Introduction
Myocardial infarction (MI) is the leading cause of
mortality among cases of coronary heart disease (1). Although long-term survival following
MI has improved over the past three decades, ~20% of patients with
MI die within 1 year of the event, with more than half of these
deaths occurring within 30 days of MI (2). In clinical practice, various
treatments, such as thrombolytic and emergent intervention therapy
and coronary artery bypass grafting, have been used to improve
timely restoration of the blood supply (3). These treatments effectively decrease
damage and death of ischemic myocardial tissue. However, the
successful restoration of cardiac blood flow also leads to
ischemia/reperfusion injury (IRI), which causes metabolic
dysfunction of cardiac muscle and cardiac disorganization (4). These changes ultimately result in
secondary damage, as well as arrhythmia and infarct expansion
(4). Therefore, is it important to
develop a novel therapeutic strategy to protect against IRI.
Previous studies have reported that activation of
hypoxia-inducible factor 1α (HIF-1α) serves a key role in the
therapy strategy of IRI (5,6). For example, it has been shown that
ischemic preconditioning of the circumflex coronary artery
decreases infarct size following transient, non-lethal prolonged
occlusion of the same vessel (5).
Przyklenk et al (6) reported
that remote ischemic preconditioning (RIP) decreases infarct size
following prolonged left anterior descending coronary artery
occlusion, and another study revealed that RIP prevents I/R-induced
endothelial dysfunction and decreases the extent of myocardial
infarction (7). Moreover, a
mechanistic study observed that HIF-1α serves a critical role in
the protective effect of RIP (8).
It has also been shown that inhibition of HIF-1α degradation is
promising in the treatment of IRI (9–11).
During hypoxia, HIF-1α is hydroxylated, resulting in its
ubiquitination and subsequent proteasomal degradation (9). A family of Fe+2 and
2-oxoglutarate-dependent dioxygenases, termed prolyl-4 hydroxylase
domain (PHD) 1–3 proteins, are responsible for the hydroxylation of
HIF-1α (10). PHDs can be inhibited
by dimethyloxalylglycine (DMOG) (12). However, it remains unknown whether
RIP and direct PHD inhibition possess synergetic protective
effects.
It has been reported that AKT/endothelial nitric
oxide synthase (eNOS) and vascular endothelial growth factor (VEGF)
serve a key role in the protective signaling pathway in IR
(13–15). For example, Wang et al
(13) revealed that the herbal drug
Tongxinluo protects against pressure overload-induced heart failure
in mice via activation of the AKT/eNOS axis. Qiao et al
(14) found that transient acidosis
during early reperfusion attenuates myocardium IRI via the AKT/eNOS
signaling pathway. In addition, VEGF serves an important role in
the protective effect in IRI. For example, apigenin protects the
brain against IRI via VEGF (15).
Another study demonstrated that prolonged cold ischemia in renal
graft causes liver pyroptosis and injury at least partially through
increased histone release, VEGF improves liver function by
suppressing histone-induced hepatocyte pyroptosis via VEGF receptor
2 (16). Furthermore, VEGF may be a
downstream regulator of the AKT/eNOS signaling pathway; Kang et
al (17) observed that cornin
induces angiogenesis via the AKT/eNOS/VEGF signaling pathway.
HIF-1α regulates the expression of downstream
proteins involved in glucose metabolism and angiogenesis, such as
VEGF and erythropoietin, to facilitate ischemic adaptation
(18). Therefore, the present study
evaluated whether RIP and DMOG exert synergetic protective effects
in I/R-induced cardiac damage and investigated the potential role
of HIF-1α and the downstream VEGF and AKT/eNOS signaling
pathways.
Materials and methods
Rabbits
All experiments were performed according to the
Institutional Guidelines for the Care and Use of Laboratory Animals
in Research and were approved by the Committee of the Affiliated
Hospital of The Second Affiliated Hospital of Nanchang
University.
In total, 40 male rabbits (age, 5–6 months; weight,
2.5-3.0 kg) were purchased from Nanchang Longping Rabbit Co., Ltd.
and raised in The Second Affiliated Hospital of Nanchang
University. The rabbits were housed at a constant room temperature
(23±2°C) and relative humidity (50±10%) with free access to food
and water and a fixed 12 h light/dark cycle. The animals were
ventilated with a small animal ventilator (Shanghai Alcott Biotech
Co., Ltd.) at a rate of 60 breaths/min, inspiratory/expiratory
ratio of 1:3 and tidal volume of 50 ml.
Remote pre-conditioning, DMOG and I/R
treatment
Male rabbits were randomly allocated into seven
groups: i) Sham (n=6); ii) I/R (n=8); iii) lung (L-)RIP + I/R
(n=7); iv) thigh (T-)RIP + I/R (n=7); v) DMOG (D) + I/R (n=7); vi)
D + L-RIP + I/R (n=7); and vii) D + T-RIP + I/R (n=7). For
establishment of the I/R model, animals were anesthetized with an
ear intravenous (i.v.) injection of 3% pentobarbital sodium (30
mg/kg body weight; supplemented as required) and deep
anesthetization was confirmed by loss of the pedal withdrawal
reflex. The chest was opened via thoracotomy through the fourth or
fifth intercostal space and the heart was exposed (19). A 5-0 prolene was placed around the
left main coronary artery (LCA) to create a reversible snare. Prior
to LCA occlusion (LCAO), animals were anti-coagulated using 150
U/kg i.v. sodium heparin (Sigma-Aldrich; Merck KGaA) received i.v.
lidocaine (4 mg/kg; Sigma-Aldrich; Merck KGaA) to attenuate
arrhythmia. All rabbits underwent a total of 30 min LCAO, followed
by 180 min reperfusion. The femoral and pulmonary artery were
carefully exposed and encircled with a bulldog clamp to form a
reversible snare for artery occlusion. Rabbits in the sham group
underwent the same thoracotomy procedure but without ligation of
the coronary artery.
L/T-RIP was performed as follows: Rabbits were
treated with left pulmonary artery (or left limb) ischemia for 25
min, followed by release for 5 min. A total of 20 mg/kg DMOG
(Sigma-Aldrich; Merck KGaA) was administered intraperitoneally 24 h
before cardiac IRI as described previously (20). At the end of the experiment, rabbits
were euthanized with an overdose of pentobarbital sodium (3%; 100
mg/kg body weight; ear i.v. injection).
