Introduction
Allergic rhinitis (AR) is a non-communicable chronic
inflammatory disease of the nasal mucosa mediated by immunoglobulin
E (IgE). AR is caused by exposure of genetically susceptible
individuals to environmental allergens (1). The prevalence of AR has increased
worldwide, affecting 10–40% of the global population (2,3);
in China, AR incidence has been reported to range from 8.7 to 24.1%
of the population (4). AR is also
associated with a variety of complications, including asthma and
otitis media, which affect quality of life and work performance,
and imposes a financial burden (3,5).
Currently, corticosteroids and antihistamines (H1 receptor
blockers) are used for AR treatment (6–8).
Although glucocorticoids are the most effective drug for most
patients at present, systemic side effects limit their use in large
doses over long periods of time (9). Moreover, glucocorticoids do not cure
AR since a rebound phenomenon occurs following drug withdrawal.
Antihistamines can attenuate sneezing, itching and runny nose, but
have no effect on congestion (10). Furthermore, anticholinergic side
effects including drowsiness, sedation and somnolence, and
depression are associated with multiple antihistamine treatment
(6,11). Therefore, providing a novel
strategy derived from natural products that are safe and effective
for AR therapy is of practical importance.
Total glucosides of paeony (TGP) is an active
ingredient extracted from the root of Paeonia alba that is
characterized by anti-inflammatory, anti-oxidative and
pain-relieving properties (9,12).
In addition, TGP regulates the balance of the T helper (h)1/Th2
ratio via two-way cellular immune regulation, which induces reverse
modulation of B lymphocyte growth (13). TGP has been reported to serves an
important role in the treatment of both inflammatory and immune
disease (14–16). Previous studies have shown that
the pathogenesis of AR is type I allergic disease caused by Th1/Th2
immune imbalance, accompanied by Th17, T regulatory and other Th
cell participation (10,17), indicating that TGP may have a
therapeutic effect on AR.
AR is an inflammatory disease with complex
pathogenesis involving a variety of signaling cascades and
regulatory pathways (18). To the
best of our knowledge, its molecular mechanism has not yet been
elucidated. The transforming growth factor-β (TGF-β) family has
been reported to serve a critical role in inflammatory and immune
response regulation (19,20). Evidence suggests that the
immunoreactivity of the TGF-β signal pathway is enhanced along with
increased nasal mast cells in AR (21), suggesting TGF-β signaling may be
involved in AR pathogenesis. As an inhibitor of TGF-β signaling,
Sma- and Mad-related protein 7 (Smad7) blocks TGF-β1-triggered
signaling by binding to the TGF-β receptor to mediate biological
activity of TGF-β1 and affect airway remodeling (22). Therefore, it was hypothesized that
TGP ameliorates the symptoms of AR by regulating the Smad7-TGF-β
signaling pathway.
The present study aimed to provide insight into the
effect of TGP in an AR mouse model by investigating the Smad7/TGF-β
signaling pathway to elucidate the potential mechanism underlying
the role of TGP and propose an optimal therapeutic strategy for
AR.
Materials and methods
Animals
A total of 88 specific-pathogen-free BALB/c male
mice (age, 6 weeks; weight, 20–22 g) were obtained from Jinan
Pengyue Laboratory Animal Breeding Co., Ltd. (Jinan, China;
Research Resource Identifier SCR_010607). All mice were
accommodated at an average temperature of 22±2°C with 55±10%
humidity in a controlled habitat with a 12-h light/dark cycle
(light on 8:00 a.m.-8:00 p.m.). In addition, free access to
standard food and water was provided. All experimental procedures
performed on animals were based on the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (23) and the study was approved by the
Institutional Animal Care and Use Committee of the First Affiliated
Hospital of Zhejiang Chinese Medical University (approval no.
AWE2020030601). All efforts were made to minimize the suffering of
animals.
Construction of AR mouse model
Mice used to establish the AR model were sensitized
via intraperitoneal injection of 75 µg ovalbumin (OVA;
Sigma-Aldrich; Merck KGaA) diluted in 200 µl sterile normal saline
supplemented with 2 mg aluminum hydroxide (Sigma-Aldrich; Merck
KGaA). Diluted OVA (total volume, 200 µl) was injected into the
mice on days 0, 7, 14 and 21, respectively. Subsequently, mice were
challenged with daily nasal instillation of 500 µg OVA diluted in
20 µl sterile saline on days 23–27 after initial sensitization. For
the AR control (con), challenge with OVA was replaced by challenge
by sterile saline.
Study grouping
A total of 88 mice were randomly assigned into seven
groups (n=8/group) as follows: i) Con (untreated); ii) AR
(OVA-induced AR); iii) AR + saline (AR mice given 60 mg/kg saline
orally); iv) AR + 10 mg/kg TGP (AR mice given 10 mg/kg TGP orally);
v) AR + 20 mg/kg TGP (AR mice given 20 mg/kg TGP orally); vi) AR +
30 mg/kg TGP (AR mice given 30 mg/kg TGP orally); vii) AR + 60
mg/kg TGP (AR mice given 60 mg/kg TGP orally); viii) AR + 120 mg/kg
TGP (AR mice given 120 mg/kg TGP orally); ix) AR + small
interfering (si)-con (AR mice injected with 60 µg/kg siRNA negative
con vector via the caudal vein); x) AR + si-Smad7 (AR mice injected
with 40 µg/kg Smad7 siRNA vector via the caudal vein) and xi) AR +
TGP + si-Smad7 (AR mice given 60 mg/kg TGP orally and injected with
40 µg/kg Smad7 siRNA via the caudal vein).
After grinding TGP (H20055058; Ningbo Lihua
Pharmaceutical Co., Ltd.) into powder, 10 mg/ml suspension was
prepared with 0.5% sodium carboxymethyl cellulose. For the TGP
treatment groups, mice were administered 60 mg/kg TGP orally after
daily intranasal challenge on days 28–42, while an equal volume of
saline instead of TGP was given to mice in the saline groups. The
siRNA negative con (forward, 5′-UUCUCCGAACGUGUCACG-UTT-3′ and
reverse, 5′-ACGUGACACGUUCGGAGAATT-3′) and Smad7 siRNA vectors
(forward, 5′-CCAAUGACCACGAGUUUA-UTT-3′ and reverse,
5′-AUAAACUCGUGGUCAU-UGGTT-3′) were designed and constructed by
Shanghai GenePharma Co., Ltd.
Measurement of nasal symptoms
Following the final OVA/sterile saline
challenge on day 27 post-initial sensitization, the severity of
nasal allergic symptoms was detected by recording the frequency of
sneezing and nose rubbing motions in all mice for 20 min.
Measurement of nasal symptoms was performed in a single-blinded
manner by three experimenters.
Blood and tissue samples
At the end of the experiment, mice in each group
were fasted overnight and anesthetized with intraperitoneal
injection of sodium pentobarbital (50 mg/kg body weight). The mice
were sacrificed by cervical dislocation following deep anesthesia.
Blood (0.5 ml) was harvested from the abdominal aorta into tubes
with EDTA followed by centrifugation at 1,000 × g for 20 min at 4°C
to prepare plasma for the biochemical analysis. The nasal mucosa
was removed immediately and rinsed with cold saline before
preserving at −70°C. Half of the nasal mucosa tissue was fixed in
4% paraformaldehyde for 24 h at room temperature and then embedded
in paraffin, followed by slicing into 5 µm sections for
histological examination. Cold Tris-HCl (10 mM) was used to
homogenize the rest of the tissue immediately, and clear
supernatant was obtained by centrifuging at 4,000 × g for 10 min at
4°C for use in biochemical analysis.
