-19: A review. Phytother Res.
35:1298–1312. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lee DYW, Li QY, Liu J and Efferth T:
Traditional Chinese herbal medicine at the forefront battle against
COVID-19: Clinical experience and scientific basis. Phytomedicine.
80:1533372021. View Article : Google Scholar : PubMed/NCBI
|
















