Introduction
Oral squamous cell carcinoma (OSCC) is the most
common subset of head and neck squamous cell carcinoma (HNSCC)
(1). Clinical treatment of OSCC
includes surgery, radiotherapy and chemotherapy (2). Multiple treatment strategies have
improved the clinical outcome for patients with OSCC, but the
overall 5-year survival rate after diagnosis is still <50%
(3). As with other solid tumors,
5-fluorouracil (5-FU) is one of the most effective and commonly
used drugs for OSCC (4), but its
clinical effectiveness is often limited due to acquired resistance
with continuous drug administration (5). Therefore, identifying an effective
therapeutic drug to improve the prognosis of patients with OSCC is
important.
Propofol (2, 6-diisopropylphenol) is a commonly used
intravenous sedative-hypnotic drug that allows for smooth induction
and a rapid recovery from anesthesia (6). Propofol also displays a number of
non-anesthetic effects, including antitumor activities (7,8).
In a variety of human cancer cells, it has been reported that
propofol triggers cell apoptosis and inhibits proliferation,
migration and invasion (9,10).
Certain retrospective studies and prospective trials have reported
that propofol treatment improves the survival outcomes for patients
with certain types of cancer after tumor resection (11–16). However, the role of propofol in
the treatment of OSCC is not completely understood.
Secreted substances are responsible for
communication between cells and may promote tumor progression
(17). Increasing evidence
demonstrates that the microenvironment is the primary driver for
cancer growth, motility and therapy resistance (18–20). Secretions from cells can also
enter the blood system and serve as serum tumor markers. A recent
study showed that for patients undergoing radical resection for
non-small cell lung cancer (NSCLC), propofol reduced postoperative
serum tumor angiogenesis-related factors, such as VEGF and TGF-β
(21), thus propofol may affect
the tumor microenvironment and serve a pivotal role in the
development and progression of cancer.
Antibody arrays are extensively utilized in cancer
research to identify candidate biomarkers and explore signaling
pathways, which benefits diagnosis, prognosis and treatment
(22). Numerous biological
websites and software can be used for bioinformatics analysis,
including gene expression profile interactive analysis (GEPIA)
(23), which has been
successfully applied in numerous cancer studies. The present study
investigated the effect of propofol on OSCC cytotoxicity and the
secreted protein profile using an antibody array. Bioinformatics
tools are used to evaluate the clinical importance of these altered
secreted proteins.
Materials and methods
Chemicals and reagents
Propofol (purity >98%) was obtained from The
United States Pharmacopeial Convention. 5-FU was purchased from
Sigma-Aldrich (Merck KGaA). Propofol and 5-FU were dissolved in
DMSO (Amresco, LLC) and stored at 4°C before use. Recombinant AREG
(rAREG) was purchased from R&D Systems, Inc.
Cell culture
Human OSCC cell lines (SAS and SCC9) were cultured
in DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin, and maintained at 37°C in a humidified
atmosphere of 5% CO2.
Cell cytotoxicity
The cytotoxic activity of propofol on OSCC cells was
assessed using an MTT assay. Cells (5×103/well) in 100
µl medium were plated in each well of a 96-well plate overnight.
Subsequently, cells were treated with the indicated concentrations
of propofol (0, 5, 10, 20, 40, 80 and 160 µM) or 5-FU (0–160 µM)
for the indicated interval (24, 48 or 72 h). Then, MTT reagent (5
mg/ml in PBS) was added to each well and incubated for 4 h. The
supernatant was removed and 100 µl DMSO was added to each well to
dissolve the formazan crystals. The absorbance was measured at a
wavelength of 550 nm using a microplate reader, and the background
value at a wavelength of 750 nm was subtracted. Cell viability is
presented as the percentage relative to untreated cells.
Detection of the apoptotic rate by
flow cytometry
Cells (1.5×105 cells/well) were seeded
into 6 well plates and cultured overnight. After treatment with 160
µM propofol for 48 h at 37°C, cells were collected and washed with
cold PBS. The Annexin V FITC/PI Apoptosis Detection kit (BD
Biosciences) was used to detect cell apoptosis. After staining at
25°C in the dark for 15 min, the apoptotic rate was determined by
BD FACSCanto™ II flow cytometer (BD Biosciences). Data acquisition
was performed using FACSDiva software version 6.1 (BD Biosciences).
The apoptotic rate (%) was calculated as the sum of
Annexin-V-FITC+/PI− (early apoptosis; Q4) and
Annexin-V-FITC+/PI+ (late apoptosis; Q4)
cells.
Western blotting
Total protein was isolated from cells using RIPA
buffer supplemented with a protease inhibitor cocktail. A Pierce
BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.) was used to
determine protein concentrations. Equal amounts of total protein
(40 µg/lane) were separated by SDS-PAGE (8–15% gel) and then
transferred to PVDF membranes (Millipore). After blocking with 5%
skimmed milk in TBS at room temperature for 1 h, the membranes were
incubated with specific primary antibodies overnight at 4°C. The
following primary antibodies were used: Cleaved PARP (1:2,000; cat.
no. ab32064; Abcam), α-tubulin (1:10,000; cat. no. 05–829; EMD
Millipore), AREG (1:200; cat. no. sc-74501; Santa Cruz
Biotechnology, Inc.), phosphorylated (p)-EGFR (1:1,000; cat. no.
6963S; Cell Signaling Technology, Inc.), and EGFR (1: 200; cat. no.
sc-53274; Santa Cruz Biotechnology, Inc.). After several washes
with TBST (0.05% Tween-20), the membranes were then incubated for 1
h with appropriate HRP-conjugated goat anti-rabbit IgG (1:5,000;
cat. no. 20202; Leadgene Biomedical, Inc.) and goat anti-mouse IgG
(1:5,000; cat. no. 115-035-003; Jackson ImmunoResearch
Laboratories, Inc.) secondary antibodies. The membranes were then
washed with TBST and protein bands were visualized using ECL
reagents (Merck KGaA) and autoradiography. The intensity of signals
was recorded using UN-SCAN-IT gel 6.1 software (Silk Scientific,
Inc.).
Preparation of conditioned media
(CM)
Cells were cultured in DMEM until they reached 70%
confluence. The adherent cells were washed three times with PBS to
remove FBS, and then cultured in serum-free DMEM for 48 h. The
culture media was collected, centrifuged at 500 × g at room
temperature for 15 min and then carefully aspirated to collect the
CM. Amicon® Ultra (Merck KGaA) with a 3 kDa cut-off
value was used to increase the protein concentration by
centrifugation at 2,000 × g at 4°C for 3 h. The resultant CM was
stored at −80°C until further use. An equal amount of conditioned
culture media (40 µg) was analyzed by 15% SDS-PAGE and transferred
to PVDF membrane, after which the membrane was stained with Ponceau
S at room temperature for 5 min as a loading control.
Human growth factor antibody
array
An Human Growth Factor Antibody Array Membrane (41
Targets; cat. no. ab134002; Abcam) containing 41 targets was used
to determine changes in secreted proteins after propofol treatment.
The membrane was blocked by incubating at room temperature for 30
min with 1X blocking buffer (provided in the kit). The array
membrane was incubated with 200 µg concentrated CM sample overnight
at 4°C. After washing (reagent provided in the kit), the membrane
was incubated with the primary antibody cocktail for overnight at
4°C, washed and then incubated with the secondary antibody for 2 h
at room temperature. Subsequently, bound antibodies were visualized
using ECL reagents. The primary antibody cocktail, secondary
antibody and ECL reagents were all provided as part of the array
kit. The relative intensity of the signal was calculated using
UN-SCAN-IT gel 6.1 software (Silk Scientific, Inc.). The background
(negative control) was subtracted from the intensity of the dot
signal and normalized using the average value for the positive
control spots.
