Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous
and chronic condition featuring hirsutism, anovulation and
polycystic ovaries with a prevalence of 10% (1–3).
Patients with PCOS are vulnerable to reproductive abnormalities
(4,5), insulin resistance (6), coronary heart disease (7), anxiety and depression (8). In addition, >30% of infertility
results from PCOS (9). It has
been revealed that there are multiple causes of PCOS and these are
attributed to genetic and environmental factors, which are
prominent contributors to the development of this disease (10). Despite the severity and high
incidence rate of PCOS, effective methods for its treatment are
limited. Additionally, an insufficient number of research studies
have examined its mechanism of development (9). Therefore, a more effective
therapeutic treatment for PCOS is required and the investigation of
its mechanism of action may contribute to this process.
Dipeptidyl peptidase 4 (DPP4), also known as cluster
of differentiation 26, is a type II transmembrane protein released
from the cell membrane in a non-classical secretory mechanism. The
release of DPP4 may be detected in plasma as well as in different
tissues, such as the liver, spleen, lung and ovaries (11). A previous study demonstrated that
DPP4 overexpression caused hepatic insulin resistance and promoted
liver steatosis, while its inhibition could improve hepatic insulin
sensitivity and prevent further accumulation of ectopic fat in the
liver (12). Kawasaki et
al (13) reported that the
inhibition of DPP4 expression could alleviate
lipopolysaccharide-induced lung injury in mice and potentially
serve as a therapeutic drug for acute respiratory distress
syndrome. Furthermore, it was revealed that DPP4 levels were
elevated in patients with PCOS and that inhibition of DPP4
expression could be used to treat the development of this condition
(14). During the progression of
PCOS, estrogen induces cAMP response element-binding protein (CREB)
expression in order to promote transcription of cytochrome P450
(CYP) 19A1 and CYP11A1. This in turn reduces the development of the
disease (15). However, the
effects of the inhibition of DPP4 expression on ovarian granulosa
cell proliferation and on the activation of the CREB/aromatase
pathway remain unknown. Therefore, the present study was designed
to explore the role of inhibition of DPP4 expression in ovarian
granulosa cell proliferation and on the activation of the
CREB/aromatase pathway.
Advanced glycation end products (AGEs) refer to the
early glycation products, which undergo further reactions, such as
rearrangements and dehydration to become irreversibly cross-linked,
fluorescent and senescent macroprotein derivatives (16). By stimulating DPP4 production,
AGEs evoke endothelial cell damage (17). AGEs significantly increase DPP4
production, which in turn induces inflammatory reactions in
proximal tubular cells (18).
Previous studies have also demonstrated that AGEs are closely
associated with the pathogenesis of PCOS, as well as its metabolic
and reproductive consequences (19,20). In addition, the expression levels
of DPP4 are upregulated in the serum and ovarian granulosa cells in
patients with PCOS (21). In
particular, glycolaldehyde (GOA)-derived AGEs are deemed toxic
AGEs, and long term-incubation of GOA with bovine serum albumin
(BSA) produces toxic AGEs (22).
Therefore, the present study explored whether AGEs could induce the
expression of DPP4 in granulosa cells and whether inhibition of
DPP4 expression could alleviate the suppressed proliferation of
GOA-BSA-treated ovarian granulosa cells.
Materials and methods
Experimental animals
A total of 20 adult female Sprague Dawley rats, aged
6 weeks, weighing 82±5 g were provided by the Animal Center of the
Chinese Academy of Sciences and were randomly divided into a
control and a PCOS group (n=10 per group) (23). Rats were housed in a room with
controlled temperature (21–22°C), and in a 12-h light/dark cycle,
with access to food and water ad libitum. The experiment was
conducted in strict accordance to the Guidelines for the Care and
Use of Laboratory Animals (24)
and the ‘3R’ principle (25). All
experiments received approval from the Experimental Animal Ethics
Committee of Zhaofenghua Biological Technology Co., Ltd. (approval
number IACUC-20201012-16).
Establishment of a PCOS animal
model
In the PCOS group (n=10), the rats were
intragastrically administered 0.5 mg/kg letrozole (Jiangsu Hengrui
Medicine Co., Ltd.) dissolved in 1% (w/w) carboxymethylcellulose
(CMC; Beyotime Institute of Biotechnology) and fed with a high fat
diet for 21 days (26). The
control group of 10 rats received vehicle only (1% aqueous solution
of CMC) once daily (p.o.). To obtain vaginal smears, rats were
restrained by hand, a sterile cotton swab moistened with normal
saline was inserted into the vagina of the animal to dip in the
exfoliated cells via gentle rotation in the vagina (27–29). The whole movement was gentle to
ensure that the rats experienced no pain. The vaginal smears of the
rats were monitored 1 week following modeling and the estrous cycle
was used to assess the successful establishment of the model. The
animal health and behavior were monitored every day, and the
following humane endpoints were used in the present study: Rapid
loss of 15% of the original body weight; continuous inability of
the rats to gain weight; and inability to eat and drink on their
own. On the 30th day, the rats were anesthetized with 2% sodium
pentobarbital (intraperitoneal injection, 40 mg/kg) and then the
blood samples (4–6 ml) from the abdominal aorta were collected,
following cervical dislocation of the spine. When the cessation of
the heartbeat and breathing of the rats was verified, and there
were no reflexes, the death of the rats was confirmed. The blood
samples were centrifuged at 1,000 × g/min for 10 min at 4°C to
obtain the serum samples. The granular cells from the ovaries of
control and PCOS rats were isolated as previously reported
(30).
Hematoxylin and eosin (H&E)
staining
The ovarian tissues of control and PCOS rats were
collected and fixed with 10% formaldehyde at room temperature.
Following dehydration, tissues were embedded in paraffin and sliced
into pieces (cross-sections, 5 µm). After being deparaffinized and
rehydrated, an H&E staining kit (product code ab245880; Abcam)
was applied to the sections following the manufacturer's protocol,
and the sections were observed by light microscope, Leica DM3000
(Leica Microsystems Ltd.; magnification, ×200).
Cell culture, treatment and
transfection
The immortalized human granulosa-like tumor cell
line, KGN, which maintained the physiological characteristics of
ovarian cells and has been widely used to study PCOS (30–33) was purchased from the American Type
Culture Collection (ATCC). The cells were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere with 5%
CO2.
In order to knockdown DPP4 expression in KGN cells,
small interfering RNA (siRNA) targeting DPP4 (siRNA-DPP4-1 and
siRNA-DPP4-2) and the corresponding negative control (siRNA-NC)
were synthesized by GenScript. The transfection of 2 µg/ml vectors
into KGN cells at 37°C for 10 h was conducted using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) as determined by the manufacturer's
protocol. At 48 h post-transfection, cells were used for subsequent
experiments. The sequence for siRNAs were as follows: siRNA-DPP4-1
forward, 5′-GUACCUCCUUAUUCAUGGATT-3′ and reverse,
5′-UCCAUGAAUAAGGAGGUACTT-3′; siRNA-DPP4-2 forward,
5′-CACCGUGGAAGGUUCUUCUTT-3′ and reverse,
5′-AGAAGAACCUUCCACGGUGTT-3′; siRNA-NC, forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-TTAAGAGGCUUGCACAGUGCA-3′.
