Introduction
Congenital heart disease (CHD) is a congenital
disease with a high incidence, particularly in America, that has
gradually increased in recent years (1,2).
CHD is the leading cause of infant mortality due to birth defects
and accounts for nearly one-third of all congenital anomalies
(3,4). CHD is often accompanied by other
complications and patients require repeated surgical procedures.
This seriously affects the quality of life of patients and is also
a burden on the family and society (5). Therefore, early prenatal diagnosis
is important to investigate the occurrence of CHD and improve the
medical management of mother and fetal outcomes (6,7).
In current obstetric care, the clinical strategy for diagnosing CHD
mainly relies on fetal echocardiography in the second trimester of
pregnancy (8,9). However, only ~50% of CHDs can be
detected by echocardiography, which means that not all CHDs are
detected during pregnancy (10).
Hence, finding potential biomarkers for strict fetal CHD screening
would contribute greatly to prenatal diagnosis.
Circular RNAs (circRNAs) have been identified as
novel noncoding RNAs (ncRNAs) that are formed during RNA maturation
through a process called splicing (11,12). CircRNAs have a closed-loop
structure without 3′ and 5′ polarity (13). CircRNAs are cyclic in structure
and contain introns of gene fragments (14). Studies have shown that microRNAs
(miRNAs) are sponged by circRNAs (15–17). Previous studies have shown that
circRNAs can bind miRNAs as competitive endogenous RNAs (ceRNAs),
preventing miRNAs from binding their target mRNAs and resulting in
the increased expression of targets. This regulatory mechanism is
the most common mechanism for circRNAs (18). Studies have shown that the circRNA
levels in the heart are diverse and closely associated with heart
disease (19,20).
Our previous study found that hsa_circ_105039 is
significantly downregulated in CHD (21). However, how hsa_circ_105039
activity regulates CHD disease progression remains to be
elucidated. The current study was designed to determine the
function of the circRNA hsa_circ_105039 in the pathogenesis of CHD,
as well as to search for its target miRNA.
Materials and methods
Cell culture and differentiation
The human induced pluripotent stem (iPS) cell line
(provided by The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences; cat. no. SCSP-1302) was cultured in
mTeSR1 media (Stemcell Technologies, Inc.) at 37°C with 5%
CO2. Following the digestion of iPS cells with trypsin,
the concentration was adjusted to 10 cells/µl and then placed at
the bottom of the culture dish at 40 µl per drop. The cells were
returned to the incubator to be used for hanging drop culture. iPS
cells were seeded into 0.1% gelatin-coated petri dishes to remove
murine embryonic fibroblasts. At 2 days following inoculation, the
iPS cells were gently aspirated. The cells were seeded in a
low-adhesion petri dish and cultured for 5 days to induce embryoid
body (EB) formation. The culture conditions were DMEM (cat. no.
11960069; Gibco; Thermo Fisher Scientific, Inc.), 10% fetal bovine
serum (cat. no. 16140071; Gibco; Thermo Fisher Scientific, Inc.),
1% L-glutamine (cat. no. 25030081; Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin-streptomycin (cat. no. SV30010; HyClone;
Cytiva). After inducing the formation of EB, the cells were
inoculated into gelatin-coated petri dishes for culture. DMSO (1%)
was added to induce induction on days 1, 3 and 5 of culture and
myocardial differentiation medium (cat. no. 5911; ScienCell
Research Laboratories, Inc.) was added 1 week following
induction.
Cell transfection
Small interfering (si)RNAs, negative controls and
miR-17 inhibitors were synthesized by Sangon Biotech Co., Ltd.
DMSO-induced cardiomyocytes were transfected with
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Briefly, 100 nM siRNA was transfected into the DMSO-induced
cardiomyocytes at 37°C with 5% CO2. After incubation for
6 h, the medium was replaced with the mTeSR1 followed by incubation
at 37°C with 5% CO2 for 48 h, and transfection
efficiency was measured via reverse transcription-quantitative PCR
(RT-qPCR). The target sequences of si-negative control (NC) and
si-hsa_circ_105039 were as follows: si-NC,
5′-GACCGGCAGATCGAAACTAAA-3′ and si-hsa_circ_105039,
5′-GCAAGACAGCAAGCAGATCTA-3′.
Cell viability assay
A
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT; Sigma-Aldrich; Merck KGaA) assay was used to evaluate
cytotoxic activity. The iPS cells were induced into cardiomyocytes
using 1% DMSO, and the resulting cells were transfected with the
si-RNAs and cultured at 37°C with 5% CO2 for 48 h. The
cells were collected and cultured in 96-well plates at a
concentration of 5,000 cells/well. Following incubation at 37°C
with 5% CO2 for 24 h, the media was aspirated, 20 µl 5
mg/ml MTT was added to each well and the cells were incubated at
37°C with 5% CO2 for another 4 h. Then, the supernatant
was completely replaced with 150 µl DMSO to dissolve the purple
crystals. Finally, following 10 min of agitation, the absorbance
was measured at 570 nm.
Transwell assay
The transfected cells were cultured in a six-well
plate until confluence reached ~100% and then 1% fetal bovine serum
was added to the culture medium. The DMSO-induced cardiomyocytes
were adjusted to 7×104 and the cells were seeded in a
24-well Transwell chamber (pore size, 8 µm; cat. no. 3422; Corning,
Inc.). At 24 h following transfection, 5×104 iPS cells
were inoculated into the upper compartment of serum-free medium and
10% fetal bovine serum was added to the lower compartment.
Following culture at 37°C for 8 h, the bottom cells were fixed with
4% paraformaldehyde at room temperature for 10 min and then stained
with 400 µl 0.4% crystal violet (Beijing Solarbio Science &
Technology Co., Ltd.) at room temperature for 10 min and images
were acquired under an inverted microscope (Ts2R; Nikon
Corporation) at ×200 magnification, and then the number of cells
stained positive for crystal violet were counted.
Flow cytometry analysis
Cells were washed three times with phosphate buffer.
Then, 1×106 cells were resuspended in 100 µl with 5 µl
FITC-Annexin V (eBioscience; Thermo Fisher Scientific, Inc.) and
propidium iodide (eBioscience; Thermo Fisher Scientific, Inc.)
antibodies in the dark. Then, the cells were incubated at 4°C for
30 min. After washing three times with PBS, the cells were analyzed
by flow cytometry (Accuri™ C6; BD Biosciences) using FlowJo version
10 software (FlowJo LLC) (22).
The percentage of late apoptotic cells was calculated.
