Introduction
During the onset and development of osteoarthritis
(OA), chondrocytes are altered via exposure to pro-inflammatory
cytokines (1–3). In addition, the upregulation of
pro-inflammatory cytokines, such as IL-1β, in the cartilage and
serum of patients with OA have been associated with OA development
(4). IL-1β can promote
degradation of the extracellular matrix (ECM) by enhancing the
generation of catabolic enzymes, such as MMPs, resulting in
cartilage disruption (5,6). The reduction of IL-1β levels was
previously proposed as an anti-OA therapeutic strategy (7). When OA is caused by the upregulation
of IL-1β, the NF-κB signal transduction pathway is presumably
activated, which could regulate the production of apoptotic and
inflammatory proteins (8).
The apoptosis of chondrocytes is a primary cause of
cartilage disruption (9,10). Endoplasmic reticulum (ER) stress,
which is caused by glucose or oxygen deprivation, certain
therapeutic agents [e.g. thapsigargin (TG)] and free radical
damage, can induce the activation of apoptotic pathways (11,12). Although the ER can initially
protect against stress caused by misfolded protein accumulation by
maintaining cell homeostasis, a severe unfolded protein response
(UPR) eventually reduces this protection (13,14). The upregulated expression of
protein disulfide isomerase and glucose-regulated protein 78
(GRP78), two markers of ER stress, is observed in OA articular
cartilage, indicating that ER stress is associated with OA
development (15).
Tetramethylpyrazine (TMP), also known as
ligustrazine, is a nitrogenous heterocyclic compound comprising of
one carbon atom in a pyrazine ring attached to one methyl group
(16). TMP is the major active
alkaloid ingredient in Chinese herbal medicines (Ligusticum
wallichii) and can dilate blood vessels, inhibit platelet
aggregation and improve microcirculation. Accordingly, it has been
extensively used in clinical settings (17). TMP ameliorates corneal
neovascularization by regulating cell infiltration into the cornea
after alkali burn (16). TMP also
exerts therapeutic effects on sepsis-induced acute lung injury in
rats by inhibiting ER stress (18). TMP can also limit ER stress
resulting from placental gestational diabetes mellitus (19). However, to the best of our
knowledge, no studies have examined the effects of TMP for the
treatment of OA, and few studies have assessed the potential
mechanism of TMP in cartilage degeneration. To address these
issues, the current study aimed to investigate the protective
effect of TMP on ER stress, inflammation and oxidative stress in OA
chondrocytes. Moreover, the effects of TMP on ECM metabolism and
the degree of apoptosis, as well as the mechanisms underlying the
effects of IL-1β exposure in chondrocytes were evaluated.
Materials and methods
Reagents
TMP (purity >98%) and TG, a compound frequently
used to induce ER stress, were provided by Sigma-Aldrich (Merck
KGaA). A caspase 3 activity kit (cat. no. K186-100) was purchased
from BioVision, Inc. and primary antibodies [CHOP (cat. no. 2895;
1:1,000) and GRP78 (cat. no. 3183; 1:1,000)] were provided by Cell
Signaling Technology, Inc. Antibody against collagen (Col) II (cat.
no. sc-52658; 1:50) was obtained from Santa Cruz Biotechnology,
Inc. Additionally, an in-situ cell death detection kit (cat.
no. 11684817910) was provided by Roche Diagnostics. The remaining
reagents were provided by Sigma-Aldrich (Merck KGaA), except as
otherwise noted.
Cell culture and drug treatment
Each animal experiment was performed in accordance
with the guidelines of the Animal Care and Use Committee of Hainan
Medical University and was approved by the Animal Care and Use
Committee of Hainan Medical College (approval no. 2019-23).
Chondrocytes were isolated and cultured as previously described
(20). The experiments were
carried out between January 2019 and November 2020. In total, 30
3-month-old male Sprague-Dawley rats (Animal Center of the Chinese
Academy of Sciences; weight, 250-300 g) were used. All rats were
housed under specific pathogen-free laboratory conditions (18-29°C;
40-70% humidity), with free access to food and water. After animals
had been sacrificed via CO2 inhalation (40% vol/min for
5 min), their knee-joint cartilage was removed under aseptic
conditions and the tissue was cut using micro-scissors. Then, 0.25%
(w/v) trypsin-ethylenediaminetetraacetic acid was used to treat the
cartilage fragments at 37°C for 30 min, followed by treatment with
0.2% (w/v) collagenase II in serum-free DMEM (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C for 4 h. The fragments were resuspended
and filtered using a 150-µm mesh. The obtained chondrocytes were
plated into high-glucose (4.5 g/l) DMEM that was supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% antibiotics
(streptomycin/penicillin) at 5×106 cells/25
cm2 flask, then incubated at 37°C (21). Chondrocytes from passage 2
(Fig. S1) were used for cell
experiments.
Cells were incubated with TMP (100 µM) (22,23) at 37°C for 24 h in the presence or
absence of TG (10 µM) (24). The
concentrations of TMP and TG were mainly selected based on our
preliminary experiments and previous reports as aforementioned.
Western blotting assay
RIPA lysis buffer (Sigma-Aldrich; Merck KGaA)
containing protease and phosphatase inhibitors was used to
homogenize the chondrocyte culture, and a BCA protein assay kit was
used to assess the protein content. Next, 8-10% SDS-PAGE was
performed to resolve the proteins (50 µg), followed by transfer
onto nitrocellulose membranes. Membranes were then incubated in 5%
skim milk powder at room temperature for 90 min, followed by
incubation with a primary antibody overnight at 4°C and then with
secondary antibody [anti-mouse IgG (cat. no. 7076; 1:1,000; Cell
Signaling Technology, Inc.) or anti-rabbit IgG (cat. no. 7074;
1:1,000; Cell Signaling Technology, Inc.)] for 60 min at room
temperature. Finally, protein bands were detected using a western
blotting detection reagent (cat. no. 32106; Thermo Fisher
Scientific, Inc.) under an imaging system (Bio-Rad Laboratories,
Inc.). Densitometric analysis was performed using Image Lab 3.0
software (Bio-Rad Laboratories, Inc.).
