Introduction
Sepsis is a life-threatening systemic inflammatory
response syndrome, which may be accompanied by multiple organ
failure and septic shock in severe cases (1,2).
Sepsis is a dominant cause of mortality worldwide, particularly in
intensive care units, with a higher mortality rate compared with
that of breast and lung cancer (3).
In recent years, research had demonstrated that dysregulation of
gene expression and dysfunction of the immune system are closely
associated with the pathogenesis and pathophysiology of sepsis, in
addition to pathogens and endotoxins (4–6).
Furthermore, sepsis induces the excessive release of
pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6 and
tumor necrosis factor (TNF)-α, as well as immunosuppression and
tissue injury, thereby promoting susceptibility to secondary
infections, these inflammatory cytokines contribute to aggressive
immunopathology, including sepsis (7,8).
Long non-coding RNA (lncRNA) is a large class of
non-protein-coding transcripts that are >200 nucleotides in
length (9). It has been
demonstrated that lncRNA plays various roles in numerous biological
processes, such as cell proliferation, apoptosis, inflammatory and
immune responses (10). Aberrant
expression of lncRNA has been implicated in several inflammatory
and immune diseases, including sepsis (11–13).
For example, lncRNA H19 functions as a competitive endogenous
(ce)RNA of microRNA (miRNA/miR)-874 to regulate the progression of
sepsis, both in septic patients and in animal models of sepsis
(14). The lncRNA HOX transcript
antisense RNA accelerates the secretion of TNF-α in mice with
lipopolysaccharide (LPS)-induced sepsis (15). The lncRNA GDP-mannose
4,6-dehydratase antisense 1 (GMDS-AS1) is a novel functional lncRNA
that has only been studied in lung adenocarcinoma, in which it was
found to inhibit cell proliferation and induce apoptosis by
targeting the miR-96-5p/CYLD axis (16). However, few studies have
investigated the role of GMDS-AS1 and its mechanism of action in
the progression of sepsis.
In the present study, the expression of GMDS-AS1 was
examined in LPS-induced THP-1 cells. The effects of GMDS-AS1 on the
production of inflammatory factors and cell apoptosis were
investigated. The study explored whether GMDS-AS1 functioned as a
ceRNA to regulate the expression of caspase-2 (CASP2) by sponging
miR-96-5p in LPS-induced THP-1 cells. The findings may contribute
to the diagnosis and treatment of sepsis in the clinical
setting.
Materials and methods
Cell culture and treatment
The human monocytic leukemia THP-1 cell line was
obtained from the American Type Culture Collection and cultured in
RPMI 1640 medium (MilliporeSigma) supplemented with 10% FBS
(HyClone; Cytiva) at 37°C in a humidified atmosphere containing 5%
CO2. To mimic sepsis in vitro, THP-1 cells were
stimulated with l µg/mlLPS (Sigma-Aldrich; Merck KGaA) for 24 h at
37°C.
Cell transfection
For the overexpression of GMDS-AS1 and miR-96-5p,
the pcDNA3.1 vector containing full-length GMDS-AS1
(pcDNA-GMDS-AS1) and empty vector (pcDNA-NC), miR-96-5p mimics
(5′-UUUGGCACAGCACAUUUUUGCUCAAAAAUGUGCUAGUGCCAAAUU-3′) and miR-NC
(cat. no. 4464061) were all designed and synthesized by Thermo
Fisher Scientific, Inc. In addition, the cells that were not
transfected with the plasmid were used as the control group.
Following stimulation with l µg/ml LPS for 24 h at 37°C, THP-1
cells were transfected with pcDNA-GMDS-AS1 or/and miR-96-5p mimic
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at a concentration of 50 ng/ml. Following
transfection for 48 h at 37°C, the transfection efficiency was
detected by reverse transcription-quantitative PCR (RT-qPCR).
RT-qPCR analysis
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The quality of the RNA was assessed using a NanoDrop 2000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.) at 260 and 280 nm, according to the manufacturer's protocol.
RT was performed using PrimeScript RT Master Mix (Takara Bio, Inc.)
at 50°C for 45 min. qPCR was then performed with SYBR Premix EX
Taq™ II (Takara Bio, Inc.) on an ABI PRISM 7300 detection system
(Thermo Fisher Scientific, Inc.) with the following thermocycling
conditions: Initial denaturation at 85°C for 30 sec, followed by 22
cycles at 55°C for 30 sec and 72°C for 30 sec. The results are
presented by using the 2−ΔΔCq method (17). U6 was used as an internal control of
miR-96-5p and GAPDH served as the internal reference of IL-6,
TNF-α, IL-1β, GMDS-AS1 and CASP2. The following primer sequences
were used: IL-6, forward 5′-GGAGACTTGCCTGGTGAAA-3′ and reverse,
5′-CTGGCTTGTTCCTCACTACTC-3′ and TNF-α, forward,
5′-AGCCGATGGGTTGTACCT-3′ and reverse, 5′-TGAGTTGGTCCCCCTTCT-3′; and
IL-1β, forward 5′-TGTGGCAGCTACCTATGTCT-3′ and reverse,
5′-GGGAACATCACACACTAGCA′; and GMDS-AS1, forward
5′-AATGCTTTGAGGCCAAGCTA-3′ and reverse, 5′-TGGGTTCATAAGGGTTGCAT-3′;
and CASP2, forward 5′-GCAAACCTCAGGGAAACATTC′ and reverse,
5′-TGTCGGCATACTGTTTCAGCA-3′; and GAPDH, forward
5′-CTGGGCTACACTGAGCACC-3′ and reverse, 5′-AAGTGGTCGTTGAGGGCAATG-3′;
and U6, forward 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′.