Immunofluorescence
The nasal mucosa slices were dehydrated and dewaxed
followed by treatment with 3% hydrogen peroxide for 10 min at room
temperature to quench the endogenous peroxidase activity. Then, 10%
goat serum (cat. no. 5425S; Cell Signaling Technology, Inc.) and
0.3% Triton X-100 PBS solution were used to block the slices for 1
h at room temperature, followed by culturing with primary
antibodies [Smad7 (cat. no. ab272928; 1:100) and TGF-β (cat. no.
ab15537; 5 µg/ml); both Abcam] overnight at 4°C. Subsequently,
corresponding fluorescent-labeled secondary antibody (cat. no.
ab150117; 1:200; Abcam) was used to treat the slices for 1 h at
room temperature. To identify the nuclei, 5 µg/ml DAPI (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to stain the slices for 5
min at room temperature. Finally, a fluorescence microscope
(Olympus Corporation; cat. no. BX 51; magnification, ×200) was used
to observe Smad7- and TGF-β-positive cells in the nasal mucosa
slices. Data were evaluated by Image ProPlus version 5.0 software
(Media Cybernetics, Inc.).
Immunohistochemistry
For immunohistochemistry assay, xylene was used to
dewax nasal mucosa tissue slices, which were then rehydrated
followed by dehydrating in graded ethanol solution. Slices were
heated with citrate (10 mmol/l, pH 6.0) in a microwave for 30 min
for antigen retrieval. To block the endogenous peroxidase activity,
0.3% hydrogen peroxide solution was utilized to incubate the slices
for 30 min at room temperature. Following 5 min washing in PBS, the
slices were cultured with primary antibody against Smad7 (cat. no.
ab216428, 1:100, Abcam,) and TGF-β (cat. no. ab15537, 1:100, Abcam)
at 4°C overnight, then treated with horseradish peroxidase
(HRP)-conjugated secondary antibody (cat. no. ab6721, 1:1,000,
Abcam,) for 30 min at room temperature. Finally, 3,
3′-diamino-benzidine tetrahydrochloride was used to develop the
signal for visualization of positive cells for examination under a
light microscope (Zeiss GmbH; magnification, ×500;). Data were
evaluated using Image ProPlus version 5.0 software (Media
Cybernetics, Inc.).
Western blot analysis
RIPA lysis buffer (Beyotime Institute of
Biotechnology) supplemented with protease inhibitors was utilized
to obtain total protein from nasal tissue homogenate and the
concentration was determined by bicinchoninic acid assay (BCA) kit
(Pierce; Thermo Fisher Scientific, Inc.). Subsequently, equal
amounts of total protein (30 mg/lane) were isolated by 10% sodium
dodecyl sulfate polyacrylamide gel electrophoresis followed by wet
transfer onto PVDF membranes (MilliporeSigma). Then, 5% non-fat
milk diluted in TBST (0.1% Tween-20) was used to block the
membranes for 1 h at room temperature, followed by treatment with
rabbit primary antibodies against Smad7 primary (cat. no. ab227309,
1:500; Abcam), TGF-β (cat. no. ab205604, 1:1,000; Abcam), Bax (cat.
no. ab182733; 1:2,000; Abcam), Bcl-2 (cat. no. ab32124, 1:1,000;
Abcam), Cleaved-caspase 3 (cat. no. ab2302, 1:200; Abcam), IL-4
(cat. no. ab62351, 1:2,000; Abcam), IL-5 (cat. no. 3432, 1:1,000;
Cell Signaling Technology, Inc.), IL-17 (cat. no. 13828, 1:2,000;
Cell Signaling Technology, Inc.), IFN-γ (cat. no. 8455, 1:1,500;
Cell Signaling Technology, Inc.) and β-actin (cat. no. ab8227;
1:1,000; Abcam) at 4°C overnight. Membranes were rinsed with PBS
for 5 min and incubated with appropriate HRP-conjugated secondary
antibody IgG (cat. no. ab15842, 1:1,000; Abcam) for 1 h at room
temperature. Protein bands were developed with ECL detection
reagent (Thermo Fisher Scientific, Inc.) and the band intensity was
quantified using Image J software (National Institutes of Health;
version 1.44o). β-actin was used as the internal control to
normalize the relative levels of protein.
Measurement of IgE and inflammatory
cytokines in serum
The levels of IgE and inflammatory cytokines in the
plasma of each mouse were measured by ELISA. ELISA kits (R&D
System) were utilized to determine the levels of IL-4 (cat. no.
PI612; Beyotime Institute of Biotechnology), IL-5 (cat. no. PI620;
Beyotime Institute of Biotechnology), IL-17 (cat. no. PI545;
Beyotime Institute of Biotechnology), IFN-γ (cat. no. PI508;
Beyotime Institute of Biotechnology) and IgE (cat. no. 747734;
Pharmingen) in the serum according to the manufacturer's protocol.
Following washing by PBS, plasma was treated with HRP and
chromogenic solution of biotin. Finally, absorbance at 450 nm was
measured.
Histopathological evaluation of nasal
mucosa tissue
For histopathological evaluation of nasal mucosa
tissue, hematoxylin-eosin (HE) staining was performed to measure
the number of eosinophils, periodic acid-Schiff (PAS) staining was
used to detect the number of goblet cells and Masson staining was
performed to determine the percentage of collagen fibers. Briefly,
nasal mucosa tissue slices underwent dewaxing, rehydration and
dehydration followed by staining with HE, PAS and Masson's
trichrome reagent in accordance with the manufacturer's
instructions. (all Sigma-Aldrich; Merck KGaA). Numbers of
eosinophils and goblet cells, as well as the percentage of collagen
fibers were observed and quantified using Image-Proplus 6.0 (Media
Cybernetics, Inc.) under a light microscope (Zeiss GmbH) in five
randomly selected fields of view at 500× magnification. The
histopathological changes in each group were assessed in a
double-blind manner. Eosinophils and gobletells are presented as
number/mm2 per section, while collagen fibers are
expressed as a percentage.
Oxidative stress detection
The concentration of malondialdehyde (MDA) and
glutathione (GSH), and activity of superoxide dismutase (SOD), and
catalase (CAT) in the nasal mucosa tissue and serum were determined
as previously described (20). A
homogenizer was used to prepare 100 g/l tissue homogenate from
fresh nasal mucosa tissue, followed by centrifugation at 4,000 × g
for 20 min at 4°C. BCA protein assay kit was used to quantify the
protein concentration in tissue using the appropriate amount of
supernatant (20 µl). In accordance with the manufacturer's
protocol, the concentrations of MDA and GSH, and the activities of
SOD and CAT in tissue supernatant and serum were measured using MDA
kit (cat. no. S0131), SOD (cat. no. S0109), GSH (cat. no. S0052)
and CAT kits (cat. no. C0016; all Beyotime Institute of
Biotechnology), respectively. The concentrations of MDA and GSH are
presented in nmol/mg protein, while the activity of SOD and CAT are
presented in U/mg protein.
TUNEL staining
Apoptosis of nasal tissue cells was detected by
TUNEL assay using an In Situ Cell Death Detection kit (cat.
no. 11684795910; Roche Diagnostics GmbH) according to the
manufacturer's protocol. Briefly, following deparaffinization of
nasal tissue slices with gradient alcohol, DNA fragments were
stained using the In Situ Cell Death Detection kit according
to the manufacturer's instructions. Then, a confocal microscope
(Leica-Leitz DM-Il; Leica Microsystems GmbH) was used to observe
the stained sections at ×200 magnification. To evaluate apoptosis,
five fields of view were randomly selected to count TUNEL-positive
cells and analyzed using Image-Pro Plus 6.0 (Media Cybernetics,
Inc.) to quantify the apoptosis rate.
Statistical analysis
Data are expressed as the mean ± standard deviation
of three experimental repeats. All statistical analysis was
performed utilizing SPSS 21.0 statistical software (IBM Corp). Data
with non-normal distribution was analyzed by Mann-Whitney U test.
