Introduction
Tetralogy of Fallot (TOF) is the most common
cyanotic congenital heart disease with a prevalence of 1/3,600 live
births (1). Its symptoms consist
of pulmonary outflow tract obstruction, ventricular septal defects
(VSDs), overriding aortic roots and right ventricular hypertrophy
(2). Although advances in
pharmacotherapy and surgical procedures have improved the survival
rate of patients with TOF, the exact pathogenesis of TOF is yet to
be elucidated. However, only 20% of total TOF cases have a known
cause, which may be associated with gene mutations or chromosomal
anomalies, thus the exact etiology for the remaining TOF cases
remains unclear (2). In addition
to genetic mechanisms, epigenetics may play an important role in
the development of TOF (3).
The Notch signaling pathway is highly conserved and
related to the formation of the atrioventricular ducts, valves,
outflow tracts and trabecula (4,5).
Mutations in the genes of the Notch signaling pathway can cause
heart defects in humans or mice, proving its important role in
cardiac development (6–8). δ like non-canonical Notch ligand 1
(DLK1) is a non-canonical Notch ligand that regulates the Notch
signaling pathway (9). Several
studies have found that DLK1 is involved in the cell
differentiation process throughout embryonic development and
adulthood (9). During cardiac
development, a previous study demonstrated that DLK1 is highly
expressed in the heart of embryos (10). In addition, DLK1 can negatively
regulate the differentiation of cardiac fibroblasts into
myofibroblasts, and therefore control myocardial fibrosis (11). Furthermore, a previous study
reported that mutations of zinc finger protein 57 homolog, the
target gene of DLK1, can cause a variety of cardiac defects,
including atrial septal defect and VSD, via the Notch signaling
pathway (10). Moreover, Page
et al (2) found that there
was a very low frequency of genetic variants in the DLK1 coding
region in 829 patients with TOF (2). Therefore, these previous studies
indicate that epigenetic changes of the DLK1 gene may be a risk
factor for the pathogenesis of TOF.
DNA methylation is the most thoroughly studied
epigenetic regulatory mechanism and can alter gene expression in
both development and disease (12). Numerous studies have shown that
aberrant DNA methylation may be associated with cardiovascular
disease (13–15). For example, abnormal DNA
methylation of several candidate genes involved in cardiac
development, such as transcription factor GATA-4, has been found in
patients with congenital heart disease (CHD) (3). In addition, aberrant methylation
levels of the homeobox protein Nkx-2.5 gene body and heart and
neural crest derivatives-expressed protein 1 gene promoter region
are negatively correlated with their mRNA expression (16). In the present study, the changes
in DNA methylation of the DLK1 promoter region in TOF were explored
and its influence on gene expression was analyzed. These findings
may provide important clues in understanding the etiology of this
disease.
Materials and methods
Clinical tissue samples
The Ethics Committee of the Children's Hospital of
Fudan University [Shanghai, China; approval no. 2015(26)] and Soochow University (Suzhou,
China) approved the collection of cardiac tissues. Written informed
consent of all study participants was obtained from their parents
or relatives, and the study was registered in the Chinese Clinical
Trial Registry (registration no. ChiCTR2100051811). TOF was
diagnosed in 25 patients recruited from the Children's Hospital of
Fudan University between January 2016 and July 2018. They were
diagnosed by echocardiogram and confirmed by surgery. The five
control samples obtained from the Department of Forensic Medicine
of Soochow University were patients who had passed away from
accidents without any known heart problems. The clinical
characteristics of the samples are summarized in Table I.
 | Table I.Demographic characteristics of TOF
cases (n=25) and controls (n=5). |
Table I.
