Introduction
Bicuspid aortic valve disease (BAV) represents the
most common congenital heart defect. BAV is generally considered to
affect 0.5 to 1.4% of the population, with a male prevalence based
on autopsy studies and small echocardiographic studies (1–3). BAV
is a complex and heterogeneous disease accounting for more
premature deaths than all other congenital heart diseases combined
(4). The mechanism of the disease
process is still unclear, and a number of questions remain
unanswered. BAV can occur as a component of genetic syndromes. For
example, 30% of women with Turner syndrome also have BAV (5). On the other hand, evidence of familial
clustering of BAV suggests that familial inherited BAV aligns with
autosomal dominant transmission with reduced penetrance (6). Analysis of particular pedigrees,
positional cloning approach, and genetic analysis have proven to be
crucial to the discovery of multiple genetic loci associated with
familial BAV, including the involvement of the Notch1 and GATA
binding protein 5 (7,8) Different phenotypes of BAV have been
identified according to cusp fusion (9): i) Phenotype I, right-left (R-L)
coronary cusp fusion, which is associated with coarctation of the
aorta, aortic stenosis and increased aortic wall shear stress; ii)
phenotype II, right-non-coronary (R-NC) cusp fusion, associated
with cusp pathology, aortic stenosis and regurgitation, aortic
aneurysm, larger aortic arch dimensions and myxomatous mitral valve
disease; and iii) phenotype III, left-non-coronary (L-NC) cusp
fusion, which is rare.
The most common abnormality in adults with BAV is
enlargement of the thoracic aorta (10) and this varies according to the
pattern of cusp fusion, with faster rates of aortic sinus and
ascending aortic dilatation associated with the L-R compared with
the R-NC morphology.
In the present study, morphological evaluations of
the ascending aortic wall from patients with BAV were performed.
This analysis was focused on elucidating the association between
the alterations in the expression levels of the selected microRNAs
(miRNAs/miRs) in tissues and recirculating blood through a
biomolecular analyses. This work may provide groundwork for the
possible identification of novel and usable biomarkers focused on
rupture and dissection, which are hallmarks of severity and
clinical complications in BAV.
Materials and methods
Clinical data
This study was approved by the Institutional Review
Board of University of Palermo (Palermo, Italy; approval no. CE
2A17-000-527-28). Written informed consent was obtained from all
participants. The present study included patients with BAV with
aneurysm (17 men and 9 women; age, 59.2±17.3 years) admitted
between January 2014 and January 2015 to the Cardiac Surgery Unit
at the University of Palermo for surgical procedures. According to
the morphology of BAV, 73% (19 cases) presented the R-L BAV
phenotype, 19% (5 cases) presented the R-NC phenotype and 8% (2
cases) presented the L-NC phenotype (Table I and Fig. S1). Evaluation of the BAV morphology
and ascending aorta diameter was performed preoperatively and in
the operating room by transthoracic echocardiography and
transesophageal echocardiography. Furthermore, measurements of
aortic root and ascending aortic diameter sizes were carried out
using helical computed tomography image analysis techniques. The
mean diameter of the aortic root was 48.5±1.8 mm, the mean diameter
of the ascending aorta was 54.03±1.5 mm. Relevant medical histories
regarding aortic disease were obtained from the patients' medical
records (Table I). The surgical
procedure used was The Button Bentall Procedure (11). Control ascending aortic specimens
were obtained from 12 patients (8 men, 4 women; age, 53.2±16.5
years) with tricuspid aortic valve not associated to aortopathy
underwent cardiac surgery for coronary artery diseases. This
surgical action allowed us to obtain a sample of the aortic wall
from subjects who had no clinical damage to the aorta. The control
cases had no history of smoking, hypertension or diabetes.
 | Table I.Clinicopathological characteristics
of the study population. |
Table I.
Clinicopathological characteristics
of the study population.
|
| Group |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristic | L-NC and R-NC
(n=7) | R-L (n=19) | P-value |
|---|
| Age, mean ± SD | 46.0±15.9 | 63.3±14.9 | 0.067a |
| Sex, male, n
(%) | 5 (71.0) | 12 (63.2) | 1.000b |
| Smoking status, n
(%) |
|
| 0.805b |
|
Ex-smoker | 3 (42.9) | 5
(26.3) |
|
|
Yes | 3 (42.9) | 6
(31.6) |
|
| No | 1 (14.2) | 8
(42.1) |
|
|
Hypercholesterolemia, n (%) | 0 (0.0) | 13 (68.4) | 0.022b |
| Hypertension, n
(%) | 5 (71.4) | 14 (73.7) | 1.000b |
| Diabetes, n
(%) | 2 (28.6) | 4
(21.1) | 1.000b |
| Renal failure, n
(%) | 0 (0.0) | 1
(5.3) | 1.000b |
| BCPO, n (%) | 0 (0.0) | 3
(15.8) | 1.000b |
| Coronary artery
disease, n (%) | 0 (0.0) | 8
(42.1) | 0.257b |
| Aneurysm, n
(%) |
|
|
<0.001b |
|
Ascending aorta | 4 (57.1) | 0
(0.0) |
|
|
Root | 0 (0.0) | 19 (100.0) |
|
| Valve dysfunction,
n (%) | 3 (42.9) | 1
(5.3) | 0.324b |
| Drugs, n (%) |
|
| 0.563b |
|
ACE-inhibitors | 4 (57.1) | 6
(31.6) |
|
|
Beta-blockers | 2 (28.6) | 10 (52.6) |
|
|
Beta-blockers/ACE
inhibitors | 2 (28.6) | 3
(15.8) |
|
Aortic specimens and histopathological
evaluation
Aortic specimens were collected from the resected
walls of the ascending aorta of both BAV and control patients at
the time of surgery and embedded in paraffin. The samples were
fixed in 10% buffered formalin for 24 h at room temperature and
embedded in paraffin. Sections (5 µm) were obtained from paraffin
blocks of samples with a cutting microtome. The sections were
stained with H&E or Alcian periodic acid-Schiff (PAS), as
described previously (12).
Masson's Trichrome staining using a Masson's Tricromica kit (cat.
no. 04-010802; Bio Optica Milano SpA) and Weigert Van Gieson
staining using an Elastic Stain kit (cat. no. HT25A-1KT;
Sigma-Aldrich; Merck KGaA) were also conducted. Following staining,
the slides were observed with an optical microscope (Leica DM 5000
B Microscope; Leica Microsystems, Inc.) connected to a digital
camera (Leica DC300 F; Leica Microsystems, Inc.). H&E staining
was performed for medionecrosis evaluation, Alcian PAS stain was
performed for the evaluation of PAS-positive mucoid material
accumulation, Masson's Trichrome stain was performed for fibrosis
evaluation, and Elastic Stain was performed for the identification
of elastic fibers alterations. The histological evaluation of the
ascending aortic wall was performed according to criteria adapted
from Schlatmann and Becker (13)
and de Sa et al (14); the histological data were analyzed
in a semi-quantitative manner. Each alteration was graded from 0
(no change) to 3 (severe change). For each patient, the sum of the
values measured was calculated as the aortic wall score. For the
histological evaluation, all specimens were evaluated independently
by two observers (FR and FC), who were blinded to the clinical data
(13,14).
Immunohistochemistry
Immunohistochemical experiments were performed on
4–5 µm thick 10% buffered formalin fixed and paraffin-embedded
sections of the ascending aortic wall, obtained from paraffin
blocks with a cutting microtome as previously described (15,16).
All sections were dewaxed in xylene for 30 min at 60°C and
rehydrated, at room temperature, by sequential immersion in a
graded series of alcohols and transferred into water for 5 min.