For the data with normal distribution, differences between two
groups were analyzed by unpaired Student's t-test; comparisons
between ≥3 groups were analyzed by one-way ANOVA followed by post
hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of Smad7/TGF-β
pathway-associated proteins is downregulated in the nasal tissue of
AR mice
First, the dose response profile of TGP effect on
the development of AR was assessed. TGP treatment relieved serum
IgE, eosinophil count and nasal symptoms (sneezing and rubbing) in
AR mice in a dose-dependent manner (Fig. S1). Thus, 60 mg/kg TGP was
selected as the best concentration for subsequent experiments.
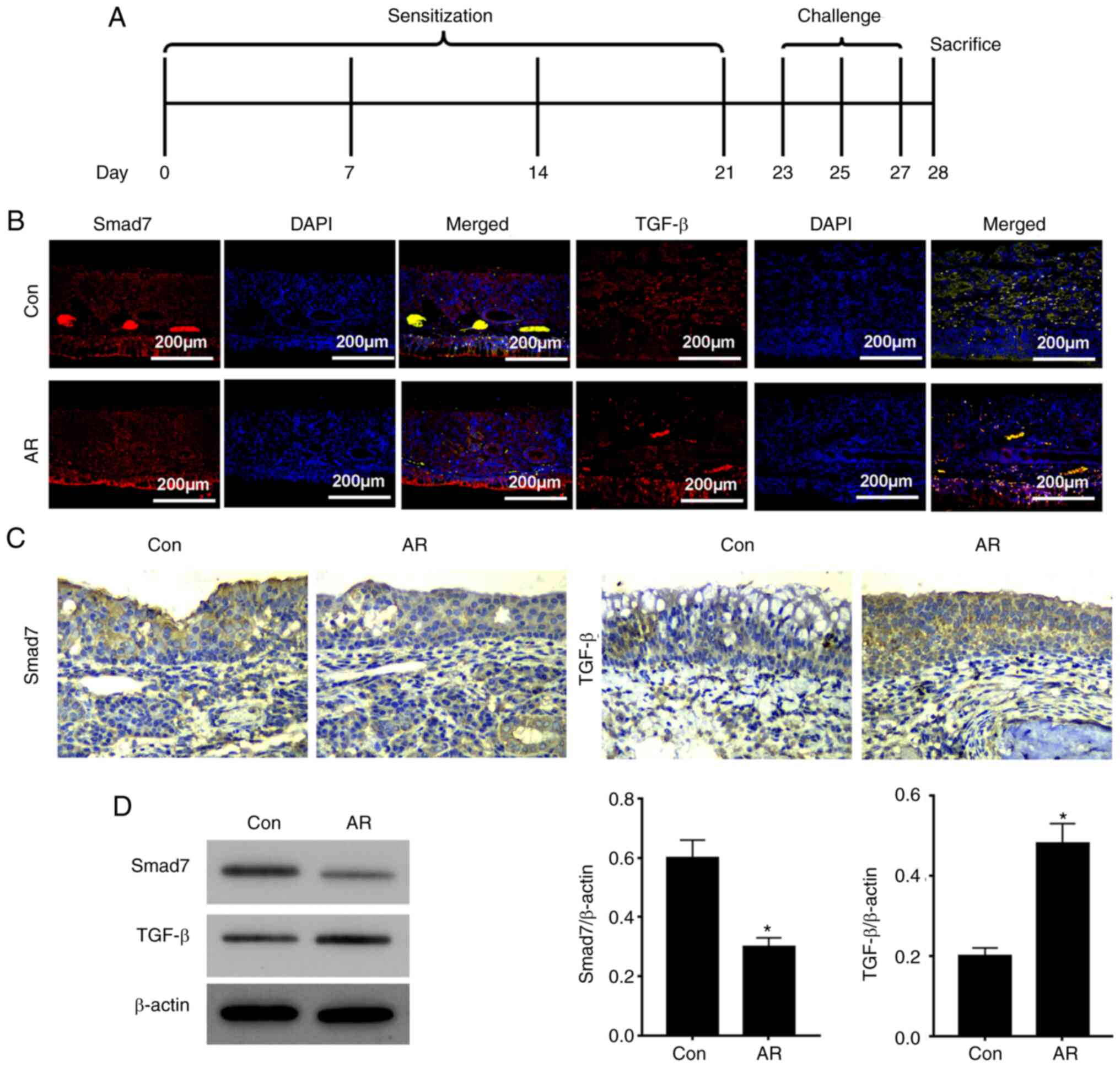
To investigate the role of the Smad7TGF-β pathway in
AR, an AR mouse model was constructed (Fig. 1A). Immunofluorescence assay
demonstrated that Smad7 expression was decreased and TGF-β
expression was increased in the AR group compared with the Con
group (Fig. 1B). Protein
expression levels of Smad7 were decreased and those of TGF-β were
enhanced in the nasal mucosa of AR mice, as shown by
immunohistochemistry and western blot analysis (Fig. 1C and D). These data indicated that
expression of Smad7 was upregulated and that of TGF-β was
downregulated in the nasal tissue of AR mice.
TGP upregulates the Smad7/TGF-β
pathway in nasal tissue of AR mice
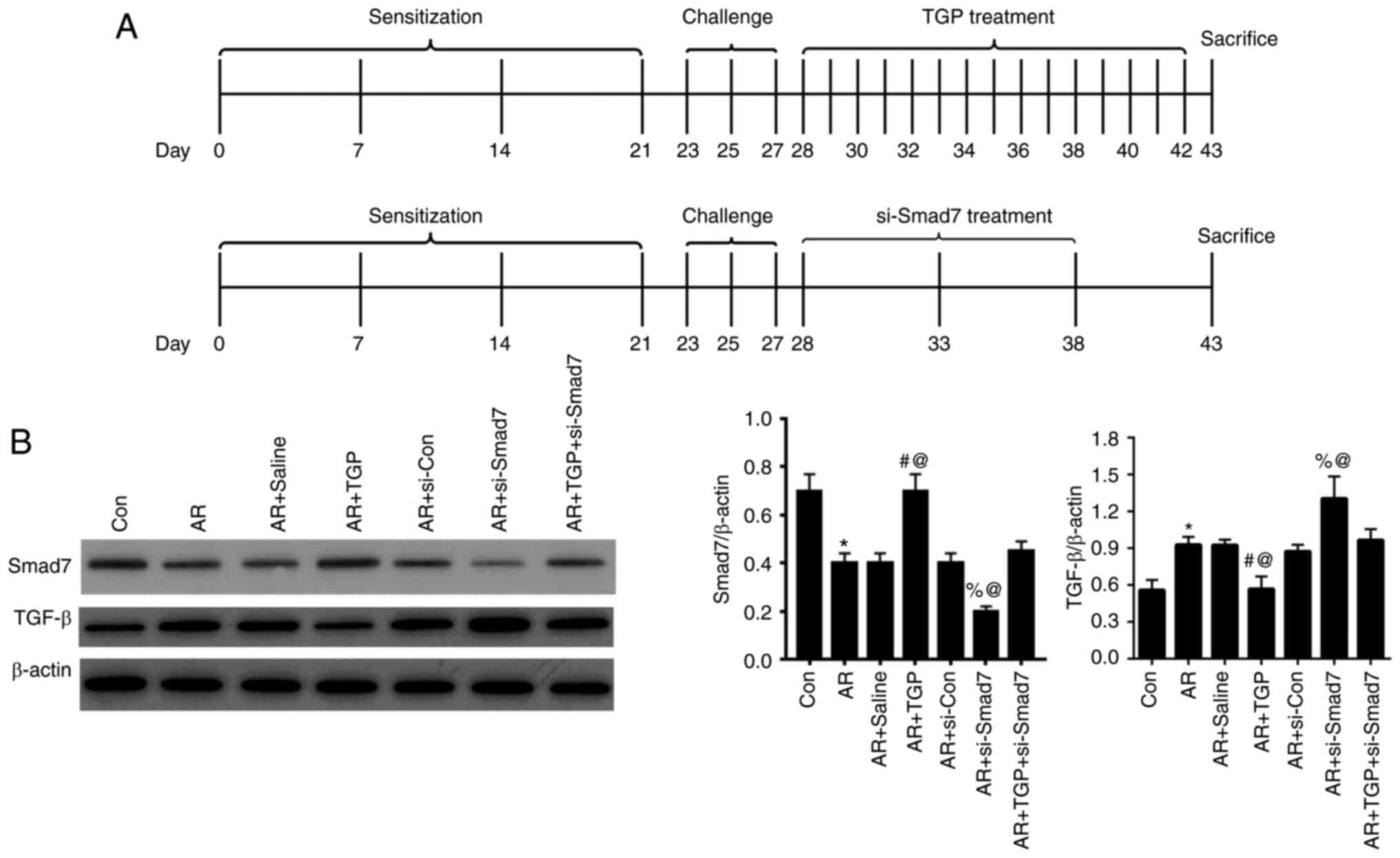
The effects of TGP on the Smad7/TGF-β pathway in AR
mice were investigated. The treatment protocol (TGP and si-Smad7)
of AR model mice is shown in Fig.
