Introduction
Endometriosis (EM), the presence of functional
endometrial glands and stroma outside the uterine cavity, is a
common gynecological disorder characterized by dysmenorrhea,
chronic pelvic pain, menstrual abnormalities and infertility
(1–4). Previous study demonstrated that EM
affects ~10% of individuals who have a uterus, and that the
probability of symptomatic perimenopause increases to 30–50%
(5). Currently, the most common
primary diagnostic method for EM is laparoscopy, supplemented with
the screening for cancer antigen 125 (CA125) and endometrium
antibody, as well as B-ultrasounds, x-ray and magnetic resonance
imaging (6). Although EM is a
benign disease, it has certain characteristics of malignant tumors,
including cell invasion, new blood vessel generation, unlimited
growth, reduced numbers of apoptotic cells, infiltration and
destruction of surrounding tissues and metastasis (7). Therefore, inhibition of the growth
and invasion of EM may be a possible treatment strategy for EM.
Chemokines are selective mediators of leukocyte
migration to inflammatory sites (8). It has been demonstrated that
chemokines are key players in a variety of physiological and
pathological events, including chemotaxis, cell proliferation,
apoptosis, angiogenesis and inflammatory processes/diseases
(9,10). C-C motif chemokine ligand (CCL)28
is a mucosa-associated epithelial chemokine that is selectively
expressed in certain mucosal tissue, such as epithelial mucosal
tissues (11,12). CCL28 is a functional ligand for CC
chemokine receptor (CCR)10, a member of the chemokine receptor
family, which belongs to the G protein-coupled receptor
superfamily, and is normally expressed by melanocytes, plasma cells
and skin-homing T cells (13).
Upregulation of CCR10 can facilitate cell proliferation and
invasion in glioma, contributing to gliomagenesis (14). Furthermore, CCR10 can stimulate
breast cancer cell invasion and migration by increasing MMP7
expression via ERK1/2 activation (15,16). In ectopic endometrial stromal
cells, depletion of CCL27 can suppress cell proliferation,
metastasis and adhesion (17).
CCL28 induces apoptosis of decidual stromal cells via binding of
CCR3/CCR10 in human spontaneous abortion (18). Furthermore, estrogen may serve a
crucial role in the protection against genital infection by
regulating mucosa-associated epithelial chemokine (MEC)/CCL28
expression in the uterus (19).
There are also several studies demonstrating that the ERK1/2
signaling pathway is associated with migration and apoptosis of
endometrial stromal cells (20–24). Another study has reported that
CCL28 can promote cell proliferation and metastasis in breast
cancer via the MAPK signaling pathway (25). However, the function of CCL28 in
endometrial stromal cells and its underlying mechanisms are still
unknown.
The present study aimed to explore the role of CCL28
and its underlying mechanism in endometrial stromal cells to
propose a novel therapy for EM treatment. It provided important
leads for designing studies in the future to understand the
mechanism of EM and aid in the development of novel therapeutic
strategies.
Materials and methods
Patient samples
Patients who met the EM diagnostic criteria (visual
inspection/laparoscopy/laparotomy) participated in the study. EM is
usually diagnosed by visual inspection of the pelvis during
laparoscopy or laparotomy (6).
After informed consent was obtained, the EM tissues from 15
patients (female, age range from 25 years to 55 years, mean age: 37
years, Shanghai, China) with deep-infiltrating EM who underwent
laparoscopic treatment at the Shanghai First Maternity and Infant
Hospital, Tongji University School of Medicine (Shanghai, China)
between February 2020 and December 2020 were removed via biopsy.
Deep-infiltrating EM is located 5 mm below the surface of the
peritoneum. Control endometrial samples were collected from 15
patients (female, age range from 23 years to 51 years, mean age: 35
years, Shanghai, China) without EM who underwent laparoscopy and
hysteroscopy surgery for benign gynecological diseases. CCL28 and
CCR10 expression was detected in these tissues by IHC. Furthermore,
80 serum samples (healthy individuals: 40; EM patients, 40) were
collected to detect CCL28 levels by ELISA. Tissue samples were
collected independent of menstrual cycle stage. The following
inclusion criteria were used: i) EM was confirmed by two
pathologists following laparoscopic biopsy; and ii) No preoperative
chemotherapy or radiotherapy was received. Patients who had
received hormonal treatment and birth control prior to enrollment
were excluded from the study. The clinical characteristics of the
patients and controls are shown in Table I. The Ethics Committee of Shanghai
First Maternity and Infant Hospital, Tongji University School of
Medicine (Shanghai, China) approved all experiments involving
patients.
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
|
Characteristics | Patients with
endometriosis (n=40) | Healthy patients
(n=40) | P-value |
|---|
| Age, year | 35.55±3.64 | 31.32±5.87 | 0.092 |
| BMI, kg/m2 | 19.10±2.53 | 21.32±2.12 | 0.061 |
| CA125, IU/ml | 23.74±2.56 | 14.57±2.04 | 0.013 |
| EM stage, n
(%) |
|
|
|
|
III | 21 (52.5) | NA |
|
| IV | 19 (47.5) | NA |
|
| Benign conditions,
n (%) |
|
|
|
| Uterine
myoma | NA | 8 (20.0) |
|
|
Endometrial hyperplasia | NA | 13 (32.5) |
|
|
Others | NA | 19 (47.5) |
|
| Menstrual phase, n
(%) |
|
| 0.171 |
|
Proliferative | 21 (52.5) | 27 (67.5) |
|
|
Secretory | 19 (47.5) | 13 (32.5) |
|
Cell culture
Primary endometrial stromal cells derived from
ectopic endometria of female patients with EM, or from normal
endometria of female patients without EM, were isolated and
cultured as previously described (26,27). Immunocytochemistry using
anti-cytokeratin 19 and anti-Vimentin antibodies was performed to
identify cell purity. The cells were cultured in DMEM/F12 (cat.no.
SH30023.01B; Hyclone; Cytiva) containing 10% FBS (cat. no.
16000-044; Gibco; Thermo Fisher Scientific, Inc.), 1% double
antibiotics (penicillin and streptomycin mixture), 2 mM L-glutamine
and 1 ng/ml fibroblast growth factor-2 at 37°C in a 5% CO2
incubator. 293T cells were cultured with DMEM containing 10% FBS
(cat. no. 16000-044; Gibco; Thermo Fisher Scientific, Inc.), 1%
double antibiotics in a 37°C, 5% CO2 incubator.
Plasmid construction and lentivirus
packaging
Targeting different sites of CCL28, three
interference sequences were synthesized (Table II). Short hairpin RNA (sh)
constructs were created using double chain annealing and inserted
into the pLKO.1-Puro vector (Addgene, Inc.) at AgeI-EcoRI
restriction sites, while a negative control shRNA (shNC) as a
negative control. Subsequently, plasmids of pLKO.1-Puro-shCCL28-1,
−2 and −3 (1,000 ng) were co-transfected with viral packaging
plasmids psPAX2 (900 ng) and pMD2.G (100 ng; Addgene, Inc.;
packaging vector:envelope vector, 1:9) into 3rd generation 293T
cells (ATCC) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) for 48 h at 37°C in a 5% CO2
incubator. Following 48 h of incubation, viral particles were
collected via ultracentrifugation at 55,000 × g, 4°C for 2.5 h, and
then the viral supernatant (MOI, 10) was used to infect EM stromal
cells (1×106). After 24 h infection, the cells were cultured for 24
h with serum-free transfer solution before further experiments were
performed.
 | Table II.CCL28 sequences for gene
silencing. |
Table II.
CCL28 sequences for gene
silencing.
| Target site
name | Sequence
(5′-3′) |
|---|
| CCL28-1 (site:
167–185) |
GCACGGAGGTTTCACATCA |
| CCL28-2 (site:
260–278) |
CTGTCATCCTTCATGTCAA |
| CCL28-3 (site:
321–339) |
GCAGTGGATGAAAGTGCAA |
ELISA
An ELISA was employed to detect CCL28 levels in the
serum of patients with EM or in the supernatant of endometrial
stromal cells (1×106 cells/ml). The Human MEC/CCL28 ELISA Kit (cat.
no. RAB0072; Sigma-Aldrich; Merck KGaA) was used according to the
manufacturer's protocol.
Immunohistochemical (IHC)
detection
Tissue sections (4 µm) were washed with 0.02 M PBS
and fixed with 4% formaldehyde for 30 min at room temperature.
After three washes with 0.02 M PBS, samples were incubated in 0.3%
H2O2 in a wet-box for 10 min and then blocked in 1% BSA (cat. no.