Apoptosis analysis
Caspase 3 and TUNEL kits were used to detect
apoptotic activity. In brief, chondrocytes were subjected to
incubation in 4% paraformaldehyde for 1 h, 3% (v/v)
H2O2 for 15 min and 0.1% Triton X-100 for 8
min, both at room temperature. Thereafter, an in-situ cell
death detection kit was used to analyze the specimens at room
temperature, and nuclei were visualized via DAPI staining for 8 min
at room temperature. The samples were examined using a Nikon
Eclipse Ti confocal microscope (Nikon Corporation). A total of
20-30 visual fields (magnification, ×40) in each group were
analyzed to count the apoptotic cells.
Apoptotic activity was evaluated using a Caspase 3
Activity Assay kit in accordance with the manufacturer's
instructions.
Immunofluorescence assay
After drug treatment, cells were subjected to
incubation in 4% paraformaldehyde for 1 h, 0.5% Triton X-100 for 8
min and 5% BSA (Thermo Fisher Scientific, Inc.) for 45 min, all at
room temperature. Next, an anti-Col II (1:50) primary antibody was
incubated with the cells overnight at 4°C, followed by another 1 h
incubation with a secondary antibody (mouse IgG - H&L Alexa
Fluor® 488; 1:200; cat. no. ab150113; Abcam) at room
temperature. Subsequently, cells were incubated with DAPI at room
temperature for 8 min to enable visualization of nuclei. Finally, a
Nikon Eclipse Ti confocal microscope (magnification, ×40; Nikon
Corporation) was used to examine samples.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was obtained from chondrocyte samples
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.) in accordance with the manufacturer's instructions. RNA was
quantified via spectrophotometry. In total, 500 ng RNA was reverse
transcribed and amplified using the PrimeScript-RT reagent kit and
SYBR Premix Ex Taq mixture (Takara Bio, Inc.), respectively. The
following thermocycling conditions were used for the qPCR: Initial
denaturation for 10 min at 95°C, followed by 40 cycles for 15 sec
at 95°C and 1 min at 60°C. The 2−∆∆Cq method was used to
calculate expression of the target gene (25). The level of target mRNA was
normalized to the level of GAPDH. The following primer sequences
were used: COX-2 forward, 5′-CCAGAGCAGAGAGATGAAATACCA-3′ and
reverse, 5′-GCAGGGCGGGATACAGTTC-3′; inducible nitric oxide synthase
forward, 5′-GATTCAGTGGTCCAACCTGCA-3′ and reverse,
5′-CGACCTGATGTTGCCACTGTT-3′; IL-1β forward,
5′-CCCTGCAGCTGGAGAGTGTGG-3′ and reverse,
5′-TGTGCTCTGCTTGAGAGGTGCT-3′; IL-6 forward,
5′-CGAGCCCACCAGGAACGAAAGTC-3′ and reverse,
5′-CTGGCTGGAAGTCTCTTGCGGAG-3′; TNF-α forward,
5′-GAAGCCCCTCCCAGTTCTAGTTC-3′ and reverse,
5′-CACTCCCCATCCTCCCTGGTC-3′; MMP-3 forward,
5′-TCCCTGTTCAGCCATCCCTTG-3′ and reverse,
5′-TCGCTCTGGTAGCCCTTCTC-3′; MMP-13 forward,
5′-TGGTCCCTGCCCCTTCCCTA-3′ and reverse,
5′-CCGCAAGAGTCACAGGATGGTAGTA-3′; thrombospondin type 1 motif 4
(ADAMTS-4) forward, 5′-GGAATGGTGGAAAGTATTGTGAGG-3′ and reverse,
5′-GAGGTCGGTTCGGTGGTTGT-3′; thrombospondin type 1 motif 5
(ADAMTS-5) forward, 5′-CAACTTGACATTTGGGCCTGA-3′ and reverse,
5′-TCCACGGCAGGCAACTTCT-3′; and GAPDH forward,
5′-AACGGCACAGTCAAGGCTGA-3′ and reverse,
5′-ACGCCAGTAGACTCCACGACAT-3′.
Statistical analysis
Statistical analysis was performed using SPSS
version 19.0 software (IBM Corp.). The results are presented as the
mean ± SD. Differences between groups were assessed using one-way
ANOVA, followed by the Tukey's post hoc test. Each experiment was
repeated at least five times. P<0.05 was considered to indicate
a statistically significant difference.
