Introduction
It is well established that cortisol is the main
hormone that regulates stress in humans and it plays an equivalent
role in rodents (1). Stress
begins when the hypothalamus releases corticotropin-releasing
factor (CRF), thereby activating the hypothalamic-pituitary-adrenal
(HPA) axis (2). In animal models,
stress is associated with abnormalities in processes that maintain
gut homeostasis, such as in visceral perception (3), the integrity of the
intestinal-epithelial barrier (4), ion transport (5) and host defense mechanisms.
Increasing gut permeability promotes an inflammatory environment,
such as that observed in chronic gastrointestinal diseases
(6). Accumulating evidence has
shown that, in adult rats, acute or chronic stress involves the
release of CRF (7); furthermore,
other models of stress, such as neonatal maternal deprivation
stress, restraint and immobilization stress (8,9),
and chronic unpredictable stress (10), mimic the epithelial response to
stress, stimulating ion secretion and enhancing permeability. Data
from chronic stress models have revealed that a region-specific
reduction in epithelial tight junction (TJ) protein expression in
the colon is induced by corticosterone (11). Recently, the same authors proposed
that chronic elevation of glucocorticoid (GC) levels may impair the
epithelial barrier function of human/rat claudin 1 promoters in the
colon via the transcription repressor HES family bHLH transcription
factor 1 and GCs natural cytotoxicity triggering receptor 3 axis
(12). Therefore, intestinal
permeability during stress is a process that is highly regulated
and is crucial for maintaining interactions between microbiota,
colonic epithelial cells, immune system cells and nervous system
cells. Although it has been hypothesized that acute or short-term
stress can be an adaptive mechanism that confers greater immune
protection after injury or infection (13,14), to date, little is known regarding
epithelial cells (goblet, mast and immune cells) and other effector
mechanisms involved in conferring this protection.
While observations have documented that mast cells
are recruited by high levels of corticosterone (15), it has been proposed that these
cells are sentinels that are responsible for supporting
stress-induced colonic mucosal pathophysiology, and they also
communicate with immune cells (16) and the central nervous system via
substance P (SP) (17). SP is a
neuropeptide that is released throughout the body by the
neuroendocrine system in all organs and inflammatory cells, such as
lymphocytes (18). SP acts by
binding to the neurokinin-1 receptor (NK1R), and it exerts
proinflammatory effects on immune and epithelial cells. In
addition, SP regulates smooth muscle contractility, hyperalgesia,
epithelial ion transport, vascular permeability and immune function
in the gastrointestinal tract (18). At present, the two types of stress
imposed on intestinal epithelial components (goblet and mast cells,
TJ proteins) and non-epithelial components (inflammatory infiltrate
and lymphocyte cytokines of the lamina propria) in the colon and
serum SP were evaluated. These changes could help elucidate the
initiation and perpetuation mechanisms of gut inflammation by
stress.
The current study used a stress model in which mice
were subjected to repeated movement restriction, including one
session (acute) or three repeated sessions over 2 h (chronic). The
objective of the present study was to evaluate the effects of these
two stress protocols on mucosal components and immune response
parameters in the colon, as well as on serum SP levels. The results
of the present study could provide a useful reference for the
management of therapies that regulate the effects of stress on
intestinal bowel disease dysfunction.
Materials and methods
Experimental animals
A total of 45 male BALB/c mice (weight, 20-30 g;
age, 10-12 weeks) were used in this study. The mice were allowed
free access to food and water (Labdiet 5013; LabDiet). All mice
were kept on a 12-h light/dark cycle (lights on at 6:00 a.m.) at
room temperature (RT) at 20°C, with a relative humidity of 55%. The
current protocol was developed based on the ARRIVE guidelines for
reporting animal research (19)
and was approved by the Ethics in Research Committee of the Escuela
Superior de Medicina (IPN). The animals were handled following the
Mexican Federal Regulations for animal experimentation and care
(Regulation-062-ZOO-1999, Ministry of Agriculture, México City,
México).
Stress protocol
The animals were randomly divided into three groups
(n=15): i) Control group without stress; ii) acute stress group (2
h of movement restriction repeated once); and iii) chronic stress
group (2 h of movement restriction repeated for 3 days). Mice were
placed in cylindrical plexiglass containers according to a
previously described method (20). The animals from the control group
remained in their home cages without water and food for the same
duration as that for which the animals in both stressed groups were
exposed to stress.
Tissue collection
Male BALB/c mice were anaesthetized by an
intraperitoneal injection of a lethal dose of 100 mg/kg body weight
pentobarbital sodium salt (cat. no. P3761; Sigma-Aldrich; Merck
KGaA). Blood was obtained via cardiac puncture (0.7-1 ml), and
serum was obtained via centrifugation at 1,660 × g for 7 min at
4°C. The serum samples were stored at −20°C until use. After
exsanguination the large intestines were dissected, a colon
segments were cut (1 cm), fixed with 4% paraformaldehyde at RT for
24 h and processed for paraffin embedding. Sections (7-µm thick)
were generated with a microtome (Rotatory Microtome; Leica
Microsystems GmbH), placed on coverslips and stained with H&E
or Alcian Blue (AB) or toluidine blue at RT.
Corticosterone assay
Plasma corticosterone concentrations were determined
using a commercially available ELISA kit for corticosterone
analysis according to the manufacturer's instructions (cat. no.
ADI-901-097; Enzo Life Sciences, Inc.). The corticosterone
concentrations in the plasma samples were calculated based on a
standard curve and are expressed in ng/ml.
Quantitative analysis of the
polymorphonuclear cells (PMN) in the colon
For the quantification of leukocyte infiltration,
colon sections were stained with H&E. After deparaffinization
with xylol and rehydration in a descending alcohol gradient, the
samples were immersed in Harris hematoxylin solution (cat. no.
H3136; Sigma-Aldrich; Merck KGaA) and incubated for 20 min at RT.
After incubation in an eosin solution for 2 min at RT (cat. no.
E4009; Sigma-Aldrich; Merck KGaA), the samples were then washed
with distilled water. Finally, the sections were dehydrated and
mounted with Entellan® (cat. no. 1079610500; Merck
KGaA). The samples were analyzed via optical microscopy, and the
PMN numbers were determined. The PMNs in the inflammatory
infiltrates were counted (12 per slide/3 slides per animal). The
percentage of PMNs in each sample was calculated. The number of
cells counted was evaluated using Image-Pro Plus version 5.1
software (Media Cybernetics, Inc.) and an E600 microscope (Eclipse
E-600; Nikon Corporation) at a ×40 magnification, and the total
number of PMNs was quantified (21).
Acid mucin staining and goblet cell
quantification
Colon samples were fixed and stained for acidic
mucins as previously described (22). Acid mucins were stained with AB
(cat. no. C.I.74240; Sigma-Aldrich; Merck KGaA). After
deparaffinization with xylene for 30 min at 60°C, the samples were
rehydrated in a descending alcohol gradient. The samples were
incubated in 3% acetic acid for 3 min at RT and then incubated with
1% AB solution in 3% acetic acid pH 2.5 for 25 min at RT. Then, the
samples were washed with warm water until the color changed.
