Introduction
Myocardial infarction (MI) is a key manifestation of
cardiovascular disease (1).
According to the Centers for Medicare & Medicaid Services
Hospital Inpatient Quality Reporting Program data on 2,363
hospitals in 2018, the average mortality of acute MI was 13.6%
(2). MI is the injury and
necrosis of myocardial cells caused by a sudden decrease in
coronary blood flow, continuous myocardial ischemia and hypoxia
(3,4). In clinical practice, the primary
application of percutaneous coronary intervention is to promptly
restore coronary blood transport and save the dying myocardium
(5,6). Left ventricular remodeling following
MI can have serious consequences, such as heart failure and
rupture, and is the primary cause of death in patients with MI
(1). Therefore, delaying or
preventing left ventricular remodeling is an important step in
preventing heart failure following MI. However, the detailed
mechanisms underlying MI remain unclear. Therefore, it is important
to investigate the mechanisms of MI injury, which may help to
develop novel therapies for myocardial injury.
Programmed cell death includes necroptosis,
parthanatos, oxytosis, ferroptosis, extracellular trap (ET)osis,
neutrophil ETosis and pyroptosis (7). Pyroptosis leads to myocardial
perforation and rupture of the cell membrane and releases
pro-inflammatory factors such as interleukin-1β (IL-1β) and IL-18,
initiated by caspase-1, causing a local inflammatory response
(8). Previous studies have found
that pyroptosis serves an important role in cardiovascular disease
progression (including atherosclerosis, MI, ischemia-reperfusion,
diabetic cardiomyopathy and heart failure) (9–11).
A previous study indicated that the inflammatory
body-caspase axis serves an important role in inflammation-induced
cell death following ischemia-reperfusion (10). The inflammasome is a complex that
can transform pro-caspase-1 into activated caspase-1. Activated
caspase-1 can convert pro-IL-1β and pro-IL-18 into mature IL-1β and
IL-18, cleave gasdermin D (GSDMD) into the GSDMD-N terminal with a
pore-forming effect and induce pyroptosis (12). GSDMD, a member of the GSDM family,
is a common substrate for caspase-1,-4, −5 and −11 and mediates
pyroptosis by producing an N-terminal fragment (13,14). Active caspase-1, −4 −11 cleave
GSDMD in response to canonical and non-canonical inflammasome
activation (15). Moreover, GSDMD
inhibition improves cardiac function and decreases infarct size by
attenuating cardiomyocyte pyroptosis (16,17). The role of pyroptosis and the
mechanism of its occurrence and development in the MI process
remain to be elucidated, which may provide novel directions and
strategies for the clinical treatment of cardiovascular
disease.
Interferon regulators (IRFs) are multifunctional
transcription factors that serve an important regulatory role in
the interferon signaling pathway, which is involved in host immune
response, cell differentiation and immune regulation (18). IRF2 is a member of the IRF family
of transcription factors (19),
which can transcriptionally induce direct target genes, including
toll-like receptor 3, Bcl-11a and GSDMD (20–22). Kayagaki et al (22) found that IRF2 transcriptionally
induces GSDMD expression in pyroptosis and directly drives GSDMD
mRNA expression. GSDMD expression is significantly decreased in
IRF2-deficient macrophages, endothelial cells and multiple types of
tissue, corresponding to decreased IL-1 secretion and inhibition of
pyroptosis (22). However, its
functional contribution to MI remains unclear and requires further
clarification.
The present study investigated the effect of IRF2 in
MI injury and clarified the potential mechanism of IRF2 involvement
in MI via GSDMD-induced pyroptosis.
Materials and methods
Bioinformatics analysis
GeneMANIA (23), a
web tool that can identify other proteins associated with a set of
input genes, was used to generate protein-protein interaction (PPI)
network images. The associations between co-expression,
colocalization, predicted related genes, shared protein domains,
genetic interactions and physical interactions were determined
using GeneMANIA.
Animals and MI model in vivo
A total of 25 male C57BL/6 mice (aged 6–8 weeks;
weight, 20–26 g) were provided by Ai Ling Fei (Nanjing, China).
Mice were maintained at 23–25°C with 60–70% humidity, 12-h
light/dark cycles and access to normal chow diet and water. The
mice were divided into the following five groups (n=5 per group):
i) Control (Con); ii) sham; iii) MI; iv) MI + sh-NC; and v) MI +
sh-IRF2. Untreated mice were set as the con group. For shRNAs
transfection in vivo, mice were injected with
lentivirus-IRF2-short hairpin (sh)RNA (sh-IRF2 forward,
GGTCCTGACTTCAACTATA; reverse, TATAGTTGAAGTCAGGACC) or
lentivirus-scramble (forward, UUCUCCGAACGUGUCACGUTT; reverse,
ACGUGACACGUUCGGAGAATT) (both 1×109 viral
particles/mouse, Shanghai GenePharma Co., Ltd.) via the tail vein.
After 7 days, a mouse model of MI was generated by ligation of the
left anterior descending coronary artery, as previously described
(24). Briefly, mice were
anesthetized with 45 mg/kg 1% pentobarbital sodium intraperitoneal
injection and then underwent open-heart surgery. The MI model was
established by threading a 7/0 surgical thread 2 mm under the left
auricle and ligating the anterior descending branch of the left
coronary artery for 24 h. In the sham group, a parallel operation
was performed, however, the thread was threaded without ligation.
After operation, 40,000 units of penicillin (Thermo Fisher
Scientific, Inc.) were injected intraperitoneally daily for 3
consecutive days.
Cell culture and MI model in
vitro
H9c2 cells were purchased from EK Biosciences GmbH
(Shanghai, China) and cultured in Dulbecco's Modified Eagle Medium
containing 10% foetal bovine serum (both Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2. To establish an MI injury
model in vitro, H9c2 cells were exposed to a hypoxia
incubator (1% oxygen, 5% CO2 and 94% N2) for
6, 12, 24 and 48 h. Untreated cells were set as Con group.
Histopathological examination and
immunohistochemical (IHC) staining
At 24 h after the surgery, the were mice were
euthanized by intraperitoneal injection of 50 mg/kg, followed by
dislocation of the cervical spine. The heart tissue samples were
fixed in 4% paraformaldehyde at room temperature for 24 h, embedded
in paraffin and sectioned into 5 µm slices. The slices were stained
with hematoxylin-eosin (H&E) for 7 min at room temperature and
photographed using a light microscope (×400 magnification; Nikon
Eclipse TE2000-U; Nikon Corporation). For IHC staining of IRF2, the
tissue slices were deparaffinized in xylene, dehydrated with graded
ethanol solutions and rehydrated in PBS. The slices then were
incubated with 3% H2O2 at 37°C for 10 min to
block endogenous peroxidase activity. For antigen retrieval, the
slices were treated with PBS (pH 9) for 15 min at 95°C in a
microwave oven and then cooled in running water. Subsequently,
slices were incubated with normal goat serum sealant (Shanghai
Haoran Biotechnology Co., Ltd.) for 20 min at room temperature.