Luciferase reporter assay
PsiCHECK-circ_105039-wild-type (WT) and mutated
(MUT) were obtained from Sangon Biotech Co., Ltd. miR-17 mimic and
miR-17-NC were synthesized by Sangon Biotech Co., Ltd. IPS cells
were cultured in mTeSR1 media. The sequences of
psiCHECK-circ_105039-WT/MUT were cloned into the pGL3-basic
reporter (Promega Corporation). For the reporter assays, cells were
co-transfected with pRT-TK Renilla plasmid (cat. no. E2241;
Promega Corporation) and pGL3 -circ_105039-WT/MUT firefly
luciferase reporter plasmids as well as miR-17 mimic or control
using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After culturing for 48 h, the luciferase
activity was measured using the dual luciferase reporter gene assay
system (GloMax 20/20; Promega Corporation) according to the
manufacturer's instructions. The sequences of miRNA mimics were as
follows: control sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and control
anti-sense, 5′-ACGUGACACGUUCGGAGAATT-3′; miR-17 mimic sense,
5′-CAAAGUGCUUACAGUGCAGGUAG-3′ and anti-sense,
5′-ACCUGCACUGUAAGCACUUUGUU-3′. The firefly luciferase activity was
normalized to Renilla luciferase activity for each sample.
The data were recorded with a SpectraMax M5e microplate reader
(Molecular Devices, LLC).
RT-qPCR
mRNA was isolated from DMSO-induced cardiomyocytes
(2×105 cells) cells by TRIzol Plus® reagent
(cat. no. 9108; Takara Biotechnology Co., Ltd.). Total cDNA was
reverse transcribed using PrimeScript™ RT reagent kit with gDNA
Eraser (cat. no. RR047A; Takara Biotechnology Co., Ltd.) according
to the manufacturer's protocols. RT-qPCR was performed with the
SYBR-Green PCR kit (cat. no. RR820; Takara Biotechnology Co., Ltd.)
on a 7900HT Fast Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Thermocycling conditions were as follows:
95°C for 30 sec and 40 cycles of 95°C for 15 sec, 60°C for 60 sec;
and melt curves were recorded with 95°C for 15 sec, 72°C for 60 sec
and 95°C for 15 sec. Changes in expression were calculated using
the 2−ΔΔCq method (23). The results were expressed as the
ratio of optimal density to glyceraldehyde-3-phosphate
dehydrogenase (GAPDH). Each experiment was performed independently
three times. Primer sequences are listed in Table I.
 | Table I.Primer sequences used in reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used in reverse
transcription-quantitative PCR.
| Gene | Forward sequences
(5′-3′) | Reverse sequences
(5′-3′) | Size, bp |
|---|
| 18S rRNA |
CTACCACATCCAAGGAAGCA |
TTTTTCGTCACTACCTCCCCG | 89 |
| circ_079265 |
CGTCGCCCACATAGGAATC |
ACTGACTTGAGACCAGTTGAATAA | 91 |
| GAPDH |
CAGGGCTGCTTTTAACTCTGGTAA |
GGGTGGAATCATATTGGAACATGT | 101 |
| Nkx2.5 |
GCAGGCGCAGGTCTATG |
TGCGTGGACGTGAGTTTC | 105 |
| GATA4 |
ATGGGACGGGTCACTATCT |
GCGCTGAGGCTTGATGA | 86 |
| α-MHC |
CCCGCTTTGGGAAATTCATTAG |
CTCCAGCAGGTAGGTCTCTAT | 83 |
| ANP |
ACGCAGACCTGATGGATTTC |
GCTTCTTCATTCGGCTCACT | 109 |
| cTnI |
GACAAGGTGGATGAAGAGAGATAC |
CTTGCCTCGAAGGTCAAAGA | 102 |
Western blotting
Total protein samples were extracted from cells
lysed with RIPA lysis buffer (Beyotime Institute of Biotechnology).
The samples were then analysed by western blotting. The proteins
were collected and centrifuged at 12,000 × g for 15 min at 4°C. A
BCA protein assay kit (Beyotime Institute of Biotechnology) was
used to determine protein concentration. The samples (20 µg/lane)
were separated via SDS-PAGE [12% for atrial natriuretic peptide
(ANP) and cardiac troponin I (cTnI); 10% for cyclinD2, GATA-binding
protein 4 (GATA4), homobox transcription factor (Nkx2.5) and GAPDH;
6% for α-myosin heavy chain (α-MHC)] and transferred onto a
polyvinylidene fluoride (PVDF, 0.22 µm; cat. no. ISEQ00010;
MilliporeSigma). The membranes were incubated in a blocking
solution containing 5% non-fat milk in PBS with 1% Tween-20 at room
temperature for 1.5 h and then incubated with the primary
antibodies diluted in blocking solution individually. The primary
antibodies used were as follows: ANP (1:1,000; cat. no. ab190001;
Abcam), cyclinD2 (1:2,000; cat. no. ab230883; Abcam), cTnI
(1:1,000; cat. no. ab209809; Abcam), GATA4 (1:1,000; cat. no.
ab84593; Abcam), Nkx2.5 (1:1,000; cat. no. ab106923; Abcam), α-MHC
(1:2,000; cat. no. ab185967; Abcam) and GAPDH (1:5,000; cat. no.
ab181603; Abcam). After incubation with the primary antibodies at
4°C for 12 h, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(1:10,000; cat. no. A0208; Beyotime Institute of Biotechnology) for
1 h at room temperature. The bound antibodies were detected using a
chemiluminescence system (cat. no. 180-5001; Tanon Science and
Technology Co., Ltd.). GAPDH was used as an internal control. Image
Lab version 3.0 (Bio-Rad Laboratories, Inc.) and Quantity One
version 4.62 (Bio-Rad Laboratories, Inc.) software were used for
densitometry.
Bioinformatics analysis
The regulatory network of hsa_circ_105039 was
evaluated by bioinformatics analysis (RegRNA 2.0 version,
http://regrna2.mbc.nctu.edu.tw). To
elucidate the potential function of miR-17, a bioinformatics
analysis was performed using miRTarBase 7.0 version (http://mirtarbase.mbc.nctu.edu.tw/php/index.php)
and TargetScan (http://www.targetscan.org/vert_72/).
Statistical analysis
All experimental data are shown as the mean ±
standard deviation. The experiments were performed independently in
triplicate. Statistical graphs were made by GraphPad Prism 8.3
software (GraphPad Software, Inc.). Differences between
experimental groups were analyzed for statistical significance
using one-way analysis of variance followed by the Tukey's post hoc
multiple comparison test. P<0.05 was considered to indicate a
significant difference.
Results
Hsa_circ_105039 knockdown suppresses
viability, enhances apoptosis and promotes migration of iPS cells
in vitro
Our previous study reported that hsa_circ_105039 was
expressed at lower levels in the heart tissue of patients with CHD
(21). To determine whether
hsa_circ_105039 has a positive effect in promoting the viability of
iPS cells, siRNA and overexpression plasmids targeting
hsa_circ_105039 were used to knockdown and overexpress
hsa_circ_105039 expression levels. Following iPS cell
differentiation by DMSO, the gene expression of hsa_circ_105039 was
significantly upregulated (Fig.
