Introduction
Osteoclasts are multinucleated cells formed by the
fusion of hematopoietic bone marrow precursors and are typically
present in the bone marrow adjacent to the bone surface (1). Osteoclasts play a pivotal role in
bone resorption in several bone-related diseases, such as
rheumatoid arthritis and periodontitis (2,3).
Receptor activator of NF-κB ligand (RANKL), macrophage colony
stimulating factor (M-CSF) and tumor necrosis factor (TNF)-α are
cytokines known to promote osteoclastogenesis in vitro and
in vivo (4–6).
Lipopolysaccharide (LPS) can induce inflammatory
cytokine production and pathological bone loss (7). Various inflammatory cytokines
induced by LPS, such as TNF-α, play important roles in the
maturation of osteoclast progenitors (8,9).
These cytokines are related to osteoclastogenesis and bone
resorption induced by LPS in vitro and in vivo
(10). Furthermore, LPS may also
lead to osteoclastogenesis and promote the fusion and survival of
osteoclasts (11). In addition,
the expression of RANKL in osteoblasts is stimulated by LPS
(12).
Chemokines, which are various small chemotactic
cytokines, are potentially related to the physiological
development, pathological recruitment and function of osteoclasts
(13–15). C-X-C motif chemokine ligand 12
(CXCL12) is widely recognized as stromal cell-derived factor 1 and
belongs to the CXC chemokine family. CXCL12 is a 68-residue
chemokine with a molecular weight of 8 kDa that exists in both
secreted and membrane-bound forms, and is abundantly expressed in
bone marrow and several other tissues (16,17). CXCL12 has strong chemotactic
effects on lymphocytes and has been shown to be associated with
osteoclast progenitor cell survival, function and fusion (18,19).
The crucial roles of CXCR4 and its ligand CXCL12
have been extensively studied (20). CXCR4 is a 352-residue G
protein-coupled receptor with seven transmembrane helices (21). CXCR4 is broadly expressed by both
mononuclear cells and progenitor cells in the bone marrow (18). CXCL12 was shown to indirectly
increase osteoclastogenesis and bone resorption in vivo by
LPS-stimulated TNF-α in macrophages, and LPS-enhanced RANKL in
osteoblasts in mice injected with LPS (22). Furthermore, CXCL12 was shown to
directly enhance both RANKL- and TNF-α-induced osteoclastogenesis
(22). As shown in studies using
the CXCR4 antagonist, AMD3100, the interaction of CXCL12 and CXCR4
induces osteoclastogenesis and regulates osteoclast function
(18,23,24).
CXCR7 is a G protein-coupled receptor with seven
membrane-spanning helices (16).
A previous study showed that a CXCR7 agonist negatively regulates
CXCL12-CXCR4-induced cellular events, such as angiogenesis
(25). However, the functions and
roles of CXCR7 in this process remain unclear. To understand the
mechanisms of bone resorption relevant to disease, it is important
to investigate the role of CXCR7 agonists in LPS-induced
osteoclastogenesis. However, to the best of our knowledge, there
have been no studies to evaluate the effects of CXCR7 agonists on
osteoclastogenesis induced by LPS in vivo. Therefore, the
present study was performed to investigate the effects of the CXCR7
agonist, VUF11207, on LPS-induced osteoclastogenesis in an animal
model in vivo.
Materials and methods
Reagents and animals
A total of 20 8–10-week-old healthy 20–25 g male
C57BL/6J mice (wild-type/WT) purchased from CLEA Japan, Inc., were
used. Mice were kept in cages that were maintained at 25°C, 50%
relative humidity under a 12 h light/dark cycle with free access to
food and water. A total of five mice were assigned to each
experimental group by simple random sampling. All experimental
procedures conformed to ‘Regulations for Animal Experiments and
Related Activities at Tohoku University’, and were reviewed by the
Institutional Laboratory Animal Care and Use Committee of Tohoku
University and approved by the President of Tohoku University
(approval no. 2019DnA-047-05; Miyagi, Japan).
CXCL12 was obtained from R&D Systems, Inc. The
CXCR7 agonist, VUF11207, was purchased from MilliporeSigma. LPS
from Escherichia coli was purchased from Sigma-Aldrich
(Merck KGaA). Recombinant mouse RANKL (26) and TNF-α (27) were produced as described
previously. Recombinant mouse M-CSF was produced by the
M-CSF-expressing cell line, CMG14-12, as described previously
(28).
Mouse experiments and histological examination. Mice
in each group received subcutaneous injections over the crown of
the head for 5 days with one of the following: i)
Phosphate-buffered saline (PBS, 100 µl); ii) LPS (100 µg/day); iii)
LPS (100 µg/day) + CXCR7 agonist (100 µg/day); or iv) CXCR7 agonist
(100 µg/day).
The mice were sacrificed by inhalation of an
overdose of 5% isoflurane on day 6. Inhalation was continued until
a pulse could not be detected, breathing had ceased and there was
an absence of reflexes observed in combination. The calvariae of
mice were isolated and cut into three pieces. After fixation with
4% formaldehyde in PBS at 4°C for 3 days, the samples were
demineralized in 14% EDTA for 3 days. The samples were embedded in
paraffin blocks and cut into sections 5-µm thick using a microtome
(REM-710; Yamato Kohki Industrial Co., Ltd.). The sections were
stained for tartrate-resistant acid phosphatase (TRAP) and
counterstained with hematoxylin according to the protocol described
previously (22,29). Osteoclasts were recognized as
TRAP-positive cells with more than three nuclei. The number of
TRAP-positive cells (cells/section) was counted in the sagittal
sutures of calvariae according to previously described methods
(22,29,30).
Measurement of bone destruction
Mice were injected as described above and sacrificed
on day 6. Bone destruction was assessed by micro-computed
tomography (CT) (ScanXmate-E090; Comscantecno Co., Ltd.). The
dissected calvariae were fixed with 4% formaldehyde in PBS at 4°C
for 3 days. The calvariae were scanned by micro-CT to create
three-dimensional images using TRI/3D-BON64 version R7.00 software
(Ratoc System Engineering Co., Ltd.). The bone resorption areas
were measured around the bregma 45 pixels in the sagittal plane and
50 pixels in the coronal plane. The bone resorption areas (%) to
total areas were measured using ImageJ version 1.51 (National
Institutes of Health) as described previously (22,29,30). Shaded areas of the same color
density were considered bone resorption areas.