Subsequently, the sections were immersed for 8 min in sodium
citrate buffer (pH 6.0) at 95°C for antigen retrieval and
subsequently in Aceton at −20°C for 8 min to prevent the detachment
of the sections from the slide. All subsequent reactions were
conducted at room temperature. After washing with PBS (pH 7.4) for
5 min, the sections were treated for 5 min with Peroxidase
Quenching Solution (reagent A of Histostain®-Plus 3rd
Gen IHC Detection Kit; Invitrogen; Thermo Fisher Scientific, Inc.)
to inhibit any endogenous peroxidase activity. After washing with
PBS for 5 min, the sections were treated with a blocking protein
(reagent B of Histostain®-Plus 3rd Gen IHC Detection
Kit) for 10 min to block non-specific antigenic sites.
Subsequently, the sections were incubated with the following
primary antibodies overnight at 4°C: Anti-MMP9 (mouse monoclonal
antibody; 1:100; cat. no. sc-21733; Santa Cruz Biotechnology, Inc.)
and anti-MMP2 (mouse monoclonal antibody; 1:100; cat. no. sc-53630;
Santa Cruz Biotechnology, Inc.). Appropriate positive and negative
(isotype) controls were run concurrently. Following washing with
PBS for 5 min, the sections were incubated with a universal
biotinylated secondary antibody (Biotinylated Secondary Antibody
Reagent C from Histostain®-Plus 3rd Gen IHC Detection
Kit) for 10 min. After a subsequent washing with PBS for 5 min, the
sections were incubated with streptavidin-peroxidase complex
(Streptavidin-Peroxidase Conjugate Reagent D
Histostain®-Plus 3rd Gen IHC Detection Kit) for 10 min,
and following a further washing in PBS for 5 min, the slides were
incubated in the dark for 5 min with the DAB chromogen reagents E1
and E2 (Histostain®-Plus 3rd Gen IHC Detection Kit).
Nuclear counterstaining was carried out using hematoxylin
(Hematoxylin aqueous formula; cat. no. S2020; Bio Optica Milano
SpA). The specimens were examined by two independent observers (FR
and FC) that performed a quantitative evaluation to determine the
percentage of smooth muscle cells of the medial tunica. The
percentage of immunopositive-stained cells was then calculated in a
high-power field (magnification, ×400) and repeated for 10 fields;
the arithmetic means of counts were used for statistical
analyses.
TUNEL assay
DNA fragmentation was examined using a TUNEL assay,
which preferentially labels DNA strand breaks generated during
apoptosis; specifically, the Fluorescein In situ Cell Death
Detection kit (cat. no. 11684809910; Roche Applied Science) was
used according to the manufacturer's protocol. The 5-µm thick
tissue sections of ascending aorta obtained from paraffin blocks,
prepared as aforementioned, were dewaxed in xylene and rehydrated
in decreasing alcohol steps (ethanol at 100, 96, 80, 50% and
distilled water). Then, the sections were incubated with proteinase
K working solution at 37°C for 30 min for permeabilization and
subsequently rinsed twice with PSB. Thereafter, the sections were
treated with the TUNEL reaction mixture for 60 min at 37°C in the
dark and rinsed three times with PBS. The nuclei were stained with
Hoechst 33342 solution (1:1,000 in PBS; cat. no. 62249; Thermo
Fisher Scientific, Inc.) for 15 min at room temperature. Finally,
the coverslip was applied using PBS as the mounting medium and the
stained sections were examined by a confocal microscope (Leica
Confocal Microscope TCS SP8; Leica Microsystems, Inc.) and analyzed
by Leica Application Suite advanced fluorescence software version
3.3.0. (Leica Microsystems, Inc.), and images of five random and
non-overlapping fields were examined at an objective magnification
of ×200 for analysis.
Extraction of miRNA from aortic
specimens and plasma
Ascending aortic wall biopsies were obtained during
the surgical procedure and immediately stored at −80°C. Blood was
withdrawn from patients before the surgical procedure and was
processed within 90 min after collection. Plasma was obtained by
centrifugation at 2,000 × g for 15 min at 4°C and stored at −80°C
until needed. Total RNA including small RNA was isolated from
aortic specimens using the RNeasy Mini Kit (cat. no. 74104; Qiagen
GmbH) and from plasma using the miRNeasy Serum/Plasma kit (cat. no.
217184; Qiagen GmbH) according to the manufacturer's instructions.
The quantity and quality of total RNA were determined by a NanoDrop
ND-1000 spectrophotometer (NanoDrop Technologies; Thermo Fisher
Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Reverse transcription was performed using the
miScript II RT kit (cat. no. 218161; Qiagen GmbH) according to the
manufacturer's instructions. cDNA was pre-amplified using the
miScript RT Kit (cat. no. 331451; Qiagen GmbH), as previously
described (17). qPCR analysis was
performed using the miScript SYBR Green PCR Kit (Qiagen GmbH) at
the following thermocycling conditions: 95°C 15 min; and 40 cycles
of 94°C 15 sec, 55°C 30 sec and 70°C 30 sec. The primers used are
reported in Table II. miRNAs,
including miR-718, miR-486, miR-130 and miR-122, were selected from
the literature based on their previously reported association with
aortopathies and vascular degeneration (18–20).
miRNA expression levels were normalized to that of U6 snRNA and
miR-16, and changes in the transcript level were calculated using
the 2−ΔΔCq method (21).
qPCR was carried out using the Rotor-Gene™ 6000 Real-Time PCR
Machine (Qiagen GmbH).
 | Table II.Primers used for reverse
transcription-quantitative PCR. |
Table II.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Catalogue
numbersa |
|---|
| Hs_miR-718_2 | MS00037856 |
| Hs_miR-486_1 | MS00004284 |
| Hs_miR-130a_1 | MS00003444 |
| Hs_miR-122a_1 | MS00003416 |
| Hs_RNU6-2_11 | MS00033740 |
| Hs_miR-16_2 | MS00031493 |
Statistical analyses
Unpaired Student's t-test and Fisher's test were
conducted to compare, according to sex, all demographic and
clinical features, comorbidity conditions and pharmacological
treatments. The data obtained for MMP expression, TUNEL evaluation
and miRNA expression were compared using one-way ANOVA followed by
Bonferroni's post hoc test for multiple comparisons. The data are
expressed as the mean ± SD. The Kruskal-Wallis test and Dunn's post
hoc test were used for comparisons of the distributions of
non-normal variables in the three patient groups [control group
(CN), low-grade medial degeneration (LGMD) and high-grade medial
degeneration (HGMD)] and for all multiple pairwise comparisons,
respectively. A multivariate ordinal logit model (proportional odds
model) was used to examine the predictors of disease. The ordinal
logit model is a frequently-used method as it enables to ordinal
variables to be modelled. In the ordinal logit model the cumulative
probability of a level is connected to explanatory variables. In
the present study, the response variable Y was classified into
three response ordinal categories CN, LGMD and HGMD, with CN as the
reference level. Indeed, in the proportional odds model CN was
considered as reference category vs. cumulative remaining
categories. The theory of logit model is reported on in previous
studies (22–24). The explanatory variables that were
considered in this model were: PL_miR-718, PL_miR-486, PL_miR-130,
PL_miR122, TIS_miR-718, TIS_miR-486, TIS_miR-130 and TIS_miR122.
Through the selection stepwise of LOGISTIC procedure of the
Statistical Analysis Software (SAS) system the significant
variables/predictors of model were identified. In stepwise
selection, an attempt was made to remove any insignificant
variables from the model before adding a significant variable to
the model: Significance level of 0.05 was required to allow a
variable into the model and a significance level of 0.05 was
required for a variable to stay in the model (the score chi-square
was used to determine entry; the Wald chi-square was used to
determine removal). Results are presented as the adjusted odds
ratios (ORs) with 95% confidence interval only for
variables/predictors that were significant in stepwise selection of
LOGISTIC procedure. Statistical analysis was performed using the
SAS 9.4 programme (SAS Institute, Inc.). α<0.05 or P<0.05
were considered to indicate a statistically significant
difference.