Demographic characteristics of TOF
cases (n=25) and controls (n=5).
|
Characteristics | TOF | Control |
|---|
| Age, years,
median | 0.59
(0.37-0.95) | 0.17
(0.01-0.79) |
| (IQR) |
|
|
| <1,
n (%) | 20 (80) | 4 (80) |
| 1~2, n
(%) | 4 (16) | 1 (20) |
| >2,
n (%) | 1 (4) | 0 (0) |
| Sex |
|
|
| Female,
n (%) | 10 (40) | 0 (0) |
| Male, n
(%) | 15 (60) | 5 (100) |
Immunohistochemistry
The right ventricular outflow tract (RVOT) tissues
of patients with TOF and controls were fixed with 10% neutral
buffered formalin for ~48 h at room temperature. The fixed tissues
were embedded in paraffin and cut into 4 µm thick sections. The
paraffin-embedded sections were baked in 56°C for ~3 h, dewaxed in
dimethylbenzene, hydrated in descending alcohol series (100%, 5
min; ~100%, 5 min; ~95%, 5 min; ~85%, 5 min; ~75%, 5 min) and
boiled (100°C) for antigen retrieval with citrate buffer (0.01
mol/l; pH, 6.0) (Beyotime Institute of Biotechnology). Next,
endogenous peroxidase activity was blocked with 3% hydrogen
peroxide for ~30 min at room temperature, and 5% bovine serum (0.05
g/ml; BioFroxx; neoFroxx GmbH) was used to reduce non-specific
staining. The sections were incubated with a primary antibody
against DLK1 (1:200; cat. no. ab210471; Abcam) overnight at 4°C,
followed by incubation with horseradish peroxidase (HRP)-conjugated
anti-rabbit/anti-mouse IgG antibodies (cat. no. GK500710; Gene Tech
Co., Ltd.) for 2 h at 25°C. Lastly, DAB and hematoxylin were
applied for staining. The intensity of DLK1 protein expression was
detected under a light microscope (Lecia Microsystems GmbH). For
each sample, three fields of view without repeating areas were
selected under the light microscope (magnification, ×200) and
images were captured in order to analyze the expression of DLK1 in
RVOT tissues using ImageJ software version 1.48 (National
Institutes of Health). Segmentation was set at a level that allowed
for the detection of positive immunostaining and the positive area
% of each image was measured. The mean value of the total of three
visual fields was calculated.
DNA extraction and bisulfite
sequencing PCR (BSP)
Genomic DNA from the RVOT tissues of controls and
patients with TOF was extracted using the E.Z.N.A.®
Tissue DNA Kit (cat. no. D3396; Omega Bio-Tek, Inc.) following the
manufacturer's instructions. Bisulfite modification of genomic DNA
was carried out using the EZ DNA Methylation-Gold kit (Zymo
Research Corp.) and amplified by PCR with ZymoTaq PreMix (Zymo
Research Corp.). The primers were designed using Methyl Primer
Express™ v1.0 software (Applied Biosystem; Thermo Fisher
Scientific, Inc.): DLK1-BSP-forward (F),
5′-TAGTTGGGTATGTGTGTTTGTG-3′ and reverse (R),
5′-TTACCCAACCATAAACATCCT-3′. Thermocycling conditions were as
follows: 95°C for 5 min; 40 cycles of 95°C for 30 sec, 58°C for 30
sec and 72°C for 40 sec; and 72°C for 7 min.
The PCR products were purified (Axygen; Corning,
Inc.), inserted into a pGEM-T easy vector (Promega Corporation),
and then transformed into E. coli DH5α competent cells (cat.
no. DL1002; Shanghai Weidi Biotechnology Co., Ltd.). A total of 10
clones were selected randomly to determine the methylation status.
The sequencing results were analyzed using the QUMA website
(http://quma.cdb.riken.jp).
Luciferase reporter plasmid
construction
pGL3-DLK1_-902/-597 was constructed by amplifying
the fragment containing DLK1-R (−899 to −641 bp) using the
following primers: DLK1-KpnI-F,
5′-ATAGGTACCAGTCAGCTGGGTATGTGTGC-3′; DLK1-XhoI-R,
5′-GATCTCGAGTATAGACACAGTCCTAGGTGGCAG-3′. Next, the amplified
fragment was inserted into the pGL3-Promoter (Promega Corporation)
vector to determine the influence of the DLK1_R region on gene
transcriptional activity.