Preparation of RNA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Mice received subcutaneous injections into the crown
of the head for 5 days as described above. The mice were then
sacrificed, and their calvariae were isolated, frozen in liquid
nitrogen and homogenized (Micro Smash MS-100R; Tomy Seiko Co.,
Ltd.). Total RNA was obtained from samples using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
purified by the RNeasy Mini Kit (Qiagen, Inc.). After purification
of total RNA, cDNA was synthesized from each total RNA sample (2
µg) with oligo(dT) primers by using the SuperScript IV First-Strand
Synthesis System according to the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc.). The levels of TRAP,
Cathepsin K, RANKL and TNF-α transcripts were quantified by qPCR
(Thermal Cycler Dice Real Time system; Takara Bio, Inc.). The
reaction consisted of a volume of 25 µl containing 2 µl cDNA as a
template, 23 µl TB Green Premix Ex Taq II (Takara Bio, Inc.) and 50
pmol/µl each primer. The thermocycling conditions consisted of an
initial denaturation step at 95°C for 10 sec, followed by 50 cycles
of denaturation for 5 sec at 95°C and annealing for 30 sec at 60°C.
The levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA
were used for normalization. Relative expression of mRNA was
analyzed by the 2−ΔΔCq method (31). All primers were designed by our
laboratory (Division of Orthodontics and Dentofacial Orthopedics,
Tohoku University Graduate School of Dentistry). The primers used
for analysis are listed in Table
I. Preparation of osteoclast precursors and cultures for
osteoclastogenesis. Mouse bone marrow cells were used for the in
vitro study. After sacrifice, the femora and tibiae of mice
were dissected aseptically. Both ends of these bones were cut off
to obtain bone marrow cells. Bone marrow cells were seeded
(5×106 cells) into 10-cm culture dishes with α-modified
minimal essential medium (α-MEM; FUJIFILM Wako Pure Chemical
Corporation) containing 100 ng/ml M-CSF, 10% fetal bovine serum
(FBS; Biowest, Inc.), 100 IU/ml penicillin G (Meiji Seika Kaisha,
Ltd.) and 100 µg/ml streptomycin (Meiji Seika Kaisha, Ltd.). Bone
marrow cells were incubated at 37°C in 5% CO2 for 4
days. Floating cells were eliminated by rinsing with PBS. After
elimination of floating cells, adherent cells were detached using
trypsin/EDTA solution (Sigma-Aldrich; Merck KGaA) and collected.
The obtained cells were recognized as osteoclast precursors
(22,29,30). Osteoclast precursors were seeded
into 96-well plates and cultured in an atmosphere of 5%
CO2 at 37°C for 5 days containing the following for
RANKL analysis: i) M-CSF (100 ng/ml); ii) M-CSF (100 ng/ml) + RANKL
(50 ng/ml); iii) M-CSF (100 ng/ml) + RANKL (50 ng/ml) + CXCL12 (100
ng/ml); iv) M-CSF (100 ng/ml) + RANKL (50 ng/ml) + CXCL12 (100
ng/ml) + CXCR7 agonist (100 ng/ml); v) M-CSF (100 ng/ml) + RANKL
(50 ng/ml) + CXCR7 agonist (100 ng/ml); or vi) M-CSF (100 ng/ml) +
CXCR7 agonist (100 ng/ml). Cultures for TNF-α analysis contained
the following: i) M-CSF (100 ng/ml); ii) M-CSF (100 ng/ml) + TNF-α
(50 ng/ml); iii) M-CSF (100 ng/ml) + TNF-α (50 ng/ml) + CXCL12 (100
ng/ml); iv) M-CSF (100 ng/ml) + TNF-α (50 ng/ml) + CXCL12 (100
ng/ml) + CXCR7 agonist (100 ng/ml); v) M-CSF (100 ng/ml) + TNF-α
(50 ng/ml) + CXCR7 agonist (100 ng/ml); or vi) M-CSF (100 ng/ml) +
CXCR7 agonist (100 ng/ml). After fixation with 4% formaldehyde at
room temperature for 1 h, the cultured cells were stained with TRAP
as described previously (22,29,30). Osteoclasts were identified as
TRAP-positive cells with three or more nuclei. The number of
osteoclasts (cells/well) was counted under a light microscope.
 | Table I.Primers used in this study. |
Table I.
Primers used in this study.
| Gene | Sequence
(5′→3′) | Genbank number | Size, bp | Tm, °C |
|---|
| GAPDH | F:
GGTGGAGCCAAAAGGGTCA | XM_017321385.1 | 138 | 67.3 |
|
| R:
GGGGGCTAAGCAGTTGGT |
|
| 64.0 |
| TRAP | F:
AACTTGCGACCATTGTTA | XM_011242384.2 | 159 | 56.5 |
|
| R:
GGGGACCTTTCGTTGATGT |
|
| 63.7 |
| Cathepsin K | F:
GCAGAGGTGTGTACTATGA | BC046320.1 | 73 | 50.3 |
|
| R:
GCAGGCGTTGTTCTTATT |
|
| 57.8 |
| RANKL | F:
CCTGAGGCCCAGCCATTT | NM_011613.3 | 107 | 63.9 |
|
| R:
CTTGGCCCAGCCTCGAT |
|
| 66.5 |
| TNF-α | F:
CTGTAGCCCACGTCGTAGC | NM_013693.3 | 97 | 56.4 |
|
| R:
TTGAGATCCATGCCGTTG |
|
| 53.9 |
Immunoblotting
Osteoclast precursors prepared from bone marrow
cells were incubated for 6 h (5×106 cells) in 60-mm cell
culture dishes (Corning, Inc.) using serum-free α-MEM for culture
under conditions of serum starvation. After serum starvation for 6
h, osteoclast precursors were cultured with the following for RANKL
analysis: i) RANKL (100 ng/ml); ii) RANKL (100 ng/ml) + CXCL12 (100
ng/ml); or iii) RANKL (100 ng/ml) + CXCL12 (100 ng/ml) + CXCR7
agonist (100 ng/ml). Cultures for TNF-α analysis contained the
following: i) TNF-α (100 ng/ml); ii) TNF-α (100 ng/ml) + CXCL12
(100 ng/ml); or iii) TNF-α (100 ng/ml) + CXCL12 (100 ng/ml) + CXCR7
agonist (100 ng/ml). They were added to the dishes for specific
periods (0, 15 or 30 min). Osteoclast precursors treated with the
specified reagents were gently rinsed twice with PBS.
Radioimmunoprecipitation (RIPA) lysis buffer (MilliporeSigma) with
phosphatase inhibitor and 1% protease (Thermo Fisher Scientific,
Inc.) was added to the cell culture dishes. The cells were scraped
from the dishes. Measurement of total protein concentrations was
performed using a Pierce BCA protein assay kit (Thermo Fisher
Scientific, Inc.). β-Mercaptoethanol and Laemmli sample buffer
(Bio-Rad Laboratories, Inc.) were added to protein samples. The
samples were denatured at 95°C for 5 min for SDS-PAGE. The same
amounts of proteins (40 µg) and marker were loaded into the wells
using 4–15% Mini-PROTEAN TGX Precast Gels (Bio-Rad Laboratories,
Inc.) and the gels were run at 120 V for 1 h. The proteins were
transferred from the gels onto polyvinylidene difluoride (PVDF)
membranes using a PVDF Trans-Blot Turbo Transfer System (Bio-Rad
Laboratories, Inc.). After transfer, nonspecific binding sites on
the membranes were blocked by incubation with Block-Ace (KAC Co.,
Ltd.) at room temperature for 120 min. After blocking, the
membranes were reacted overnight at 4°C with the following primary
antibodies (all, 1:1,000): Monoclonal anti-β-actin mouse antibody
(cat. no. A1978; Sigma-Aldrich; Merck KGaA), phosphorylated
(p)-p44/42 MAPK (Erk1/2) antibody (cat. no. 9101), p44/42 (Erk1/2)
antibody (cat. no. 9102), p-p38 MAPK rabbit monoclonal antibody
(cat. no. 4511), p38 MAPK antibody cat. no. 9212, p-SAPK/JNK rabbit
monoclonal antibody (cat. no. 4671) and SAPK/JNK antibody (cat. no.