GEPIA analysis
GEPIA is an online web server that uses The Cancer
Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) data. The
TCGA included the RNA-Seq data of 9,736 tumor samples with 33
cancer types and 726 adjacent normal tissues. GTEx included the
RNA-Seq data of >8,000 normal samples. The datasets were both
stored in a MySQL relational database (version 5.7.17) (23). The present study used GEPIA to
determine the clinical importance of the candidate genes identified
by the antibody array. The gene name was set as ‘AREG’ and ‘HNSCC’
datasets were used for the analysis. The expression data were the
first log2 (TPM+1) transformed data that were used for differential
analysis. The log2FC was defined as median (tumor) - median
(normal). The |Log2FC| cutoff was 1. The cutoff value for P was
0.05. Log2 (TPM+1) where TPM = Transcripts Per Million was used for
the log-scale. Whisker plots show the relative RNA expression for
candidate genes between HNSCC tissues (n=519) and normal tissues
(n=44). GEPIA was also used to determine the relationship between
the expression level for candidate genes and overall survival (OS)
for patients with HNSCC.
Reverse transcription-quantitative PCR
(qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.). RNA
was reverse transcribed into cDNA using the PrimeScript RT Reagent
kit (Takara Bio, Inc.) according to the manufacturer's protocol.
Subsequently, qPCR was performed using Kapa SYB® FAST
qPCR Master Mix (Takara Bio, Inc.) and the MyGo PCR detection
system (IT-IS Life Science Ltd.). The thermocycling conditions were
as follows: Initial denaturation at 95°C for 180 sec, followed by
40 cycles at 95°C for 10 sec, 60°C for 20 sec and 72°C for 30 sec.
The following primers were used for qPCR: AREG forward,
5′-ACCTACTCTGGGAAGCGTGA-3′ and reverse, 5′-GGACTTTTCCCCACACCGTT-3′;
and GAPDH forward, 5′-CACCCATGGCAAATTCCATGGCA-3′ and reverse,
5′-TCTAGACGGCAGGTCAGGTCCACC-3′. AREG mRNA expression was quantified
using the 2−∆∆Cq method and normalized to the internal
reference gene GAPDH (24).
Human EGFR phosphorylation antibody
array
Cells were pre-starved in serum-free DMEM for 4 h,
and then stimulated for 15 min with serum-free DMEM containing
human rAREG protein (50 ng/ml; R&D Systems, Inc.) at 37°C. A
Human EGFR Phosphorylation Antibody Array-Membrane (17 Targets;
cat. no. ab134005; Abcam) was used to determine changes in EGFR
activation after rAREG treatment. The membrane was blocked by
incubating at room temperature for 1 h with 1X blocking buffer
(provided in the kit). The array membrane was then incubated with
cell lysates (200 µg/ml) overnight at 4°C. After washing (reagent
provided in the kit), the membrane was incubated with a
biotin-conjugated anti-EGFR cocktail (reagent provided in the kit)
overnight at 4°C, washed (reagent provided in the kit) and
subsequently incubated with the HRP-conjugated streptavidin for 2 h
at room temperature. The signals are visualized using ECL reagents
(provided in the kit) and autoradiography. In addition, SAS and
SCC9 cells were treated with rAREG (50–100 ng/ml) and analyzed by
western blot to verify the activation of EGFR.
Establishment of 5-FU-resistant (FUR)
sublines
To determine the mechanism underlying 5-FU
resistance, SCC9 cells were cultured in the completed medium with
gradually increasing concentrations of 5-FU from 0.5 to 160 µM.
After ~6 months, cells survived in three different concentrations
of 5-FU (40, 80 and 160 µM). Cells with different levels of
5-FU-resistance were named SCC9-FUR40, SCC9-FUR80 and SCC9-FUR160
cells, respectively.
AREG overexpression
SCC9 cells were seeded at a density of
3×105 cells/6 cm culture dish, after which cells were
cultured in completed medium overnight at 37°C with 5%
CO2. SCC9 cells were transfected with AREG
overexpression plasmid (1 µg; pCMV3-AREG) or empty vector (1 µg;
pCMV3) using TurboFect transfection reagent (Thermo Fisher
Scientific, Inc.). Following overnight incubation at 37°C, the
medium was replaced with fresh complete medium, and the cells
received a second incubation of 24 h at 37°C. All plasmids were
purchased from Sino Biological Inc. The amino acid sequence of AREG
overexpression was as follows:
MRAPLLPPAPVVLSLLILGSGHYAAGLDLNDTYSGKREPFSGDHSADGFEVTSRSEMSSGSEISPVSEMPSSSEPSSGADYDYSEEYDNEPQIPGYIVDDSVRVEQVVKPPQNKTESENTSDKPKRKKKGGKNGKNRRNRKKKNPCNAEFQNFCIHGECKYIEHLEAVTCKCQQEYFGERCGEKSMKTHSMIDSSLSKIALAAIAAFMSAVILTAVAVITVQLRRQYVRKYEGEAEERKKLRQENGNVHAIA.
After transfection, cells were cultured for 48 h and then harvested
to determine the transfection efficiency via western blotting. The
effect of AREG overexpression on 5-FU sensitivity was also
determined using a MTT assay.
Statistical analysis
Data are presented as the mean ± SEM of at least
three independent experiments. Statistical analyses were performed
using GraphPad Prism software (version 6.0; GraphPad Software,
Inc.). Comparisons between two groups were analyzed using the
unpaired Student's t-test. Comparisons among multiple groups were
analyzed using one-way ANOVA followed by Bonferroni's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
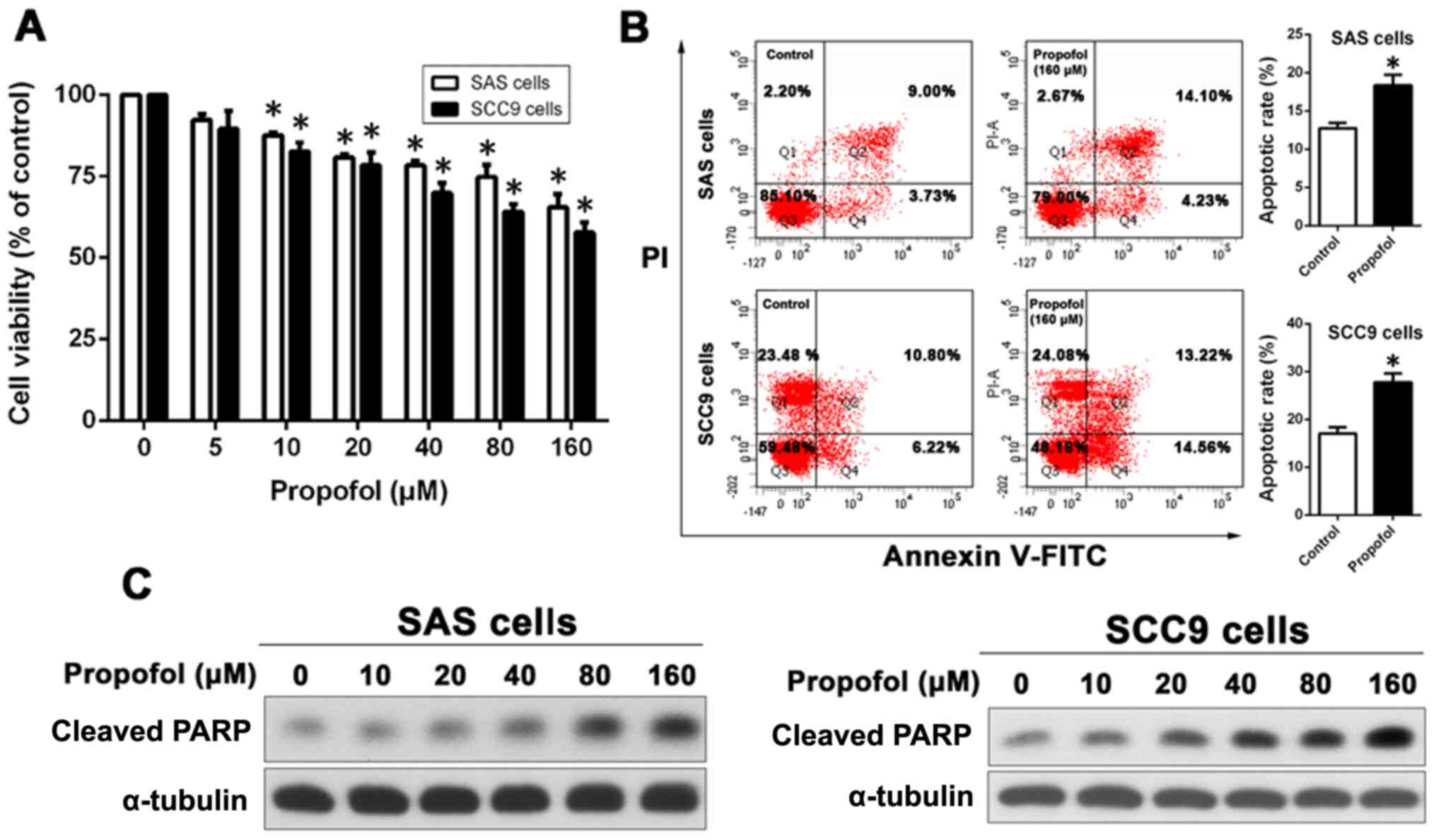
Effect of propofol on anticancer
activity in OSCC cells
To determine the cytotoxic effect of propofol on
OSCC cells, SAS and SCC9 cells were incubated in media containing
0–160 µM propofol for 48 h, and then cell viability was determined
using an MTT assay. As shown in Fig.