Results
Clinical data and histological
evaluation
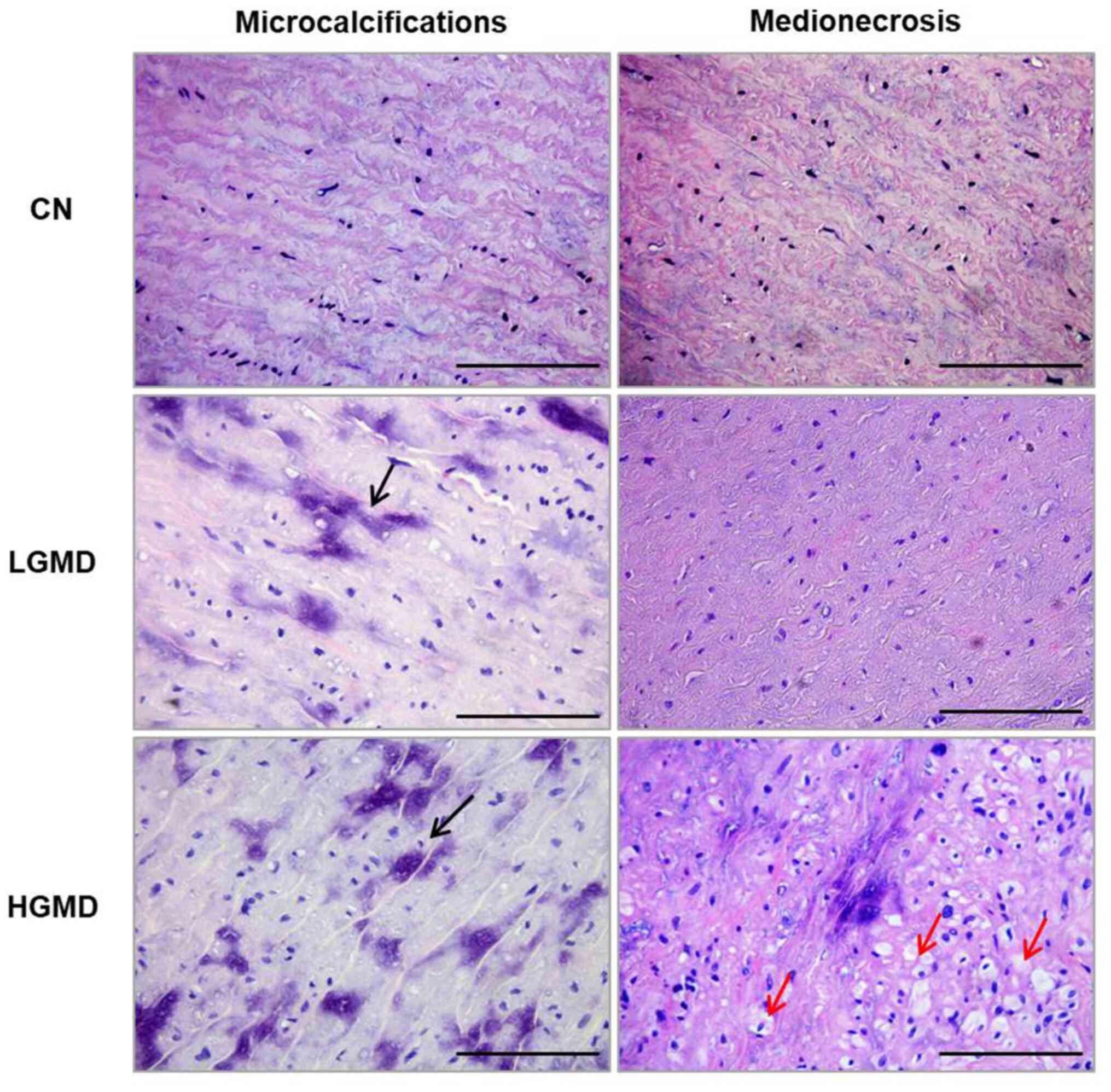
The histological evaluation of the ascending aortic
wall showed focal to diffuse microcalcifications of the medial
tunica, absent to multifocal areas of the destruction of the smooth
muscle, such as medionecrosis (Fig.
1), focal to multicentric elastic fragmentation (Fig. 2A), low levels to large increase of
collagen content (Fig. 2B) and
sporadic to consistent accumulation of PAS-positive material
(Fig. 2C). Using the criteria
adapted from Schlatmann and Becker (13) and de Sa et al (14), an ascending aortic wall score was
determined and according to this score two groups of patients with
BAV were identified: i) LGMD (mean aortic wall score, 5.75±0.96),
with focal or absent medionecrosis, foci of elastic fragmentation,
faint and sporadic accumulation of mucoid material, and faint or
focal increase of interstitial collagen in some areas of the media;
and ii) HGMD (mean aortic wall score, 9.25±1.5), with severe
medionecrosis, a number of foci of elastic fragmentation,
consistent accumulation of mucoid material and accumulation of
collagen in more than a third of the media. Both groups consisted
of 13 patients, the LGD group consisted of 9 R-L, 3 R-NC and 1 L-NC
BAV phenotypes, whereas the HGD group consisted of 10 R-L, 2 R-NC
and 1 L-NC BAV phenotypes. The morphological analyses indicated
that the grade of medial degeneration was independent from the BAV
phenotype. In the samples of the CN group, no morphological
alterations were observed in the media of the ascending aortic wall
(Figs. 1 and 2; Table
III).
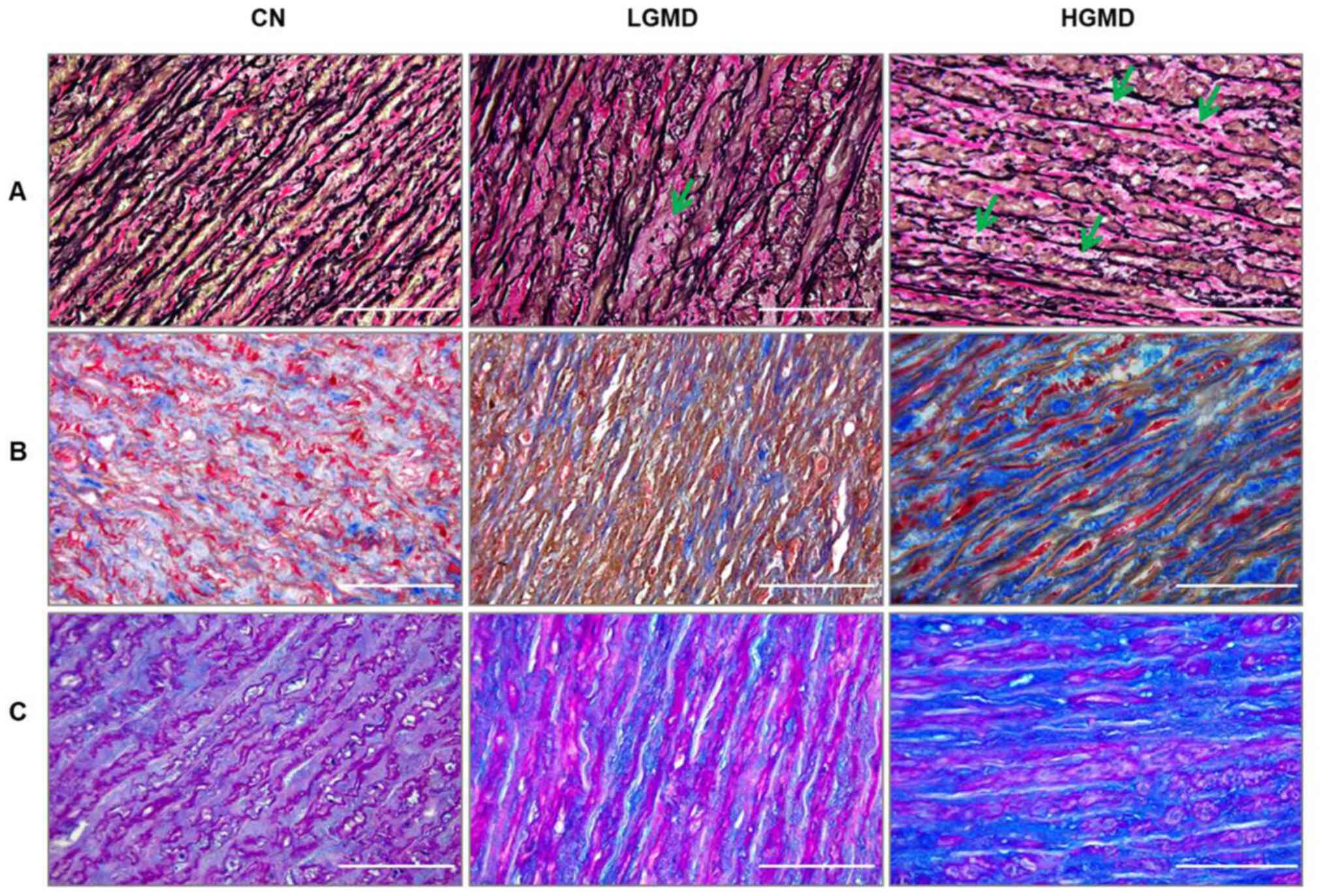 | Figure 2.Weigert-van Gieson, Masson's
Trichrome and Alcian PAS staining of the ascending aortic wall. (A)
Representative images of elastic Weigert-van Gieson staining of the
ascending aortic wall. The specimens showed focal elastic
fragmentation in the LGD group (green arrow), and multicentric
areas of elastic fragmentation in the HGD group (green arrows),
thus indicating elastic degeneration of the medial tunica. (B)