Results
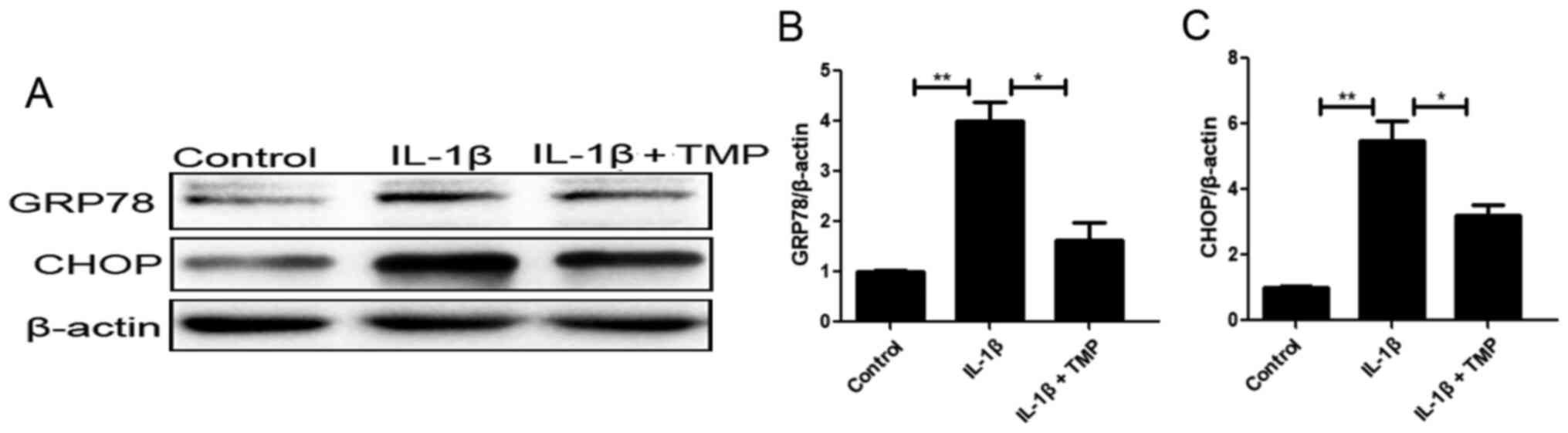
TMP alleviates ER stress and
subsequent apoptosis of IL-1β-treated chondrocytes
ER stress plays an important role in the
pathological mechanism of OA. In this process, UPR sensors separate
from the chaperone protein GRP78 and are then phosphorylated, which
contributes to activation of CHOP and subsequent apoptosis
(26,27). To determine the sensitivity of ER
stress to TMP exposure in IL-1β-exposed chondrocytes, the levels of
ER stress-related factors, including GRP78 and CHOP (downstream
apoptotic protein), were assessed. Western blot analysis revealed
that the aforementioned proteins were upregulated upon IL-1β
exposure, while TMP had the opposite effects (Fig. 1).
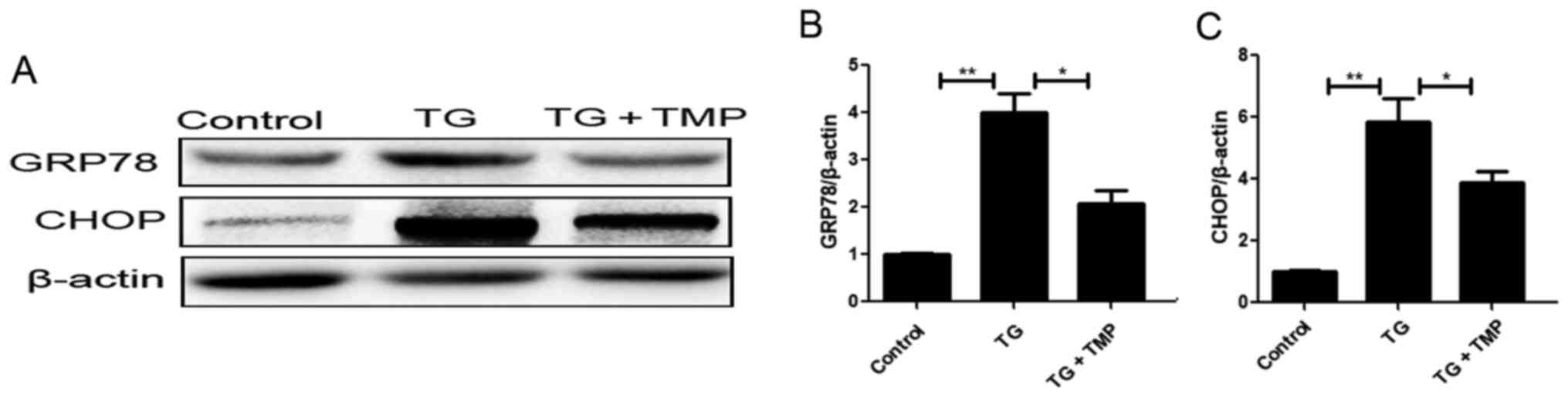
TMP inhibits TG-induced ER stress and
subsequent expression of apoptotic proteins in chondrocytes
In order to clarify whether the cytoprotective
effects of TMP were associated with its regulation of ER stress,
chondrocytes were treated with TG, a commonly used inducer of ER
stress. Western blot analysis indicated that compared with
controls, the expression levels of CHOP and GRP78 were upregulated
in TG-exposed chondrocytes. Furthermore, TMP treatment partly
abolished this upregulated CHOP and GRP78 expression (Fig. 2A-C). Collectively, these findings
indicated that TMP markedly attenuated ER stress level in OA
chondrocytes.