A8010; Beijing Solarbio Science & Technology Co., Ltd.) for 1 h
at room temperature. Subsequently, samples were incubated with
primary antibodies against CCL28 (1:200; cat. no. 18214-1-AP;
ProteinTech Group, Inc.) and CCR10 (1:100; cat. no. 22071-1-AP;
ProteinTech Group, Inc.) for 1 h at room temperature. The primary
incubation was then followed by a 30-min incubation with
HRP-labeled secondary antibodies (cat. no. D-3004; Shanghai
Changdao Biological Technology Co., Ltd.) at room temperature.
Samples were then subjected to 3, 3′-diaminobenzidine (DAB)
staining (cat. no. FL-6001; Shanghai Changdao Biological Technology
Co., Ltd.), 3 min of hematoxylin staining (cat. no. 714094; Zhuhai
Besso Biotechnology Co., Ltd.) and alcohol differentiation with 1%
hydrochloric acid at room temperature, followed by washing with tap
water for 10 min and drying in a 65°C oven for 15 min. Finally,
samples were made transparent in xylene for 3 min and sealed with
neutral gum (cat. no. G8590; Solarbio) at room temperature. After
drying in a 65°C oven for 15 min, samples were imaged using an
upright fluorescence microscope (ECLIPSE Ni; Nikon Corporation).
CCL28 and CCR10 expression was analyzed using an image analysis
system version 11.0 (IMS; Beijing Changheng Rongchuang
Technology).
Immunocytochemical detection
Endometrial cells were cultured on coverslips for 24
h. The cells were washed with 0.02 M PBS to remove the medium,
fixed with 4% formaldehyde for 30 min at room temperature and
washed with 0.02 M PBS. Cells were permeated with 0.5% Triton X-100
(cat. no. T8200; Beijing Solarbio Science & Technology Co.,
Ltd.) for 10 min at room temperature and then blocked with 1% BSA
for 1 h at room temperature. Subsequently, cells were incubated
with primary antibodies against CK19 (1:200; cat. no. ab52625;
Abcam) and vimentin (1:500; cat. no. ab92547; Abcam) at 4°C
overnight. Following primary incubation, cells were incubated for
30 min with HRP-labeled secondary antibodies (cat. no. D-3004;
Shanghai Changdao Biological Technology Co., Ltd.). Cells were then
subjected to DAB staining. Finally, cells were imaged using an
upright fluorescence microscope and the expression levels of CK19
and vimentin were analyzed using an image analysis system version
11.0 (IMS; Beijing Changheng Rongchuang Technology).
Flow cytometry analysis
The EM markers of CD10 and CD90 have been detected
to verify the purity of endometrial stromal cells. Endometrial
stromal cells in the logarithmic growth phase were digested,
resuspended and counted. Resuspended cells (5,000,000-10,000,000)
were centrifuged at 1,000 × g for 5 min to obtain the cell
precipitants, and then incubated with the following antibodies:
FITC Mouse Anti-Human CD10 (1:50; cat. no. 340925; BD Biosciences);
FITC Mouse Anti-Human CD90 (1:100; cat. no. 561969; BD
Biosciences); and FITC Mouse IgG1 (1:100; cat. no. 555748; BD
Biosciences). After 30 min of incubation at 4°C in the dark, the
cells were detected using a Flow cytometer (CytoFLEX; Beckman
Coulter, Inc.) and analyzed using BD Accuri™ C6 Software (Version
1.0.264.21; BD Biosciences).
Cell proliferation assay
Endometrial stromal cells in the logarithmic growth
phase were digested with trypsin and cultured overnight in 96-well
plates (cat. no. TR4001; TrueLine) at a density of 3,000 cells/well
in a 37°C, 5% CO2 incubator. At 0, 12, 24 and 48 h of treatment of
shNC, shCCL28-1and shCCL28-2, or different concentrations of CCL28
recombinant protein (0, 5, 10, 20 and 40 ng/ml), or vehicle + DMSO,
CCL28 + DMSO, vehicle + PD98059 and CCL28 + PD98059, Cell Counting
Kit-8 (CCK-8; cat. no. CP002; SAB Biotherapeutics, Inc.) reagent
and serum-free medium were mixed at a volume ratio of 1:10.
Subsequently, 100 µl CCK-8 mixture was added to the aforementioned
groups. After 1 h incubation at 37°C, the optical density at 450 nm
was measured using a microplate reader.
Cell invasion assay
Endometrial stromal cells in the logarithmic growth
phase were digested with trypsin and seeded into 6-well plates at a
density of 300,000 cells/well. After 24 h of culture at 37°C, the
stromal cells were transduced with shCCL28 (shCCL28-1, shCCL28-2)
lentivirus for 48 h, or pre-treated with PD98059 (an ERK inhibitor;
10 µmol/l; S1177; Selleck) for 30 min at 37°C. Subsequently, cells
were treated with CCL28 recombinant protein for 48 h at 37°C and
then collected for Transwell detection. For the cell invasion
assay, a 24-well Transwell plate was used (pore size, 8 µm;
MilliporeSigma; Merck KGaA). The upper chamber of the Transwell
plate was coated with 30 µl Matrigel at 37°C for 30 min and 2×105
cells in 200 µl DMEM/F12 were added. DMEM/F12 containing 10% FBS
was added to the lower chamber. After a 48-h incubation at37°C, the
membrane was fixed with 4% formaldehyde and stained with 0.5%
crystal violet (1 ml) for 30 min at room temperature. The number of
invasive cells was counted at a magnification of ×200 via a light
microscope (XDS-500C; Shanghai Caikang Optical Instrument Co.,
Ltd.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from endometrial tissues or
stromal cells using TRIzol® reagent (cat. no. 1596-026;
Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
quantified and underwent RNA integrity confirmation. Total RNA (1
µg) was reverse transcribed into complementary DNA using a Reverse
Transcription Kit (cat. no. K1622; Fermentas; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions as
follows: 37°C for 60 min; 85°C for 5 min; 4°C for 5 min. qPCR was
subsequently performed using an ABI-7300 (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and a SYBR-Green PCR Kit (cat. no.
K0223; Thermo Fisher Scientific, Inc.). The following thermocycling
conditions were used for qPCR: Initial denaturation: 10 min at
95°C; followed by 40 cycles of denaturation, elongation and
annealing at 15 sec at 95°C and 45 sec at 60°C. CCL28, CCR10, MMP2,
MMP9 and ITGB1 mRNA expression levels were quantified using the
2-ΔΔCq method (28) and
normalized to the internal reference gene GAPDH. The primers are
listed in Table III.
 | Table III.Sequences of primers for reverse
transcription-quantitative PCR. |
Table III.
Sequences of primers for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′-3′) |
|---|
| CCL28 | F:
CTGATGGGGATTGTGACTTG |
|
| R:
TGGTGTTTCTTCCTGTGGC |
| CCR10 | F:
AGGGCTGGAGTCTGGGAAGTG |
|
| R:
CACGATGACGGAGACCAAGTGT |
| MMP2 | F:
GGGAGTACTGCAAGTTCCCCTTCTT |
|
| R:
TGGAAGCGGAATGGAAAC |
| MMP9 | F:
AGGACGGCAATGCTGATG |
|
| R:
TCGTAGTTGGCGGTGGTG |
| ITGB1 | F:
AATGTAACCAACCGTAGC |
|
| R:
GGTCAATGGGATAGTCTTC |
| GAPDH | F:
AATCCCATCACCATCTTC |
|
| R:
AGGCTGTTGTCATACTTC |
Western blotting
Total protein was extracted from endometrial stromal
cells using RIPA buffer (containing protease and phosphatase
inhibitors; cat. no. R0010; Beijing Solarbio Science &
Technology Co., Ltd.). Following total protein quantification using
a BCA kit (cat. no. 23223; Thermo Fisher Scientific, Inc.), 25 µl
of protein/lane was separated via SDS-PAGE using 10 and 12% gels
before being transferred onto polyvinylidene fluoride membranes
(cat. no. HATF00010; MilliporeSigma; Merck KGaA). Membranes were
blocked in 5% skimmed milk (BD Biosciences) for 1 h at room
temperature. Subsequently, membranes were incubated overnight at
4°C, with gentle shaking, with primary antibodies against CCL28
(dilution, 1:500; cat. no. ab196567; Abcam), CCR10 (dilution,
1:250; cat. no. ab3904; Abcam), MMP2 (dilution, 1:5,000; cat. no.
ab37150; Abcam), MMP9 (dilution, 1:1,000; cat. no. ab194316;
Abcam), ITGB1 (1:1,000; cat. no. ab24693; Abcam), phosphorylated
(p)-ERK (dilution, 1:1,000; cat. no. ab214362; Abcam), ERK
(dilution, 1:10,000; cat. no. ab184699; Abcam) and GAPDH (dilution,
1:2,000; cat. no. 5174; Cell Signaling Technology, Inc.). The
membranes were washed three times with TBS-0.05% Tween-20 (TBST),
followed by a 2-h incubation at room temperature with
HRP-conjugated goat anti-rabbit (cat. no. A0208) and goat
anti-mouse (catalog no. A0216) secondary antibody (dilution,
1:1,000; Beyotime Institute of Biotechnology). Membranes were
washed with TBST and visualized using a chemiluminescent reagent
(cat. no. WBKLS0100; MilliporeSigma; Merck KGaA) and ECL imaging
system (Tanon-5200; Tanon Science and Technology Co., Ltd.). ImageJ
version 1.47 (National Institutes of Health) was used to
semi-quantify protein expression levels using GAPDH as a loading
control.