Representative images of Masson's Trichrome staining of the
ascending aortic wall that show the collagen content (blue
staining) in the medial tunica. In particular, there was a large
increase in the collagen content observed in the HGD group, which
suggested the connective tissue remodeling of the aortic wall. (C)
Representative images of Alcian PAS staining of the ascending
aortic wall that shows the accumulation of mucoid material (blue
staining). The LGD group presented with the sporadic accumulation
of mucoid material, whereas the HGD group showed a consistent
accumulation of mucoid material. Magnification, ×400; scale bar,
100 µm. CN, control; HGMD, high-grade medial degeneration; LGMD,
low-grade medial degeneration; PAS, periodic acid-Schiff. |
 | Table III.Histopathological features of the
ascending aortic wall. |
Table III.
Histopathological features of the
ascending aortic wall.
|
| Control group
(n=12) | Bicuspid aortic
valve group (n=26) |
|---|
|
|
|
|
|---|
| Histopathological
features | n | % | n | % |
|---|
|
Microcalcifications |
|
|
|
|
| 0 | 8 | 67 | 0 | 0 |
| I | 4 | 33 | 6 | 23 |
| II | 0 | 0 | 12 | 46 |
|
III | 0 | 0 | 8 | 31 |
| Medionecrosis |
|
|
|
|
| 0 | 12 | 100 | 7 | 27 |
| I | 0 | 0 | 5 | 19 |
| II | 0 | 0 | 6 | 23 |
|
III | 0 | 0 | 8 | 31 |
| Elastic fiber
fragmentation |
|
|
|
|
| 0 | 11 | 92 | 0 | 0 |
| I | 1 | 8 | 8 | 31 |
| II | 0 | 0 | 11 | 42 |
|
III | 0 | 0 | 7 | 27 |
| Collagen
accumulation |
|
|
|
|
| 0 | 10 | 83 | 3 | 11 |
| I | 2 | 17 | 10 | 39 |
| II | 0 | 0 | 8 | 31 |
|
III | 0 | 0 | 5 | 19 |
| PAS-positive
material accumulation |
|
|
|
|
| 0 | 12 | 100 | 0 | 0 |
| I | 0 | 0 | 12 | 46 |
| II | 0 | 0 | 4 | 15 |
|
III | 0 | 0 | 10 | 39 |
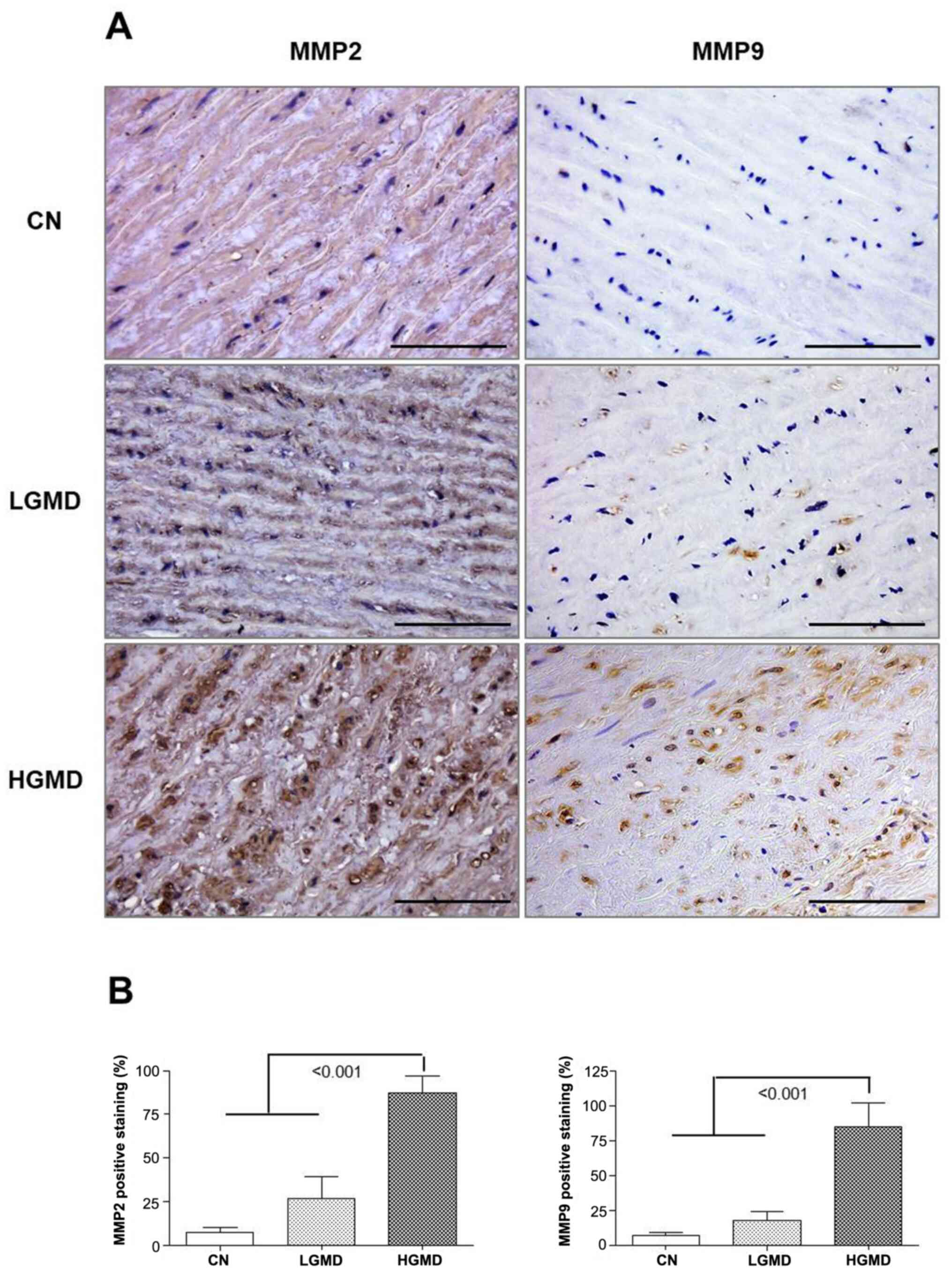
Ascending aorta from the HGD group
showed increased expression of MMP-9 and MMP-2
The percentage of MMP2- and MMP9-stained cells was
calculated following immunohistochemistry. The levels of MMP2 and
MMP9 immunoreactivity were both significantly increased in the HGD
group compared with the LGMD and CN groups (both P<0.001;
Fig. 3A and B).