1A, propofol decreased OSCC cell viability in a dose-dependent
manner; however, significance was only recorded at concentrations
>5 µM. To determine whether propofol induced OSCC cell
apoptosis, Annexin V-FITC/PI staining-based flow cytometry was
conducted to detect SAS and SCC9 cell apoptosis following treatment
with propofol. Compared with the control groups (12.73±0.7126 and
17.02±1.337%, respectively), propofol significantly increased the
proportion of apoptotic cells in both SAS (18.33±1.410%) and SCC9
(27.78±1.827%) cells (Fig.
1B).
The expression levels of cleaved- poly(ADP-ribose)
polymerase (PARP), a protein that is associated with apoptosis
(25), were determined by western
blotting to assess the effect of propofol on cell apoptosis. The
results demonstrated that propofol treatment increased the
expression of cleaved PARP increased in a concentration-dependent
manner (Fig. 1C).
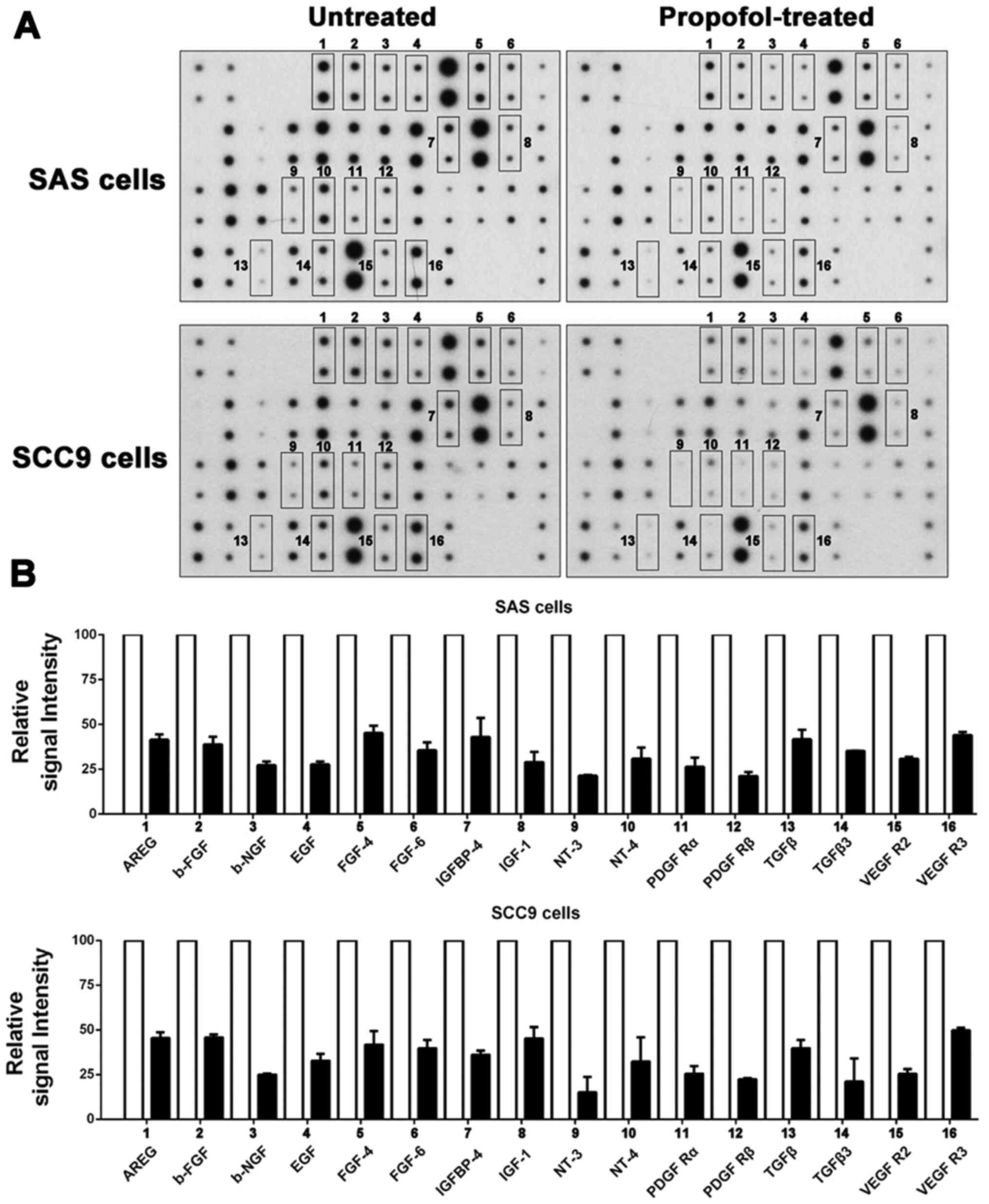
Propofol attenuates the secretion of
multiple growth factors in OSCC cells
Numerous studies have demonstrated that growth
factors secreted by tumor cells into the microenvironment are
related to antiapoptosis effects (19,26). To determine the effect of propofol
on the secretion of growth factors, CM was used for an antibody
array to detect changes in the secretion of growth factors by OSCC
cells after treatment with propofol (Fig. 2A). The relative signal intensity
for the growth factors was compared between untreated and
propofol-treated SAS and SCC9 cells. SAS and SCC9 cells displayed
similar results, with a total of 16 growth factors [AREG, basic
fibroblast growth factor, beta-nerve growth factor, EGF, FGF-4,
FGF-6, insulin like growth factor binding protein-4, insulin like
growth factor-1, neurotrophin (NT)-3, NT-4, platelet derived growth
factor receptor (PDGF R)α, PDGF Rβ, TGFβ, TGFβ3, VEGFR2 and VEGFR3]
displaying a >2-fold reduction in secretion in the
propofol-treated group compared with the untreated group (Fig. 2B).