9252; Cell Signaling Technology, Inc.). The membranes were washed
with Tris buffered saline (TBS) and TBS with Triton X-100 (TBST)
with gentle agitation. The membranes were incubated at room
temperature for 60 min with HRP-conjugated anti-rabbit IgG antibody
(cat. no. 7074; Cell Signaling Technology, Inc.; 1:3,000) and
anti-mouse antibody (cat. no. NA931; GE Healthcare; 1:10,000) as
secondary antibodies. The membranes were washed in TBST and TBS
with gentle agitation. After washing, SuperSignal West Femto
Maximum Sensitivity Substrate (Thermo Fisher Scientific, Inc.) was
added and incubated for 5 min. The signals on blots were imaged
using the FUSION-FX7.EDGE Chemiluminescence Imaging System (Vilber
Lourmat) (32).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean (SEM) of more than three independent experiments. All
data were analyzed using Statcel version 3 software (OMS Publishing
Co., Ltd.). Differences between groups were examined using one-way
ANOVA followed by Bonferroni/Dunn's test. F-values are shown in
Table SI. P<0.05 was
considered to indicate a statistically significant difference.
Results
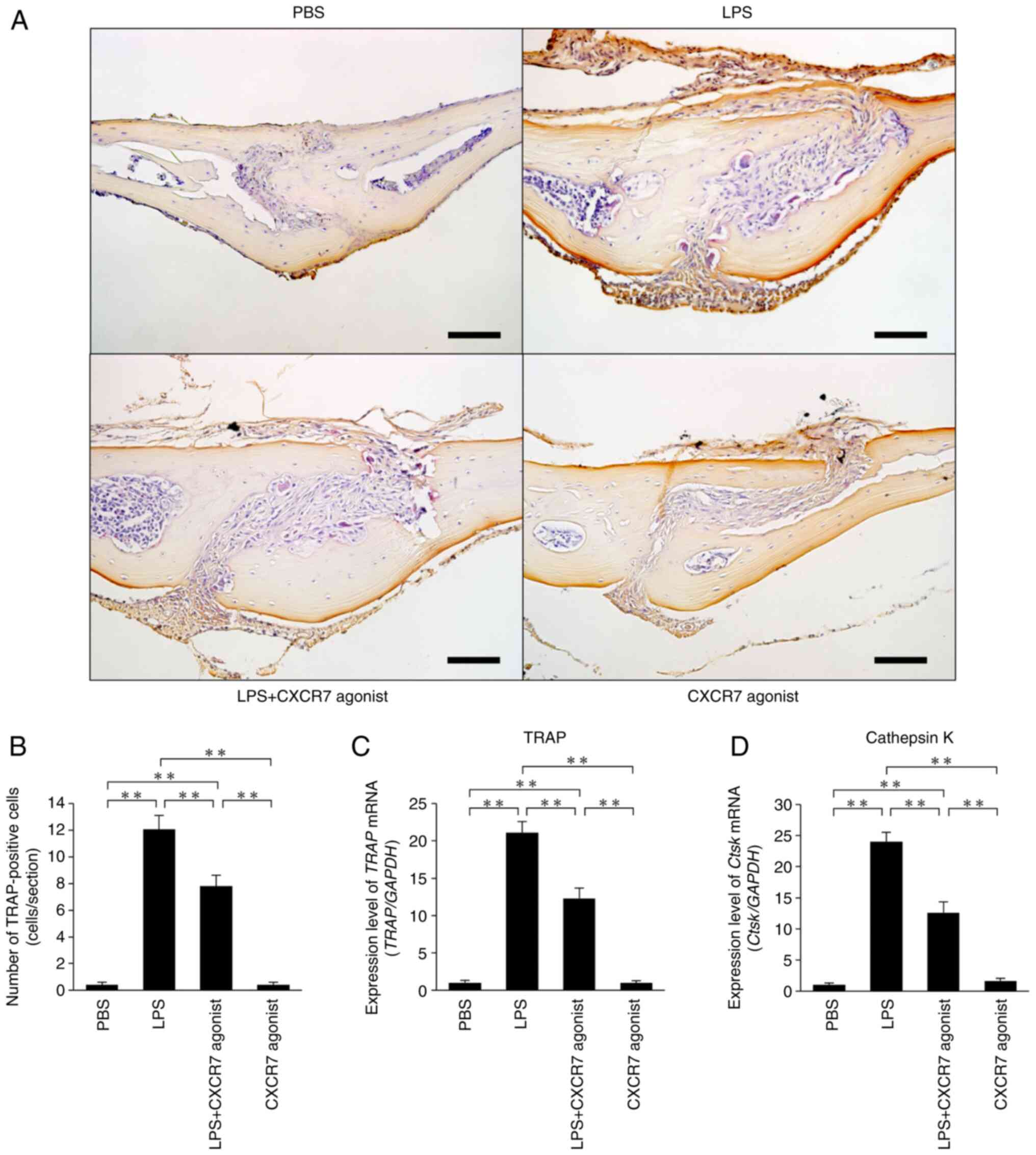
CXCR7 agonist inhibits LPS-induced
osteoclastogenesis in vivo
LPS was administered for 5 consecutive days and
calvariae were stained with TRAP to reveal osteoclast formation
(Fig. 1A). Large numbers of
osteoclasts formed in the sutures of calvariae in LPS-injected mice
compared with the PBS-injected mice. The number of osteoclasts was
significantly lower in mice treated with LPS+CXCR7 agonist than in
mice treated with LPS alone (Fig.
1B). Both TRAP and Cathepsin K mRNA expression levels were
significantly lower in mice injected with LPS+CXCR7 agonist than in
mice injected with LPS alone (Fig. 1C
and D).