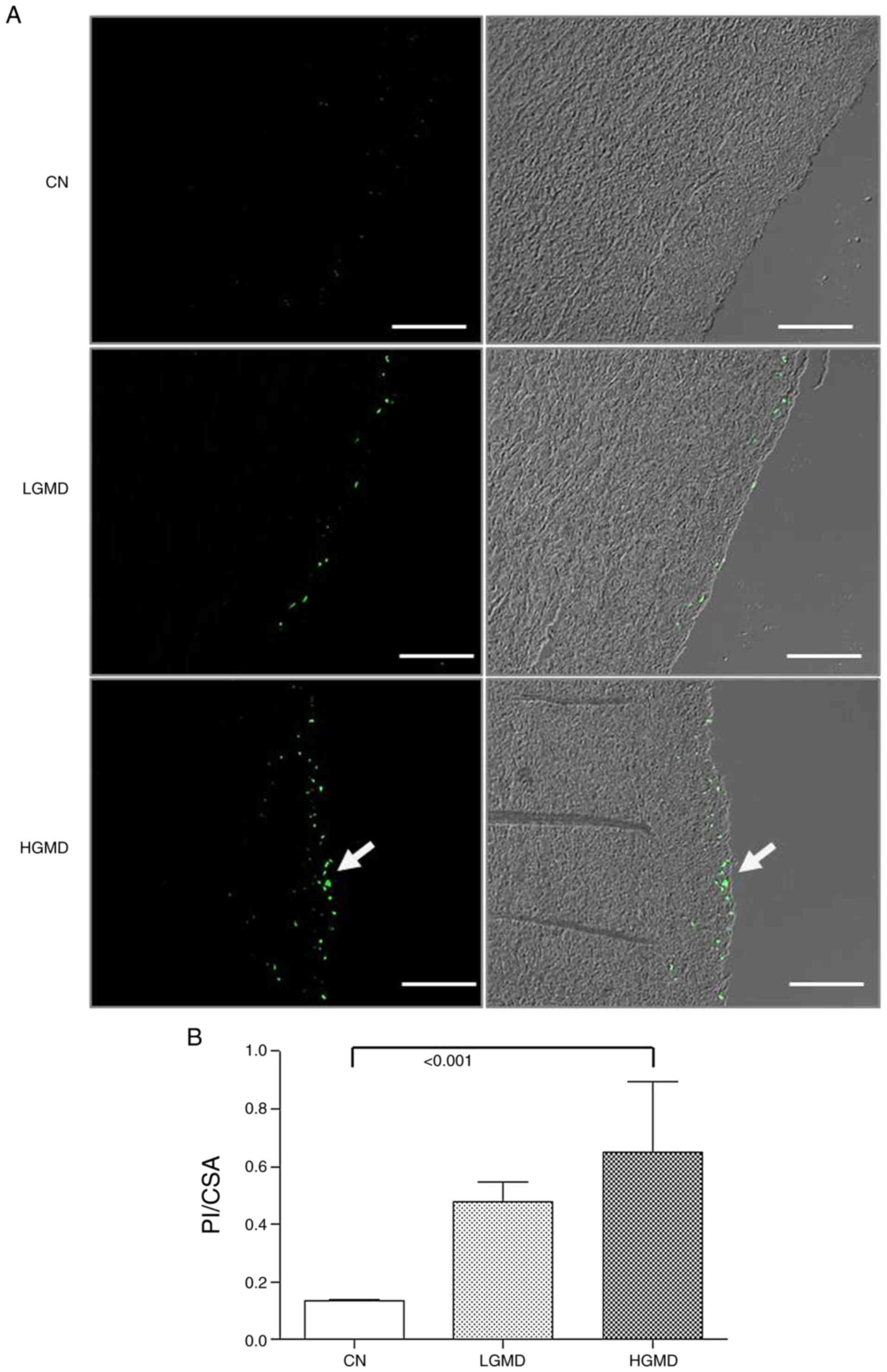
Ascending aorta from HGD group have
apoptotic cells in the medial tunica
TUNEL assay results showed an increase of the number
of apoptotic cells in the HGD group and focal apoptotic cells were
observed in the LGD group compared with the control. The apoptotic
cells were observed in the subintimal region of the media, which
confirmed the severity of damage of the ascending aortic wall.
Apoptosis in the HGD group was significantly increased compared
with the CN group (P<0.001; Fig. 4A
and B).
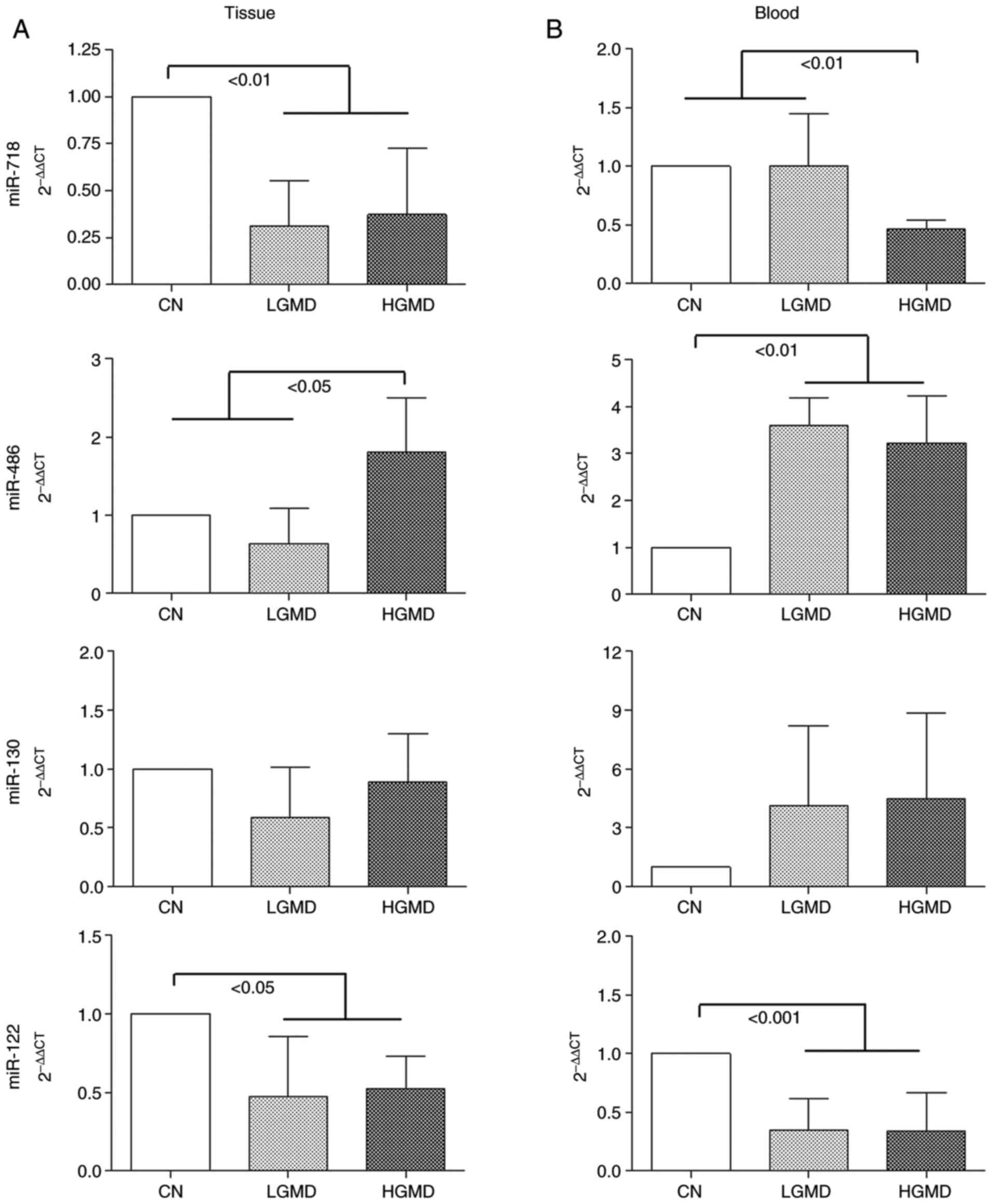
Significance of tissue and plasma
miR-718, miR-486, miR-130 and miR-122 expression levels in relation
to the ascending aortic wall score
miRNA expression analysis of the ascending aortic
wall showed a significant downregulation of the tissue expression
levels of miR-718 and miR-122 in the LGMD and HGD groups compared
with the CN group (P<0.01 and P<0.05, respectively; Fig. 5A). Notably, the HGD group exhibited
a significant increase of the tissue expression levels of miR-486
compared with those of the LGMD and CN groups (P<0.05; Fig. 5A). No significant difference was
identified for the tissue expression levels of miR-130 between the
three groups (Fig. 5A).
