Introduction
Doxorubicin is one of the most widely used
chemotherapeutic drugs (1), which
acts primarily by triggering apoptosis via inhibition of
topoisomerase activity and generation of reactive oxygen species
(ROS) (2). Cardiotoxicity and
congestive heart failure (CHF) are prominent side effects of
doxorubicin because of its toxic effects on cardiomyocytes
(3) and this limits its clinical
use. Notably, doxorubicin is widely used to produce animal and
cellular models of heart failure (4,5).
Apoptosis is known to be one of the key pathological processes of
CHF (6); therefore, a strategy to
prevent cardiomyocyte apoptosis could effectively delay and treat
CHF (7).
Substance P (SP) belongs to the tachykinin family of
sensory neuropeptides, which serves an important role in activating
tachykinin receptor 1. SP functions in the repair of sensory
injury, regulation of smooth muscle contraction and modulation of
inflammation/immune responses (8–12).
SP is mainly expressed in the central nervous system and peripheral
afferent sensory neurons, especially in C-fibers (13). In addition to neurons, SP is also
found in heart tissue (14).
Expression of SP has been reported to markedly increase following
ischemic injury, which SP has previously been shown to limit
(15,16). In addition, SP is hypothesized to
promote inflammation and cardiac hypertrophy in myocarditis
(17,18). SP may also be involved in heart
failure caused by hypertension or stress by promoting expression of
MMPs (19). However, the effects
of SP on doxorubicin-induced heart failure remain to be
elucidated.
Autophagy is one type of cellular degradation, which
functions to remove unnecessary or damaged components (20,21). Inappropriate autophagy is
associated with several diseases, including cardiac and
neurodegenerative diseases (22).
The present study used cellular and animal experiments to
investigate the effects of SP on cardiomyocyte injury caused by
doxorubicin. Additionally, the potential mechanisms involving
autophagy were investigated. The data demonstrated that SP limited
doxorubicin-induced cardiomyocyte injury, probably by regulating
apoptosis and autophagy. These results have implications for the
prevention of doxorubicin-induced heart failure.
Materials and methods
Ethics statement
The animal protocols performed in the present study
were approved by the Ethics Committee of People's Hospital
Affiliated to Nanchang University (Nanchang, China; approval no.
2019-037). After the experiments, animals were anesthetized by 5%
isoflurane, followed by decapitation.
Cell culture and treatments
H9c2 myocardial cells were purchased from BeNa
Culture Collection (Beijing Beina Chunglian Institute of
Biotechnology; cat. no. BNCC295057) and were cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
in an incubator with 5% CO2 at 37°C. The effects of SP
on the viability of H9c2 cells were determined using the Cell
Counting Kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology).
The protective effects of SP on doxorubicin-induced cell injury
were evaluated as follows: After cells had adhered to the
substrate, culture medium was discarded and the cells were washed
with 1X phosphate buffered saline (PBS). Doxorubicin (2 µM; cat.
no. D107159; Shanghai Aladdin Biochemical Technology Co., Ltd.;
model group) or 2 µM doxorubicin + 1 µg/ml exogenous SP (cat. no.
HY-P0201A; MedChemExpress) medium (SP group) were then added to
paired wells; after 24 h at 37°C, flow cytometry and western
blotting were performed. The cells in the control group did not
receive any treatment.
CCK-8 assay
After H9c2 cells had completely adhered to the well,
the supernatant was discarded and different concentrations of SP
(0, 0.1, 0.5, 1, 5 and 10 µg/ml) suspended in fresh culture medium
were added to the cells. The cells were cultured in an incubator at
37°C for 24 h and cell viability was assessed to screen for safe
concentrations of SP. Briefly, following treatment, 10 µl CCK-8
solution was added to each well and incubated at 37°C for 1.5 h.
Optical density was measured at a 450 nm using a microplate reader
to calculate cell viability at each SP concentration and 1 µg/ml SP
was selected to investigate its effect on doxorubicin-induced
cardiomyocyte injury.
Flow cytometry
Following treatment for 24 h, cells were collected,
washed with PBS and centrifuged at 897 × g for 3 min at 4°C.
Annexin V-fluorescein isothiocyanate (3 µl) and propidium iodide (5
µl) were added to the cells according to the instructions of the
assay kit (cat. no. C1062S; Beyotime Institute of Biotechnology).
After gentle mixing, the cells were incubated at room temperature
for 10 min in the dark and apoptosis was measured by flow cytometry
(NovoCyte® 2060R; ACEA Biosciences, Inc.) and analyzed
using FlowJo 7.6 (FlowJo, LLC), The percentage of early + late
apoptotic cells were counted.
Preparation of a heart-failure model
and treatments
A total of 18 male Sprague Dawley rats (age, 2
months; weight, 200±20 g) were purchased from Hunan Slake Jingda
Experimental Animal Co., Ltd. [license no. SCXK (Xiang) 2019-0004].
The animals were housed in a specific pathogen-free condition that
was automatically maintained at a temperature of 23±2°C, a relative
humidity of 45–65%, and with a controlled 12 h light/dark cycle and
free to access to food and water. The animals were divided into
three groups (n=6/group): i) Control group; ii) model group; and
iii) SP treatment group. The heart-failure rat model was prepared
as previously described (23).
Briefly, doxorubicin was injected intraperitoneally once every 3
days (3 mg/kg) with a cumulative total of 15 mg/kg. The injections
were completed within 2 weeks to establish the rat myocardial
injury model. SP was injected via the caudal vein at a dose of 6.7
µg/kg once every 4 days as previously described (24). The rats in the control and model
groups were injected with the same amount of saline. An
electrocardiogram (ECG) was used to monitor heart function after
treatment and heart rate was automatically recorded. Thereafter,
the rats were decapitated following anesthesia (5% isoflurane).
Myocardial tissue was collected and fixed in 4% paraformaldehyde at
4°C overnight for determination of pathological changes.
Hematoxylin and eosin (H&E)
staining
Fixed tissue was washed with running water for
several hours, serially dehydrated in 70, 80 and 90% ethanol, a
mixture of ethanol and xylene for 15 min, and then xylene for 30
min. The tissues were subsequently immersed in a mixture of xylene
and paraffin for 15 min, and then in paraffin for 50–60 min. The
paraffin-embedded tissues were then sectioned (10 µm). After
warming, dewaxing and rehydrating, the sections were stained with
hematoxylin (3%) and eosin (3%) for 5 min at room temperature. The
sections were observed under a light microscope (BX53, Olympus
Corporation).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
After dewaxing (xylene treatment for 10 min) and
rehydrating in a gradient ethanol series at room temperature, 50
µg/ml proteinase K was added and the sections were incubated at
37°C for 30 min. The tissues were then washed with PBS three times
(5 min/wash). The PBS was removed and TUNEL solution (5 µl/ml;
Beyotime Institute of Biotechnology) was added to each slide and
incubated at 45°C for 2 h in the dark, followed by DAPI (5 µg/ml)
staining at room temperature for 5 min. The liquid on the slide was
dried using absorbent paper and the slide was sealed and observed
under a fluorescence microscope.
Transmission electron microscopy
(TEM)
Myocardial tissues were placed in 2.5%
glutaraldehyde at 4°C for 4 h followed by fixation with 1% osmium
tetroxide for 1.5 h. and washed three times with pre-cooled PBS.