Gelatinase zymography
Total protein from cells was isolated using RIPA
buffer, quantified by a BCA kit (cat. no. 23223; Thermo Fisher
Scientific, Inc.) and 25 µl protein per lane was separated via 10%
SDS-PAGE containing 1% gelatin. The gels were then washed with
eluent (2.5% Triton X-100, 50 mM Tris-HCl, 5 mM CaCl2;
pH 7.6) twice for 30 min, and rinsed with rinsing solution (eluent
without Triton X-100) twice for 20 min. The gels were subsequently
incubated in incubation solution (50 mM Tris-HCL, 5 mM
CaCl2, 0.02% brij-35; pH 7.6) for 20 h at 37°C. The gels
were stained using Coomassie brilliant blue staining solution for 3
h at room temperature in a low-speed shaker, and the staining
solution was recovered. A decolorizing solution (30% methanol and
10% acetic acid) was added to highlight clear bands on a blue
background for 30 min at room temperature. Images of the gels were
then captured for observation using a gel imager.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 7.0 software (GraphPad Software, Inc.). One-way ANOVA
followed by Tukey's post hoc test was used for statistical
comparisons among more than two groups, whereas unpaired Student's
t-tests were used for statistical comparisons between two groups.
For Table I, the t-test was used
for age, BMI and cancer antigen-125 comparisons between the patient
and control groups. The χ2 test was used for comparisons between
menstrual phases of the patient and control groups. Data are
presented as the mean ± standard deviation of ≥3 independent
experimental repeats. P<0.05 was considered to indicate a
statistically significant difference.
Results
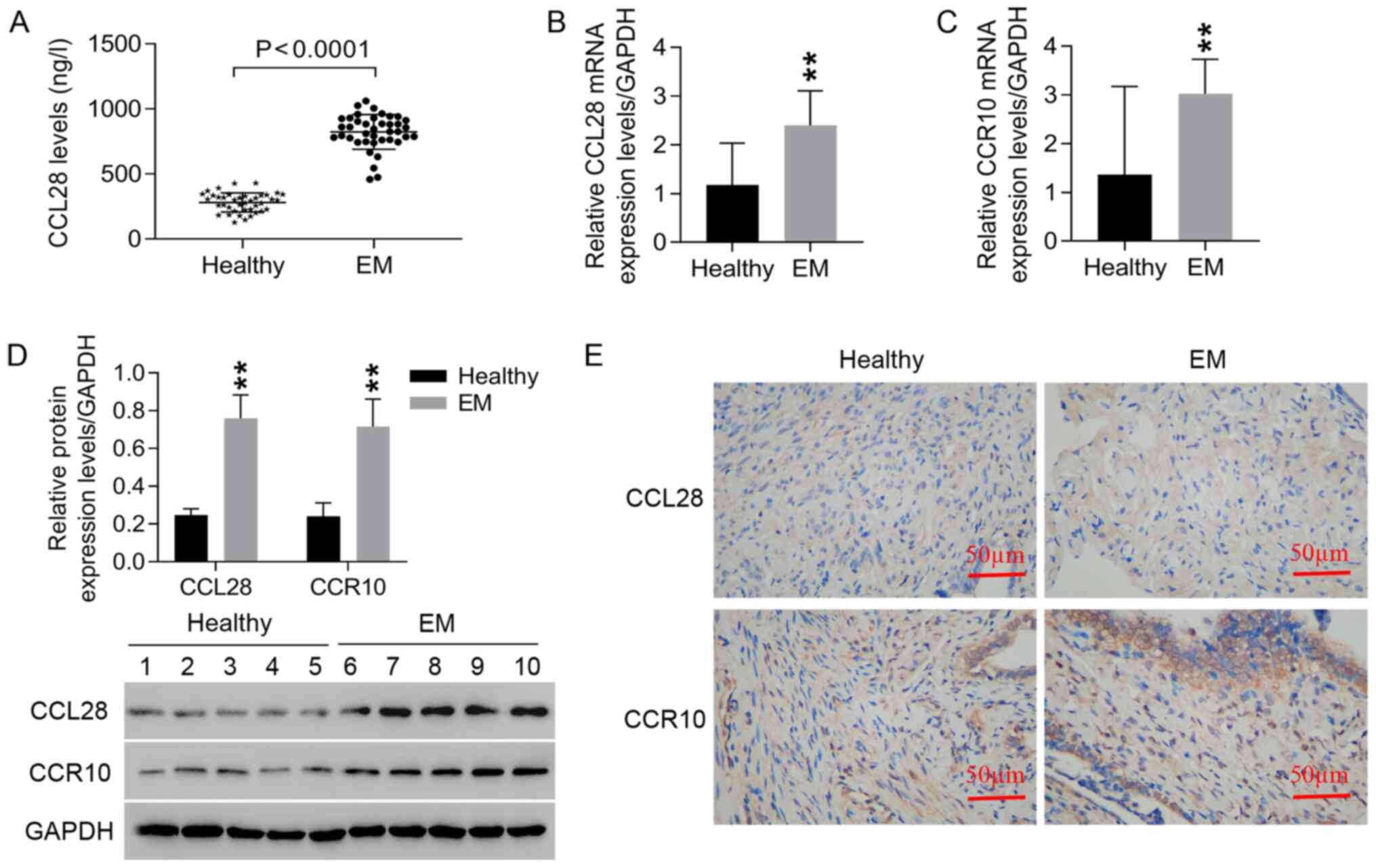
CCL28 and CCR10 are highly expressed
in the serum and endometrial tissues of patients with EM
CCL28 levels in the serum of patients with EM were
significantly higher compared with those of the healthy controls
(Fig. 1A). Furthermore,
significantly higher mRNA (Fig. 1B
and C) and protein (Fig. 1D)
expression levels of CCL28 and CCR10 were observed in EM tissues
compared with healthy tissues. IHC staining also demonstrated the
high expression levels of CCL28 and CCR10 in EM tissues compared
with healthy tissues (Fig. 1E).
These results indicated that increased expression levels of CCL28
and CCR10 may contribute to the progression of EM.
Knockdown of CCL28 expression in
endometrial stromal cells via lentiviral transduction
Positive vimentin expression was observed in ectopic
endometrial stromal cells, whereas CK19 expression was negative
(Fig. 2A). Vimentin is a marker
of endometrial stromal cells and CK19 is a marker of epithelial
cells (29,30). CD10 and CD90 detection using flow
cytometry verified that >95% of the isolated cells were EM
stromal cells (Fig. 2B). To
understand the function of CCL28 in EM, lentiviral transduction was
used to downregulate CCL28 expression in ectopic endometrial
stromal cells. Both mRNA and protein expression levels of CCL28
were significantly downregulated by shCCL28-1, −2 and −3 compared
with negative control (shNC; Fig. 2C
and D). Among the three, shCCL28-1 and shCCL28-2 were more
efficient and selected for use in subsequent experiments.