Significant differences among the LGMD, HGMD and CN
groups for miR-718, miR-486, miR-130 and miR-122 in both tissue and
plasma were analyzed by Kruskal-Wallis test (Table IV). To improve the understanding of
the significance of these markers, Dunn's post hoc test and
multivariate (multivariate analysis adjusted OR, 95% CI) analyses
were also performed. The comparison of the tissue expression levels
of miR-718, miR-486, miR-130 and miR-122 between CN, LGMD and HGD
groups showed significant differences (Table IV). In particular the Dunn's post
hoc test showed a significant decrease of miR-718 tissue expression
levels in the LGMD and HGD groups compared with the CN group
(P<0.001 and P<0.05, respectively; Table IV). In addition, the analysis
confirmed the data regarding the upregulation of the tissue
expression levels of miR-486 in the HGD group compared with the LGD
group (P<0.001; Table IV). The
Dunn's post hoc test showed the decrease of miR-486 tissue
expression levels in the LGD group compared with the CN group
(P<0.05). Moreover, miR-130 tissue expression levels were
decreased in the LGD group compared with the CN and HGD groups
(P<0.001 and P<0.05, respectively). Finally, miR-122 tissue
expression levels were decreased in the LGMD and HGD groups
compared with the CN group (P<0.01 and P<0.05,
respectively).
 | Table IV.Comparison of miRNA expression levels
in the patient groups. |
Table IV.
Comparison of miRNA expression levels
in the patient groups.
|
|
|
| Univariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Predictor | Patient group | Median (range) |
P-valuea | Comparison |
P-valueb | Multivariate
analysis, adjusted ORc
(95% CI) |
|---|
| PL_miR-718 | CN | 1.000
(1.000–1.000) | <0.001 | CN vs. LGMD | <0.001 | NS |
|
| LGMD | 0.393
(0.259–0.591) |
| CN vs. HGMD | NS |
|
|
| HGMD | 0.686
(0.384–1.084) |
| LGMD vs. HGMD | NS |
|
| PL_miR-486 | CN | 1.000
(1.000–1.000) | <0.001 | CN vs. LGMD | <0.010 | 2.467
(1.407–4.327) |
|
| LGMD | 4.714
(2.027–9.459) |
| CN vs. HGMD | <0.001 |
|
|
| HGMD | 9.541
(4.869–14.075) |
| LGMD vs. HGMD | NS |
|
| PL_miR-130 | CN | 1.000
(1.000–1.000) | <0.001 | CN vs. LGMD | <0.001 | NS |
|
| LGMD | 3.441
(1.913–10.193) |
| CN vs. HGMD | NS |
|
|
| HGMD | 1.260
(0.350–12.010) |
| LGMD vs. HGMD | NS |
|
| PL_miR122 | CN | 1.000
(1.000–1.000) | <0.002 | CN vs. LGMD | <0.010 | NS |
|
| LGMD | 0.124
(0.012–1.491) |
| CN vs. HGMD | <0.010 |
|
|
| HGMD | 0.185
(0.042–1.295) |
| LGMD vs. HGMD | NS |
|
| TIS_miR-718 | CN | 1.000
(1.000–1.000) | <0.001 | CN vs. LGMD | <0.001 | NS |
|
| LGMD | 0.362
(0.271–0.428) |
| CN vs. HGMD | <0.050 |
|
|
| HGMD | 0.575
(0.294–1.070) |
| LGMD vs. HGMD | NS |
|
| TIS_miR-486 | CN | 1.000
(1.000–1.000) | <0.001 | CN vs. LGMD | <0.050 | NS |
|
| LGMD | 0.590
(0.398–1.179) |
| CN vs. HGMD | NS |
|
|
| HGMD | 2.229
(0.796–3.266) |
| LGMD vs. HGMD | <0.001 |
|
| TIS_miR-130 | CN | 1.000
(1.000–1.000) | <0.001 | CN vs. LGMD | <0.001 | NS |
|
| LGMD | 0.544
(0.187–0.877) |
| CN vs. HGMD | NS |
|
|
| HGMD | 0.821
(0.523–1.380) |
| LGMD vs. HGMD | <0.050 |
|
| TIS_miR122 | CN | 1.000
(1.000–1.000) | <0.007 | CN vs. LGMD | <0.010 | NS |
|
| LGMD | 0.513
(0.145–1.235) |
| CN vs. HGMD | <0.050 |
|
|
| HGMD | 0.693
(0.356–1.216) |
| LGMD vs. HGMD | NS |
|
Plasma circulating levels of miR-718 were
significantly decreased in the HGD group compared with the CN and
LGD groups (P<0.01; Fig. 5B),
whereas miR-122 levels were significantly decreased in the LGMD and
HGD groups compared with the CN group (P<0.001; Fig. 5B). Notably, plasma expression levels
of miR-486 were significantly increased in the LGMD and HGD groups
compared with the CN group (P<0.01). No significant differences
were identified for miR-130 plasma expression levels between the
three groups.
Kruskal-Wallis and Dunn's post hoc test (Table IV) showed that miR-718 plasma
expression levels were significantly decreased in the LGD group
compared with the CN group (P<0.001), whereas miR-486 plasma
expression levels were increased in the LGMD and HGD groups
compared with the CN group (P<0.01 and P<0.001,
respectively). miR-130 plasma expression levels were increased only
in the LGD group compared with the CN group (P<0.001) and
miR-122 expression levels were decreased in both the LGMD and HGD
groups compared with the CN group (P<0.01).
In addition, it was observed that in the
multivariate analysis, although plasma and tissue expression levels
of miR-718, miR-486, miR-130 and miR-122 were included in the
model, only the plasmid levels of miR-486 showed a significant
change [odds ratio (OR) 2.467; 95% CI, 1.407–4.327; Table IV]. The OR analysis obtained with
the logit model, suggested that statistically the increase of
miR-486 increased the risk of damage in the ascending aortic wall
(increase of a point of plasma miR-486 produced an increase equal
to 2.467 of the risk of being LGMD or HGMD compared with the CN
group).
