Introduction
As the main type of primary liver cancer,
hepatocellular carcinoma (HCC) is the most common malignant tumor
and one of the most fatal types of cancer in the world today
(1) According to the latest global
cancer burden data released by the International Agency for
Research on Cancer, primary liver cancer is the sixth leading cause
of cancer, with 906,000 new cases per year, and is the third
leading cause of mortality, with 830,000 cases per year (2). A liver cancer microenvironment, the
tissue around the tumor, contains a complex mixture of non-HCC
cells and extracellular matrix that has the potential to promote
the occurrence and development of liver cancer (3). Epidemiological studies have revealed
that HCC displays a unique background of chronic liver injury
(4). Globally, 80% of liver cancer
is caused by occult hepatitis and viral infection-induced chronic
hepatitis (5). The resulting liver
inflammatory response and the development of liver fibrosis and
liver nodules are directly related to the formation of liver cancer
(6). Moreover, before the formation
of liver cancer, there is long-term development of precancerous
lesions (7).
Previous evidence has suggested that the cytological
origin of HCC is derived from the abnormal differentiation of stem
or oval cells in the liver (8).
Hepatic oval cells (HOCs) are endogenous stem cells of the liver
that can differentiate into liver cells for organ regeneration or
transform into liver cancer cells to form malignant tumors
(9). When the liver is severely and
permanently damaged and the regeneration ability of mature
hepatocytes is inhibited, HOCs proliferate in large quantities,
thus replacing hepatocytes to repair liver tissue (10). Under certain circumstances, atypical
hyperplasia occurs. At this time, the morphological features,
ultrastructure, enzymology and surface markers of liver tissue
suggest that normal liver cells gradually transform to cancer
cells, then to fibrosis or nodular lesions, and finally to HCC
(11). The Wnt-1/β-catenin
signaling pathway serves an important role in regulating the
proliferation of various types of cells and the maintenance and
differentiation of stem cells (12). It has been confirmed that the
abnormal activation of the Wnt-1/β-catenin signaling pathway is one
important factor in hepatocarcinogenesis induction (13). Under normal circumstances, Wnt-1
signaling is necessary for tissue repair and regeneration (14). β-catenin is a key downstream
component of Wnt-1 signaling pathways and can regulate hepatocyte
proliferation (15).
Kangxianruangan granule (KXRG) is a compound granule
of Traditional Chinese Medicine (TCM) that is composed of Seaweed,
Hawthorn, Salvia miltiorrhiza, Rhizoma Curcumae, Carapax
Trionycis and Oyster. In TCM it is considered that KXRG can
treat liver fibrosis and cirrhosis (data not available). However,
whether KXRG alters the liver cancer microenvironment via
anti-liver fibrosis mechanisms, promotes HOC differentiation into
normal hepatocytes, inhibits abnormal HOC proliferation or induces
HOC apoptosis, thereby delaying or blocking the occurrence and
development of liver cancer precancerous lesions is not completely
understood. The molecular pharmacological mechanism underlying KXRG
function in liver protection requires clarification.
In the present study, serum pharmacological methods
were adopted to investigate the effects of KXRG on HOCs and the
molecular changes that are related to the Wnt-1/β-catenin signaling
pathway. The aim was to therefore clarify the internal mechanisms
underlying KXRG and its role in blocking the formation of hepatic
precancerous lesions. The present study provided a foundation for
understanding the mechanisms underlying inhibition of fibrosis in
liver cancer via the Wnt/β-catenin signaling pathway, identifying a
potential treatment option for patients with HCC.
Materials and methods
Reagents
The rat HOC cell line WB-F344 was purchased from
China Center for Type Culture Collection. KXRG was bought from
Hubei Provincial Hospital of Traditional Chinese Medicine. DMEM and
FBS were purchased from Gibco (Thermo Fisher Scientific, Inc.).
N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) was purchased from
Sigma-Aldrich (Merck KGaA). The Cell Counting Kit-8 (CCK-8) assay
was bought from Dojindo Molecular Technologies, Inc.
BugBuster® 10X Protein Extraction Reagent was purchased
from Sigma-Aldrich (cat. no. 70921). α fetoprotein (AFP) ELISA kit
was purchased from Elabscience, Inc. (cat. no. E-EL-R0153c).
Cytokeratin-19 (CK-19) antibody was obtained from Elabscience, Inc.
(cat. no. E-AB-70083). Rabbit anti-rat primary antibodies targeted
against Wnt-1 (cat. no. 27935-1-AP), β-catenin (cat. no.
66379-1-Ig), Cyclin D1 (cat. no. 60186-1-Ig), C-myc (cat. no.
67447-1-Ig), matrix metalloproteinase-7 (MMP-7; cat. no.
10374-2-AP), Axin2 (cat. no. 20540-1-AP), epithelial cell adhesion
molecule (EpCAM; cat. no. 21050-1-AP) and GAPDH (cat. no.
10494-1-AP), as well as HRP-labeled goat anti-rabbit secondary
antibodies (cat. no. PR30009) were purchased from Wuhan Sanying
Biotechnology. FITC-labeled goat anti-mouse secondary antibodies
(cat. no. BA1101) were purchased from Boster Biological Technology.
RNAiso Plus, PrimeScript™ reverse transcription (RT) reagent and
SYBR Premix Ex Taq kits were purchased from Takara Biotechnology
Co., Ltd. The Annexin V-FITC/PI cell apoptosis detection kit and
cell cycle assay kits were bought from NanJing KeyGen Biotech Co.,
Ltd. (cat. no. KGA108). X-tremeGENE TM (cat. no. XTGHP-RO) was
bought from Merck Sharp & Dohme-Hoddesdon. The Wnt-1 plasmid
was purchased from Guangzhou RiboBio Co., Ltd.
Preparation of KXRG-containing drug
serum
A total of 20 male Sprague-Dawley rats weighing
200–220 g (7–8 weeks old) were obtained from the Hubei Provincial
Center for Disease Control and Prevention. Rats were adapted to the
new experimental environment for 3 days and received humanistic
care based on the Guide for the Care and Use of Laboratory Animals
of Institutional Animal Care and Use Committee of Hubei Provincial
Hospital of Chinese Medicine. The housing conditions were as
follows: Temperature, 20–25°C; relative humidity, 40–70%; 12-h
light/dark cycle; fixed water supply system; and feed rate of three
times per day. The present study was approved by the Hubei
Provincial Hospital of Traditional Chinese Medicine Laboratory
Animal Ethics Committee (Wuhan, China; approval no. 2019005).
KXRG was formulated into a 4.06 g/ml stock solution
with 0.9% NaCl. The rats were administered a dose that was l ml/100
g via gavage twice a day for 7 consecutive days. On the 7th day,
the rats were anesthetized by the intraperitoneal injection of 2%
pentobarbital sodium (40 mg/kg). After successful anesthesia, 3–5
ml blood was drawn from the inferior vena cava of each rat. After
1–5 min, the rats were sacrificed by dislocation of the cervical
vertebrae. The blood was then immediately poured into sterile EP
tubes, and left to stand at room temperature for 0.5-1 h before
centrifugation at 3,000 rpm for 10 min. Finally, the supernatant
(serum) was removed and placed at −80°C.
Cell culture and KXRG
administration
The rat HOC cell line WB-F344 was cultured in DMEM
containing 10% FBS at 37°C with 5% CO2. The medium was
replaced every 2–3 days. The cells were divided into the following
groups: i) normal; ii) model; iii) high concentration of
KXRG-containing serum (KXRG-H); iv) middle concentration of
KXRG-containing serum (KXRG-M); and v) low concentration of
KXRG-containing serum (KXRG-L) (16). With the exception of the normal
group, cells were treated with 3 µg/ml MNNG prior to treatment with
KXRG, which was used to induce the transformation of HOCs into
hepatoma cells, for 24 h (17). and
the cells in the normal group were treated with an equal amount of
DMEM. Then, the original KXRG-containing drug serum was applied to
the KXRG-H group, half of the concentration of the original
KXRG-containing drug serum was applied to the KXRG-M group and a
quarter of the concentration of the original KXRG-containing drug
serum was applied to the KXRG-L group; FBS was used to prepare the
half and one-quarter concentrations of the drug-containing serum.
The cells in normal group and model group were treated with DMEM.
The dosage of drug in each group was 20% of the culture medium.