1A). When the plasmid was transfected for 48 h, the expression
of hsa_circ_105039 was analyzed by RT-qPCR (Fig. 1B). The MTT assay suggested that
iPS cell viability was significantly promoted when hsa_circ_105039
was overexpressed, but was suppressed when hsa_circ_105039 was
knocked down (Fig. 1C). Flow
cytometry showed that the apoptosis of iPS cells was induced
following hsa_circ_105039 knockdown (Fig. 1D and E). However, it was
significantly suppressed when hsa_circ_105039 was overexpressed
(Fig. 1D and E). In addition, the
migration of iPS cells was suppressed after hsa_circ_105039 was
knocked down (Fig. 1F and G). By
contrast, it was promoted when hsa_circ_105039 was overexpressed
(Fig. 1F and G).
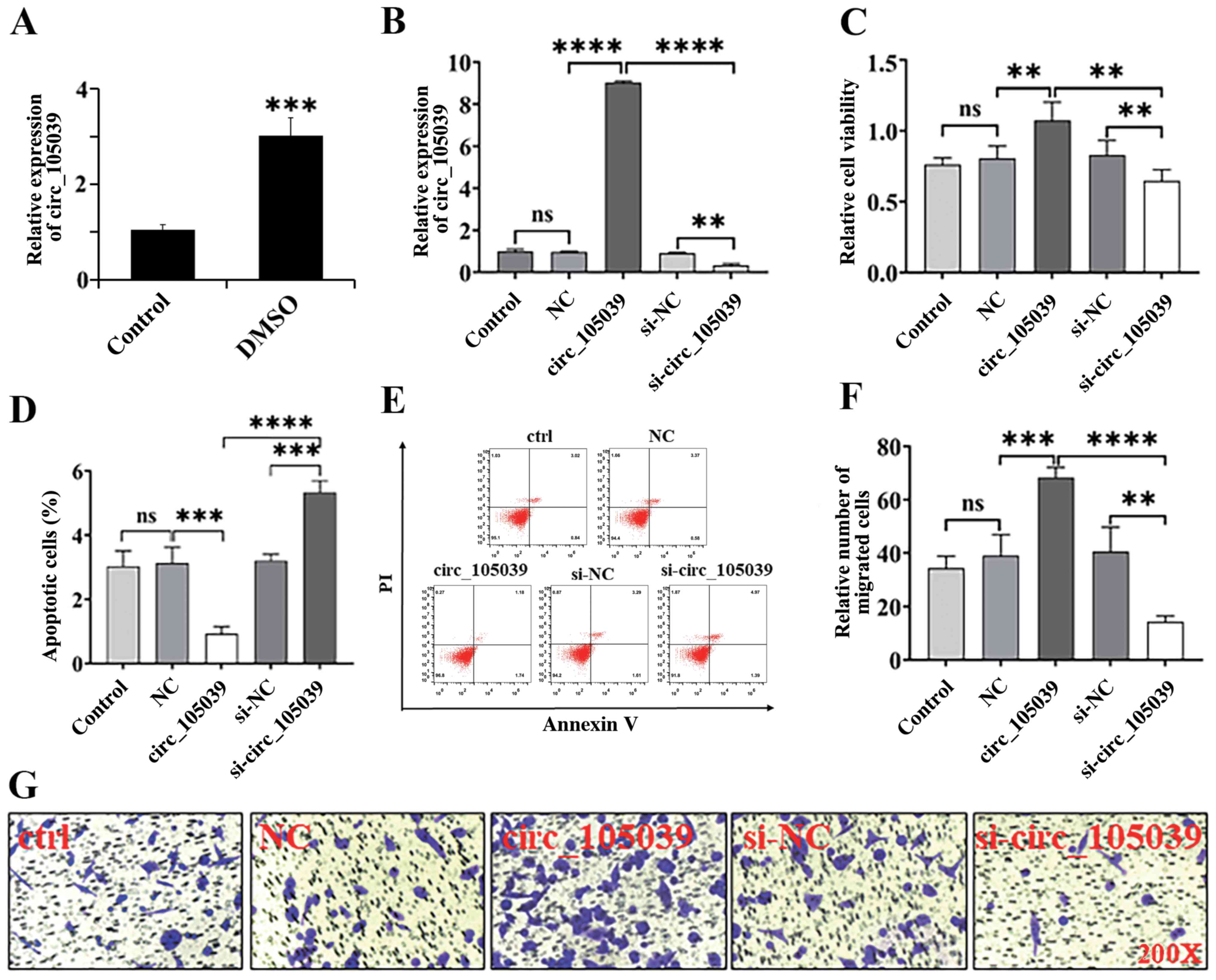 | Figure 1.Expression of hsa_circ_105039 and
cell viability, migration and apoptosis in DMSO-induced iPS
cardiomyocytes. (A and B) Reverse transcription-quantitative PCR
assay for the expression of hsa_circ_105039. (C)
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
assay of viability of iPS cells. (D) Statistical analysis histogram
of flow cytometry. (E) Scatter plot of flow cytometry. (F and G)
Transwell assay of iPS cell migration. n=3. **P<0.01,
***P<0.005 and ****P<0.001. ns, not significant; iPS, induced
pluripotent stem; DMSO, dimethyl sulfoxide; NC, negative control;
circ, circular RNA; si, small interfering RNA. |
Hsa_circ_105039 knockdown suppresses
the expression of differentiation-related genes and proteins in iPS
cells
To verify the role of hsa_circ_105039 in regulating
iPS cell differentiation, the gene and protein expression levels of
ANP, Nkx2.5, cTnI, α-MHC and GATA4 were detected by RT-qPCR and
western blotting, respectively (Fig.
2A-F). Following iPS cell differentiation by DMSO, the gene
expression of ANP (Fig. 2B),
Nkx2.5 (Fig. 2C), cTnI (Fig. 2D), α-MHC (Fig. 2E) and GATA4 (Fig. 2F) was significantly increased
following hsa_circ_105039 overexpression, but decreased when
hsa_circ_105039 was knocked down. Western blot analysis showed that
the protein levels of ANP, Nkx2.5, cTnI, α-MHC and GATA4 were also
increased or decreased following hsa_circ_105039 overexpression or
knockdown, respectively (Fig.
2G-L). The results indicated that hsa_circ_105039 had a
positive effect on the differentiation of iPS cells.