Immunohistochemistry and
immunofluorescence
Immunohistochemistry and immunofluorescence staining
were performed as previously described (21). Hearts were removed and fixed in 4%
paraformaldehyde for 30 min at 4°C. Then, tissues were embedded in
paraffin and cut into 5-µm sections. For hematoxylin and eosin
staining, slides were stained with 0.5% hematoxylin for 10 min to
stain the nuclei and stained with 1% eosin for 3 min at room
temperature. For immunofluorescence, slices blocked with 1% non-fat
milk for 2 h at room temperature. The slides were incubated with
rabbit anti-caspase-3 primary antibody (1:100; cat. no. ab44976;
Abcam) overnight at 4°C, then with Alexa fluor 555-coupled
anti-rabbit secondary antibody (4 µg/ml; cat. no. A-21429; Thermo
Fisher Scientific, Inc.) for 1 h at room temperature. Images were
acquired on a confocal spinning disk microscope (400×
magnification; Leica Microsystems GmbH).
Area at risk (AAR)
The AAR was determined as described previously
(22). After clamping the aorta and
re-occlusion of the left anterior descending coronary artery, Evans
blue dye was injected at 1 ml/kg body weight and the ventricles
were serially sectioned from the apex to base in 1 cm slices. AAR
was determined using ImageJ software (Version 1.51K, National
Institutes of Health) and myocardial infarct size was expressed as
a percentage of AAR.
TUNEL
For apoptosis detection, paraffin-embedded heart
sections were stained with a TUNEL kit (Roche Diagnostics). A
portion of heart tissue was fixed in 4% paraformaldehyde solution
at 4°C for 6 h and cut into 5 µm-thick paraffin-embedded sections.
These samples were deparaffinized in xylene for 5 min and
rehydrated with 100 and 95% ethanol for 3 min at room temperature.
Slides were then digested with proteinase K and peroxidase activity
was blocked with 3% H2O2. Following
incubation with PBS/0.2% Tween-20 at room temperature for 30 min,
sections were incubated in TdT Reaction Buffer at 37°C for 10 min
and TdT Reaction Mixture at 37°C for 1 h. Next, sections were
incubated with Streptavidin-horseradish peroxidase for 20 min at
room temperature, washed with PBS/0.2% Tween-20 and incubated with
0.66 mg/ml diaminobenzidine (DAB) substrate at room temperature for
1–2 min. Sections were counterstained with Gill's hematoxylin
(0.5%) at room temperature for 30 sec. Sections were mounted with
anti-fading mounting medium and frozen sections were analyzed with
a confocal laser scanning microscope (400× magnification; cat. no.
LSM 710; Carl Zeiss GmbH). A total of four fields/slide was viewed
and the average was calculated.
Creatine kinase (CK) activity
Serum CK activity was measured using a colorimetric
assay kit (cat. no. K777; BioVision, Inc.) according to the
manufacturer's instruction.
Reverse transcription-quantitative
(RT-q)PCR
RNA samples were extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) and cDNA was
synthesized using a SuperScript™ III First-Strand Synthesis system
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instruction. PCR primers used for qPCR were as
follows: HIF-1α forward, 5′-ATTTTACCCATCCGTGTGAC-3′ and reverse,
5′-CTTCCAGGTGGCAGACTTTA-3′; VEGF forward,
5′-ATCATGCGGATCAAACCTCA-3′ and reverse, 5′-CTCGGCTTGTCACATTTTTC-3′;
AKT forward, 5′-CTCATTCCAGACCCACGAC-3′ and reverse,
5′-ACAGCCCGAAGTCCGTTA-3′; eNOS forward, 5′-GAGAACGGAGAGAGTTTTGC-3′
and reverse, 5′-CTGTTGAAGCGGATTTTGTA-3′; and β-actin forward,
5′-GCTGTCCCTGTACGCCTCTGG-3′ and reverse,
5′-GCTTCTCCTTGATGTCCCGC-3′. The transcript levels of HIF-1α, AKT
and eNOS were measured using an ABI 7900 system with a SYBR Green
qRT-PCR kit (both Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Thermocycling
conditions were as follows: 94°C for 10 min; 40 cycles at 94°C for
5 sec, 60°C for 15 sec and 72°C for 2 min; and final step of 72°C
for 10 min. The relative expression level was normalized to β-actin
using the Cq method (23).
Western blotting
Tissues were minced with an electric homogenizer and
lysed in RIPA buffer (Beyotime Institute of Biotechnology) with
constant agitation for 2 h at 4°C. Samples were centrifuged for 20
min at 15,000 × g at 4°C and the supernatant was aspirated into new
tubes. The protein concentration was determined using a Bradford
assay kit. Protein extracts (10 µg/lane) were fractionated on a
Tris-HCl 5–15% gradient gel (Bio-Rad Laboratories, Inc.) and were
then transferred to PVDF membranes (MilliporeSigma). Following
blocking with 5% non-fat milk for 1 h at room temperature,
membranes were incubated using the following antibodies:
Anti-HIF-1α (1:1,000; cat. no. A11945; ABclonal Biotech Co., Ltd.),
anti-phosphorylated (p)-AKT (1:2,000; cat. no. 4060; Cell Signaling
Technology, Inc.), anti-AKT (1:1,000; cat. no. 4685; Cell Signaling
Technology, Inc.) and anti-eNOS (1:1,000; cat. no. 32027; Cell
Signaling Technology, Inc.) at 4°C overnight. Then, membranes were
incubated with horseradish peroxidase-labeled anti-rabbit (cat. no.
AS014; ABclonal Biotech Co., Ltd.) or anti-mouse (both 1:2,000;
cat. no. AS003; ABclonal Biotech Co., Ltd.) secondary antibodies
for 1 h at room temperature. Anti-β-actin (1:5,000; cat. no. AC028;
ABclonal Biotech Co., Ltd.) served as loading control. Signals were
detected with ECL Detection Reagent (Cytiva). Semi-quantification
of western blotting bands was performed using ImageJ software
(Version 1.51K; National Institutes of Health).