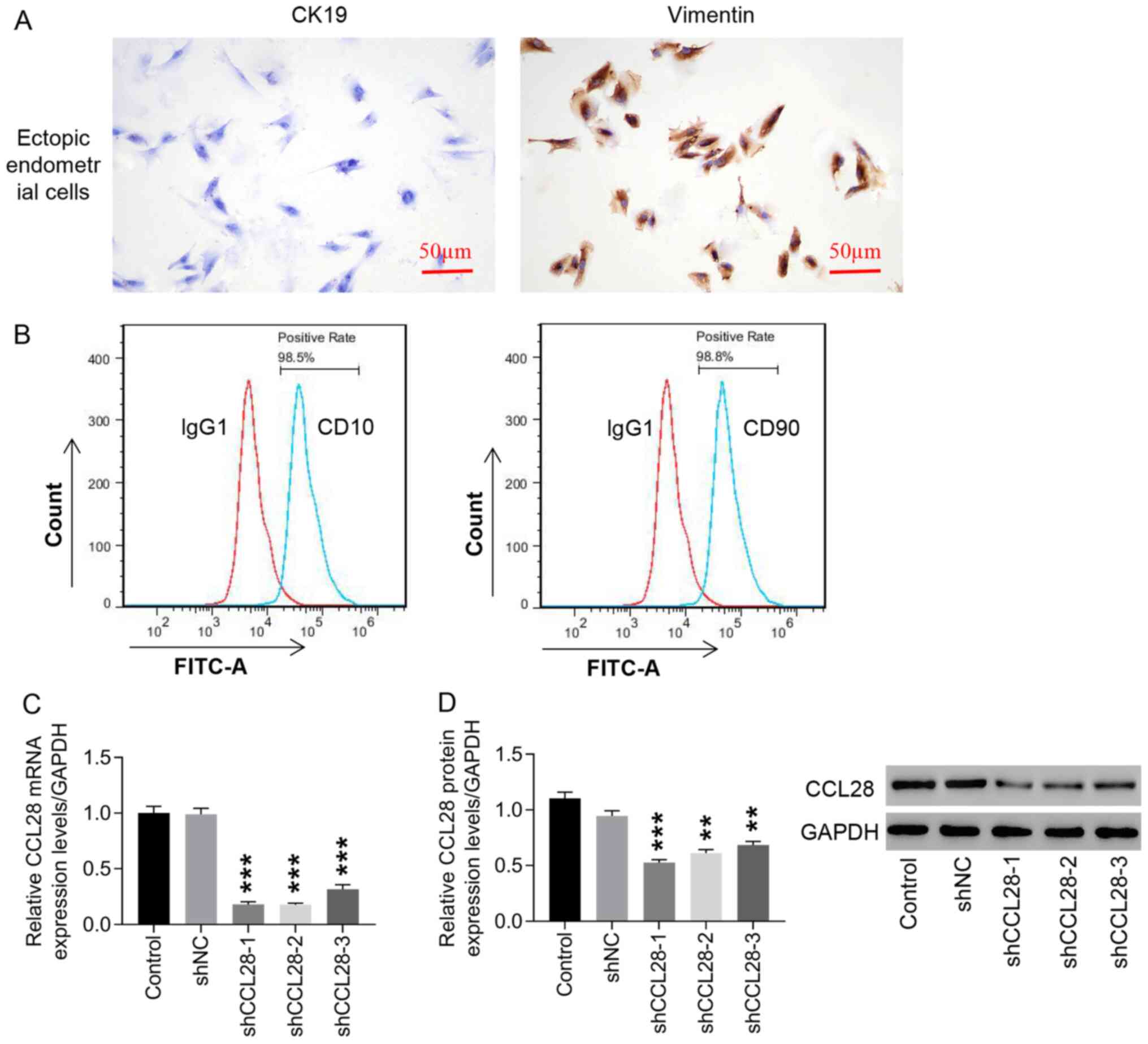 | Figure 2.Knockdown of CCL28 expression in
endometrial stromal cells by lentiviral transduction. (A)
endometrial stromal cells were identified via immunocytochemistry,
which was used to analyze CK19 and vimentin expression (×200, 50
µm). (B) CD10 and CD90 were detected using flow cytometry to
identify the percentage of EM stromal cells. shCCL28-1, −2 and −3
were constructed to transduce endometrial stromal cells, CCL28 (C)
mRNA and (D) protein expression levels were detected to determine
the knockdown efficiency of the constructs. **P<0.01 and
***P<0.001 vs. shNC. Control, cells cultured with medium; shNC,
cells infected with negative control lentivirus; shCCL28-1, −2 and
−3, cells transduced with shCCL28 lentivirus-1, −2 and −3; CCL28,
C-C motif chemokine ligand 28; sh, short hairpin RNA; CK19,
cytokeratin-19. |
Knockdown of CCL28 in endometrial
stromal cells significantly suppresses cell proliferation and
invasion
Following CCL28 knockdown, cell proliferation and
invasion were evaluated. Cell proliferation (0–48 h) and cell
invasion in shCCL28 endometrial stromal cells were significantly
decreased compared with those in the shNC group (Fig. 3A and B). Compared with the shNC
group, a significant decrease in CCL28 levels in the supernatant of
shCCL28 endometrial stromal cells was observed (Fig. 3C). Both mRNA (Fig. 3D) and protein (Fig. 3F) expression levels of CCL28,
CCR10, MMP2, MMP9 and ITGB1 in shCCL28 endometrial stromal cells
were significantly reduced compared with those of shNC endometrial
stromal cells. Knockdown of CCL28 also decreased the activities of
MMP2 and MMP9 compared with the shNC group (Fig. 3E). Furthermore, CCL28 knockdown
significantly decreased the protein expression levels of p-ERK/ERK
ratio, compared with shNC (Fig.
3G). These results suggested that knockdown of CCL28 attenuated
EM progression by inhibiting cell proliferation and invasion via
the regulation of CCR10, MMP2, MMP9 and ITGB1 expression, and this
may involve the ERK signaling pathway.
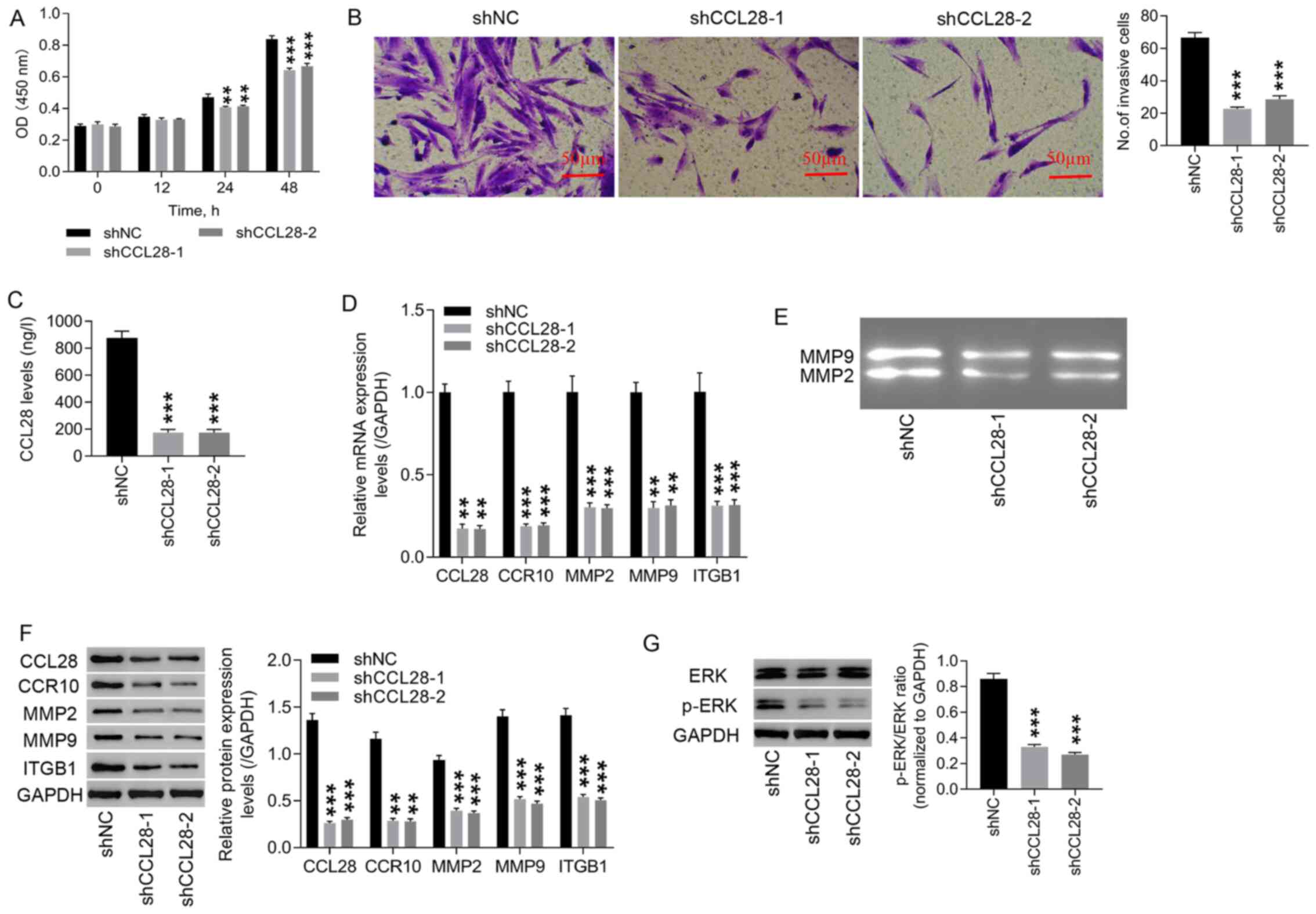 | Figure 3.Knockdown of CCL28 in endometrial
stromal cells significantly suppresses cell proliferation and
invasion. (A) Following CCL28 knockdown in endometrial stromal
cells, cell proliferation was detected using a Cell Counting Kit-8
assay at 0, 12, 24 and 48 h. (B) Cell invasion at 48 h was detected
using a Transwell invasion assay (×200, 50 µm). (C) CCL28 levels in
endometrial stromal cell supernatants were detected using an ELISA.