Bioinformatics analysis of the
clinical significance of the downregulation of secreted proteins in
patients with HNSCC
To determine the clinical importance of these
secreted proteins, GEPIA were used to determine differences in
expression patterns between HNSCC tissues and healthy tissues.
Compared with those in healthy tissues, the mRNA expression levels
of AREG, PDGF Rβ, TGF-β and TGF-β3 were significantly upregulated
in HNSCC tissues, whereas NT-3 was significantly downregulated
(Fig. 3A).
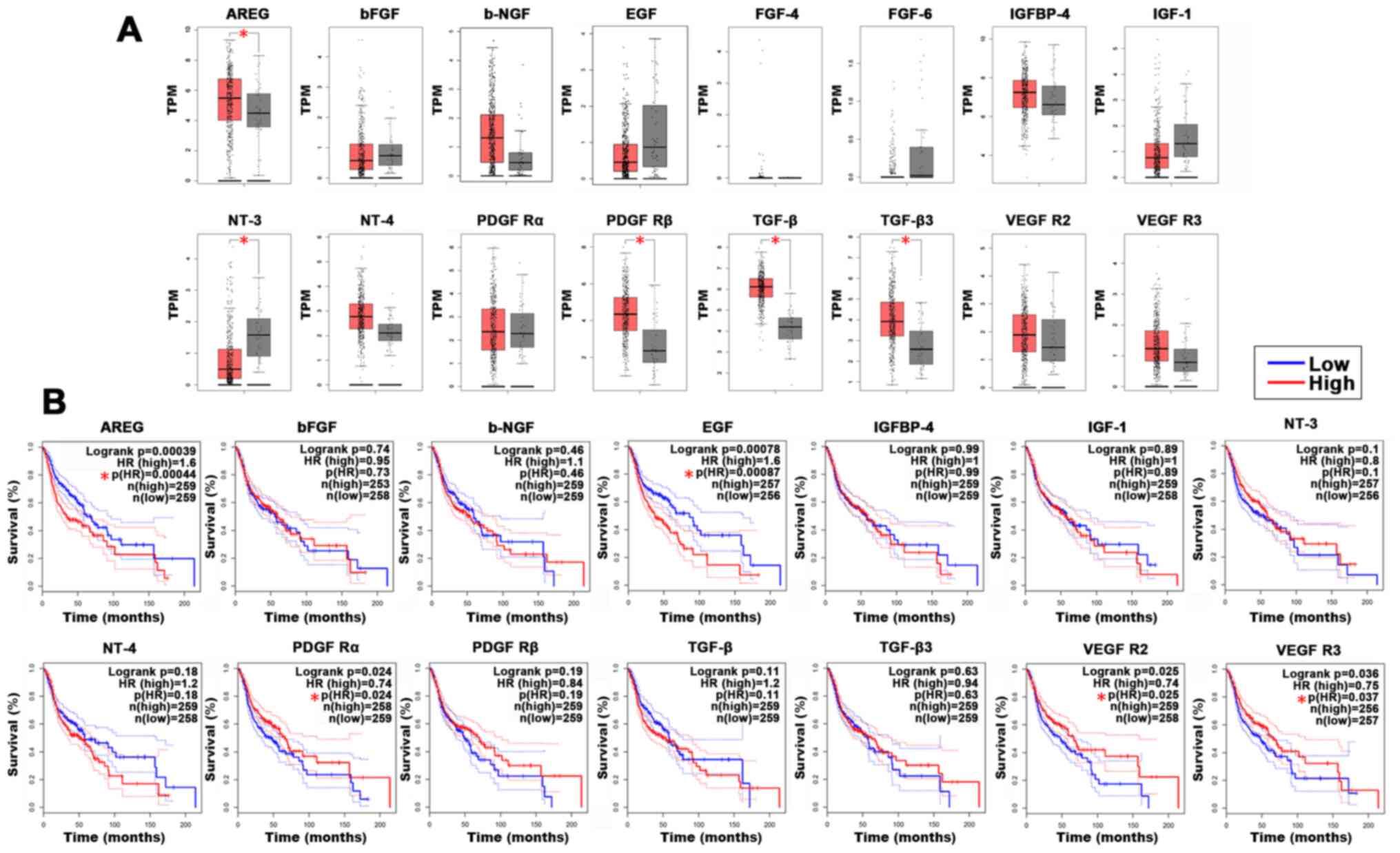 | Figure 3.Association between decreased protein
secretion and the clinical features of HNSCC. (A) Box plots
indicating mRNA expression levels for candidate genes in HNSCC
tissues (red) and normal tissue (gray) for The Cancer Genome Atlas
datasets from GEPIA. The y-axes represent transformed log2 (TPM+1).
The |log2FC| cutoff was 1 and the P-value cutoff was 0.05. (B)
Association between target genes and overall survival was
determined using GEPIA. The solid line represents the survival
curve and the dashed lines represents the 95% confidence interval.
Log-rank P<0.05 was considered to indicate a statistically
significant difference. Patients with expression levels higher than
the median are indicated by a red line and patients with expression
levels lower than the median are indicated by a blue line.
*P<0.05. HNSCC, head and neck squamous cell carcinoma; GEPIA,
gene expression profile interactive analysis; TPM, transcripts per
million; FC, fold change; HR, hazard ratio; b-FGF, basic fibroblast
growth factor; b-NGF, beta-nerve growth factor; IGFBP, insulin like
growth factor binding protein; IGF, insulin like growth factor; NT,
neurotrophin; PDGF R, platelet derived growth factor receptor. |
GEPIA was also used to perform an OS analysis to
determine the clinical relevance of these secreted proteins in
terms of prognosis. The results demonstrated that high mRNA
expression of AREG (log-rank P=0.00039) and EGF (log-rank
P=0.00087) was significantly associated with a worse OS (Fig. 3B). However, high expression levels
of PDGF Rα (log-rank P=0.024), VEGFR2 (log-rank P=0.025) and VEGFR
(log-rank P=0.036) were significantly associated with an improved
prognosis for patients with HNSCC. The bioinformatics analysis
results demonstrated that AREG was highly expressed in cancer
tissues and associated with a poor prognosis, thus AREG was
selected as the target gene for the present study.