Statistical analysis
Data are presented as the mean ± SD of 6–8
independent repeats. All data were compared with one-way ANOVA
followed by Tukey's post hoc test using GraphPad Prism 6 (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
L/T-RIP and DMOG exert a synergetic
protective effect on I/R-induced myocardial injury
Firstly, the present study evaluated the effect of
L/T-RIP on myocardium morphology and infarct size in I/R-treated
rabbits. Myocardial cells were neatly arranged in the Sham group,
with clear boundaries and intact nuclei (Fig. 1A). By contrast, myocardial cells in
the I/R-treated rabbits were disorderly arranged, with unclear
boundaries, myofiber rupture and nuclei disappearance. In the
L/T-RIP + D groups, the severity of myocardial injuries was notably
improved (Fig. 1A). The myocardium
infarct size was significantly increased by 8.75 fold in I/R
rabbits compared with Sham rabbits; this increase was significantly
inhibited in I/R D + L/T-RIP rabbits (Fig. 1B). Moreover, L/T-RIP and DMOG
exerted a synergetic effect on decreasing myocardium infarct size,
as indicated by changes in AAR in I/R rabbits (Fig. 1B). Consistently, the plasma CK
levels, a biochemical marker of cell injury during reperfusion
(24), were significantly increased
by 3.15 fold, which was significantly decreased by L/T-RIP or DMOG
in I/R rabbits (Fig. 1C). L/T-RIP
and DMOG had an additive protective effect on CK levels (Fig. 1C). These data indicated that L/T-RIP
and DMOG exerted a synergetic protective effect on I/R-induced
cardiac damage.
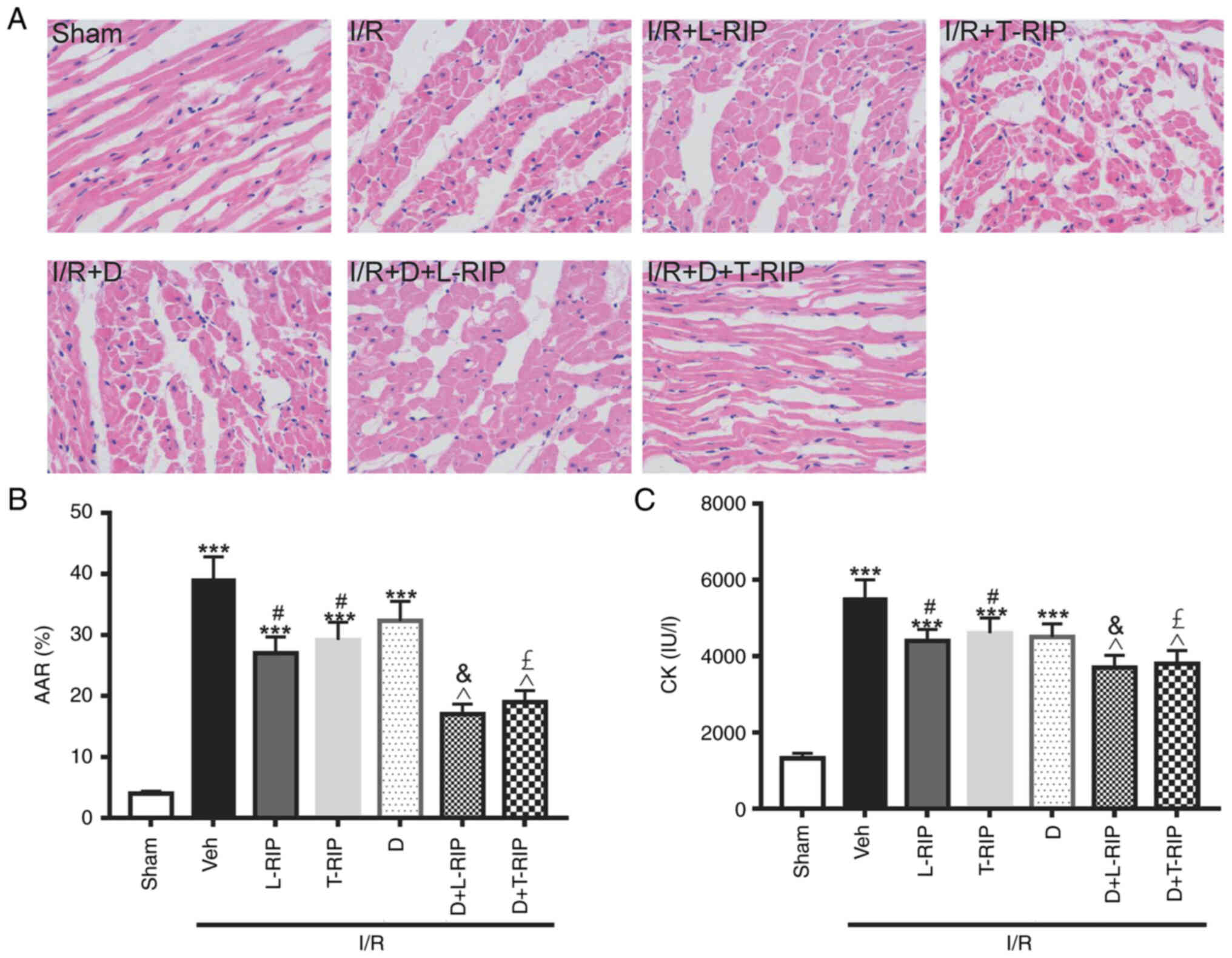 | Figure 1.L/T-RIP and D exhibit a synergetic
protective effect on cardiac IRI. (A) Representative hematoxylin
and eosin staining. Magnification, 400×. Inhibitory effect of L/T
RIP and/or D on (B) AAR and (C) CK. ***P<0.001 vs. Sham;
#P<0.05 vs. Veh; &P<0.05 vs.
L-TRIP; £P<0.05 vs. T-RIP; ^P<0.05 vs.