2A. Following nasal cavity challenge, AR mice were treated with
TGP on days 28–42 and/or si-Smad7 on days 28, 33 and 38. Finally,
on day 43, mice were sacrificed. Protein expression levels of Smad7
and TGF-β were detected by western blot analysis. The decreased
expression of Smad7 induced by AR was reversed in the AR + TGP
group but further decreased in the AR + si-Smad7 group; this effect
was partially restored in the AR + TGP + si-Smad7 group. Enhanced
expression of TGF-β induced by AR was rescued in the AR + TGP group
but further increased in the AR + si-Smad7 group; this effect was
partially restored in the AR + TGP + si-Smad7 group (Fig. 2B). Taken together, these findings
indicated that TGP regulated the Smad7/TGF-β pathway in the nasal
tissue of AR mice.
TGP alleviates increased serum IgE,
nasal symptoms and histopathological changes in AR mice
The increased serum IgE concentration induced by AR
was attenuated in the AR + TGP group but further enhanced in the AR
+ si-Smad7 group; this effect was reversed in AR + TGP + si-Smad7
group (Fig. 3A). The aggravated
nasal symptoms (sneezing and rubbing) induced by AR were lessened
in the AR + TGP group but further increased in the AR + si-Smad7
group; this effect was partially reversed in the AR + TGP +
si-Smad7 group (Fig. 3B and C).
The increased number of eosinophil, goblet cell count and
percentage of collagen fibers induced by AR were decreased in the
AR + TGP group but further increased in the AR + si-Smad7 group;
this effect was rescued in the AR + TGP + si-Smad7 group (Fig. 3D). These data suggested that TGP
decreased serum IgE concentration, nasal symptoms and
histopathological changes in AR mice.
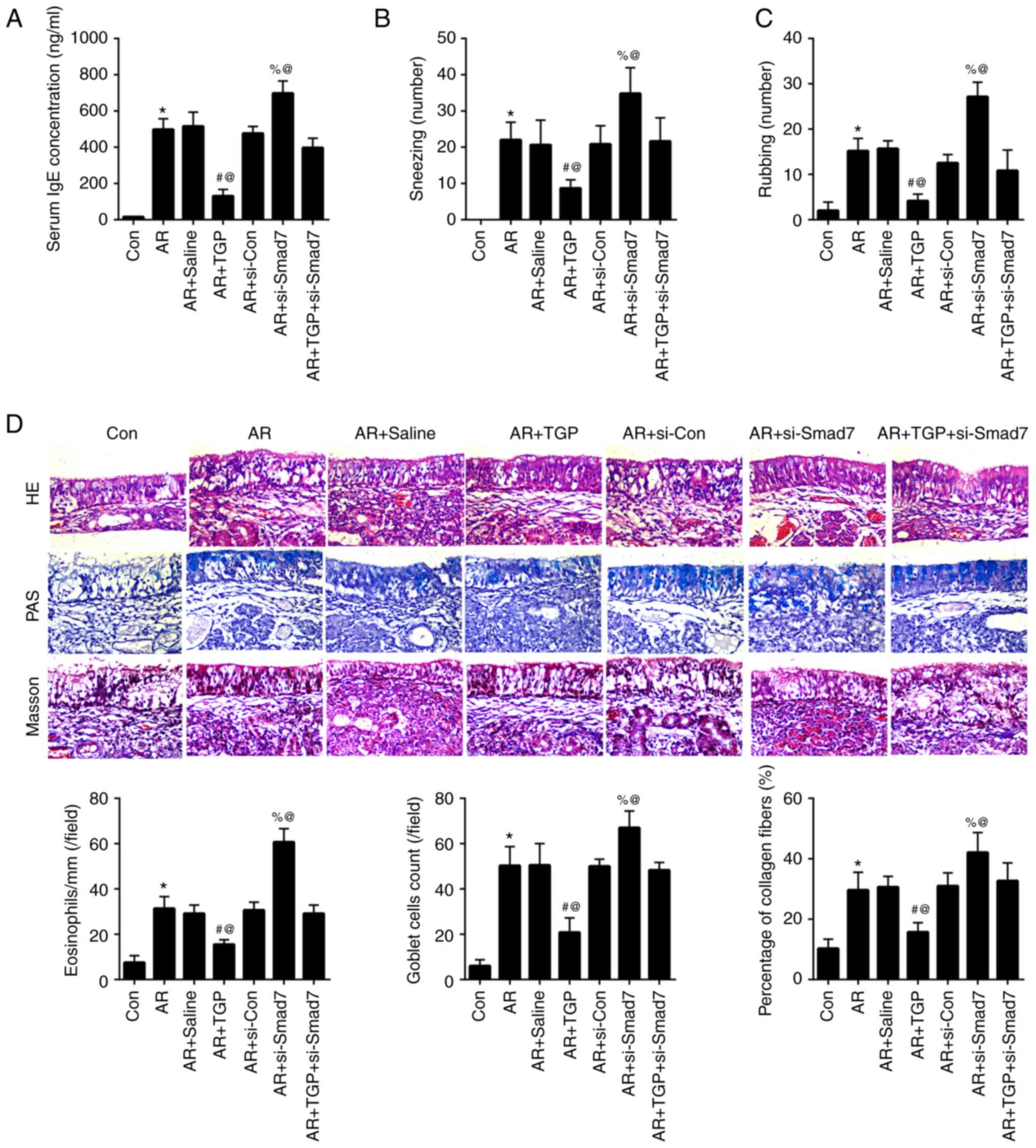 | Figure 3.Effects of TGP on serum IgE, nasal
symptoms and histopathological changes in AR mice. (A) IgE
concentration in the blood was determined by ELISA. (B) Sneezing
and (C) rubbing frequency were observed in AR mice for 30 min. (D)
Level of eosinophils, goblet cell count and percentage of collagen
fibers were determined by HE, PAS and Masson staining, respectively
(magnification, ×500). *P<0.05 vs. Con; #P<0.05
vs. AR + Saline; %P<0.05 vs. AR + si-Con;
@P<0.05 vs. AR + TGP + si-Smad7. Smad7, Sma- and
Mad-related protein 7; AR, allergic rhinitis; Con, control; si,
small interfering; TGP, total glucosides of paeony; HE,
hematoxylin-eosin; PAS, period acid-Schiff. |
TGP alleviates oxidative stress in AR
mice
Next, the effects of TGP on oxidative stress in AR
mice were determined. The increased MDA induced by AR in the blood
was decreased in the AR + TGP group but further heightened in the
AR + si-Smad7 group; this effect was rescued in the AR + TGP +
si-Smad7 group (Fig. 4A). The
weakened blood levels of GSH, SOD and CAT caused by AR were
enhanced in the + TGP group but further decreased in the AR +
si-Smad7 group; this effect was partially restored in the AR + TGP
+ si-Smad7 group (Fig. 4B-D). The
enhanced MDA levels resulting from AR in nasal mucosa were
decreased in the AR + TGP group but further increased in the AR +
si-Smad7 group; this effect was reversed in the AR + TGP + si-Smad7
group (Fig. 4E). The decreased
GSH, SOD and CAT levels induced by AR in the nasal mucosa were
increased in the AR+ TGP group but further weakened in the AR +
si-Smad7 group; this effect was reversed in the AR + TGP + si-Smad7
group (Fig. 4F-H). These results
indicated that TGP ameliorated oxidative stress in AR mice.