After dehydration with a gradient ethanol series and acetone, the
tissues were incubated in epoxy resin overnight at room temperature
prior to sectioning (2 nm slices). After that, the slices were
stained with 2% uranyl acetate and 0.5% lead citrate for 5 min at
room temperature. Autophagic ultrastructure was observed by
transmission electron microscopy (HT7700; Hitachi High-Technologies
Corporation; magnification, ×8,000).
Western blotting
Myocardial tissue was ground into powder in liquid
nitrogen. Proteins were extracted from tissues and H9c2 cells using
a protein isolation kit (cat. no. 28-9425-44; Cytiva) and protein
concentration was determined by the bicinchoninic acid method. The
proteins (25 µg/lane) were denatured and separated by sodium
dodecyl sulfate polyacrylamide gel electrophoresis for 2 h (12%
gel), followed by transfer to a nitrocellulose membrane (300 mA was
applied for 80 min) as described previously (25,26). The membranes were blocked in 5%
skimmed milk for 2 h at room temperature. Thereafter, the membrane
was incubated with primary antibodies at 4°C overnight, and then
after washing, the membrane was incubated with a secondary antibody
(horseradish peroxidase-labeled goat anti-rabbit IgG; 1:100; cat.
no. ab6721; Abcam) at room temperature for 2 h. Dye solution from
an enhanced chemiluminescence kit (cat. no. RPN2133; Cytiva) was
added to the membrane and staining was visualized using a gel
imaging system (Bio-Rad Laboratories, Inc.). The gray value was
analyzed by Quantity One software (version 4.62; Bio-Rad
Laboratories, Inc.). The primary antibodies included mouse
monoclonal anti-β-actin (1:2,000; cat. no. TA-09; OriGene
Technologies, Inc.), mouse anti-B-cell lymphoma 2 (Bcl-2; 1:500;
cat. no. ab692; Abcam), rabbit anti-Bcl-2-associated X protein
(Bax; 1:500; cat. no. A0207; ABclonal Biotech Co., Ltd.), rabbit
anti-Beclin-1 (1:1,000; cat. no. ab62557; Abcam) and rabbit
anti-microtubule-associated protein 1A/1B-light chain 3 (LC3-II;
1:500; cat. no. bs-8878R; BIOSS).
Statistical analysis
All data were expressed as the mean ± standard
deviation with six repeats in both the animal and cell culture
experiments. Statistical analysis was carried out with GraphPad
Prism 7 (GraphPad Software, Inc.) using one-way ANOVA followed by
the Bonferroni test. P<0.05 was considered to indicate a
statistically significant difference.
Results
SP inhibits doxorubicin-induced
apoptosis of H9c2 cells
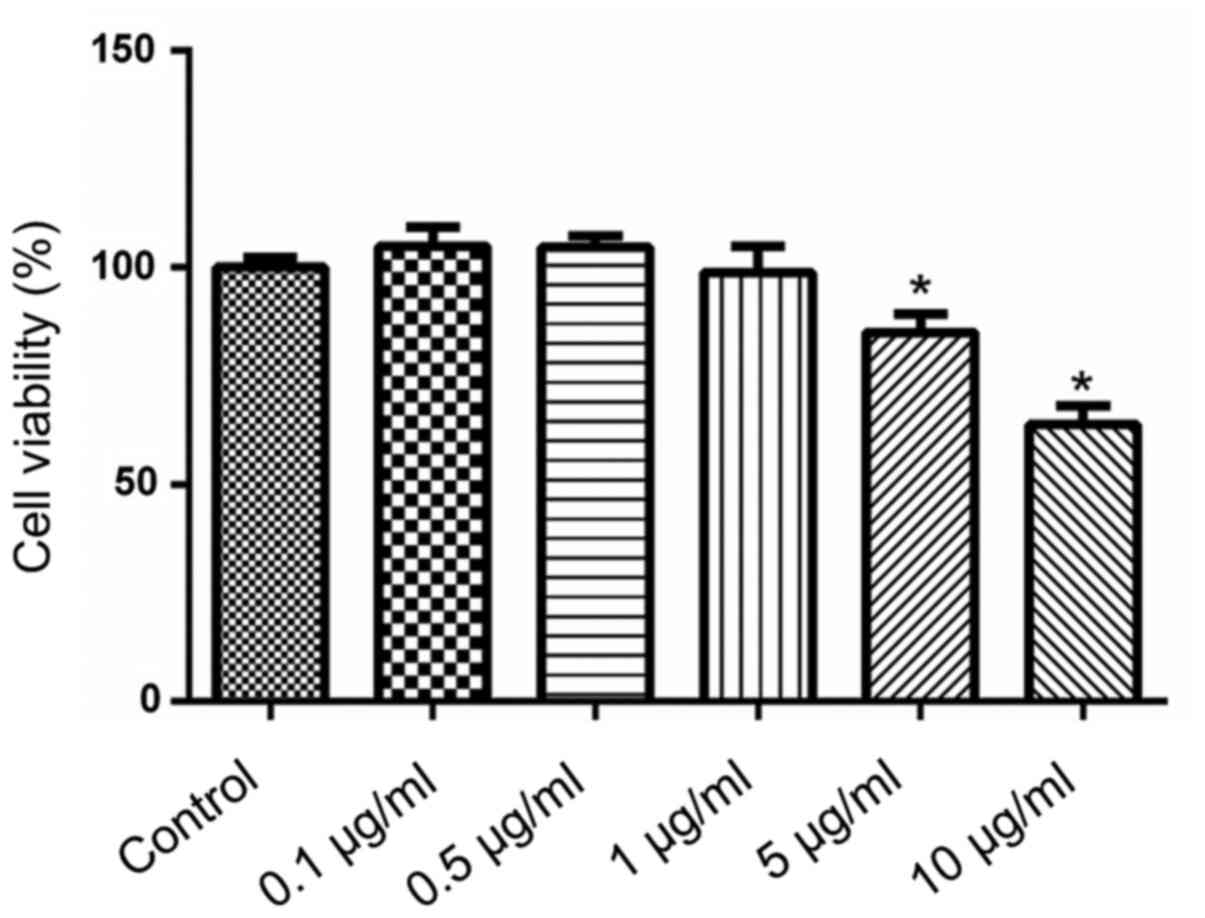
H9c2 myocardial cells were treated with different
concentrations of SP for 24 h. Viability of H9c2 cells was detected
using the CCK-8 method. SP at concentrations ranging between 0.1
and 1.0 µg/ml did not affect H9c2 cell viability (Fig. 1). By contrast, 5 or 10 µg/ml SP
significantly reduced cell viability compared with untreated
controls.