D. L, lung; T, thigh; RIP, remote ischemic preconditioning; D,
dimethyloxalylglycine; IRI, I/R injury; I/R, ischemia/reperfusion;
AAR, area at risk; CK, creatine kinase; Veh, Vehicle. |
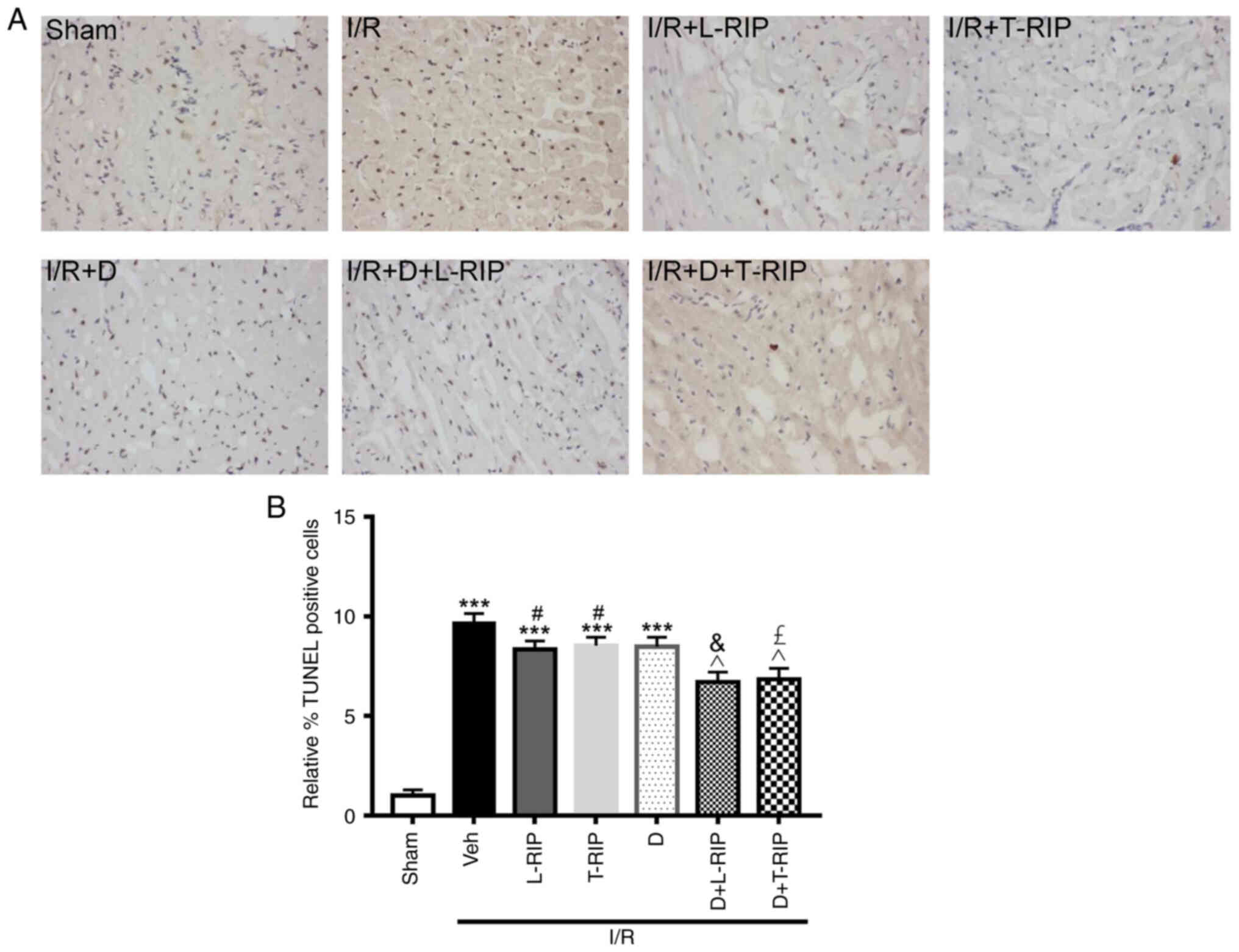
L/T-RIP and DMOG have a synergetic
effect on myocardial apoptosis, as indicated by TUNEL and caspase 3
assay
Myocardial apoptosis in cardiac tissue was
identified via TUNEL staining. The number of apoptotic cells was
significantly increased in the I/R group compared with the Sham
group (Fig. 2A and B). Furthermore,
L/T-RIP and DMOG exerted a synergetic protective effect and
decreased the number of apoptotic myocardiocytes in I/R
rabbits.
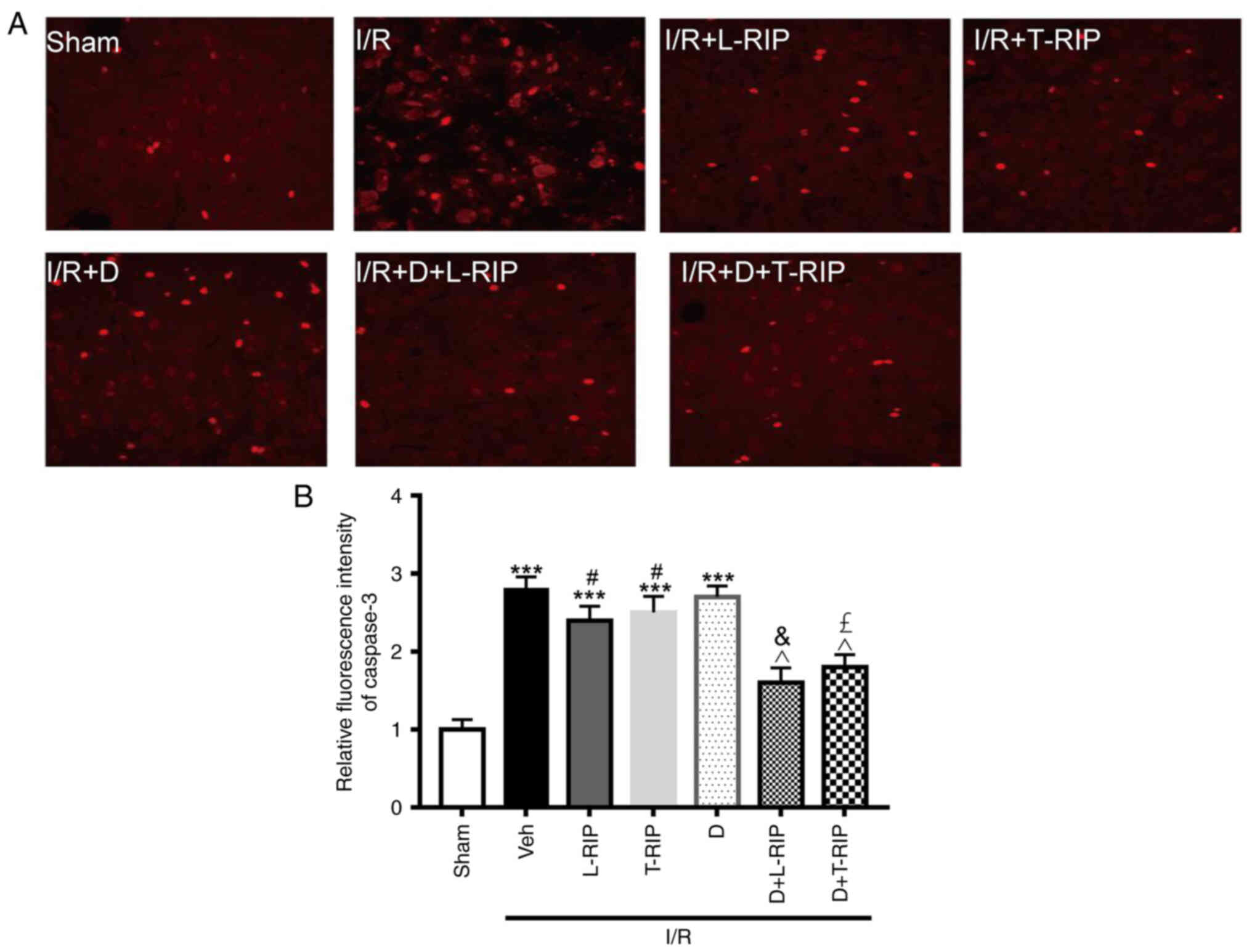
The effect of L/T-RIP and DMOG on caspase 3, an
apoptosis marker, was evaluated using immunofluorescence staining.