Slices were then incubated with anti-IRF2 antibody (cat. no.
PA5-83159; 1:200; Sigma-Aldrich; Merck KGaA) in a humidified
chamber at 4°C overnight and were visualized with DAB at room
temperature for 2 min and counterstained with hematoxylin at room
temperature for 5 min.
Echocardiographic measurement
Transthoracic echocardiography was performed 24 h
after surgery in mice post-MI using an echocardiography system
equipped with a 12-MHz phased-array transducer. The left
ventricular ejection fraction (EF) and fractional shortening (FS)
were measured using M-mode tracing. All experiments were performed
by professional technicians who were blinded to the experimental
groups.
Western blotting
Total protein samples from H9c2 cells and heart
tissue were extracted by using RIPA lysis buffer containing 1 mM
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology). The total protein concentrations were determined
using a bicinchoninic acid Protein Assay kit (Beyotime Institute of
Biotechnology). Proteins (30 µg per lane) were separated using 10%
SDS-PAGE (Beyotime Institute of Biotechnology) and then transferred
to PVDF membranes. The membranes were blocked in 5% skimmed milk
for 1 h and then incubated overnight at 4°C with primary
antibodies. The blot was probed using the following primary
antibodies: IRF2 (cat. no. A303-380A-T; 1:1,000; Thermo Fisher
Scientific, Inc.), cleaved caspase-1 (c-caspase-1; cat. no.
PA5-77886; 1:1,000; Thermo Fisher Scientific, Inc.), GSDMD (cat.
no. PA5-30823; 1:1,000; Thermo Fisher Scientific, Inc.), GSDMD-N
(cat. no. ab215203; 1:1,000; Abcam), IL-1β (cat. no. P420B;
1:1,000; Thermo Fisher Scientific, Inc.), IL-18 (cat. no.
PA5-79477; 1:1,000; Thermo Fisher Scientific, Inc.), and GAPDH
(cat. no. ab8245; 1:5,000; Abcam), followed by incubation with goat
anti-rabbit IgG secondary antibody (cat. no. G-21234; 1:10,000;
Thermo Fisher Scientific, Inc.) at room temperature for 1 h.
Protein bands were visualized by enhanced chemiluminescence Pierce™
ECL Western Blotting Substrate (cat. no. 32209; Thermo Fisher
Scientific, Inc.). The densitometry analysis was performed using
ImageJ software (version 1.8.0; National Institutes of Health).
TUNEL staining
Myocardial tissue apoptosis was measured using a
In Situ Cell Death Detection Kit (Sigma Aldrich; Merck KGaA)
according to the manufacturer's instructions. For the TUNEL
staining, the heart tissue samples were fixed in 4%
paraformaldehyde at room temperature for 24 h, embedded in paraffin
and sectioned into 5-µm slices. The slices were deparaffinized in
xylene, dehydrated with graded ethanol solutions and rehydrated in
PBS, and then treated with 25 µg/ml proteinase K for 8 min at room
temperature. Then, the slices were stained with the TUNEL reaction
mixture containing TdT and TMR-dUTP for 1 h at 37°C in the dark.
After applying stop solution for 10 min at room temperature,
streptavidin-HRP working solution (50 µl) was added at room
temperature for 30 min, followed by incubation with DAB (50 µl)
solution at room temperature for 10 min. The color was then
developed using 3,3-diaminobenzidine (Sigma-Aldrich; Merck KGaA). A
total of five fields of view were randomly selected to count the
TUNEL-positive cells under a light microscope (×400 magnification)
and the proportion of TUNEL-positive cells was calculated.
Determination of cellular
apoptosis
H9c2 cells were cultured at room temperature in
6-well plates at a density of 1×105 cells/well to
evaluate pyroptosis. After hypoxia treatment, the treated H9c2
cells were harvested and incubated with Annexin V-fluorescein
isothiocyanate and propidium iodide for 15 min at 4°C. The samples
were analyzed using a flow cytometer (BD Accuri C6 Plus; BD
Biosciences) with FlowJo software (v10.6.2; FlowJo, LLC) according
to the manufacturer's instructions.
Chromatin immunoprecipitation (ChIP)
assay
The ChIP assay was performed with the ChIP Assay kit
(cat. no. P2078; Beyotime Institute of Biotechnology) according to
the manufacturer's protocol. Briefly, H9c2 cells (1×105)
were incubated with 1% formaldehyde for 10 min at 25°C to crosslink
the protein and DNA. Glycine (Beijing Solarbio Technology Co.,
Ltd.) was then used to terminate the crosslinking. H9c2 cells were
centrifugated at 1,000 × g for 2 min at 4°C, resuspended in the
lysis buffer (0.2 ml) at at 4°C for 10 min. The Ch fragments were
obtained by shearing crosslinked complex DNA with ultrasound,
followed by IP with the antibody directed against anti-IRF2 (cat.
no. ab245658; 1:100; Abcam) overnight at 4°C, taking IgG as a
negative control. Ch fragments were detected by PCR.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from H9c2 cells using
RNAsimple Total RNA Kit (cat. no. DP419; Tiangen Biotech Co., Ltd.)
and RNA was reverse transcribed into cDNA using a
PrimeScript® RT reagent kit (Takara Bio, Inc.) according
to the manufacturer's protocol. Subsequently, qPCR was performed
using the SYBR Premix Ex Taq™ II kit (Thermo Fisher Scientific,
Inc.) and an ABI Prism 7700 sequence detector (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The sequences of the PCR primers
used were as follows: GSDMD forward, 5′-TCTGCCCTCAACACTTCTGG-3′ and
reverse, 5′-TGCAGCCACAAATAACTCAGC-3′; and GAPDH forward,
5′-GAAGGTCGGAGTCAACGGATT-3′ and reverse, 5′-TTCCCGTTCTCAGCCATGT-3′.
The following thermocycling conditions were used: 90°C for 5 min;
then 90°C for 15 sec and 60°C for 30 sec for 45 cycles. Expression
levels were quantified using the 2−∆∆Cq method (25) and normalized to the internal
reference gene GAPDH.
Dual-luciferase reporter assay
The recombinant luciferase reporter plasmids
containing the GSDMD promoter (pEZX-PG04-GSDMD), pcDNA3.1 vector
(pc-NC, 100 nM), IRF2 overexpression vector (IRF2, 100 nM), IRF2
short hairpin (sh)RNA (sh-IRF2 forward, GGTCCTGACTTCAACTATA;
reverse, TATAGTTGAAGTCAGGACC; 100 nM) or negative control (sh-NC
forward, UUCUCCGAACGUGUCACGUTT; reverse, ACGUGACACGUUCGGAGAATT; 100
nM) were co-transfected into H9c2 cells by Lipofectamine™ 3000
transfection reagent (cat. no. L3000001; Thermo Fisher Scientific,
Inc.) for 48 h at 37°C. Following 48 h transfection, the luciferase
activity was detected using a Dual-Luciferase Reporter Gene Assay
Kit (cat. no. 11402ES60; Shanghai Qcbio Science & Technologies
Co., Ltd.) according to the manufacturer's instructions. The
firefly luciferase activity was normalized to Renilla
luciferase activity for each sample.