Using the results of the CCK-8 assay, 1 µg/ml SP was
selected to investigate the effect of SP on doxorubicin-induced
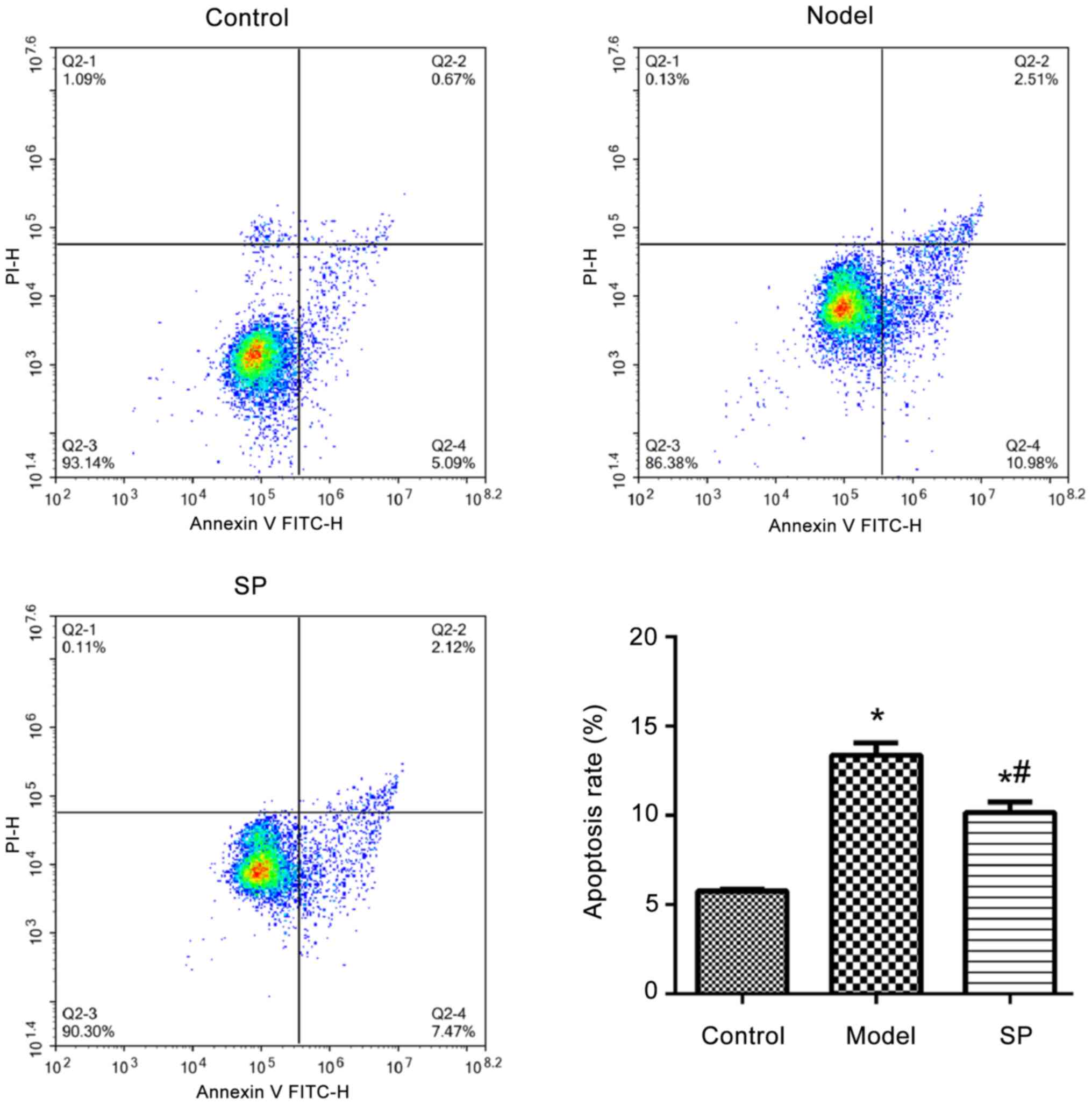
injury of H9c2 cells. The apoptosis of H9c2 cells treated with
doxorubicin was significantly higher compared with that detected in
the control H9c2 cells, whereas SP significantly reduced this
effect (Fig. 2).
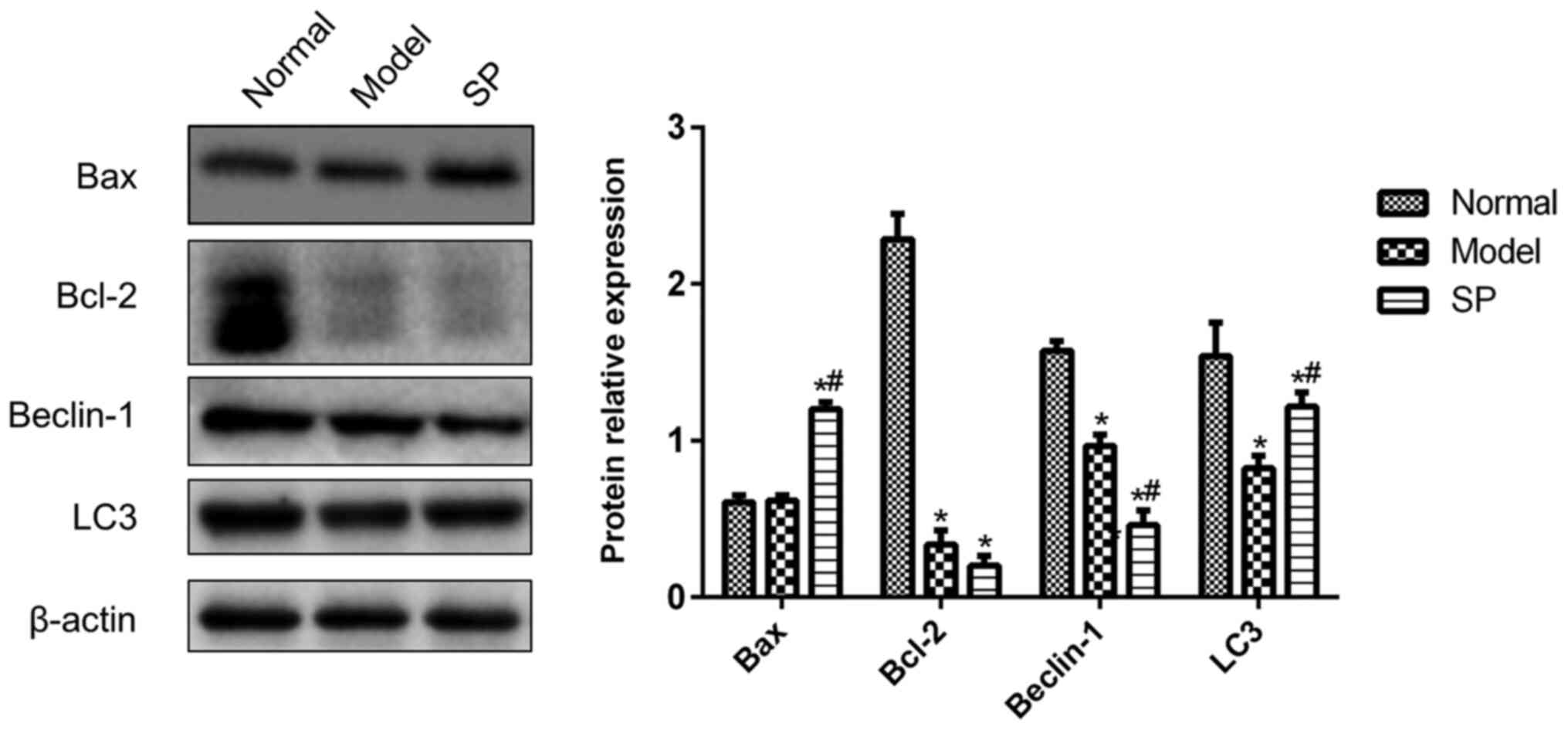
Effects of SP on the expression levels
of Bax, Bcl-2, Beclin-1 and LC3 in doxorubicin-treated H9c2
cells
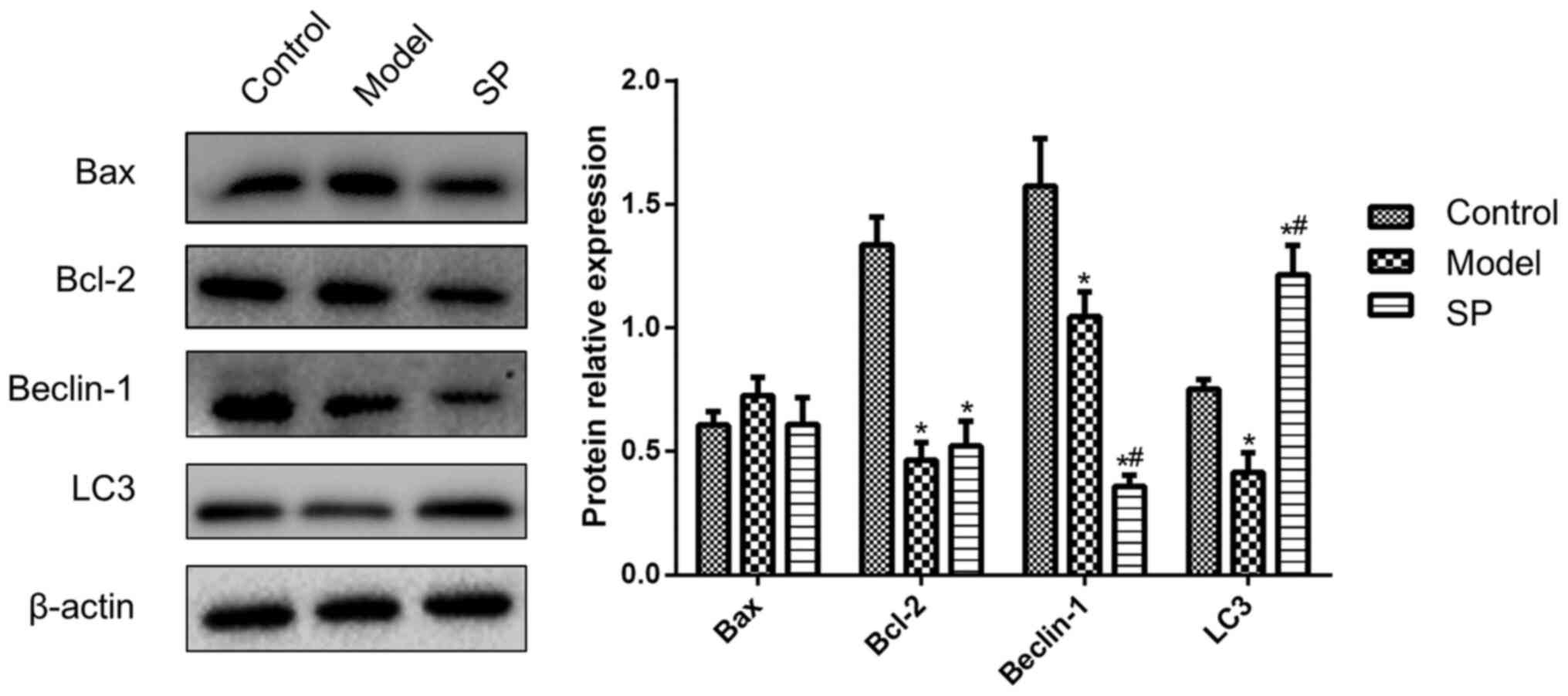
In order to further explore the specific effects of
SP on apoptosis and autophagy, the protein expression levels of
Bcl-2, Bax, Beclin-1 and LC3 were monitored in H9c2 cells. As shown
in Fig. 3, Bcl-2 expression in
doxorubicin-treated H9c2 cells was significantly lower compared