Finally, the sections were dehydrated and mounted with Entellan.
The samples were observed via light microscopy, and the positive
staining of five randomly selected Lieberkühn crypts in each colon
sample was observed (n=5). The number of goblet cells per group was
determined (magnification, ×40; Eclipse E-600; Nikon Corporation)
and analyzed using Image-Pro Plus 5.1 software (Media Cybernetics,
Inc.) and an E600 microscope; the average numbers of goblet cells
per crypt and per group were determined (22).
Mast cell staining and
quantification
Mast cells were observed using toluidine blue
staining (cat. no. 198161; Sigma-Aldrich; Merck KGaA). After
deparaffinization with xylol and rehydration in a descending
alcohol gradient, the samples were immersed in a 0.5% toluidine
blue solution and incubated for 30 min at RT. Then, the samples
were washed with distilled water, dehydrated and mounted with
Entellan. The numbers of mast cells in the intestinal lamina
propria of each group were determined. The mast cells were
identified and counted randomly via light microscopy at a ×40
magnification with an E600 microscope (Eclipse E-600; Nikon
Corporation). The average numbers of mast cells per section per
group were determined (23).
Epithelial cell isolation
Claudin-1 and E-cadherin expression in isolated
epithelial cells from the large intestine was determined. Briefly,
fragments of the large intestine were incubated in RPMI-1640 medium
(cat. no. R7388; Sigma-Aldrich; Merck KGaA) with 1 mM
dithiothreitol (cat. no. 20290; Thermo Fisher Scientific, Inc.) and
1.5 mM EDTA (cat. no. E6511; Sigma-Aldrich; Merck KGaA), with
continuous shaking at 415 × g for 30 min at 37°C. The cell
suspension was passed through organza to remove the mucus and
centrifuged at 415 × g for 10 min at 4°C. The pellet was suspended
in 15 ml RPMI-1640 medium, passed through an organza filter and
washed twice with 15 ml RPMI-1640 medium followed by centrifugation
at 415 × g for 10 min at 4°C. The washed pellet was suspended in
20% Percoll® (cat. no. P1644; Sigma-Aldrich; Merck KGaA)
and centrifuged over a discontinuous Percoll gradient at 1,160 × g
for 30 min at 25°C. Epithelial cells were recovered from the
interphase between 20 and 40%. The cells were washed with PBS and
centrifuged as aforementioned. The cells were resuspended in
RPMI-1640 medium. The purity of the samples was analyzed via light
microscopy based on the normal morphology of epithelial cells. Cell
viability was determined using a Neubauer chamber and an optical
microscope (magnification, ×20). Then 10 µl of cell suspension were
added to an equal volume of 0.4% trypan blue. The number of cells
were counted and their viability (viable cells excluding trypan
blue; cat. no. T8154; Sigma-Aldrich; Merck KGaA) was determined to
be 90%. The samples contained up to 85% epithelial cells (24).
Western blot analysis of claudin-1 and
E-cadherin expression
The protein expression levels of claudin-1 and
E-cadherin were determined via western blotting. Samples were
homogenized in 100 µl lysis buffer [10 mmol Tris pH 7.4, containing
1% SDS (cat. no. 1610301; Bio-Rad Laboratories, Inc.), 2 mmol/l
sodium orthovanadate (cat. no. S6508; Sigma-Aldrich; Merck KGaA)
and 12.55 µg/ml phenylmethylsulphonyl fluoride (cat. no. P7626;
Sigma-Aldrich; Merck KGaA)]. The samples were sonicated four times
for 15 sec with 30 sec of rest at 4°C (22). Proteins were quantified by
Nanodrop Lite (Thermo Fisher Scientific, Inc.). Proteins were
equally loaded (20 µg per well), separated via 12% SDS-PAGE and
transferred onto nitrocellulose membranes (Bio-Rad Laboratories,
Inc.). After blocking with 3% powdered skimmed milk, 0.1% Tween-20
PBS for 2 h at RT, the membranes were incubated for 1 h at RT with
polyclonal antibodies against claudin-1 (cat. no. GTX134842;
polyclonal; 23 kDa; GeneTex, Inc.) and E-cadherin (cat. no.
GTX100443; polyclonal; 97 kDa; GeneTex, Inc.), followed by
incubation for 1 h at RT with HRP-conjugated secondary antibodies
(cat. no. A27036; Oligoclonal; Thermo Fisher Scientific, Inc.). The
immunoreactive bands were detected using an ECL detection kit
(Amersham; Cytiva) and semi-quantified via densitometry using
ImageJ 1.49 software (National Institutes of Health). The membranes
were stripped and reprobed with an anti-β-actin primary antibody
(cat. no. GTX109639; polyclonal; GeneTex, Inc.) to ensure equal
loading (25).
Lamina propria lymphocytes
After treatment with EDTA 30 min at 37°C, the large
intestines were washed twice with 25 ml RPMI-1640 medium and then
transferred to 50-ml tubes containing 25 ml RPMI-1640 medium with
60 U/ml type IV collagenase (cat. no. C5138; Sigma-Aldrich; Merck
KGaA), 1% fetal calf serum and 50 g/ml gentamicin (cat. no. G1397;
Sigma-Aldrich; Merck KGaA). The tubes were incubated horizontally
for 30 min at 37°C in a shaking water bath. The contents of each
tube were then transferred to Petri dishes. The intestinal mucosa
samples were filtered through an organza filter with a syringe
plunger; then, the single cell suspensions containing lamina
propria cells were filtered and centrifuged at 415 × g for 10 min
at 4°C. The cells were resuspended, collected and centrifuged in a
discontinuous 40/75% Percoll gradient at 1,160 × g for 30 min at
4°C. The cells were recovered from the interphase and then washed
with RPMI-1640 medium. Viability was determined using trypan blue
exclusion, and it was found to be >90% (24).
Flow cytometry assays
The isolation of lymphocytes from the lamina propria
of the large intestine was carried out as previously described with
some modifications (24,26). Cells from the lamina propria of
the large intestine were resuspended, and the concentration was
adjusted to 1×106 cells/ml in PBS for cytofluorometric
analysis (26).
Anti-CD4+/PerCP (cat. no. GTX79970; MEM-241; GeneTex
Inc.) was used to determine the predominant cytokines produced by
the CD4+ T cell population. The CD4+ cells
were fixed and permeabilized with 200 µl Cytofix/Cytoperm
Fixation/Permeabilization Solution kit (cat. no. BD 554714; Thermo
Fisher Scientific, Inc.) and then incubated for 20 min at RT in the
dark. Subsequently, the cells were centrifuged at 415 × g for 5 min
at 4°C, the excess solution was removed and the obtained pellet was
resuspended again. In total, 500 µl 1X Perm/Wash solution was
added, and the samples were centrifuged again at 415 × g for 5 min
at 4°C. Then, the supernatants were decanted and the cells were
resuspended again. Antibody cocktails (10 µl) were added and
incubated for 20 min in the dark at RT. Markers of the T helper
(Th)1 profile were detected with anti-IL-12/APC (cat. no. 554480;
C15-6; BD Pharmingen; BD Biosciences), anti-IFN-γ/FITC (cat. no.