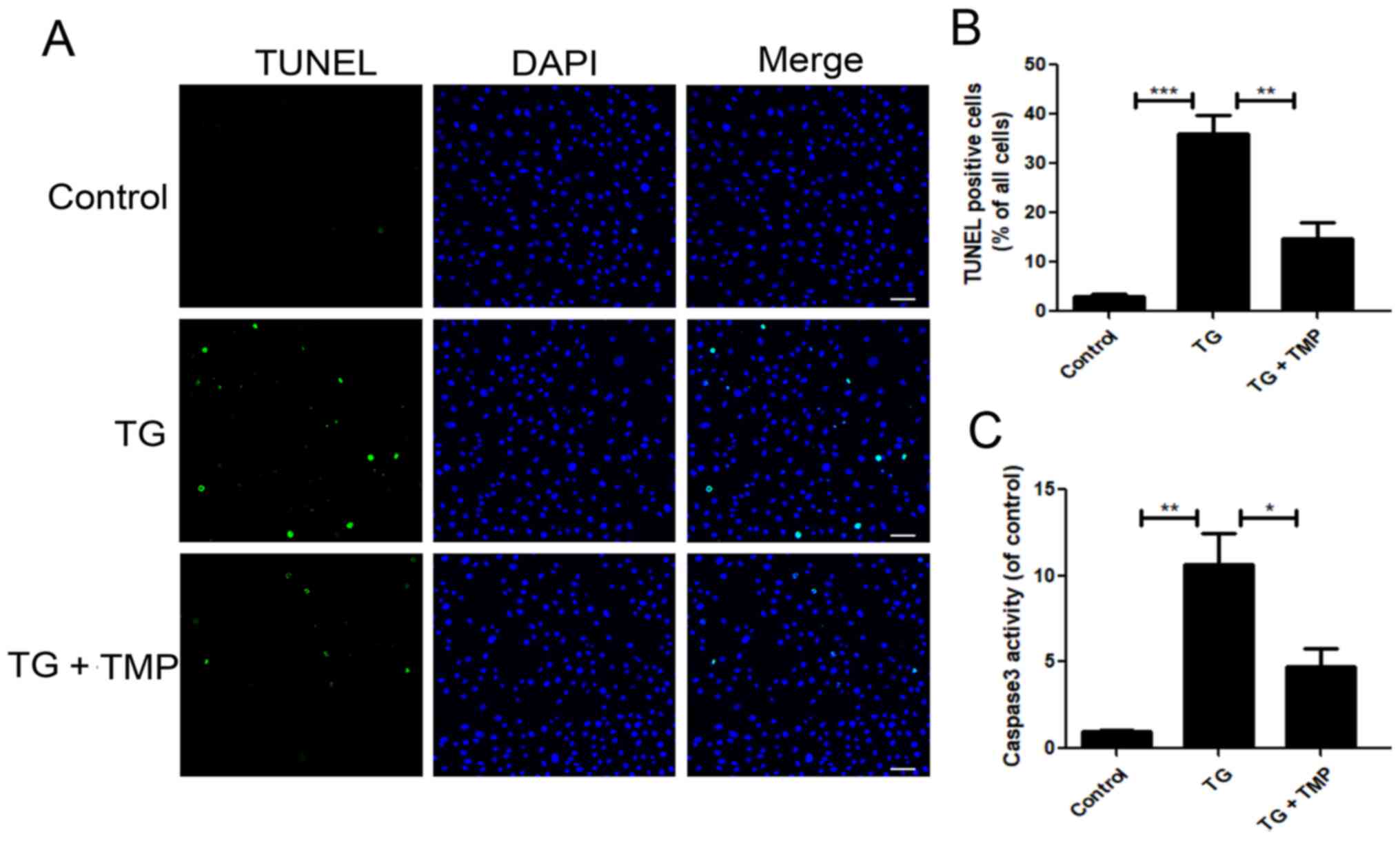
TMP inhibits TG-induced apoptosis in
vitro
To further investigate the relationship between ER
stress and the role of TMP in resisting apoptosis in TG-treated
chondrocytes, a caspase 3 activity assay and TUNEL staining of
chondrocytes were conducted. TUNEL staining identified that TMP
significantly reduced the number of apoptotic cells among
TG-treated chondrocytes (Fig. 3A and
B). The results of the caspase 3 activity assay were consistent
with the suppression of TG-mediated apoptosis upon TMP treatment
(Fig. 3C). Collectively, these
findings indicated that the anti-apoptotic effects of TMP were
mediated by the suppression of ER stress.
TMP suppresses the mRNA expression
levels of inflammatory cytokines induced by TG in chondrocytes
Next, RT-qPCR analysis was conducted to examine the
mRNA expression levels of inflammatory factors (including inducible
nitric oxide synthase, IL-6, IL-1β, cyclooxygenase-2 and TNF-α).
Upregulated expression of inflammatory factors (IL-6, IL-1β, iNOS,
COX-2 and TNF-α) were observed in TG-exposed chondrocytes.