Discussion
Current guidelines on the treatment of ascending
aortic aneurysm associated with non-BAV disease suggest surgical
repair at aortic diameter ≥55 or ≥50 mm when additional risk
factors or coarctation are present; when surgery is primarily
indicated for BAV, replacement of aortic root or tubular ascending
aorta should be considered at ≥45 mm maximum diameter (25). The risk of rupture and acute
dissection increases significantly once a diameter of 50 mm is
reached (26), nevertheless, acute
aortic dissection in patients with BAV can occur at smaller sizes
than generally perceived. Svensson and Khitin (27) assessed that in patients with BAV
dissected at a maximum diameter of <50 mm (18). In addition, the risk of dissection
in BAV appears to be comparable to patients with Marfan syndrome,
but it can occur more frequently as BAV can present in 1–2% of the
population (28). Acute aortic
dissection may occur at a younger age in patients with BAV; data
from The International Register for Acute Aortic Dissection
suggested that patients under the age of 40 who suffered type A
aortic dissection more often had BAV compared with those dissecting
over the age of 40 (9 vs. 1%; P<0.01) and these patients
dissected at ascending aortic diameter <50 mm (29). There is, in our opinion, a
discrepancy between current guidelines, and increasing data from
international literature (30) has
suggested that surgical repair of ascending aortic aneurysm in
patients with BAV should be performed at a smaller diameter than
the ones suggested (≥55 or ≥50 mm). Aortic dissection and rupture
is ‘often’ associated with severe aortic wall degeneration and, to
be able to link the grade of medial tunica degeneration to specific
plasma biomarkers, might be of help in preventing deadly
complications, such as dissection and rupture. The present study
investigated the involvement of miR-718, miR-486, miR-130 and
miR-122 in patients with BAV with regard to aortic wall
degeneration. This research started from the assumption that, in
patients with BAV with ascending aortic aneurysm or dissection, the
pathological changes may involve the ascending aortic portion, as
well as the non-dilated aortic root (31). The presence of BAV may lead to
abnormal blood flow and shear stress at the ascending aorta, thus
causing apoptosis of the endothelial cells, inflammation, oxidative
stress and extracellular matrix (ECM) remodeling (32). Moreover, in our hypothesis,
circulating miRNAs may be involved in the paracrine communication
between cells contributing to the regulation of the disease
progression. The determination of circulating biomarkers of aortic
histological abnormalities may be useful for the identification of
novel biomarkers that could significantly improve the diagnosis,
prognosis and treatment of BAV.
Previously, different types of aneurysm related to
BAV morphology have been described (33,34).
In particular, it seems that a specific aneurysm characterized by
root enlargement is frequently associated with the R-L BAV
phenotype. This form of aneurysm involves the young BAV population,
and being male increases the risk of developing this condition. A
previous study supported the predominant genetic origin of BAV root
aneurysm type, reporting a TGF-β receptor type-2 gene mutation
(35). The histological analysis
conducted in the present study showed that the severity of medial
degeneration of the ascending aortic wall was not associated with a
specific BAV phenotype; thus, after morphological evaluation,
patients were divided into two groups according to the grade of
their medial degeneration score. The two groups were composed of
patients with different types of BAV phenotypes. However, the
prevailing phenotype of BAV was the R-L phenotype. The HGD group
showed increased immunoreactivity for MMP2 and MMP9, which is in
accordance with other studies that have reported an association
between the overexpression of MMPs in medial and aortic aneurysm
(36–38). The risk of aortic dissection could
be enforced by the presence of multicentric areas of elastic
fragmentation, massive increases of collagen content and apoptotic
cells in the media, as demonstrated by the results of the present
study. Furthermore, the identification of easily detectable
biomarkers of the grade of ascending aortic wall degeneration could
reduce mortality related to aortic complications.
The miRNAs examined in the current study were chosen
based on their previously reported association with aortopathies
(18–20). The expression levels of miR-718,
miR-486, miR-130 and miR-122 were first investigated in the tissue
specimens of the ascending aortic wall, followed by detection in
circulation (plasma) to determine whether there was an association
between aortic damage and variation in the expression levels of
miRNAs. The data obtained showed an association between tissue and
circulating levels of these miRNAs. In multivariate analysis, the
estimate of odds ratio for miR-486 indicated that high levels of
miR-486 might be associated with a high risk of having high scores
of damage in the ascending aortic wall. It has been demonstrated
that miR-122, miR-130a and miR-486 regulation is dependent on the
tricuspid or bicuspid morphology of the aortic valve, and miR-718
may be considered as a biomarker of aortic dilatation (20). Moreover, miR-122 and miR-130a are
molecular effectors in the BAV-associated dysregulation of TGF-β1
receptor. TGF-β1 mediates tissue fibrosis and ECM remodeling in
ascending aorta (20). The present
study results support the idea that the regulation of these miRNAs
may be associated with ascending aortic wall damage.
miRNAs are known to be versatile master regulators
of signaling pathways owing to their capacity to modulate the
expression of crucial components of signal transduction at multiple
levels. Thus, these candidate miRNAs, and in particular miR-486 and
miR-122, may be potential biomarkers of the risk of rupture and
dissections in patients with BAV. Moreover, the evaluation of miRNA
levels in plasma is reproducible and consistent among individuals,
being protected from endogenous ribonuclease-induced degradation
(39,40). As an added value, plasma is a
biological specimen more easily accessible by non-invasive means
compared to other tissues, which might improve the feasibility of
these molecules as useful biomarkers in routine clinical
practice.
In conclusion, we proposed that miR-122, miR-130,
miR-718 and miR-486 are novel molecular features associated with
severe medial degeneration in patients with BAV with aortic
dilatation. In particular, as aforementioned, miR-486 could be
considered as a new non-invasive biomarker of aortic wall
degeneration in BAV owing to its association with the morphological
features of the vessel. A significant dysregulation of this
biomarkers might be associated with high risk of dissection and
rupture of the ascending aortic diameter.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by CARDIOSERVICE
SAS.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CP, AMG, FR, VAg and FC were responsible for the
conception of the present study. CP, AT, VAg, GR and VAr acquired
the clinical and surgical data. AMG, FR, RB, AP and FC acquired the
histological, immunohistochemical and mRNA data. CP, AMG, FR, FC,
GR and VAr analyzed and interpreted the data. RA was responsible
for statistical analysis. All authors drafted the work and revised
it critically for important intellectual content. All authors have
read and approved the final manuscript. CP, AMG, FR, FC and VAr
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of University of Palermo (approval no. CE 2A17-000-527-28;
Palermo, Italy). Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Masri A, Svensson LG, Griffin BP and Desay
MY: Contemporary natural history of bicuspid aortic valve disease:
A systematic review. Heart. 103:1323–1330. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Michelena HI, Desjardins VA, Avierinos JF,
Russo A, Nkomo VT, Sundt TM, Pellikka PA, Tajik AJ and
Enriquez-Sarano M: Natural history of asymptomatic patients with
normally functioning or minimally dysfunctional bicuspid aortic
valve in the community. Circulation. 117:2776–2784. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abdulkareem N, Smelt J and Jahangiri M:
Bicuspid aortic valve aortopathy: Genetics, pathophysiology and
medical therapy. Interact Cardiovasc Thorac Surg. 7:554–559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu T, Xie M, Lv Q, Li Y, Fang L, Zhang L,
Deng W and Wang J: Bicuspid aortic valve: An update in morphology,
genetics, biomarker, complications, imaging diagnosis and
treatment. Front Physiol. 9:19212018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niaz T and Hagler DJ: Is there a genetic
basis to the different morphological subtypes of bicuspid aortic
valve? Ann Transl Med. 6 (Suppl 2):S1172018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harrison OJ, Visan AC, Moorjani N, Modi A,
Salhiyyah K, Torrens C, Ohri S and Cagampang FR: Defective NOTCH
signaling drives increased vascular smooth muscle cell apoptosis
and contractile differentiation in bicuspid aortic valve
aortopathy: A review of the evidence and future directions. Trends
Cardiovasc Med. 29:61–68. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller RL, Diamonstein CJ and Benheim A:
The importance of genetics and genetic counselors in the evaluation
of patients with bicuspid aortic valve and aortopathy. Curr Opin
Cardiol. 34:73–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balistreri CR, Crapanzano F, Schirone L,
Allegra A, Pisano C, Ruvolo G, Forte M, Greco E, Cavarretta E,
Marullo AG, et al: Deregulation of notch1 pathway and circulating
endothelial progenitor cell (EPC) number in patients with bicuspid
aortic valve with and without ascending aorta aneurysm. Sci Rep.