Caspase 3 was significantly increased, and L/T-RIP and DMOG showed
an additive effect on decreasing caspase 3 expression in I/R
rabbits (Fig. 3A and B). These
results suggested that L/T-RIP and DMOG exerted an enhanced effect
on decreasing myocardial apoptosis in I/R rabbits.
L/T-RIP and DMOG have a synergetic
effect on activating HIF-1α, VEGF and AKT/eNOS
Next, the changes in HIF-1α protein expression
levels following different treatments were evaluated. Both the mRNA
and protein expression levels of HIF-1α were significantly
increased by I/R; the increase was further enhanced by L/T-RIP and
DMOG (Fig. 4A and B). Moreover, L/T
RIP and DMOG had synergetic effects on increasing HIF-1α mRNA and
protein expression levels hearts from I/R rabbits. These results
suggested that the additive effect of L/T RIP and DMOG may be
caused by increasing HIF-1α expression.
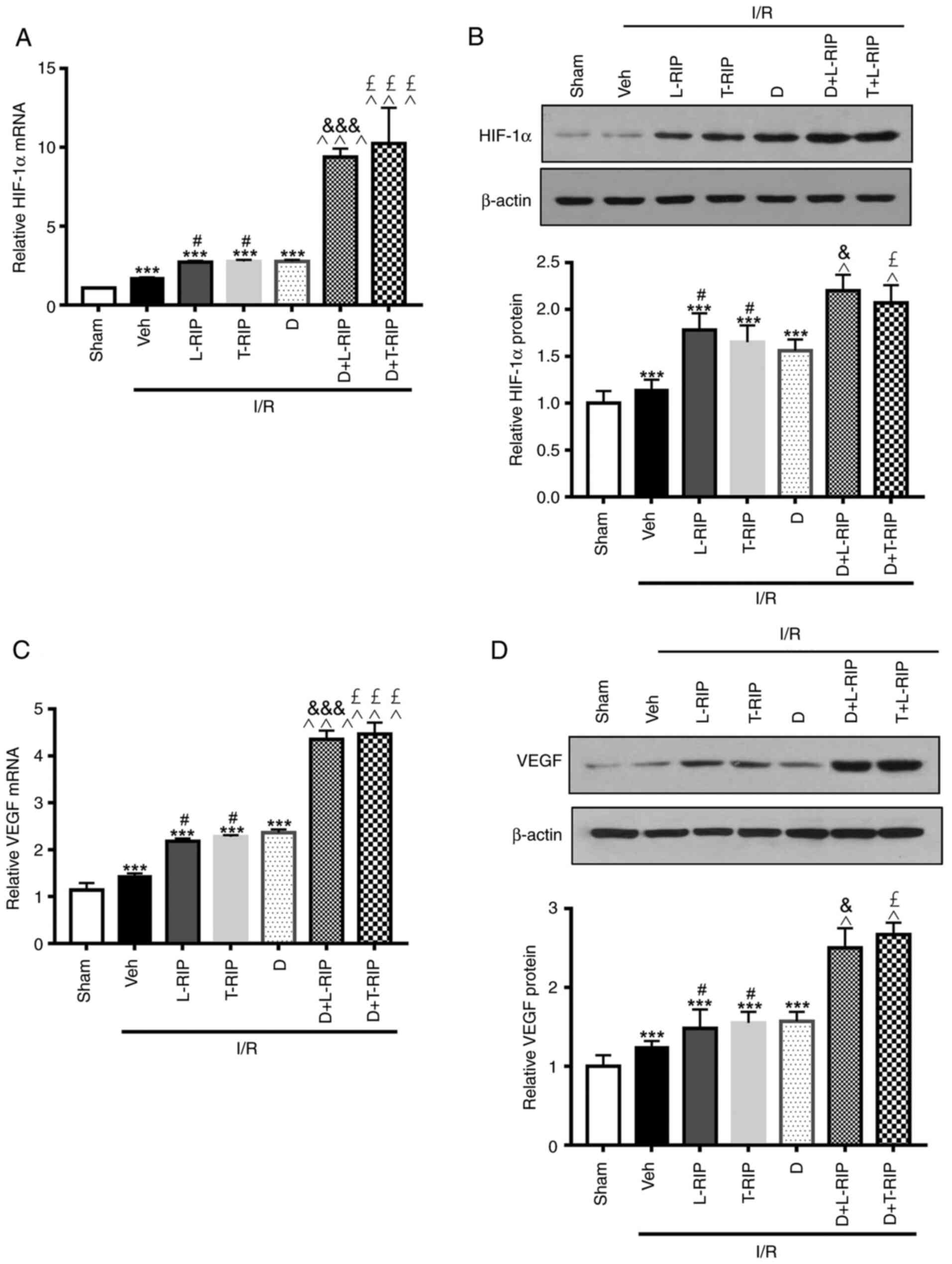 | Figure 4.L/T RIP and D show a synergetic
effect on I/R-induced expression of HIF-1α and VEGF. (A) Inhibitory
effect of L/T-RIP and/or D on HIF-1α mRNA levels. (B)
Representative western blot images showing the inhibitory effect of
L/T-RIP and/or D on HIF-1α protein levels. (C) Inhibitory effect of
L/T-RIP and/or D on VEGF mRNA levels. (D) Representative western
blot images showing the inhibitory effect of L/T-RIP and/or D on
VEGF protein levels. ***P<0.001 vs. Sham; #P<0.05
vs. I/R; &P<0.05,
&&&P<0.001 vs. L-TRIP;
£P<0.05, £££P<0.001 vs. T-RIP;
^P<0.05, ^^^P<0.001 vs. D. L, lung; T,
thigh; RIP, remote ischemic preconditioning; D,
dimethyloxalylglycine; IRI, I/R injury; I/R, ischemia/reperfusion;
Veh, Vehicle. |
Similarly, both the mRNA and protein expression
levels of VEGF, a downstream effector of HIF-1α, were significantly
increased by I/R; this upregulation was further enhanced by L/T RIP
and DMOG in I/R rabbits. As expected, L/T-RIP and DMOG had a
greater effect on increasing VEGF mRNA and protein expression
levels.