KXRG-treated cells were harvested after a 24-h incubation.
Flow cytometry
Apoptosis, the cell cycle and the expression of
CK-19 protein were determined via flow cytometry. Cells were
digested with 0.25% pancreatin (MedChemExpress; cat. no. HY-B2118)
without EDTA. Rat HOC WB-F344 cells were collected and washed with
PBS. Cell apoptosis was assessed using the AnnexinV-FITC/PI cell
apoptosis detection kit according to the manufacturer's protocol.
Briefly, cells were re-suspended in 500 µl binding buffer mixed
with 5 µl AnnexinV-FITC, then mixed with 5 µl PI and incubated at
room temperature in the dark for 15 min. The cell cycle was
assessed using the cell cycle detection kit according to the
manufacturer's protocol. Cells were washed with PBS, centrifuged
with 350 × g for 5 min at 4°C, fixed with pre-cooled 70% ethanol at
4°C for 1–2 h, washed for a second time and the cell suspension was
stained at 37°C for 15 min with 1 ml PI/Triton X-100 (20 µg PI/0.1%
Triton X-100) containing 0.2 mg RNase. In terms of CK-19 protein
detection, the cells were washed with PBS, fixed with 4%
paraformaldehyde at room temperature for 15 min, washed for a
second time with PBS, permeabilized with 0.5% Triton X-100 for 10
min and added with CK-19 antibody at 4°C for 24 h. The apoptotic
rate, cell cycle and expression of CK-19 protein were detected by
flow cytometry (BD Biosciences).
Cell transfection
When the cells grow to 70–80% confluence,
1:1/plasmid volume: DNA quality was selected to transfect cells. A
serum-free medium of 8 ml was added to a transfection tube. The DNA
of Wnt-1 was shaken and added with 2 ml X-tremeGENE TM (cat. no.
XTGHP-RO) transfection reagent. The mixture was left at room
temperature for 10–15 min. The medium was removed from the culture
plate and wash it once with PBS. The mixture was added and the
cells were returned to the incubator for 1 h. The mixture was
removed, then the complete medium was added and incubated at 37°C
for 24 h. The cells were subcultured again, and then were
re-cultured in a 35-mm Petri dish with a suitable density of
0.8×105 cells and underwent immunostaining. Cells were
cultured under normal conditions for 48 h and then observed under a
fluorescence microscope.
Immunofluorescence assays
To detect the expression of Wnt-1 and β-catenin in
cells, circular slides were placed in 24-well plates. Subsequently,
WB-F344 cells were seeded (1×104) with 400 µl DMEM per
slide. After treatment with KXRG-containing serum, cells were fixed
with 4% paraformaldehyde at 37°C for 30 min. Cells were then
permeabilized with 0.2% Triton X-100 at 37°C for 20 min, blocked
with 5% BSA (Thermo Fisher Scientific, Inc.; cat. no. 37520) at
37°C for 30 min, and then incubated with primary antibodies
targeted against Wnt-1 (1:100) and β-catenin (1:100) at 4°C
overnight. Slides were incubated with Cyanine 3- and FITC-labeled
fluorescent secondary antibodies (both 1:100) at 37°C for 1 h in
the dark, followed by staining with DAPI at 37°C for 5 min in the
dark. Finally, slides were observed under a fluorescence microscope
(magnification, ×400).
ELISA
Cells were collected and centrifuged at 350 × g for
10 min at 4°C, and the supernatant was suctioned. AFP levels in
cell supernatants were determined by performing an ELISA using a
rat AFP ELISA kit according to the manufacturer's protocol.
RT-quantitative PCR (RT-qPCR)
Total RNA was extracted from WB-F344 cells using
RNAiso Plus according to the manufacturer's protocol. To detect
Wnt-1, β-catenin, Cyclin D1, C-myc, MMP-7, Axin2 and EpCAM
expression levels, total RNA was reverse transcribed into cDNA
using the PrimeScript RT Reagent Kit. The following temperature
protocol was used for RT: 7°C for 15 min and 85°C for 5 sec. The
StepOne Plus device (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used to perform the qPCR reactions using the SYBR Premix
Ex Taq kit according to the manufacturer's protocol. The following
thermocycling conditions were used for qPCR: Initial denaturation
at 95°C for 10 sec; and 40 cycles of 95°C for 5 sec and 60°C for 20
sec. Relative mRNA expression levels were quantified using the
2−ΔΔCq method (18) and
normalized to the internal reference gene GAPDH. All primers are
listed in Table I and were
synthesized by TsingKe Biological Technology.
 | Table I.Gene primer sequences used for
reverse transcription-quantitative PCR. |
Table I.
Gene primer sequences used for
reverse transcription-quantitative PCR.
| Gene | Sequence
(5′→3′) |
|---|
| GAPDH | F:
ACAGCAACAGGGTGGTGGAC |
|
| R:
TTTGAGGGTGCAGCGAACTT |
| Wnt-1 | F:
GAAACCGCCGCTGGAACT |
|
| R:
GAGGTGATTGCGAAGATAAACG |
| β-catenin | F:
CTCTAGTGCAGCTTCTGGGTT |
|
| R:
AGATGGCAGGCTCGGTAATG |
| Cyclin D1 | F:
CCCTGACACCAATCTCCTCAACGAC |
|
| R:
CTCCTCGCAGACCTCTAGCATCCAG |
| C-myc | F:
CGAGCTGAAGCGTAGCTTTT |
|
| R:
CTCGCCGTTTCCTCAGTAAG |
| MMP-7 | F:
GTGGACAAACTGAGGGAA |
|
| R:
CTAAGAACCGAGGCAAGT |
| Axin2 | F:
CTATGCCTGTCTCCTCTAA |
|
| R:
GGTATCCACACATTTCTCC |
| EpCAM | F:
ACGACGGTCTGTATGATCCC |
|
| R:
TAGGTCCTCACTCTCTCGGA |
Western blotting
The protein concentration was determined using the
BCA method and the mass of protein loaded per lane was 1.0, 0.8,
0.6, 0.4 and 0.2 mg/ml. After extracting proteins, the protein was
loaded into SDS-PAGE gel (5% concentrated, 12% separator) for
separation. Separated proteins were transferred onto PVDF
membranes, which were blocked at 4°C overnight with 5% non-fat milk
in PBS. The membranes were washed three times in Tris-buffered
saline [Trise-Base (cat. no. 1115GR500), HCl (cat. no. GB622-89),
DTT (cat. no. 1111GR005), SDS (cat. no. 30166428), bromophenol blue
(cat. no. 71008060), glycerine (cat. no. 10010618)] with 5%
Tween-20 (TBST), and then incubated with primary antibodies at 4°C
overnight. After being washed with TBST, the membranes were then
incubated with HRP-labeled secondary antibodies at 3°C in a shaking
table for 2 h. Following a further wash with TBST, ECL (cat. no.
P1050; Applygen Technologies, Inc.) was adopted to identify the
immunoreactive bands. The densitometry analyses of the
immunoreactive bands were performed using a Fuji ultrasonic-Doppler
velocity profile (UVP) system and ImageJ software. GAPDH was used
as the loading control. The dilutions of the primary and secondary
antibodies were as follows: Wnt-1, 1:1,000; β-catenin, 1:800;
Cyclin D1, 1:1,000; C-myc, 1:1,000; MMP-7, 1:1,000; Axin2, 1:1,000;
EpCAM, 1:1,000; and GAPDH, 1:2,000.