8:138342018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sievers HH, Stierle U, Hachmann RMS and
Charitos El: New insights in the association between bicuspid
aortic valve phenotype, aortic configuration and valve
haemodynamics. Eur J Cardiothorac Surg. 49:439–446. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McKusick VA: Association of congenital
bicuspid aortic valve and Erdheim's cystic medial necrosis. Lancet.
1:1026–1027. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kouchoukos NT, Wareing TH, Murphy SF and
Perrillo JB: Sixteen-year experience with aortic root replacement.
Results of 172 operations. Ann Surg. 214:308–318. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bellavia M, Rappa F, Lo Bello M, Brecchia
G, Tomasello G, Leone A, Spatola G, Uzzo ML, Bonaventura G, Davis
S, et al: Lactobacillus casei and Bifidobacterium lactis
supplementation reduces tissue damage of intestinal mucosa and
liver after 2,4,6-trinitrobenzenesulfonic acid treatment in mice. J
Biol Regul Homeost Agents. 28:251–261. 2014.PubMed/NCBI
|
|
13
|
Schlatmann TJ and Becker AE: Histologic
changes in the normal aging aorta: Implications for dissecting
aortic aneurysm. Am J Cardiol. 39:13–20. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Sa M, Moshkovitz Y, Butany J and David
TE: Histologic abnormalities of the ascending aorta and pulmonary
trunk in patients with bicuspid aortic valve disease: Clinical
relevance to the ross procedure. J Thorac Cardiovasc Surg.
118:588–594. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barone R, Rappa F, Macaluso F, Bavisotto
CC, Sangiorgi C, Di Paola G, Tomasello G, Di Felice V, Marcianò V,
Farina F, et al: Alcoholic liver disease: A mouse model reveals
protection by lactobacillus fermentum. Clin Transl Gastroenterol.
7:e1382016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rappa F, Sciume C, Lo Bello M, Bavisotto
CC, Gammazza AM, Barone R, Campanella C, Davis S, Carini F, Zarcone
F, et al: Comparative analysis of Hsp10 and Hsp90 expression in
healthy mucosa and adenocarcinoma of the large bowel. Anticancer
Res. 34:4153–4159. 2014.PubMed/NCBI
|
|
17
|
Barone R, Pitruzzella A, Gammazza AM,
Rappa F, Salerno M, Barone F, Sangiorgi C, D'Amico D, Locorotondo
N, Di Gaudio F, et al: Nandrolone decanoate interferes with
testosterone biosynthesis altering blood-testis barrier components.
J Cell Mol Med. 21:1636–1647. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu J, Song HF, Li SH, Guo J, Tsang K,
Tumiati L, Butany J, Yau TM, Ouzounian M, Fu S, et al: Progressive
aortic dilation is regulated by mir-17-associated miRNAs. J Am Coll
Cardiol. 678:2965–2977. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ikonomidis JS, Ivey CR, Wheeler JB,
Akerman AW, Rice A, Patel RK, Stroud RE, Shah AA, Hughes CG,
Ferrari G, et al: Plasma biomarkers for distinguishing etiologic
subtypes of thoracic aortic aneurysm disease. J Thorac Cardiovasc
Surg. 145:1326–1333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martínez-Micaelo N, Beltrán-Debón R,
Baiges I, Faiges M and Alegret JM: Specific circulating microRNA
signature of bicuspid aortic valve disease. J Transl Med.
15:762017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Agresti A: Analysis of Ordinal Categorical
Data. 2nd edition. Wiley; New York, NY: 2010, View Article : Google Scholar
|
|
23
|
Allison PD: Logistic Regression Using SAS:
Theory and Application. 2nd edition. SAS Institute Inc.; Cary, NC:
2012
|
|
24
|
Hosmer DW Jr and Lemeshow S: Applied
Logistic Regression. 3rd edition. John Wiley & Sons; New York,
NY: 2013, View Article : Google Scholar
|
|
25
|
Baumgartner H, Falk V, Bax JJ, De Bonis M,
Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Muñoz DR, et al:
ESC/EACTS Guidelines for the management of valvular heart disease.
Eur Heart J. 38:2739–2791. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elefteriades JA: Natural history of
thoracic aortic aneurysms: Indications for surgery, and surgical
versus non-surgical risks. Ann Thorac Surg. 74:S1877–S1880. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Svensson LG and Khitin L: Aortic
cross-sectional area/height ratio timing of aortic surgery in
asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc
Surg. 123:360–361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gott VL, Greene PS, Alejo DE, Cameron DE,
Naftel DC, Miller DC, Gillinov AM, Lashinger JC and Pyeritz RE:
Replacement of the aortic root in patients with Marfan's syndrome.
New Engl J Med. 340:1307–1313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pape LA, Tsai TT, Isselbacher EM, Oh JK,
O'Gara PT, Evangelista A, Fattori R, Meinhardt G, Trimarchi S,
Bossone E, et al: Aortic diameter ≥5.5 cm is not a good predictor
of type A aortic dissection: Observations from the international
registry of acute aortic dissection (IRAD). Circulation.
116:1120–1127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Etz CD, Haunschild J, Girdauskas E, Corte
AD, Fedak PWM, Schäfers HJ, Sundt TM and Borger MA: Surgical
management of the aorta in BAV patients. Prog Cardiovasc Dis.
63:475–481. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pisano C, Maresi E, Balistreri CR, Candore
G, Merlo D, Fattouch K, Bianco G and Ruvolo G: Histological and
genetic studies in patients with bicuspid aortic valve and
ascending aorta complications. Interact Cardiovas Thorac Surg.
14:300–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Siu SC and Silversides CK: Bicuspid aortic
valve disease. J Am Coll Cardiol. 55:2789–2800. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cotrufo M and Corte AD: The association of
bicuspid aortic valve disease with asymmetric dilatation of the
tubular ascending aorta: Identification of a definite syndrome. J
Cardiovasc Med (Hagerstown). 10:291–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Corte AD, Bancone C, Quarto C, Dialetto G,
Covino FE, Scardone M, Caianello G and Cotrufo M: Predictors of
ascending aortic dilatation with bicuspid aortic valve: A wide
spectrum of disease expression. Eur J Cardiothorac Surg.
31:397–404. 2007. View Article : Google Scholar
|
|
35
|
Girdauskas E, Schulz S, Borger MA, Mierzwa
M and Kuntze T: Transforming growth factor-beta receptor type II
mutation in a patient with bicuspid aortic valve disease and
intraoperative aortic dissection. Ann Thorac Surg. 91:e70–e71.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thompson RW, Holmes DR, Mertens RA, Liao
S, Botney MD, Mecham RP, Welgus HG and Parks WC: Production and
localization of 92-kilodalton gelatinase in abdominal aortic
aneurysms. An elastolytic metalloproteinase expressed by
aneurysm-infiltrating macrophages. J Clin Invest. 96:318–326. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Longo GM, Xiong W, Greiner TC, Zhao Y,
Fiotti N and Baxter BT: Matrix metalloproteinases 2 and 9 work in
concert to produce aortic aneurysms. J Clin Invest. 110:625–632.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tamarina NA, McMillan WD, Shively VP and
Pearce WH: Expression of matrix metalloproteinases and their
inhibitors in aneurysms and normal aorta. Surgery. 122:264–271.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fedak PW, de Sa MP, Verma S, Nili N,
Kazemian P, Butany J, Strauss BH, Weisel RD and David TE: Vascular
matrix remodeling in patients with bicuspid aortic valve
malformations: Implications for aortic dilatation. J Thorac
Cardiovasc Surg. 126:797–806. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oury C, Servais L, Bouznad N, Hego A,
Nchimi A and Lancellotti P: MicroRNAs in valvular heart diseases:
Potential role as markers and actors of valvular and cardiac
remodeling. Int J Mol Sci. 17:11202016. View Article : Google Scholar : PubMed/NCBI
|