554411; XMG1.2; BD Pharmingen; BD Biosciences), anti-TNF-α/PE (cat.
no. 554419; MX6-XT22; BD Pharmingen; BD Biosciences) and
anti-IL-1β/FITC (cat. no. IC4013F; NJTEN3; R&D Systems, Inc.).
Then, two more washes and centrifugation cycles were performed with
300 µl 1X Perm/Wash solution at 415 × g for 5 min at 4°C. Markers
of the Th2/T regulatory (Treg) profiles were detected with
anti-IL-4/PE (cat. no. 554435; 11B11; BD Pharmingen; BD
Biosciences), anti-IL-6/APC (cat. no. 561367; MP5-20F3; BD
Pharmingen; BD Biosciences), anti-IL-10/FITC (cat. no. 554466;
JE55-E3; BD Pharmingen; BD Biosciences), anti-CD25/FITC (cat. no.
553072; BD Pharmingen; BD Biosciences) and anti-FoxP3/PE (cat. no.
50-5773-U100; G3 Tonbo Biosciences) antibodies. Then, two more
washes and spin cycles were performed with 300 µl 1X Perm/Wash
solution at 415 × g for 5 min at 4°C. Finally, the samples were
stored at 4°C in the dark until analysis. The fluorescent signal
intensities were recorded and analyzed using a FACSArial flow
cytometer (Becton, Dickinson and Company). Events were collected
from the lymphocyte gate on the FSC/SSC dot plot. Overall, 20,000
gated events were acquired from each sample using BD FACSDIVA™
software 6.1 (Becton, Dickinson and Company). The data were
analyzed using Summit software v4.3 (Dako; Agilent Technologies,
Inc.) and are reported as percentages. The data from five mice per
group are reported as the mean ± SD.
Serum SP ELISA
Serum SP concentrations were measured using a
competitive ELISA kit according to the recommendations of the
manufacturer (cat. no. 583751; Cayman Chemical Company). A total of
50 µl per well of serum samples from the acute, chronic and control
groups were added, and the assay was performed in triplicate. The
plate was read with a microplate reader (BioTek Instruments, Inc.)
at 420 nm. The SP concentrations were calculated based on a
standard curve and are expressed in pg/ml.
Statistical analysis
The experimental assays were repeated for ≥3
independent assays (n=5 mice per group). The data are expressed as
the mean ± SD, and multiple comparisons between groups were
analyzed using one-way ANOVA, and the means of the respective
groups were compared using the post hoc Tukey's multiple comparison
test. Statistical analysis was performed using GraphPad Prism
Version 9 software (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
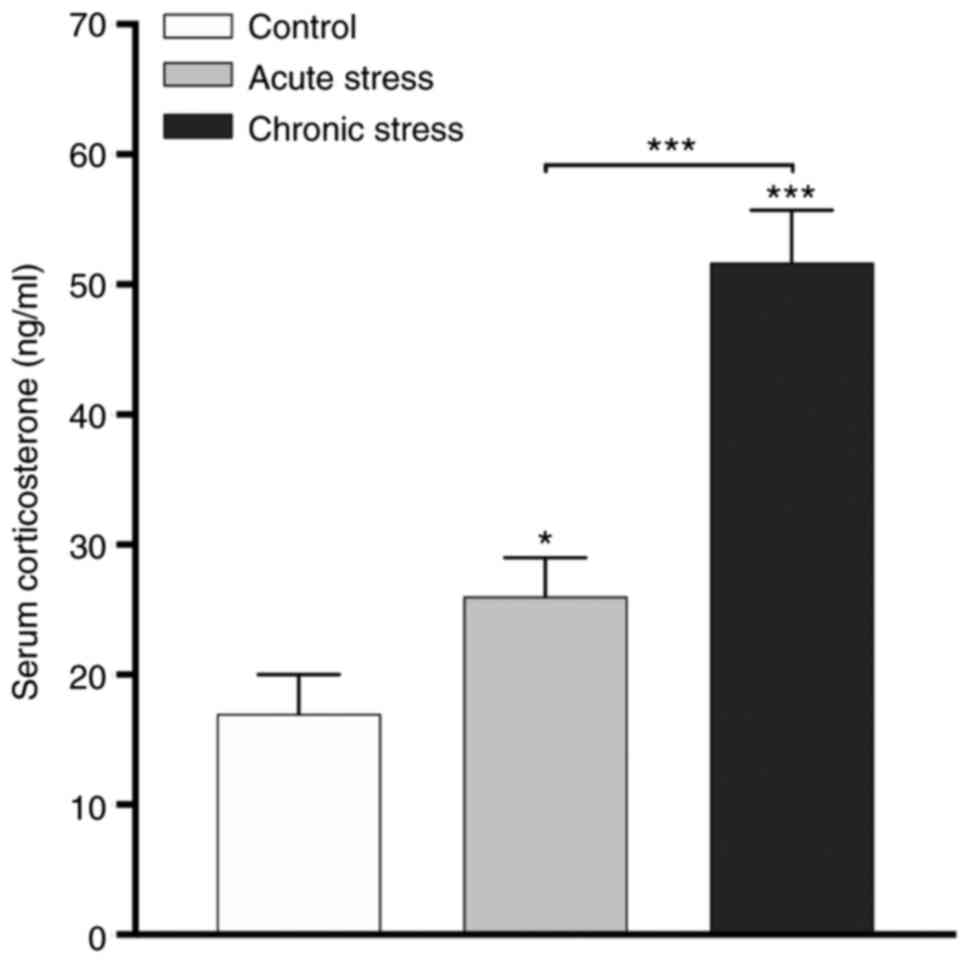
Serum corticosterone concentrations
are increased in the stressed animal models
The serum corticosterone concentration is an
indicator of stress, and different stressors are associated with
increases in serum corticosterone concentrations (27). The results demonstrated that the
serum corticosterone hormone concentrations were increased in the
acute (P<0.05) and chronic (P<0.001) stress groups compared
with the control group (Fig. 1).
Additionally, the concentration of corticosterone was increased in
the chronic stress group compared with the acute group
(P<0.001). These results indicated that serum corticosterone
concentrations are modified with respect to the type of stress,
acute or chronic.