GOA-BSA was prepared as previously reported
(22), and KGN cells were treated
with 400 µg/ml GOA-BSA for 24, 48, 72 and 96 h with or without
sitagliptin (100 nM; APeXBIO Technology LLC) co-treatment for 24
h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from cells or samples was extracted using
TRlzol® reagent (Thermo Fisher Scientific, Inc.). The
samples were reverse transcribed into cDNA using a RevertAid First
Strand cDNA Synthesis kit (cat. no. K1622; Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
qPCR was performed using a SYBR-Green kit (QuantiNova SYBR Green
RT-PCR kit, cat. no. 208152; Qiagen AB). The thermocycling
conditions were: Initial denaturation at 95°C for 8 min;
denaturation at 95°C for 25 sec, annealing at 60°C for 1 min (37
cycles); and final extension at 72°C for 10 min. GAPDH was used as
an endogenous control and the calculation of the relative gene
expression was performed using the 2−ΔΔCq method
(34). Primers are as follows:
DPP4 (rat) forward, 5′-ATTCCGTACCCAAAGGCAGG-3′ and reverse,
5′-AGGCCACGTCACACAAGTAG-3′; DPP4 (human) forward,
5′-TCTGCTGAACAAAGGCAATGA-3′ and reverse,
5′-CTGTTCTCCAAGAAAACTGAGC-3′; CREB forward,
5′-TAGTGCCCAGCAACCAAGT-3′ and reverse,
5′-ACATGTTACCATCTTCAAACTGAC-3′; CYP19A1, forward,
5′-ACTTATCCTATCAGGACGGAAGGT-3′ and reverse,
5′-AGGGGGCAATTTAGAGTCGC-3′; CYP11A1 forward,
5′-GCTGAAGTGGAGCAGGTACA-3′ and reverse, 5′-CTTTGACCAGGACTGAGCGT-3′;
GAPDH (rat) forward, 5′-GCATCTTCTTGTGCAGTGCC-3′ and reverse,
5′-TACGGCCAAATCCGTTCACA-3′; GAPDH (human) forward,
5′-AATGGGCAGCCGTTAGGAAA-3′ and reverse,
5′-GCGCCCAATACGACCAAATC-3′.
Western blot analysis
Total proteins from cells or samples were extracted
using RIPA lysis buffer (Beijing Solarbio Science & Technology
Co., Ltd.). A BCA kit (Beyotime Institute of Biotechnology) was
used to quantify the protein concentration. Subsequently, the
samples (30 µg per lane) were separated with 12% SDS-PAGE and
transferred to PVDF membranes (MilliporeSigma). Following blocking
with 5% non-fat milk for 2 h at room temperature, the membranes
were incubated with the corresponding primary antibodies at 4°C
overnight. Subsequently, a HRP-conjugated goat anti-rabbit
secondary antibody (1:10,000; cat. no. ab205718; Abcam) was used to
incubate the membranes for 2 h at room temperature. Finally, an ECL
kit (Beyotime Institute of Biotechnology) was used to determine the
relative protein levels. The rabbit primary antibodies (Abcam)
included: DPP4 (1:2,000; cat. no. ab187048), cyclin-dependent
kinases (CDKs) CDK2 (1:2,000; cat. no. ab32147), CDK4 (1:2,000;
cat. no. ab108357), CDK6 (1:2,000; cat. no. ab124821), cell cycle
proteins (cyclins) cyclin B (1:2,000; cat. no. ab32053), cyclin E
(1:2,000; cat. no. ab33911), CREB (1:2,000; cat. no. ab32515),
CYP19A1 (1:1,000; cat. no. ab106168), CYP11A1 (1:1,000; cat. no.
ab272494), GAPDH (1:2,500; cat. no. ab9485) and β-actin (1:2,000;
cat. no. ab8227). The densitometry analysis was performed using
ImageJ software (version 1.8.0; National Institutes of Health).
Cell Counting Kit-8 (CCK-8) assay
The effects of silencing DPP4 expression were
investigated on KGN cell viability using the CCK-8 assay. The cells
(2,000 cells per well) were incubated in 96-well plates for 24, 48,
72 and 96 h at 37°C. Subsequently, 10% CCK-8 solution (Beyotime
Institute of Biotechnology) was added to each well and the cells
were incubated for an additional 2 h at 37°C. Subsequently, the
absorbance was determined at 450 nm using a microplate reader
(Thermo Fisher Scientific Inc.).
5-Ethynyl-2′-deoxyuridine (EdU)
staining
The transfected KGN cells were seeded into 96-well
plates and incubated with EdU solution (Beyotime Institute of
Biotechnology) for 4 h at room temperature. Following fixation with
4% paraformaldehyde at room temperature for 1 h, the cells were
stained with 1X Apollo567 solution for 30 min at room temperature
in the dark and DAPI solution was used to stain the cell nuclei at
room temperature for 5 min. A fluorescent microscope was employed
to observe the results (magnification, ×200) and ImageJ software
(version 1.8.0; National Institutes of Health) was used to analyze
the percentage of EdU-positive nuclei (green) compared with the
total percentage of the nuclei (stained with blue fluorescence),
and then the data was revealed as relative to the control group
(normal KGN cells).
Flow cytometry
The transfected KGN cells were centrifuged at 1,000
× g/min for 30 sec at 4°C and the supernatant was discarded.
Subsequently, l ml PBS was used to resuspend the cells. The
precipitated cells were resuspended using 100 µl RNase A at 37°C
for 30 min. A total of 400 µl PI staining solution was added and
the cells were stained in the dark at room temperature for 10 min.
Finally, flow cytometry (BD FACS; BD Biosciences) was used to
assess the cell cycle and the data was analyzed by FlowJo version
7.6 (FlowJo LLC).
Statistical analysis
All data are presented as the mean ± SD of three
independent experiments after normalization to the control group,
and statistical analysis was performed using SPSS 20.0 (IBM Corp.).
Statistical differences between two groups were determined using an
unpaired Student's t-test, while one-way ANOVA was carried out with
Tukey's multiple comparison post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
DPP4 expression is increased in serum
samples of rats with PCOS and KGN cells
To validate the success of the PCOS animal model,
ovarian tissues from control and PCOS rats were observed by H&E
staining. As illustrated in Fig.
1A, the structure of ovarian tissues in the control rats was
well arranged, while in the PCOS rats it was arranged in disorder.
In addition, control rats exhibited ovarian follicles at a variety
of stages, whereas ovarian cystic expansion and a significant drop
in the number of granular cells was observed in the ovaries of PCOS
rats. The aforementioned results indicated the successful
establishment of the PCOS animal model. Subsequently, the relative
mRNA expression levels of DPP4 were determined in the serum samples
of rats with PCOS using RT-qPCR analysis. DPP4 expression was
significantly increased in rats with PCOS (~3.4-fold of the
control) compared with that of the control group (Fig. 1B). In addition, DPP4 expression in
granular cells from the ovaries of control and PCOS rats was
detected using RT-qPCR and western blot analyses. The relative
expression levels of DPP4 were significantly increased in granular
cells from the ovaries of PCOS rats compared with those of the
control rats (Fig. 1C). The
aforementioned results suggested that DPP4 exhibited high
expression in the PCOS group.