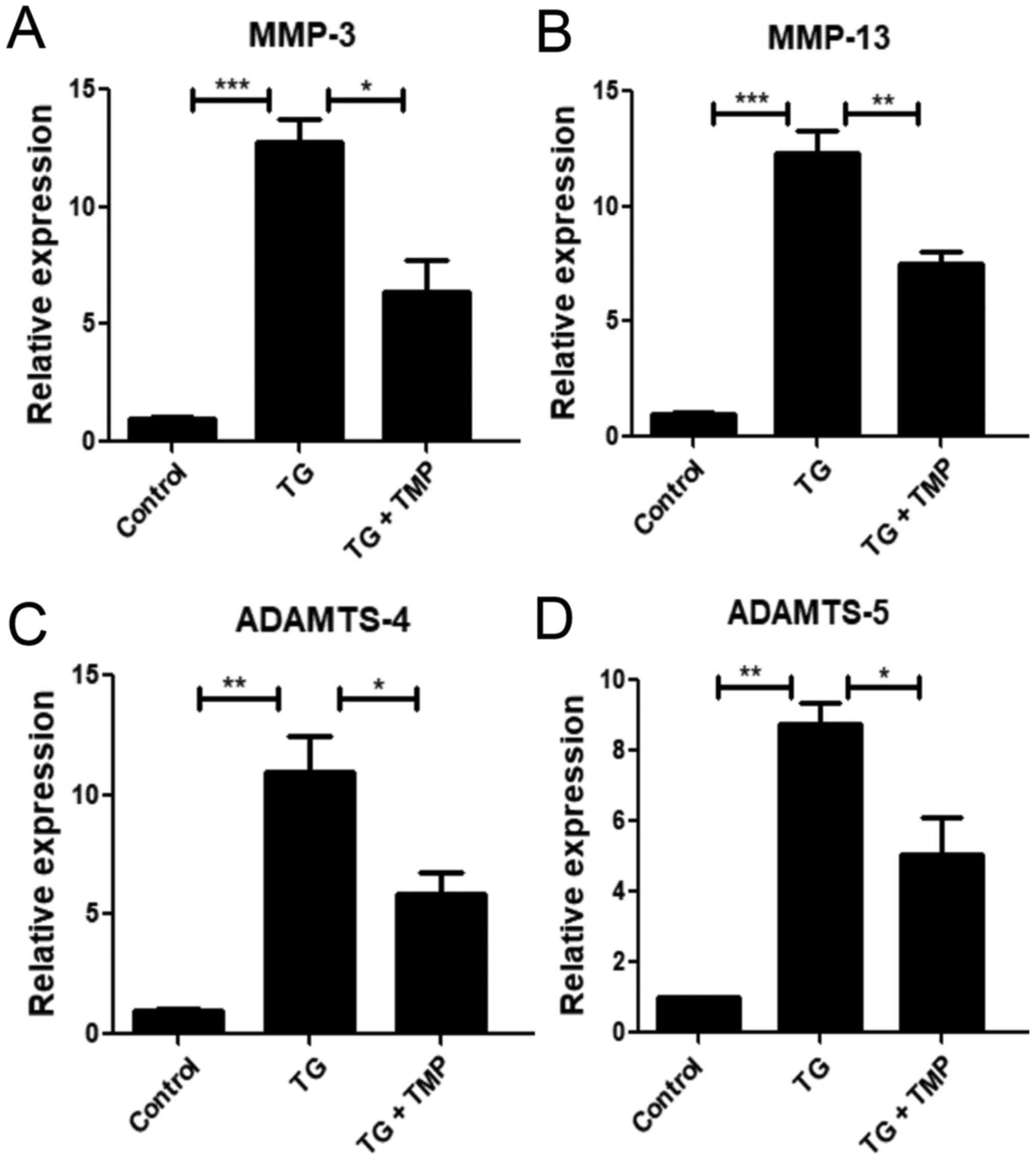
TMP suppresses TG-activated catabolic
activity in chondrocytes
Catabolic enzymes, including MMP-3 and −13, as well
as ADAM metallopeptidase with thrombospondin type 1 motif 4
(ADAMTS-4) and −5, are closely associated with ECM decomposition in
chondrocytes (5). As shown in
Fig. 5, the expression levels of
ADAMTS-4 and −5 were markedly increased in the TG group, and this
pattern was reversed by TMP. Similarly, TMP significantly decreased
the enhanced expression levels of MMP-3 and −13 in the TG group.
These findings suggested that TMP exposure suppressed TG-mediated
chondrocyte catabolism.
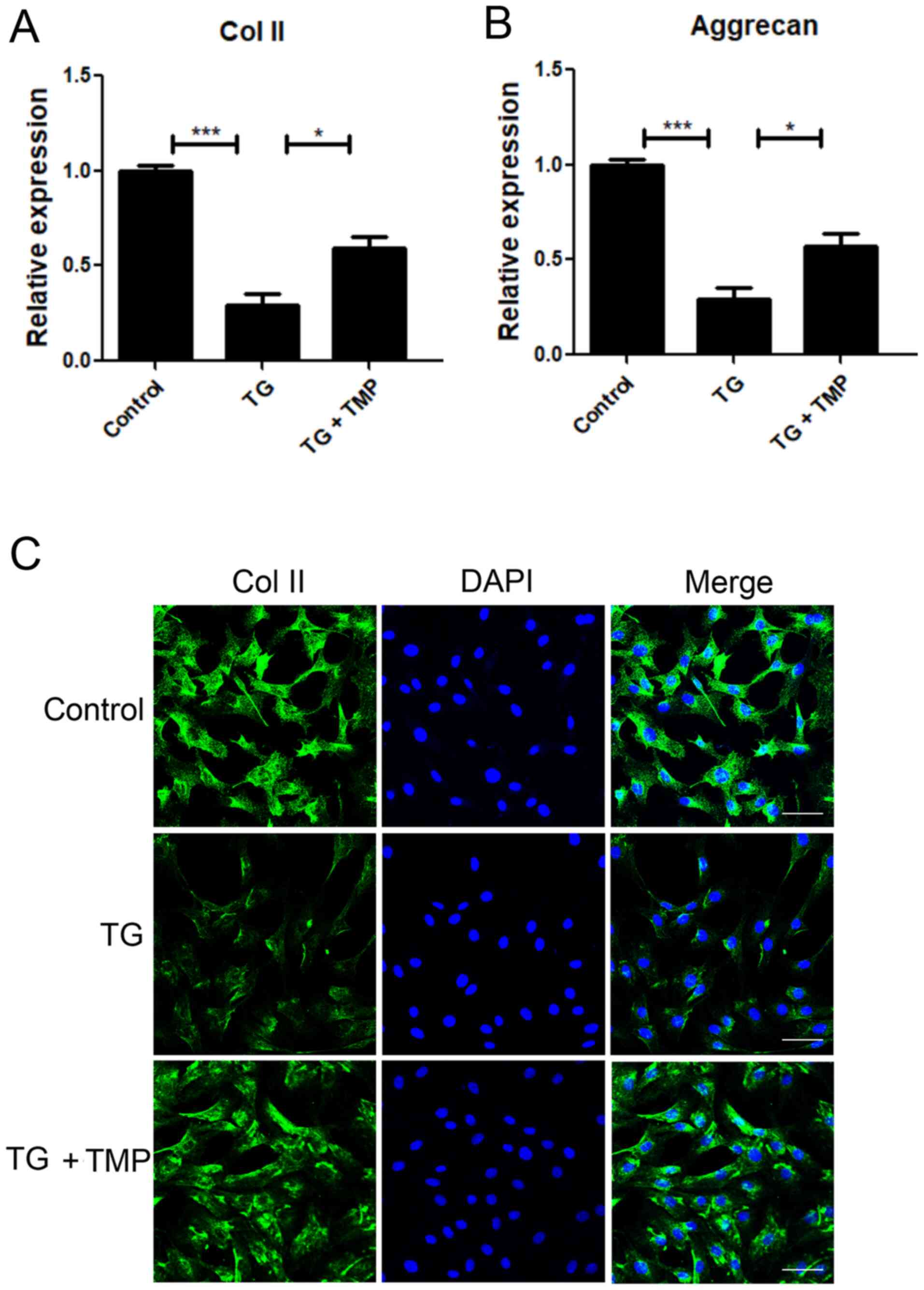
TMP suppresses TG-activated ECM
degradation in chondrocytes
Immunofluorescence and PCR assays were performed to
measure ECM degradation-associated mRNAs and proteins. The
expression levels of aggrecan and Col II, the two main ECM
components, were significantly downregulated after TG exposure
(Fig. 6A and B). Nonetheless,
TMP-exposed chondrocytes exhibited increased mRNA expression levels
of aggrecan and Col II, compared with TG-exposed cells.