with that in the control group, whereas the addition of SP to the
doxorubicin-treated cells had no effect on Bcl-2 expression. Bax
expression was comparable in all three groups, which indicated that
Bax was unaffected by doxorubicin and SP.
Compared with in the control H9c2 cells, doxorubicin
treatment reduced the expression levels of Beclin-1 and LC3. SP
further reduced Beclin-1 expression, but inhibited the
doxorubicin-induced decrease in LC3 (Fig. 3). These data suggested that SP
might rescue doxorubicin-induced autophagy dysfunction.
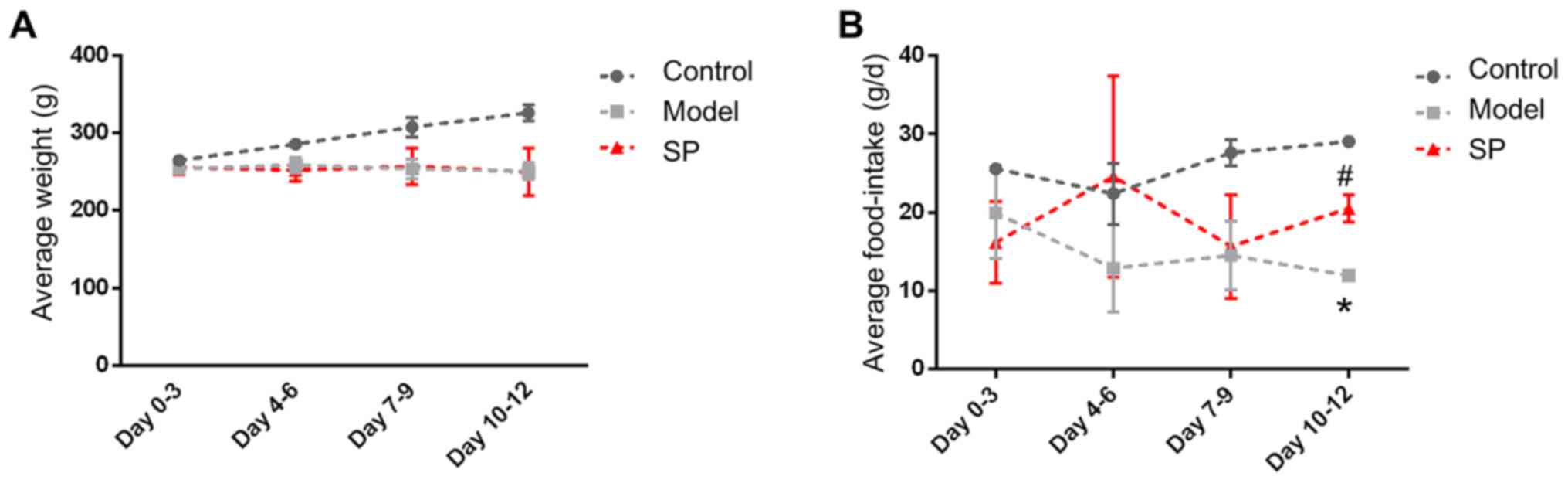
SP increases food intake in rats with
heart failure
Of the 12 rats used in the present study, none died
during the experiments. Compared with the control rats, the food
intake and body weight of the rats in the heart-failure group were
significantly decreased (Fig. 4).