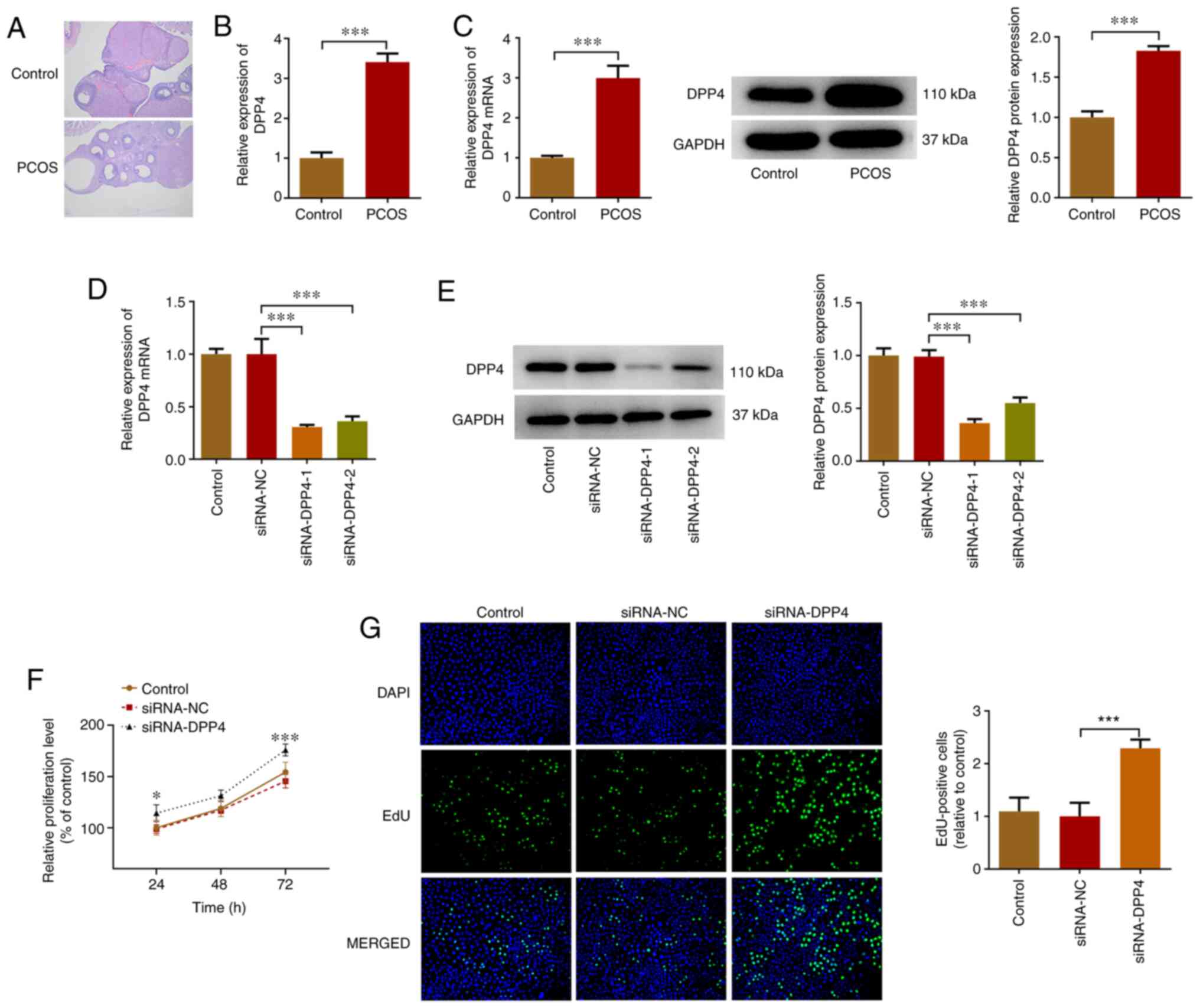 | Figure 1.Expression levels of DPP4 are
increased in the serum of rats with PCOS and KGN cells, while
inhibition of DPP4 expression promotes the proliferation of KGN
cells. (A) Ovarian tissues from control and PCOS rats were
subjected to H&E staining (magnification, ×200). (B) RT-qPCR
was used to assess the relative mRNA expression levels of DPP4 in
the serum of rats with PCOS. (C) DPP4 RNA and protein expression
levels in granular cells from the ovaries of control and PCOS rats
were determined using RT-qPCR and western blot analyses. The
relative mRNA and protein expression levels of DPP4 in KGN cells
that were transfected with indicated vectors were detected using
(D) RT-qPCR and (E) western blot analyses, respectively. The
proliferation of DPP4-silenced KGN cells was detected using (F)
Cell Counting Kit-8 and (G) 5-ethynyl-2′-deoxyuridine staining
assays. *P<0.05, ***P<0.001. DPP4, dipeptidyl peptidase 4;
PCOS, polycystic ovary syndrome; RT-qPCR, reverse
transcription-quantitative PCR; EdU, 5-ethynyl-2′-deoxyuridine;
siRNA, small interfering RNA; NC, negative control. |
Knockdown of DPP4 expression promotes
the proliferation of KGN cells
The effects of silencing DPP4 expression were
investigated on KGN cell proliferation. KGN cells were transfected
with siRNA-DPP4 and the relative mRNA and protein expression levels
were detected using RT-qPCR and western blot analyses,
respectively. The expression levels of DPP4 were significantly
decreased compared with those noted in the siRNA-NC group, whereas
siRNA-DPP4-1 contributed to the lowest DPP4 expression (0.3 vs.
0.36; Fig. 1D and E). Therefore,
siRNA-DPP4-1 was selected for subsequent experiments. In addition,
the proliferation of DPP4-silenced KGN cells was detected using the
CCK-8 and EdU staining assays. The results demonstrated that the
proliferation of KGN cells was significantly increased due to DPP4
silencing in comparison with that of the siRNA-NC group (Fig. 1F and G).
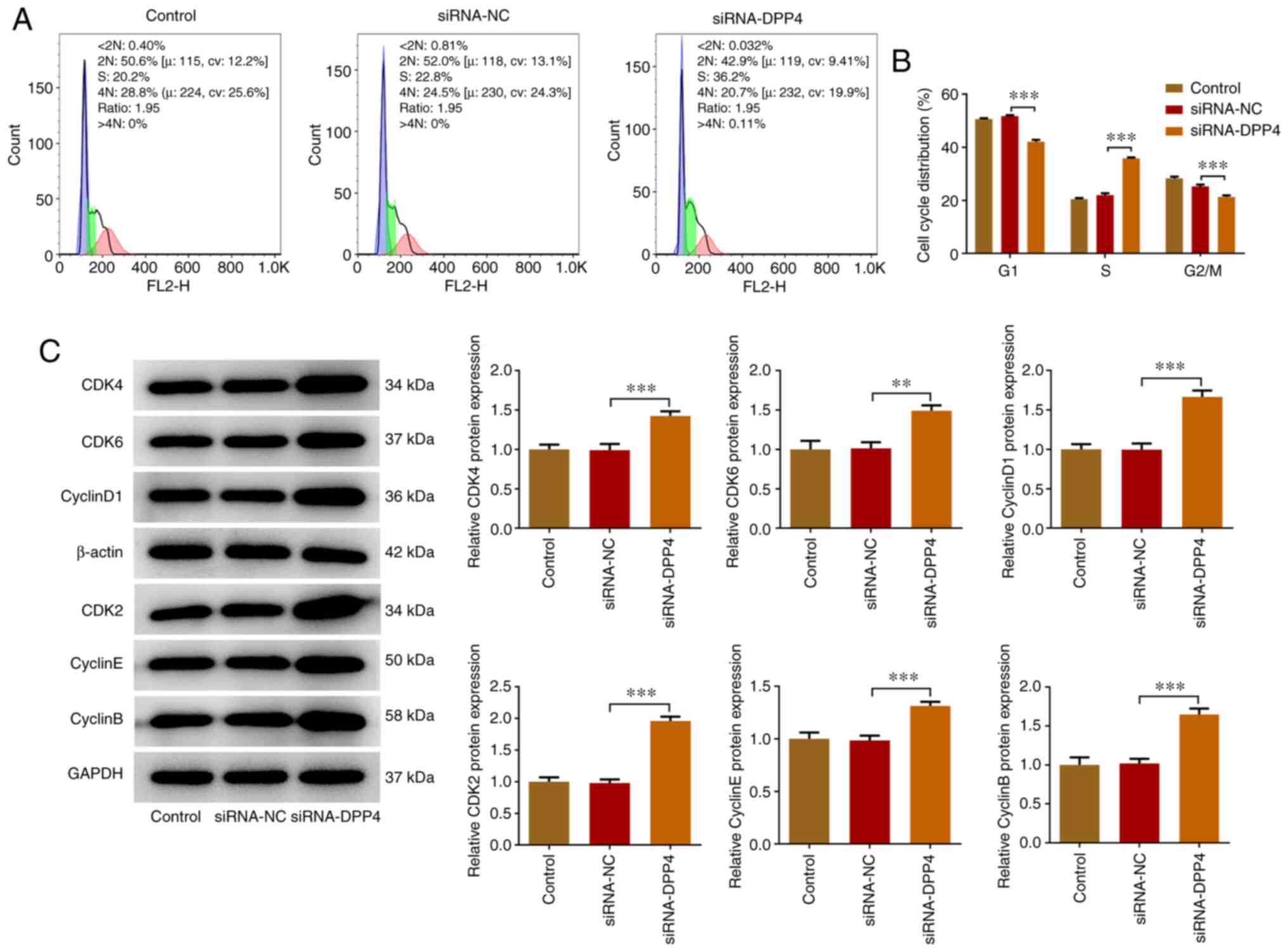
Knockdown of DPP4 expression
accelerates the cell cycle of KGN cells
Flow cytometry was employed to assess the role of
the inhibition of DPP4 expression on the cell cycle of KGN cells.