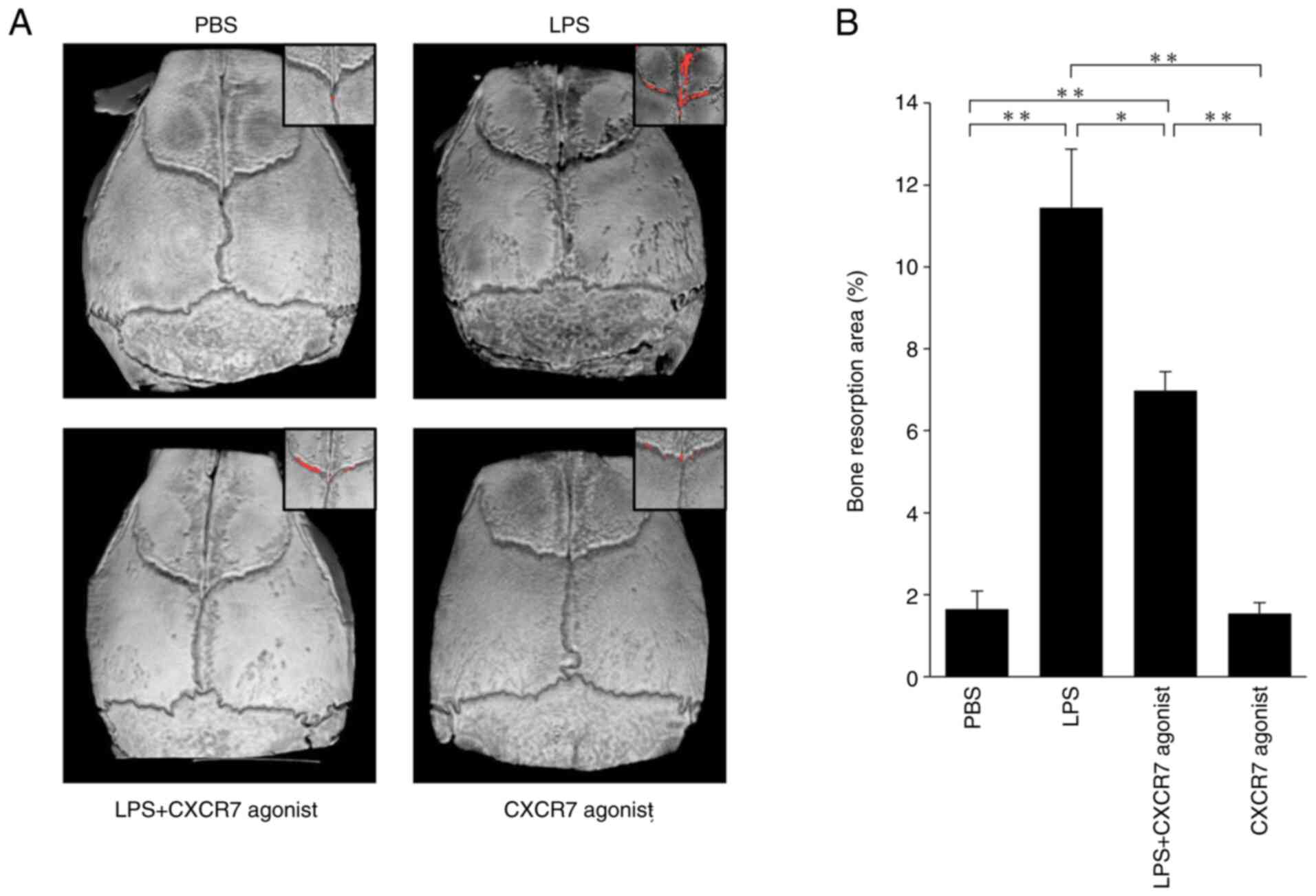
CXCR7 agonist inhibits LPS-induced
bone resorption in vivo
The bone resorption areas on mouse calvariae in each
treated mouse were evaluated by micro-CT (Fig. 2A). LPS-treated mice showed a large
area bone of resorption compared with the PBS-injected mice. The
area of bone resorption was significantly smaller in mice treated
with LPS+CXCR7 agonist than in mice injected with LPS alone
(Fig. 2B).
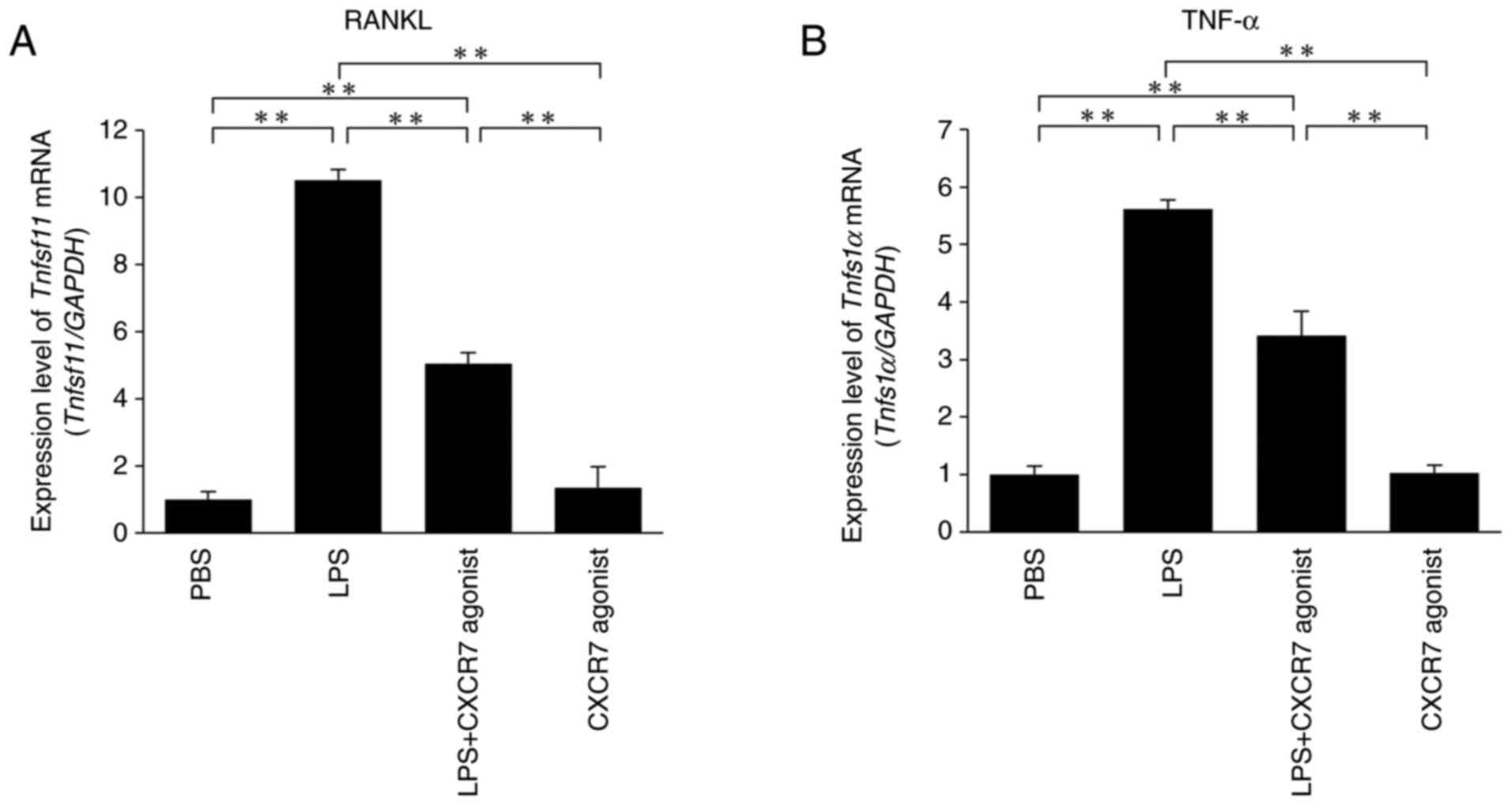
CXCR7 agonist inhibits LPS-induced
production of RANKL and TNF-α in vivo
The expression levels of RANKL and TNF-α mRNAs were
significantly increased in LPS-treated mice compared with the
PBS-injected mice. Furthermore, RANKL and TNF-α mRNA levels were
significantly lower in mice treated with LPS+CXCR7 agonist compared
with those administered with LPS alone (Fig. 3A and B).