Administration of SP to the heart-failure rats was associated with
no significant change in body weight for 12 days; however, food
intake rose on days 10–12.
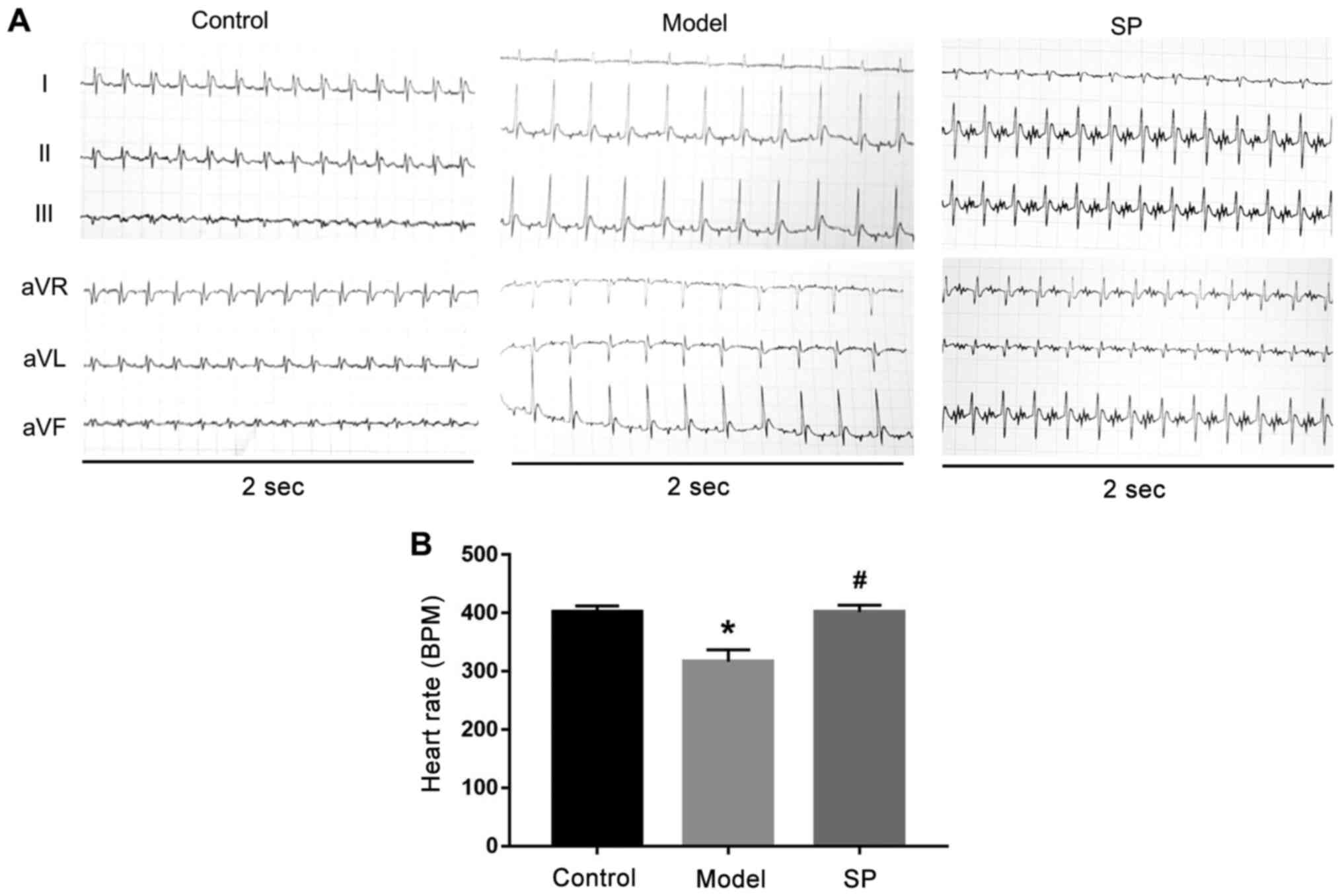
Subsequently, a six-lead ECG was used to monitor
heart function and representative traces are shown in Fig. 5. Heart rate was also quantified in
the three groups. Compared with in the control group, heart rate in
the heart-failure group was significantly decreased, whereas SP
treatment inhibited this decrease (Fig. 5).
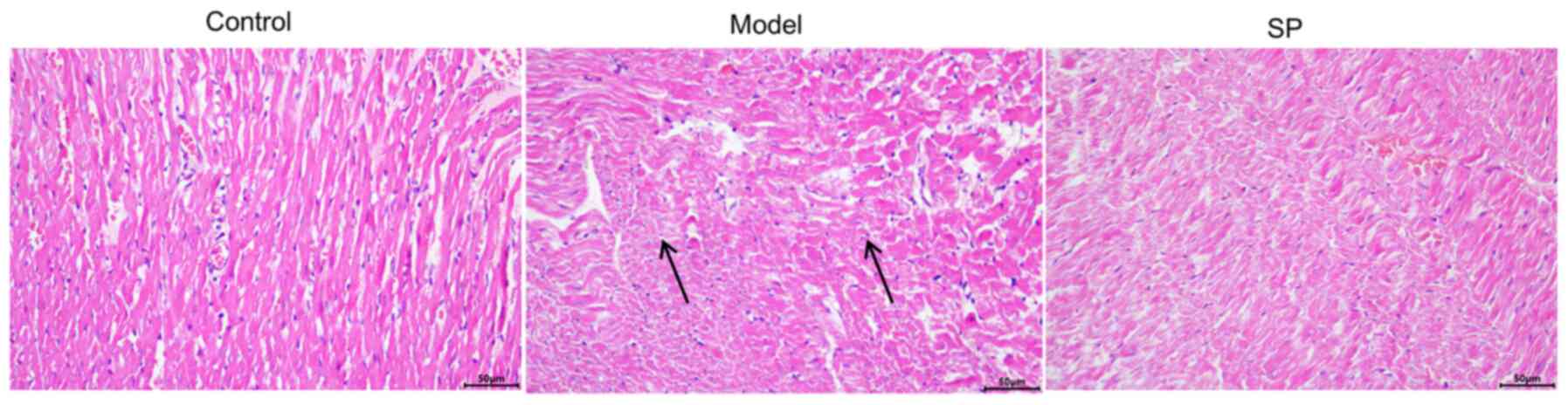
Histological observation of rat
myocardium
The results of H&E staining of rat myocardial
tissue are shown in Fig. 6.
Compared with in the control group, the cardiomyocytes in the
doxorubicin-induced heart-failure group were arranged in a
disordered pattern and were loosely connected. SP treatment
ameliorated these pathological changes caused by doxorubicin.