The cell cycle is divided into the G1, S, G2
and M phases and each phase exhibits different characteristics
(23). Different functions are
required for the transition from one phase to the next (35). The number of DPP4-silenced KGN
cells at the G1 and G2/M phases was slightly
diminished compared with that of the siRNA-NC group, whereas the
number of cells at the S phase was significantly increased by DPP4
silencing in comparison with that of siRNA-NC (Fig. 2A and B). The cell cycle is
regulated via the activation of cell cycle proteins (cyclins) and
CDKs (36,37). Therefore, the expression levels of
CDK2, CDK4, CDK6, cyclin D1, cyclin E and cyclin B were detected
using western blot analysis. The results indicated that the
expression levels of the cell cycle proteins (cyclin D1, cyclin E
and cyclin B) and CDKs (CDK2, CDK4, CDK6) were upregulated in the
DPP4-silenced KGN cells compared with those of the siRNA-NC group
(Fig. 2C). The aforementioned
results revealed that silencing of DPP4 expression could
effectively accelerate the cell cycle of KGN cells.
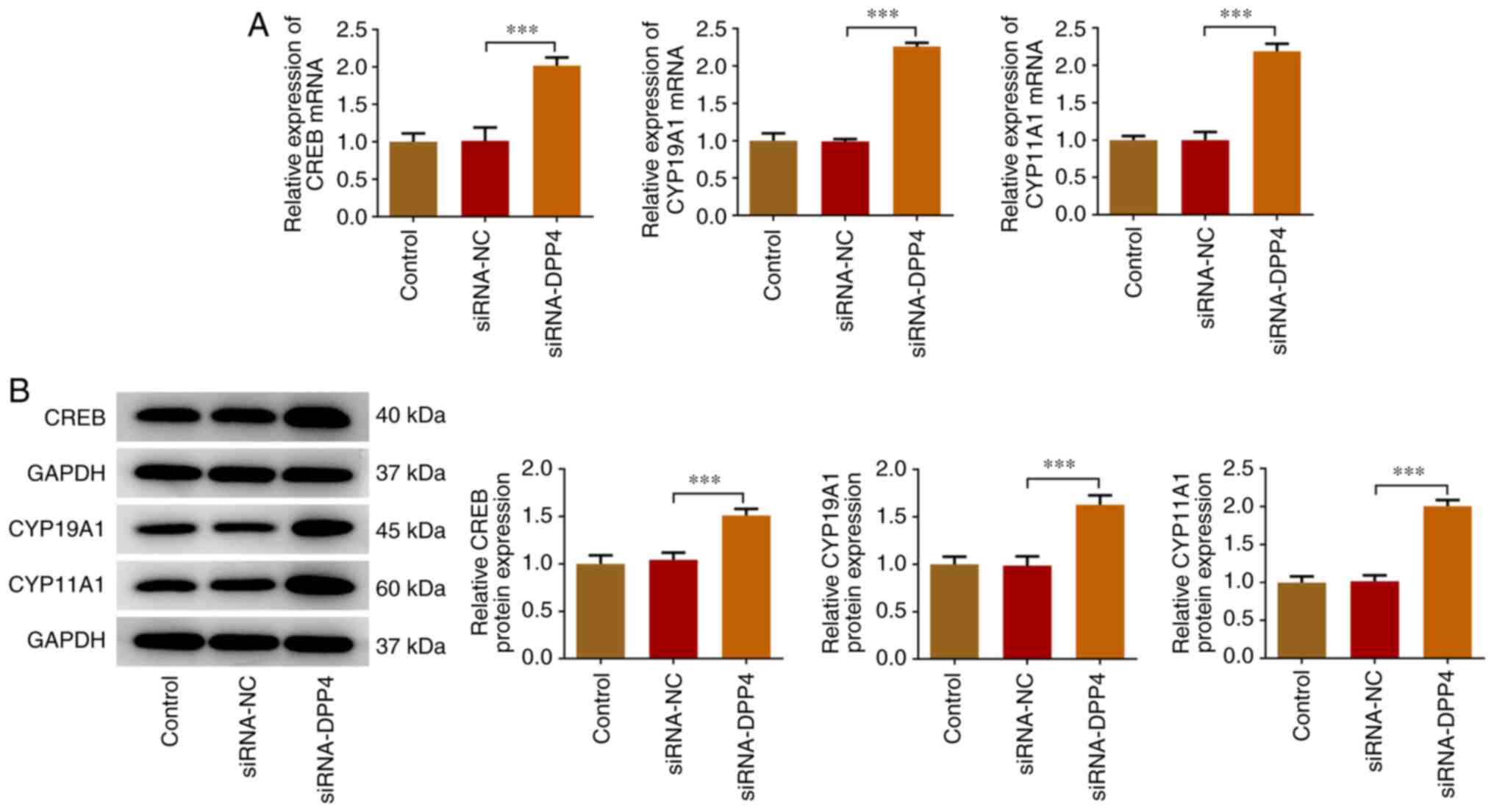
Knockdown of DPP4 expression induces
upregulation of the expression levels of CREB, CYP19A1 and
CYP11A1
Subsequently, the expression levels of CREB, CYP19A1
and CYP11A1 were detected via RT-qPCR and western blot analyses.
The transcription factor CREB and the enzymes CYP19A1 and CYP11A1
had higher expression levels in DPP4-silenced KGN cells compared
with those of the siRNA-NC group, revealing that DPP4 silencing
induced the activation of CREB and the transcription of aromatase
(Fig. 3).