(D) mRNA expression levels of CCL28, CCR10, MMP2, MMP9 and ITGB1
were examined using reverse transcription-quantitative PCR. (E)
Activities of MMP2 and MMP9 were detected using gelatinase
zymography. (F) Protein expression levels of CCL28, CCR10, MMP2,
MMP9 and ITGB1 were detected via western blotting. (G) Protein
expression levels of p-ERK/ERK ratio were detected via western
blotting. **P<0.01 and ***P<0.001 vs. shNC. shNC, cells
infected with negative control lentivirus; shCCL28-1 and −2, cells
transduced with shCCL28 lentivirus-1 and −2; CCL28, C-C motif
chemokine ligand 28; CCR10, CC chemokine receptor 10; ITGB1,
integrin β1; sh, short hairpin RNA; p, phosphorylated; OD, optical
density. |
CCL28 recombinant proteins
significantly increase CCL28 and CCR10 expression in healthy
endometrial stromal cells
The results demonstrated that positive vimentin
expression was observed in endometrial stromal cells from healthy
controls, whereas CK19 expression was negative (Fig. 4A). Detection of CD10 and CD90 by
flow cytometry verified that >95% of the isolated cells were
healthy endometrial stromal cells (Fig. 4B). A series of CCL28 recombinant
protein concentrations (0, 5, 10, 20 and 40 ng/ml) were used to
treat healthy endometrial stromal cells. At 48 h, CCL28 could
significantly promote healthy endometrial stromal cell
proliferation in a dose-dependent manner compared with 0 ng/ml,
whereas at 0, 12 and 24 h no significant change was observed
(Fig. 4C). In most cases there is
still a small increase at 40 ng/ml and in general the increase in
cell proliferation at different concentrations is small.
Furthermore, after CCL28 recombinant protein treatment, both mRNA
and protein expression levels of CCL28 and CCR10 were significantly
increased in a dose-dependent manner compared with 0 ng/ml
(Fig. 4D-F). Although there is
still a small increase at 40 ng/ml and in general the increase of
40 ng/ml in cell proliferation and expression of CCL28 and CCR10 is
smaller than that of 20 ng/ml. Based on these results, 20 ng/ml
CCL28 recombinant protein was selected for the subsequent
experiments.
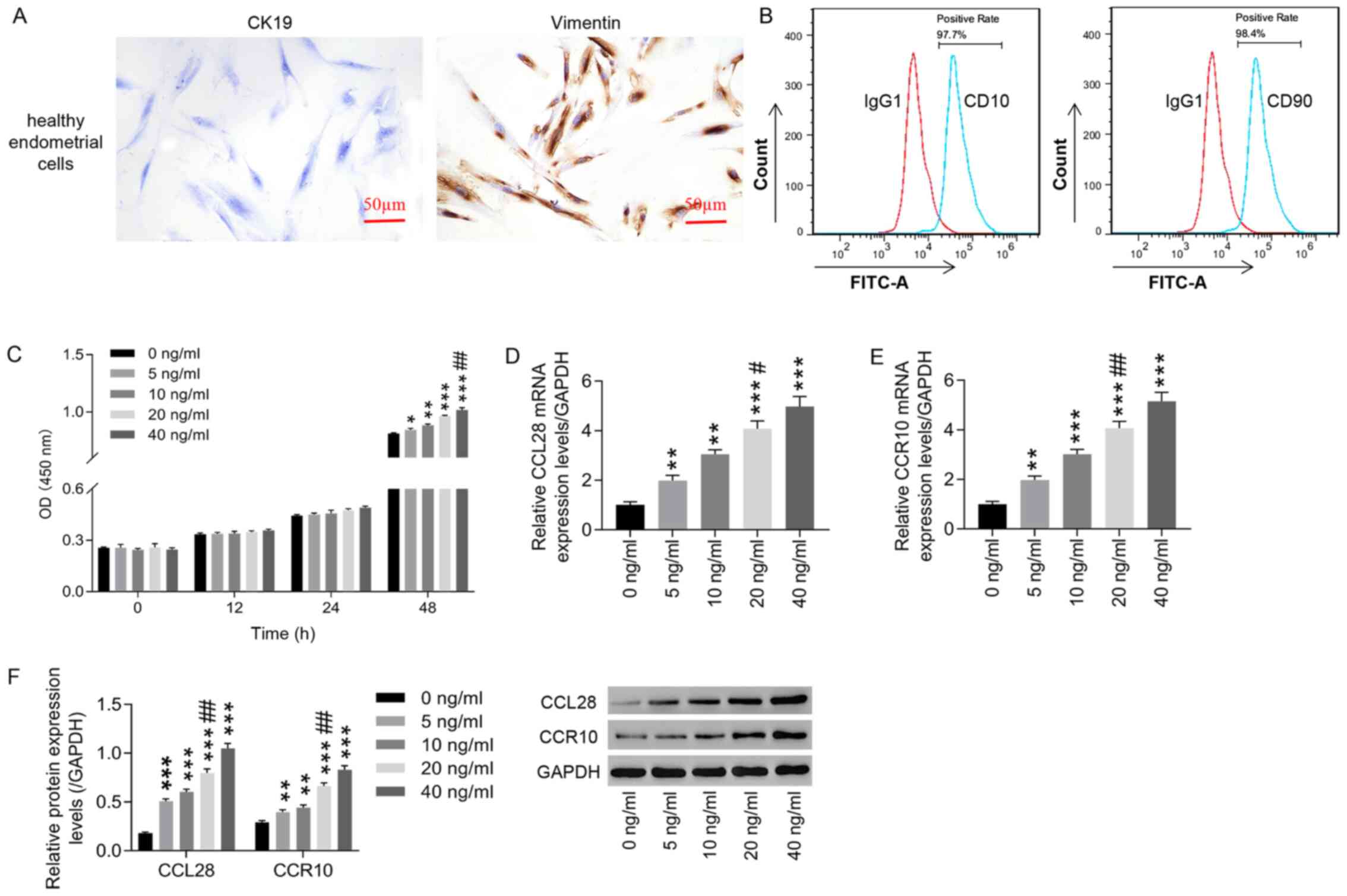 | Figure 4.Treatment with CCL28 recombinant
protein increases CCL28 and CCR10 expression in healthy human
endometrial stromal cells. (A) Healthy human endometrial stromal
cells were identified using immunocytochemistry to analyze CK19 and
vimentin expression (×200, 50 µm). (B) CD10 and CD90 were detected
via flow cytometry to identify the percentage of healthy
endometrial stromal cells. Healthy human endometrial stromal cells
were then treated with CCL28 recombinant protein at concentrations
of 0, 5, 10, 20 and 40 ng/ml. (C) Cell proliferation was detected
using a Cell Counting Kit-8 assay to determine the effect of CCL28
recombinant protein. Subsequently, at 48 h after CCL28 recombinant
protein treatment, the mRNA expression levels of (D) CCL28 and (E)
CCR10 were detected using reverse transcription-quantitative PCR.