Apoptosis analysis
Apoptosis was evaluated by flow cytometry using the
FITC Annexin V/propidium iodide (PI) Apoptosis Detection Kit I
(Guangzhou RiboBio Co., Ltd.). After transfection, the cells were
harvested and re-suspended in binding buffer, then incubated with
Annexin V-FITC and PI (10 mg/ml) for 20 min at 37°C in the dark.
The samples were then placed in an ice bath and data were obtained
by flow cytometer (FACSCalibur; BD Biosciences). FlowJo software
(BD Biosciences; Version 7.6) was used to analyze the
double-stained cells Q1, Q2 and Q3 regions represented early
apoptosis rate, late apoptosis rate and dead cell rate,
respectively; cell apoptosis (%)=Q1+ Q2 + Q3.
Luciferase reporter assay
The wild-type and mutant CASP2 3′-untranslated
region (UTR) fragments, including putative miR-96-5p-binding sites,
were synthesized and cloned into the pGL3 vector (Promega
Corporation). THP-1 cells were co-transfected with the wild-type
and mutant constructs and miRNA (miR-NC; cat. no. 4464061 or
miR-96-5p mimic
(5′-UUUGGCACAGCACAUUUUUGCUCAAAAAUGUGCUAGUGCCAAAUU-3; Thermo Fisher
Scientific, Inc.) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). The relative luciferase activities
were measured 48 h after transfection using a Dual-Luciferase
Reporter Assay System (Promega Corporation) Renilla
luciferase activity served as the internal reference.
Western blot analysis
Cells were lysed using RIPA lysis buffer (Beyotime
Institute of Biotechnology), and the concentration of the proteins
extracted from the cells was detected using a BCA Protein Assay kit
(Beijing Dingguo Changsheng Biotechnology Co., Ltd.). A total of 20
µg protein samples were separated on 10% gels using SDS-PAGE and
transferred onto PVDF membranes. After being blocked using 5%
non-fat milk at room temperature for 1 h, the membranes were
incubated with anti-caspase-2 primary antibody (1:500; cat. no.
ab32021; Abcam) and anti-GAPDH primary antibody (1:2,500; cat. no.
ab9485; Abcam) at 4°C overnight. The membranes were then incubated
with a goat anti-rabbit secondary antibody (1:5,000; cat. no.
ab6721; Abcam) for 1 h at room temperature. Proteins bands were
visualized using an ECL reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). and analyzed with Image J software (version 3.0;
National Institutes of Health).
Bioinformatics analysis
The Targetscan DataBase (version 7.2;
targetscan.org/vert_72/) was used to screen the target genes of
miR-96-5p. PCT (probability of preferentially conserved
targeting) was used to evaluate the conservative targeting
probability of all highly conservative miRNA families.
Statistical analysis
SPSS 23.0 software (IBM Corp.) and GraphPad Prism 6
(GraphPad Software, Inc.) were used for statistical analysis. The
data are presented as the mean ± SD. Two-tailed Student's t-tests
(unpaired) were used to compare the differences between two groups.
Comparisons among multiple groups were performed with one-way ANOVA
followed by Tukey's or Dunnett's post hoc test. P<0.05 was
considered to indicate a statistically significant difference. All
experiments were performed in triplicate.
Results
miR-96-5p is downregulated in an in
vitro model of LPS-induced sepsis
An in vitro sepsis model was first
established using LPS treatment of THP-1 cells, and the expression
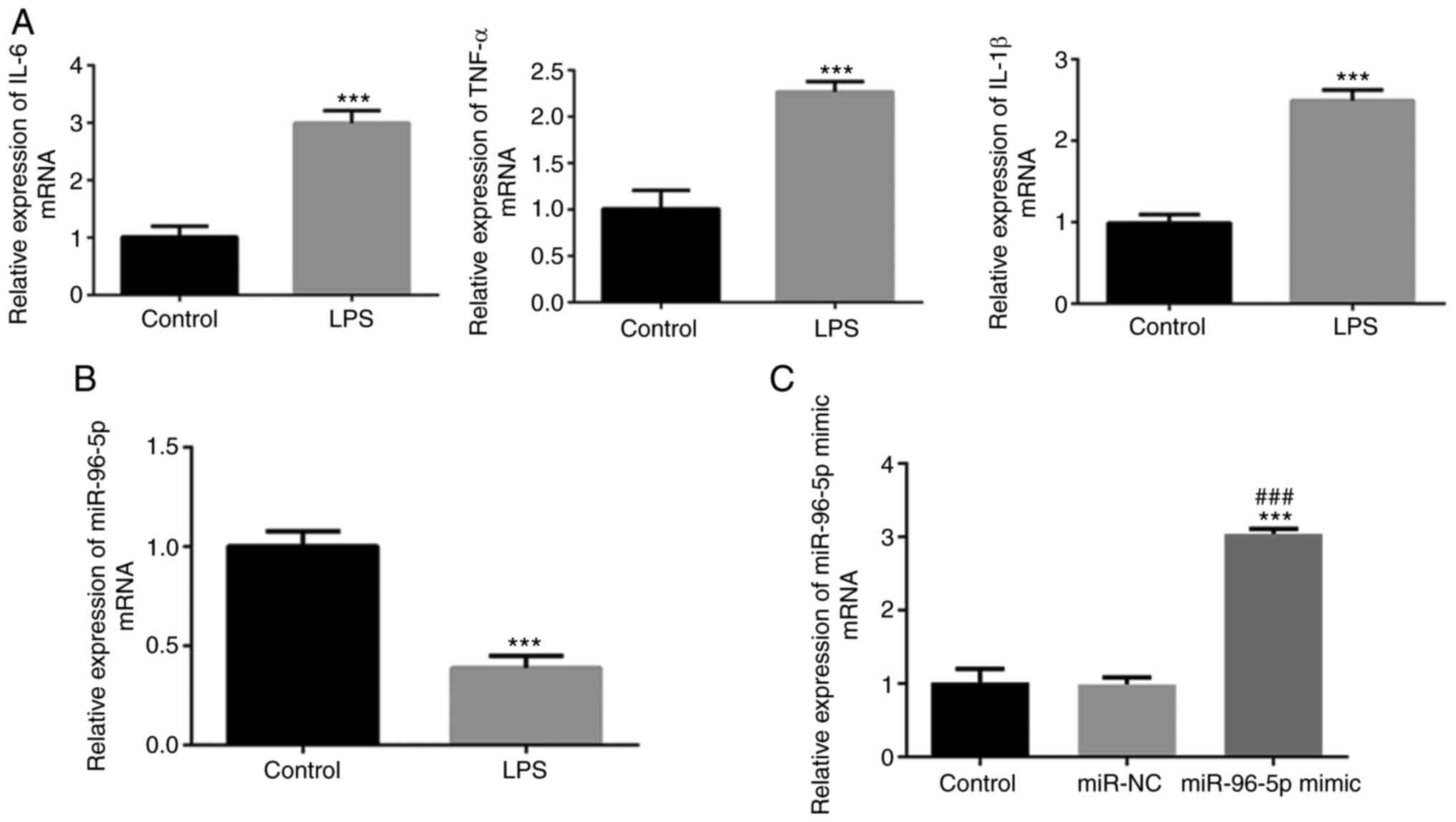
levels of IL-1β, IL-6 and TNF-α were detected. As shown in Fig. 1A, a significant increase in the
secretion of IL-1β, IL-6 and TNF-α was observed following LPS
stimulation. Additionally, miR-96-5p expression in THP-1 cells
significantly decreased following induction with LPS (Fig. 1B). These results indicated that the
septic cell model was successfully constructed and that miR-96-5p
may be associated with the pathophysiology of sepsis.