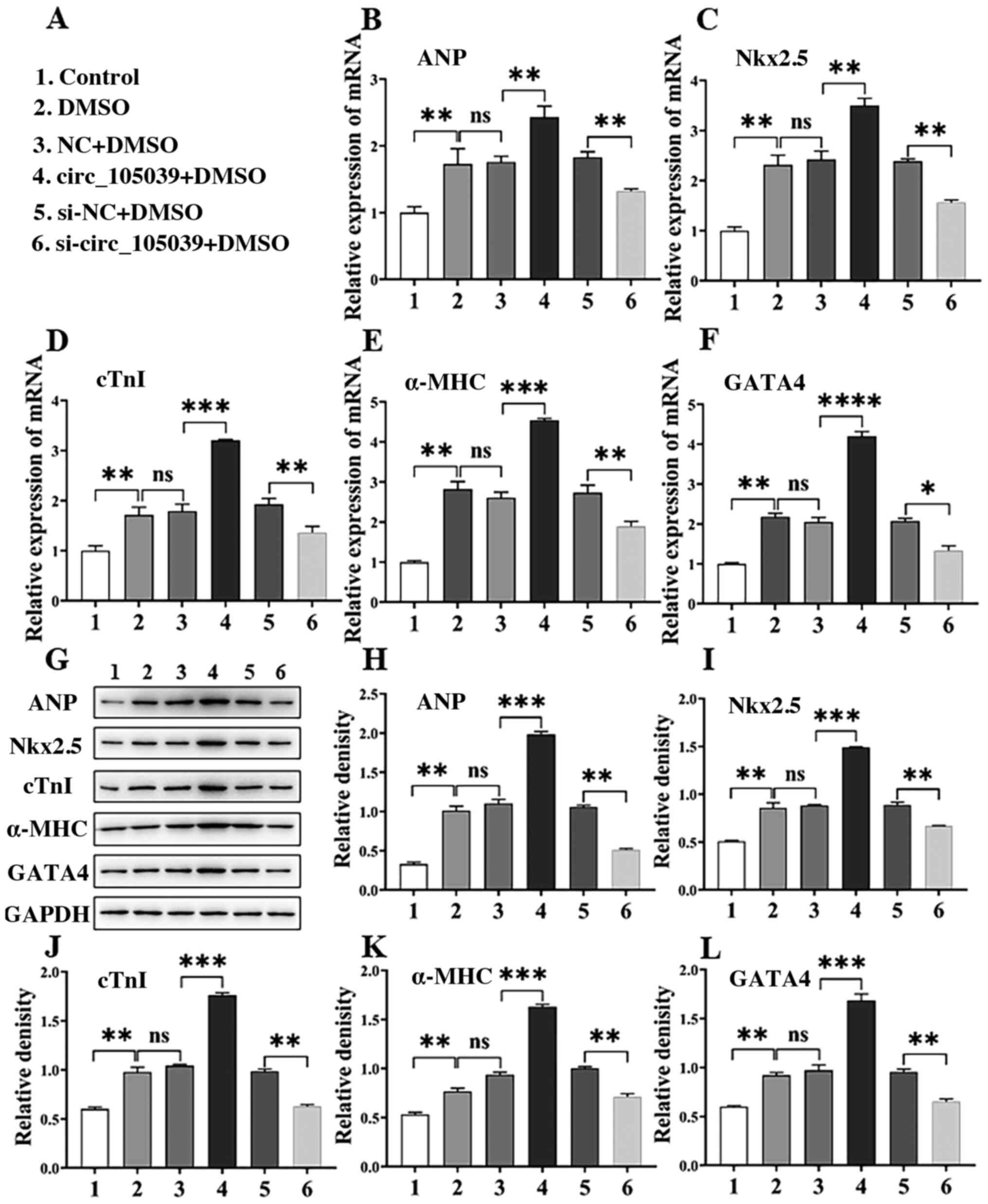 | Figure 2.DMSO induces iPS cells to
differentiate into cardiomyocytes and express
differentiation-related genes and proteins in iPS cells. (A)
Experimental grouping. Reverse transcription-quantitative PCR assay
for the expression of (B) ANP, (C) Nkx2.5, (D) cTnI, (E) α-MHC and
(F) GATA4. (G) Western blotting assay for (H) ANP, (I) Nkx2.5, (J)
cTnI, (K) α-MHC and (L) GATA4. n=3. *P<0.05, **P<0.01,
***P<0.005 and ****P<0.001. ns, not significant; iPS, induced
pluripotent stem; DMSO, dimethyl sulfoxide; NC, negative control;
circ, circular RNA; si, small interfering RNA; ANP, atrial
natriuretic peptide; Nkx2.5, homobox transcription factor; cTnI,
cardiac troponin I; α-MHC, α-myosin heavy chain; GATA4,
GATA-binding protein 4. |
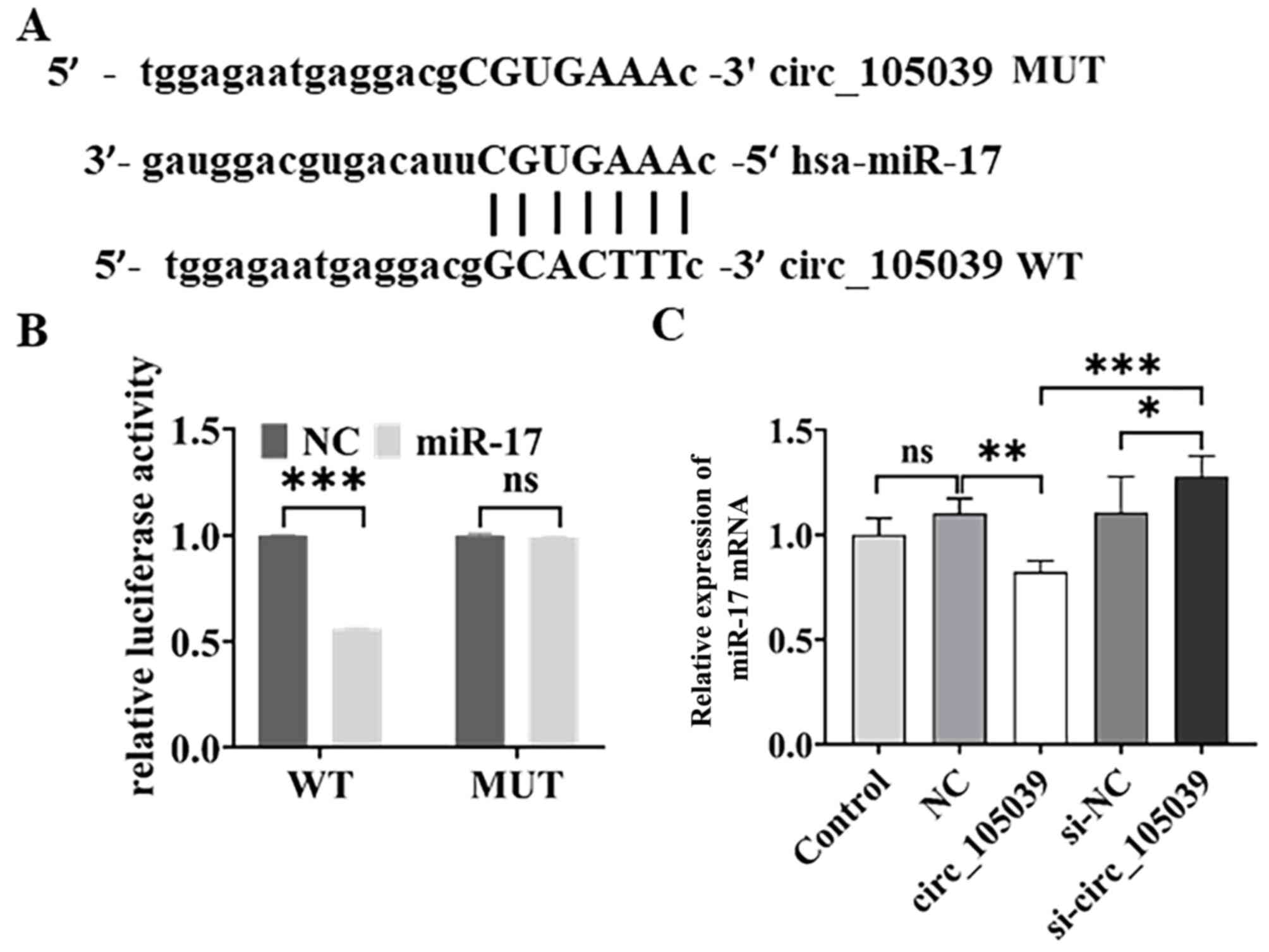
Hsa_circ_105039 directly binds to
miR-17
It is known that circRNAs act as competing
endogenous RNAs by sponging miRNAs (15–17). Thus, the regulatory network of
hsa_circ_105039 was evaluated by bioinformatics analysis
(RegRNA2.0). The results showed that miRNA-17 had a potential
binding site for hsa_circ_105039 (Fig. 3A). Therefore, in iPS cells
co-transfected with WT hsa_circ_105039 and miR-17 mimics, the
relative intensity of luciferase decreased (Fig. 3B). In addition, the expression of
miR-17 in iPS cells was detected by RT-qPCR. The results showed
that the expression of miR-17 decreased when the hsa_circ_105039
gene was overexpressed (Fig. 3C).
By contrast, the expression of miR-17 increased when the
hsa_circ_105039 gene was knocked down (Fig. 3C). These results indicated that
hsa_circ_105039 had a negative effect on miR-17.
miR-17 targets cyclinD2
To elucidate the potential function of miR-17, a
bioinformatics analysis was performed using miRTarBase and
TargetScan and a potential target gene of miR-17, cyclinD2, was
identified. The 3′-UTR sequences of cyclinD2 mRNA were
complementary to the miR-17 seed sequence (Fig. 4B). Plasmids containing the WT or