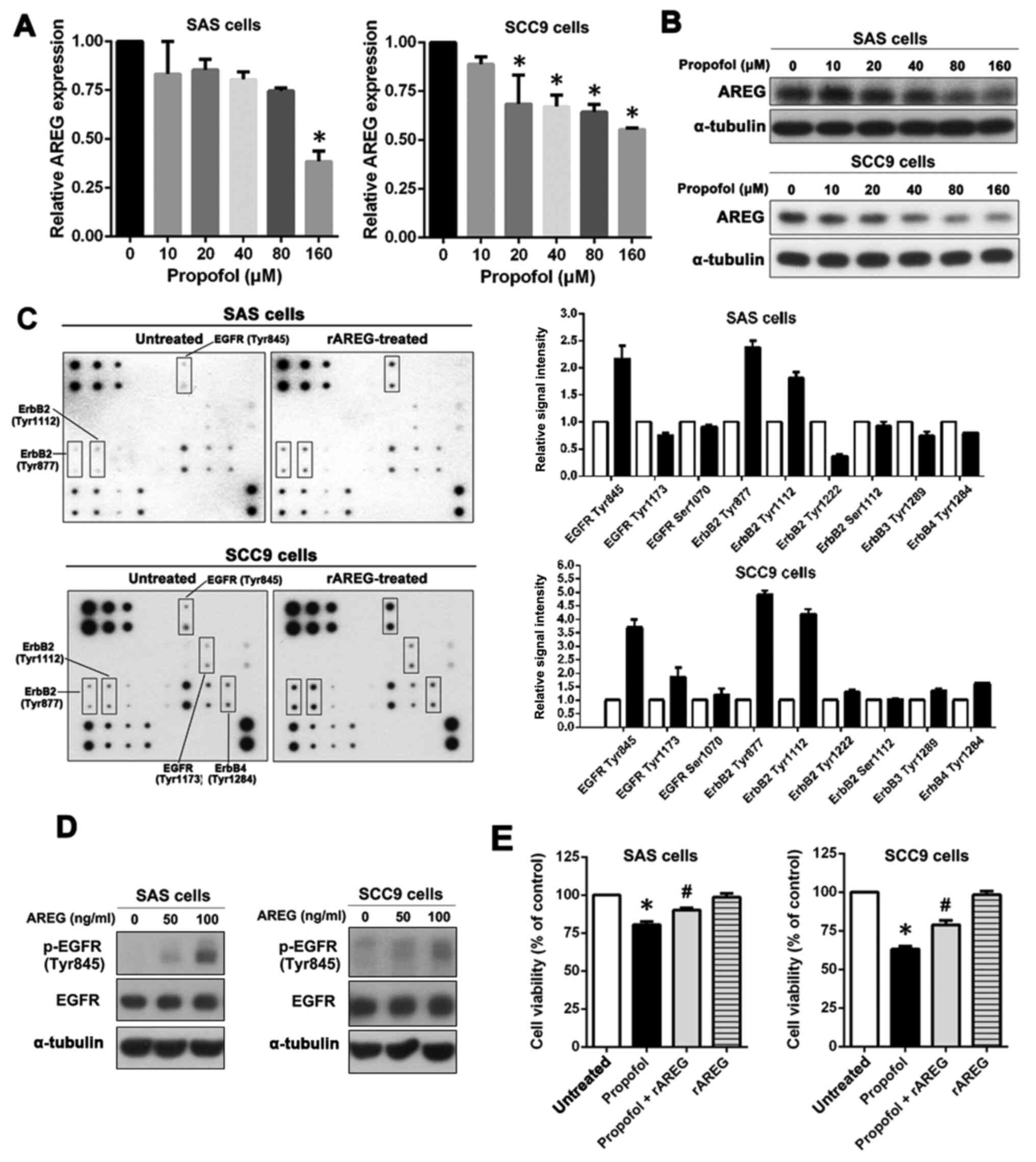
Propofol reduces AREG expression in
OSCC cells and may limit the activation of its related signaling
pathways
The effect of propofol on AREG expression was
investigated. SAS and SCC9 cells were treated with different
concentrations of propofol (0–160 µM). The results demonstrated
that the mRNA expression level of AREG was significantly reduced at
160 µM in SAS cells and significantly reduced at 20 µM in SCC9
cells. (Fig. 4A). After propofol
treatment, the effect of propofol on AREG protein expression was
significantly reduced in a dose-dependent manner in both SAS and
SCC9 cells. (Fig. 4B). The
activation of specific kinases via extracellular stimulation of
AREG may serve a crucial role in mediating cell functions.
Therefore, OSCC cells were treated with rAREG, and a human EGFR
phosphorylation antibody array was used to determine the relative
phosphorylation levels for 17 different EGF receptors.
In rAREG-treated SAS cells, there was more than
twice the level of phosphorylation of EGFR (Tyr845) and ErbB2
(Tyr877) and 1.5 times the level of ErbB2 (Tyr1112) compared with
that in untreated SAS cells (Fig.
4C). SCC9 cells displayed similar activation behavior, with an
activation efficiency greater than that of SAS cells. As shown in
Fig. 4C, the phosphorylation
level of EGFR (Tyr1173) and ErbB4 (Tyr1284) increased >1.5 times
and the phosphorylation level for EGFR (Tyr845), ErbB2 (Tyr877) and
ErbB2 (Tyr1112) increased >3-fold.
AREG is the main ligand that is responsible for EGFR
signal activation (27). AREG
also induced Tyr845 phosphorylation of EGFR in both SAS and SCC9
cells, so SAS and SCC9 cells were treated with increasing doses of
rAREG (50–100 ng/ml) and the level of phosphorylated EGFR (Tyr845)
was assessed via western blotting. AREG markedly induced EGFR
(Tyr845) phosphorylation in SAS and SCC9 cells in a
concentration-dependent manner (Fig.
4D).
To verify that propofol induced OSCC cell apoptosis
by decreasing the expression of AREG, OSCC cells were pretreated
with rAREG to assess whether this increased resistance to propofol.
The results demonstrated that pretreatment with rAREG significantly
increased the resistance of SAS and SCC9 cells to propofol
(Fig. 4E). These results
indicated that propofol suppressed the expression and secretion of
AREG, which may limit AREG-induced EGFR activation and promote cell
apoptosis.
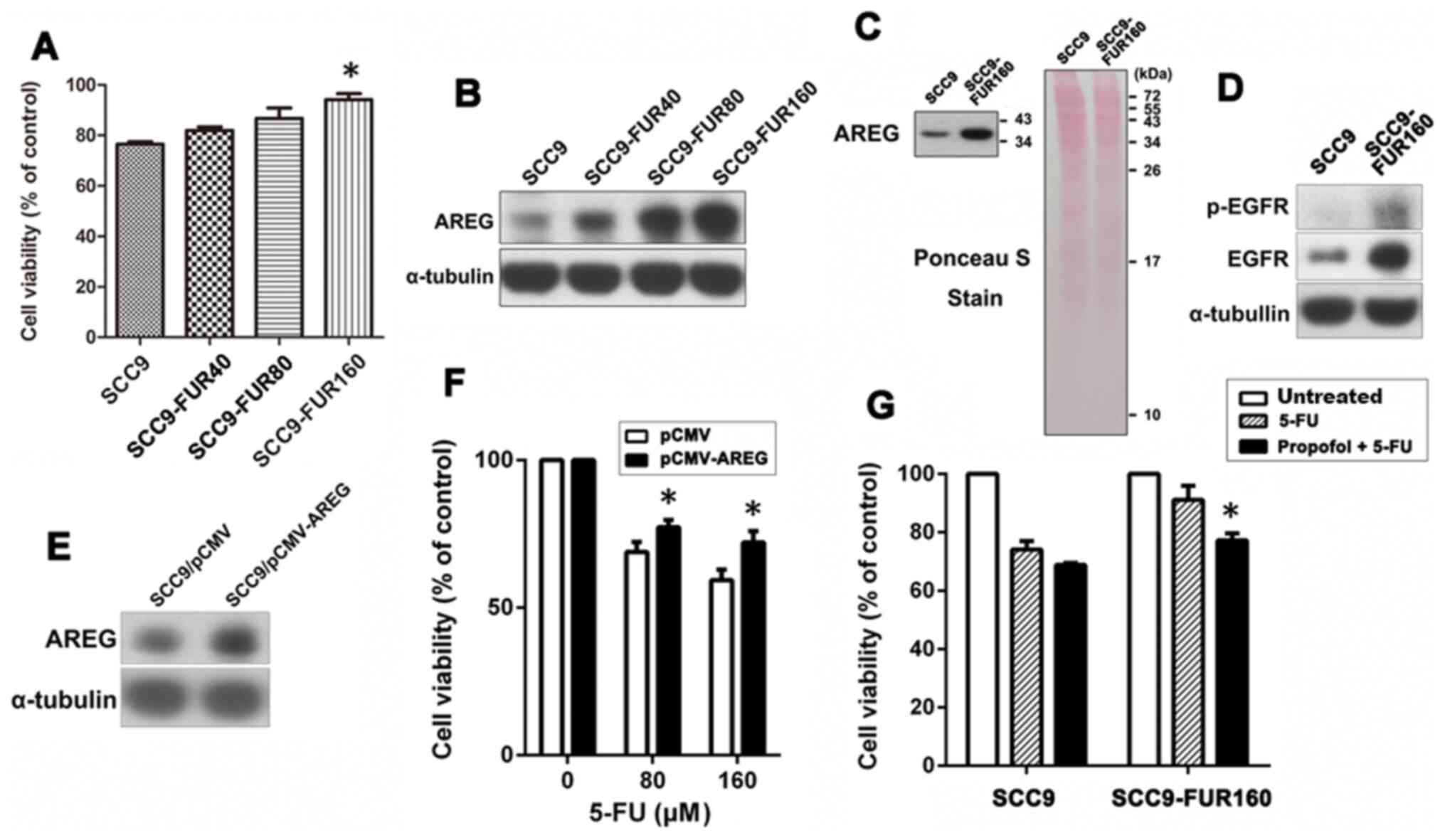
Elevated AREG expression and its
associated activation are related to the development of 5-FU
resistance, but propofol alleviates resistance to 5-FU
To determine the relationship between AREG
expression and 5-FU sensitivity in OSCC cells, different
concentrations of 5-FU were used to establish cell lines with
different degrees of 5-FU resistance. The results indicated that
5-FU resistance was gradually increased from SCC9, SCC9-FUR40,
SCC9-FUR80 to SCC9-FUR160 cells, displaying significantly increased
cell viability in SCC9-FUR160 cells compared with SCC9 cells
(Fig. 5A).