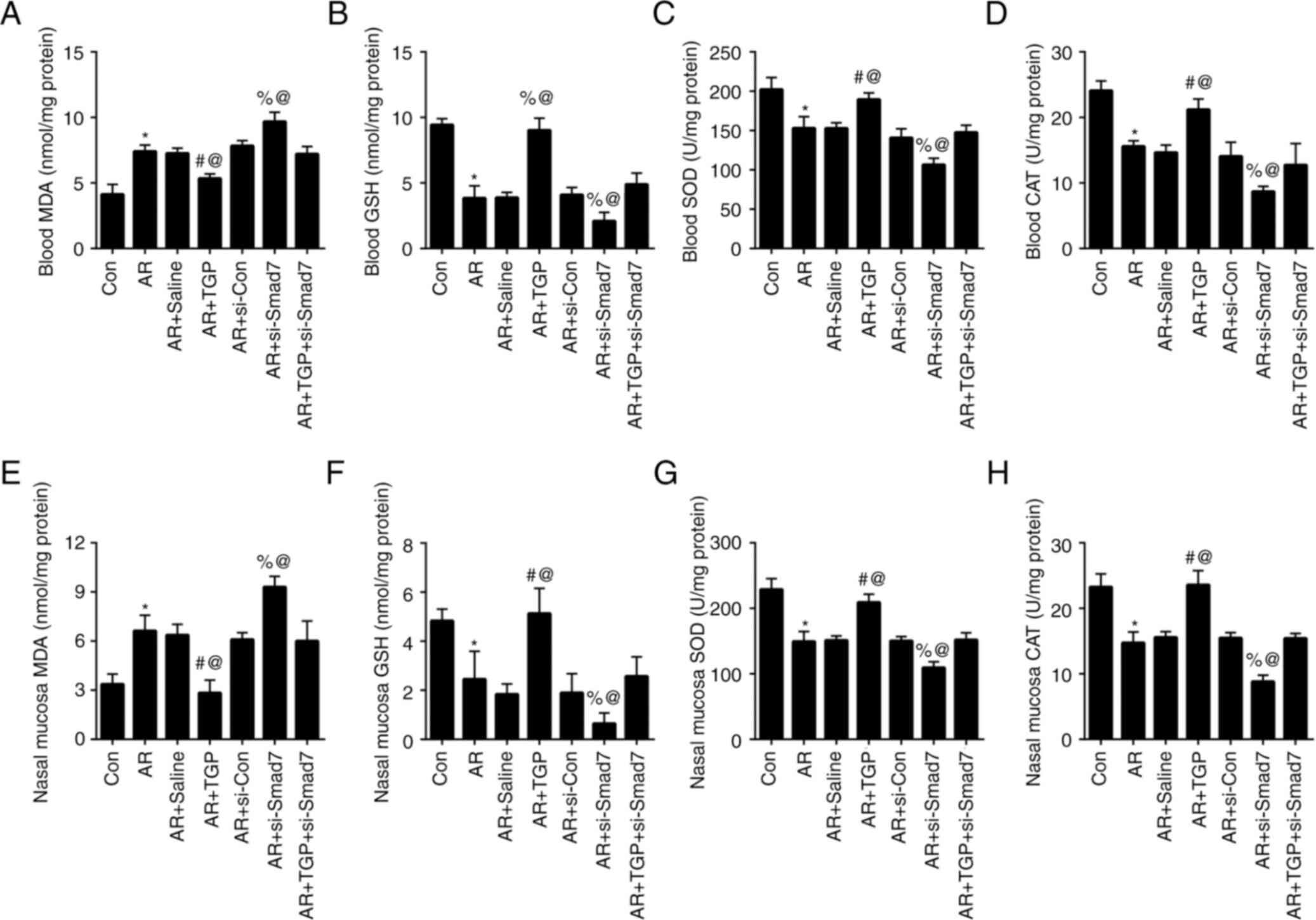 | Figure 4.Effects of TGP on oxidative stress in
AR mice. Levels of (A) MDA, (B) GSH, (C) SOD and (D) CAT were
measured in the blood of AR mice. Levels of (E) MDA, (F) GSH, (G)
SOD and (H) CAT were measured in the nasal mucosa of AR mice.
*P<0.05 vs. Con; #P<0.05 vs. AR + Saline;
%P<0.05 vs. AR + si-Con; @P<0.05 vs. AR
+ TGP + si-Smad7. Smad7, Sma- and Mad-related protein 7; AR,
allergic rhinitis; Con, control; si, small interfering; TGP, total
glucosides of paeony; MDA, malondialdehyde; GSH, glutathione; SOD,
superoxide dismutase; CAT, catalase. |
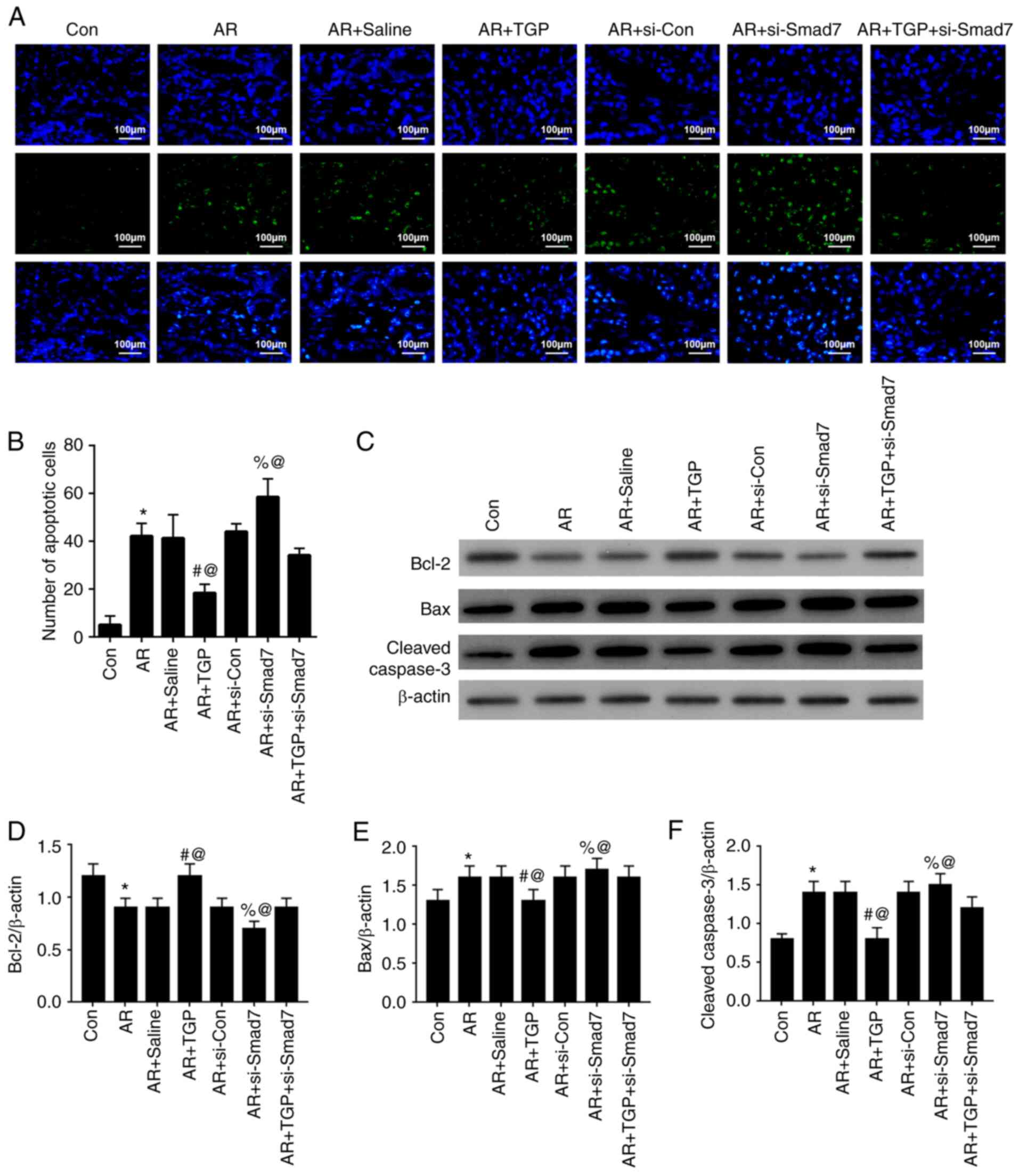
TGP alleviates cell apoptosis in AR
mice
The effects of TGP on cell apoptosis in AR mice were
investigated. The increased cell apoptosis induced by AR was
lessened in the AR + TGP group but further enhanced in the AR +
si-Smad7 group; this effect was partially restored in the AR + TGP
+ si-Smad7 group (Fig. 5A and B).