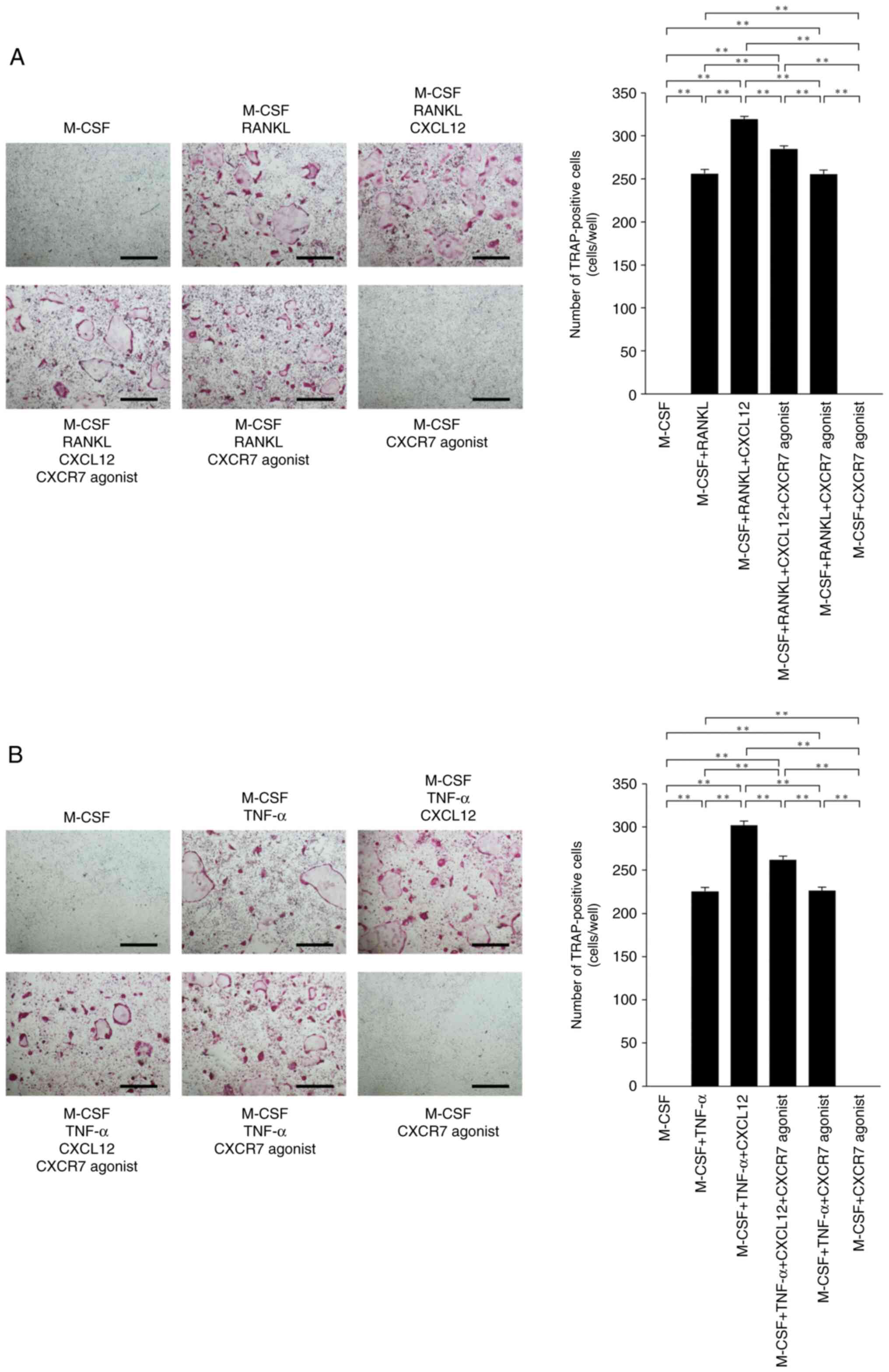
CXCR7 agonist inhibits RANKL- and
TNF-α-induced osteoclastogenesis through CXCL12 inhibition in
vitro
The effects of CXCR7 agonist on RANKL- and
TNF-α-induced osteoclastogenesis were assessed to investigate
whether CXCR7 agonist affects osteoclast precursor cells via
CXCL12. CXCL12 enhanced RANKL- and TNF-α-induced
osteoclastogenesis. The number of osteoclasts was decreased in
osteoclast precursor cells cultured with M-CSF+RANKL+CXCL12+CXCR7
agonist compared with M-CSF+RANKL+CXCL12 (Fig. 4A). The number of TRAP-positive
osteoclasts was also decreased in cultures with
M-CSF+TNF-α+CXCL12+CXCR7 agonist compared with M-CSF+TNF-α+CXCL12
(Fig. 4B).