Cell culture, transfection and
dual-luciferase reporter gene assay
293 cells (The Cell Bank of Type Culture Collection
of The Chinese Academy of Sciences) were plated in 96-well plates
at 2–4×105 cells/well and grown in DMEM (Gibco; Thermo
Fisher Scientific, Inc). containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and penicillin-streptomycin (1:100; Gibco, Thermo
Fisher Scientific, Inc.) at 37°C under 5% CO2. Once
cells reached 60% confluency, 100 ng pGL3-basic, pGL3-promoter and
pGL3-DLK1_-902/-597 were separately co-transfected with 4 ng
internal reference pRL-TK plasmids (Promega Corporation) using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 25°C. The transfection process lasted 30–60
min.
The JASPAR database (version JASPAR 2020) was used
to analyze the sequence of the DLK1_R region and binding sites for
the protein C-ets-1 (ETS1) transcription factor containing the CpG
site 11 were found (17).
Therefore, 100 ng pcDNA3.1-ETS1 and 100 ng constructed plasmids
were co-transfected into cells. Luciferase activity was detected
using the dual-luciferase reporter assay system (Promega
Corporation) 48 h after transfection. Three independent luciferase
activity assays were conducted. PGL3-basic vector served as a
negative control, and the pGL3-promoter vector was a positive
control. PRL-TK plasmids were used to normalize the luciferase
activity to avoid errors caused by differences in the number of
cell and efficiency of plasmid transfection to cells. To compare
the differences between the two groups, data of three times
experiments were normalized relative to the pGL3-promoter
group.
Electrophoretic mobility shift assay
(EMSA) and Shift-western blotting
293 cells were plated in 10 cm dishes at
2×106 cells/well. Once cells reached 70% confluency,
12.5 µg pcDNA3.1 and pcDNA3.1-ETS1 were separately transfected into
cells using Lipofectamine 3000 at 25°C according to the
manufacturer's protocol. The transfection process lasted 30–60 min.
The pcDNA3.1 plasmid (the empty vector) was used as the negative
control. After 48 h, the nuclear protein was extracted by using
cytoplasmic extraction reagent and nuclear extraction reagent
(Thermo Fisher Scientific, Inc.), and the concentration of the
nuclear protein was measured using BCA protein assay (Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol.
Furthermore, western blotting was performed to verify the
overexpression of ETS1. Samples containing equal amounts of protein
(15 µg) were separated by SDS-PAGE on 10% gels (Epizyme, Inc.), and
subsequently transferred to nitrocellulose membranes
(MilliporeSigma) and blocked with 5% skimmed milk (0.05 g/ml) for
~1 h at room temperature. The membranes were probed with primary
antibodies against ETS1 (1:1,000; cat. no. D8O8A; Cell Signaling
Technology, Inc.) and PCNA (1:5,000; cat. no. 10205-2-AP;
ProteinTech Group, Inc.) at 4°C overnight. Then, membranes were
incubated with HRP-conjugated anti-rabbit secondary antibody
(1:5,000; cat. no. M21002; Abmart Pharmaceutical Technology Co.,
Ltd.) for ~2 h at room temperature. The blots were visualized using
enhanced chemiluminescence reagents (Thermo Fisher Scientific,
Inc.).
Biotin-labeled oligonucleotide probes containing the
binding sequence of ETS1 were synthesized and labeled with biotin
at the 5′ end. Unlabeled probes used for the competition were also
synthesized and the methylated probes were modified at CpG sites.
These probes were purchased from Generay Biotech Co., Ltd. The
sequences of the DNA probes are shown in Table II. ETS1 protein (10 µg) was
co-incubated with 4 pmol unlabeled probes (1:100 dilution) for ~30
min at room temperature and subsequently with 20 fmol
biotin-labeled probes (1:10,000 dilution) for ~30 min at room
temperature. The protein-DNA complexes were separated from free DNA
probes on a 6% polyacrylamide gel in 0.5X TBE at 100 V for ~50 min
and then transferred to a nylon membrane (MilliporeSigma).