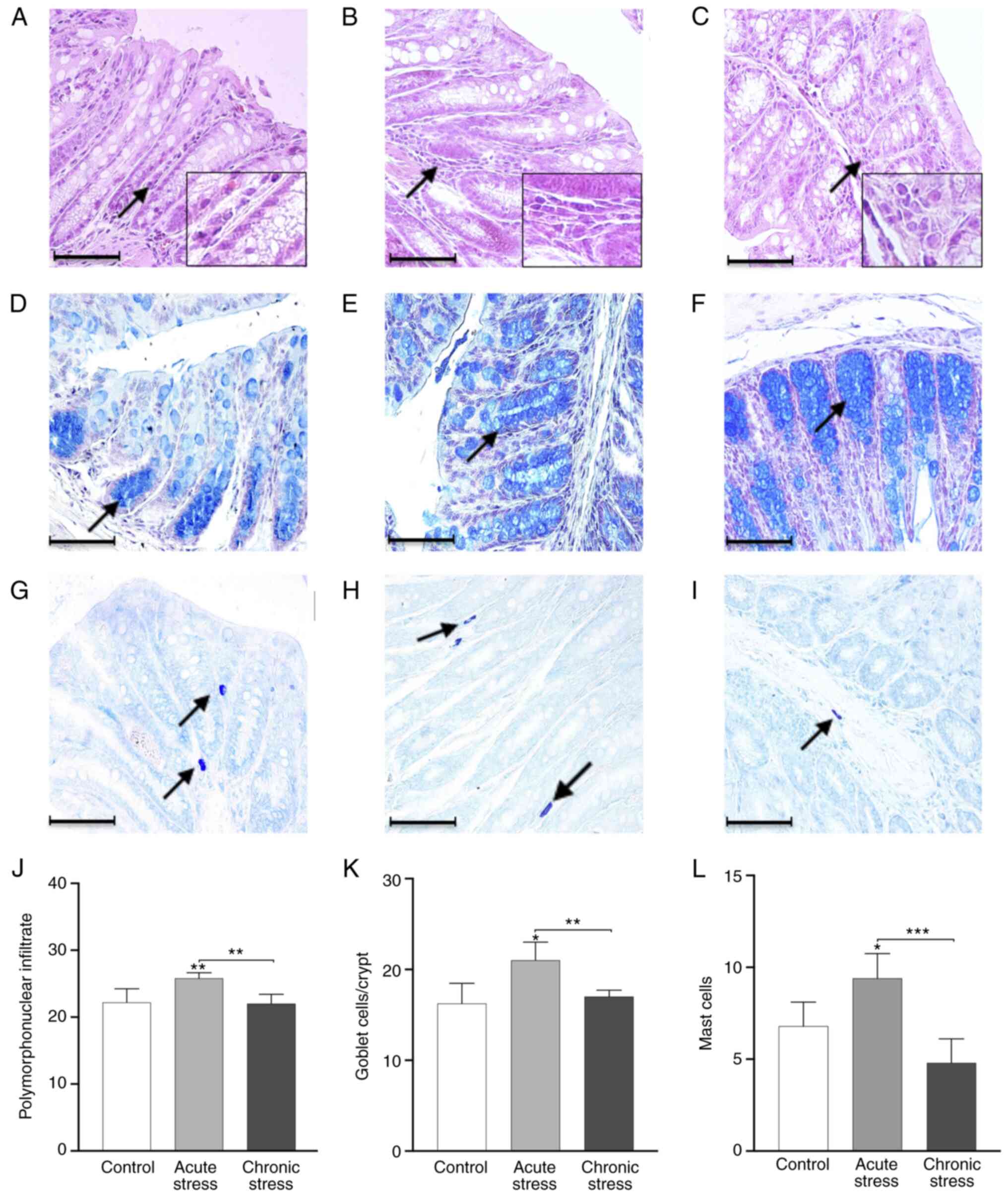
Acute stress induces changes in
inflammatory cell infiltration
The acute stress group (Fig. 2B) exhibited an accumulation and
increase in crypt inflammatory cell infiltration compared with the
control and chronic stress groups (Fig. 2A and C). The quantification of the
infiltrating PMN showed a significant increase in the acute stress
group compared with the control and chronic stress groups
(P<0.05; Fig. 2J). However, no
significant differences were observed between the chronic stress
and control groups. The infiltrating PMN was significantly higher
in the acute stress group compared with in the chronic stress group
(P<0.01; Fig. 2J). These
results indicated that acute stress induced differential
modifications of the inflammatory infiltrate in mice under
stress-induced conditions.
Acute stress increases the number of
goblet cells
Acute stress altered goblet cell numbers, and an
increase was observed in the acute stress group compared with the
control group (Fig. 2D and E).
The chronic stress group showed a similar goblet cell number as the
control group (Fig. 2F).
Furthermore, goblet cell quantification revealed a statistically
significant increase in the acute stress group compared with the
control group (P<0.05; Fig.
2K). Additionally, the goblet cell number was increased in the
acute stress group compared with the chronic group (P<0.01). The
results demonstrated that acute stress stimulates an increase in
the number of goblet cells.
Mast cell numbers increase during
acute stress
The presence of metachromatic mast cells in colon
was detected via toluidine blue staining. The number of mast cells
was markedly increased in the acute stress group (Fig. 2H) compared with the chronic stress
(Fig. 2I) and control groups
(Fig. 2G). The quantification of
mast cells showed a significant increase in the acute stress group
compared with the control and chronic stress groups (P<0.05 and
P<0.001; Fig. 2L). No
significant differences were found between the chronic stress and
control groups. These results showed a differential number of mast
cells in acute or chronic stress.
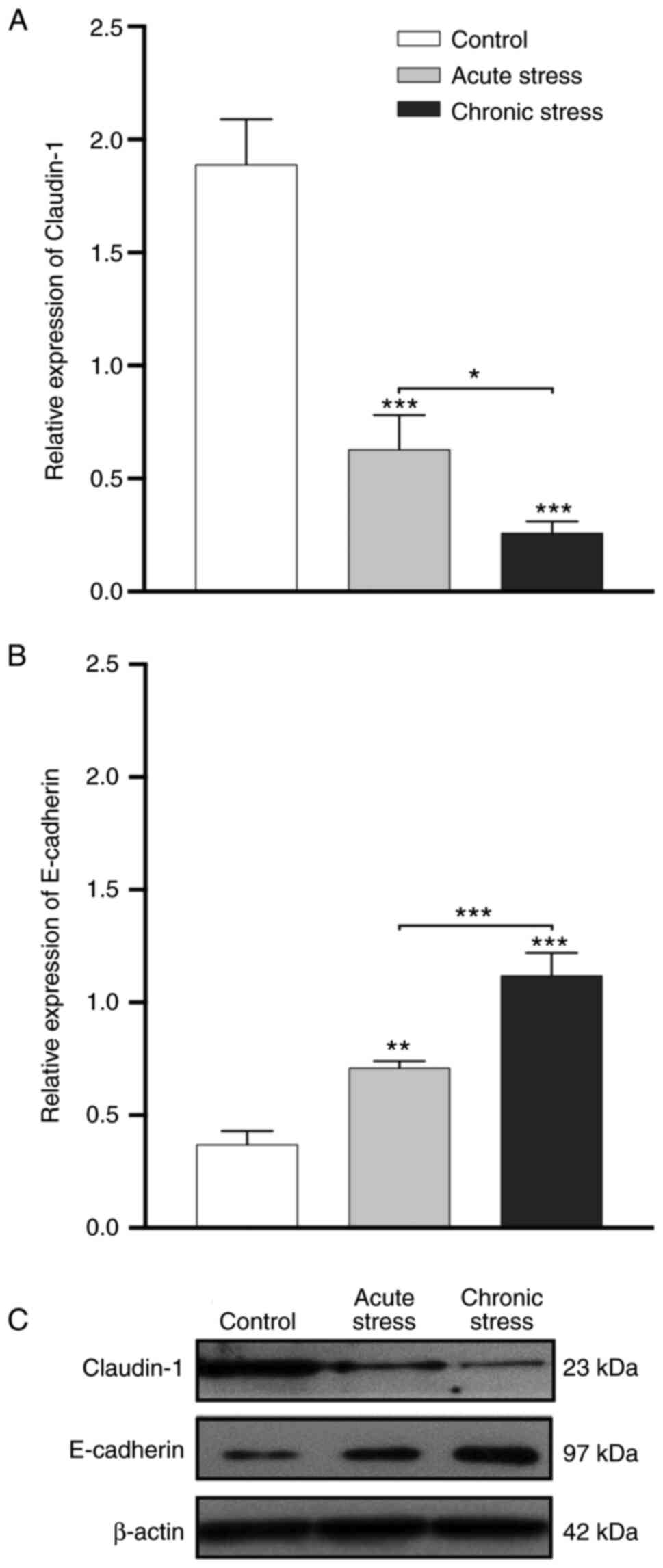
Claudin-1 and E-cadherin expression is
altered under stress
Western blot analysis demonstrated different protein
expression patterns under stress conditions. Densitometric analysis
revealed lower levels of claudin-1 expression in the acute and
chronic stress groups compared with those in the control group
(P<0.001). Claudin-1 expression was also significantly higher in
the acute stress group compared with in the chronic stress group
(P<0.05; Fig. 3A and C). By
contrast, densitometric analysis showed higher expression levels of
E-cadherin in the acute (P<0.01) and chronic (P<0.001) stress
groups compared with in the control group. E-cadherin expression
was also significantly higher in the chronic stress group than in
the acute stress group (P<0.001; Fig. 3B and C). These data indicated that
stress conditions generated changes in claudin-1 and E-cadherin
protein expression.