Inhibitory effect of CXCR7 agonist on
osteoclastogenesis via phosphorylation of MAPKs
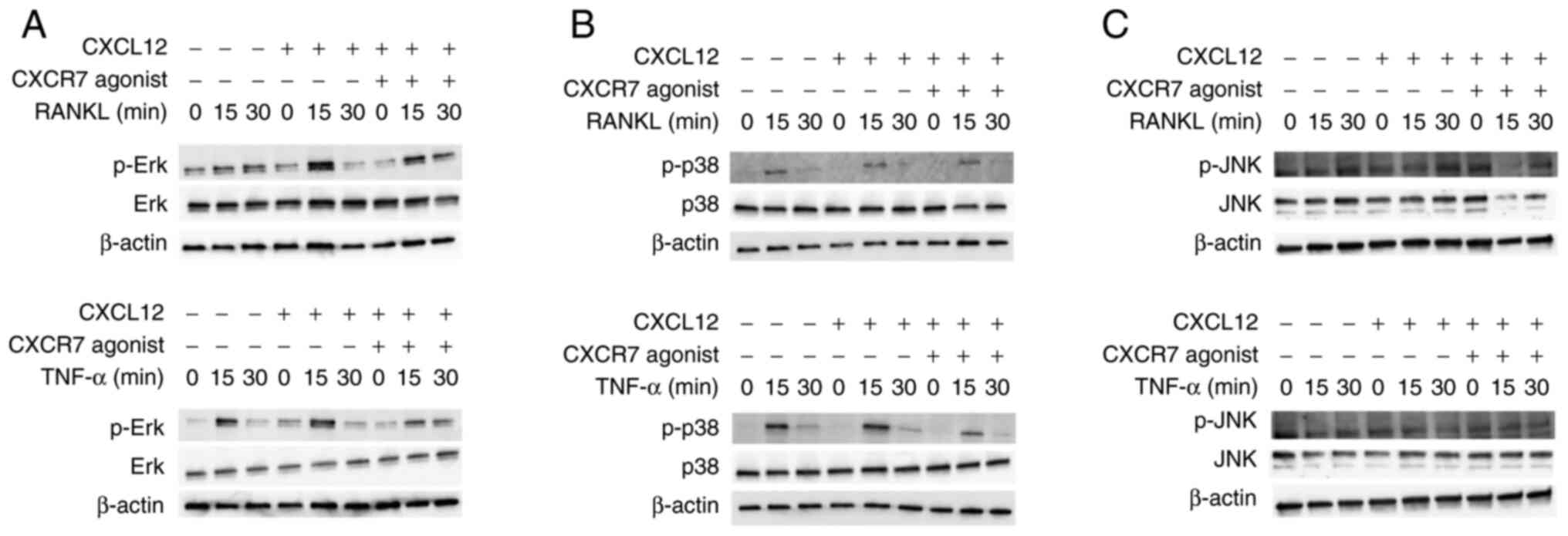
The signal transduction pathway by which CXCR7
agonist inhibits osteoclastogenesis was examined. When RANKL or
TNF-α was added to osteoclast precursors, the phosphorylation of
MAPKs increased at 15 min. CXCL12 enhanced the phosphorylation of
Erk when cells were treated with RANKL, but p-Erk expression was
only slightly enhanced when cells were treated with TNF-α (Fig. 5A). Moreover, CXCR7 agonist reduced
phosphorylation of Erk when cells were treated with RANKL or TNF-α
and CXCL12 (Fig. 5A). However, no
effect was observed on the phosphorylation of p38 and JNK (Fig. 5B and C).
Discussion
In the present study, the effects of a CXCR7 agonist
as a CXCL12 inhibitor on osteoclastogenesis and bone resorption
induced by LPS was analyzed in vivo. The CXCR7 agonist
ameliorated osteoclastogenesis and bone resorption induced by LPS.
Moreover, it was found that CXCR7 agonist inhibited the induction
of RANKL and TNF-α expression by LPS in vivo. CXCR7 agonist
inhibited RANKL-induced and TNF-α-induced osteoclastogenesis by
inhibiting CXCL12 stimulation in vitro. CXCL12-mediated
enhancement of osteoclastogenesis was inhibited by the CXCR7
agonist reducing the phosphorylation of Erk.
It has been reported that CXCL12-CXCR4-induced
cellular events, such as angiogenesis, are negatively regulated by
CXCR7 agonist (25). There have
been no studies of the effects of CXCR7 agonists on
osteoclastogenesis and bone resorption. To the best of our
knowledge, the present study was the first to elucidate the effects
of a CXCR7 agonist on LPS-induced osteoclastogenesis and bone
resorption. A previous study showed that VUF11207 was a high
affinity and potent ligand of CXCR7 (33), and this compound was used as a
CXCR7 agonist in the present study.
CXCL12 plays important roles in patients with
periodontitis and rheumatoid arthritis. CXCL12-CXCR4 signaling
accelerates alveolar bone resorption (34). The interaction of CXCL12 and CXCR4
in patients with rheumatoid arthritis is important for cytokine
production, angiogenesis and local inflammatory cell recruitment
(35). Thus, CXCR4 signaling via
CXCL12 enhances the differentiation and function of osteoclasts.
However, whether CXCR7 agonists can inhibit the effects of CXCL12
is still unknown. VUF11207 is highly selective for CXCR7,
suggesting that CXCR7 agonists may inhibit CXCL12-CXCR4 signaling
(25).
LPS can induce systemic inflammation through
interaction with CXCR4, activating the CXCL12/CXCR4 pathway
(36). LPS can also modulate
production of endogenous CXCL12 and CXCR4 (7). Our previous study showed that both
CXCL12 and CXCR4 mRNA levels were increased in LPS-injected mice
(22). In the present study, 100
µg/day of CXCR7 agonist was administered subcutaneously over the
calvariae for 5 days. CXCR7 agonist inhibited osteoclastogenesis in
the suture of the calvariae induced by LPS injection in
vivo. TRAP and Cathepsin K mRNA levels were also lower in mice
co-administered LPS and CXCR7 agonist compared with mice
administered with LPS alone. The current study further investigated
the inhibitory effect of CXCR7 agonist on bone resorption induced
by LPS. The severity of bone destruction was evaluated by micro-CT
through calculation of the ratio of bone destruction area to total
area. It was found that the area of bone resorption was
significantly lower in LPS and CXCR7 agonist-treated mice. These
results suggested that CXCR7 agonist could attenuate
osteoclastogenesis and bone resorption induced by LPS in
vivo. These results regarding the effects of a CXCR7 agonist on
osteoclastogenesis were similar to those of a previous study
indicating that a CXCR7 agonist inhibited CXCL12-induced
angiogenesis (25).
LPS induces RANKL expression from osteoblasts and
production of proinflammatory cytokines, such as TNF-α and IL-1,
from macrophages and other cells (37). It has been reported that
periodontal ligament cells also express numerous types of
proinflammatory cytokines to regulate osteoclastogenesis by LPS
(38). RANKL and TNF-α contribute
to LPS-induced osteoclastogenesis and bone resorption (39). In the present study, RANKL and
TNF-α mRNA levels were significantly lower in mice co-administered
LPS and CXCR7 agonist compared with mice administered with LPS
alone. Furthermore, our previous study showed that CXCL12 directly
enhanced both RANKL- and TNF-α-induced osteoclast differentiation
(22). In the present study,
CXCR7 agonist was added to this culture system to investigate the
effects of CXCR7 agonist on RANKL- and TNF-α-induced
osteoclastogenesis. It was found that the CXCR7 agonist suppressed
osteoclastogenesis enhanced by CXCL12. These results suggested that
one of the mechanisms underlying the inhibitory effect of CXCR7 on
osteoclastogenesis induced by LPS may be decreased levels of
osteoclast-associated cytokines induced by LPS in vivo.