miR-96-5p overexpression decreases
LPS-induced inflammatory cytokine production and apoptosis
To explore the biological role of miR-96-5p in
sepsis, a miR-96-5p mimic was transfected into THP-1 cells to
upregulate miR-96-5p expression, and transfection efficiency was
verified by RT-qPCR. The expression levels of miR-96-5p were
significantly increased in the miR-96-5P mimic group compared with
the control and miR-NC groups, demonstrating efficient transfection
(Fig. 1C).
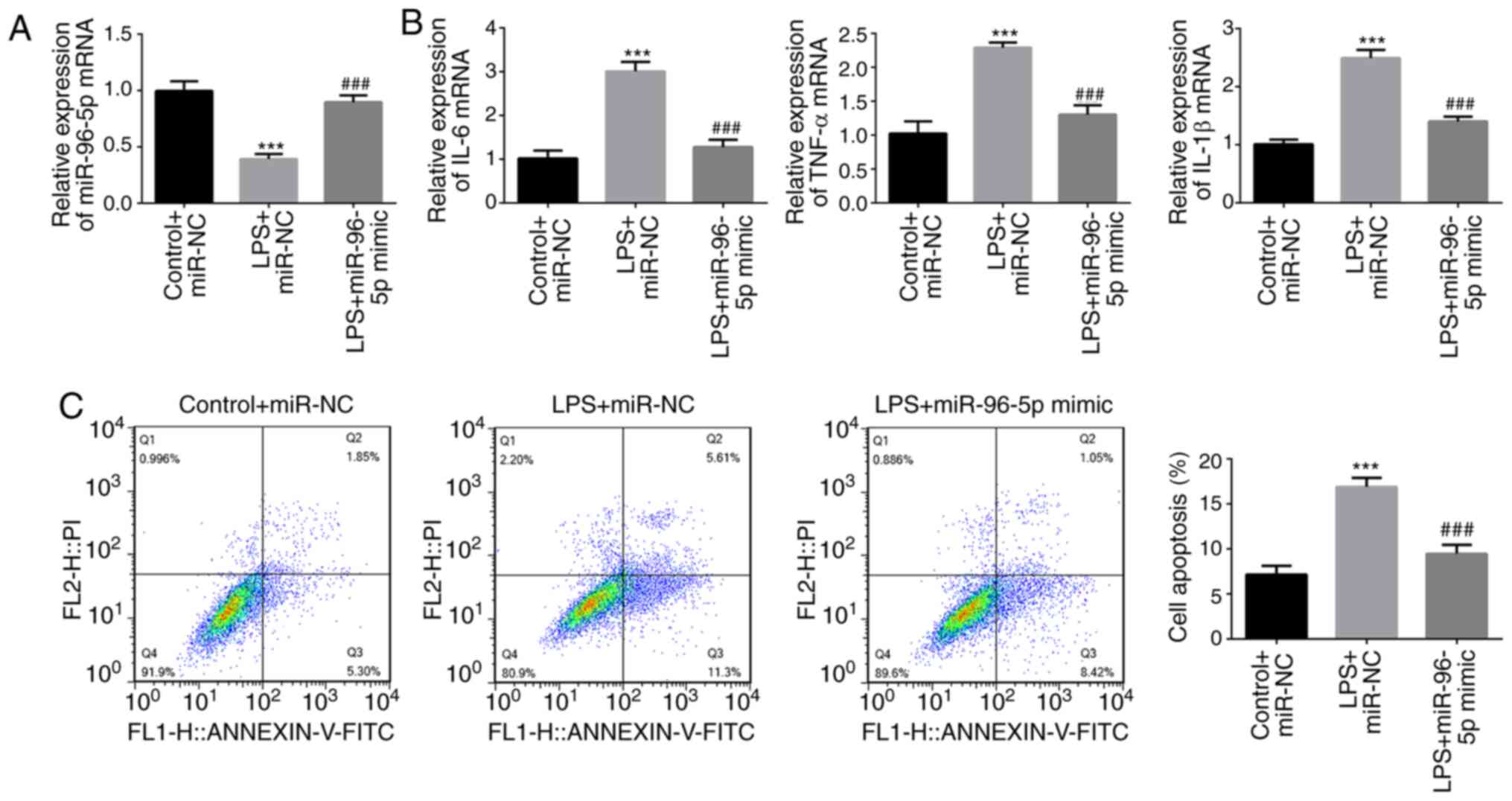
THP-1 cells were stimulated by LPS and transfected
with miR-96-5p mimic or miR-NC (Fig.