Subsequently, the membrane was detected with the LightShift™ EMSA
kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The Shift-western blotting was performed
using the same methods as EMSA, except that EMSA involved the use
of nylon membranes, while Shift-western blotting required
nitrocellulose membranes for protein transfer. The membrane was
blocked with 5% skimmed milk (0.05 g/ml) for ~1 h at room
temperature, incubated with the primary antibody against ETS1
(1:1,000; cat. no. D8O8A; Cell Signaling Technology, Inc.) at 4°C
overnight, and then incubated with a HRP-conjugated anti-rabbit
secondary antibody (1:5,000; cat. no. M21002; Abmart Pharmaceutical
Technology Co., Ltd.) for ~2 h at room temperature. Finally, the
blots were visualized using enhanced chemiluminescence reagents
(Thermo Fisher Scientific, Inc.).
 | Table II.Sequences of oligonucleotide probes
used for electrophoretic mobility shift assay. |
Table II.
Sequences of oligonucleotide probes
used for electrophoretic mobility shift assay.
| Probe | Sequences
(5′→3′) |
|---|
|
Biotin-DLK1_-744/-720 | F:
TCTGTTTATACGTGTGTTTGCGTGT |
|
| R:
ACACGCAAACACACGTATAAACAGA |
|
Unlabeled-DLK1_-744/-720 | F:
TCTGTTTATACGTGTGTTTGCGTGT |
|
| R:
ACACGCAAACACACGTATAAACAGA |
|
MU-DLK1_-744/-720 | F:
TCTGTTTATACGTGTGGCGATATGT |
|
| R:
ACATATCGCCACACGTATAAACAGA |
|
Me-DLK1_-744/-720 | F:
TCTGTTTATACGTGTGTTTGCGTGT |
|
| R:
ACACGCAAACACACGTATAAACAGA |
Chromatin
immunoprecipitation-quantitative (ChIP-q)PCR
293 cells were seeded in two 10 cm dishes at a
density of 2×106 cells/well. After 24 h, the
experimental group was treated with 40 µM 5-Aza-2′-deoxycytidine
(Sigma Aldrich; Merck KGaA), and the control group was treated with
the same volume of DMSO. The treatment of both groups lasted for
~48 h at 37°C under 5% CO2. The ChIP assay was performed
using EZ-Magna ChIP™ A/G Chromatin Immunoprecipitation kit
(MilliporeSigma) according to the manufacturer's instructions.
Briefly, cells were incubated with 225 µl 37% formaldehyde (final
concentration, 1%) for ~10 min at room temperature, then 1 ml 10X
glycine was added to stop crosslinking for ~5 min at room
temperature and collected with cold 1X PBS. After resuspension in
500 µl SDS lysis buffer, samples were then sonicated by ultrasonic
disruptor (Diagenode Bioruptor; intensity 5, 30 sec on and 30 sec
off, 25 cycles) and centrifuged at 10,000 × g at 4°C for 10 min to
remove insoluble material. Sheared chromatin (50 µl) was incubated
with 450 µl ChIP dilution buffer and antibody overnight at 4°C with
rotation. Antibodies used for ChIP included: Anti-ETS1 antibody
Chip grade (1:50; cat. no. D8O8A; Cell Signaling Technology, Inc.)
and anti-rabbit IgG Chip grade (5 µg; cat. no. 2729S; Cell
Signaling Technology, Inc.). The antibody of IgG was used as the
negative control. The next day, the magnetic beads were washed with
low salt wash buffer, high salt wash buffer, LiCl wash buffer and
TE buffer at room temperature. Each sample was incubated with 100
µl ChIP elution buffer at 62°C for 2 h with shaking, at 95°C for 10
min and then cooled down to room temperature. Beads were then
separated using magnets, and the supernatant was purified using
FastPure Gel DNA Extraction Mini Kit (cat. no. DC301; Vazyme
Biotech Co., Ltd.) to acquire DNA fragments. The enriched DNA
fragments were quantified via qPCR using TB Green®
Premix Ex Taq™ (cat. no. RR420A; Takara Bio, Inc.). Thermocycling
conditions were as follows: 95°C for 30 sec; 40 cycles of 95°C for
5 sec and 60°C for 20 sec; and 95°C for 15 sec and 60°C for 60 sec.