MUT form of the 3′-UTR of cyclinD2 were constructed and expressed
into iPS cells together with miR-con (NC group) or miR-17 mimics
separately and a quantitative luciferase reporter was conducted. As
shown in Fig. 4C, the relative
intensity of luciferase activity was reduced in iPS cells
co-transfected with WT hsa_circ_105039, but not in cells
co-transfected with miR-17 mimics.
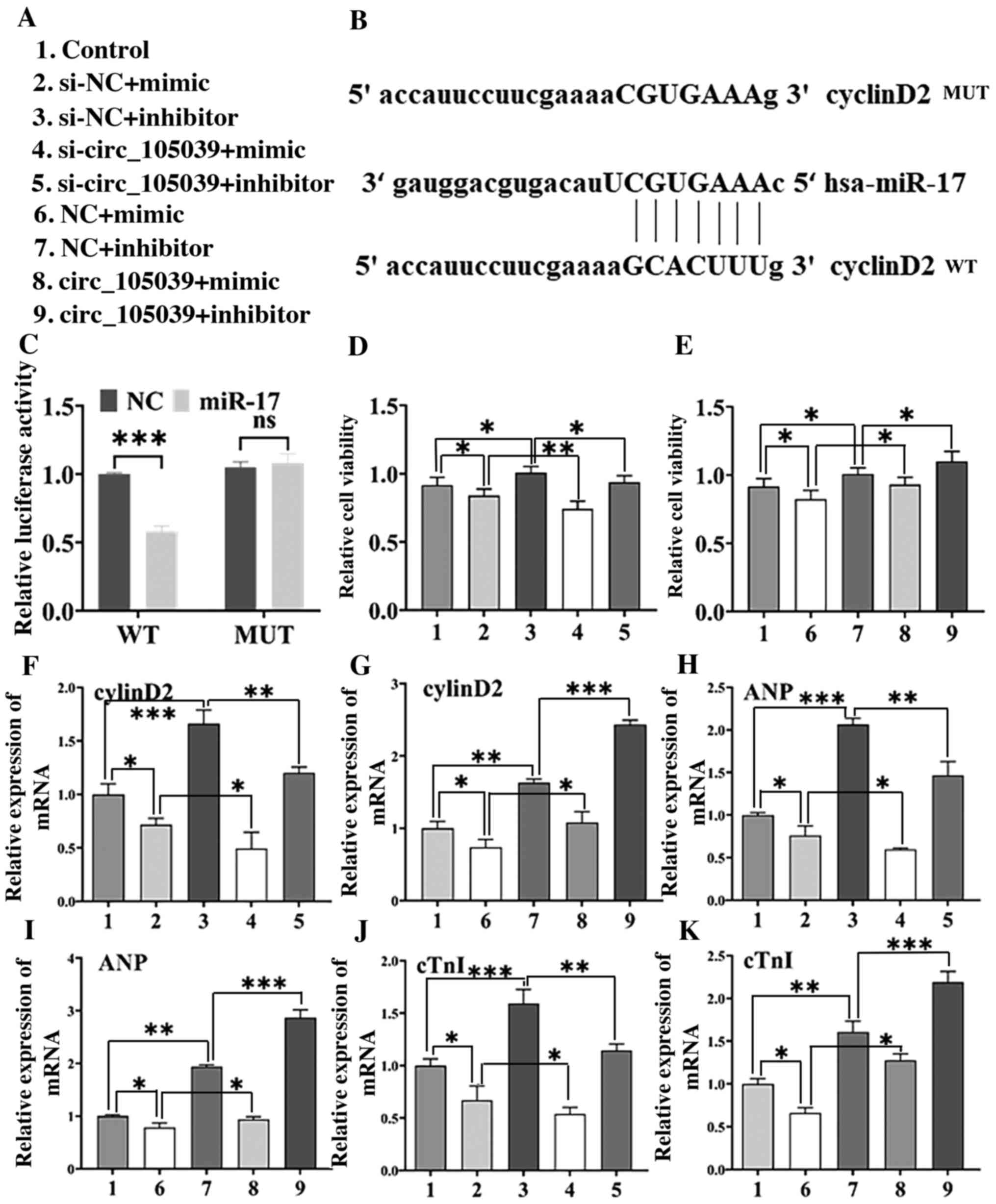 | Figure 4.CyclinD2 is a direct target of miR-17
and hsa_circ_105039-mediated regulation of cell viability and
differentiation is reversed by the restoration of cyclinD2, ANP and
cTnI levels in DMSO-induced iPS cardiomyocytes. (A) Experimental
grouping. (B) Sequence alignment of cyclinD2 and the potential
binding site in the 3′-UTR of miR-17. (C) Luciferase reporter assay
was performed in iPS cells co-transfected with the cyclinD2-WT
plasmid or cyclinD2 plasmid. (D and E)
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
assay of iPS cell viability. Reverse transcription-quantitative PCR
assay of the expression of (F and G) cyclinD2, (H and I) ANP and (J
and K) cTnI. n=3. *P<0.05, **P<0.01 and ***P<0.005. ns,
not significant; miR, microRNA; circ, circular RNA; ANP, atrial
natriuretic peptide; cTnI, cardiac troponin I; iPS, induced
pluripotent stem; DMSO, dimethyl sulfoxide; WT, wild-type; MUT,
mutated; NC, negative control; si, small interfering RNA. |
Hsa_circ_105039 regulates the
differentiation of iPS cells by inhibiting miR-17
In addition, the viability of iPS cells following
hsa_circ_105039 overexpression or knockdown (Fig. 4A) was analyzed and the results are
shown in Fig. 4D and E. Compared
with the control group, the viability of iPS cells was suppressed
when hsa_circ_105039 was knocked down (Fig. 4D) in the si-circ_105039 + mimic
group. However, compared with the NC + mimic group, the viability
of iPS cells was promoted when hsa_circ_105039 was overexpressed in
the circ_105039 + mimic group (Fig.