Previous studies have reported that the AKT/eNOS
signaling pathway serves a protective role in experimental IRI
(14,25). Here, L/T-RIP and DMOG increased mRNA
and protein expression levels of AKT, eNOS and VEGF in I/R rabbits.
Notably, L/T-RIP and DMOG exerted an enhanced effect on the mRNA
and protein expression levels of AKT in the cardiac tissue of
rabbits with I/R (Fig. 5).
Furthermore, L/T-RIP and DMOG had a synergetic effect on the mRNA
and protein expression levels of eNOS in the cardiac tissue of
rabbits with I/R. These results suggested that both L/T-RIP and
DMOG exerted a cardiac protective effect via activation of the VEGF
and AKT/eNOS signaling pathway.
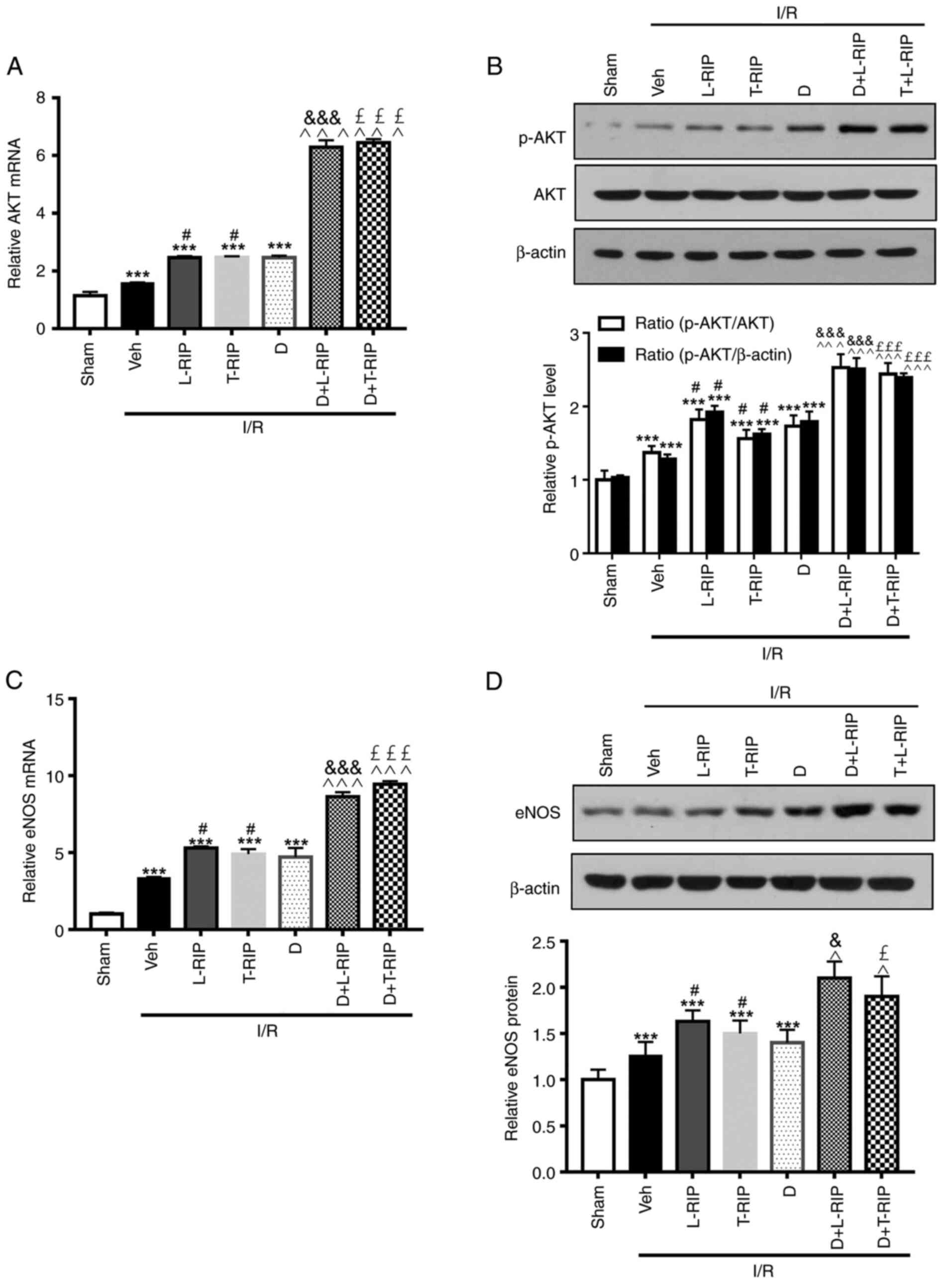 | Figure 5.L/T-RIP and D exert a synergetic
effect on I/R-induced expression of AKT and eNOS. (A) Inhibitory
effect of L/T-RIP and/or D on AKT mRNA levels. (B) Representative
western blot images showing the inhibitory effect of L/T-RIP and/or
D on AKT protein levels. (C) Inhibitory effect of L/T-RIP and/or D
on eNOS mRNA levels. (D) Representative western blot images showing
the inhibitory effect of L/T-RIP and D on eNOS protein levels.