Statistical analysis
SPSS 13.0 (SPSS, Inc.) statistical software was used
for data analysis. Data are presented as the mean ± SD, and the
experiments were repeated three times. Comparisons among multiple
groups were analyzed using one-way ANOVA followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
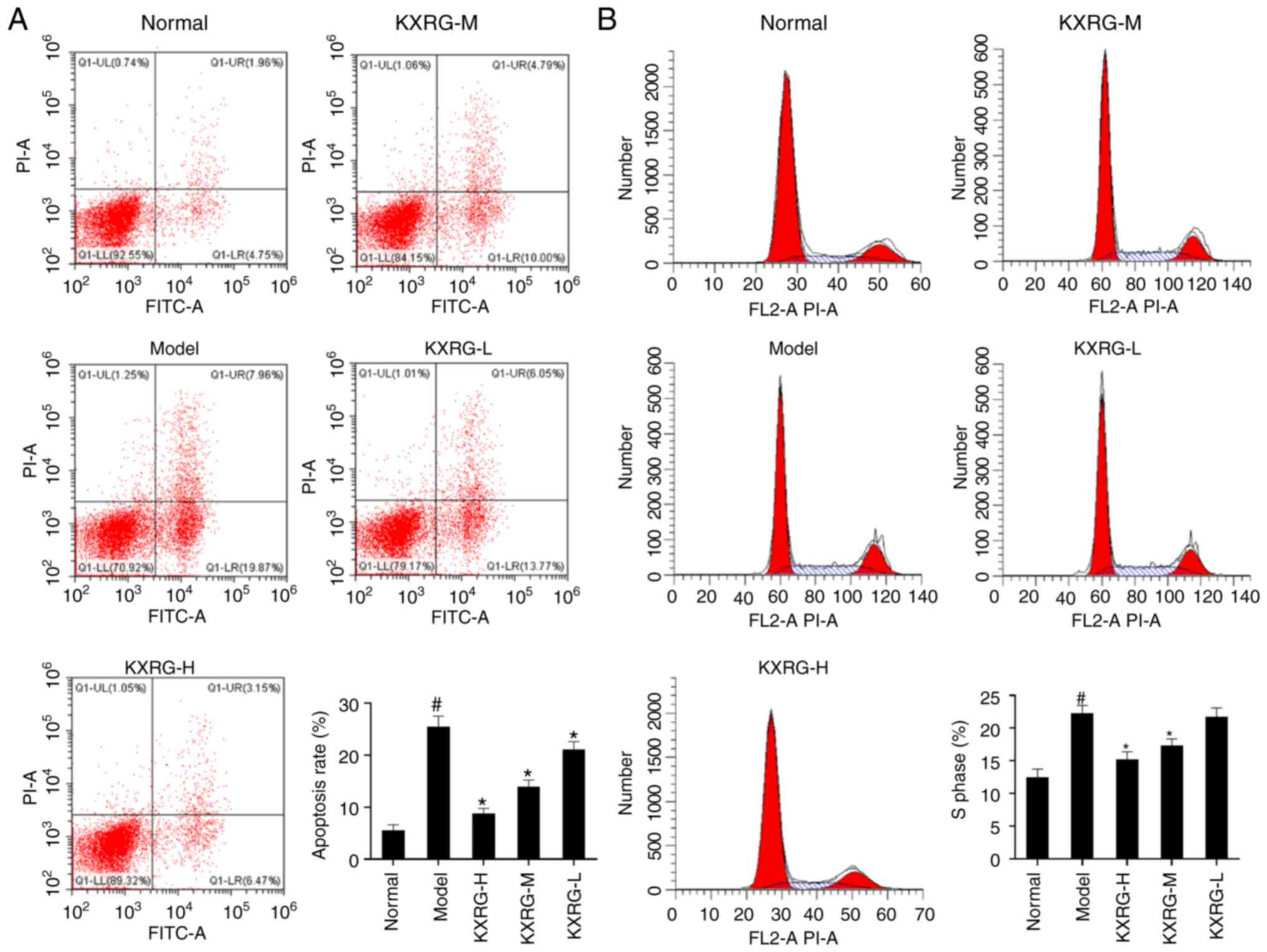
Effect of KXRG-containing serum on the
apoptotic rate and cell cycle in MNNG-stimulated WB-F344 cells
As shown in Fig. 1A and
B, compared with the normal group, the apoptotic rate and
proportion of S phase cells were significantly increased in the
model group (P<0.05). Following the addition of high and medium
concentrations of KXRG-containing serum, the apoptotic rate and the
proportion of S phase cells were significantly reduced compared
with the model group (P<0.01). Furthermore, the apoptotic
rate in the KXRG-L group was also significantly reduced compared
with the model group (P<0.01).
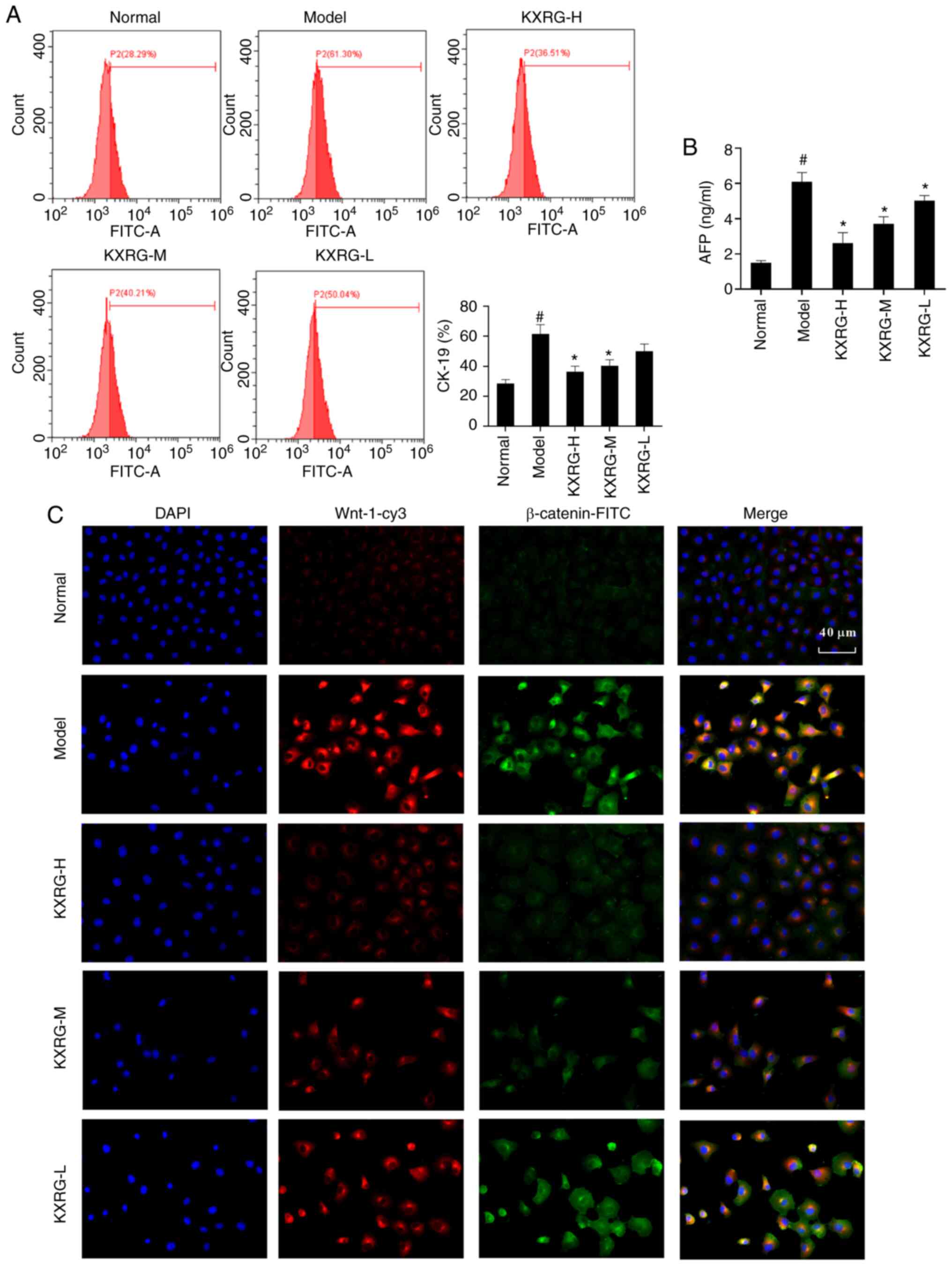
Effect of KXRG-containing serum on
CK-19 and AFP in MNNG-stimulated WB-F344 cells
As shown in Fig. 2A,
compared with the normal group, the level of CK-19 protein in the
model group was significantly higher (P<0.05). Following
treatment with KXRG-containing serum, the level of CK-19 protein in
cells decreased significantly (P<0.01). As shown in Fig. 2B, compared with the normal group,
the concentration of AFP in the cell supernatant of the model group
significantly increased (P<0.05). Following the addition of high
and medium concentrations of KXRG-containing serum, the
concentration of AFP decreased significantly compared with the
model group (P<0.01).