4E). These results indicated that hsa_circ_105039 promoted iPS
cell viability by inhibiting miR-17.
To investigate whether hsa_circ_105039 regulated the
differentiation of iPS cells by inhibiting miR-17, hsa_circ_105039
mimic and miR-17 inhibitor were co-transfected into the iPS cells.
RT-qPCR results showed that the gene expression of cyclinD2
(Fig. 4F and G), ANP (Fig. 4H and I) and cTnI (Fig. 4J and K) were decreased in the
si-circ_105039 + mimic group and recovered in the circ_105039 +
mimic inhibitor group compared with the control group. In addition,
western blotting (Fig. 5A and B)
was performed to determine the protein expression of cyclinD2
(Fig. 5C and D), ANP (Fig. 5E and F) and cTnI (Fig. 5G and H), the results were
consistent with the results of the RT-qPCR assay. Altogether, these
results indicated that hsa_circ_105039 promoted iPS cell viability
and suppressed cell apoptosis by regulating the expression of
cyclinD2 by inhibiting miR-17.
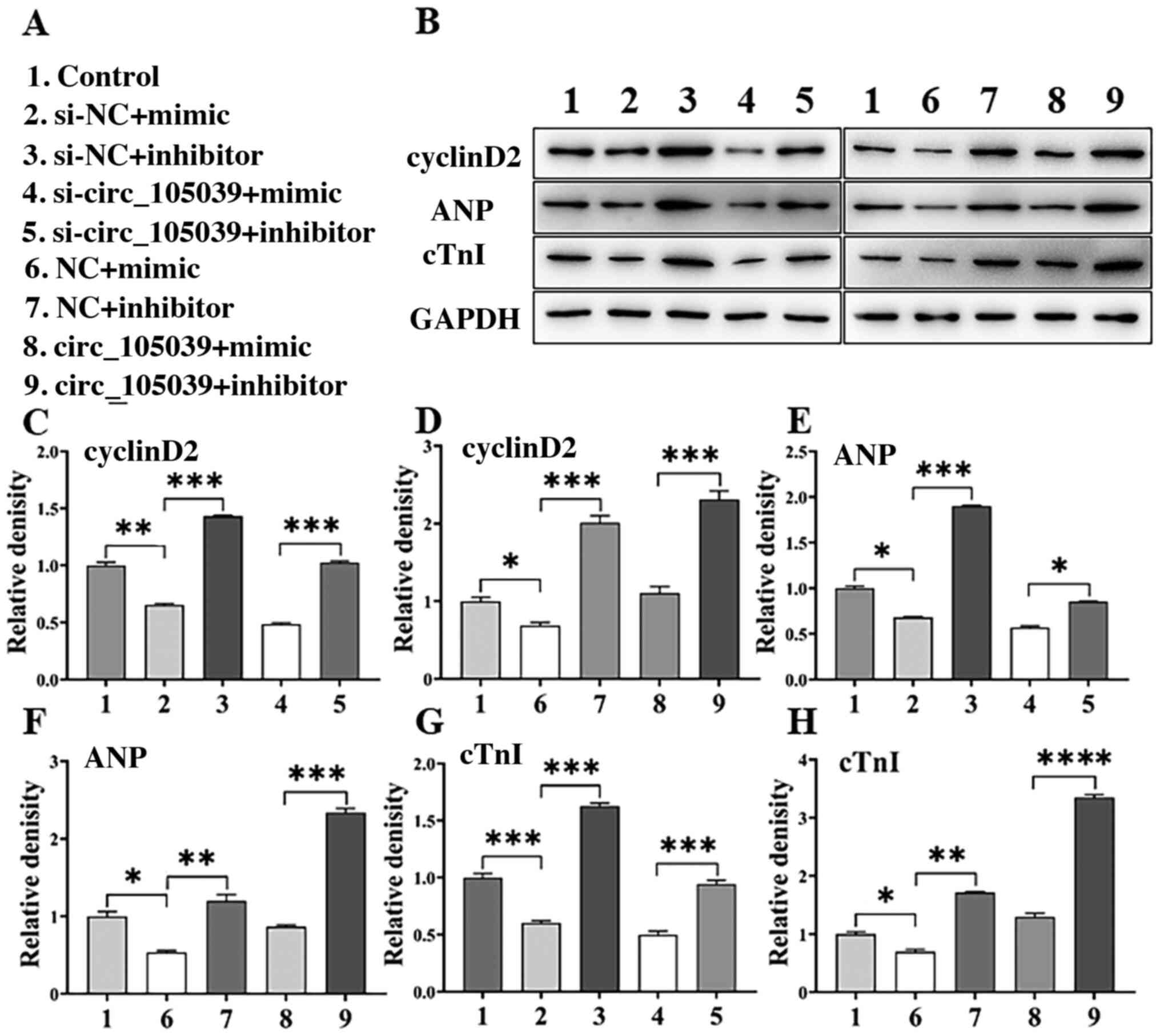 | Figure 5.cyclinD2 is a direct target of miR-17
and hsa_circ_105039-mediated regulation of cell viability and
differentiation is reversed by the restoration of cyclinD2, ANP and
cTnI levels in DMSO-induced iPS cardiomyocytes. (A) Experimental
grouping. (B) Western blotting bands. The analysis histogram of (C
and D) cyclinD2, (E and F) ANP and (G and H) cTnI. n=3. *P<0.05,
**P<0.01, ***P<0.005 and ****P<0.001. ns, not significant;
miR, microRNA; circ, circular RNA; ANP, atrial natriuretic peptide;
cTnI, cardiac troponin I; iPS, induced pluripotent stem; NC,
negative control; si, small interfering RNA. |
Discussion
IPS cells have characteristics similar to embryonic
stem cells (24). They have the
ability to differentiate into a number of types of cells (including
cardiomyocytes). Cardiomyocytes derived from iPS cells recapitulate
the phenotypic differences caused by genetic variation, making it
an attractive human disease model (25). In addition, cardiomyocytes derived
from iPS cells can also be used as source cells for heart
regeneration in animal models. DMSO has both hydrophilic and
lipophilic properties. It is a commonly used solvent for
water-insoluble substances (26).