The levels of AREG expression were elevated as 5-FU
resistance increased (Fig. 5B).
SCC9 and SCC9-FUR160 cells were then compared to determine whether
highly resistant cells displayed a high level of AREG secretion and
activation of the related signaling pathways. SCC9-FUR160 cells
displayed higher protein expression levels of AREG compared with
SCC9 cells (Fig. 5C). The
relative expression of total EGFR and the phosphorylation of EGFR
(Tyr845) was also notably higher in SCC9-FUR160 cells compared with
that in SCC9 cells (Fig. 5D).
To verify that AREG mediated 5-FU resistance, AREG
overexpression vector or empty vector was used to transiently
transfect SCC9 cells and then the sensitivity to 5-FU was assessed.
AREG overexpression was confirmed by western blotting (Fig. 5E). The results demonstrated that
AREG overexpression significantly increased resistance to 5-FU for
SCC9-FUR160 cells (Fig. 5F).
To determine whether propofol increased sensitivity
to 5-FU for OSCC cells, SCC9 and SCC9-FUR160 cells were pretreated
with propofol for 24 h and then combined with 5-FU for another 48
h. As the antibody array analysis results showed that treatment of
cells with 10 µM propofol markedly reduced the secretion of a
number of growth factors, cells were treated with 10 µM propofol.
Propofol slightly increased 5-FU cytotoxicity in SCC9 cells, but
significantly increased sensitivity to 5-FU in SCC9-FUR160 cells
compared with 5-FU alone (Fig.
5G). These results demonstrated that propofol alleviated
resistance to 5-FU, potentially by decreasing the expression and
secretion of AREG.
Discussion
Propofol is one of the most commonly used
intravenous anesthetics in clinical practice (28). The present study aimed to
determine the effect of propofol on the biological behavior and
secretion of growth factors in OSCC cells. The results demonstrated
that propofol was cytotoxic to OSCC cells and downregulated the
secretion of several growth factors. The GEPIA database results
indicated that AREG was highly expressed in cancer tissues and
associated with poor prognosis for patients with OSCC, thus AREG
was selected for further investigation. AREG is the ligand of EGFR
(27). The results of the current
study revealed that propofol downregulated the secretion of AREG,
which may have reduced the activation of the EGFR pathway. To the
best of our knowledge, the present study was the first to
demonstrate that propofol regulated AREG to exert an antitumor
effect.
AREG is one of the ligands of EGFR that is shed from
the membrane through the proteolytic process of disintegrin and
metalloprotease 17 (ADAM17) and is converted into an active soluble
form, which activates receptors (29). Previous studies have reported that
AREG upregulation is related to drug resistance and a failure of
treatment for multiple types of cancer, including OSCC (30–32). EGFR in HNSCC is also 15%
upregulated, and high EGFR expression is associated with a low
survival rate (33). Similarly,
>80% of OSCC cases exhibit EGFR upregulation, which is highly
correlated with carcinogenic, antiapoptotic and aggressive
phenotypes (34–36).
Increasing evidence shows that anesthetics can
affect tumor progression (37)
and long-term outcomes for patients (38), especially in terms of cancer
recurrence (39). It has been
reported that different anesthetics can have opposite effects on
cancer development (40).
Therefore, the identification of an appropriate anesthetic to
provide adequate anesthesia management for patients with cancer is
important.
Previous studies show that propofol can have an
anticancer effect in different human cancer cells, including
osteosarcoma (41),
hepatocellular carcinoma (42),
lung cancer (43), ovarian cancer
(44), cervical cancer (45), glioma (46), gastric cancer (47), breast cancer (48) and colorectal cancer cells
(49). A previous study showed
that propofol displayed an anticancer effect on OSCC cells by
inhibiting cell proliferation and promoting cell apoptosis
(50). Retrospective analyses
demonstrated that propofol-based total intravenous anesthesia
significantly reduces postoperative mortality for patients with
cancer, so propofol may be involved in tumor suppression (51,52).
Tumors are heterogeneous tissues that are surrounded
by the tumor microenvironment. The complex microenvironment for a
tumor is composed of cancer cells, stromal cells, immune cells and
extracellular matrix. The tumor microenvironment is related to
tumor progression and affects tumor growth, metastasis, drug
resistance and recurrence (53).
The regulation of the microenvironment for tumors has also become a
new strategy for cancer treatment (54).
At present, it is known that propofol displays
anti-inflammatory properties (55). The present study demonstrated that
propofol decreased the secretion of a variety of growth factors,
which implied that the anti-inflammatory effect of propofol may be
achieved via the secretion of inflammatory factors. However, the
antibody array used in the present study only analyzed the
secretion of 41 growth factors in OSCC cells. To further assess the
anti-inflammatory effects of propofol, future studies should select
a suitable immune cell line for further analysis.
Previous studies have reported that propofol
inhibits the proliferation, metastasis and progress of pancreatic
cancer by inhibiting the expression of ADAM (56,57). The ADAM family is involved in the
process of proteolytic shedding of membrane-associated proteins,
which causes cleavage of transmembrane proteins and solubilizes the
complete ectodomain of cytokines, growth factors, receptors and
adhesion molecules, which changes in the tumor microenvironment
(58).
A previous study also showed that propofol inhibits
the release of VEGF-C, which is induced by breast surgery (59). VEGF-C has been shown to be
involved in lymphangiogenesis to promote cancer metastasis. VEGF-C
has also been observed to increase cell proliferation and
migration, contributing to OSCC progression (60). Extracellular vesicles (EVs) are
also important facilitators of malignant cell communication.
Propofol has been shown to exhibit anticancer activity by
inhibiting the release of EVs during a cancer resection, which is
related to tumor progression and prognosis (61,62).