Statistical analysis
All data were analyzed using GraphPad Prism 9
(GraphPad Software, Inc.) and are expressed as the mean ± SD. All
experiments were repeated three times. One-way ANOVA followed by
Tukey's multiple comparisons test was used for comparisons between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
IRF2 expression is associated with MI
in mice
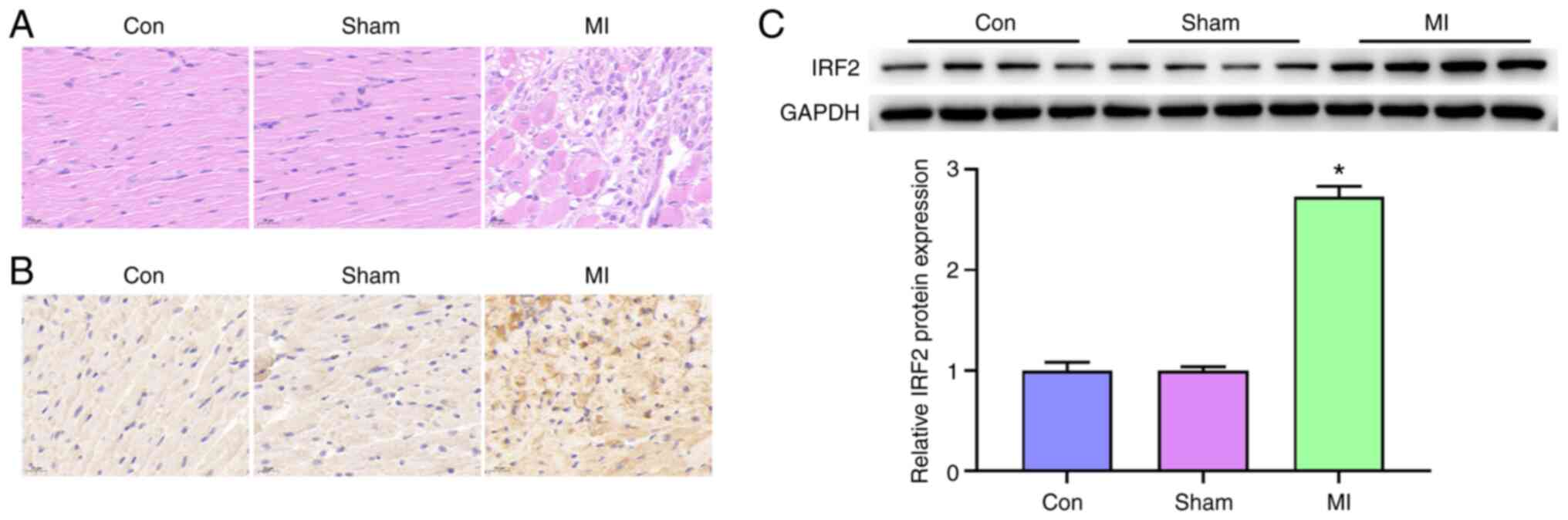
H&E and IHC staining for IRF2 showed severe MI
in MI model mice. IHC analysis revealed a positive association
between increased IRF2 expression and MI progression (Fig. 1A and B). Western blotting
indicated that IRF2 expression was increased in MI samples compared
with that in the con (Fig. 1C).
Taken together, these results indicated that IRF2 expression was
positively associated with MI progression.
IRF2 silencing attenuates MI in
mice
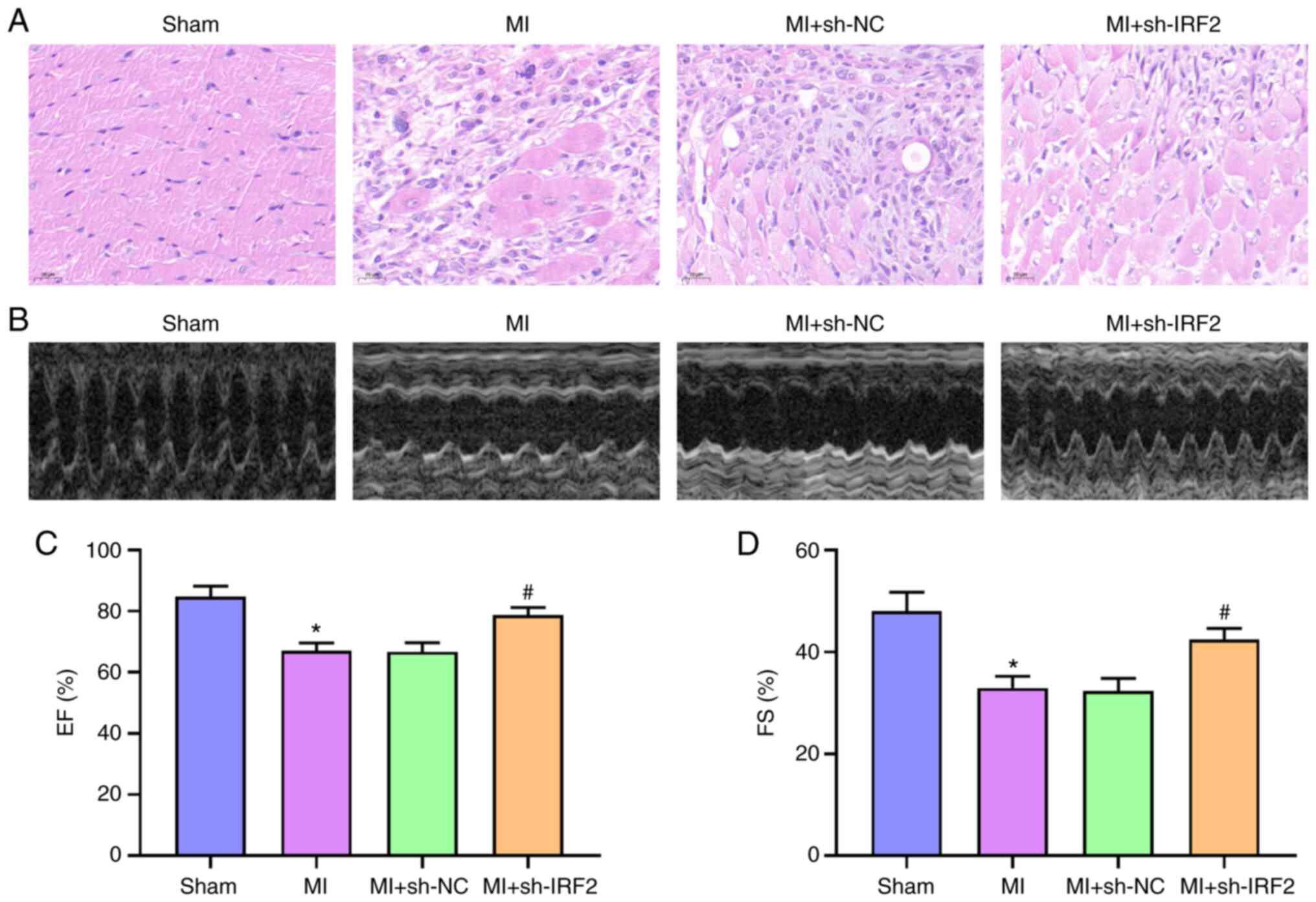
The effect of IRF2 on MI was investigated. IRF2
shRNA was transmitted to mice using a lentivirus. IRF2 silencing
alleviated histological heart damage in the MI + sh-IRF2 group
compared with the MI group (Fig.