DMSO has a great impact on cell cycle and cell apoptosis, can
change cell metabolism process and thus affect cell growth
(27). The differentiation of iPS
cells into cardiomyocytes has three stages: First differentiation
into mesoderm cells, then cardiomyocyte progenitor cells and
finally mature cardiomyocytes (28). Most of the available protocols for
using DMSO as a cardiogenic drug focus on its use in the first few
days of EB culture (29). Studies
have confirmed that DMSO can improve the efficiency of
cardiomyocyte differentiation (27,30). However, the underlying mechanism
of this molecular action is unclear. The present study chose DMSO
(1%) to induce iPS cell differentiation into cardiomyocytes. The
gene and protein expression of ANP, Nkx2.5, cTnI, α-MHC and GATA4
was detected to verify whether the differentiation was successful.
ANP, Nkx2.5, cTnI, α-MHC and GATA4 serve an important role in the
maturation of cardiomyocytes. Cardiac contractile proteins (cTnI,
α-MHC and ANP) are specific markers marking the differentiation and
maturation of cardiomyocytes and the transcription factors Nkx2.5
and GATA4 are early markers of the cardiac lineage and expressed
throughout the duration of cardiac development (31–33). Aberrant expression of these
specific molecular markers (cTnI, ANP, α-MHC, Nkx2.5 and GATA4),
including upregulation and downregulation, will contribute to
abnormal cardiac cell differentiation and further result in failure
of the early primitive heart tube formation, cardiomyocyte
differentiation and heart morphogenesis (33–35).
CircRNAs serve a key role in a number of types of
biological processes. However, initially, these molecules were only
deemed to be missplicing products or intermediates of
transcriptional processes and were not given much attention.
Studies have shown that circRNAs can regulate the function of
miRNAs by acting as miRNA sponges (12,36). Our previous study discovered that
hsa_circ_105039 was underexpressed in the heart tissues of patients
with CHD (21). To the best of
the authors' knowledge, this is the first time that hsa_circ_105039
has been reported in CHD. The present study analyzed the potential
function of hsa_circ_105039 in the viability, migration and
apoptosis of iPS cells. It was shown that hsa_circ_105039 was a
mediator in the viability, migration and apoptosis of iPS cells. In
addition, it was found that hsa_circ_105039 acted as a sponge for
miR-17, which could be regulated in this biological process. miRNAs
possess positive effects on the progression of CHD (37) but potentially act as sensitizing
agents in breast cancer (38),
tumorigenesis (39) and chronic
pancreatitis (40). By performing
software prediction analysis and luciferase reporter assays, the
present study further found that miR-17 targeted cyclinD2. The
miRNA target prediction database predicted that cyclinD2 is a
direct target of miR-17. The present study revealed the existence
and functional importance of the hsa_circ_105039/miR-17/cyclinD2
axis in iPS cells for the first time, to the best of the authors'
knowledge, and provided important insights into the role of this
pathway in CHD. It showed that has_circRNA_105039 acted as a sponge
for miR-17 to inhibit its regulation of cyclinD2 transcription.
These results indicated the protective functions of hsa_circ_105039
upregulation in iPS cells exposed to CHD.
In conclusion, by acting as a sponge for miR-17,
hsa_circ_105039 may be a potential biomarker for prognosis and
therapeutic target for CHD. Due to its functions including
regulating the viability, migration, apoptosis and differentiation
of iPS cells, hsa_circ_105039 may be a potential key molecule in
the diagnosis of CHD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81870240), Nanjing
Municipality Health Bureau (grant no. JQX18010), Jiangsu Provincial
Medical Youth Talent (grant no. QNRC2016114) and Foundation of
Liaoning Province, China (grant no. 201602879).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BY performed the experiments and data analysis. ML
conceived and designed the study and wrote the manuscript. SH
performed the RT-qPCR and western blotting experiments, analyzed
the related data, and reviewed and edited the manuscript. ZY and JZ
designed the work, gave final approval of the version to be
published, and agree to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of
any part of the work are appropriately investigated and resolved.
ZY and JZ confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CHD
|
congenital heart disease
|
|
DMSO
|
dimethyl sulfoxide
|
|
iPS
|
induced pluripotent stem
|
|
ANP
|
atrial natriuretic peptide
|
|
α-MHC
|
α-myosin heavy chain
|
|
cTnI
|
cardiac troponin I
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
GATA4
|
GATA-binding protein 4
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
|
|
Nkx2.5
|
homobox transcription factor
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
References
|
1
|
Fernandes SM, Marelli A, Hile DM and
Daniels CJ: Access and delivery of adult congenital heart disease
care in the United States: Quality driven team based care. Cardiol
Clin. 38:295–304. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burchill LJ, Lee MGY, Nguyen VP and Stout
KK: Heart failure in adult congenital heart disease. Cardiol Clin.
38:457–469. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schulkey CE, Regmi SD, Magnan RA, Danzo
MT, Luther H, Hutchinson AK, Panzer AA, Grady MM, Wilson DB and Jay
PY: The maternal-age-associated risk of congenital heart disease is
modifiable. Nature. 520:230–233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Klena NT, Gabriel GC, Liu X, Kim AJ,
Lemke K, Chen Y, Chatterjee B, Devine W, Damerla RR, et al: Global
genetic analysis in mice unveils central role for cilia in
congenital heart disease. Nature. 521:520–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruneau BG: The developmental genetics of
congenital heart disease. Nature. 451:943–948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakhi R, Kauling RM, Theuns DA,
Szili-Torok T, Bhagwandien RE, van den Bosch AE, Cuypers JAAE,
Roos-Hesselink JW and Yap SC: Early detection of ventricular
arrhythmias in adults with congenital heart disease using an
insertable cardiac monitor (EDVA-CHD study). Int J Cardiol.