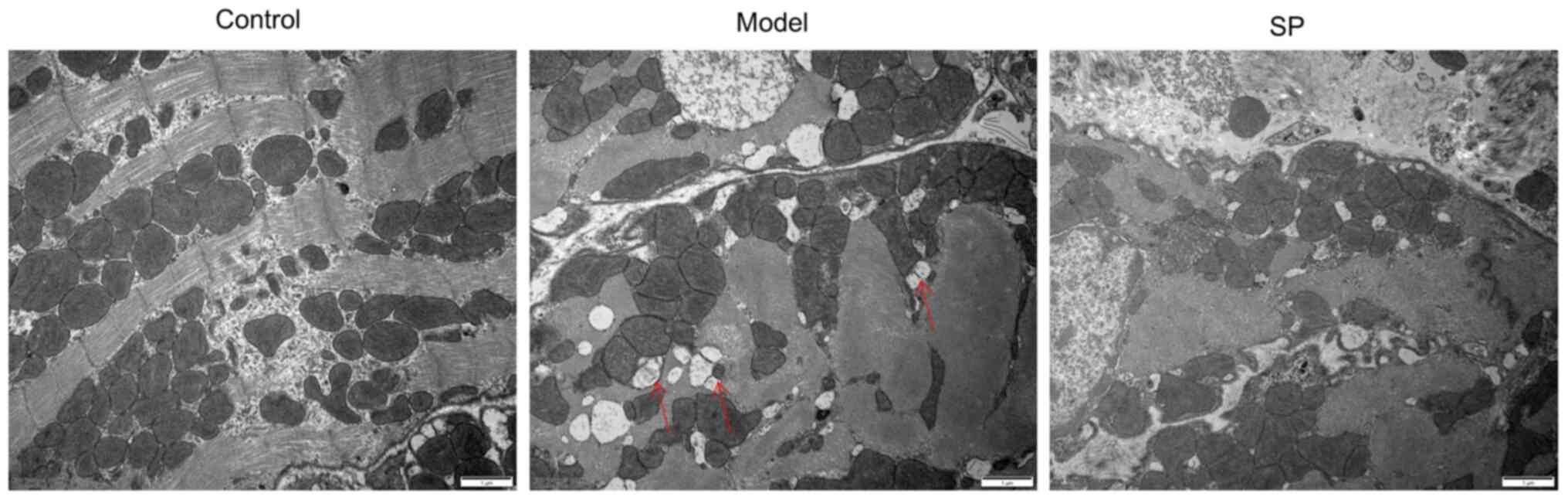
Compared with in the control group, cardiomyocytes
in the heart-failure group exhibited a loss of striations,
vacuolation with damaged organelle, indicating dysfunction of
autophagy. By contrast, SP reduced the loss of striations, as well
as vacuolation (Fig. 7).
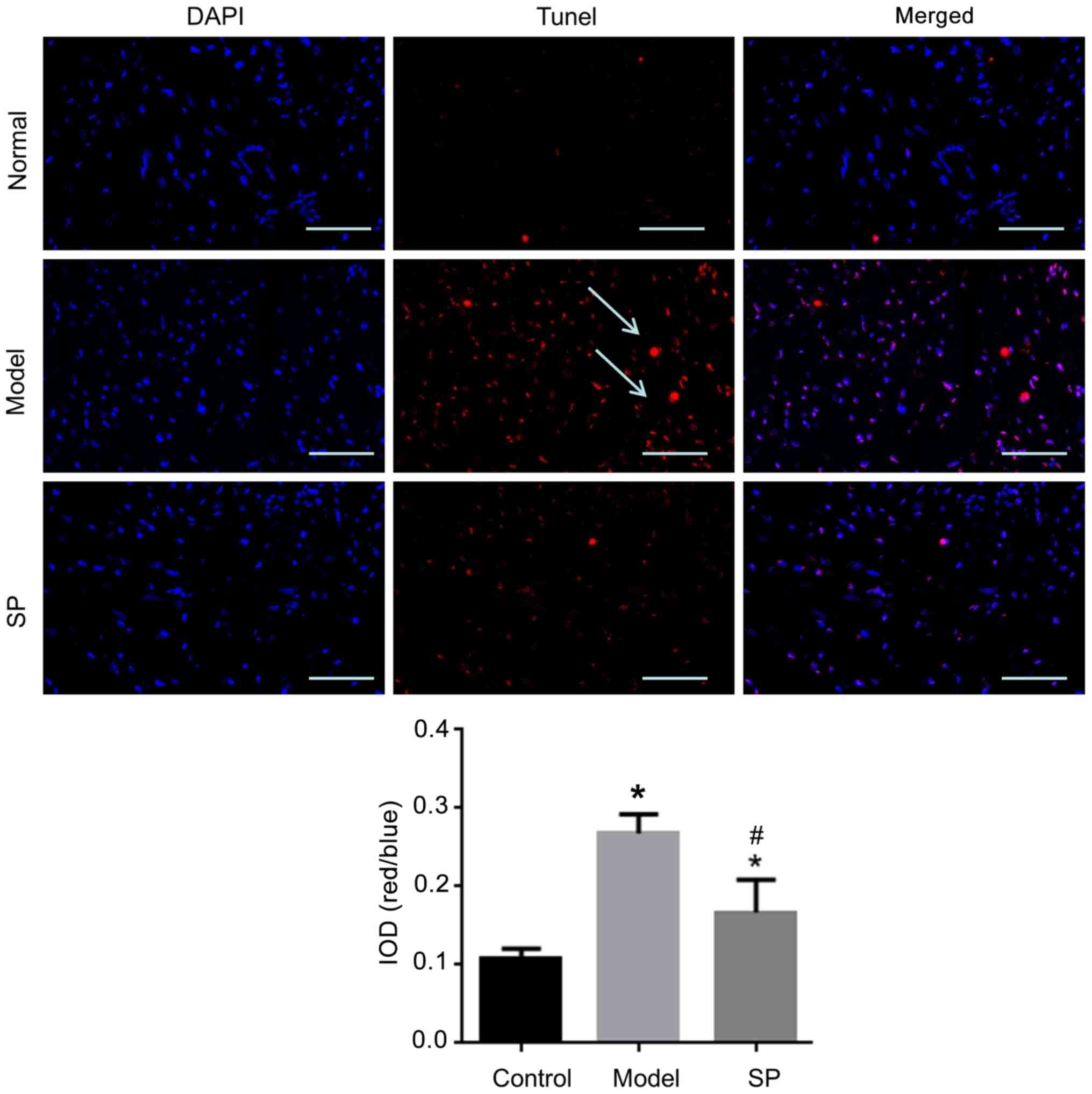
SP reduces cardiomyocyte apoptosis in
rats with heart failure
TUNEL staining was used to determine the level of
apoptosis in myocardial tissue. Apoptosis in myocardial tissue from
the heart-failure group was significantly higher compared with that
in the control group, whereas this effect was inhibited by SP
treatment (Fig. 8).
Effects of SP on the expression levels
of Bax, Bcl-2, Beclin-1 and LC3 in heart-failure rats
Compared with in the control group, there was a
significant decrease in the protein expression levels of Bcl-2,
Beclin-1 and LC3, but not Bax, in the heart-failure group (Fig. 9). SP inhibited these changes in
LC3, although Beclin-1 expression was further reduced.
Additionally, SP increased Bax expression, while it did not affect
Bcl-2 expression compared with the model group. These results
indicated that SP promoted autophagy, while reducing apoptosis.