Immunofluorescence staining for Col II showed results similar to
those of PCR analysis (Fig. 6C).
These findings indicated that TMP exposure protected ECM proteins
within TG-treated nucleus pulposus cells.
Discussion
Cartilage contains chondrocytes and ECM, which
comprise a small portion of the overall tissue volume. However,
chondrocytes have been implicated in the generation and maintenance
of ECM proteins (e.g., Cols II, IX and XI), proteoglycans (i.e.,
aggrecan) and glycoproteins (5).
Mutations of genes that encode cartilage components can trigger
skeletal dysplasia and potentially impair the production of
cartilage components, leading to the accumulation of protein
aggregates within the ER. Cartilage damage is induced by mutant
variants in the ER, rather than a lack of certain ECM proteins
(15). Such mutated ECM
components can aggregate and destroy chondrocyte homeostasis, by
means of ER stress, thereby promoting the pathogenic mechanisms of
chondrodysplasias (28). In the
ColIITgcog mouse model, the expression of thyroglobulin (the
mutated thyroid protein) within chondrocytes indicates that ER
stress can sufficiently induce a short stature disorder, similar to
chondrodysplasia (29).
Irrespective of the presence or absence of mutated thyroglobulin,
ER stress is aggravated by destabilization of the medial
meniscus-mediated OA induction within chondrocytes. Typically,
susceptibility to ER stress in the ColIITgcog mouse model has a
beneficial effect. Specifically, it can mitigate OA due to the
effective management of ER stress within articular chondrocytes
(30).
Certain stress sensors can recognize misfolded
proteins, thus initiating the UPR. For this reason, all cells
contain the necessary components, such as activating transcription
factor 4, activating transcription factor 6α, GRP78, protein kinase
RNA-like ER kinase, inositol-required enzyme 1 α, CHOP and X-box
binding protein 1 (31). When
CHOP transcription is initiated, apoptosis is induced in response
to severe ER stress, thus activating death effectors, such as Bim
and Bcl-2 (32,33). Reversal of
Ca2+-activated K+ channel suppression by
inhibition of ER stress-induced β1 subunit loss can protect TMP
from the impact of homocysteine on coronary dilation (34). In a gestational diabetes mellitus
mouse model, TMP can mitigate placental oxidative stress,
inflammation and ER stress (35).
Furthermore, TMP alleviates sepsis-triggered acute lung injury by
inhibiting the apoptosis of pulmonary microvascular endothelial
cells via the protein kinase RNA-like ER kinase/eIF2a/activating
transcription factor 4/CHOP apoptosis signal transduction pathway
during ER stress (18). The
results of the present study indicated that TMP has a protective
effect on chondrocytes, a possible relationship with ER stress and
a role in apoptosis signal transduction. The current results
demonstrated that TMP treatment decreased CHOP and GRP78 expression
levels in cells exposed to IL-1β, suggesting a possible mechanism
for TMP signaling. To optimize the investigation into the function
of ER stress in OA, the present study used TG, which is known to
induce ER stress in in vitro models (24). It was found that TMP exposure
markedly decreased TG-triggered ER stress within chondrocytes.
Moreover, exposure to TMP partly abolished TG-induced chondrocyte
apoptosis. These results indicated that ER stress was closely
associated with the protection of TMP in chondrocytes.
The aggravation of OA over time may be attributed to
decreased ER folding- and UPR-associated protein activities and
expression (36). Older adults
are more likely to have ER stress at the ECM protein synthesis
stage, which can resemble the initial stage of OA (37). The present study found that
TG-exposed chondrocytes showed characteristics of cartilage
degeneration, such as downregulation of ECM proteins (aggrecan and
Col II) and upregulation of degrading enzymes (MMP-3 and −13, and
ADAMTS-4 and −5) secreted by chondrocytes, although TMP partly
abolished these effects.
Inflammation contributes to the variability in OA
characteristics, and may be associated with both joint symptoms and
OA deterioration (38,39). The upregulation of
matrix-degrading enzymes and pro-inflammatory factors can destroy
cartilage and enhance chondrocyte senescence, thereby promoting OA
deterioration (40). The
upregulation of inflammatory cytokines (e.g., TNF-α and IL-1β) can
increase the expression levels of catabolic proteins, such as MMPs
and ADAMTS, thereby accelerating the progressive losses of
proteoglycans and Cols (41,42). IL-1β is widely applied in studies
of the pathophysiology of OA to trigger the release of inflammatory
cytokines, as well as chondrocyte apoptosis (6). Regulation of the levels of several
catabolic enzymes (MMPs or ADAMTS) is achieved by transcription
factors, especially NF-κB (7,8).
In an OA rat model, p65 knockdown suppressed disease
deterioration at the early stage (43). TMP mitigates endotoxin-mediated
retinal inflammation by suppressing the activation of microglial
cells via the Toll-like receptor 4/NF-κB signal transduction
pathway (44). TMP also decreases
inflammation in liver fibrosis, while suppressing the production of
inflammatory cytokines within hepatic stellate cells via regulation
of the NLR family pyrin domain containing 3 inflammasome pathway
(45). The current findings
indicated that the mechanisms underlying the effects of TMP on
TG-exposed chondrocytes included the inhibition of inflammatory
mediators. The TG-exposed chondrocytes showed inflammatory
characteristics, while TMP markedly reversed these effects.