Effect of KXRG-containing serum on the
Wnt-1/β-catenin signaling pathway in MNNG-stimulated WB-F344
cells
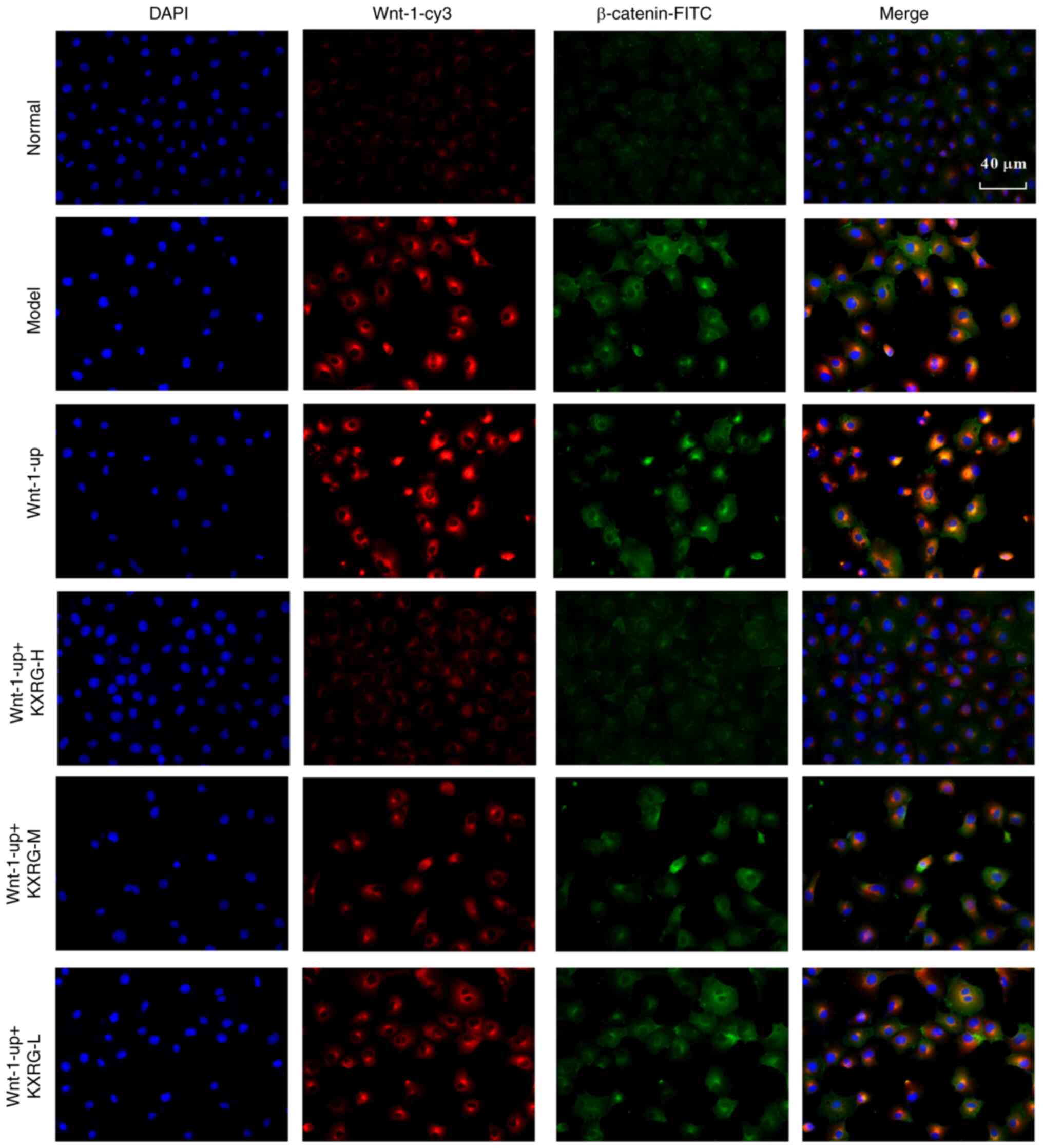
Subsequently, an immunofluorescence assay was
performed to simultaneously detect the expression of Wnt-1 and
β-catenin in cells. As illustrated in Fig. 2C, compared with the normal group,
the level of Wnt-1 and β-catenin in the model group was increased.
Following the addition of KXRG-containing serum, the level of Wnt-1
and β-catenin in cells decreased compared with the model group. As
demonstrated in Fig. 3A and B,
compared with the normal group, the mRNA expression levels of
Wnt-1, β-catenin, Cyclin D1, C-myc, MMP-7, Axin2 and EpCAM in the
model group were significantly increased (P<0.05). Addition of
high and medium concentrations of KXRG-containing serum resulted in
the mRNA expression levels of Wnt-1, β-catenin, Cyclin D1, C-myc,
MMP-7, Axin2 and EpCAM reducing significantly compared with the
model group (P<0.01). Moreover, the mRNA expression levels of
Cyclin D1, C-myc and Axin2 in the KXRG-L group were also
significantly decreased compared with the model group
(P<0.01).
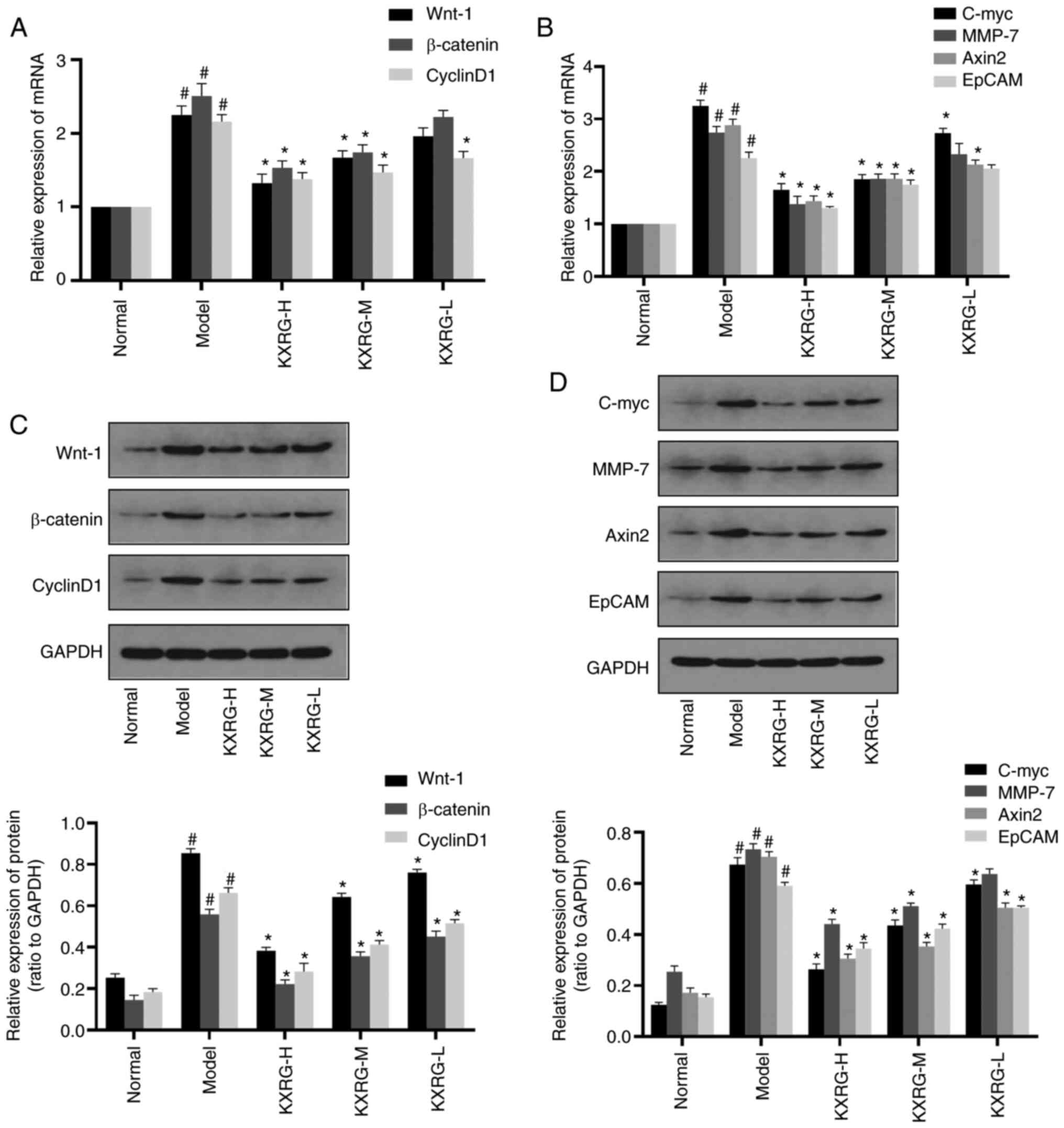 | Figure 3.Effect of KXRG-containing serum on
the Wnt-1/β-catenin signaling pathway in MNNG-stimulated WB-F344
cells. mRNA expression levels of (A) Wnt-1, β-catenin, Cyclin D1,
(B) C-myc, MMP-7, Axin2 and EpCAM were detected via reverse
transcription-quantitative PCR. Protein expression levels of (C)
Wnt-1, β-catenin, Cyclin D1, (D) C-myc, MMP-7, Axin2 and EpCAM were
detected by performing western blotting. The data of the normal
group are set to 1 and data are presented as the mean ± SD (n=3).