305:63–69. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meller CH, Grinenco S, Aiello H, Córdoba
A, Sáenz-Tejeira MM, Marantz P and Otaño L: Congenital heart
disease, prenatal diagnosis and management. Arch Argent Pediatr.
118:e149–e161. 2020.(In English, Spanish). PubMed/NCBI
|
|
8
|
Bishop L, Lansbury A and English K: Adult
congenital heart disease and pregnancy. BJA Educ. 18:23–29. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shum KK, Gupta T, Canobbio MM, Durst J and
Shah SB: Family planning and pregnancy management in adults with
congenital heart disease. Prog Cardiovasc Dis. 61:336–346. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmidt AB, Lund M, Corn G, Halldorsson
TI, Øyen N, Wohlfahrt J, Olsen SF and Melbye M: Dietary glycemic
index and glycemic load during pregnancy and offspring risk of
congenital heart defects: A prospective cohort study. Am J Clin
Nutr. 111:526–535. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Costello A, Lao NT, Barron N and Clynes M:
Reinventing the wheel: Synthetic circular RNAs for mammalian cell
engineering. Trends Biotechnol. 38:217–230. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verduci L, Strano S, Yarden Y and Blandino
G: The circRNA-microRNA code: Emerging implications for cancer
diagnosis and treatment. Mol Oncol. 13:669–680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Yang T and Xiao J: Circular RNAs:
Promising biomarkers for human diseases. EBioMedicine. 34:267–274.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bolha L, Ravnik Glavac M and Glavac D:
Circular RNAs: Biogenesis, function, and a role as possible cancer
biomarkers. Int J Genomics. 2017:62183532017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Y, Zhang C, Xiong J and Ren H:
Emerging important roles of circRNAs in human cancer and other
diseases. Genes Dis. 8:412–423. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thum T, Gross C, Fiedler J, Fischer T,
Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, et
al: MicroRNA-21 contributes to myocardial disease by stimulating
MAP kinase signalling in fibroblasts. Nature. 456:980–984. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu N, Bezprozvannaya S, Williams AH, Qi
X, Richardson JA, Bassel-Duby R and Olson EN: MicroRNA-133a
regulates cardiomyocyte proliferation and suppresses smooth muscle
gene expression in the heart. Genes Dev. 22:3242–3254. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu J, Li J, Liu H, Yin J, Zhang M, Yu Z
and Miao H: Circulating plasma circular RNAs as novel diagnostic
biomarkers for congenital heart disease in children. J Clin Lab
Anal. 33:e229982019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clavijo PE, Friedman J, Robbins Y, Moore
EC, Smith E, Zauderer M, Evans EE and Allen CT: Semaphorin4D
inhibition improves response to immune-checkpoint blockade via
attenuation of MDSC recruitment and function. Cancer Immunol Res.
7:282–291. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wada N, Wang B, Lin NH, Laslett AL,
Gronthos S and Bartold PM: Induced pluripotent stem cell lines
derived from human gingival fibroblasts and periodontal ligament
fibroblasts. J Periodontal Res. 46:438–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rowntree RK and McNeish JD: Induced
pluripotent stem cells: Opportunities as research and development
tools in 21st century drug discovery. Regen Med. 5:557–568. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Casiraghi A, Ardovino P, Minghetti P,
Botta C, Gattini A and Montanari L: Semisolid formulations
containing dimethyl sulfoxide and alpha-tocopherol for the
treatment of extravasation of antiblastic agents. Arch Dermatol
Res. 299:201–207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Santos NC, Figueira-Coelho J,
Martins-Silva J and Saldanha C: Multidisciplinary utilization of
dimethyl sulfoxide: Pharmacological, cellular, and molecular
aspects. Biochem Pharmacol. 65:1035–1041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kehat I, Kenyagin-Karsenti D, Snir M,
Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J
and Gepstein L: Human embryonic stem cells can differentiate into
myocytes with structural and functional properties of
cardiomyocytes. J Clin Invest. 108:407–414. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inamdar MS, Venu P, Srinivas MS, Rao K and
VijayRaghavan K: Derivation and characterization of two sibling
human embryonic stem cell lines from discarded grade III embryos.
Stem Cells Dev. 18:423–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pal R, Mamidi MK, Das AK and Bhonde R:
Diverse effects of dimethyl sulfoxide (DMSO) on the differentiation
potential of human embryonic stem cells. Arch Toxicol. 86:651–661.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Behrens AN, Iacovino M, Lohr JL, Ren Y and
Martin CM: Nkx2 5 mediates differential cardiac differentiation
through interaction with Hoxa10. Stem Cells Dev. 22:2211–2220.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cao N, Huang Y, Zheng J, Spencer CI, Zhang
Y, Fu JD, Nie B, Xie M, Zhang M, Wang H, et al: Conversion of human
fibroblasts into functional cardiomyocytes by small molecules.
Science. 352:1216–1220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Z and Huang J: Neuregulin-1 increases
connexin-40 and connexin-45 expression in embryonic stem
cell-derived cardiomyocytes. Appl Biochem Biotechnol. 174:483–493.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Behrens AN, Iacovino M, Lohr JL, Ren Y,
Zierold C, Harvey RP, Kyba M, Garry DJ and Martin CM: Nkx2-5
mediates differential cardiac differentiation through interaction
with Hoxa10. Stem Cells Dev. 22:2211–2220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao N, Huang Y, Zheng J, Spencer CI, Zhang
Y, Fu JD, Nie B, Xie M, Zhang M, Wang H, et al: Conversion of human
fibroblasts into functional cardiomyocytes by small molecules.
Science. 352:1216–1220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Solé C and Lawrie CH: Circular RNAs and
cancer: Opportunities and challenges. Adv Clin Chem. 99:87–146.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sucharov CC, Miyamoto SD and Garcia AM:
Circulating microRNAs as biomarkers in pediatric heart diseases.
Prog Pediatr Cardiol. 49:50–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kandettu A, Radhakrishnan R, Chakrabarty
S, Sriharikrishnaa S and Kabekkodu SP: The emerging role of miRNA
clusters in breast cancer progression. Biochim Biophys Acta Rev
Cancer. 1874:1884132020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu KJ: The role of miRNA biogenesis and
DDX17 in tumorigenesis and cancer stemness. Biomedical Journal.
43:107–114. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tesfaye AA, Azmi AS and Philip PA: miRNA
and gene expression in pancreatic ductal adenocarcinoma. Am J
Pathol. 189:58–70. 2019. View Article : Google Scholar : PubMed/NCBI
|