2A). To understand the effects of IRF2 silencing on MI mice,
in vivo cardiac function was measured using transthoracic
echocardiography (Fig. 2B).
Compared with the sham group, MI significantly decreased EF and FS;
this was improved in the MI + sh-IRF2 group (Fig. 2C and D). These data suggested that
IRF2 silencing alleviated systolic dysfunction in MI mice.
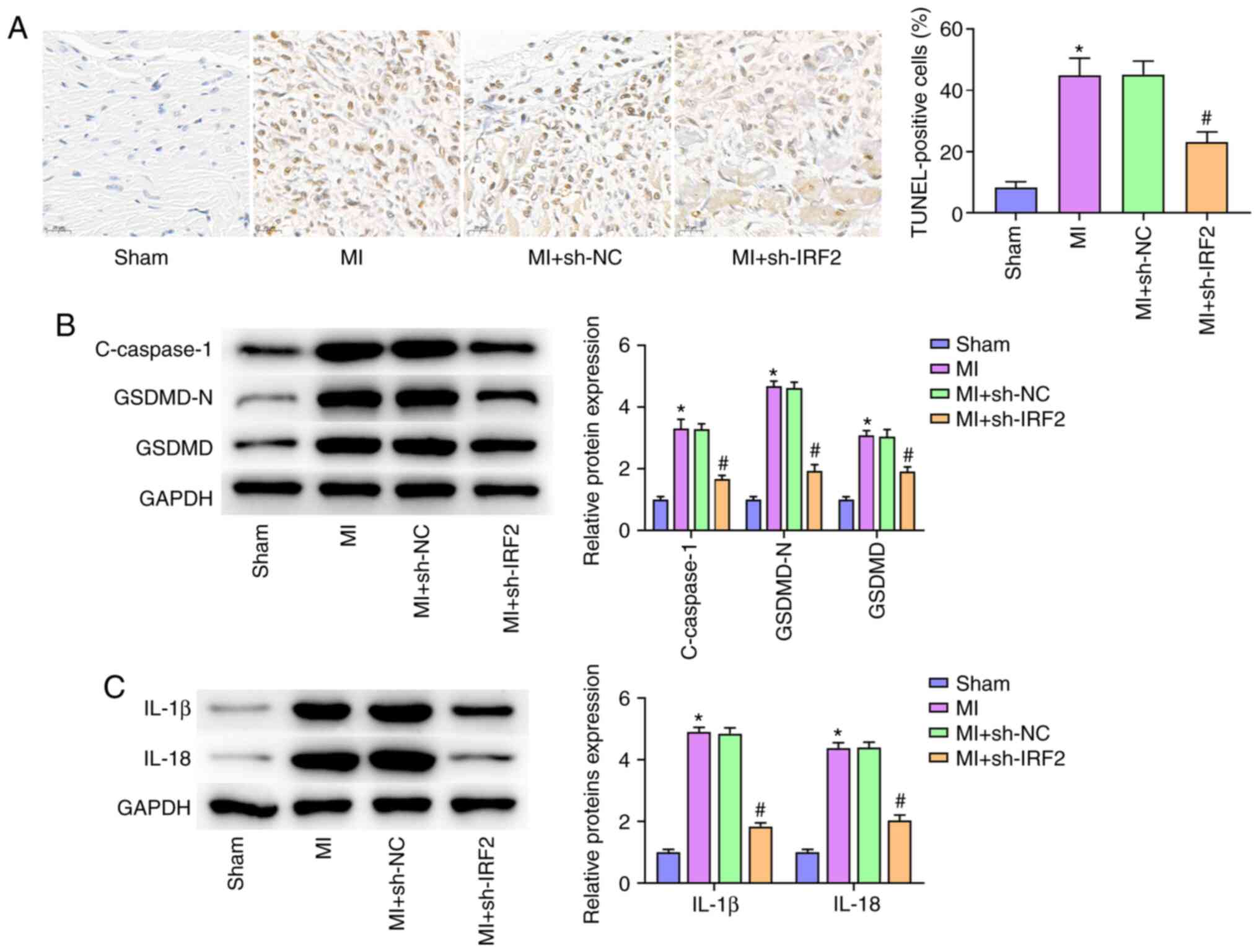
IRF2 silencing attenuates
GSDMD-induced pyroptosis in vivo
Pyroptosis, a new form of inflammatory programmed
cell death, has been shown to aggravate MI following cardiomyocyte
loss (16). To investigate the
protective effect of IRF2 silencing on MI by decreasing pyroptosis,
TUNEL staining was used to evaluate pyroptotic cell death in the MI
model. Cardiac pyroptosis and the number of TUNEL-positive cells
increased in the MI group and IRF2 silencing significantly
inhibited cardiac pyroptosis (Fig.
3A). Western blot analysis revealed that MI resulted in the
upregulation of c-caspase-1, IL-1β, IL-18, GSDMD-N and GSDMD 24 h
after MI; IRF2 silencing significantly inhibited expression of
c-caspase-1, IL-1β, IL-18, GSDMD-N and GSDMD protein levels induced
by MI (Fig. 3B and C).
IRF2 silencing attenuates
GSDMD-induced pyroptosis in vitro
One of the key events in MI is hypoxia, which also
promotes pyroptosis (26,27). To determine whether IRF2 silencing
prevented pyroptosis in cardiomyocytes, H9c2 cells were cultured
under hypoxic conditions for 6, 12, 24 and 48 h. Exposure of H9c2
cells to hypoxia (6–48 h) induced a time-dependent increase in IRF2
expression (Fig. 4A). Compared
with the NC group, IRF2 expression was significantly decreased in
the sh-IRF2 group and significantly increased in the IRF2
overexpression group (Fig. 4B).
Hypoxia markedly elevated the rate of programmed cell death, and
IRF2 silencing significantly decreases the rate of programmed cell
death, and IRF2 overexpression aggravated the rate of programmed
cell death compared with the hypoxia group (Fig. 4C). Western blot analysis showed
that hypoxia resulted in the upregulation of c-caspase-1, IL-1β,
IL-18, GSDMD-N and GSDMD (Fig. 4D and
E). Moreover, IRF2 silencing significantly inhibited the
protein expression of c-caspase-1, IL-1β, IL-18, GSDMD-N and GSDMD
induced by hypoxia. These results suggested that IRF2 silencing
attenuated GSDMD-induced pyroptosis in vitro.