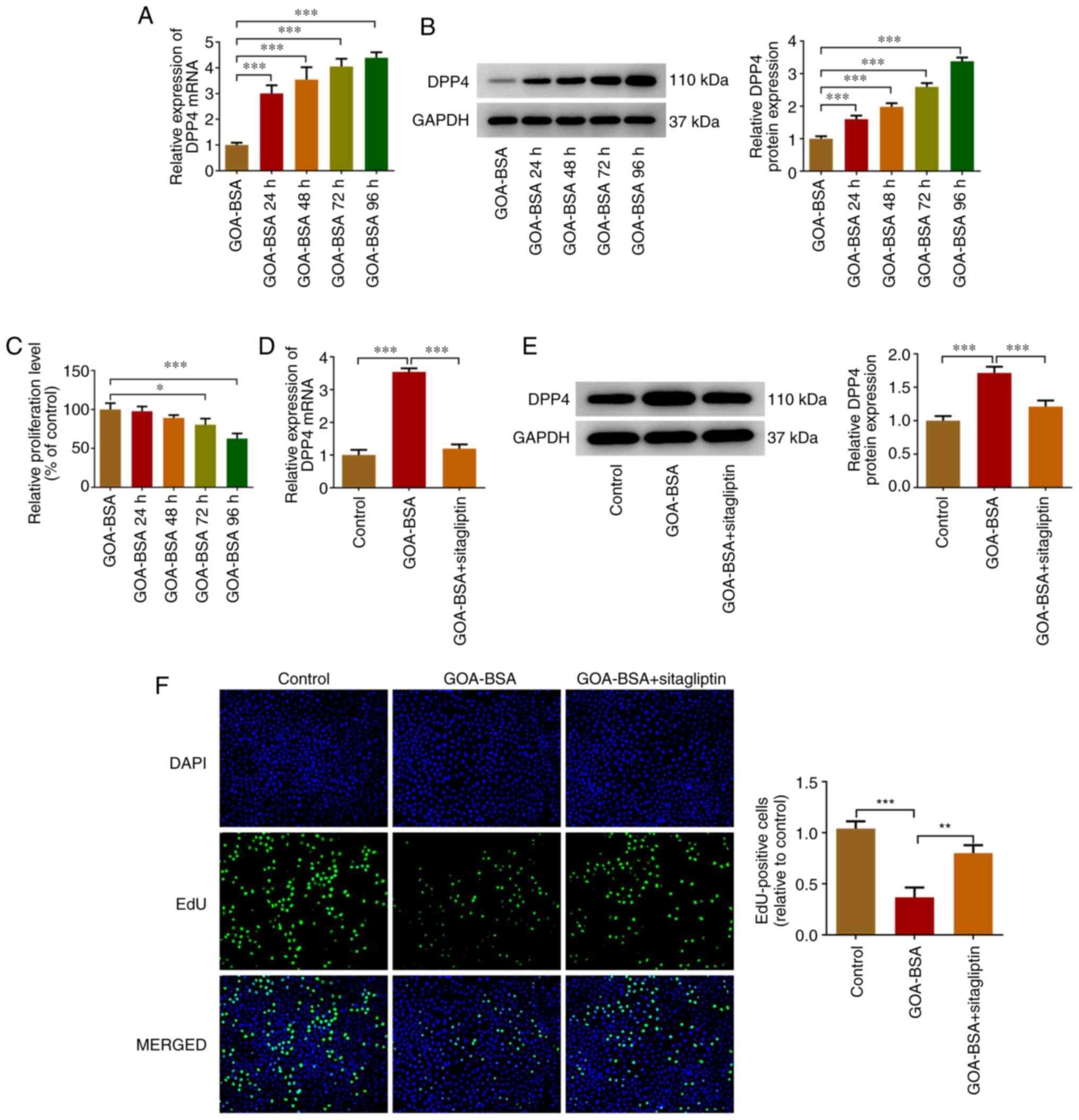
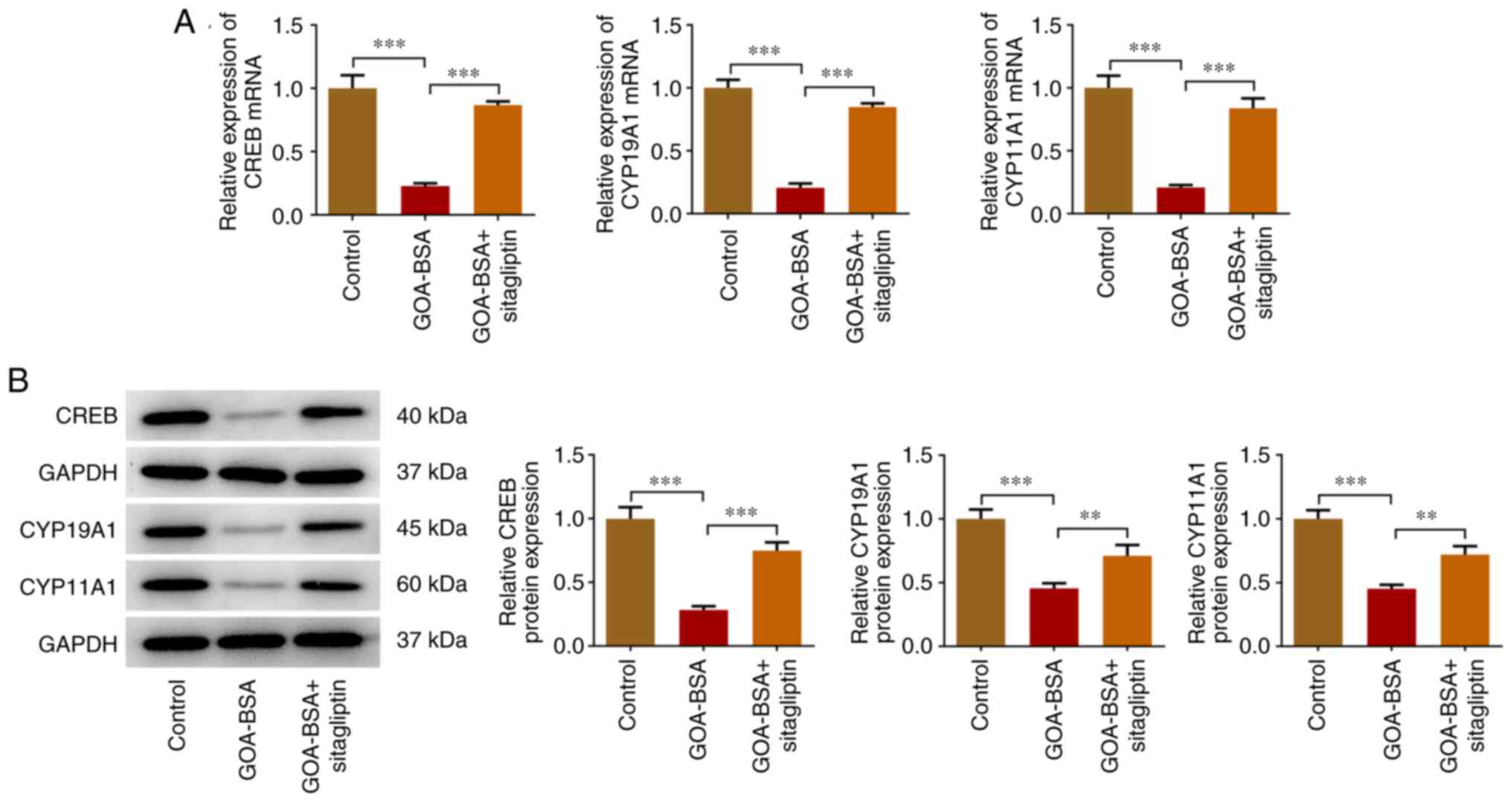
GOA-BSA upregulates DPP4 expression
and inhibits proliferation of KGN cells, whereas sitagliptin
reverses these effects
Previous studies have demonstrated that AGEs are
closely associated with the pathogenesis of PCOS as well as its
reproductive consequences (18,19). Additionally, DPP4 expression was
increased in KGN cells and in serum samples derived from rats with
PCOS (19,20). Toxic AGEs were prepared by long
term-incubation of GOA with BSA (22). Therefore, GOA-BSA was used to
treat KGN cells. DPP4 expression was increased following treatment
of the cells with GOA-BSA and this process occurred in a
time-dependent manner (Fig. 4A and
B). The CCK-8 assay indicated that treatment of KGN cells with
GOA-BSA decreased their proliferation in a time-dependent manner
(Fig. 4C). Subsequently, 96 h was
selected as the action time of GOA-BSA considering that GOA-BSA
treatment for 96 h resulted in the largest effect (Fig. 4A-C). Increased DPP4 expression was
decreased following the addition of sitagliptin, which is an
inhibitor of DPP4 (Fig. 4D and
E). In addition, the proliferation of KGN cells in GOA-BSA
group was increased following concomitant treatment of the cells
with GOA-BSA and stimulation by sitagliptin, suggesting that the
latter partly abolished the inhibitory effects of GOA-BSA on KGN
cells (Fig. 4F).