2A), and the effects of miR-96-5p overexpression on the
inflammatory responses and apoptosis of THP-1 cells were
determined. As shown in Fig. 2B,
LPS treatment significantly increased the mRNA expression of IL-6,
TNF-α and IL-1β, whereas miR-96-5p mimic decreased the levels of
these inflammatory cytokines. Moreover, flow cytometry revealed a
significantly increased rate of apoptosis in LPS-stimulated cells,
whereas the opposite results were observed in
miR-96-5p-overexpressing cells (Fig.
2C). These results suggested a protective role for miR-96-5p in
LPS-induced THP-1 cells.
GMDS-AS1 is highly expressed and
regulates miR-96-5p expression in LPS-induced THP-1 cells
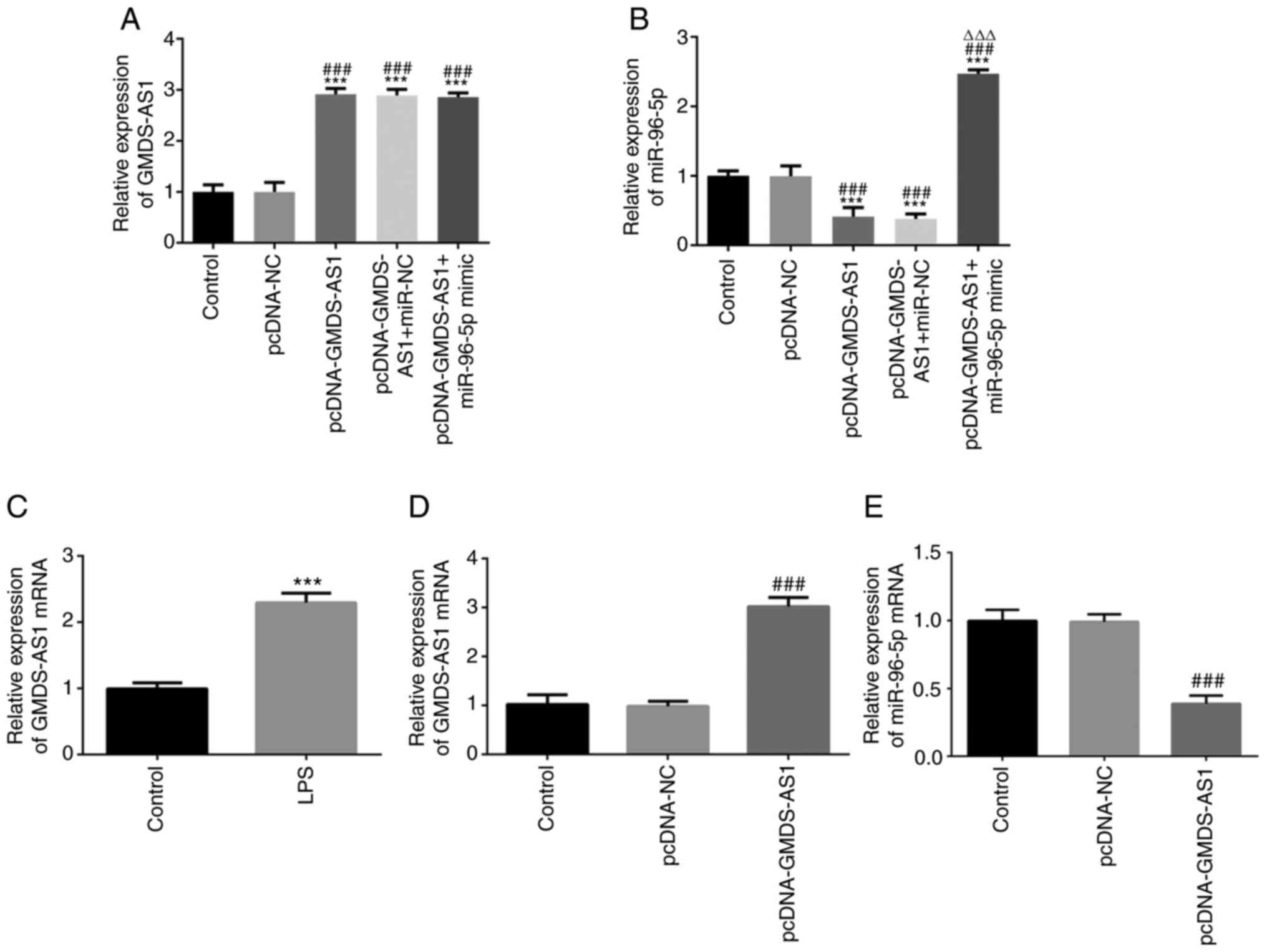
RT-qPCR showed that the expression levels of
GMDS-AS1 and mir-96-5p were significantly increased after
co-transfection (Fig. 3A-B). The
GMDS-AS1 level was then examined in THP-1 cells following LPS
stimulation. As shown in Fig. 3C,
the expression of GMDS-AS1 significantly increased following LPS
stimulation. A GMDS-AS1 overexpression vector was transfected into
THP-1 cells, and the transfection efficiency was verified by
RT-qPCR. The expression levels of GMDS-AS1 were significantly
increased in the pcDNA-GMDS-AS1 group compared with those in the
control and pcDNA-NC groups, suggesting that the transfection was
efficient (Fig. 3D). After
transfection with pcDNA-GMDS-AS1 in THP-1 cells, miR-96-5p
expression was significantly downregulated compared with that in
the control and pcDNA-NC groups (Fig.