Discussion
In the present study, the rate of apoptosis of H9c2
cells was increased following doxorubicin treatment. Pathological
changes of myocardial tissue in doxorubicin-treated rats were
observed. By contrast, SP protected against doxorubicin-induced
injury of H9c2 cells and heart tissue. The present study provided
evidence that SP limited apoptosis and triggered autophagy, which
potentially has implications for therapeutic applications.
Doxorubicin is a common chemotherapeutic drug, which
acts primarily through the induction of apoptosis by inhibiting
topoisomerase activity and generating ROS (1). Cardiomyocytes are susceptible to
doxorubicin; therefore, cardiotoxicity is a prominent side effect
(3), and doxorubicin is widely
used to produce animal and cellular models of heart failure
(4,5). The present study found that
doxorubicin triggered cardiotoxicity, as evidenced by an increase
in apoptosis and apoptosis-related protein (Bcl-2) expression.
Notably, it was found that a safe concentration of SP prevented
doxorubicin-induced apoptosis of H9c2 cells. These data suggested
that SP at specific concentrations may prevent doxorubicin-induced
cardiotoxicity.
The present study demonstrated that 1 µg/ml SP had
no significant effect on the viability of H9c2 cells, but reduced
doxorubicin-induced apoptosis of H9c2 cells. In addition, the
experimental results demonstrated that doxorubicin could reduce the
expression levels of Beclin-1 and LC3 in H9c2 cells, whereas SP
further reduced the expression of Beclin-1 but increased the
expression of LC3. As the mammalian ortholog of the yeast Atg6
gene, Beclin-1 is an essential mediator of autophagy. In addition,
it has also been reported that Beclin 1 may have a proapoptotic
role (27). By contrast, LC3 can
be used to indicate autophagy (28). Therefore, the present study
indicated that SP may promote autophagy and reduce apoptosis.
In the apoptosis cascade, Bcl-2 and Bax balance each
other in controlling release of cytochrome c from mitochondria to
determine cell death (29).
Therefore, the expression levels of Bcl-2 and Bax are considered to
be representative of apoptosis (30). The present study detected Bcl-2
and Bax expression in H9c2 cells and heart tissue that had
experienced doxorubicin-induced injury. It was observed that
doxorubicin reduced Bcl-2 expression in vitro and in
vivo, whereas it did not affect Bax, which implicated apoptosis
in doxorubicin-induced injury of cardiomyocytes (31,32). Notably, SP could reverse the
effect of doxorubicin on the Bcl-2/Bax ratio. These data further
support the conclusion that SP can prevent doxorubicin-induced
apoptosis of cardiomyocytes.
The present study also investigated the effects of
SP on food intake and body weight in rats with heart failure. The
data demonstrated that SP could increase food intake following
heart failure. Reduction of heart rate is an important functional
index of heart failure (33). The
present study used an ECG to monitor heart function. Heart rate was
reduced in model group, indicating the heart function was impaired.
By contrast, SP could reduce the impairment. The results of H&E
staining also suggested that SP could repair doxorubicin-induced
morphological changes in heart tissue. Taken together, these data
support the conclusion that SP can block doxorubicin-induced heart
malfunction.
Autophagy is considered to be an important mechanism
of organelle and protein turnover in cells (20). Autophagy can prevent apoptosis in
cases of mild external stimulation, and the activation of
apoptosis-related caspase-3 can block autophagy (34–36). The present findings indicated that
SP could reduce doxorubicin-induced cardiomyocyte apoptosis by
increasing autophagy in myocardial tissue, which suggested a
potential for SP in the treatment of heart failure.
The data in the present study contradicted some
earlier publications (37,38)
in which SP antagonists inhibited doxorubicin-induced cardiomyocyte
apoptosis and triple-negative breast cancer chemoresistance. These
discrepancies might be caused in part by bidirectional regulation
of SP signaling pathways, as discussed in a previous review in
which SP was shown to have beneficial as well as detrimental
effects on heart failure (39).
The duration and dose of SP might also control its various
functions. The present study revealed that high doses of SP were
detrimental to H9c2 cells, whereas low doses exerted protection
against doxorubicin-induced cardiomyocyte injury. This observation
might have important implications for clinical applications of SP
and warrants further investigation.
The present study demonstrated a mechanism by which
SP reduced cardiomyocyte apoptosis in doxorubicin-induced
cardiomyocyte injury, potentially by promoting autophagy. However,
the effects of SP on doxorubicin-induced oxidative stress deserve
future investigation. Additionally, the direct relationship between
apoptosis and autophagy still requires investigation.
In conclusion, the present study indicated that SP
reduced cardiomyocyte apoptosis, potentially by promoting
autophagy, in a rat model of doxorubicin-induced heart failure,
which indicated that SP might be a potential therapeutic substance
for heart failure.
Acknowledgements
Not applicable.
Funding
This work was supported by the Jiangxi Science and Technology
Project (grant no. 20192BBGL70031).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FXC, QW, QLL, JF and LP performed the experiments
and analyzed the data. FC and JH designed the study, wrote the
manuscript and confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Ethics Committee of People's Hospital Affiliated to Nanchang
University (Nanchang, China; approval no. 2019-037).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Taheri M, Mahmud Hussen B, Tondro Anamag
F, Shoorei H, Dinger ME and Ghafouri-Fard S: The role of miRNAs and
lncRNAs in conferring resistance to doxorubicin. J Drug Target. Apr
15–2021.(Epub ahead of print.). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Conklin KA: Chemotherapy-associated
oxidative stress: Impact on chemotherapeutic effectiveness. Integr
Cancer Ther. 3:294–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shabalala S, Muller CJF, Louw J and
Johnson R: Polyphenols, autophagy and doxorubicin-induced
cardiotoxicity. Life Sci. 180:160–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitry MA and Edwards JG: Doxorubicin
induced heart failure: Phenotype and molecular mechanisms. Int J
Cardiol Heart Vasc. 10:17–24. 2016.PubMed/NCBI
|
|
5
|
Jiang Y, Liu Y, Xiao W, Zhang D, Liu X,
Xiao H, You S and Yuan L: Xinmailong attenuates doxorubicin-induced
lysosomal dysfunction and oxidative stress in H9c2 cells via HO-1.
Oxid Med Cell Longev. 2021:58969312021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Narula J, Haider N, Arbustini E and
Chandrashekhar Y: Mechanisms of disease: Apoptosis in heart failure
- seeing hope in death. Nat Clin Pract Cardiovasc Med. 3:681–688.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Empel VP, Bertrand AT, Hofstra L,
Crijns HJ, Doevendans PA and De Windt LJ: Myocyte apoptosis in
heart failure. Cardiovasc Res. 67:21–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pennefather JN, Lecci A, Candenas ML,
Patak E, Pinto FM and Maggi CA: Tachykinins and tachykinin
receptors: A growing family. Life Sci. 74:1445–1463. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brain SD and Cox HM: Neuropeptides and
their receptors: Innovative science providing novel therapeutic
targets. Br J Pharmacol. 147 (Suppl 1):S202–S211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Massaad CA, Safieh-Garabedian B, Poole S,
Atweh SF, Jabbur SJ and Saadé NE: Involvement of substance P, CGRP
and histamine in the hyperalgesia and cytokine upregulation induced
by intraplantar injection of capsaicin in rats. J Neuroimmunol.