#P<0.05 vs. normal; *P<0.01 vs. model. KXRG,
kangxianruangan granule; MNNG,
N-methyl-N′-nitro-N-nitrosoguanidine; KXRG-H, high concentration of
KXRG-containing serum; KXRG-M, middle concentration of
KXRG-containing serum; KXRG-L, low concentration of KXRG-containing
serum; MMP, matrix metalloproteinase; EpCAM, epithelial cell
adhesion molecule. |
As displayed in Fig. 3C
and D, compared with the normal group, the protein expression
levels of Wnt-1, β-catenin, Cyclin D1, C-myc, MMP-7, Axin2 and
EpCAM in the model group were significantly increased (P<0.05).
Following the application of KXRG-containing serum, the protein
expression levels of Wnt-1, β-catenin, Cyclin D1, C-myc, MMP-7,
Axin2 and EpCAM were significantly reduced (P<0.01), with the
exception of MMP-7 in the KXRG-L group.
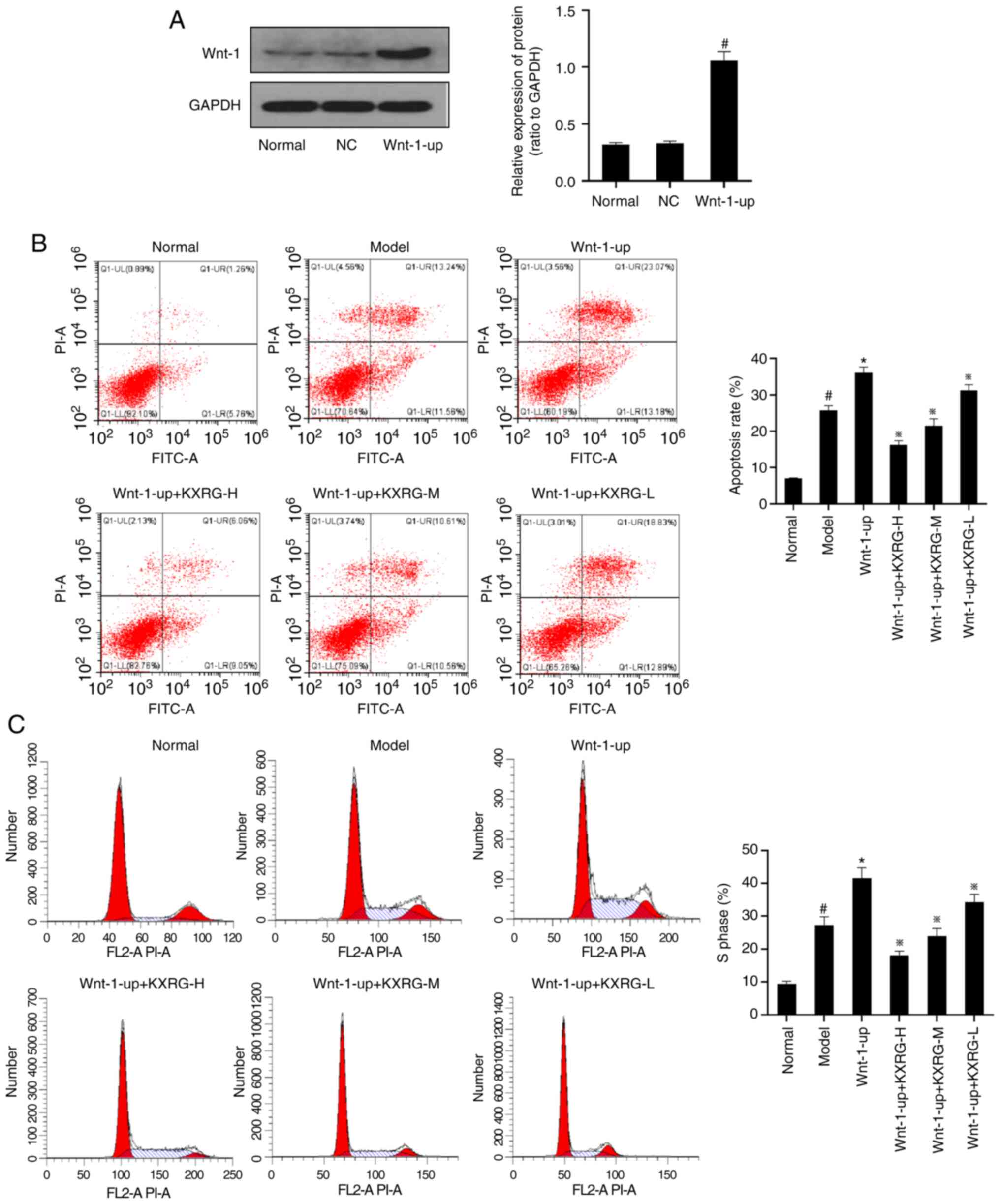
Effect of KXRG on the apoptotic rate,
cell cycle and AFP in MNNG-stimulated WB-F344 cells following Wnt-1
overexpression
To determine the effects of Wnt-1 overexpression in
WB-F344 cells, western blotting was performed to detect the Wnt-1
protein expression level in each group of cells. As exhibited in
Fig. 4A, compared with the normal
group, there was no significant change in Wnt-1 expression in the
negative control group, whereas the Wnt-1 protein expression level
in the Wnt1 overexpression group (Wnt-1-up) was significantly
increased (P<0.05). As demonstrated in Fig. 4B and C, compared with the normal
group, the cell apoptotic rate and the proportion of S phase cells
were significantly increased in the model group (P<0.05).
Compared with the control group, the cell apoptotic rate and the
proportion of S phase cells were further increased in the Wnt-1-up
group (P<0.01). Following the addition of KXRG-containing serum,
the cell apoptotic rate and the proportion of S phase cells were
significantly reduced compared with the model group
(P<0.01).