Numerous studies have shown that propofol may be
involved in tumor suppression, displaying an important effect on
the process of tumor spread and chemotherapy (8,9,28,63,64); however, the underlying molecular
mechanisms are not completely understood. The present study
investigated the effect of propofol on the secretion profile of
growth factors by OSCC cells. To determine the clinical
significance of the altered proteins, the GEPIA database was used
and the results indicated that AREG was significantly elevated in
cancer tissues and associated with a poor prognosis for patients
with OSCC. The present study also determined that AREG expression
and the activation of its related signaling pathways was involved
in 5-FU-resistance in OSCC. Pretreatment with propofol increased
5-FU sensitivity in 5-FU resistant cells. Therefore, the results of
the present study provided a theoretical basis for the combined use
of propofol and 5-FU for the treatment of OSCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Ditmanson Medical
Foundation of Chia-Yi Christian Hospital (grant no. R109-16).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JCC and MSC conceived and designed the current
study. KSY and PCC performed data analysis and wrote the
manuscript. YPW performed the experiments. MJH and INL were
responsible for data interpretation and manuscript revision. JCC
revised the manuscript critically for important intellectual
content. All authors have read and approved the final manuscript.
JCC and MSC confirmed the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Curado MP and Hashibe M: Recent changes in
the epidemiology of head and neck cancer. Curr Opin Oncol.
21:194–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scully C and Bagan J: Oral squamous cell
carcinoma overview. Oral Oncol. 45:301–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chi AC, Day TA and Neville BW: Oral cavity
and oropharyngeal squamous cell carcinoma - an update. CA Cancer J
Clin. 65:401–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagata M, Nakayama H, Tanaka T, Yoshida R,
Yoshitake Y, Fukuma D, Kawahara K, Nakagawa Y, Ota K, Hiraki A, et
al: Overexpression of cIAP2 contributes to 5-FU resistance and a
poor prognosis in oral squamous cell carcinoma. Br J Cancer.
105:1322–1330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sahinovic MM, Struys MMRF and Absalom AR:
Clinical Pharmacokinetics and Pharmacodynamics of Propofol. Clin
Pharmacokinet. 57:1539–1558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vasileiou I, Xanthos T, Koudouna E, Perrea
D, Klonaris C, Katsargyris A and Papadimitriou L: Propofol: A
review of its non-anaesthetic effects. Eur J Pharmacol. 605:1–8.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song J, Shen Y, Zhang J and Lian Q: Mini
profile of potential anticancer properties of propofol. PLoS One.
9:e1144402014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong T, Ning X, Deng Z, Liu M, Zhou B,
Chen X, Huang S, Xu Y, Chen Z and Luo R: Propofol-induced
miR-219-5p inhibits growth and invasion of hepatocellular carcinoma
through suppression of GPC3-mediated Wnt/β-catenin signalling
activation. J Cell Biochem. 120:16934–16945. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye LL, Cheng ZG, Cheng XE and Huang YL:
Propofol regulates miR-1-3p/IGF1 axis to inhibit the proliferation
and accelerates apoptosis of colorectal cancer cells. Toxicol Res
(Camb). 10:696–705. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jun IJ, Jo JY, Kim JI, Chin JH, Kim WJ,
Kim HR, Lee EH and Choi IC: Impact of anesthetic agents on overall
and recurrence-free survival in patients undergoing esophageal
cancer surgery: A retrospective observational study. Sci Rep.
7:140202017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng X, Wang Y, Dong L, Zhao S, Wang L,
Chen H, Xu Y and Wang G: Effects of propofol-based total
intravenous anesthesia on gastric cancer: A retrospective study.
OncoTargets Ther. 11:1141–1148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu ZF, Lee MS, Wong CS, Lu CH, Huang YS,
Lin KT, Lou YS, Lin C, Chang YC and Lai HC: Propofol-based Total
Intravenous Anesthesia Is Associated with Better Survival Than
Desflurane Anesthesia in Colon Cancer Surgery. Anesthesiology.
129:932–941. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai HC, Lee MS, Lin C, Lin KT, Huang YH,
Wong CS, Chan SM and Wu ZF: Propofol-based total intravenous
anaesthesia is associated with better survival than desflurane
anaesthesia in hepatectomy for hepatocellular carcinoma: A
retrospective cohort study. Br J Anaesth. 123:151–160. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai HC, Lee MS, Lin KT, Chan SM, Chen JY,
Lin YT and Wu ZF: Propofol-based total intravenous anesthesia is
associated with better survival than desflurane anesthesia in
intrahepatic cholangiocarcinoma surgery. Medicine (Baltimore).
98:e184722019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guerrero Orriach JL, Raigon Ponferrada A,
Malo Manso A, Herrera Imbroda B, Escalona Belmonte JJ, Ramirez
Aliaga M, Ramirez Fernandez A, Diaz Crespo J, Soriano Perez AM,
Fontaneda Heredia A, et al: Anesthesia in Combination with Propofol
Increases Disease-Free Survival in Bladder Cancer Patients Who
Undergo Radical Tumor Cystectomy as Compared to Inhalational
Anesthetics and Opiate-Based Analgesia. Oncology. 98:161–167. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nazemi M and Rainero E: Cross-Talk Between
the Tumor Microenvironment, Extracellular Matrix, and Cell
Metabolism in Cancer. Front Oncol. 10:2392020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilson TR, Fridlyand J, Yan Y, Penuel E,
Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, et al:
Widespread potential for growth-factor-driven resistance to
anticancer kinase inhibitors. Nature. 487:505–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nisar S, Yousuf P, Masoodi T, Wani NA,
Hashem S, Singh M, Sageena G, Mishra D, Kumar R, Haris M, et al:
Chemokine-Cytokine Networks in the Head and Neck Tumor
Microenvironment. Int J Mol Sci. 22:45842021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mughees M, Sengupta A, Khowal S and Wajid
S: Mechanism of tumour microenvironment in the progression and
development of oral cancer. Mol Biol Rep. 48:1773–1786. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sen Y, Xiyang H and Yu H: Effect of
thoracic paraspinal block-propofol intravenous general anesthesia
on VEGF and TGF-β in patients receiving radical resection of lung
cancer. Medicine (Baltimore). 98:e180882019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Z, Dodig-Crnković T, Schwenk JM and
Tao SC: Current applications of antibody microarrays. Clin
Proteomics. 15:72018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res.
45W:W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Castells M, Thibault B, Delord JP and
Couderc B: Implication of tumor microenvironment in
chemoresistance: Tumor-associated stromal cells protect tumor cells
from cell death. Int J Mol Sci. 13:9545–9571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berasain C and Avila MA: Amphiregulin.
Semin Cell Dev Biol. 28:31–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bateman BT and Kesselheim AS: Propofol as
a transformative drug in anesthesia: Insights from key early
investigators. Drug Discov Today. 20:1012–1017. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Busser B, Sancey L, Brambilla E, Coll JL
and Hurbin A: The multiple roles of amphiregulin in human cancer.
Biochim Biophys Acta. 1816:119–131. 2011.PubMed/NCBI
|
|
30
|
Gao J, Ulekleiv CH and Halstensen TS:
Epidermal growth factor (EGF) receptor-ligand based molecular
staging predicts prognosis in head and neck squamous cell carcinoma
partly due to deregulated EGF- induced amphiregulin expression. J
Exp Clin Cancer Res. 35:1512016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsieh MJ, Chen YH, Lee IN, Huang C, Ku YJ
and Chen JC: Secreted amphiregulin promotes vincristine resistance
in oral squamous cell carcinoma. Int J Oncol. 55:949–959.
2019.PubMed/NCBI
|
|
32
|
Bourova-Flin E, Derakhshan S, Goudarzi A,
Wang T, Vitte AL, Chuffart F, Khochbin S, Rousseaux S and
Aminishakib P: The combined detection of Amphiregulin, Cyclin A1
and DDX20/Gemin3 expression predicts aggressive forms of oral
squamous cell carcinoma. Br J Cancer. 125:1122–1134. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bossi P, Resteghini C, Paielli N, Licitra
L, Pilotti S and Perrone F: Prognostic and predictive value of EGFR
in head and neck squamous cell carcinoma. Oncotarget.