(F) Relative protein expression levels of CCL28 and CCR10 were
analyzed via western blotting at 48 h after CCL28 recombinant
protein treatment. *P<0.05, **P<0.01 and ***P<0.001 vs. 0
ng/ml; and #P<0.05 and ##P<0.01 vs. 10 ng/ml. CCL28, C-C
motif chemokine ligand 28; CCR10, CC chemokine receptor 10; CK19,
cytokeratin-19; OD, optical density. |
CCL28 may contribute to EM progression
by regulating MMP2, MMP9 and ITGB1 expression via activation of the
ERK signaling pathway
The mechanism of the ERK signaling pathway in EM
progression was explored. The results demonstrated that
CCL28-induced proliferation and invasion of healthy endometrial
stromal cells were markedly attenuated by PD98059 (ERK inhibitor;
Fig. 5A and B). Furthermore,
compared with vehicle + DMSO, CCL28 induced the activities of MMP2
and MMP9, which were markedly inhibited by PD98059 (Fig. 5C). CCL28-induced MMP2, MMP9 and
ITGB1 protein expression levels, as well as p-ERK/ERK ratio, were
significantly decreased by PD98059 (Fig. 5D and E). These results indicated
that CCL28 may contribute to EM progression by regulating MMP2,
MMP9 and ITGB1 expression via the ERK signaling pathway.
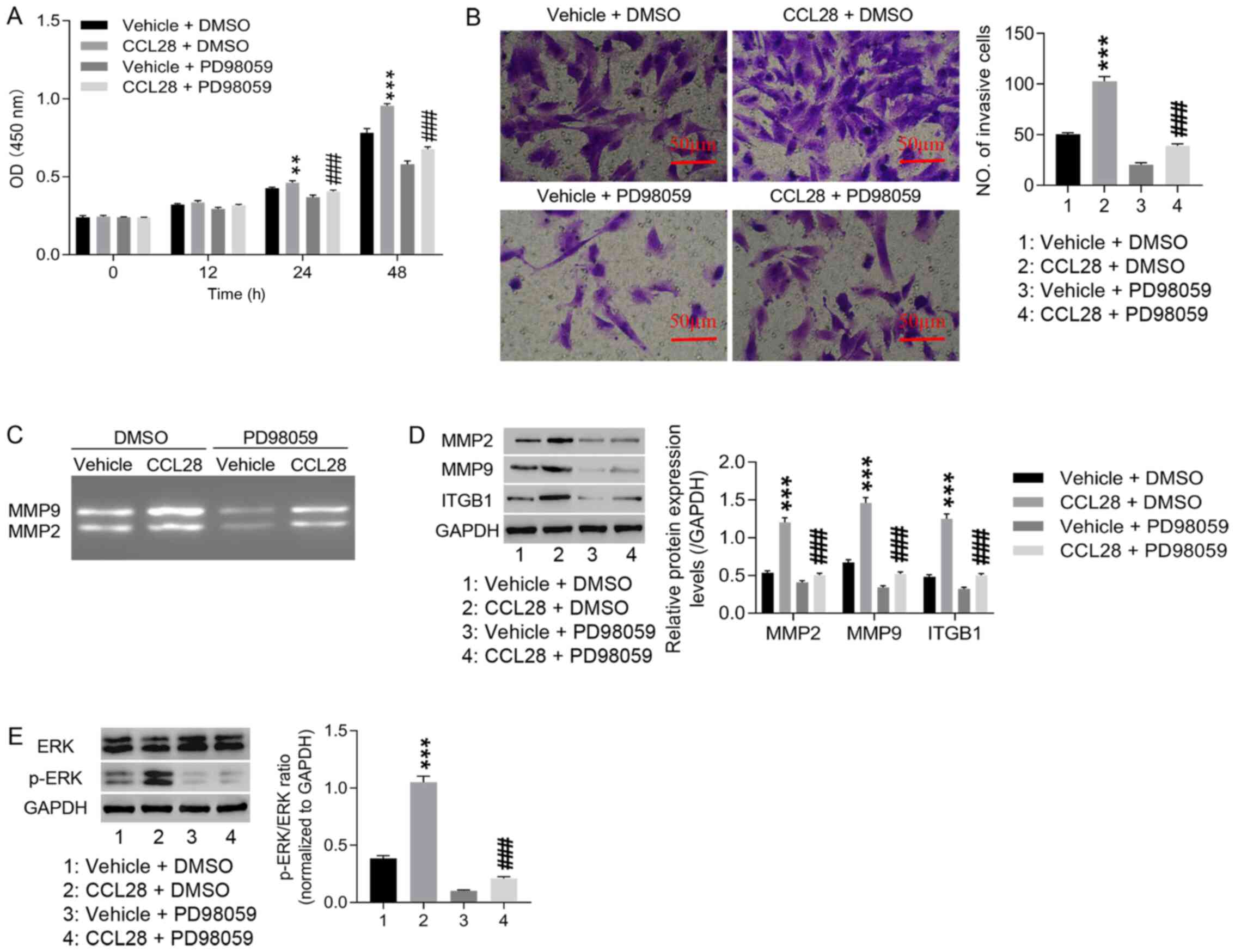 | Figure 5.CCL28 may contribute to endometriosis
progression by regulating MMP2, MMP9 and ITGB1 expression via
activating the ERK signaling pathway. Healthy endometrial stromal
cells were pre-treated with 10 µmol/l PD98059 (ERK inhibitor) for
30 min, and then treated with 20 ng/ml CCL28 recombinant protein
for 48 h. (A) Cell proliferation was detected using the Cell
Counting Kit-8 assay at 0, 12, 24 and 48 h. (B) Cell invasion was
detected using the Transwell invasion assay at 48 h (×200, 50 µm).
(C) MMP2 and MMP9 activity was detected via gelatinase zymography.
Relative protein expression levels of (D) MMP2, MMP9 and ITGB1 and
(E) the p-ERK/ERK ratio were analyzed via western blotting.
**P<0.01, ***P<0.001 vs. vehicle + DMSO; and ###P<0.001
vs. CCL28 + vehicle. Vehicle, solvent of CCL28 recombinant
proteins; DMSO, solvent of PD98059; CCL28, C-C motif chemokine
ligand 28; ITGB1, integrin β1; p, phosphorylated; OD, optical
density. |
Discussion
Previous research has suggested that chemokine
ligands serve important roles in the development and progression of
EM. For example, the levels of CCL2 are elevated in the peritoneal
fluid of female patients with EM (31,32), and can enhance endometrial stromal
cell survival and invasion (23).
A previous study also demonstrated that proinflammatory cytokines
contribute to the development of EM by upregulating the secretion
of CCL20 in endometrial stromal cells (33). Furthermore, it has been reported
that certain chemokines, such as CCL2 and CCL5, have the potential
to be biomarkers for EM (34,35). As for chemokine CCL28, a study has
revealed that it is elevated in the serum of patients with celiac
disease and decreases following treatment (36). In Helicobacter pylori infection,
upregulated CCL28 expression is associated with a risk of gastritis
and peptic ulcer disease (37).
In the present study, significantly elevated CCL28 expression was
observed in the serum and endometrial tissues of patients with EM,
alongside significantly increased CCR10 expression. Knockdown of
CCL28 in endometrial stromal cells significantly suppressed cell
proliferation and invasion. These results are consistent with a
report that depletion of CCL27 can suppress cell proliferation and
metastasis in ectopic endometrial stromal cells (17). Therefore, these results suggested
that CCL28 may serve a critical role in the progression of EM, and
knockdown of CCL28 may attenuate EM by inhibiting cell
proliferation and invasion.