Another mechanism may involve CXCR7 agonist-mediated inhibition of
RANKL- and TNF-α-induced osteoclastogenesis via inhibition of
CXCL12. Furthermore, CXCR7 agonists may indirectly inhibit
osteoclastogenesis in vivo.
CXCR7 transduces signals via the β-arrestin pathway
rather than the G protein-mediated pathway (40). It has been reported that CXCL12
leads to the phosphorylation of Erk in CXCR7-positive glioma cells
(41). We hypothesized that
CXCL12 and CXCR7 agonist may regulate the phosphorylation of MAPKs
in osteoclast precursors. In the present study, immunoblotting was
performed to investigate signal transduction. CXCL12 enhanced
phosphorylation of Erk when osteoclast precursors were treated with
RANKL or TNF-α. This result suggested that CXCL12 enhanced
phosphorylation in osteoclast precursors as well as in glioma
cells. Moreover, CXCR7 agonist reduced phosphorylation of Erk.
These results were consistent with the inhibitory effect of CXCR7
agonist on osteoclastogenesis in vitro. Therefore,
suppression of CXCL12-enhanced phosphorylation of Erk was
considered to play a major role in the inhibitory effect of CXCR7
agonist on osteoclastogenesis.
CXCL12 is associated with various diseases, and
CXCR7 agonists have been shown to be useful in these diseases
(42). The findings of the
present study suggested that CXCR7 agonists have the potential to
suppress inflammation by CXCL12-enhanced osteoclastogenesis. To
confirm the role of CXCR7 agonists in more detail, further analysis
including knockout mice should be performed in the future. It was
concluded that CXCR7 agonists inhibited osteoclastogenesis and bone
resorption induced by LPS in vivo. Furthermore, CXCR7
agonists also inhibited RANKL- and TNF-α-induced osteoclastogenesis
by inhibiting CXCL12-mediated enhancement of osteoclastogenesis
in vitro. The underlying mechanisms through which CXCR7
agonists attenuated osteoclastogenesis and bone destruction induced
by LPS in vivo appeared to be related to inhibitory effects
on LPS-induced TNF-α and RANKL expression in vivo, as well
as on RANKL- and TNF-α-induced osteoclastogenesis in vitro
via inhibition of CXCL12-mediated upregulation of
osteoclastogenesis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was funded by the Japan Society for the Promotion
of Science Grants-in-Aid for Scientific Research (JSPS KAKENHI;
grant nos. 16K11776, 19K10397 and 18K09862).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
APN, HK, FO and IM contributed to the conception and
design of this study, data acquisition, analysis and
interpretation, and drafting of the manuscript. HK, APN and FO
contributed to critical revision of the manuscript. APN, HK and FO
confirm the authenticity of all the raw data. AP, SO, TN, AM, YN
and RK collected the samples and performed data analysis. HK and IM
supervised the project. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures conformed to
‘Regulations for Animal Experiments and Related Activities at
Tohoku University’, and were reviewed by the Institutional
Laboratory Animal Care and Use Committee of Tohoku University, and
finally approved by the President of University (approval no.
2019DnA-047-05; Miyagi, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boyce BF, Li J, Xing L and Yao Z: Bone
remodeling and the role of TRAF3 in osteoclastic bone resorption.
Front Immunol. 9:22632018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crotti TN, Dharmapatni AA, Alias E and
Haynes DR: Osteoimmunology: Major and costimulatory pathway
expression associated with chronic inflammatory induced bone loss.
J Immunol Res. 2015:2812872015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Azuma Y, Kaji K, Katogi R, Takeshita S and
Kudo A: Tumor necrosis factor-alpha induces differentiation of and
bone resorption by osteoclasts. J Biol Chem. 275:4858–4864. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi K, Takahashi N, Jimi E, Udagawa
N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima
N, et al: Tumor necrosis factor alpha stimulates osteoclast
differentiation by a mechanism independent of the ODF/RANKL-RANK
interaction. J Exp Med. 191:275–286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kitaura H, Zhou P, Kim HJ, Novack DV, Ross
FP and Teitelbaum SL: M-CSF mediates TNF-induced inflammatory
osteolysis. J Clin Invest. 115:3418–3427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xing Q, de Vos P, Faas MM, Ye Q and Ren Y:
LPS promotes pre-osteoclast activity by up-regulating CXCR4 via
TLR-4. J Dent Res. 90:157–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Islam S, Hassan F, Tumurkhuu G, Dagvadorj
J, Koide N, Naiki Y, Mori I, Yoshida T and Yokochi T: Bacterial
lipopolysaccharide induces osteoclast formation in RAW 264.7
macrophage cells. Biochem Biophys Res Commun. 360:346–351. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mörmann M, Thederan M, Nackchbandi I,
Giese T, Wagner C and Hänsch GM: Lipopolysaccharides (LPS) induce
the differentiation of human monocytes to osteoclasts in a tumour
necrosis factor (TNF) alpha-dependent manner: A link between
infection and pathological bone resorption. Mol Immunol.
45:3330–3337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou W and Bar-Shavit Z: Dual modulation of
osteoclast differentiation by lipopolysaccharide. J Bone Miner Res.
17:1211–1218. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimura K, Kitaura H, Fujii T, Hakami ZW
and Takano-Yamamoto T: Anti-c-Fms antibody inhibits
lipopolysaccharide-induced osteoclastogenesis in vivo. FEMS Immunol
Med Microbiol. 64:219–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kikuchi T, Matsuguchi T, Tsuboi N, Mitani
A, Tanaka S, Matsuoka M, Yamamoto G, Hishikawa T, Noguchi T and
Yoshikai Y: Gene expression of osteoclast differentiation factor is
induced by lipopolysaccharide in mouse osteoblasts via Toll-like
receptors. J Immunol. 166:3574–3579. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee J, Park C, Kim HJ, Lee YD, Lee ZH,
Song YW and Kim HH: Stimulation of osteoclast migration and bone
resorption by C-C chemokine ligands 19 and 21. Exp Mol Med.
49:e3582017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Votta BJ, White JR, Dodds RA, James IE,
Connor JR, Lee-Rykaczewski E, Eichman CF, Kumar S, Lark MW and
Gowen M: CKbeta-8 [CCL23], a novel CC chemokine, is chemotactic for
human osteoclast precursors and is expressed in bone tissues. J
Cell Physiol. 183:196–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watanabe K, Penfold ME, Matsuda A,
Ohyanagi N, Kaneko K, Miyabe Y, Matsumoto K, Schall TJ, Miyasaka N
and Nanki T: Pathogenic role of CXCR7 in rheumatoid arthritis.
Arthritis Rheum. 62:3211–3220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Puchert M and Engele J: The peculiarities
of the SDF-1/CXCL12 system: In some cells, CXCR4 and CXCR7 sing
solos, in others, they sing duets. Cell Tissue Res. 355:239–253.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen D, Xia Y, Zuo K, Wang Y, Zhang S,
Kuang D, Duan Y, Zhao X and Wang G: Crosstalk between SDF-1/CXCR4
and SDF-1/CXCR7 in cardiac stem cell migration. Sci Rep.