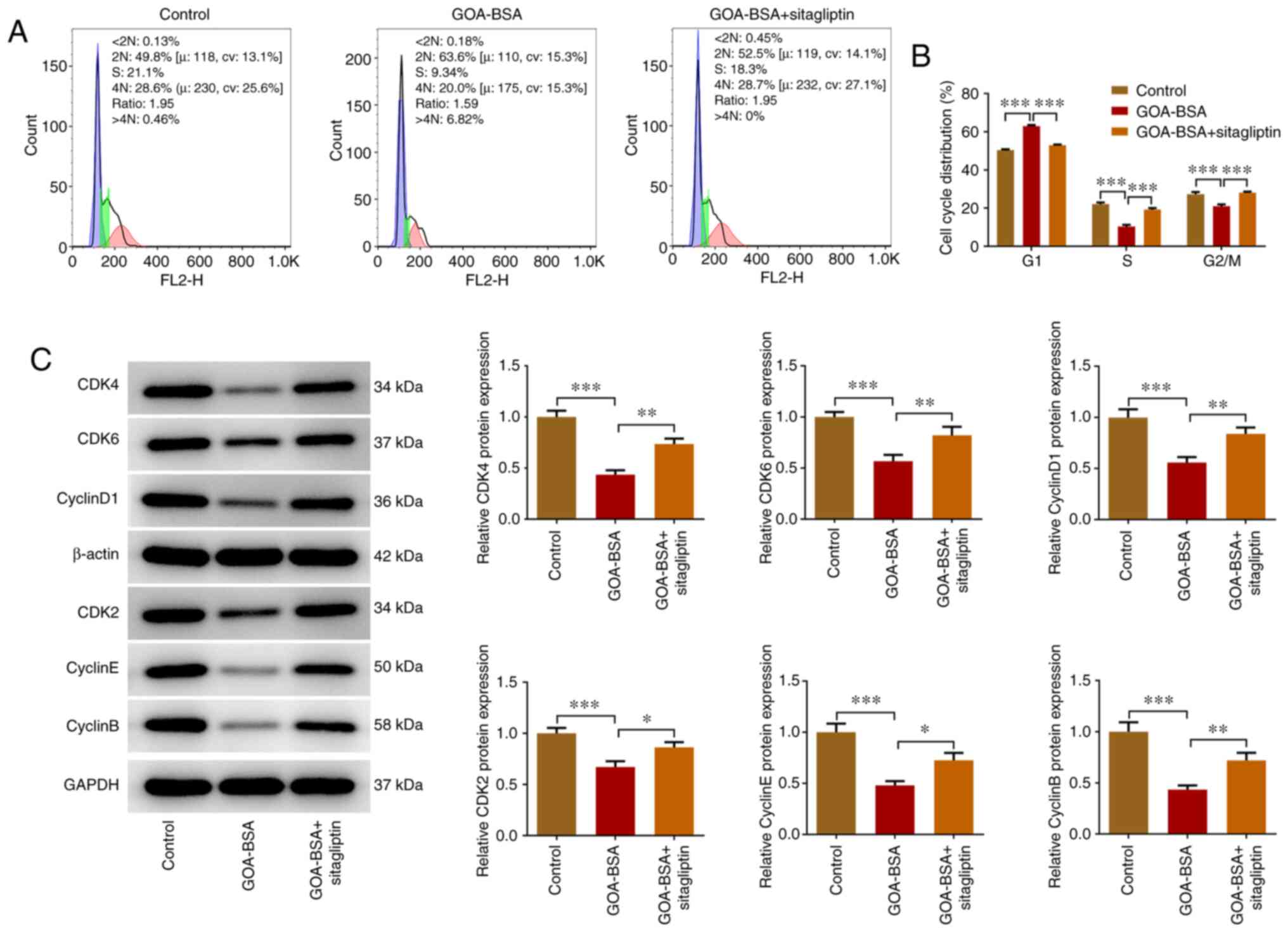
Suppression of DPP4 expression
alleviates the inhibitory effects of GOA-BSA on the cell cycle of
KGN cells
The number of cells in the S and G2/M
phases was decreased following their treatment with GOA-BSA, while
the addition of sitagliptin alleviated the inhibitory effects of
GOA-BSA (Fig. 5A and B). In
contrast to these observations, the increased number of cells at
the G1 phase was diminished by sitagliptin treatment. In
addition, sitagliptin promoted the effects on the cell
cycle-associated proteins, as demonstrated by the increased
expression levels of CDK4, CDK6, cyclin D1, CDK2, cyclin E and
cyclin B compared with those noted in the GOA-BSA group (Fig. 5C).
Suppression of DPP4 expression
upregulates the expression levels of CREB, CYP19A1 and CYP11A1 in
GOA-BSA-treated KGN cells
The mRNA and protein expression levels of CREB,
CYP19A1 and CYP11A1 were significantly decreased due to GOA-BSA
treatment compared with those noted in the control group. However,
the addition of sitagliptin partially increased the expression
levels of CREB, CYP19A1 and CYP11A1, indicating that it could
abolish the inhibitory effects of GOA-BSA on KGN cells (Fig. 6A and B).
Discussion
PCOS is a common endocrinopathy affecting a large
part of the global population. It contributes to ovulatory
infertility in women, accounting for 75% of non-ovulatory infertile
women of childbearing age (38,39). It has been reported that one in
5-6 women has severe infertility and irregular menstrual cycles
(40). In recent years, PCOS has
been a research hotspot and numerous studies have been conducted to
examine its mechanism of action (6–10).
However, a limited number of studies have investigated the role of
DPP4 in the development of PCOS. Therefore, the aim of the present
study was to detect DPP4 expression and explore its role in PCOS.
First, a PCOS model was established in rats via intragastrical
administration of letrozole (26). PCOS is commonly manifested as
ovulatory dysfunction, clinical and biochemical excess of androgen
levels, and polycystic ovaries (41). In the present study, disarranged
structure of ovarian tissues, ovarian cystic expansion and a
significant drop in the number of granular cells were observed in
the ovary of PCOS rats, suggesting the successful establishment of
the PCOS animal model. Subsequently, DPP4 expression was detected
in serum samples derived from rats with PCOS and ovarian granulosa
cells, indicating that the expression levels of DPP4 were
significantly upregulated in this model. To further assess the
effects of DPP4 on PCOS, KGN cells were transfected with siRNA-DPP4
and the data indicated that knockdown of DPP4 expression promoted
the proliferation and cell cycle progression of KGN cells and the
induction of CREB, CYP19A1 and CYP11A1 expression.
DPP4 is a ubiquitous multifunctional type II
transmembrane protease and a member of the S9 protease family
(29,30). It was initially identified in 1963
(42,43). DPP4 has been revealed to
participate in several biological processes, such as inflammation
and tumor immunity (44,45). The mRNA and protein expression
levels of DPP4 have notably been revealed to be increased in the
ovaries (43). Jensterle et
al (46) reported that DPP4
expression was increased in PCOS and that treatment of the cells
with DPP4 inhibitors, such as alogliptin and pioglitazone, could be
a therapeutic therapy for the treatment of this condition. In
addition, DPP4 expression was revealed to be upregulated in rats
with PCOS (47). In the present
study, RT-qPCR and western blot analyses indicated that DPP4
expression was significantly increased in ovarian granulosa cells,
which was consistent with the results reported in the
aforementioned studies.
AGEs, also known as ‘glycotoxins’, are the products
of non-enzymatic glycation and oxidation of proteins and lipids
that are formed during endogenous and exogenous reactions (48–50). A previous study demonstrated that
AGEs were an important player in aging, diabetes, atherosclerosis,
female fertility and the pathogenesis of cancer (48). Azhary et al (51) reported that AGEs were accumulated
in granulosa cells from patients with PCOS. AGEs were also revealed
to be associated with the pathogenesis of PCOS (52,53). In addition, it has been
demonstrated that induction of AGEs stimulates the release of DPP4
from endothelial cells (54). In
the present study, it was revealed that AGEs (GOA-BSA) increased
the expression levels of DPP4 and inhibited the proliferation of
KGN cells in a time-dependent manner.
Sitagliptin is an effective selective inhibitor of
DPP4 specifically designed to inhibit the DPP4 enzyme (55). Ferjan et al (56) demonstrated that sitagliptin was an
optimal therapeutic strategy for the treatment of PCOS.