***P<0.001 vs. Sham; #P<0.05 vs. Veh;
&P<0.05, &&&P<0.001 vs.
L-TRIP; £P<0.05, £££P<0.001 vs. T-RIP;
^P<0.05, ^^^P<0.001 vs. D. L, lung; T,
thigh; RIP, remote ischemic preconditioning; D,
dimethyloxalylglycine; IRI, I/R injury; I/R, ischemia/reperfusion;
Veh, Vehicle; p, phosphorylated; eNOS, endothelial nitric oxide
synthase. |
Discussion
The present results demonstrated that L/T-RIP and
DMOG decreased myocardial infarct size in experimental rabbits.
Moreover, L/T-RIP and DMOG exerted a significant synergetic
protective effect on myocardial injury and cardiac apoptosis.
Mechanistically, the two treatments exhibited additive effects on
HIF-1α expression, thereby activating the VEGF and eNOS/HIF-1α
axis. The present study indicated that the combination of RIP and
PHD inhibition may be used as a therapy for IRI.
Accumulating evidence has revealed that remote organ
preconditioning decreases I/R-induced damage in the heart and
kidney (26–28) and that HIF-1α serves a key role in
the protective effect of RIP, which may be mediated by systemic
modulation of the inflammatory response to I/R-induced cardiac and
lung damage in rats (20,29). Another study observed that RIP
protects the heart by increasing expression of HIF-1α and
subsequent activation of IL-10 (30). In line with these studies, the
present study demonstrated that L/T IP protected I/R-induced
cardiac injury by increasing HIF-1α expression.
Similar to L/T-RIP, the present study identified
that DMOG administration decreased cardiac injury and significantly
increased HIF-1α expression. This was consistent with a previous
study showing that systemic administration of DMOG in rats
increases both the mRNA and protein expression levels of HIF-1α in
blood vessels (31). Furthermore,
the present results suggested that RIP and DMOG exerted significant
synergetic protective effects and by increasing HIF-1α expression;
their combination may be a favorable strategy for the prevention
and treatment of I/R-induced cardiac injury.
Previous studies have revealed that NO is involved
in protecting the myocardium following pulmonary IP, which
decreases free radical and NO release (32,33).
Bai et al (34) reported
that Baicalin protects against myocardial IRI via activation of the
PI3K/AKT/eNOS pathway. In addition, RIP increases p-ERK expression;
inhibition of p-ERK prevents neuronal NOS expression and remote
preconditioning-mediated neuroprotection (35). In the present study, the mRNA and
protein expression levels of AKT and eNOS were significantly
increased in the combined L/T-RIP and DMOG groups. These results
suggested that the protective role of L/T-RIP and DMOG was exerted
via activation of HIF-1α and the downstream VEGF and AKT/eNOS axis
(20,30).
In conclusion, the present study demonstrated that
L/T-RIP and DMOG administration immediately before the onset of
coronary artery reperfusion decreased myocardial infarct size and
that these factors exerted synergetic protective effects on HIF-1α
and the downstream VEGF and AKT-eNOS pathway. The clinical
application of L/T-RIP and DMOG in combination may provide a
potential strategy for prevention and treatment of I/R-induced
cardiac injury.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jiangxi Provincial
Natural Science Foundation Project (grant nos. 20171BBG70067 and
20181074) and National Natural Science Foundation of China (grant
nos. 81160019 and 81360031).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY and YT participated in the design of the study
and performed the statistical analysis. JY, JX, SW, HX and LT
performed the experiments. JY and YT drafted the manuscript. JY and
YT confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed according to the
Institutional Guidelines for the Care and Use of Laboratory Animals
in Research and were approved by the Committee of the Affiliated
Hospital of The Second Affiliated Hospital of Nanchang
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao S, Zhu L, Tinzin L, Huang F, Ma L and
Zhou Y: Acute myocardial infarction in a young woman: Unexpected
findings of a coronary occlusion. Leg Med (Tokyo). 42:1016622019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ye Q, Zhang J and Ma L: Predictors of
all-cause 1-year mortality in myocardial infarction patients.
Medicine (Baltimore). 99:e212882020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berger JS: Platelet-directed therapies and
coronary artery bypass grafting. Am J Cardiol. 104 5 Suppl:44C–48C.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang S, Wotzkow C, Bongoni AK, Shaw-Boden
J, Siegrist M, Taddeo A, Blank F, Hofstetter W and Rieben R: Role
of the plasma cascade systems in ischemia/reperfusion injury of
bone. Bone. 97:278–286. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Przyklenk K, Bauer B, Ovize M, Kloner RA
and Whittaker P: Regional ischemic ‘preconditioning’ protects
remote virgin myocardium from subsequent sustained coronary
occlusion. Circulation. 87:893–899. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kharbanda RK, Mortensen UM, White PA,
Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K,
Redington AN and MacAllister R: Transient limb ischemia induces
remote ischemic preconditioning in vivo. Circulation.
106:2881–2883. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo JY, Yang T, Sun XG, Zhou NY, Li FS,
Long D, Lin T, Li PY and Feng L: Ischemic postconditioning
attenuates liver warm ischemia-reperfusion injury through
Akt-eNOS-NO-HIF pathway. J Biomed Sci. 18:792011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M
and Kaelin WG Jr: von Hippel-Lindau protein mutants linked to type
2C VHL disease preserve the ability to downregulate HIF. Hum Mol
Genet. 10:1019–1027. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bruick RK and McKnight SL: A conserved
family of prolyl-4-hydroxylases that modify HIF. Science.