Stress induces changes in the
percentage of cytokine/IL CD4+ T cells and
CD4+ Treg cells from the lamina propria
Compared with the control, acute and chronic stress
diminished the percentages of CD4+ T cells and
CD4+ Treg cells in the lamina propria (Table I). Acute stress significantly
increased IL-6 and IL-4 cytokine CD4+ lymphocyte
percentages in the lamina propria compared with the control
(P<0.05; Table I). Compared
with the control group, the acute and chronic stress groups had
significantly decreased percentages of CD4+
lymphocytes/IFN-γ, TNF-α, IL-1β and IL-10 in the lamina propria
(P<0.05; Table I). A
significant reduction in the IL-6 and IL-4 cytokine CD4+
lymphocyte and IL-12 cytokine CD4+ lymphocyte
percentages in the lamina propria of the chronic stress group
compared with those in the lamina propria of the acute and control
groups was observed (P<0.05; Table
I). Comparison of the acute stress and control groups did not
reveal differences with respect to the IL-12 cytokine
CD4+ lymphocytes. Both groups of stressed animals showed
a significant reduction in FoxP3/CD4+ Treg cell
expression compared with the control group without stress
(P<0.05). Based on these results, stress induced a differential
percentage of cytokine/IL CD4+ T cells and
CD4+ Treg cells. The gating strategy and a
representative dot-plots from lamina propria lymphocytes from large
intestine are shown in the supplementary material (Fig. S1).
 | Table I.Cytokine/IL CD4+ T and
Treg cell responses in the lamina propria of the large intestine
under acute stress and chronic stress. |
Table I.
Cytokine/IL CD4+ T and
Treg cell responses in the lamina propria of the large intestine
under acute stress and chronic stress.
| Cytokine/IL | Control | Acute stress | Chronic stress |
|---|
| IFN-γ | 2.77±0.15 |
0.50±0.10a |
0.23±0.06a |
| TNF-α | 1.93±0.15 |
0.73±0.12a |
0.70±0.10a |
| IL-1β | 1.47±0.15 |
0.43±0.15a |
0.30±0.10a |
| IL-12 | 4.20±0.20 | 4.10±0.20 |
0.57±0.12a |
| IL-6 | 2.60±0.20 |
11.60±1.18a |
0.70±0.10a |
| IL-4 | 2.03±0.25 |
8.03±0.15a |
0.23±0.12a |
| IL-10 | 2.70±0.20 |
0.63±0.15a |
0.13±0.06a |
| FoxP3 | 5.17±0.21 |
1.07±0.31a |
0.93±0.15a |
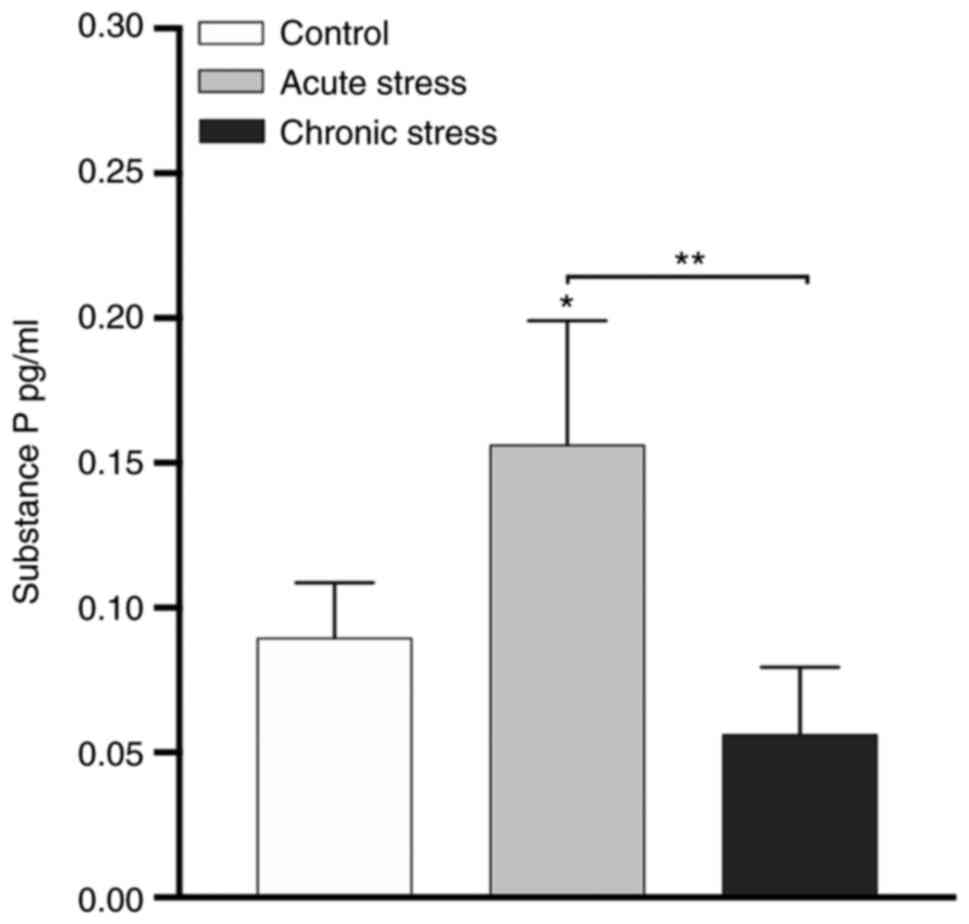
Influence of acute stress on serum SP
concentration
Stimulation of the sensory nerve causes an axonal
reflex, producing neuropeptides, especially SP, that activate
inflammatory cells. Next, the effect of stress on serum SP
concentration was examined. As shown in Fig. 4, acute stress significantly
increased the serum SP concentration compared with the chronic
stress and control groups (P<0.05 and P<0.01). No significant
difference was observed between the chronic stress and control
groups. These data indicate that the release of serum SP was
stimulated during acute stress.