In addition, decreased Bcl-2 expression levels induced by AR were
enhanced in the AR + TGP group but further weakened in the AR +
si-Smad7 group; this effect was reversed in the AR + TGP + si-Smad7
group. The increased Bax and Cleaved-caspase 3 expression levels
caused by AR were decreased in the AR + TGP group but further
enhanced in the AR + si-Smad7 group; this effect was reversed in
the AR + TGP + si-Smad7 group (Fig.
5C-F). These findings indicated that TGP ameliorated cell
apoptosis in AR mice.
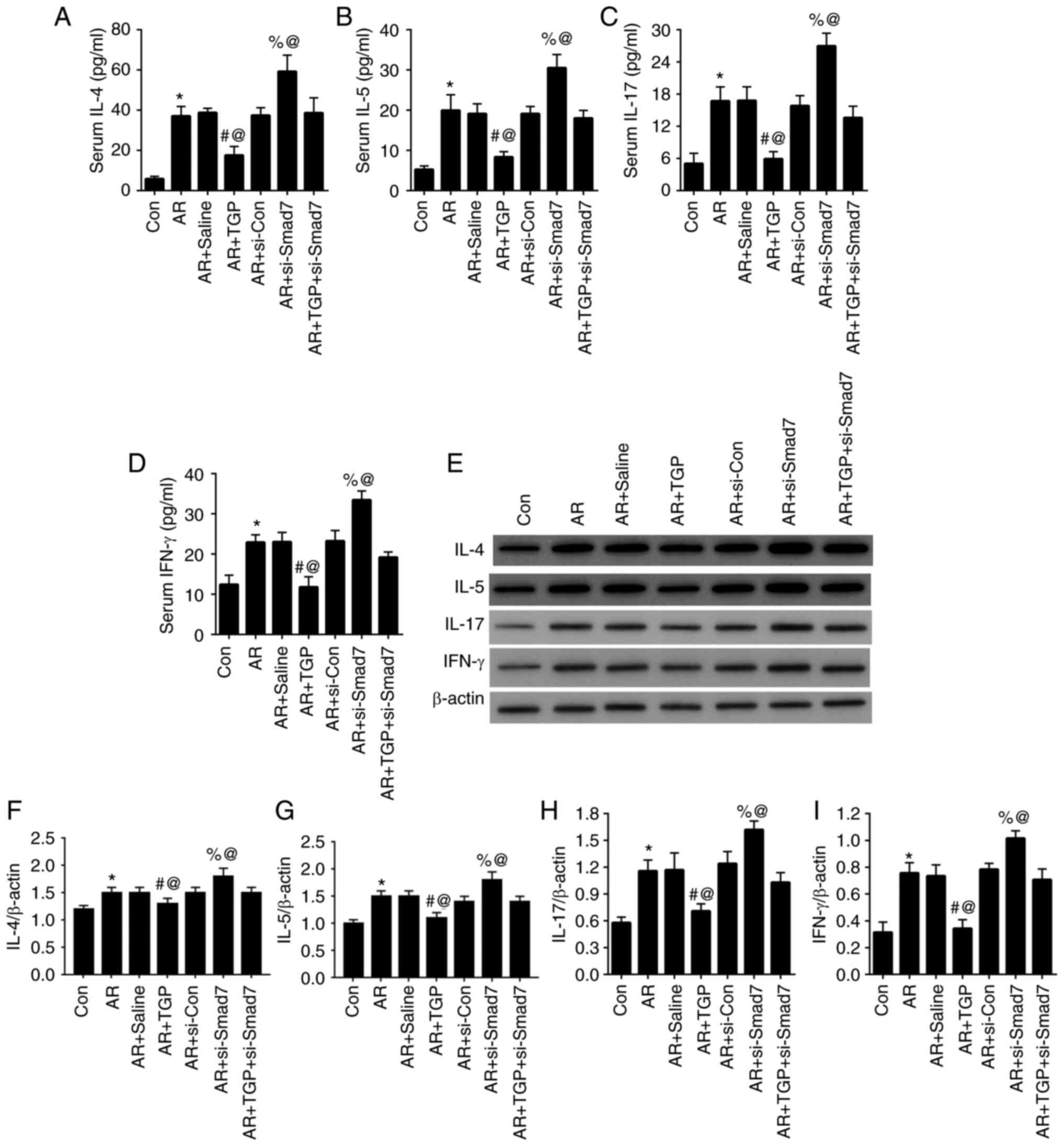
TGP alleviates inflammatory response
in AR mice
Lastly, the effect of TGP on inflammatory response
in AR mice was investigated. ELISA demonstrated that increased
levels of IL-4, IL-5, IL-17 and IFN-γ caused by AR were decreased
in the AR + TGP group but further increased in the AR + si-Smad7
group; this effect was reversed in the AR + TGP + si-Smad7 group
(Fig. 6A-D). Similar changes in
protein levels of IL-4, IL-5, IL-17 and IFN-γ were also detected by
western blot analysis (Fig. E-I). These findings demonstrated that
TGP ameliorated the inflammatory response in AR mice.
Discussion
AR is an allergen-induced allergic inflammatory
response in the nasal mucosa that is initiated by release of
IgE-mediated mediators and involves a variety of immunoreactive
cells and cytokines including, Th2 lymphocytes, M2a macrophages and
IL-4 (24–26). The Smad7/TGF-β signaling pathway
is not only involved in regulation of numerous types of disease,
including spinal cord ischemia reperfusion, anaplastic thyroid
cancer and pancreatic cancer (22,27,28), but has also been reported to be
associated with AR processes (21). In the present study, expression of
Smad7 was upregulated and that of TGF-β was downregulated in the
nasal tissue of AR mice.