Three independent qPCRs were performed. The primers used for
ChIP-qPCR were as follows: ChIP-qPCR-F,
5′-TTTGTGTTTCAGCGCGGCTAAG-3′ and ChIP-qPCR-R,
5′-TTACCCAACCATGGGCATCCTCC-3′. The obtained results were expressed
as % input (18).
Statistical analysis
All data are presented as the median (interquartile
range) of three independent experiments. Data were analyzed using
GraphPad Prism software (version 7.0, GraphPad Software, Inc.). The
differences between DLK1 expression and the methylation status of
the DLK1 gene promoter in the two groups were determined with a
Mann-Whitney test. Pearson's correlation was used to test the
correlation between methylation levels and protein expression. The
differences in luciferase activity and the enrichment of the
amplified fragments between multiple groups were tested by one-way
ANOVA, followed by the LSD post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of DLK1 protein in the RVOT
tissues of patients with TOF and controls
To determine the clinical relevance of DLK1 in the
development of TOF, immunohistochemistry was used to detect the
expression level of DLK1 protein in the RVOT tissues of 25 patients
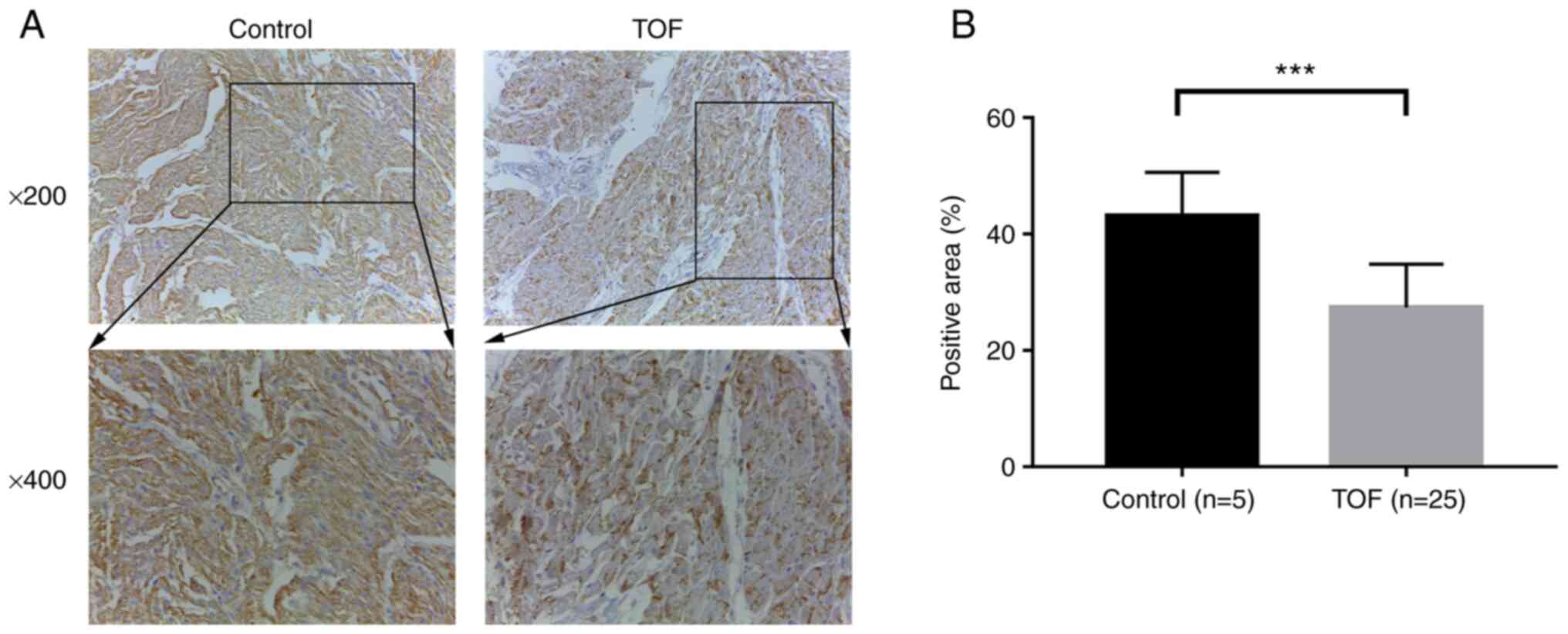
with TOF and five controls. As shown in Fig. 1A, the intensity of positive
yellow-brown immunohistochemical staining of DLK1 protein in the
RVOT tissues was markedly weaker in patients with TOF than that in
controls (Fig. 1A). The further
semi-quantitative analysis showed that the expression of DLK1
protein in the patients with TOF was significantly lower than that
in the controls (P<0.001; Fig.