Discussion
The present study identified an association between
plasma corticosterone and the stress type in the mouse model, and
similar results have been reported previously under different
stress conditions (27–30). The current study observed
significantly increased inflammatory infiltration during acute
stress, and this increase has been observed since pioneering
studies (13,14); such studies propose that the
increases in the corticosterone levels in the plasma and intestinal
lamina propria are associated with immune cell trafficking, which
is a crucial event for the surveillance and effector functions of
the immune system (13).
Furthermore, the influence of GCs on leukocyte redistribution is
likely the most important factor in supporting the immune response
(31,32). Conversely, the present study also
demonstrated an increase in the number of goblet cells in the
lamina propria; these cells are responsible for synthesizing mucus
(22), and an increase in their
numbers can enhance the thickness of the mucosal layer to prevent
contact with luminal bacteria. By contrast, another report has
shown that chronic stress reduced the number of goblet cells, and
neutrophil cellularity was unaltered (22). Moreover, chronic stress studies
(33) have shown that this type
of stress promotes an increase in the immunopathology, long term
goblet cell depletion and elevations in local and/or systemic
inflammatory mediators (33).
Unlike other studies, the number of mast cells was increased during
chronic stress, but the current study did not observe significant
changes during chronic stress. This result could be consistent with
those from an overcrowding stress model, in which the increase in
plasma corticosterone levels was associated with the recruitment of
mast cells over time (28). In
models of acute immobilization stress, it has been widely accepted
that mast cells are activated by corticotropin releasing factor
(34,35) and that they play a role in
mediating colonic goblet cell secretion (35).
Despite the decreased claudin-1 protein expression
in the TJ region during acute and chronic stress, the current study
observed that the mucosa in those experimental groups was similar
to that in the control group. A previous study (11) showed that stress mainly promotes
functional and morphological changes in the colonic epithelium, and
increases colonic permeability due to a decrease in claudin-1
expression in the TJ region (11). The decrease in claudin-1
expression in the present acute stress model was consistent with
these studies. The current study also identified an increase in
E-cadherin expression. E-cadherin is the core component of
epithelial adherent junctions and is essential for tissue
development, differentiation and maintenance of tissue barrier
formation, a critical function of epithelial tissues (36–38). A previous study investigated the
viability and cell-cell adhesion in sodium lauryl sulphate
(SLS)-treated human keratinocytes pretreated, co-treated and
post-treated with SP, and proposed that SP-treated cells had
increased E-cadherin expression. These author suggested that
E-cadherins on the membrane of keratinocytes are shifted to
desmosomes under physiological conditions and therein may mediate
an adhesion function in association with other desmosomal
cadherins. SP could provide protection against SLS-induced toxicity
by maintaining E-cadherin expression, as well as by exerting
anti-inflammatory effects. Therefore, with a low dose of SP may
protect against this condition (39).
The present results revealed an increase in the
CD4+/IL-4+ lymphocyte percentage under acute
stress. Although it is well established that stress in the colon
increases the number of CD4 lymphocytes (40), to the best of our knowledge, there
is no evidence of the effects of repeated restriction stress on the
cytokine lymphocytes profiles in the lamina propria of mouse colon.
The current data demonstrated that the predominant phenotype in
lymphocytes during acute restriction stress consisted of
anti-inflammatory Th2 cells, and increase in
CD4+/IL-6+ and
CD4+/IL4+ lymphocytes in the lamina propria
was observed. Additionally, decreases in the numbers of γδ T
lymphocytes and CD4+ and CD8+ T lymphocytes
in the epithelium of the small intestine of mice have been reported
due to the combined action of higher concentrations of
catecholamines and GCs (41).
During stress, GCs, such as corticosterone, bind to GC response
elements and suppress the expression of proinflammatory genes or
induce the expression of suppressive factors, such as NFκB
inhibitor α, dual specificity phosphatase 1 (MKP-1),
glucocorticoid-induced leucine zipper and ZFP36 ring finger protein
(TTP). For example, TTP binds to AU-rich elements in the
3′untranslatef region of the mRNA of several inflammatory cytokines
to destabilize these mRNAs. GC also reduces the stability of mRNAs
encoding IL-1β, IL-2, IL-6 and TNF-α (42). However, data on the effects of
different types of stress on T lymphocyte/cytokine profiles in the
lamina propria of the mouse colon are lacking.
The delicate balance between pro- and
anti-inflammatory mechanisms, essential for intestinal immune
homeostasis, is regulated by Treg cells that express the
transcription factor FoxP3 and play role in limiting inflammatory
responses in the intestine (43).
Moreover, evidence has shown that butyrate, produced by commensal
microorganisms during starch fermentation, facilitates extrathymic
generation of Treg cells (44).
The present study demonstrated a decrease in the percentage of
FoxP3+/CD25+/CD4+ Treg lymphocytes
in the lamina propria of the large intestine under acute and
chronic stress vs. the control group.
Additionally, in the current study, the serum SP
concentration after repeated exposure to restriction stress
increased during acute stress, and this observation differed from
the results after exposure to chronic stress. This phenomenon could
be consistent with a study showing that SP reduces the duration of
acute stress, and therefore, SP plays an essential role in the
transition between acute and chronic stress (45). Conversely, SP exerts antiapoptotic
effects on colon epithelial cells, thereby promoting accelerated
intestinal healing (46,47). Serum SP levels have been
associated with injury severity and mortality in patients with
severe traumatic brain injury (TBI), indicating that serum SP
levels could be used as a biomarker to predict mortality in
patients with severe TBI and may be of great pathophysiological
significance in these patients (48). During inflammation and injury in
the colon, sensory nerves release SP locally within tissues to
promote ‘neurogenic inflammation’, and it is well established that
SP and the NK1R may initiate this inflammation. The cell-surface
enzyme neutral endopeptidase (NEP) degrades SP in the extracellular
fluid and may terminate its proinflammatory effects. Evidence has
shown that NEP in the colon may contribute to uncontrolled
intestinal inflammation (49).
Thus, it was suggested that the increase in the serum SP
concentration during acute stress could be a mechanism via which
HPA activation is regulated.
The increase in the serum SP concentration during
acute stress may be associated with two factors that are not
necessarily contradictory. First, a previous study has proposed
that SP does not inhibit the initial activation of the HPA axis in
response to restraint stress, but does act via NK1R at a central
level (50) to reduce the
duration of the stress response; conversely, SP can promote
epithelial cell proliferation (antiapoptotic effect) at the site of
injury in the large intestine, which promotes intestinal healing
(47).