153:171–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vergnolle N, Bunnett NW, Sharkey KA,
Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI,
Andrade-Gordon P, et al: Proteinase-activated receptor-2 and
hyperalgesia: A novel pain pathway. Nat Med. 7:821–826. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meléndez GC, Li J, Law BA, Janicki JS,
Supowit SC and Levick SP: Substance P induces adverse myocardial
remodelling via a mechanism involving cardiac mast cells.
Cardiovasc Res. 92:420–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johnson MB, Young AD and Marriott I: The
Therapeutic potential of targeting substance P/NK-1R interactions
in inflammatory CNS disorders. Front Cell Neurosci. 10:2962017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hua F, Ricketts BA, Reifsteck A, Ardell JL
and Williams CA: Myocardial ischemia induces the release of
substance P from cardiac afferent neurons in rat thoracic spinal
cord. Am J Physiol Heart Circ Physiol. 286:H1654–H1664. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang LL, Guo Z, Han Y, Wang PF, Zhang RL,
Zhao YL, Zhao FP and Zhao XY: Implication of Substance P in
myocardial contractile function during ischemia in rats. Regul
Pept. 167:185–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amadesi S, Reni C, Katare R, Meloni M,
Oikawa A, Beltrami AP, Avolio E, Cesselli D, Fortunato O, Spinetti
G, et al: Role for substance p-based nociceptive signaling in
progenitor cell activation and angiogenesis during ischemia in mice
and in human subjects. Circulation. 125:1774–1786, S1-S9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robinson P, Garza A, Moore J, Eckols TK,
Parti S, Balaji V, Vallejo J and Tweardy DJ: Substance P is
required for the pathogenesis of EMCV infection in mice. Int J Clin
Exp Med. 2:76–86. 2009.PubMed/NCBI
|
|
18
|
Mak IT, Chmielinska JJ, Kramer JH, Spurney
CF and Weglicki WB: Loss of neutral endopeptidase activity
contributes to neutrophil activation and cardiac dysfunction during
chronic hypomagnesemia: Protection by substance P receptor
blockade. Exp Clin Cardiol. 16:121–124. 2011.PubMed/NCBI
|
|
19
|
Cury PR, Canavez F, de Araújo VC, Furuse C
and de Araújo NS: Substance P regulates the expression of matrix
metalloproteinases and tissue inhibitors of metalloproteinase in
cultured human gingival fibroblasts. J Periodontal Res. 43:255–260.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu G, Wang X, Wu S, Li X and Li Q:
Neuroprotective effects of puerarin on
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson's
disease model in mice. Phytother Res. 28:179–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y and Klionsky DJ: Autophagy and
disease: Unanswered questions. Cell Death Differ. 27:858–871. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu X, Zhang N, Kan J, Tang S, Sun R, Wang
Z, Chen M, Liu J and Jin C: Polyphenols from Arctium lappa L
ameliorate doxorubicin-induced heart failure and improve gut
microbiota composition in mice. J Food Biochem. Apr 17–2021.(Epub
ahead of print). View Article : Google Scholar
|
|
24
|
Piao J, Park JS, Hwang DY, Son Y and Hong
HS: Substance P blocks ovariectomy-induced bone loss by modulating
inflammation and potentiating stem cell function. Aging (Albany
NY). 12:20753–20777. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Z, Song Z, Shen F, Xie P, Wang J,
Zhu AS and Zhu G: Ginsenoside Rg1 prevents PTSD-like behaviors in
mice through promoting synaptic proteins, reducing Kir4.1 and TNF-α
in the hippocampus. Mol Neurobiol. 58:1550–1563. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen F, Song Z, Xie P, Li L, Wang B, Peng
D and Zhu G: Polygonatum sibiricum polysaccharide prevents
depression-like behaviors by reducing oxidative stress,
inflammation, and cellular and synaptic damage. J Ethnopharmacol.
275:1141642021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang X, Qi Q, Hua X, Li X, Zhang W, Sun
H, Li S, Wang X and Li B: Beclin 1, an autophagy-related gene,
augments apoptosis in U87 glioblastoma cells. Oncol Rep.
31:1761–1767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Runwal G, Stamatakou E, Siddiqi FH, Puri
C, Zhu Y and Rubinsztein DC: LC3-positive structures are prominent
in autophagy-deficient cells. Sci Rep. 9:101472019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Q and Zhang L, Yuan X, Ou Y, Zhu X,
Cheng Z, Zhang P, Wu X, Meng Y and Zhang L: The Relationship
between the Bcl-2/Bax proteins and the mitochondria-mediated
apoptosis pathway in the differentiation of adipose-derived stromal
cells into neurons. PLoS One. 11:e01633272016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Xu W, Chen H, Li W, Li W and Zhu
G: Astragaloside IV prevents Abeta1–42 oligomers-induced memory
impairment and hippocampal cell apoptosis by promoting
PPARgamma/BDNF signaling pathway. Brain Res.
147041:20201747.PubMed/NCBI
|
|
31
|
Korsmeyer SJ, Shutter JR, Veis DJ, Merry
DE and Oltvai ZN: Bcl-2/Bax: A rheostat that regulates an
anti-oxidant pathway and cell death. Semin Cancer Biol. 4:327–332.
1993.PubMed/NCBI
|
|
32
|
Andreu-Fernández V, Sancho M, Genovés A,
Lucendo E, Todt F, Lauterwasser J, Funk K, Jahreis G, Pérez-Payá E,
Mingarro I, et al: Bax transmembrane domain interacts with
prosurvival Bcl-2 proteins in biological membranes. Proc Natl Acad
Sci USA. 114:310–315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu S, Cao J, Zhang T, Zhou Y, Wang K, Zhu
G and Zhou M: Electroacupuncture ameliorates the coronary occlusion
related tachycardia and hypotension in acute rat myocardial
ischemia model: potential role of hippocampus. Evid Based
Complement Alternat Med. 2015:9259872015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Youle RJ and Narendra DP: Mechanisms of
mitophagy. Nat Rev Mol Cell Biol. 12:9–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pagliarini V, Wirawan E, Romagnoli A,
Ciccosanti F, Lisi G, Lippens S, Cecconi F, Fimia GM, Vandenabeele
P, Corazzari M, et al: Proteolysis of Ambra1 during apoptosis has a
role in the inhibition of the autophagic pro-survival response.
Cell Death Differ. 19:1495–1504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Legi A, Rodriguez E, Eckols TK, Mistry C
and Robinson P: Substance P antagonism prevents
chemotherapy-induced cardiotoxicity. Cancers (Basel). 13:17322021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Robinson P, Kasembeli M, Bharadwaj U,
Engineer N, Eckols KT, Tweardy DJ and Substance P: Substance P
receptor signaling mediates doxorubicin-induced cardiomyocyte
apoptosis and triple-negative breast cancer chemoresistance. BioMed
Res Int. 2016:19592702016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dehlin HM and Levick SP: Substance P in
heart failure: The good and the bad. Int J Cardiol. 170:270–277.
2014. View Article : Google Scholar : PubMed/NCBI
|