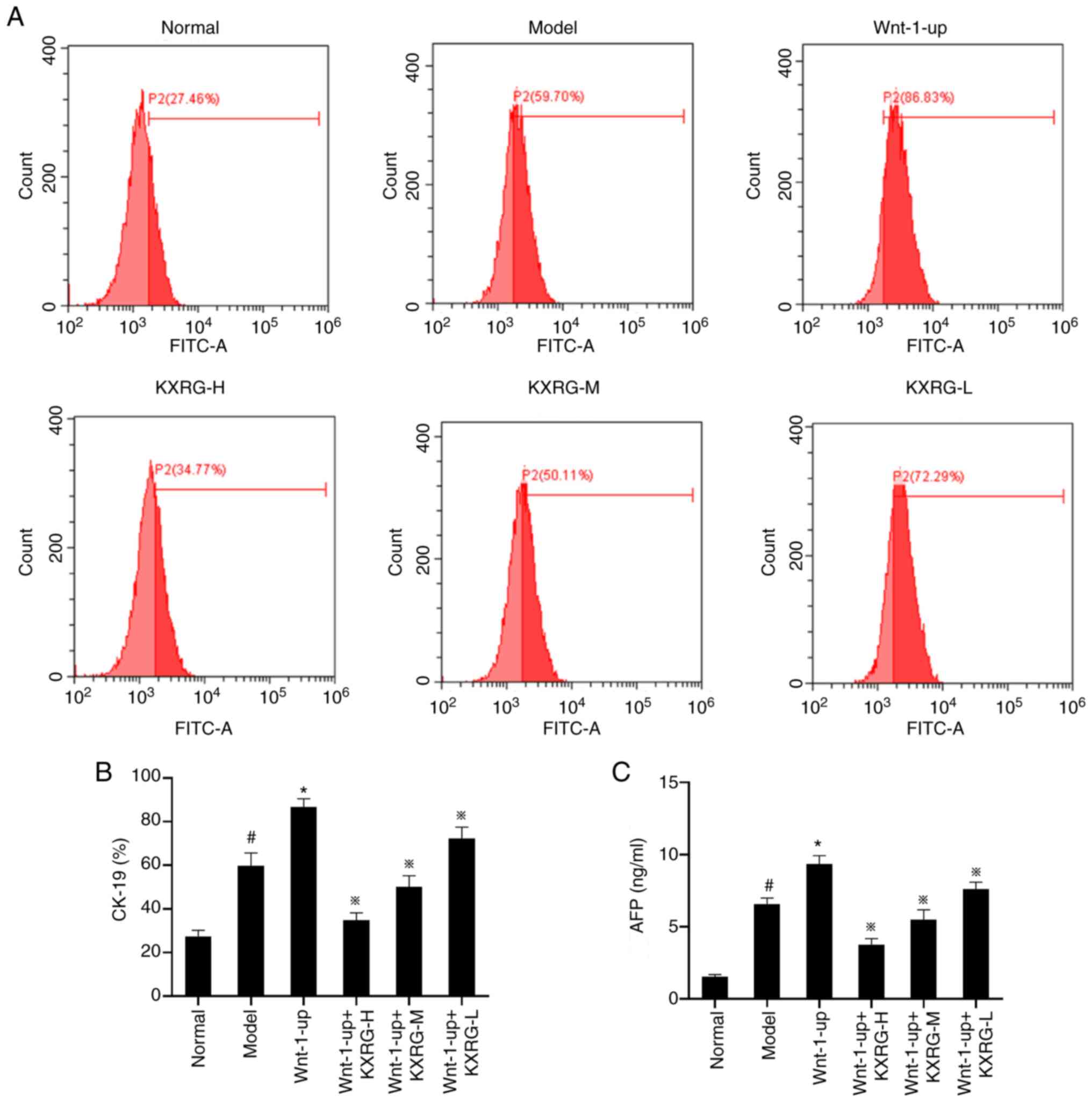
As displayed in Fig.
5, compared with the normal group, the level of intracellular
CK-19 protein and the concentration of AFP in the cell supernatants
were significantly increased in the model group (P<0.05).
Compared with the model group, the level of intracellular CK-19
protein and the concentration of AFP in the cell supernatant were
further increased in the Wnt-1-up group (P<0.01). Compared with
the model group, the level of CK-19 protein and the concentration
of AFP were significantly decreased after adding KXRG-containing
serum (P<0.01).
Effect of KXRG on the Wnt-1/β-catenin
signaling pathway in MNNG-stimulated WB-F334 cells following Wnt-1
overexpression
As illustrated in Fig.
6, compared with the normal group, the levels of Wnt-1 and
β-catenin in the model group were increased. Compared with the
model group, the levels of Wnt-1 and β-catenin were further
increased in the Wnt-1-up group. Following the addition of
KXRG-containing serum, the levels of Wnt-1 and β-catenin in cells
decreased compared with the model group.
As shown in Fig. 7A and
B, compared with the normal group, the mRNA expression levels
of Wnt-1, β-catenin, Cyclin D1, C-myc, MMP-7, Axin2 and EpCAM in
the model group were all significantly increased (P<0.05).
Compared with the model group, the mRNA expression levels of Wnt-1,
β-catenin, Cyclin D1, C-myc, MMP-7, Axin2 and EpCAM were further
increased in the Wnt-1-up group (P<0.01). Following treatment
with KXRG-containing serum, the mRNA expression levels of Wnt-1,
β-catenin, Cyclin D1, C-myc, MMP-7, Axin2 and EpCAM were all
significantly reduced compared with the model group
(P<0.01).
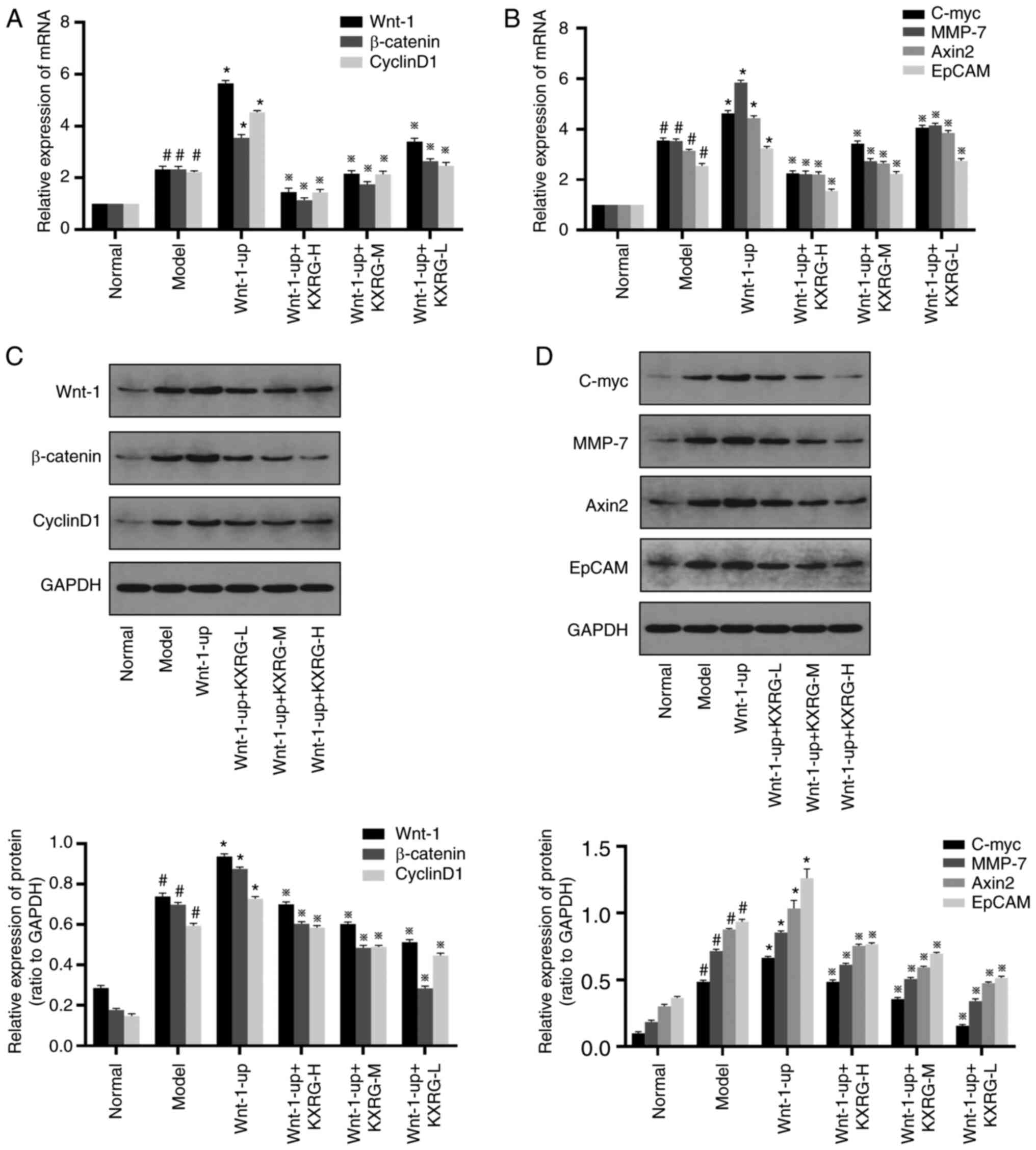 | Figure 7.Effect of KXRG-containing serum on
the Wnt-1/β-catenin signaling pathway in MNNG-stimulated,
Wnt-1-overexpression WB-F344 cells. mRNA expression levels of (A)
Wnt-1, β-catenin, Cyclin D1, (B) C-myc, MMP-7, Axin2 and EpCAM were
detected by reverse transcription-quantitative PCR. Protein
expression levels of (C) Wnt-1, β-catenin, Cyclin D1, (D) C-myc,
MMP-7, Axin2, and EpCAM detected by western blotting. The data of
the normal group are set to 1 and data are presented as the mean ±
SD (n=3). #P<0.05 vs. normal; *P<0.01 vs. model;
※P<0.01 vs. Wnt-1-up. KXRG, kangxianruangan granule; MNNG,
N-methyl-N′-nitro-N-nitrosoguanidine; KXRG-H, high concentration of
KXRG-containing serum; KXRG-M, middle concentration of
KXRG-containing serum; KXRG-L, low concentration of KXRG-containing
serum; MMP, matrix metalloproteinase; EpCAM, epithelial cell
adhesion molecule. |
As demonstrated in Fig.