5:168132015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okada K, Kawao N, Yano M, Tamura Y,
Kurashimo S, Okumoto K, Kojima K and Kaji H: Stromal cell-derived
factor-1 mediates changes of bone marrow stem cells during the bone
repair process. Am J Physiol Endocrinol Metab. 310:E15–E23. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teixido J, Martinez-Moreno M,
Diaz-Martinez M and Sevilla-Movilla S: The good and bad faces of
the CXCR4 chemokine receptor. Int J Biochem Cell Biol. 95:121–131.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong Y, Liu H, Zhang X, Xu F, Qin L, Cheng
P, Huang H, Guo F, Yang Q and Chen A: Inhibition of SDF-1α/CXCR4
signalling in subchondral bone attenuates post-traumatic
osteoarthritis. Int J Mol Sci. 17:9432016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pawig L, Klasen C, Weber C, Bernhagen J
and Noels H: Diversity and inter-connections in the CXCR4 chemokine
receptor/ligand family: Molecular perspectives. Front Immunol.
6:4292015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shima K, Kimura K, Ishida M, Kishikawa A,
Ogawa S, Qi J, Shen WR, Ohori F, Noguchi T, Marahleh A and Kitaura
H: C-X-C Motif Chemokine 12 enhances lipopolysaccharide-induced
osteoclastogenesis and bone resorption in vivo. Calcif Tissue Int.
103:431–442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo T, Liu H, Feng W, Liu D, Du J, Sun J,
Wang W, Han X, Guo J, Amizuka N, et al: Adipocytes enhance
expression of osteoclast adhesion-related molecules through the
CXCL12/CXCR4 signalling pathway. Cell Prolif. 50:e123172017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hatano K, Ishida Y, Yamaguchi H, Hosomichi
J, Suzuki JI, Usumi-Fujita R, Shimizu Y, Shibutani N, Kaneko S and
Ono T: The chemokine receptor type 4 antagonist, AMD3100,
interrupts experimental tooth movement in rats. Arch Oral Biol.
86:35–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uto-Konomi A, McKibben B, Wirtz J, Sato Y,
Takano A, Nanki T and Suzuki S: CXCR7 agonists inhibit the function
of CXCL12 by down-regulation of CXCR4. Biochem Biophys Res Commun.
431:772–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McHugh KP, Hodivala-Dilke K, Zheng MH,
Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO and Teitelbaum
SL: Mice lacking beta3 integrins are osteosclerotic because of
dysfunctional osteoclasts. J Clin Invest. 105:433–440. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kitaura H, Sands MS, Aya K, Zhou P,
Hirayama T, Uthgenannt B, Wei S, Takeshita S, Novack DV, Silva MJ,
et al: Marrow stromal cells and osteoclast precursors
differentially contribute to TNF-alpha-induced osteoclastogenesis
in vivo. J Immunol. 173:4838–4846. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takeshita S, Kaji K and Kudo A:
Identification and characterization of the new osteoclast
progenitor with macrophage phenotypes being able to differentiate
into mature osteoclasts. J Bone Miner Res. 15:1477–1488. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saeed J, Kitaura H, Kimura K, Ishida M,
Sugisawa H, Ochi Y, Kishikawa A and Takano-Yamamoto T: IL-37
inhibits lipopolysaccharide-induced osteoclast formation and bone
resorption in vivo. Immunol Lett. 175:8–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishida M, Kitaura H, Kimura K, Sugisawa H,
Aonuma T, Takada H and Takano-Yamamoto T: Muramyl dipeptide
enhances lipopolysaccharide-induced osteoclast formation and bone
resorption through increased RANKL expression in stromal cells. J
Immunol Res. 2015:1327652015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohori F, Kitaura H, Ogawa S, Shen WR, Qi
J, Noguchi T, Marahleh A, Nara Y, Pramusita A and Mizoguchi I:
IL-33 inhibits TNF-α-induced osteoclastogenesis and bone
resorption. Int J Mol Sci. 21:11302020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wijtmans M, Maussang D, Sirci F, Scholten
DJ, Canals M, Mujić-Delić A, Chong M, Chatalic KL, Custers H,
Janssen E, et al: Synthesis, modeling and functional activity of
substituted styrene-amides as small-molecule CXCR7 agonists. Eur J
Med Chem. 51:184–192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nagashima H, Shinoda M, Honda K, Kamio N,
Hasuike A, Sugano N, Arai Y, Sato S and Iwata K: CXCR4 signaling
contributes to alveolar bone resorption in Porphyromonas
gingivalis-induced periodontitis in mice. J Oral Sci.
59:571–577. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pablos JL, Santiago B, Galindo M, Torres
C, Brehmer MT, Blanco FJ and García-Lázaro FJ: Synoviocyte-derived
CXCL12 is displayed on endothelium and induces angiogenesis in
rheumatoid arthritis. J Immunol. 170:2147–2152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang L, Wang M, Guo YY, Sun T, Li YJ, Yang
Q, Zhang K, Liu SB, Zhao MG and Wu YM: Systemic inflammation
induces anxiety disorder through CXCL12/CXCR4 pathway. Brain Behav
Immun. 56:352–362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kitaura H, Ishida M, Kimura K, Sugisawa H,
Kishikawa A, Shima K, Ogawa S, Qi J and Shen WR: Role of muramyl
dipeptide in lipopolysaccharide-mediated biological activity and
osteoclast activity. Anal Cell Pathol (Amst).
2018:80476102018.PubMed/NCBI
|
|
38
|
Lee SY, Moon JS, Yang DW, Yoo HI, Jung JY,
Kim OS, Kim MS, Koh JT, Chung HJ and Kim SH: SLPI in periodontal
Ligament is not sleepy during biophysical force-induced tooth
movement. J Clin Periodontol. 48:528–540. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ishida M, Shen WR, Kimura K, Shima K,
Ogawa S, Qi J, Ohori F, Noguchi T, Marahleh A and Kitaura H: DPP-4
inhibitor impedes lipopolysaccharide-induced osteoclast formation
and bone resorption in vivo. Biomed Pharmacother. 109:242–253.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rajagopal S, Kim J, Ahn S, Craig S, Lam
CM, Gerard NP, Gerard C and Lefkowitz RJ: Beta-arrestin-but not G
protein-mediated signaling by the ‘decoy’ receptor CXCR7. Proc Natl
Acad Sci USA. 107:628–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hattermann K, Held-Feindt J, Lucius R,
Müerköster SS, Penfold ME, Schall TJ and Mentlein R: The chemokine
receptor CXCR7 is highly expressed in human glioma cells and
mediates antiapoptotic effects. Cancer Res. 70:3299–3308. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lounsbury N: Advances in CXCR7 modulators.
Pharmaceuticals (Basel). 13:332020. View Article : Google Scholar : PubMed/NCBI
|