TGP is the primary active ingredient of Paeonia
lactiflora Pall. and exerts anti-inflammation, anti-oxidation
and analgesic effects (12,29). In recent years, it has been
reported that TGP has numerous immunomodulatory effects in various
types of disease, including rheumatoid arthritis, autoimmune
hepatitis and Sjögren's syndrome (SS) (30–32). Therefore, the present study aimed
to determine the role of TGP in AR. TGP regulated the Smad7/TGF-β
pathway in nasal tissue of AR mice by upregulating Smad7 and
downregulating TGF-β. Moreover, TGP relieved serum IgE, nasal
symptoms and histopathological changes in AR mice.
Oxidative stress refers to the imbalance between
production of oxygen free radicals and endogenous antioxidants that
offset their harmful effects, resulting in irreversible tissue
damage (33,34). Oxygen free radicals interact with
DNA, proteins and lipids, inducing conformational changes of cell
structures and causing cellular derangement and dysfunction
(35). Oxidative stress is a key
feature in the pathology of AR (36–38). The present study demonstrated that
TGP ameliorated oxidative stress in AR mice by decreasing MDA
levels and enhancing GSH, SOD and CAT levels.
Cell apoptosis refers to spontaneous and orderly
death of cells to maintain homeostasis, involving the activation,
expression and regulation of a series of genes including Bax and
Bcl-2 (39,40). Cell apoptosis is a key process in
AR that is regulated by numerous factors. For example, inactivation
of the PD-1/PD-L1 pathway increases apoptosis of CD19+
CD25+ B regulatory cells and inhibits secretion of IL-10
in patients with AR (41).
MicroRNA (miR)-375 suppresses the JAK2/STAT3 pathway to decrease
nasal mucosa cell apoptosis and relieve AR (8). Tumor necrosis factor α and IL-5
regulate the apoptosis rate of olfactory sphere cells to weaken
olfactory regeneration in AR mice (42). In the present study, TGP
downregulated Bax and Cleaved-caspase 3, but upregulated Bcl-2 to
ameliorate cell apoptosis in AR mice.
In the later stage of AR, inflammatory cells
(primarily eosinophil granulocytes) accumulate in the nasal polyps
and secrete cytokines and inflammatory mediators, such as IgE,
IL-4, IL-5, IL-17 and IFN-γ, which serve an important role in the
development of local inflammation (43). miR-345-5p affects the Toll-like
receptor 4/NF-κB pathway in AR by serving as an anti-inflammatory
regulator (44). Adrenoreceptor
β2 inhibits AR inflammatory cytokine production induced by IL-13
(45). IL-37 suppresses the C-C
motif chemokine ligand 11 signaling pathway to alleviate allergic
inflammation in AR mice (46).
The present study verified that TGP ameliorated the inflammatory
response in AR mice by decreasing IL-4, IL-5, IL-17 and IFN-γ
levels. Smad7 inhibition aggravated the symptoms of AR mice via
activation of the TGF-β pathway and reversed the protective effect
of TGP in AR mice.
There are certain limitations in the present study.
Although TGP significantly inhibited inflammatory cytokine levels
in plasma of AR mice, levels of inflammatory cytokines in nasal
lavage fluid or mucosal extract were not investigated. TPG contains
>15 monoterpene glycosides; however, the most effective
component of TGP to alleviate AR is still unknown. Paeoniflorin and
albiflorin are the most abundant ingredients and account for the
pharmacological effects observed for TPG in both in vitro
and in vivo studies (47–49). Therefore, it was speculated that
paeoniflorin and albiflorin may be effective components of TGP in
inhibiting AR; however, further investigations are required.
In summary, the present study demonstrated that TGP
ameliorated oxidative stress, apoptosis and inflammatory response
by regulating the Smad7/TGF-β pathway in AR.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ was responsible for study concept and design and
drafting the manuscript. AZ performed experiments, analyzed and
collected data and reviewed the manuscript. All authors have read
and approved the final manuscript. YJ and AZ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All experimental procedures performed on animals in
this study were based on the National Institutes of Health Guide
for the Care and Use of Laboratory Animals. The study was approved
by the Institutional Animal Care and Use Committee (approval no.
AWE2020030601).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Al Suleimani YM and Walker MJ: Allergic
rhinitis and its pharmacology. Pharmacol Ther. 114:233–260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng L, Chen J, Fu Q, He S, Li H, Liu Z,
Tan G, Tao Z, Wang D, Wen W, et al: Chinese society of allergy
guidelines for diagnosis and treatment of allergic rhinitis.
Allergy Asthma Immunol Res. 10:300–353. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kakli HA and Riley TD: Allergic Rhinitis.
Prim Care. 43:465–475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang L, Han D, Huang D, Wu Y, Dong Z, Xu
G, Kong W and Bachert C: Prevalence of self-reported allergic
rhinitis in eleven major cities in China. Int Arch Allergy Immunol.
149:47–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bao Y, Chen J, Cheng L, Guo Y, Hong S,
Kong W, Lai H, Li H, Li H, Li J, et al: Chinese Guideline on
allergen immunotherapy for allergic rhinitis. J Thorac Dis.
9:4607–4650. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Du K, Qing H, Zheng M, Wang X and Zhang L:
Intranasal antihistamine is superior to oral H1
antihistamine as an add-on therapy to intranasal corticosteroid for
treating allergic rhinitis. Ann Allergy Asthma Immunol.
125:589–596.e3. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McColl A, Michlewska S, Dransfield I,
Dransfield I and Rossi AG: Effects of glucocorticoids on apoptosis
and clearance of apoptotic cells. ScientificWorldJournal.
7:1165–1181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mims JW: Epidemiology of allergic
rhinitis. Int Forum Allergy Rhinol. 4 (Suppl 2):S18–S20. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmadiafshar A and Ahmadiafshar S:
Efficacy and safety of inhaled and intranasal corticosteroids.
Antiinflamm Antiallergy Agents Med Chem. 13:83–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wheatley LM and Togias A: Clinical
practice. Allergic rhinitis. N Engl J Med. 372:456–463. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hossenbaccus L, Linton S, Garvey S and
Ellis AK: Towards definitive management of allergic rhinitis: Best
use of new and established therapies. Allergy Asthma Clin Immunol.
16:392020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang H, Li J, Wang L, Wang S, Nie X, Chen
Y, Fu Q, Jiang M, Fu C and He Y: Total glucosides of paeony: A
review of its phytochemistry, role in autoimmune diseases, and
mechanisms of action. J Ethnopharmacol. 258:1129132020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang XT, Wang B, Zhang WH, Peng MQ and
Lin D: Total glucosides of paeony suppresses experimental
autoimmune uveitis in association with inhibition of Th1 and Th2
cell function in mice. Int J Immunopathol Pharmacol.
32:3946320177515472018.PubMed/NCBI
|
|
14
|
Lin J, Xiao L, Ouyang G, Shen Y, Huo R,
Zhou Z, Sun Y, Zhu X, Zhang J, Shen B and Li N: Total glucosides of
paeony inhibits Th1/Th17 cells via decreasing dendritic cells
activation in rheumatoid arthritis. Cell Immunol. 280:156–163.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou L, Cao T, Wang Y, Yao H, Du G, Tian Z
and Tang G: Clinical observation on the treatment of oral lichen
planus with total glucosides of paeony capsule combined with
corticosteroids. Int Immunopharmacol. 36:106–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Y, Jin L, Kong F, Zhang H, Fang X,
Chen Z, Wang G and Li X and Li X: Clinical and immunological
consequences of total glucosides of paeony treatment in Sjögren's
syndrome: A randomized controlled pilot trial. Int Immunopharmacol.