3E). These data indicated that GMDS-AS1 exerted a regulatory
effect on miR-96-5p expression in LPS-induced THP-1 cells.
CASP2 is the target gene of
miR-96-5p
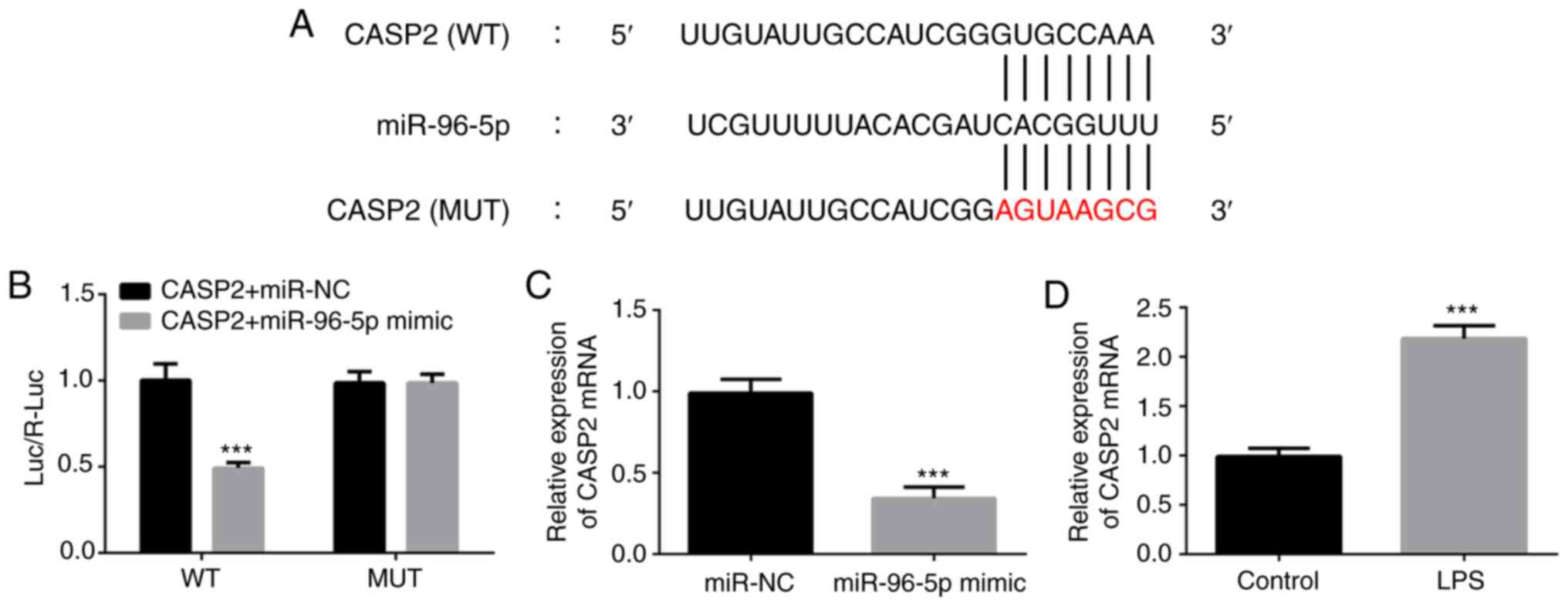
To investigate the target gene of miR-96-5p in
LPS-induced THP-1 cells, bioinformatics analysis was performed
using TargetScan (www.targetscan.com). CASP2 was identified as one of
the targets of miR-96-5p and the binding sequence is shown in
Fig. 4A. Luciferase reporter assays
demonstrated that the luciferase activity of the wild-type CASP2
construct was significantly inhibited by the miR-96-5p mimic,
whereas that of the mutated CASP2 was not (Fig. 4B). In addition, the mRNA expression
level of CASP2 significantly decreased after overexpression of
miR-96-5p (Fig. 4C). Furthermore,
LPS exposure significantly increased the expression of CASP2
(Fig. 4D). These findings confirmed
the interaction between CASP2 and miR-96-5p in LPS-stimulated THP-1
cells.
GMDS-AS1/miR-96-5p affects
inflammatory responses and apoptosis by modulating CASP2
expression
The subsequent experiments investigated how the
GMDS-AS1/miR-96-5p axis might regulate inflammatory responses and
apoptosis by CASP2. CASP2 and miR-96-5p mimic were transfected into
THP-1 cells, and transfection efficiency was verified by RT-qPCR.
The expression of CASP2 was significantly increased in the
pcDNA-CASP2 group compared with that in the control and pcDNA-NC
groups, demonstrating that the transfection was efficient (Fig. 5A). Moreover, the expression of CASP2
and miR-96-5p was maintained following co-transfection with
miR-96-5p mimic and pcDNA-CASP2 (Fig.
5B).