Furthermore, a recent study provided encouraging data on a
beneficial effect of sitagliptin in reproduction, demonstrating
that sitagliptin could improve the maturation of oocytes and
quality of embryos in PCOS patients undergoing intracytoplasmic
sperm injection (57). In the
present study, increased expression of DPP4 caused by treatment of
the cells with GOA-BSA was decreased following application of
sitagliptin. The proliferation of GOA-BSA-treated KGN cells was
diminished, while the addition of sitagliptin partially reversed
the effects of GOA-BSA on KGN cells. In addition, sitagliptin
alleviated the cell cycle of GOA-BSA-treated KGN cells, while
upregulating the expression levels of the cell cycle-associated
proteins. Furthermore, sitagliptin promoted the transcription of
aromatase in GOA-BSA-induced KGN cells, as demonstrated by the
increased expression levels of CREB, CYP19A1 and CYP11A1. However,
the present study contains certain limitations, such as the
verification of the dose and safety of sitagliptin along with the
repeated experiments using rat primary granular cells and the PCOS
rat model. Therefore, further studies are required to confirm and
expand these conclusions. In addition, to improve the reliability
of these preliminary results, further studies may be performed to
evaluate other aspects such as apoptosis, autophagy and DNA
methylation, in addition to the vitality/proliferation/cell cycle
in DPP4-silenced KGN cells.
In summary, the present study indicated that the
expression levels of DPP4 were upregulated in rats with PCOS.
Treatment of KGN cells with GOA-BSA decreased cell proliferation,
the expression levels of the cell cycle-associated proteins and the
transcription of aromatase. These effects were reversed following
treatment of the cells with sitagliptin. The present study, to the
best of our knowledge, for the first time demonstrated the
protective effect of DPP4 inhibitor, sitagliptin, against PCOS,
providing evidence for the potential value of sitagliptin in
serving as an ideal therapy for the treatment of PCOS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LW contributed to study conception and design. LL
and LW contributed to acquisition, analysis and interpretation of
data. LL drafted the initial manuscript and LW revised it
critically for important intellectual content. LL and LW confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The experiments were conducted in strict accordance
with the Guidelines for the Care and Use of Laboratory Animals and
the ‘3R’ principle. All experiments received approval from the
Experimental Animal Ethics Committee of Zhaofenghua Biological
Technology Co., Ltd. (Nanjing, China; approval no.
IACUC-20201012-16).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Meier RK: Polycystic ovary syndrome. Nurs
Clin North Am. 53:407–420. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nandi A, Chen Z, Patel R and Poretsky L:
Polycystic ovary syndrome. Endocrinol Metab Clin North Am.
43:123–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goodarzi MO, Dumesic DA, Chazenbalk G and
Azziz R: Polycystic ovary syndrome: Etiology, pathogenesis and
diagnosis. Nat Rev Endocrinol. 7:219–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferriman D and Purdie AW: The aetiology of
oligomenorrhoea and/or hirsuties: A study of 467 patients. Postgrad
Med J. 59:17–20. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balen AH, Conway GS, Kaltsas G,
Techatrasak K, Manning PJ, West C and Jacobs HS: Polycystic ovary
syndrome: The spectrum of the disorder in 1741 patients. Hum
Reprod. 10:2107–2111. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DeUgarte CM, Bartolucci AA and Azziz R:
Prevalence of insulin resistance in the polycystic ovary syndrome
using the homeostasis model assessment. Fertil Steril.
83:1454–1460. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krentz AJ, von Muhlen D and Barrett-Connor
E: Searching for polycystic ovary syndrome in postmenopausal women:
Evidence of a dose-effect association with prevalent cardiovascular
disease. Menopause. 14:284–292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jedel E, Waern M, Gustafson D, Landén M,
Eriksson E, Holm G, Nilsson L, Lind AK, Janson PO and
Stener-Victorin E: Anxiety and depression symptoms in women with
polycystic ovary syndrome compared with controls matched for body
mass index. Hum Reprod. 25:450–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barthelmess EK and Naz RK: Polycystic
ovary syndrome: Current status and future perspective. Front Biosci
(Elite Ed). 6:104–119. 2014.PubMed/NCBI
|
|
10
|
Rothenberg SS, Beverley R, Barnard E,
Baradaran-Shoraka M and Sanfilippo JS: Polycystic ovary syndrome in
adolescents. Best Pract Res Clin Obstet Gynaecol. 48:103–114. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nargis T and Chakrabarti P: Significance
of circulatory DPP4 activity in metabolic diseases. IUBMB Life.
70:112–119. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baumeier C, Schlüter L, Saussenthaler S,
Laeger T, Rödiger M, Alaze SA, Fritsche L, Häring HU, Stefan N,
Fritsche A, et al: Elevated hepatic DPP4 activity promotes insulin
resistance and non-alcoholic fatty liver disease. Mol Metab.
6:1254–1263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawasaki T, Chen W, Htwe YM, Tatsumi K and
Dudek SM: DPP4 inhibition by sitagliptin attenuates LPS-induced
lung injury in mice. Am J Physiol Lung Cell Mol Physiol.
315:L834–l845. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ozturk B, Gurbuz AS, Durak ZE and Ozturk
HS: Dipeptidyl peptidase-4 and adenosine deaminase enzyme levels in
polycystic ovary syndrome. Gynecol Endocrinol. 35:138–141. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wawrzkiewicz-Jałowiecka A, Kowalczyk K,
Trybek P, Jarosz T, Radosz P, Setlak M and Madej P: In search of
New therapeutics-molecular aspects of the PCOS pathophysiology:
Genetics, hormones, metabolism and beyond. Int J Mol Sci.
21:70542020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamagishi S, Nakamura N, Suematsu M,
Kaseda K and Matsui T: Advanced glycation end products: A molecular
target for vascular complications in diabetes. Mol Med. 21 (Suppl
1):S32–S40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishibashi Y, Matsui T, Maeda S,
Higashimoto Y and Yamagishi S: Advanced glycation end products
evoke endothelial cell damage by stimulating soluble dipeptidyl
peptidase-4 production and its interaction with mannose
6-phosphate/insulin-like growth factor II receptor. Cardiovasc
Diabetol. 12:1252013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaifu K, Ueda S, Nakamura N, Matsui T,
Yamada-Obara N, Ando R, Kaida Y, Nakata M, Matsukuma-Toyonaga M,
Higashimoto Y, et al: Advanced glycation end products evoke
inflammatory reactions in proximal tubular cells via autocrine
production of dipeptidyl peptidase-4. Microvasc Res. 120:90–93.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Diamanti-Kandarakis E, Alexandraki K,
Piperi C, Aessopos A, Paterakis T, Katsikis I and Panidis D: Effect
of metformin administration on plasma advanced glycation end
product levels in women with polycystic ovary syndrome. Metabolism.
56:129–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Merhi Z, Kandaraki EA and
Diamanti-Kandarakis E: Implications and future perspectives of AGEs
in PCOS pathophysiology. Trends Endocrinol Metab. 30:150–162. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tahara N, Yamagishi S, Takeuchi M, Tahara
A, Kaifu K, Ueda S, Okuda S and Imaizumi T: Serum levels of
advanced glycation end products (AGEs) are independently correlated
with circulating levels of dipeptidyl peptidase-4 (DPP-4) in
humans. Clin Biochem. 46:300–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miki Y, Dambara H, Tachibana Y, Hirano K,
Konishi M and Beppu M: Macrophage recognition of toxic advanced
glycosylation end products through the macrophage surface-receptor
nucleolin. Biol Pharm Bull. 37:588–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kafali H, Iriadam M, Ozardali I and Demir
N: Letrozole-induced polycystic ovaries in the rat: A new model for
cystic ovarian disease. Arch Med Res. 35:103–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Research Council. Subcommittee on
Amphibian Standards. Amphibians: Guidelines for the breeding, care,
and management of laboratory animals. National Academies Press;
Washington, DC: 1974, PubMed/NCBI
|
|
25
|
Flecknell P: Replacement, reduction and
refinement. Altex. 19:73–78. 2002.PubMed/NCBI
|
|
26
|
Zhang S, Tu H, Yao J, Le J, Jiang Z, Tang
Q, Zhang R, Huo P and Lei X: Combined use of Diane-35 and metformin
improves the ovulation in the PCOS rat model possibly via
regulating glycolysis pathway. Reprod Biol Endocrinol. 18:582020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Torres PJ, Skarra DV, Ho BS, Sau L, Anvar
AR, Kelley ST and Thackray VG: Letrozole treatment of adult female
mice results in a similar reproductive phenotype but distinct
changes in metabolism and the gut microbiome compared to pubertal
mice. BMC Microbiol. 19:572019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Venegas B, De León Gordillo LY, Rosas G,
Espinoza JA, Morán C, Domínguez R and Morales-Ledesma L: In rats
with estradiol valerate-induced polycystic ovary syndrome, the
acute blockade of ovarian β-adrenoreceptors improve ovulation.