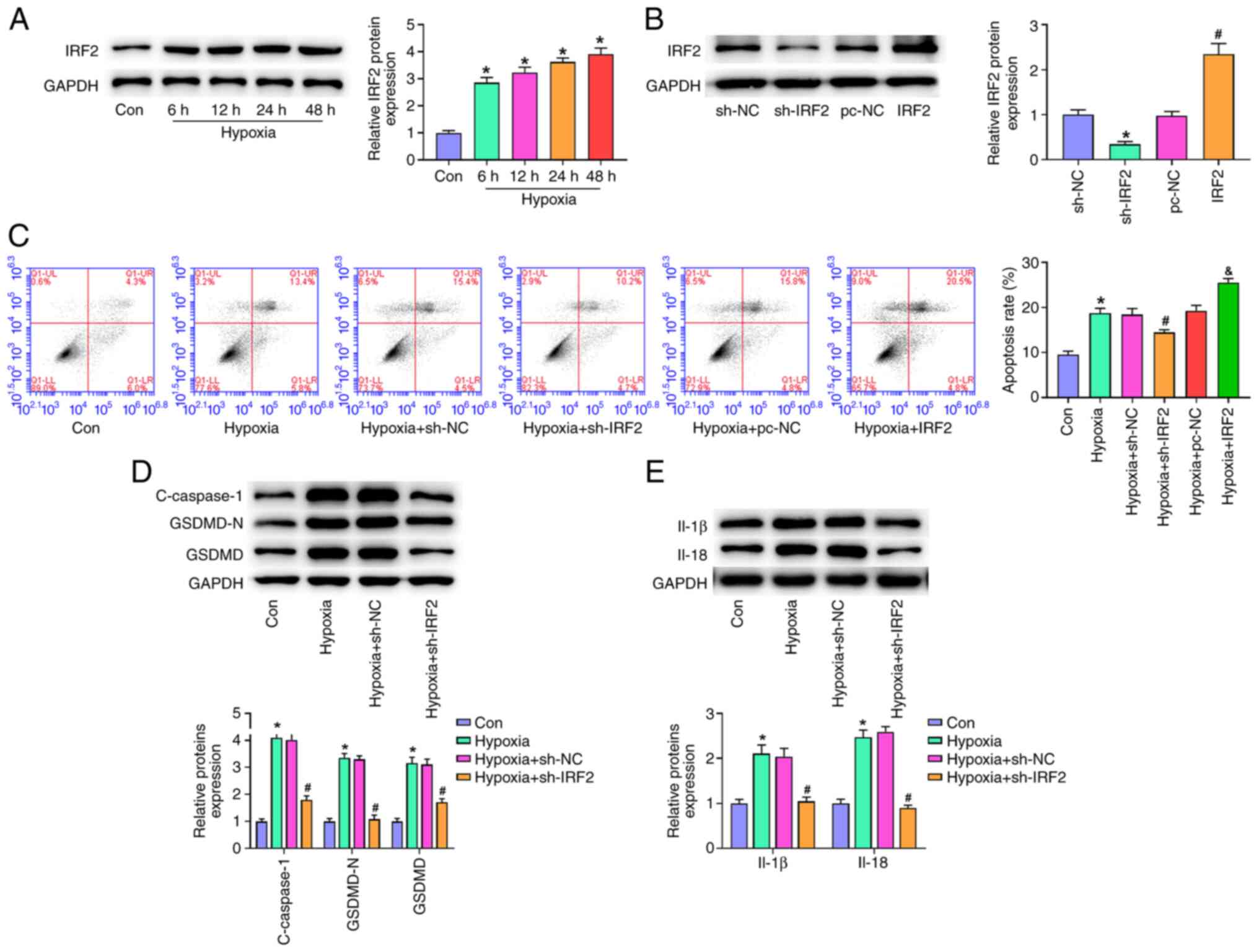 | Figure 4.IRF2 silencing attenuates
GSDMD-induced pyroptosis in vitro. (A) IRF-2 expression in
H9c2 cells stimulated by hypoxia (6, 12, 24 and 48 h) was detected
by reverse transcription-quantitative PCR. *P<0.05 vs. Con. (B)
IRF2 expression was detected by western blotting. *P<0.05 vs.
sh-NC; #P<0.05 vs. pc-NC. (C) Following transfection
of sh-IRF2 and IRF2, apoptosis of H9c2 cells was detected by flow
cytometry under hypoxic conditions. &P<0.05 vs.
Hypoxia + pc-NC, *P<0.05 vs. Con; #P<0.05 vs.
Hypoxia+sh-NC. Following transfection of sh-IRF2 and IRF2, the
expression of (D) c-caspase-1, GSDMD-N, GSDMD, (E) IL-1β, and IL-18
was detected by western blotting under hypoxic conditions.
*P<0.05 vs. Con; #P<0.05 vs. Hypoxia + sh-NC. All
data are expressed as the mean ± SD (n=3). IRF2, interferon
regulatory factor 2; GSDMD, gasdermin D; c-, cleaved; MI,
myocardial infarction; sh, short hairpin; NC, negative control;
Con, control. |
IRF2 is key for the transcriptional
activation of GSDMD
Co-expression of IRF2 and upstream genes recorded in
GeneMANIA (28) (genemania.org/)
showed that IRF2 may interact with HIF-1, indicating an association
between HIF-1 and IRF2 (Fig. 5A).
Exposure of H9c2 cells to hypoxia (6–48 h) induced a time-dependent
increase in the expressions of IRF2 and HIF-1 (Fig. 5B). These data suggested that IRF2
expression may be regulated by the HIF-1 pathway in
hypoxia-stimulated H9c2 cells. To verify whether IRF2 directly
drives GSDMD in hypoxia-induced H9c2 cells, ChIP-PCR and
dual-luciferase reporter assay were used to evaluate effect of IRF2
on GSDMD transcriptional activity. ChIP-PCR analysis of H9c2 cells
using specific antibodies against IRF2 showed IRF2 was bound to the
GSDMD promoter (Fig. 5C).
Furthermore, dual-luciferase reporter assay showed that IRF2
overexpression increased GSDMD promoter activity and IRF2 silencing
decreased GSDMD promoter activity (Fig. 5D). These findings demonstrated
that IRF2 bound directly to the GSDMD promoter. Western blot
analysis showed that treatment of cells with hypoxia and sh-IRF2
inhibited c-caspase-1, GSDMD-N, and GSDMD protein expression in
H9c2 cells (Fig. 5E). Moreover,
GSDMD overexpression increased the expression levels of
c-caspase-1, GSDMD-N and GSDMD. These data suggested that IRF2
silencing alleviated MI and hypoxia-induced cardiomyocyte
pyroptosis via transcriptional activation of GSDMD.