294:1337–1340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng J, Chen P, Zhong J, Cheng Y, Chen H,
He Y and Chen C: HIF1α in myocardial ischemiareperfusion injury
(Review). Mol Med Rep. 23:3522021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hams E, Saunders SP, Cummins EP, O'Connor
A, Tambuwala MT, Gallagher WM, Byrne A, Campos-Torres A, Moynagh
PM, Jobin C, et al: The hydroxylase inhibitor dimethyloxallyl
glycine attenuates endotoxic shock via alternative activation of
macrophages and IL-10 production by B1 cells. Shock. 36:295–302.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang B, Yang Q, Bai WW, Xing YF, Lu XT,
Sun YY and Zhao YX: Tongxinluo protects against pressure
overload-induced heart failure in mice involving VEGF/Akt/eNOS
pathway activation. PLoS One. 9:e980472014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiao X, Xu J, Yang QJ, Du Y, Lei S, Liu
ZH, Liu X and Liu H: Transient acidosis during early reperfusion
attenuates myocardium ischemia reperfusion injury via PI3k-Akt-eNOS
signaling pathway. Oxid Med Cell Long. 2013:1260832013.PubMed/NCBI
|
|
15
|
Pang Q, Zhao Y, Chen X, Zhao K, Zhai Q and
Tu F: Apigenin protects the brain against ischemia/reperfusion
injury via caveolin-1/VEGF in vitro and in vivo. Oxid Med Cell
Longev. 2018:70172042018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao H, Huang H, Alam A, Chen Q, Suen KC,
Cui J, Sun Q, Ologunde R, Zhang W, Lian Q and Ma D: VEGF mitigates
histone-induced pyroptosis in the remote liver injury associated
with renal allograft ischemia-reperfusion injury in rats. Am J
Transplant. 18:1890–1903. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang Z, Jiang W, Luan H, Zhao F and Zhang
S: Cornin induces angiogenesis through PI3K-Akt-eNOS-VEGF signaling
pathway. Food Chem Toxicol. 58:340–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Z, Yao L, Yang J, Wang Z and Du G:
PI3K/Akt and HIF1 signaling pathway in hypoxiaischemia (Review).
Mol Med Rep. 18:3547–3554. 2018.PubMed/NCBI
|
|
19
|
Subramanian VA, McCabe JC and Geller CM:
Minimally invasive direct coronary artery bypass grafting: Two-year
clinical experience. Ann Thorac Surg. 64:1648–1653. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ockaili R, Natarajan R, Salloum F, Fisher
BJ, Jones D, Fowler AA III and Kukreja RC: HIF-1 activation
attenuates postischemic myocardial injury: Role for heme
oxygenase-1 in modulating microvascular chemokine generation. Am J
Physiol Heart Circ Physiol. 289:H542–H548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He S, Cheng J, Sun L, Wang Y, Wang C, Liu
X, Zhang Z, Zhao M, Luo Y, Tian L, et al: HMGB1 released by
irradiated tumor cells promotes living tumor cell proliferation via
paracrine effect. Cell Death Dis. 9:6482018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jazbutyte V, Stumpner J, Redel A, Lorenzen
JM, Roewer N, Thum T and Kehl F: Aromatase inhibition attenuates
desflurane-induced preconditioning against acute myocardial
infarction in male mouse heart in vivo. PLoS One. 7:e420322012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo Y, Pan YZ, Zeng C, Li GL, Lei XM, Liu
Z and Zhou SF: Altered serum creatine kinase level and cardiac
function in ischemia-reperfusion injury during percutaneous
coronary intervention. Med Sci Monit. 17:CR474–CR479. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujimoto H, Ohno M, Ayabe S, Kobayashi H,
Ishizaka N, Kimura H, Yoshida KI and Nagai R: Carbon monoxide
protects against cardiac ischemia-reperfusion injury in vivo via
MAPK and Akt-eNOS pathways. Arterioscler Thromb Vasc Biol.
24:1848–1853. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Billah M, Ridiandries A, Allahwala U,
Mudaliar H, Dona A, Hunyor S, Khachigian LM and Bhindi R:
Circulating mediators of remote ischemic preconditioning: Search
for the missing link between non-lethal ischemia and
cardioprotection. Oncotarget. 10:216–244. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zarbock A and Kellum JA: Remote ischemic
preconditioning and protection of the kidney-a novel therapeutic
option. Crit Care Med. 44:607–616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lim SY and Hausenloy DJ: Remote ischemic
conditioning: From bench to bedside. Front Physiol. 3:272012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin Y and Zhao X, Zhang H, Li Q, Lu G and
Zhao X: Modulatory effect of silymarin on pulmonary vascular
dysfunction through HIF-1α-iNOS following rat lung
ischemia-reperfusion injury. Exp Ther Med. 12:1135–1140. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai Z, Luo W, Zhan H and Semenza GL:
Hypoxia-inducible factor 1 is required for remote ischemic
preconditioning of the heart. Proc Natl Acad Sci USA.
110:17462–17467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan Q, Bleiziffer O, Boos AM, Sun J,
Brandl A, Beier JP, Arkudas A, Schmitz M, Kneser U and Horch RE:
PHDs inhibitor DMOG promotes the vascularization process in the AV
loop by HIF-1a up-regulation and the preliminary discussion on its
kinetics in rat. BMC Biotechnol. 14:1122014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nagasaka Y, Fernandez BO, Steinbicker AU,
Spagnolli E, Malhotra R, Bloch DB, Bloch KD, Zapol WM and Feelisch
M: Pharmacological preconditioning with inhaled nitric oxide (NO):
Organ-specific differences in the lifetime of blood and tissue NO
metabolites. Nitric Oxide. 80:52–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Totzeck M, Hendgen-Cotta U and Rassaf T:
Concepts of hypoxic NO signaling in remote ischemic
preconditioning. World J Cardiol. 7:645–651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bai J, Wang Q, Qi J, Yu H, Wang C, Wang X,
Ren Y and Yang F: Promoting effect of baicalin on nitric oxide
production in CMECs via activating the PI3K-AKT-eNOS pathway
attenuates myocardial ischemia-reperfusion injury. Phytomedicine.
63:1530352019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu ZJ, Chen C, Li XR, Ran YY, Xu T, Zhang
Y, Geng XK, Zhang Y, Du HS, Leak RK, et al: Remote ischemic
preconditioning-mediated neuroprotection against stroke is
associated with significant alterations in peripheral immune
responses. CNS Neurosci Ther. 22:43–52. 2016. View Article : Google Scholar : PubMed/NCBI
|