7C and D, compared with the normal group, the protein
expression levels of Wnt-1, β-catenin, Cyclin D1, C-myc, MMP-7,
Axin2 and EpCAM in the model group were all significantly increased
(P<0.05). Compared with the model group, the protein expression
levels of Wnt-1, β-catenin, Cyclin D1, C-myc, MMP-7, Axin2 and
EpCAM were further increased in the Wnt-1-up group (P<0.01).
Following the addition of KXRG-containing serum, the protein
expression levels of Wnt-1, β-catenin, Cyclin D1, C-myc, MMP-7,
Axin2 and EpCAM were all significantly reduced compared with the
Wnt-1-up group (P<0.01).
Discussion
The development of liver cancer is a multi-stage
process that can be divided into three stages-initiation, promotion
and progression (19). In the
initial stages, irreversible gene mutations arise (20). In the promotion phase, clonal cell
hyperplasia occurs but is generally considered to be reversible,
which makes it an ideal period for the prevention of liver cancer
(21). This phase also corresponds
to the precancerous stage of liver cancer, with HOC proliferation
being the primary pathological feature (22). It should be noted that liver
cirrhosis is considered a precancerous lesion (23). Previously, it has been demonstrated
that HOCs can transform into HCC cells, resulting in liver cancer,
a phenomenon that is referred to as the ‘cancer stem cell
hypothesis’ (24). As previously
mentioned, when the liver is damaged, HOCs can either differentiate
into liver cells for organ regeneration or transform into liver
cancer cells (11,24). Changes in the liver microenvironment
may also lead to the malignant transformation of HOCs during the
process of hyperplasia, which is involved in the initiation and
promotion stages of hepatocarcinogenesis (25). The rat HOC line WB-F344 has
previously been widely used in the modeling of precancerous lesions
of liver. MNNG is an experimental carcinogen. Through its
stimulation on WB-F344 cells, MNNG reproduces some of the
conditions that appear during the transition from ad HOCs to HCC
cells (17).
A recent study confirmed that abnormal activation of
the Wnt-1/β-catenin signaling pathway is a crucial factor in the
induction of hepatocarcinogenesis (26). It has been shown that the expression
of Wnt proteins (Wnt-1 and Wnt-3) is increased in liver cancer
tissues and cell lines (27).
Therapeutic antibodies against Wnt proteins can reduce the
proliferation and viability of liver cancer cells, inducing the
apoptosis of tumor cells and inhibiting the activation of the
Wnt-1/β-catenin signaling pathway (28). The aberrant activation of the
Wnt-1/β-catenin signaling pathway is a signature in multiple types
of cancer, including HCC, making it a viable therapeutic target
(29). β-catenin, a crucial
component in this pathway (especially in the liver), displays a
variety of functions, serving as a transcriptional co-activator and
a cell-cell adhesion protein (26).
Several proof-of-principle pre-clinical studies clearly
demonstrated the potential of the therapeutic inhibition of
β-catenin as a means of treatment for HCC (30). β-catenin serves a role in regulating
cell proliferation, regeneration, differentiation and tumor
migration, entering the cytoplasm from the cell membrane to the
nucleus (31). In the nucleus,
β-catenin interacts with the T-cell factor/lymphoid enhancer factor
transcription factor family, stimulating the transcription of
target genes, including Cyclin D1, C-myc, MMP-7, Axin2 and EpCAM,
thus regulating cell proliferation and apoptosis (32,33).
The Wnt protein binds to the frizzled receptor, thereby affecting
the Wnt-1/β-catenin signaling pathway, and the activation and
proliferation of HOCs (34).AFP and
CK19 are markers of HOCs. When HOCs over-proliferate and transform
into hepatoma cells, the expression levels of AFP and CK19
increase.
KXRG is a compound granule used in Chinese medicine,
which contains seaweed, hawthorn, Salvia miltiorrhiza, Rhizoma
Curcumae, Carapax Trionycis and oyster. These medicines
together create a unique combination of Traditional Chinese
Medicine, which has been used to treat liver fibrosis and cirrhosis
in clinic and has a good curative effect (35,36).
The present study further explore its mechanism and potential
effect.
In the present study, different concentrations of
KXRG were applied to treat MNNG-induced WB-F344 cells. Compared
with the normal group, the apoptotic rate, proportion of S phase
cells, concentration of AFP in the cell supernatant, level of CK-19
protein, mRNA and protein expression levels of Wnt-1, β-catenin,
Cyclin D1, C-myc, MMP-7, Axin2 and EpCAM in the model group were
significantly increased. Following the application of KXRG, the
aforementioned indicators were significantly reduced in
MNNG-stimulated WB-F344 cells, suggesting that KXRG may inhibit the
transformation of rat WB-F344 HOCs into liver cancer cells,
potentially via inhibiting the expression of key genes and proteins
in the Wnt-1/β-catenin signaling pathway. Overexpression of Wnt-1
further increased the aforementioned indicators compared with the
model group. The inhibitory effects of KXRG continued to be present
in cells overexpressing Wnt-1. These results suggested that the
Wnt-1/β-catenin signaling pathway may serve an important role in
the development of HCC.
In conclusion, the present study indicated that KXRG
activated the Wnt-1/β-catenin signaling pathway, potentially
inhibiting the transformation of rat WB-F344 HOCs to liver cancer
cells. These findings lay a foundation for the study of the
anti-fibrosis-hepatocarcinoma mechanism of drugs via the
Wnt/β-catenin pathway, and suggested a novel treatment option for
patients with liver disease. However, due to the uncertainty of the
composition of the serum containing the drug, it is not possible to
determine the specific role of the monomer composition, which may
be the focus of further research.
Acknowledgements
The authors would like to thank Professor Lei Zhao
of the Wuhan Union Hospital for guiding the construction of
lentiviral vector and cell transfection.
Funding
The present study was supported by the Chinese Medicine Research
Project grant from Health and Family Planning Commission of Hubei
Province (grant no. ZY2019Z016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
As the leader of the whole project, FY designed the
whole process of the experiment, participated in the data
collection and analysis, and made a great contribution to the
manuscript writing and revision. WT, JX and LL made important
contributions to the design and operation of experiments, the
processing of data, the production of images and the writing and
revision of manuscripts. YW, XC and YL made important contributions
to the experimental design, the optimization of the experimental
procedure, the instruction of the experiment and data collection
and analysis. DH, XW, TH and DL made important contributions to
experimental design, data proofreading and manuscript writing and
manuscript revision. FY and YW confirmed the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Hubei
Provincial Hospital of Traditional Chinese Medicine Laboratory
Animal Ethics Committee (Wuhan, China; approval no. 2019005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang JD, Hainaut P, Gores GJ, Amadou A,
Plymoth A and Roberts LR: A global view of hepatocellular
carcinoma: Trends, risk, prevention and management. Nat Rev
Gastroenterol Hepato. l16:589–604. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. Apr 5–2021.(Epub ahead of
print). doi: 10.1002/ijc.33588. View Article : Google Scholar
|
|
3
|
Wang G, Wang Q, Liang N, Xue H, Yang T,
Chen X, Qiu Z, Zeng C, Sun T, Yuan W, et al: Oncogenic driver genes
and tumor microenvironment determine the type of liver cancer. Cell
Death Dis. 11:3132020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lokau J, Schoeder V, Haybaeck J and
Garbers C: Jak-stat signaling induced by interleukin-6 family
cytokines in hepatocellular carcinoma. Cancers (Basel).