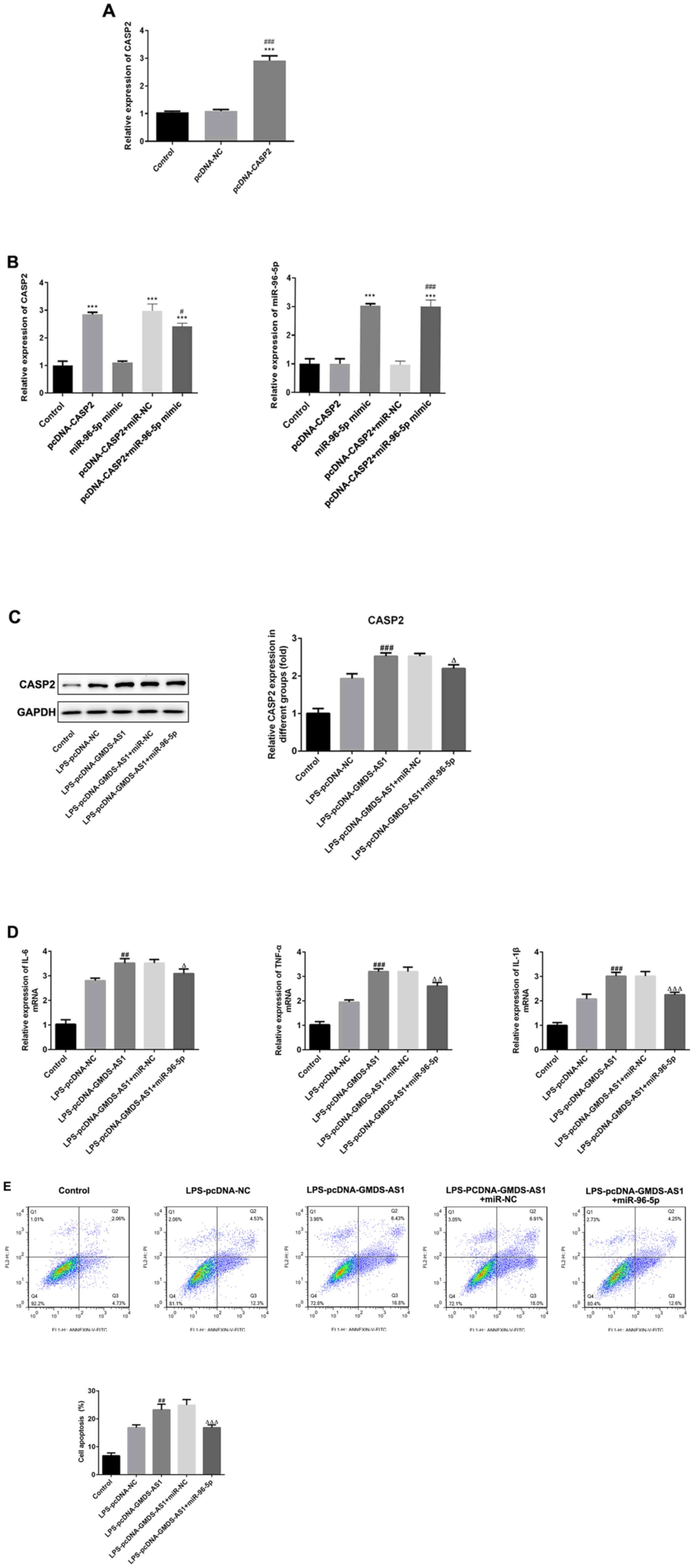 | Figure 5.GMDS-AS1/miR-96-5p axis modulates
LPS-induced inflammatory responses and apoptosis by regulating
CASP2. RT-qPCR was used to detect (A) CASP2 expression in the
pcDNA-CASP2 group. (B) Expression of CASP2 and miR-96-5p in the
pcDNA-CASP2 + miR-96-5p mimic group. (C) Western blot analysis was
used to determine the protein levels of CASP2 in LPS-induced THP-1
cells transfected with pcDNA-GMDS-AS1 and miR-NC or miR-96-5p
mimic. (D) IL-6, TNF-α and IL-1β levels in LPS-treated THP-1 cells
transfected with pcDNA-GMDS-AS1 and miR-NC or miR-96-5p mimic. (E)
Apoptosis in THP-1 cells treated with LPS and transfected with
pcDNA-GMDS-AS1 and miR-NC or miR-96-5p mimic. The data are
presented as the mean ± SD. ***P<0.001 vs. control;
#P<0.05, ##P<0.01,
###P<0.001 vs. LPS-pcDNA-NC or CASP2 + miR-NC;
∆P<0.05, ∆∆P<0.01,
∆∆∆P<0.001 vs.LPS-pcDNA-GMDS-AS1 + miR-NC. LPS,
lipopolysaccharide; miR, microRNA; NC, negative control;
GDP-mannose 4,6-dehydratase antisense 1; CASP2, caspase 2; PI,
propidium iodide; FITC, fluorescein isothiocyanate; IL,
interleukin; TNF-α, tumor necrosis factor α. |
THP-1 cells were stimulated by LPS, then
co-transfected with pcDNA-GMDS-AS1 or pcDNA-NC and miR-96-5p or
miR-NC. As shown in Fig. 5C,
western blotting results revealed that following LPS stimulation,
GMDS-AS1 overexpression significantly increased the protein levels
of CASP2 compared with the control and pcDNA-NC groups; however,
miR-96-5p mimic transfection significantly inhibited the CASP2
levels compared with pcDNA-GMDS-AS1 + miR-NC. Moreover, GMDS-AS1
increased, whereas miR-96-5p decreased, the levels of IL-6, TNF-α
and IL-1β, indicating that CASP2 overexpression accelerated the
production of inflammatory factors while downregulation of CASP2
exerted the opposite effects (Fig.
5D). In addition, the apoptosis rate significantly increased
following GMDS-AS1 overexpression. However, this increase was
inhibited by transfection with the miR-96-5p mimic (Fig. 5E). Thus, it may be concluded that
the GMDS-AS1/miR-96-5p/CASP2 axis regulates the levels of
inflammatory cytokines and apoptosis in THP-1 cells following LPS
exposure.