1B).
Methylation status analysis for DLK1
gene in patients with TOF and controls
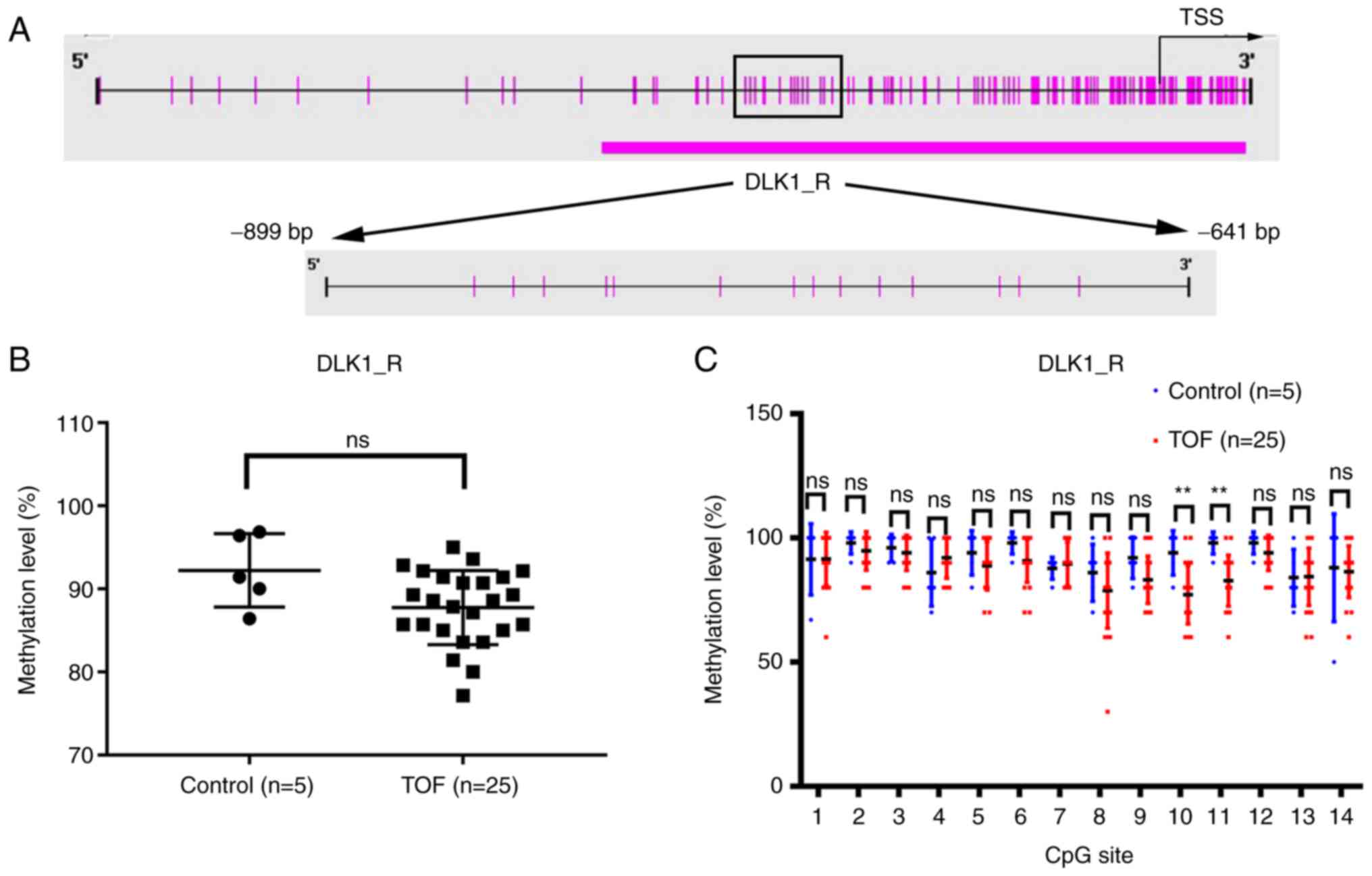
BSP was performed to measure the methylation level
of the DLK1 gene promoter. The amplicon (DLK1_R, −899 to −641 bp)
from the DLK1 gene promoter was analyzed in 25 patients with TOF
and five age-matched controls (Fig.