7:74362–74379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sarkis SA, Abdullah BH, Abdul Majeed BA
and Talabani NG: Immunohistochemical expression of epidermal growth
factor receptor (EGFR) in oral squamous cell carcinoma in relation
to proliferation, apoptosis, angiogenesis and lymphangiogenesis.
Head Neck Oncol. 2:132010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Son HK, Kim D, Lim Y, Kim J and Park I: A
novel TGF-β receptor II mutation (I227T/N236D) promotes aggressive
phenotype of oral squamous cell carcinoma via enhanced EGFR
signaling. BMC Cancer. 20:11632020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsou HH, Tsai HC, Chu CT, Cheng HW, Liu
CJ, Lee CH, Liu TY and Wang HT: Cigarette Smoke Containing Acrolein
Upregulates EGFR Signaling Contributing to Oral Tumorigenesis In
Vitro and In Vivo. Cancers (Basel). 13:35442021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang W, Cai J, Zabkiewicz C, Zhang H, Ruge
F and Jiang WG: The Effects of Anesthetics on Recurrence and
Metastasis of Cancer, and Clinical Implications. World J Oncol.
8:63–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cassinello F, Prieto I, del Olmo M, Rivas
S and Strichartz GR: Cancer surgery: How may anesthesia influence
outcome? J Clin Anesth. 27:262–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Snyder GL and Greenberg S: Effect of
anaesthetic technique and other perioperative factors on cancer
recurrence. Br J Anaesth. 105:106–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Plein LM and Rittner HL: Opioids and the
immune system - friend or foe. Br J Pharmacol. 175:2717–2725. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ye Z, Jingzhong L, Yangbo L, Lei C and
Jiandong Y: Propofol inhibits proliferation and invasion of
osteosarcoma cells by regulation of microRNA-143 expression. Oncol
Res. 21:201–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang J, Wu GQ, Zhang Y, Feng ZY and Zhu
SM: Propofol induces apoptosis of hepatocellular carcinoma cells by
upregulation of microRNA-199a expression. Cell Biol Int.
37:227–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cui WY, Liu Y, Zhu YQ, Song T and Wang QS:
Propofol induces endoplasmic reticulum (ER) stress and apoptosis in
lung cancer cell H460. Tumour Biol. 35:5213–5217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Su Z, Hou XK and Wen QP: Propofol induces
apoptosis of epithelial ovarian cancer cells by upregulation of
microRNA let-7i expression. Eur J Gynaecol Oncol. 35:688–691.
2014.PubMed/NCBI
|
|
45
|
Zhang D, Zhou XH, Zhang J, Zhou YX, Ying
J, Wu GQ and Qian JH: Propofol promotes cell apoptosis via
inhibiting HOTAIR mediated mTOR pathway in cervical cancer. Biochem
Biophys Res Commun. 468:561–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu J, Xu W and Zhu J: Propofol suppresses
proliferation and invasion of glioma cells by upregulating
microRNA-218 expression. Mol Med Rep. 12:4815–4820. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peng Z and Zhang Y: Propofol inhibits
proliferation and accelerates apoptosis of human gastric cancer
cells by regulation of microRNA-451 and MMP-2 expression. Genet Mol
Res. 15:gmr70782016. View Article : Google Scholar
|
|
48
|
Yu B, Gao W, Zhou H, Miao X, Chang Y, Wang
L, Xu M and Ni G: Propofol induces apoptosis of breast cancer cells
by downregulation of miR-24 signal pathway. Cancer Biomark.
21:513–519. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Y, Dong W, Yang H and Xiao G: Propofol
suppresses proliferation and metastasis of colorectal cancer cells
by regulating miR-124-3p.1/AKT3. Biotechnol Lett. 42:493–504. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao C, Ren C, Liu Z, Zhang L, Tang R and
Li X: GAS5, a FoxO1-actived long noncoding RNA, promotes
propofol-induced oral squamous cell carcinoma apoptosis by
regulating the miR-1297-GSK3β axis. Artif Cells Nanomed Biotechnol.
47:3985–3993. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wigmore TJ, Mohammed K and Jhanji S:
Long-term Survival for Patients Undergoing Volatile versus IV
Anesthesia for Cancer Surgery: A Retrospective Analysis.
Anesthesiology. 124:69–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lai HC, Lee MS, Lin KT, Huang YH, Chen JY,
Lin YT, Hung KC and Wu ZF: Propofol-based total intravenous
anesthesia is associated with better survival than desflurane
anesthesia in robot-assisted radical prostatectomy. PLoS One.
15:e02302902020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu T and Dai Y: Tumor microenvironment and
therapeutic response. Cancer Lett. 387:61–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang Y, Ho SH, Li B, Nie G and Li S:
Modulating the tumor microenvironment with new therapeutic
nanoparticles: A promising paradigm for tumor treatment. Med Res
Rev. 40:1084–1102. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen RM, Chen TG, Chen TL, Lin LL, Chang
CC, Chang HC and Wu CH: Anti-inflammatory and antioxidative effects
of propofol on lipopolysaccharide-activated macrophages. Ann N Y
Acad Sci. 1042:262–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gao Y, Yu X, Zhang F and Dai J: Propofol
inhibits pancreatic cancer progress under hypoxia via ADAM8. J
Hepatobiliary Pancreat Sci. 26:219–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu X, Shi J, Wang X and Zhang F: Propofol
affects the growth and metastasis of pancreatic cancer via ADAM8.
Pharmacol Rep. 72:418–426. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Murphy G: The ADAMs: Signalling scissors
in the tumour microenvironment. Nat Rev Cancer. 8:929–941. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yan T, Zhang GH, Wang BN, Sun L and Zheng
H: Effects of propofol/remifentanil-based total intravenous
anesthesia versus sevoflurane-based inhalational anesthesia on the
release of VEGF-C and TGF-β and prognosis after breast cancer
surgery: A prospective, randomized and controlled study. BMC
Anesthesiol. 18:1312018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shigetomi S, Imanishi Y, Shibata K, Sakai
N, Sakamoto K, Fujii R, Habu N, Otsuka K, Sato Y, Watanabe Y, et
al: VEGF-C/Flt-4 axis in tumor cells contributes to the progression
of oral squamous cell carcinoma via upregulating VEGF-C itself and
contactin-1 in an autocrine manner. Am J Cancer Res. 8:2046–2063.
2018.PubMed/NCBI
|
|
61
|
Zhang J, Shan WF, Jin TT, Wu GQ, Xiong XX,
Jin HY and Zhu SM: Propofol exerts anti-hepatocellular carcinoma by
microvesicle-mediated transfer of miR-142-3p from macrophage to
cancer cells. J Transl Med. 12:2792014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Buschmann D, Brandes F, Lindemann A,
Maerte M, Ganschow P, Chouker A, Schelling G, Pfaffl MW and
Reithmair M: Propofol and Sevoflurane Differentially Impact
MicroRNAs in Circulating Extracellular Vesicles during Colorectal
Cancer Resection: A Pilot Study. Anesthesiology. 132:107–120. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang J, Cheng CS, Lu Y, Ding X, Zhu M,
Miao C and Chen J: Novel Findings of Anti-cancer Property of
Propofol. Anticancer Agents Med Chem. 18:156–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cramer JD, Burtness B, Le QT and Ferris
RL: The changing therapeutic landscape of head and neck cancer. Nat
Rev Clin Oncol. 16:669–683. 2019. View Article : Google Scholar : PubMed/NCBI
|