Furthermore, the potential mechanisms by which CCL28
regulated EM stromal cell proliferation and invasion were
investigated. A significant decrease in mRNA and protein expression
levels of CCR10, MMP2, MMP9 and ITGB1, as well as decreased ERK1/2
phosphorylation, were observed in shCCL28 endometrial stromal
cells. Subsequently, various concentrations of CCL28 recombinant
proteins (between 5 and 40 ng/ml) were tested on healthy
endometrial stromal cells. Although there is still a small increase
at 40 ng/ml and in general the increase of 40 ng/ml in cell
proliferation and expression of CCL28 and CCR10 is smaller than
that of 20 ng/ml. Thus, the concentration of 20 ng/ml was chosen
for subsequent experiments. Treatment with 20 ng/ml CCL28
recombinant proteins significantly induced cell proliferation and
invasion at 48 h, and relative protein levels of MMP2, MMP9, ITGB1
and p-ERK were significantly attenuated by the ERK inhibitor,
PD98059. MMPs are important in tumor metastasis as a result of
their degradation capacity of extracellular matrix (38). Gelatinases MMP2 and MMP9 are used
as prognostic factors in numerous types of solid tumors (39,40). A previous study reported that
compared with that in the normal endometrium, expression of MMPs is
much greater in ectopic endometrium (41). MMP9 has been reported to be
associated with the grade and stage of endometrial cancer, whereas
MMP2 expression is related to CA125 expression and clinical
progression in endometrial carcinoma (42,43). MMP9 overexpression enhances the
invasion of the endometrium and the ability to degrade the
extracellular matrix, loosening the connection between cells,
thereby providing allowing ectopic endometrial tissue to enter the
myometrium (44). MMP9 may also
be involved in the formation of lesion blood vessels, providing
nutrition for ectopic endometrium (45–47). Furthermore, previous studies have
reported that the ERK1/2 signaling pathway is linked to EM
progression, such as in endometriotic cell migration and apoptosis
(20,22). It has also been demonstrated that
estrogen can upregulate the expression levels of MMP2/MMP9 in
endometrial epithelial cells via the VEGF-ERK1/2 signaling pathway
(48). Furthermore, long
non-coding RNA BRAF-activated non-protein coding RNA, can promote
cell proliferation and invasion in endometrial cancer cells by
regulating MMP2 and MMP1 via the ERK/MAPK signaling pathway
(49). The present study
demonstrated that CCL28 knockdown markedly inhibited the activities
of MMP2 and MMP9, whereas CCL28 induced MMP2 and MMP9 activity,
which was counteracted by the ERK inhibitor PD98059. Therefore, it
can be inferred that CCL28 may promote endometrial stromal cell
proliferation and invasion by regulating MMP2 and MMP9 via the ERK
signaling pathway.
Integrins are important adhesion molecules on the
surface of endometrial cells, are closely related to the
pathogenesis of EM and serve important roles in signal transduction
(50). ITGB1 is a member of the
integrins. ITGB1 expression has been demonstrated to be upregulated
in the endometrium of patients with EM, with microRNA-183 impacting
EM progression by regulating stromal cell ITGB1 expression and
function (51).
In the present study, ITGB1 expression was
positively regulated by CCL28, and this was counteracted by the ERK
inhibitor PD98059. Therefore, these results indicated that CCL28
may also regulate ITGB1 expression and function in endometrial
stromal cells via the ERK signaling pathway. However, the effect of
CCL28 on the development of EM was only investigated at the
cellular level. Therefore, in vivo experiments are required to
further confirm the therapeutic effect of CCL28 on EM and further
explore underlying molecular mechanisms to expand the clinical
application of CCL28. Although additional studies are required to
further verify the function and mechanisms of CCL28 in the
development of EM, the present study implied that CCL28 could be
considered as a potential target for EM treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shanghai Youth Clinical
Medical Personnel (Clinical Laboratory Specialty) Training Scheme
(grant no. 201605).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HC and QZ conceived and designed the study. YW, FZ,
WSu and WSh performed the experiments and collected and analyzed
the data. HC and QZ wrote and revised the manuscript. HC and QZ
have confirmed the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments conducted in the present study were
approved by the Ethics Committee of Shanghai First Maternity and
Infant Hospital, School of Medicine, Tongji University (approval
no. KS2154; Shanghai, China), and written informed consent was
obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kennedy S, Bergqvist A, Chapron C,
D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A and
Saridogan E; ESHRE Special Interest Group for Endometriosis and
Endometrium Guideline Development Group, : ESHRE guideline for the
diagnosis and treatment of endometriosis. Hum Reprod. 20:2698–2704.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eskenazi B and Warner ML: Epidemiology of
endometriosis. Obstet Gynecol Clin North Am. 24:235–258. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zondervan KT, Yudkin PL, Vessey MP,
Jenkinson CP, Dawes MG, Barlow DH and Kennedy SH: The community
prevalence of chronic pelvic pain in women and associated illness
behaviour. Br J Gen Pract. 51:541–547. 2001.PubMed/NCBI
|
|
4
|
Zondervan KT, Cardon LR and Kennedy SH:
What makes a good case-control study? Design issues for complex
traits such as endometriosis. Hum Reprod. 17:1415–1423. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rogers PA, D'Hooghe TM, Fazleabas A,
Gargett CE, Giudice LC, Montgomery GW, Rombauts L, Salamonsen LA
and Zondervan KT: Priorities for endometriosis research:
Recommendations from an international consensus workshop. Reprod
Sci. 16:335–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rolla E: Endometriosis: Advances and
controversies in classification, pathogenesis, diagnosis, and
treatment. F1000 Res. 8:82019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Wang Y, Chen P, Ma Y, Wang S, Tian
Y, Wang A and Wang D: AC002454.1 and CDK6 synergistically promote
endometrial cell migration and invasion in endometriosis.
Reproduction. 157:535–543. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bakogiannis C, Sachse M, Stamatelopoulos K
and Stellos K: Platelet-derived chemokines in inflammation and
atherosclerosis. Cytokine. 122:1541572019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Korbecki J, Grochans S, Gutowska I,
Barczak K and Baranowska-Bosiacka I: CC Chemokines in a tumor: A
review of pro-cancer and anti-cancer properties of receptors CCR5,
CCR6, CCR7, CCR8, CCR9, and CCR10 ligands. Int J Mol Sci.
21:212020. View Article : Google Scholar
|
|
10
|
Bian X, Xiao YT, Wu T, Yao M, Du L, Ren S
and Wang J: Microvesicles and chemokines in tumor microenvironment:
Mediators of intercellular communications in tumor progression. Mol
Cancer. 18:502019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hieshima K, Ohtani H, Shibano M, Izawa D,
Nakayama T, Kawasaki Y, Shiba F, Shiota M, Katou F, Saito T, et al:
CCL28 has dual roles in mucosal immunity as a chemokine with
broad-spectrum antimicrobial activity. J Immunol. 170:1452–1461.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berri M, Virlogeux-Payant I, Chevaleyre C,
Melo S, Zanello G, Salmon H and Meurens F: CCL28 involvement in
mucosal tissues protection as a chemokine and as an antibacterial
peptide. Dev Comp Immunol. 44:286–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moed H, Boorsma DM, Tensen CP, Flier J,
Jonker MJ, Stoof TJ, von Blomberg BM, Bruynzeel DP, Scheper RJ,
Rustemeyer T, et al: Increased CCL27-CCR10 expression in allergic
contact dermatitis: implications for local skin memory. Journal
Pathol. 204:39–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L, Liu X, Zhang H-Y, Du W, Qin Z, Yao
Y, Mao Y and Zhou L: Upregulation of chemokine receptor CCR10 is
essential for glioma proliferation, invasion and patient survival.
Oncotarget. 5:6576–6583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong N, Fu Y, Hu S, Xia M and Yang J:
CCR10 and its ligands in regulation of epithelial immunity and
diseases. Protein Cell. 3:571–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin HY, Sun SM, Lu XF, Chen PY, Chen CF,
Liang WQ and Peng CY: CCR10 activation stimulates the invasion and
migration of breast cancer cells through the ERK1/2/MMP-7 signaling
pathway. Int Immunopharmacol. 51:124–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ruan F, Ma J and Zhou J: Depletion of
CCL27 inhibits cell proliferation, metastasis and adhesion in
ectopic endometrial stromal cells. Int J Clin Exp Med.
9:19074–19083. 2016.
|
|
18
|
Sun C, Zhang YY, Tang CL, Wang SC, Piao
HL, Tao Y, Zhu R, Du MR and Li DJ: Chemokine CCL28 induces
apoptosis of decidual stromal cells via binding CCR3/CCR10 in human
spontaneous abortion. Mol Hum Reprod. 19:676–686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cha HR, Ko HJ, Kim ED, Chang SY, Seo SU,
Cuburu N, Ryu S, Kim S and Kweon MN: Mucosa-associated epithelial
chemokine/CCL28 expression in the uterus attracts CCR10+ IgA plasma
cells following mucosal vaccination via estrogen control. J
Immunol. 187:3044–3052. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gentilini D, Busacca M, Di Francesco S,
Vignali M, Viganò P and Di Blasio AM: PI3K/Akt and ERK1/2
signalling pathways are involved in endometrial cell migration
induced by 17β-estradiol and growth factors. Mol Hum Reprod.