Reprod Biol Endocrinol. 17:952019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marcondes FK, Bianchi FJ and Tanno AP:
Determination of the estrous cycle phases of rats: Some helpful
considerations. Braz J Biol. 62:609–614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He T, Sun Y, Zhang Y, Zhao S, Zheng Y, Hao
G and Shi Y: MicroRNA-200b and microRNA-200c are up-regulated in
PCOS granulosa cell and inhibit KGN cell proliferation via
targeting PTEN. Reprod Biol Endocrinol. 17:682019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishi Y, Yanase T, Mu Y, Oba K, Ichino I,
Saito M, Nomura M, Mukasa C, Okabe T, Goto K, et al: Establishment
and characterization of a steroidogenic human granulosa-like tumor
cell line, KGN, that expresses functional follicle-stimulating
hormone receptor. Endocrinology. 142:437–445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yi S, Zheng B, Zhu Y, Cai Y, Sun H and
Zhou J: Melatonin ameliorates excessive PINK1/Parkin-mediated
mitophagy by enhancing SIRT1 expression in granulosa cells of PCOS.
Am J Physiol Endocrinol Metab. 319:E91–E101. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Geng X, Zhao J, Huang J, Li S, Chu W, Wang
WS, Chen ZJ and Du Y: lnc-MAP3K13-7:1 inhibits ovarian GC
proliferation in PCOS via DNMT1 downregulation-mediated CDKN1A
promoter hypomethylation. Mol Ther. 29:1279–1293. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abouzeid AH and Torchilin VP: The role of
cell cycle in the efficiency and activity of cancer nanomedicines.
Expert Opin Drug Deliv. 10:775–786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakanishi M: Regulation of cell cycle
checkpoints in mammalian cells. Seikagaku. 73:343–350. 2001.(In
Japanese). PubMed/NCBI
|
|
37
|
Barnum KJ and O'Connell MJ: Cell cycle
regulation by checkpoints. Methods Mol Biol. 1170:29–40. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Q, Jiang J, Shi Y, Mo Z and Li M:
Apelin/Apelin receptor: A new therapeutic target in polycystic
ovary syndrome. Life Sci. 260:1183102020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khan MJ, Ullah A and Basit S: Genetic
basis of polycystic ovary syndrome (PCOS): Current perspectives.
Appl Clin Genet. 12:249–260. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ajmal N, Khan SZ and Shaikh R: Polycystic
ovary syndrome (PCOS) and genetic predisposition: A review article.
Eur J Obstet Gynecol Reprod Biol X. 3:1000602019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abdalla M, Deshmukh H, Atkin SL and
Sathyapalan T: miRNAs as a novel clinical biomarker and therapeutic
targets in polycystic ovary syndrome (PCOS): A review. Life Sci.
259:1181742020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Braga LDDC, Godoy-Matos AF, Siciliano PO,
Corrêa JODA and Carvalho DP: Is DPP4 activity increased in PCOS?
Diabetes Metab Syndr. 12:673–675. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang F, Zhang ZF, He YR, Wu HY and Wei SS:
Effects of dipeptidyl peptidase-4 inhibitors on transforming growth
factor-beta1 signal transduction pathways in the ovarian fibrosis
of polycystic ovary syndrome rats. J Obstet Gynaecol Res.
45:600–608. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kajiyama H, Kikkawa F, Maeda O, Suzuki T,
Ino K and Mizutani S: Increased expression of dipeptidyl peptidase
IV in human mesothelial cells by malignant ascites from ovarian
carcinoma patients. Oncology. 63:158–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Barreira da Silva R, Laird ME, Yatim N,
Fiette L, Ingersoll MA and Albert ML: Dipeptidylpeptidase 4
inhibition enhances lymphocyte trafficking, improving both
naturally occurring tumor immunity and immunotherapy. Nat Immunol.
16:850–858. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jensterle M, Goricar K and Janez A: Add on
DPP-4 inhibitor alogliptin alone or in combination with
pioglitazone improved β-cell function and insulin sensitivity in
metformin treated PCOS. Endocr Res. 42:261–268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Y, Hu M, Jia W, Liu G, Zhang J, Wang
B, Li J, Cui P, Li X, Lager S, et al: Hyperandrogenism and insulin
resistance modulate gravid uterine and placental ferroptosis in
PCOS-like rats. J Endocrinol. 246:247–263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rutkowska AZ and Diamanti-Kandarakis E: Do
advanced glycation end products (AGEs) Contribute to the
comorbidities of polycystic ovary syndrome (PCOS)? Curr Pharm Des.
22:5558–5571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Garg D and Merhi Z: Relationship between
advanced glycation end products and steroidogenesis in PCOS. Reprod
Biol Endocrinol. 14:712016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Diamanti-Kandarakis E, Katsikis I, Piperi
C, Kandaraki E, Piouka A, Papavassiliou AG and Panidis D: Increased
serum advanced glycation end-products is a distinct finding in lean
women with polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf).
69:634–641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Azhary JMK, Harada M, Kunitomi C, Kusamoto
A, Takahashi N, Nose E, Oi N, Wada-Hiraike O, Urata Y, Hirata T, et
al: Androgens increase accumulation of advanced glycation end
products in granulosa cells by activating ER stress in PCOS.
Endocrinology. 161:bqaa0152020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pertynska-Marczewska M,
Diamanti-Kandarakis E, Zhang J and Merhi Z: Advanced glycation end
products: A link between metabolic and endothelial dysfunction in
polycystic ovary syndrome? Metabolism. 64:1564–1573. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Diamanti-Kandarakis E, Piperi C, Patsouris
E, Korkolopoulou P, Panidis D, Pawelczyk L, Papavassiliou AG and
Duleba AJ: Immunohistochemical localization of advanced glycation
end-products (AGEs) and their receptor (RAGE) in polycystic and
normal ovaries. Histochem Cell Biol. 127:581–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yamagishi S, Fukami K and Matsui T:
Crosstalk between advanced glycation end products (AGEs)-receptor
RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic
vascular complications. Cardiovasc Diabetol. 14:22015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Herman GA, Bergman A, Stevens C, Kotey P,
Yi B, Zhao P, Dietrich B, Golor G, Schrodter A, Keymeulen B, et al:
Effect of single oral doses of sitagliptin, a dipeptidyl
peptidase-4 inhibitor, on incretin and plasma glucose levels after
an oral glucose tolerance test in patients with type 2 diabetes. J
Clin Endocrinol Metab. 91:4612–4619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ferjan S, Janez A and Jensterle M: DPP4
inhibitor sitagliptin as a potential treatment option in
metformin-intolerant obese Women with polycystic ovary syndrome: A
pilot randomized study. Endocr Pract. 24:69–77. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Daneshjou D, Zadeh Modarres S, Soleimani
Mehranjani M and Shariat Zadeh SMA: Comparing the effect of
sitagliptin and metformin on the oocyte and embryo quality in
classic PCOS patients undergoing ICSI. Ir J Med Sci. 190:685–692.
2021. View Article : Google Scholar : PubMed/NCBI
|