39:314–319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berker M, Frank LJ, Geßner AL, Grassl N,
Holtermann AV, Höppner S, Kraef C, Leclaire MD, Maier P, Messerer
DA, et al: Allergies-A T cells perspective in the era beyond the
TH1/TH2 paradigm. Clin Immunol. 174:73–83.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eifan AO and Durham SR: Pathogenesis of
rhinitis. Clin Exp Allergy. 46:1139–1151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sporn MB and Roberts AB: Transforming
growth factor-beta: Recent progress and new challenges. J Cell
Biol. 119:1017–1021. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lawrence DA: Transforming growth
factor-beta: A general review. Eur Cytokine Netw. 7:363–374.
1996.PubMed/NCBI
|
|
21
|
Salib RJ, Kumar S, Wilson SJ and Howarth
PH: Nasal mucosal immunoexpression of the mast cell
chemoattractants TGF-beta, eotaxin, and stem cell factor and their
receptors in allergic rhinitis. J Allergy Clin Immunol.
114:799–806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie L, Yu S, Yang K, Li C and Liang Y:
Hydrogen sulfide inhibits autophagic neuronal cell death by
reducing oxidative stress in spinal cord ischemia reperfusion
injury. Oxid Med Cell Longev. 2017:86402842017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chaudhury D, Walsh JJ, Friedman AK, Juarez
B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ,
et al: Rapid regulation of depression-related behaviours by control
of midbrain dopamine neurons. Nature. 493:532–536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naclerio RM, Meier HL, Kagey-Sobotka A,
Adkinson NF Jr, Meyers DA, Norman PS and Lichtenstein LM: Mediator
release after nasal airway challenge with allergen. Am Rev Respir
Dis. 128:597–602. 1983.PubMed/NCBI
|
|
25
|
Zhang Y, Lan F and Zhang L: Advances and
highlights in allergic rhinitis. Allergy. 76:3383–3389. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geng B, Dilley M and Anterasian C:
Biologic therapies for allergic rhinitis and nasal polyposis. Curr
Allergy Asthma Rep. 21:362021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiao C, Li L, Zhang P, Zhang L, Li K, Fang
R, Yuan L, Shi K, Pan L, Guo Q, et al: REGү ablation impedes
dedifferentiation of anaplastic thyroid carcinoma and accentuates
radio-therapeutic response by regulating the Smad7-TGF-β pathway.
Cell Death Differ. 27:497–508. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu Z, Xu Y, Zhao J, Liu Q, Feng W, Fan J
and Wang P: MiR-367 promotes epithelial-to-mesenchymal transition
and invasion of pancreatic ductal adenocarcinoma cells by targeting
the Smad7-TGF-β signalling pathway. Br J Cancer. 112:1367–1375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L, Yu J, Wang C and Wei W: The
effects of total glucosides of paeony (TGP) and paeoniflorin (Pae)
on inflammatory-immune responses in rheumatoid arthritis (RA).
Funct Plant Biol. 46:107–117. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu G, Wang Z, Li X, Liu R, Li B, Huang L,
Chen Y, Zhang C, Zhang H, Li Y, et al: Total glucosides of paeony
(TGP) alleviates constipation and intestinal inflammation in mice
induced by SjC6gren's syndrome. J Ethnopharmacol. 260:1130562020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen H, Wen Y, Pan T and Xu S: Total
glucosides of paeony improve complete freund's adjuvant-induced
rheumatoid arthritis in rats by inhibiting toll-like receptor
2-mediated tumor necrosis factor receptor-associated factor
6/nuclear factor-kappa B pathway activation. J Tradit Chin Med.
39:566–574. 2019.PubMed/NCBI
|
|
32
|
Shen M, Men R, Fan X, Wang T, Huang C,
Wang H, Ye T, Luo X and Yang L: Total glucosides of paeony
decreases apoptosis of hepatocytes and inhibits maturation of
dendritic cells in autoimmune hepatitis. Biomed Pharmacother.
124:1099112020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sies H: Oxidative stress: A concept in
redox biology and medicine. Redox Biol. 4:180–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hybertson BM, Gao B, Bose SK and McCord
JM: Oxidative stress in health and disease: The therapeutic
potential of Nrf2 activation. Mol Aspects Med. 32:234–246. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burton GJ and Jauniaux E: Oxidative
stress. Best Pract Res Clin Obstet Gynaecol. 25:287–299. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sim CS, Lee JH, Kim SH, Han MW, Kim Y, Oh
I, Yun SC and Lee JC: Oxidative stress in schoolchildren with
allergic rhinitis: Propensity score matching case-control study.
Ann Allergy Asthma Immunol. 115:391–395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Celik M, Tuncer A, Soyer OU, Sac'kesen C,
Tanju Besler H and Kalayci O: Oxidative stress in the airways of
children with asthma and allergic rhinitis. Pediatr Allergy
Immunol. 23:556–561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei Choo CY, Yeh KW, Huang JL, Su KW, Tsai
MH, Hua MC, Liao SL, Lai SH, Chen LC and Chiu CY: Oxidative stress
is associated with atopic indices in relation to childhood rhinitis
and asthma. J Microbiol Immunol Infect. 54:466–473. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu X, Lai Y and Hua ZC: Apoptosis and
apoptotic body: Disease message and therapeutic target potentials.
Biosci Rep. 39:BSR201809922019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Du L, Meng Q, Chen Y and Wu P: Subcellular
location prediction of apoptosis proteins using two novel feature
extraction methods based on evolutionary information and LDA. BMC
Bioinformatics. 21:2122020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang ZA and Tan F: The blockade of
PD-1/PD-L1 pathway promotes the apoptosis of CD19+
CD25+ Bregs and suppresses the secretion of IL-10 in
patients with allergic rhinitis. Scand J Immunol. 91:e128362020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim DK, Choi SA, Eun KM, Kim SK, Kim DW
and Phi JH: Tumour necrosis factor alpha and interleukin-5 inhibit
olfactory regeneration via apoptosis of olfactory sphere cells in
mice models of allergic rhinitis. Clin Exp Allergy. 49:1139–1149.
2019.PubMed/NCBI
|
|
43
|
Ventura MT, Bruno LM, Iacobelli A and
Tursi A: Eosinophils in allergic diseases: Immunopharmacological
regulation. Immunopharmacol Immunotoxicol. 19:405–423. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu J, Jiang Y, Han M, Jiang L, Liang D,
Li S, Xu Z, Wang L and Li N: MicroRNA-345-5p acts as an
anti-inflammatory regulator in experimental allergic rhinitis via
the TLR4/NF-κB pathway. Int Immunopharmacol. 86:1065222020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang L, Lv Q, Song X, Jiang K and Zhang J:
ADRB2 suppresses IL-13-induced allergic rhinitis inflammatory
cytokine regulated by miR-15a-5p. Hum Cell. 32:306–315. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lei H, Sun Y and Quan S: IL-37 relieves
allergic inflammation by inhibiting the CCL11 signaling pathway in
a mouse model of allergic rhinitis. Exp Ther Med. 20:3114–3121.
2020.PubMed/NCBI
|
|
47
|
Xu W, Zhao Y, Qin Y, Ge B, Gong W, Wu Y,
Li X, Zhao Y, Xu P and Xue M: Enhancement of exposure and reduction
of elimination for paeoniflorin or albiflorin via co-administration
with total peony glucosides and hypoxic pharmacokinetics
comparison. Molecules. 21:8742016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tabata K, Matsumoto K, Murakami Y and
Watanabe H: Ameliorative effects of paeoniflorin, a major
constituent of peony root, on adenosine A1 receptor-mediated
impairment of passive avoidance performance and long-term
potentiation in the hippocampus. Biol Pharm Bull. 24:496–500. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen DM, Xiao L, Cai X, Zeng R and Zhu XZ:
Involvement of multitargets in paeoniflorin-induced
preconditioning. J Pharmacol Exp Ther. 319:165–180. 2006.
View Article : Google Scholar : PubMed/NCBI
|