11:17042019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung MW, Ha SY, Choi JH, Park HJ, Myung
DS, Cho SB, Lee WS, Kim JW, Oh HH and Joo YE: Cardiac tamponade
after radiofrequency ablation for hepatocellular carcinoma: Case
report and literature review. Medicine (Baltimore). 97:e135322018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang YM, Kim SY and Seki E: Inflammation
and liver cancer: Molecular mechanisms and therapeutic targets.
Semin Liver Dis. 39:26–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niu ZS, Niu XJ, Wang WH and Zhao J: Latest
developments in precancerous lesions of hepatocellular carcinoma.
World J Gastroenterol. 22:3305–3314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng T, Wang J, Jiang H and Liu LX: Hippo
signaling in oval cells and hepatocarcinogenesis. Cancer Lett.
302:91–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen Y and Cao DL: Hepatocellular
carcinoma stem cells: Origins and roles in hepatocarcinogenesis and
disease progression. Front Biosci (Elite Ed). 4:1157–1169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen JM, Chen L, Zern MA, Theise ND, Diehl
AM, Liu P and Duan YY: The diversity and plasticity of adult
hepatic progenitor cells and their niche. Liver Int. 37:1260–1271.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu RH, Zheng LY, He DL, Meng J, Xia LP,
Hao XB and Zhang ZZ: Profiling of differentially expressed
microRNAs (miRNAs) during differentiation of rat hepatic oval cells
(HOCs) into hepatocellular carcinoma (HCC) cells. Clin Transl
Oncol. 17:230–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lecarpentier Y, Schussler O, Hebert JL and
Vallee A: Multiple targets of the canonical WNT/β-catenin signaling
in cancers. Front Oncol. 9:12482019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vilchez V, Turcios L, Marti F and Gedaly
R: Targeting Wnt/β-catenin pathway in hepatocellular carcinoma
treatment. World J Gastroenterol. 22:823–832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong S, Qu X, Yang S, Zhou S, Li P and
Zhang Q: RFC3 induces epithelialmesenchymal transition in lung
adenocarcinoma cells through the Wnt/β-catenin pathway and
possesses prognostic value in lung adenocarcinoma. Int J Mol Med.
44:2276–2288. 2019.PubMed/NCBI
|
|
15
|
Cruz-Lozano M, Gonzalez-Gonzalez A,
Marchal JA, Muñoz-Muela E, Molina MP, Cara FE, Brown AM,
García-Rivas G, Hernández-Brenes C, Lorente JA, et al:
Hydroxytyrosol inhibits cancer stem cells and the metastatic
capacity of triple-negative breast cancer cell lines by the
simultaneous targeting of epithelial-to-mesenchymal transition,
Wnt/β-catenin and TGFβ signaling pathways. Eur J Nutr.
58:3207–3219. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Y and Lin Y, Wu Y, Zeng J, Huang M,
Guo S, Luo W, Lin H and Lin Y: Molecular mechanisms of the
inhibitory effects of jiangu granule-containing serum on
RANKL-induced osteoclastogenesis. Mol Med Rep. 16:8420–8426. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bi YH, Han WQ, Li RF, Wang YJ, Du ZS, Wang
XJ and Jiang Y: Signal transducer and activator of transcription 3
promotes the Warburg effect possibly by inducing pyruvate kinase M2
phosphorylation in liver precancerous lesions. World J
Gastroenterol. 25:1936–1949. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shang W, Adzika G, Li Y, Huang Q, Ding N,
Chinembiri B, Rashid MSI and Machuki JO: Molecular mechanisms of
circular RNAs, transforming growth factor-beta, and long noncoding
RNAs in hepatocellular carcinoma. Cancer Med. 8:6684–6699. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hilmi M, Neuzillet C, Calderaro J, Lafdil
F, Pawlotsky JM and Rousseau B: Angiogenesis and immune checkpoint
inhibitors as therapies for hepatocellular carcinoma: Current
knowledge and future research directions. J Immunother Cancer.
7:3332019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chao J, Zhao S and Sun H:
Dedifferentiation of hepatocellular carcinoma: Molecular mechanisms
and therapeutic implications. Am J Transl Res. 12:2099–2109.
2020.PubMed/NCBI
|
|
22
|
Yu XT, Wang PY, Shi ZM, Dong K, Feng P,
Wang HX and Wang XJ: Urotensin-II-mediated reactive oxygen species
generation via NADPH oxidase pathway contributes to hepatic oval
cell proliferation. PLoS One. 10:e01444332015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu DB and Tang H: Advances in the study of
cirrhosis and precancerous lesions of liver cancer. Zhonghua Gan
Zang Bing Za Zhi. 27:483–486. 2019.(In Chinese). PubMed/NCBI
|
|
24
|
Xu RH, Zheng LY, He DL, Meng J, Xia LP,
Hao XB and Zhang ZZ: Retraction note to: Profiling of
differentially expressed microRNAs (miRNAs) during differentiation
of rat hepatic oval cells (HOCs) into hepatocellular carcinoma
(HCC) cells. Clin Transl Oncol. 17:9352015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Ruan B, You N, Huang Q, Liu W, Dang
Z, Xu W, Zhou T, Ji R, Cao Y, et al: Downregulation of miR-200a
induces EMT phenotypes and CSC-like signatures through targeting
the beta-catenin pathway in hepatic oval cells. PLoS One.
8:e794092013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perugorria MJ, Olaizola P, Labiano I,
Esparza-Baquer A, Marzioni M, Marin JJG, Bujanda L and Banales JM:
Wnt-beta-catenin signalling in liver development, health and
disease. Nat Rev Gastroenterol Hepatol. 16:121–136. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang JL, Fu YP, Gan W, Liu G, Zhou PY,
Zhou C, Sun BY, Guan RY, Zhou J, Fan J, et al: Hepatic stellate
cells promote the progression of hepatocellular carcinoma through
microRNA-1246-RORα-wnt/β-catenin axis. Cancer Lett. 476:140–151.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li N, Wei L, Liu X, Bai HJ, Ye Y, Li D, Li
N, Baxa U, Wang Q, Lv L, et al: A frizzled-like cysteine-rich
domain in glypican-3 mediates wnt binding and regulates
hepatocellular carcinoma tumor growth in mice. Hepatology.
70:1231–1245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei W, Chua MS, Grepper S and So SK:
Blockade of Wnt-1 signaling leads to anti-tumor effects in
hepatocellular carcinoma cells. Mol Cancer. 8:762009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu X, Zhu X, Qin F, Zhang Y, Lin J, Ding
Y, Yang Z, Shang Y, Wang L, Zhang QX and Gao Q: Linc00210 drives
Wnt/β-catenin signaling activation and liver tumor progression
through CTNNBIP1-dependent manner. Mol Cancer. 17:732018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Monga SP: β-catenin signaling and roles in
liver homeostasis, injury, and tumorigenesis. Gastroenterology.
148:1294–1310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Doumpas N, Lampart F, Robinson MD, Lentini
A, Nestor C, Cantù C and Basler K: TCF/LEF dependent and
independent transcriptional regulation of Wnt/beta-catenin target
genes. EMBO J. 38:e988732019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang TW, Gao YH, Ma SY, Qiang W and Li ZF:
Low-grade slightly elevated and polypoid colorectal adenomas
display differential beta-catenin-TCF/LEF activity, c-Myc, and
cyclin D1 expression. World J Gastroenterol. 23:3066–3076. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rana MA, Ijaz B, Daud M, Tariq S, Nadeem T
and Husnain T: Interplay of Wnt β-catenin pathway and miRNAs in HBV
pathogenesis leading to HCC. Clin Res Hepatol Gastroenterol.
43:373–386. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan JS, Zhou JH and Cheng LB: Effect of
Kangxian ruangan granule on hepatic fibrosis induced by
tetrachloromethane and ethanol in rats. Chinese Journal of
Integrated Traditional and Western Medicine on Liver Disease.
27:40–41. 2017.
|
|
36
|
Zhang CZ, Yan HM and Wang L: 31 cases of
liver cirrhosis treated with Kangxian ruangan granule. Chinese
Journal of Integrated Traditional and Western Medicine on Liver
Disease. 2:19–20. 1999.(In Chinese).
|