An important limitation of the present study was to
analyze substance P in serum and not in colonic tissue, this would
have allowed the present study to relate in situ possible
effects of substance P in the cells studied. In the future
experimental and therapeutic studies will be performed that will
allow us to relate stress and the immune system, with molecules
such as neurotransmitters or neuropeptides.
In conclusion, the parameters evaluated in the
current study suggest that acute stress may facilitate stress
management therapy to benefit the resolution of intestinal
diseases.
Supplementary Material
Supporting Data
Acknowledgements
The authors thank Dr Rosa Adriana Jarillo-Luna for
her laboratory facilities; Postgraduate Studies and Research
Section, Superior School of Medicine, National Polytechnic
Institute, Mexico City, Mexico.
Funding
This work was supported by grants from Research and Postgraduate
Secretariat of the National Polytechnic Institute (grant nos.
20180058 and 20181107).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JPY and EAR conceptualized the study. IMAM, AARA,
LMCJ, BMA, RFV, JMGM, MYO, JPY and EAR designed the study. IMAM,
AARA, LMCJ, JPY and EAR utilised the software. IMAM, AARA, LMCJ,
JPY and EAR performed formal analysis. JPY, AARA and EAR provided
the resources. JPY and EAR contributed to data curation. IMAM,
AARA, JPY and EAR wrote initial draft of the manuscript. IMAM,
AARA, LMCJ, BMA, RFV, JMGM, MYO, JPY and EAR wrote, reviewed and
edited the manuscript. JPY and EAR supervised the study. JPY and
EAR performed project administration and acquired the funding. IMAM
and EAR confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
the Mexican Federal Regulations for animal experimentation and care
(Regulation-062-ZOO-1999; Ministry of Agriculture, Mexico City,
México) and was approved by the Superior School of Medicine,
National Polytechnic Institute.
Patient consent for publication
Not applicable.
Authors' information
Drs Judith Pacheco-Yépez, Edgar Abarca-Rojano and
Aldo A. Reséndiz-Albor are fellows of Commission for the Operation
and Promotion of Academic Activities of the National Polytechnic
Institute (COFAA) and Stimulus to the Performance of
Researchers-National Polytechnic Institute (EDI–IPN). Professor
Ivonne Maciel Arciniega-Martínez is a fellow of EDI–IPN.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McEwen BS: Physiology and neurobiology of
stress and adaptation: Central role of the brain. Physiol Rev.
87:873–904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chrousos GP: The
hypothalamic-pituitary-adrenal axis and immune-mediated
inflammation. N Engl J Med. 332:1351–1362. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grundy D, Al-Chaer ED, Aziz Q, Collins SM,
Ke M, Taché Y and Wood JD: Fundamentals of neurogastroenterology:
Basic science. Gastroenterology. 130:1391–1411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barreau F, Cartier C, Leveque M, Ferrier
L, Moriez R, Laroute V, Rosztoczy A, Fioramonti J and Bueno L:
Pathways involved in gut mucosal barrier dysfunction induced in
adult rats by maternal deprivation: Corticotrophin-releasing factor
and nerve growth factor interplay. J Physiol. 580:347–356. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barclay GR and Turnberg LA: Effect of
psychological stress on salt and water transport in the human
jejunum. Gastroenterology. 93:91–97. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Camilleri M, Madsen K, Spiller R,
Greenwood-Van Meerveld B and Verne GN: Intestinal barrier function
in health and gastrointestinal disease. Neurogastroenterol Motil.
24:503–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santos J, Saunders PR, Hanssen NP, Yang
PC, Yates D, Groot JA and Perdue MH: Corticotropin-releasing
hormone mimics stress-induced colonic epithelial pathophysiology in
the rat. Am J Physiol. 277:G391–G399. 1999.PubMed/NCBI
|
|
8
|
Campos AC, Ferreira FR, Guimaraes FS and
Lemos JI: Facilitation of endocannabinoid effects in the ventral
hippocampus modulates anxiety-like behaviors depending on previous
stress experience. Neuroscience. 167:238–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Padovan CM and Guimaraes FS:
Restraint-induced hypoactivity in an elevated plus-maze. Braz J Med
Biol Res. 33:79–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Willner P: Validity, reliability and
utility of the chronic mild stress model of depression: A 10-year
review and evaluation. Psychopharmacology (Berl). 134:319–329.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng G, Wu SP, Hu Y, Smith DE, Wiley JW
and Hong S: Corticosterone mediates stress-related increased
intestinal permeability in a region-specific manner.
Neurogastroenterol Motil. 25:e127–e139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng G, Victor Fon G, Meixner W,
Creekmore A, Zong Y, K Dame M, Colacino J, Dedhia PH, Hong S and
Wiley JW: Chronic stress and intestinal barrier dysfunction:
Glucocorticoid receptor and transcription repressor HES1 regulate
tight junction protein Claudin-1 promoter. Sci Rep. 7:45022017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dhabhar FS and McEwen BS: Stress-induced
enhancement of antigen-specific cell-mediated immunity. J Immunol.
156:2608–2615. 1996.PubMed/NCBI
|
|
14
|
Dhabhar FS, Miller AH, McEwen BS and
Spencer RL: Effects of stress on immune cell distribution. Dynamics
and hormonal mechanisms. J Immunol. 154:5511–5527. 1995.PubMed/NCBI
|
|
15
|
Keita AV, Soderholm JD and Ericson AC:
Stress-induced barrier disruption of rat follicle-associated
epithelium involves Corticotropin-releasing hormone, acetylcholine,
substance P, and mast cells. Neurogastroenterol Motil. 22:770–778.
e221–e222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Santos J, Yates D, Guilarte M, Vicario M,
Alonso C and Perdue MH: Stress neuropeptides evoke epithelial
responses via mast cell activation in the rat colon.
Psychoneuroendocrinology. 33:1248–1256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vergnolle N: The enteric nervous system in
inflammation and pain: The role of proteinase-activated receptors.