However, the present study was not without its
limitations. First, no further animal experiments were conducted on
the effect of TMP on OA, and should be performed in further
studies. Second, this work did not evaluate the effect of TMP on
the upstream pathway related to ER stress. Further research should
be performed to reveal the pharmacological mechanisms of TMP in
OA.
In conclusion, the present study demonstrated that
TMP enhanced cell viability, reduced inflammatory cytokine
production and suppressed apoptosis. Such protection was associated
with TMP-mediated ER stress regulation within chondrocytes. Further
investigations are required to determine whether TMP has potential
as a therapeutic agent for the prevention of cartilage destruction
in patients with OA.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH and YB designed the study. JH and SH contributed
to statistical analysis, data interpretation and manuscript
preparation. SW and SH performed the experiments and
interpretation. SH and YB confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Hainan Medical College.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goldring MB: Chondrogenesis, chondrocyte
differentiation, and articular cartilage metabolism in health and
osteoarthritis. Ther Adv Musculoskelet Dis. 4:269–285. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suri S and Walsh DA: Osteochondral
alterations in osteoarthritis. Bone. 51:204–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sui C, Zhang L and Hu Y: MicroRNA-let-7a
inhibition inhibits LPS-induced inflammatory injury of chondrocytes
by targeting IL6R. Mol Med Rep. 20:2633–2640. 2019.PubMed/NCBI
|
|
4
|
Liu-Bryan R and Terkeltaub R: Emerging
regulators of the inflammatory process in osteoarthritis. Nat Rev
Rheumatol. 11:35–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bondeson J, Wainwright S, Hughes C and
Caterson B: The regulation of the ADAMTS4 and ADAMTS5 aggrecanases
in osteoarthritis: A review. Clin Exp Rheumatol. 26:139–145.
2008.PubMed/NCBI
|
|
6
|
Lefebvre V, Peeters-Joris C and Vaes G:
Modulation by interleukin 1 and tumor necrosis factor alpha of
production of collagenase, tissue inhibitor of metalloproteinases
and collagen types in differentiated and dedifferentiated articular
chondrocytes. Biochim Biophys Acta. 1052:366–378. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang S, Tang Q, Jin J, Zheng G, Xu J,
Huang W, Li X, Shang P and Liu H: Polydatin inhibits the
IL-1β-induced inflammatory response in human osteoarthritic
chondrocytes by activating the Nrf2 signaling pathway and
ameliorates murine osteoarthritis. Food Funct. 9:1701–1712. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng Z, Li X, Lin J, Zheng W, Hu Z, Xuan
J, Ni W and Pan X: Oleuropein inhibits the IL-1β-induced expression
of inflammatory mediators by suppressing the activation of NF-κB
and MAPKs in human osteoarthritis chondrocytes. Food Funct.
8:3737–3744. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharif M, Whitehouse A, Sharman P, Perry M
and Adams M: Increased apoptosis in human osteoarthritic cartilage
corresponds to reduced cell density and expression of caspase-3.
Arthritis Rheum. 50:507–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou Y, Liu Q, Guo P, Huang Y, Ye Z and Hu
J: Anti-chondrocyte apoptosis effect of genistein in treating
inflammation-induced osteoarthritis. Mol Med Rep. 22:2032–2042.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cláudio N, Dalet A, Gatti E and Pierre P:
Mapping the crossroads of immune activation and cellular stress
response pathways. EMBO J. 32:1214–1224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wictome M, Henderson I, Lee AG and East
JM: Mechanism of inhibition of the calcium pump of sarcoplasmic
reticulum by thapsigargin. Biochem J. 283:525–529. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bernales S, Papa FR and Walter P:
Intracellular signaling by the unfolded protein response. Annu Rev
Cell Dev Biol. 22:487–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nugent AE, Speicher DM, Gradisar I,
McBurney DL, Baraga A, Doane KJ and Horton WE Jr: Advanced
osteoarthritis in humans is associated with altered collagen VI
expression and upregulation of ER-stress markers Grp78 and bag-1. J
Histochem Cytochem. 57:923–931. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Xu Z, Yang Y, Qiu J, Yang M, Wu C,
Lai Z, Tang M, Ge J, Yu K, et al: Tetramethylpyrazine (TMP)
ameliorates corneal neovascularization via regulating cell
infiltration into cornea after alkali burn. Biomed Pharmacother.
109:1041–1051. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao Y, Liu Y and Chen K: Mechanisms and
clinical application of tetramethylpyrazine (an interesting natural
compound isolated from Ligusticum wallichii): Current status
and perspective. Oxid Med Cell Longev. 2016:21246382016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu W, Liu K, Zhang S, Shan L and Tang J:
Tetramethylpyrazine showed therapeutic effects on sepsis-induced
acute lung injury in rats by inhibiting endoplasmic reticulum
stress protein kinase RNA-like endoplasmic reticulum kinase (PERK)
signaling-induced apoptosis of pulmonary microvascular endothelial
cells. Med Sci Monit. 24:1225–1231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yung HW, Alnæs-Katjavivi P, Jones CJ,
El-Bacha T, Golic M, Staff AC and Burton GJ: Placental endoplasmic
reticulum stress in gestational diabetes: The potential for
therapeutic intervention with chemical chaperones and antioxidants.
Diabetologia. 59:2240–2250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang LB, Lee S, Wang Y, Xu QT, Meng DH
and Zhang J: Adipose-derived stem cells induce autophagic
activation and inhibit catabolic response to pro-inflammatory
cytokines in rat chondrocytes. Osteoarthritis Cartilage.
24:1071–1081. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue EX, Lin JP, Zhang Y, Sheng SR, Liu HX,
Zhou YL and Xu H: Pterostilbene inhibits inflammation and ROS
production in chondrocytes by activating Nrf2 pathway. Oncotarget.