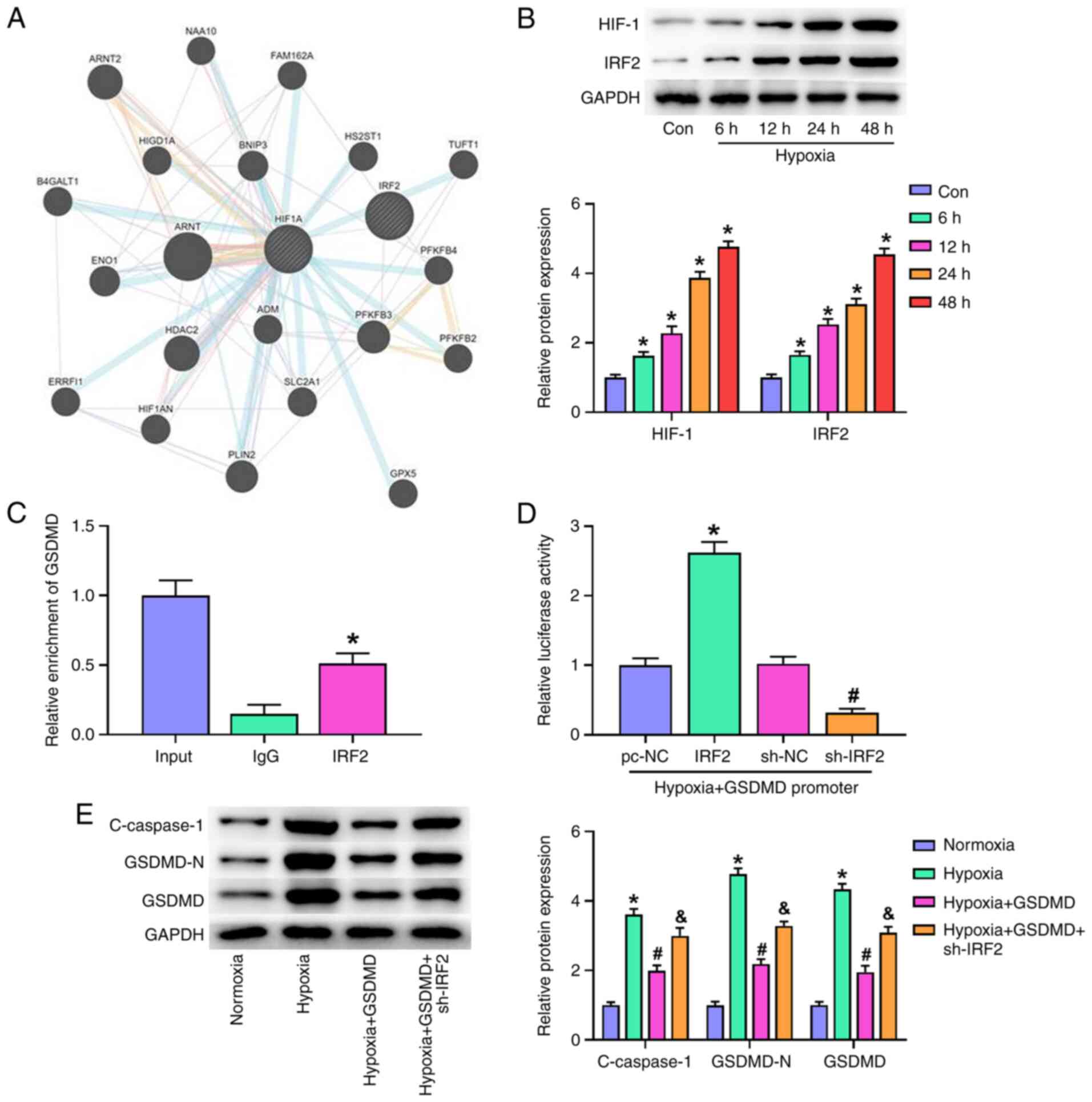 | Figure 5.IRF2 is key for the transcriptional
activation of GSDMD. (A) Co-expression of IRF2 and upstream genes
was detected in GeneMANIA database. (B) HIF-1 and IRF-2 expression
in H9c2 cells were stimulated following hypoxia (6, 12, 24 and 48
h). *P<0.05 vs. Con. (C) Chromatin immunoprecipitation-PCR was
used to evaluate the transcriptional activity of IRF2 on GSDMD.
*P<0.05 vs. IgG. (D) Dual-luciferase reporter assay was used to
evaluate the effect of IRF2 on GSDMD transcriptional activity.
*P<0.05 vs. pc-NC; #P<0.05 vs. sh-NC. (E)
Following transfection of sh-IRF2 and GSDMD, the expression of
c-caspase-1, GSDMD-N and GSDMD was detected by western blotting
under hypoxic conditions. *P<0.05 vs. Normoxia;
#P<0.05 vs. Hypoxia; &P<0.05 vs.
Hypoxia + GSDMD. All data are expressed as the mean ± SD (n=3).
IRF2, interferon regulatory factor 2; GSDMD, gasdermin D; HIF-1,
hypoxia inducible factor-1; Con, control; sh, short hairpin; NC,
negative control; c-, cleaved. |
Discussion
The present study addressed the key role of IRF2 in
MI. First, IRF2 expression was significantly increased in an MI
mouse model and hypoxia-treated H9c2 cells, and the level of IRF2
expression was positively associated with duration of hypoxia in
H9c2 cells. Second, IRF2 silencing alleviated MI and
hypoxia-induced cardiomyocyte pyroptosis in vivo and in
vitro. Third, IRF2 was key for the transcriptional activation
of GSDMD in cardiomyocytes. Therefore, IRF2 is a novel therapeutic
target for treating MI.
Despite the use of advanced therapeutic
interventions (29,30), MI remains the leading cause of
death from cardiovascular disease (31). Pyroptosis is a newly identified
form of programmed cell death that is mediated by inflammatory
caspase-1, accompanied by release of a large number of
pro-inflammatory factors and induces a cascade of amplified
inflammatory responses (32); it
is associated with numerous diseases, including MI and myocardial
cell loss following ischemia-reperfusion (33,34). IRF2 is a member of the IRF family
and combines with interferon gene promoters to serve a key role in
immune response and promote cell proliferation (35). Previous studies found that IRF2 is
involved in various cancer mechanisms, including liver, lung and
gastric cancer (36–38). To the best of our knowledge,
however, there are few reports of its involvement in cardiovascular
disease, particularly in MI. Recently, Kayagaki et al
(22) found that IRF2
transcription induces GSDMD expression to cause pyroptosis.
Therefore, it was hypothesized that IRF2 may erve a role in MI by
regulating GSDMD-induced pyroptosis. In the present study, IRF2
expression was positively associated with MI progression and IRF2
silencing attenuated MI in mice.