13:317–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tong JS, Zhang QH, Huang X, Fu XQ, Qi ST,
Wang YP, Hou Y, Sheng J and Sun QY: Icaritin causes sustained
ERK1/2 activation and induces apoptosis in human endometrial cancer
cells. PLoS One. 6:e167812011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Banu SK, Lee J, Speights VO Jr,
Starzinski-Powitz A and Arosh JA: Selective inhibition of
prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human
endometriotic cells through suppression of ERK1/2, AKT, NFkappaB,
and β-catenin pathways and activation of intrinsic apoptotic
mechanisms. Mol Endocrinol. 23:1291–1305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li MQ, Li HP, Meng YH, Wang Zhu XY, Mei J
and Li DJ: Chemokine CCL2 enhances survival and invasiveness of
endometrial stromal cells in an autocrine manner by activating Akt
and MAPK/Erk1/2 signal pathway. Fertil Steril. 97:919–929. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li MQ, Shao J, Meng YH, Mei J, Wang Y, Li
H, Zhang L, Chang KK, Wang XQ, Zhu XY, et al: NME1 suppression
promotes growth, adhesion and implantation of endometrial stromal
cells via Akt and MAPK/Erk1/2 signal pathways in the endometriotic
milieu. Hum Reprod. 28:2822–2831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang XL, Liu KY, Lin FJ, Shi HM and Ou ZL:
CCL28 promotes breast cancer growth and metastasis through
MAPK-mediated cellular anti-apoptosis and pro-metastasis. Oncol
Rep. 38:1393–1401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu J, Zhang Z, Liu J and Wang D: LIM
Kinase 1 Mediates estradiol effects on the phosphorylation of
Cofilin1 in eutopic endometrial stromal cells during the invasion
and proliferation of endometriosis. Reprod Sci. 26:1499–1505. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Q, Hang Y, Zhang T, Tan L, Li S and
Jin Y: USP10 promotes proliferation and migration and inhibits
apoptosis of endometrial stromal cells in endometriosis through
activating the Raf-1/MEK/ERK pathway. Am J Physiol Cell Physiol.
315:C863–C872. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Minkwitz C, Schoon HA, Zhang Q and
Schöniger S: Plasticity of endometrial epithelial and stromal
cells-A new approach towards the pathogenesis of equine
endometrosis. Reprod Domest Anim. 54:835–845. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng X, Zhang X, Li W, Feng RX, Li L, Yi
GR, Zhang XN, Yin C, Yu HY, Zhang JP, et al: Chronic Liver Injury
Induces Conversion of Biliary Epithelial Cells into Hepatocytes.
Cell Stem Cell. 23:114–122.e3. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gou Y, Li X, Li P, Zhang H, Xu T, Wang H,
Wang B, Ma X, Jiang X and Zhang Z: Estrogen receptor β upregulates
CCL2 via NF-κB signaling in endometriotic stromal cells and
recruits macrophages to promote the pathogenesis of endometriosis.
Hum Reprod. 34:646–658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mei J, Zhou WJ, Li SY, Li MQ and Sun HX:
Interleukin-22 secreted by ectopic endometrial stromal cells and
natural killer cells promotes the recruitment of macrophages
through promoting CCL2 secretion. Am J Reprod Immunol.
82:e131662019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hirata T, Osuga Y, Takamura M, Kodama A,
Hirota Y, Koga K, Yoshino O, Harada M, Takemura Y, Yano T, et al:
Recruitment of CCR6-expressing Th17 cells by CCL20 secreted from
IL-1 β-, TNF-α-, and IL-17A-stimulated endometriotic stromal cells.
Endocrinology. 151:5468–5476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo P, Bi K, Lu Z, Wang K, Xu Y, Wu H, Cao
Y and Jiang H: CCR5/CCR5 ligand-induced myeloid-derived suppressor
cells are related to the progression of endometriosis. Reprod
Biomed Online. 39:704–711. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jaiswal U, Yadav RK, Bhat MA, Kriplani A,
Roy KK and Netam RK: Cytokine and growth factor profile in
endometriosis: a multiplex analysis of peritoneal fluid to assess
diagnostic utility. Gynecol Endocrinol. 36:718–722. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rashidiani S, Jalili A, Babaei E,
Sheikhesmaeili F, Fakhari S, Ataee P and Parhizkar B: The chemokine
CCL28 is elevated in the serum of patients with celiac disease and
decreased after treatment. Am J Clin Exp Immunol. 6:60–65.
2017.PubMed/NCBI
|
|
37
|
Sanaei MJ, Shirzad H, Soltani A,
Abdollahpour-Alitappeh M, Shafigh MH, Rahimian G, Mirzaei Y and
Bagheri N: Up-regulated CCL18, CCL28 and CXCL13 expression is
associated with the risk of gastritis and peptic ulcer disease in
Helicobacter pylori infection. Am J Med Sci. 361:43–54. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lv Y, Zhao X, Zhu L, Li S, Xiao Q, He W
and Yin L: Targeting intracellular MMPs efficiently inhibits tumor
metastasis and angiogenesis. Theranostics. 8:2830–2845. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang X, Yang B, She Y and Ye Y: The lncRNA
TP73-AS1 promotes ovarian cancer cell proliferation and metastasis
via modulation of MMP2 and MMP9. J Cell Biochem. 119:7790–7799.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Farina P, Tabouret E, Lehmann P, Barrie M,
Petrirena G, Campello C, Boucard C, Graillon T, Girard N and Chinot
O: Relationship between magnetic resonance imaging characteristics
and plasmatic levels of MMP2 and MMP9 in patients with recurrent
high-grade gliomas treated by Bevacizumab and Irinotecan. J
Neurooncol. 132:433–437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pino M, Galleguillos C, Torres M, Sovino
H, Fuentes A, Boric MA and Johnson MC: Association between MMP1 and
MMP9 activities and ICAM1 cleavage induced by TNF in stromal cell
cultures from eutopic endometria of women with endometriosis.
Reproduction. 138:837–847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aglund K, Rauvala M, Puistola U, Angström
T, Turpeenniemi-Hujanen T, Zackrisson B and Stendahl U: Gelatinases
A and B (MMP-2 and MMP-9) in endometrial cancer-MMP-9 correlates to
the grade and the stage. Gynecol Oncol. 94:699–704. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Honkavuori M, Talvensaari-Mattila A, Soini
Y, Turpeenniemi-Hujanen T and Santala M: MMP-2 expression
associates with CA 125 and clinical course in endometrial
carcinoma. Gynecol Oncol. 104:217–221. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Barbe AM, Berbets AM, Davydenko IS, Koval
HD, Yuzko VO and Yuzko OM: Expression and significance of matrix
metalloproteinase-2 and matrix metalloproteinas-9 in Endometriosis.
J Med Life. 13:314–320. 2020.PubMed/NCBI
|
|
45
|
Freitas S, Meduri G, Le Nestour E, Bausero
P and Perrot-Applanat M: Expression of metalloproteinases and their
inhibitors in blood vessels in human endometrium. Biol Reprod.
61:1070–1082. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yoshiji H, Harris SR, Raso E, Gomez DE,
Lindsay CK, Shibuya M, Sinha CC and Thorgeirsson UP: Mammary
carcinoma cells over-expressing tissue inhibitor of
metalloproteinases-1 show enhanced vascular endothelial growth
factor expression. Int J Cancer. 75:81–87. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kodarahmian M, Amidi F, Moini A, Kashani
L, Nashtaei MS, Pazhohan A, Bahramrezai M, Berenjian S and Sobhani
A: The modulating effects of resveratrol on the expression of MMP-2
and MMP-9 in endometriosis women: A randomized exploratory trial.
Gynecol Endocrinol. 35:719–726. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shan B, Li W, Yang SY and Li ZR: Estrogen
up-regulates MMP2/9 expression in endometrial epithelial cell via
VEGF-ERK1/2 pathway. Asian Pac J Trop Med. 6:826–830. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang D, Wang D, Wang N, Long Z and Ren X:
Long non-coding RNA BANCR promotes endometrial cancer cell
proliferation and invasion by regulating MMP2 and MMP1 via ERK/MAPK
signaling pathway. Cell Physiol Biochem. 40:644–656. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lei Y, Huang K, Gao C, Lau QC, Pan H, Xie
K, Li J, Liu R, Zhang T, Xie N, et al: Proteomics identification of
ITGB3 as a key regulator in reactive oxygen species-induced
migration and invasion of colorectal cancer cells. Mol Cell
Proteomics. May 27–2011.(Epub ahead of print). doi:
10.1074/mcp.M110.005397. View Article : Google Scholar
|
|
51
|
Chen J, Gu L, Ni J, Hu P, Hu K and Shi YL:
MiR-183 regulates ITGB1P expression and promotes invasion of
endometrial stromal cells. Biomed Res Int.
2015:3402182015.PubMed/NCBI
|