Can J Gastroenterol. 17:589–592. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Connor TM, O'Connell J, O'Brien DI,
Goode T, Bredin CP and Shanahan F: The role of substance P in
inflammatory disease. J Cell Physiol. 201:167–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8:e10004122010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martinez-Carrillo BE, Godinez-Victoria M,
Jarillo-Luna A, Oros-Pantoja R, Abarca-Rojano E, Rivera-Aguilar V,
Yépez JP, Sánchez-Torres LE and Campos-Rodríguez R: Repeated
restraint stress reduces the number of IgA-producing cells in
Peyer's patches. Neuroimmunomodulation. 18:131–141. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cruz-Baquero A, Cardenas Jaramillo LM,
Gutierrez-Meza M, Jarillo-Luna RA, Campos-Rodríguez R,
Rivera-Aguilar V, Miliar-García A and Pacheco-Yepez J: Different
behavior of myeloperoxidase in two rodent amoebic liver abscess
models. PLoS One. 12:e01824802017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Machorro-Rojas N, Sainz-Espuñes T,
Godínez-Victoria M, Castañeda-Sánchez JI, Campos-Rodríguez R,
Pacheco-Yepez J and Drago-Serrano ME: Impact of chronic
immobilization stress on parameters of colonic homeostasis in
BALB/c mice. Mol Med Rep. 20:2083–2090. 2019.PubMed/NCBI
|
|
23
|
Santana T, Nagata G, Saturno JL and
Trierveiler M: Histopathological features of photodamage and mast
cell infiltrate in actinic Cheilitis with different grades of
epithelial dysplasia. J Cutan Pathol. 47:592–600. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Resendiz-Albor AA, Reina-Garfias H,
Rojas-Hernández S, Jarillo-Luna A, Rivera-Aguilar V, Miliar-García
A and Campos-Rodríguez R: Regionalization of pIgR expression in the
mucosa of mouse small intestine. Immunol Lett. 128:59–67. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lafuente N, Matesanz N, Azcutia V, Romacho
T, Nevado J, Rodríguez-Mañas L, Moncada S, Peiró C and
Sánchez-Ferrer CF: The deleterious effect of high concentrations of
D-glucose requires pro-inflammatory preconditioning. J Hypertens.
26:478–485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Resendiz-Albor AA, Esquivel R,
Lopez-Revilla R, Verdin L and Moreno-Fierros L: Striking phenotypic
and functional differences in lamina Propria lymphocytes from the
large and small intestine of mice. Life Sci. 76:2783–2803. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gong S, Miao YL, Jiao GZ, Sun MJ, Li H,
Lin J, Luo MJ and Tan JH: Dynamics and correlation of serum
cortisol and corticosterone under different physiological or
stressful conditions in mice. PLoS One. 10:e01175032015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vicario M, Guilarte M, Alonso C, Yang P,
Martínez C, Ramos L, Lobo B, González A, Guilà M, Pigrau M, et al:
Chronological assessment of mast cell-mediated gut dysfunction and
mucosal inflammation in a rat model of chronic psychosocial stress.
Brain Behav Immun. 24:1166–1175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Flores CM, Hernandez MC, Hargreaves KM and
Bayer BM: Restraint stress-induced elevations in plasma
corticosterone and beta-endorphin are not accompanied by
alterations in immune function. J Neuroimmunol. 28:219–225. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kvetnansky R, Fukuhara K, Pacak K, Cizza
G, Goldstein DS and Kopin IJ: Endogenous glucocorticoids restrain
catecholamine synthesis and release at rest and during
immobilization stress in rats. Endocrinology. 133:1411–1419. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dhabhar FS and McEwen BS: Enhancing versus
suppressive effects of stress hormones on skin immune function.
Proc Natl Acad Sci USA. 96:1059–1064. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Straub RH, Dhabhar FS, Bijlsma JW and
Cutolo M: How psychological stress via hormones and nerve fibers
may exacerbate rheumatoid arthritis. Arthritis Rheum. 52:16–26.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dhabhar FS: Enhancing versus suppressive
effects of stress on immune function: Implications for
Immunoprotection versus immunopathology. Allergy Asthma Clin
Immunol. 4:2–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Castagliuolo I, Lamont JT, Qiu B, Fleming
SM, Bhaskar KR, Nikulasson ST, Kornetsky C and Pothoulakis C: Acute
stress causes mucin release from rat colon: Role of corticotropin
releasing factor and mast cells. Am J Physiol. 271:G884–G892.
1996.PubMed/NCBI
|
|
35
|
Castagliuolo I, Wershil BK, Karalis K,
Pasha A, Nikulasson ST and Pothoulakis C: Colonic mucin release in
response to immobilization stress is mast cell dependent. Am J
Physiol. 274:G1094–G1100. 1998.PubMed/NCBI
|
|
36
|
Daulagala AC, Bridges MC and Kourtidis A:
E-cadherin beyond structure: A signaling hub in colon homeostasis
and disease. Int J Mol Sci. 20:27562019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hwang DY, Kim S and Hong HS: Substance-P
ameliorates dextran sodium sulfate-induced intestinal damage by
preserving tissue barrier function. Tissue Eng Regen Med. 15:63–73.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sayer B, Lu J, Green C, Soderholm JD,
Akhtar M and McKay DM: Dextran sodium Sulphate-induced colitis
perturbs muscarinic cholinergic control of colonic epithelial ion
transport. Br J Pharmacol. 135:1794–1800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi HW, Ahn HJ, Shin MK, Son YS and Kim
KS: Pretreatment with substance P alleviates irritation due to
sodium lauryl sulphate exposure by maintaining E-cadherin
expression on human keratinocytes. Clin Exp Dermatol. 43:291–295.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qiu BS, Vallance BA, Blennerhassett PA and
Collins SM: The role of CD4+ lymphocytes in the susceptibility of
mice to stress-induced reactivation of experimental colitis. Nat
Med. 5:1178–1182. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jarillo-Luna A, Rivera-Aguilar V,
Martinez-Carrillo BE, Barbosa-Cabrera E, Garfias HR and
Campos-Rodriguez R: Effect of restraint stress on the population of
intestinal intraepithelial lymphocytes in mice. Brain Behav Immun.
22:265–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shimba A and Ikuta K: Glucocorticoids
regulate circadian rhythm of innate and adaptive immunity. Front
Immunol. 11:21432020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Josefowicz SZ, Niec RE, Kim HY, Treuting
P, Chinen T, Zheng Y, Umetsu DT and Rudensky AY: Extrathymically
generated regulatory T cells control mucosal TH2 inflammation.
Nature. 482:395–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Arpaia N, Campbell C, Fan X, Dikiy S, van
der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ and
Rudensky AY: Metabolites produced by commensal bacteria promote
peripheral regulatory T-cell generation. Nature. 504:451–455. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mello DM, Marcinichen DR, Madruga D,
Branco R, Paschoalini MA and De Lima TC: Involvement of NK1
receptors in metabolic stress markers after the central
administration of substance P. Behav Brain Res. 181:232–238. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kang MH, Kim DY, Yi JY and Son Y:
Substance P accelerates intestinal tissue regeneration after
gamma-irradiation-induced damage. Wound Repair Regen. 17:216–223.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Koon HW, Zhao D, Zhan Y, Moyer MP and
Pothoulakis C: Substance P mediates antiapoptotic responses in
human colonocytes by Akt activation. Proc Natl Acad Sci USA.
104:2013–2018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lorente L: New prognostic biomarkers in
patients with traumatic brain injury. Arch Trauma Res.
4:e301652015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sturiale S, Barbara G, Qiu B, Figini M,
Geppetti P, Gerard N, Gerard C, Grady EF, Bunnett NW and Collins
SM: Neutral endopeptidase (EC 3.4.24.11) terminates colitis by
degrading substance P. Proc Natl Acad Sci USA. 96:11653–11658.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jessop DS, Renshaw D, Larsen PJ, Chowdrey
HS and Harbuz MS: Substance P is involved in terminating the
hypothalamo-pituitary-adrenal axis response to acute stress through
centrally located neurokinin-1 receptors. Stress. 3:209–220. 2000.
View Article : Google Scholar : PubMed/NCBI
|