Studies have found that the GSDM family induces cell
death and inflammation; in particular, GSDMD is associated with
pyroptosis (39–41). Activated caspase-1, −4, −5 and −11
cleave GSDMD protein into the GSDMD-N terminal with pore-forming
activity (42). GSDMD-N combines
with the cell inner membrane to form a pore, which causes cell
swelling and osmotic lysis and induces pyroptosis (42). The present study showed that IRF2
silencing significantly suppressed pyroptosis-associated proteins,
including GSDMD, GSDMD-N and c-caspase-1. The inflammatory response
caused by MI is key to healing of heart trauma, however, it can
also aggravate heart damage and dysfunction (12). MI activates the NLRP3
inflammasome, leading to the conversion of IL-1β and IL-18 into
active mature forms in a caspase-1-dependent manner (43). Pro-inflammatory cytokines IL-1 and
IL-18 expand infarct size and promote cardiac dysfunction (44). The present results showed that the
expression levels of pro-inflammatory cytokines IL-1β and IL-18
increased in the MI group, and IRF2 silencing notably inhibited the
expression levels of IL-1β and IL-18. Collectively, these data
suggested that IRF2 silencing decreased myocardial injury by
attenuating the pro-inflammatory response and decreasing
pyroptosis.
Hypoxia stimulates myocardial cell damage and
secretion of inflammatory factors, which can partially mimic heart
damage in MI (26). Numerous
studies have found that hypoxic stimulation activates the NLRP3
inflammasome and pyroptosis to induce cardiomyocyte death (26,27). The present results showed that
IRF2 silencing decreased the expression levels of IL-1β, IL-18 and
pyroptosis-associated proteins. H9c2 cells exposed to hypoxia were
positively associated with IRF2 expression. The association between
HIF1 and IRF2 was verified and it was shown that IRF2 expression
may be regulated by the HIF-1 pathway. The present ChIP-PCR and
luciferase reporter assays further demonstrated that IRF2 directly
bound to the GSDMD promoter to drive GSDMD transcription for the
execution of pyroptosis in hypoxia-stimulated H9c2 cells.
In conclusion, the present study demonstrated that
IRF2 was key for MI and acts via increasing inflammasomes and
pyroptosis. These findings confirmed that IRF2 was a key regulator
of MI and provided novel insights into the mechanism of MI
progression.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL conceptualized and designed the study and wrote
the manuscript. YL, YW and YH performed the experiments. HG and QW
analyzed and interpreted the data. YL and YW confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The experimental protocol of the present study was
performed in accordance with the Guide for the Care and Use of
Laboratory Animals. All institutional and national guidelines for
the care and use of laboratory animals were followed and the study
was approved by the Ethics Committee of Cangzhou Central Hospital
(approval no. CZSZ20200113).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IRF
|
interferon regulatory factor
|
|
MI
|
myocardial infarction
|
|
GSDMD
|
gasdermin D
|
|
EF
|
ejection fraction
|
|
FS
|
fractional shortening
|
|
c-
|
cleaved
|
|
ChIP
|
chromatin immunoprecipitation
|
References
|
1
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: Heart Disease and stroke statistics-2018 update:
A report from the American heart association. Circulation.
137:e67–e492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Virani SS, Alonso A, Aparicio HJ, Benjamin
EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng
S, Delling FN, et al: Heart disease and stroke statistics-2021
update. Circulation. 143:e254–e743. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Endorsed by the Latin American Society of
Interventional Cardiology; PCI WRITING COMMITTEE, ; Levine GN,
Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers
CE, Ellis SG, Guyton RA, et al: 2015 ACC/AHA/SCAI focused update on
primary percutaneous coronary intervention for patients with
ST-elevation myocardial Infarction: An update of the 2011
ACCF/AHA/SCAI guideline for percutaneous coronary intervention and
the 2013 ACCF/AHA guideline for the management of ST-elevation
myocardial infarction: A report of the American College of
Cardiology/American Heart Association Task Force on Clinical
Practice Guidelines and the Society for Cardiovascular Angiography
and Interventions. Catheter Cardiovasc Interv. 87:1001–1019. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anderson JL, Adams CD, Antman EM, Bridges
CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS,
Levin TN, et al: 2012 ACCF/AHA focused update incorporated into the
ACCF/AHA 2007 guidelines for the management of patients with
unstable angina/non-ST-elevation myocardial infarction: A report of
the American College of Cardiology Foundation/American Heart
Association Task Force on Practice Guidelines. J Am Coll Cardiol.
61:e179–347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Weg K, Prinzen FW and Gorgels AP:
Editor's Choice-Reperfusion cardiac arrhythmias and their relation
to reperfusion-induced cell death. Eur Heart J Acute Cardiovasc
Care. 8:142–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vanden Berghe T, Linkermann A,
Jouan-Lanhouet S, Walczak H and Vandenabeele P: Regulated necrosis:
The expanding network of non-apoptotic cell death pathways. Nat Rev
Mol Cell Biol. 15:135–147. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji N, Qi Z, Wang Y, Yang X, Yan Z, Li M,
Ge Q and Zhang J: Pyroptosis: A new regulating mechanism in
cardiovascular disease. J Inflamm Res. 14:2647–2666. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han Y, Qiu H, Pei X, Fan Y, Tian H and
Geng J: Low-dose sinapic acid abates the pyroptosis of macrophages
by downregulation of lncRNA-MALAT1 in rats with diabetic
atherosclerosis. J Cardiovasc Pharmacol. 71:104–112. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ibáñez B, Heusch G, Ovize M and Van de
Werf F: Evolving therapies for myocardial ischemia/reperfusion
injury. J Am Coll Cardiol. 65:1454–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Toldo S and Abbate A: The NLRP3
inflammasome in acute myocardial infarction. Nat Rev Cardiol.
15:203–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Hout GP, Bosch L, Ellenbroek GH, de
Haan JJ, van Solinge WW, Cooper MA, Arslan F, de Jager SC,
Robertson AA, Pasterkamp G and Hoefer IE: The selective
NLRP3-inflammasome inhibitor MCC950 reduces infarct size and
preserves cardiac function in a pig model of myocardial infarction.
Eur Heart J. 38:828–836. 2017.PubMed/NCBI
|
|
13
|
He WT, Wan H, Hu L, Chen P, Wang X, Huang
Z, Yang ZH, Zhong CQ and Han J: Gasdermin D is an executor of
pyroptosis and required for interleukin-1β secretion. Cell Res.