8:41988–42000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muralidharan P, Acosta MF, Gomez AI,
Grijalva C, Tang H, Yuan JXJ and Mansour HM: Design and
comprehensive characterization of tetramethylpyrazine (TMP) for
targeted lung delivery as inhalation aerosols in pulmonary
hypertension (PH): In vitro human lung cell culture and in vivo
efficacy. Antioxidants (Basel). 10:4272021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang Y, Chu L, Li L, Wang J, Yang Y, Gu J
and Zhang J: Tetramethylpyrazine protects bone marrow-derived
mesenchymal stem cells against hydrogen peroxide-induced apoptosis
through PI3K/Akt and ERK1/2 pathways. Biol Pharm Bull.
40:2146–2152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu D, Gu Y, Wang W and Chen W: Astragalin
alleviates ischemia/reperfusion-induced brain injury via
suppression of endoplasmic reticulum stress. Mol Med Rep.
22:4070–4078. 2020.PubMed/NCBI
|
|
25
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng K, Ge Y, Chen Z, Li X, Liu Z, Li X,
Li H, Tang T, Yang F and Wang X: Curcumin inhibits the
PERK-eIF2α-CHOP pathway through promoting SIRT1 expression in
oxidative stress-induced rat chondrocytes and ameliorates
osteoarthritis progression in a rat model. Oxid Med Cell Longev.
2019:85743862019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Husa M, Petursson F, Lotz M, Terkeltaub R
and Liu-Bryan R: C/EBP homologous protein drives pro-catabolic
responses in chondrocytes. Arthritis Res Ther. 15:R2182013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kung LH, Rajpar MH, Briggs MD and
Boot-Handford RP: Hypertrophic chondrocytes have a limited capacity
to cope with increases in endoplasmic reticulum stress without
triggering the unfolded protein response. J Histochem Cytochem.
60:734–748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rajpar MH, McDermott B, Kung L, Eardley R,
Knowles L, Heeran M, Thornton DJ, Wilson R, Bateman JF, Poulsom R,
et al: Targeted induction of endoplasmic reticulum stress induces
cartilage pathology. PLoS Genet. 5:e10006912009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kung LHW, Mullan L, Soul J, Wang P, Mori
K, Bateman JF, Briggs MD and Boot-Handford RP: Cartilage
endoplasmic reticulum stress may influence the onset but not the
progression of experimental osteoarthritis. Arthritis Res Ther.
21:2062019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hetz C, Zhang K and Kaufman RJ:
Mechanisms, regulation and functions of the unfolded protein
response. Nat Rev Mol Cell Biol. 21:421–438. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oyadomari S, Koizumi A, Takeda K, Gotoh T,
Akira S, Araki E and Mori M: Targeted disruption of the Chop gene
delays endoplasmic reticulum stress-mediated diabetes. J Clin
Invest. 109:525–532. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Puthalakath H, O'Reilly LA, Gunn P, Lee L,
Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin
J, Motoyama N, et al: ER stress triggers apoptosis by activating
BH3-only protein Bim. Cell. 129:1337–1349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun WT, Wang XC, Novakovic A, Wang J, He
GW and Yang Q: Protection of dilator function of coronary arteries
from homocysteine by tetramethylpyrazine: Role of ER stress in
modulation of BKCa channels. Vascul Pharmacol. 113:27–37. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiao Y, Zhang S, Zhang J and Du J:
Tetramethylpyrazine attenuates placental oxidative stress,
inflammatory responses and endoplasmic reticulum stress in a mouse
model of gestational diabetes mellitus. Arch Pharm Res.
42:1092–1100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brown MK and Naidoo N: The endoplasmic
reticulum stress response in aging and age-related diseases. Front
Physiol. 3:2632012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Venn G, Billingham ME and Hardingham TE:
Increased proteoglycan synthesis in cartilage in experimental
canine osteoarthritis does not reflect a permanent change in
chondrocyte phenotype. Arthritis Rheum. 38:525–532. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng G, Zhan Y, Tang Q, Chen T, Zheng F,
Wang H, Wang J, Wu D, Li X, Zhou Y, et al: Monascin inhibits IL-1β
induced catabolism in mouse chondrocytes and ameliorates murine
osteoarthritis. Food Funct. 9:1454–1464. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jia J, Sun J, Liao W, Qin L, Su K, He Y,
Zhang J, Yang R, Zhang Z and Sun Y: Knockdown of long non-coding
RNA AK094629 attenuates the interleukin-1β induced expression of
interleukin-6 in synovium-derived mesenchymal stem cells from the
temporomandibular joint. Mol Med Rep. 22:1195–1204. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Greene MA and Loeser RF: Aging-related
inflammation in osteoarthritis. Osteoarthritis Cartilage.
23:1966–1971. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Irie K, Uchiyama E and Iwaso H:
Intraarticular inflammatory cytokines in acute anterior cruciate
ligament injured knee. Knee. 10:93–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Speziali A, Delcogliano M, Tei M, Placella
G, Chillemi M, Tiribuzi R and Cerulli G: Chondropenia: Current
concept review. Musculoskelet Surg. 99:189–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Csaki C, Mobasheri A and Shakibaei M:
Synergistic chondroprotective effects of curcumin and resveratrol
in human articular chondrocytes: Inhibition of IL-1beta-induced
NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther.
11:R1652009. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu PF, Sun FF, Jiang LF, Bao JP and Wu LD:
Paeoniflorin inhibits IL-1β-induced MMP secretion via the NF-κB
pathway in chondrocytes. Exp Ther Med. 16:1513–1519.
2018.PubMed/NCBI
|
|
45
|
Zhuang Z, Ye G and Huang B: Kaempferol
alleviates the interleukin-1β-induced inflammation in rat
osteoarthritis chondrocytes via suppression of NF-κB. Med Sci
Monit. 23:3925–3931. 2017. View Article : Google Scholar : PubMed/NCBI
|