Discussion
Severe sepsis is as a life-threatening medical
emergency (18). Although a
standardized approach and new strategies for sepsis treatment have
gradually developed, the pathogenesis of sepsis has yet to be fully
elucidated (19,20). In the present study, the expression
level of miR-96-5p and effects of miR-96-5p overexpression on
inflammatory cytokine production and apoptosis were investigated in
LPS-induced THP-1 cells. Moreover, the mechanism through which
GMDS-AS1 regulates miR-96-5p and CASP2 to affect inflammatory
responses and apoptosis.
miRNA participates in a variety of cellular
processes, such as cell proliferation, metastasis and apoptosis
(21,22). The dysregulation of miRNA
contributes to the occurrence and development of multiple diseases,
including sepsis (23–25). miR-96-5p has been reported to be
involved in several cancer types. For example, Ress et al
(26) reported that miR-96-5p
affected the proliferation of colorectal cancer (CRC) cells and was
associated with poor survival of patients with CRC. Furthermore,
miR-96-5p accelerated ovarian cancer cell proliferation and
migration by targeting Caveolae1 (27). In addition, a previous study
revealed that miR-96-5p expression was significantly downregulated
in clinical samples from patients with sepsis (28). In the present study, the expression
level of miR-96-5p was decrased in an LPS-induced THP-1 cell model,
which is consistent with previous reports (29,30).
Moreover, upregulation of miR-96-5p inhibited the secretion of
inflammatory factors, including IL-6, TNF-α and IL-1β, as well as
apoptosis.
A previous study have reported that lncRNAs may act
as ceRNAs that bind to sites similar to the 3′-UTR region of mRNA
to regulate biological processes (31). In this regard, in the present study,
the expression of GMDS-AS1 was detected in THP-1 cells exposed to
LPS, revealing higher expression of GMDS-AS1 compared with that in
untreated THP-1 cells. Subsequent experiments revealed that
GMDS-AS1 overexpression inhibited miR-96-5p expression,
highlighting the regulatory effect of GMDS-AS1 on miR-96-5p. In
addition, the target gene of miR-96-5p was also examined in order
to further elucidate the mechanisms underlying sepsis. Through
bioinformatics analysis, CASP2 was predicted as the target gene of
miR-96-5p in sepsis, and a luciferase reporter assay verified the
association between miR-96-5p and CASP2. The subsequent experiments
also demonstrated that CASP2 expression was altered in LPS-treated
cells and was negatively modulated by miR-96-5p.
Excessive inflammatory responses and cell apoptosis
are two major characteristics of sepsis (32,33).
Anti-inflammatory and anti-apoptosis strategies have been
considered as effective approaches for relieving or treating sepsis
(34,35). Previous studies demonstrated that
certain lncRNAs and miRNAs play regulatory roles in inflammatory
responses and apoptosis to slow down the development of sepsis
(36,37). Yong et al (38) demonstrated that the lncRNA
metastasis-associated lung adenocarcinoma transcript 1 decreased
the expression of breast cancer susceptibility gene 1 and recruited
zeste homolog 2 to promote skeletal muscle cell apoptosis and
inflammatory response in sepsis. Lu et al (39) reported that sepsis-induced kidney
injury associated transcript 1 (SIKIAT1) was highly expressed both
in sepsis patients and an LPS-treated sepsis cell model. SIKIAT1
silencing repressed cell apoptosis, whereas its overexpression
promoted apoptosis by regulating the miR-96/forkhead box A1 axis
(39). In the present study,
upregulation of GMDS-AS1 increased the CASP2 level and this effect
was reversed by miR-96-5p overexpression. The protein levels of
IL-6, TNF-α and IL-1β and cell apoptosis were increased after
GMDS-AS1 overexpression and decreased after treatment with
miR-96-5p mimic, suggesting that GMDS-AS1 and miR-96-5p jointly
regulate the inflammatory response and cell apoptosis by targeting
CASP2.
In summary, the present study uncovered the
significance of the lncRNA GMDS-AS1 in sepsis. GMDS-AS1 was
demonstrated to facilitate inflammatory responses and cell
apoptosis by targeting miR-96-5p/CASP2 in LPS-induced THP-1 cells.
This ceRNA mechanism may provide novel evidence and a new research
direction for the clinical treatment of sepsis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ and JL designed the experiments. LJ was involved
in the collection, interpretation and analysis of the data and
wrote the manuscript. JL designed the study and was involved in
data collection, analysis and interpretation, as well as
preparation of manuscript. LJ and JL confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chaudhry H, Zhou J, Zhong Y, Ali MM,
McGuire F, Nagarkatti PS and Nagarkatti M: Role of cytokines as a
double-edged sword in sepsis. In vivo. 27:669–684. 2013.PubMed/NCBI
|
|
2
|
Parrillo JE, Parker MM, Natanson C,
Suffredini AF, Danner RL, Cunnion RE and Ognibene FP: Septic shock
in humans. Advances in the understanding of pathogenesis,
cardiovascular dysfunction, and therapy. Ann Intern Med.
113:227–242. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Becker KL, Snider R and Nylen ES:
Procalcitonin in sepsis and systemic inflammation: A harmful
biomarker and a therapeutic target. Br J Pharmacol. 159:253–264.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bickler SW and De Maio A: Dysfunction of
the innate immune system during sepsis: A call for research. Crit
Care Med. 41:364–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Delano MJ and Ward PA: The immune system's
role in sepsis progression, resolution, and long-term outcome.