25:1285–1298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia C, Chen H, Zhang J, Zhou K, Zhuge Y,
Niu C, Qiu J, Rong X, Shi Z, Xiao J, et al: Role of pyroptosis in
cardiovascular diseases. Int Immunopharmacol. 67:311–318. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lei Q, Yi T and Chen C: NF-κB-Gasdermin D
(GSDMD) axis couples oxidative stress and NACHT, LRR and PYD
domains-containing protein 3 (NLRP3) inflammasome-mediated
cardiomyocyte pyroptosis following myocardial infarction. Med Sci
Monit. 24:6044–6052. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han B, Xu J, Shi X, Zheng Z, Shi F, Jiang
F and Han J: DL-3-n-butylphthalide attenuates myocardial
hypertrophy by targeting gasdermin D and inhibiting gasdermin D
mediated inflammation. Front Pharmacol. 12:6881402021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yanai H, Negishi H and Taniguchi T: The
IRF family of transcription factors: Inception, impact and
implications in oncogenesis. Oncoimmunology. 1:1376–1386. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamura T, Yanai H, Savitsky D and
Taniguchi T: The IRF family transcription factors in immunity and
oncogenesis. Annu Rev Immunol. 26:535–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heinz S, Haehnel V, Karaghiosoff M,
Schwarzfischer L, Müller M, Krause SW and Rehli M: Species-specific
regulation of Toll-like receptor 3 genes in men and mice. J Biol
Chem. 278:21502–21509. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun L, Jiang Z, Acosta-Rodriguez VA,
Berger M, Du X, Choi JH, Wang J, Wang KW, Kilaru GK, Mohawk JA, et
al: HCFC2 is needed for IRF1- and IRF2-dependent Tlr3 transcription
and for survival during viral infections. J Exp Med. 214:3263–3277.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kayagaki N, Lee BL, Stowe IB, Kornfeld OS,
O'Rourke K, Mirrashidi KM, Haley B, Watanabe C, Roose-Girma M,
Modrusan Z, et al: IRF2 transcriptionally induces GSDMD expression
for pyroptosis. Sci Signal. 12:eaax49172019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Warde-Farley D, Donaldson SL, Comes O,
Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT,
et al: The GeneMANIA prediction server: Biological network
integration for gene prioritization and predicting gene function.
Nucleic Acids Res. 38:W214–W220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bock-Marquette I, Saxena A, White MD,
Dimaio JM and Srivastava D: Thymosin beta4 activates
integrin-linked kinase and promotes cardiac cell migration,
survival and cardiac repair. Nature. 432:466–472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Z, Wang Z, Guan Q, Qiu F, Li Y, Liu
Z, Zhang H, Dong H and Zhang Z: PEDF inhibits the activation of
NLRP3 inflammasome in hypoxia cardiomyocytes through PEDF
receptor/phospholipase A2. Int J Mol Sci. 17:20642016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen A, Chen Z, Xia Y, Lu D, Yang X, Sun
A, Zou Y, Qian J and Ge J: Liraglutide attenuates NLRP3
inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS
pathway in H9c2 cells. Biochem Biophys Res Commun. 499:267–272.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Montojo J, Zuberi K, Rodriguez H, Bader GD
and Morris Q: GeneMANIA: Fast gene network construction and
function prediction for Cytoscape. F1000Res. 3:1532014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bellis A, Di Gioia G, Mauro C, Mancusi C,
Barbato E, Izzo R, Trimarco B and Morisco C: Reducing cardiac
injury during ST-elevation myocardial infarction: A reasoned
approach to a multitarget therapeutic strategy. J Clin Med.
10:29682021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rymer JA, Fonseca E, Bhandary DD, Kumar D,
Khan ND and Wang TY: Difference in medication adherence between
patients prescribed a 30-day versus 90-day supply after acute
myocardial infarction. J Am Heart Assoc. 10:e0162152021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
England RN and Autieri MV:
Anti-inflammatory effects of interleukin-19 in vascular disease.
Int J Inflam. 2012:2535832012.PubMed/NCBI
|
|
32
|
Cookson BT and Brennan MA:
Pro-inflammatory programmed cell death. Trends Microbiol.
9:113–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nazir S, Gadi I, Al-Dabet MM, Elwakiel A,
Kohli S, Ghosh S, Manoharan J, Ranjan S, Bock F, Braun-Dullaeus RC,
et al: Cytoprotective activated protein C averts Nlrp3
inflammasome-induced ischemia-reperfusion injury via mTORC1
inhibition. Blood. 130:2664–2677. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dolunay A, Senol SP, Temiz-Resitoglu M,
Guden DS, Sari AN, Sahan-Firat S and Tunctan B: Inhibition of NLRP3
inflammasome prevents LPS-induced inflammatory hyperalgesia in
mice: Contribution of NF-κB, Caspase-1/11, ASC, NOX, and NOS
Isoforms. Inflammation. 40:366–386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Birnbaum MJ, van Zundert B, Vaughan PS,
Whitmarsh AJ, van Wijnen AJ, Davis RJ, Stein GS and Stein JL:
Phosphorylation of the oncogenic transcription factor interferon
regulatory factor 2 (IRF2) in vitro and in vivo. J Cell Biochem.
66:175–183. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li YR, Wen LQ, Wang Y, Zhou TC, Ma N, Hou
ZH and Jiang ZP: MicroRNA-520c enhances cell proliferation,
migration, and invasion by suppressing IRF2 in gastric cancer. FEBS
Open Bio. 6:1257–1266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yongyu Z, Lewei Y, Jian L and Yuqin S:
[ARTICLE WITHDRAWN] MicroRNA-18a Targets IRF2 and CBX7 to promote
cell proliferation in hepatocellular carcinoma. Oncol Res.
26:1327–1334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang C, Zhang X, Wang HM, Liu XM, Zhang
XJ, Zheng B, Qian GR and Ma ZL: MicroRNA-18a-5p functions as an
oncogene by directly targeting IRF2 in lung cancer. Cell Death Dis.
8:e27642017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuan B, Zhou XM, You ZQ, Xu WD, Fan JM,
Chen SJ, Han YL, Wu Q and Zhang X: Inhibition of AIM2 inflammasome
activation alleviates GSDMD-induced pyroptosis in early brain
injury after subarachnoid haemorrhage. Cell Death Dis. 11:762020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao L, Dong X, Gong W, Huang W, Xue J, Zhu
Q, Ma N, Chen W, Fu X, Gao X, et al: Acinar cell NLRP3 inflammasome
and gasdermin D (GSDMD) activation mediates pyroptosis and systemic
inflammation in acute pancreatitis. Br J Pharmacol. 178:3533–3552.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli
VG, Wu H and Lieberman J: Inflammasome-activated gasdermin D causes
pyroptosis by forming membrane pores. Nature. 535:153–158. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Deten A, Volz HC, Briest W and Zimmer HG:
Cardiac cytokine expression is upregulated in the acute phase after
myocardial infarction. Experimental studies in rats. Cardiovasc
Res. 55:329–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abbate A, Van Tassell BW, Seropian IM,
Toldo S, Robati R, Varma A, Salloum FN, Smithson L and Dinarello
CA: Interleukin-1beta modulation using a genetically engineered
antibody prevents adverse cardiac remodelling following acute
myocardial infarction in the mouse. Eur J Heart Fail. 12:319–322.
2010. View Article : Google Scholar : PubMed/NCBI
|