Immunol Rev. 274:330–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maslove DM and Wong HR: Gene expression
profiling in sepsis: Timing, tissue, and translational
considerations. Trends Mol Med. 20:204–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin GS: Sepsis, severe sepsis and
septic shock: Changes in incidence, pathogens and outcomes. Expert
Rev Anti Infect Ther. 10:701–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Wang H, Zhu R, Liu Q, Fei J and
Wang S: Anti-inflammatory activity of curcumin-loaded solid lipid
nanoparticles in IL-1β transgenic mice subjected to the
lipopolysaccharide-induced sepsis. Biomaterials. 53:475–483. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Egranov SD, Yang L and Lin C:
Molecular mechanisms of long noncoding RNAs-mediated cancer
metastasis. Genes Chromosomes Cancer. 58:200–207. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang Y and Fullwood MJ: Roles, functions,
and mechanisms of long Non-coding RNAs in cancer. Genomics
Proteomics Bioinformatics. 14:42–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai Y, Liang Z, Li Y, Li C and Chen L:
Circulating Long Noncoding RNAs as potential biomarkers of sepsis:
A preliminary study. Genet Test Mol Biomarkers. 21:649–657. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang TN, Li D, Xia J, Wu QJ, Wen R, Yang
N and Liu CF: Non-coding RNA: A potential biomarker and therapeutic
target for sepsis. Oncotarget. 8:91765–91778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun L, Li L and Yan J: Progress in
relationship of the long non-coding RNA and sepsis. Zhonghua Wei
Zhong Bing Ji Jiu Yi Xue. 29:181–183. 2017.(In Chinese). PubMed/NCBI
|
|
14
|
Fang Y, Hu J, Wang Z, Zong H, Zhang L,
Zhang R and Sun L: LncRNA H19 functions as an Aquaporin 1
competitive endogenous RNA to regulate microRNA-874 expression in
LPS sepsis. Biomed Pharmacother. 105:1183–1191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang HJ, Wei QF, Wang SJ, Zhang HJ, Zhang
XY, Geng Q, Cui YH and Wang XH: LncRNA HOTAIR alleviates rheumatoid
arthritis by targeting miR-138 and inactivating NF-kappaB pathway.
Int Immunopharmacol. 50:283–290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao M, Xin XF, Zhang JY, Dai W, Lv TF and
Song Y: LncRNA GMDS-AS1 inhibits lung adenocarcinoma development by
regulating miR-96-5p/CYLD signaling. Cancer Med. 9:1196–1208. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jain S: Sepsis: An update on current
practices in diagnosis and management. Am J Med Sci. 356:277–286.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rello J, Valenzuela-Sanchez F,
Ruiz-Rodriguez M and Moyano S: Sepsis: A review of advances in
management. Adv Ther. 34:2393–2411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hamers L, Kox M and Pickkers P:
Sepsis-induced immunoparalysis: Mechanisms, markers, and treatment
options. Minerva Anestesiol. 81:426–439. 2015.PubMed/NCBI
|
|
21
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Varshney J and Subramanian S: MicroRNAs as
potential target in human bone and soft tissue sarcoma
therapeutics. Front Mol Biosci. 2:312015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu D, Dong J, Li P, Tang C, Cheng W, Xu Z,
Zhou W, Ge J, Xia C and Zhang Z: MiRNA-21 has effects to protect
kidney injury induced by sepsis. Biomed Pharmacother. 94:1138–1144.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ge C, Liu J and Dong S: MiRNA-214 protects
sepsis-induced myocardial injury. Shock. 50:112–118. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Ruan Z, Mao Y, Dong W, Zhang Y,
Yin N and Jiang L: MiR-27a is up regulated and promotes
inflammatory response in sepsis. Cell Immunol. 290:190–195. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ress AL, Stiegelbauer V, Winter E,
Schwarzenbacher D, Kiesslich T, Lax S, Jahn S, Deutsch A,
Bauernhofer T, Ling H, et al: MiR-96-5p influences cellular growth
and is associated with poor survival in colorectal cancer patients.
Mol Carcinog. 54:1442–1450. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu B, Zhang J and Yang D: MiR-96-5p
promotes the proliferation and migration of ovarian cancer cells by
suppressing Caveolae1. J Ovarian Res. 12:572019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen J, Jiang S, Cao Y and Yang Y: Altered
miRNAs expression profiles and modulation of immune response genes
and proteins during neonatal sepsis. J Clin Immunol. 34:340–348.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng Q, Tang L and Wang Y: Regulatory
role of miRNA-26a in neonatal sepsis. Exp Ther Med. 16:4836–4842.
2018.PubMed/NCBI
|
|
30
|
How CK, Hou SK, Shih HC, Huang MS, Chiou
SH, Lee CH and Juan CC: Expression profile of MicroRNAs in
gram-negative bacterial sepsis. Shock. 43:121–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hotchkiss RS and Nicholson DW: Apoptosis
and caspases regulate death and inflammation in sepsis. Nat Rev
Immunol. 6:813–822. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gotts JE and Matthay MA: Sepsis:
Pathophysiology and clinical management. BMJ. 353:i15852016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matsuda A, Jacob A, Wu R, Aziz M, Yang WL,
Matsutani T, Suzuki H, Furukawa K, Uchida E and Wang P: Novel
therapeutic targets for sepsis: Regulation of exaggerated
inflammatory responses. J Nippon Med Sch. 79:4–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oberholzer C, Oberholzer A, Clare-Salzler
M and Moldawer LL: Apoptosis in sepsis: A new target for
therapeutic exploration. FASEB J. 15:879–892. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jia Y, Li Z, Cai W, Xiao D, Han S, Han F,
Bai X, Wang K, Liu Y, Li X, et al: SIRT1 regulates inflammation
response of macrophages in sepsis mediated by long noncoding RNA.
Biochim Biophys Acta Mol Basis Dis. 1864:784–792. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng D, Yu Y, Li M, Wang G, Chen R, Fan
GC, Martin C, Xiong S and Peng T: Inhibition of MicroRNA 195
prevents apoptosis and multiple-organ injury in mouse models of
sepsis. J Infect Dis. 213:1661–1670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yong H, Wu G, Chen J, Liu X, Bai Y, Tang
N, Liu L and Wei J: lncRNA MALAT1 accelerates skeletal muscle cell
apoptosis and inflammatory response in sepsis by decreasing BRCA1
expression by recruiting EZH2. Mol Ther Nucleic Acids. 19:97–108.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu S, Wu H, Xu J, He Z, Li H and Ning C:
SIKIAT1/miR-96/FOXA1 axis regulates sepsis-induced kidney injury
through induction of apoptosis. Inflamm Res. 69:645–656. 2020.
View Article : Google Scholar : PubMed/NCBI
|