2A). The results underwent quality control to remove unreliable
methylation data.
The integral methylation level of DLK1_R was not
significantly different in 25 TOF patients with a median of 88.57%
(IQR, 85–91.43%), compared with the five controls with a median of
91.43% (IQR, 88.21-96.64%) (P=0.0716; Fig. 2B). Of note, the subsequent
analysis of the methylation status of each CpG site in the DLK1_R
region revealed that the methylation level of CpG site 10 and CpG
site 11 was significantly lower in the TOF group than in the
control group (P<0.01; Fig.
2C).
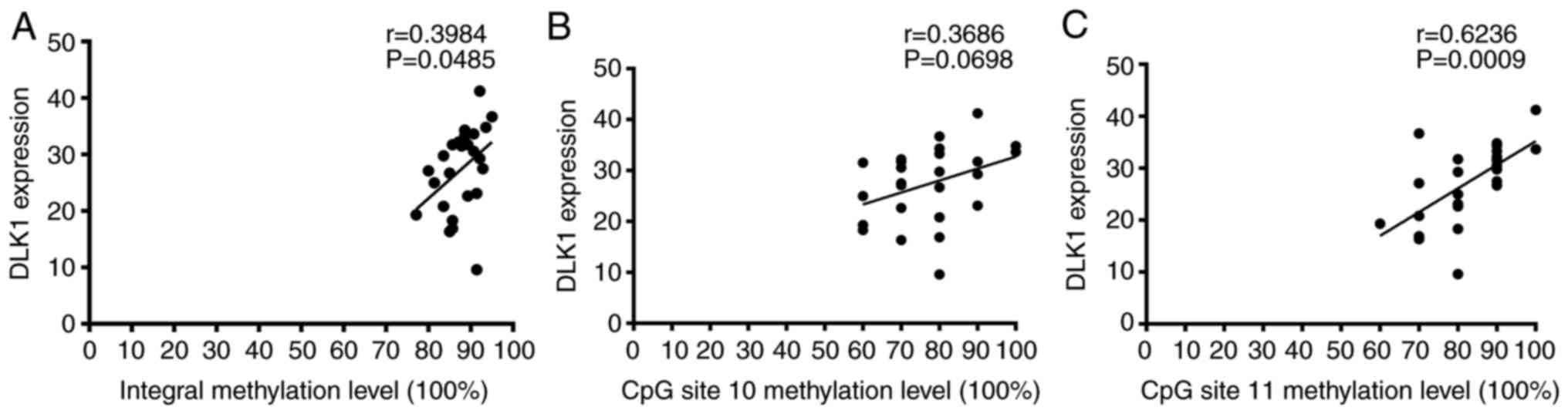
Pearson's correlation analysis was used to determine
whether DLK1_R methylation level was related to the expression
level of DLK1 protein. There was a moderate positive correlation
between the integral methylation status of DLK1_R and DLK1
expression in 25 patients with TOF (r=0.3984, P=0.0485; Fig. 3A). DLK1 protein expression was not
significantly correlated with the methylation level of CpG site 10
(r=0.3686, P=0.0698; Fig. 3B).
However, a moderate positive correlation was also observed between
DLK1 expression and the methylation status of CpG site 11 in
patients with TOF (r=0.6236, P=0.0009; Fig. 3C).
ETS1 transcription factor binds to the
promoter region of DLK1 and inhibits gene transcription
activity
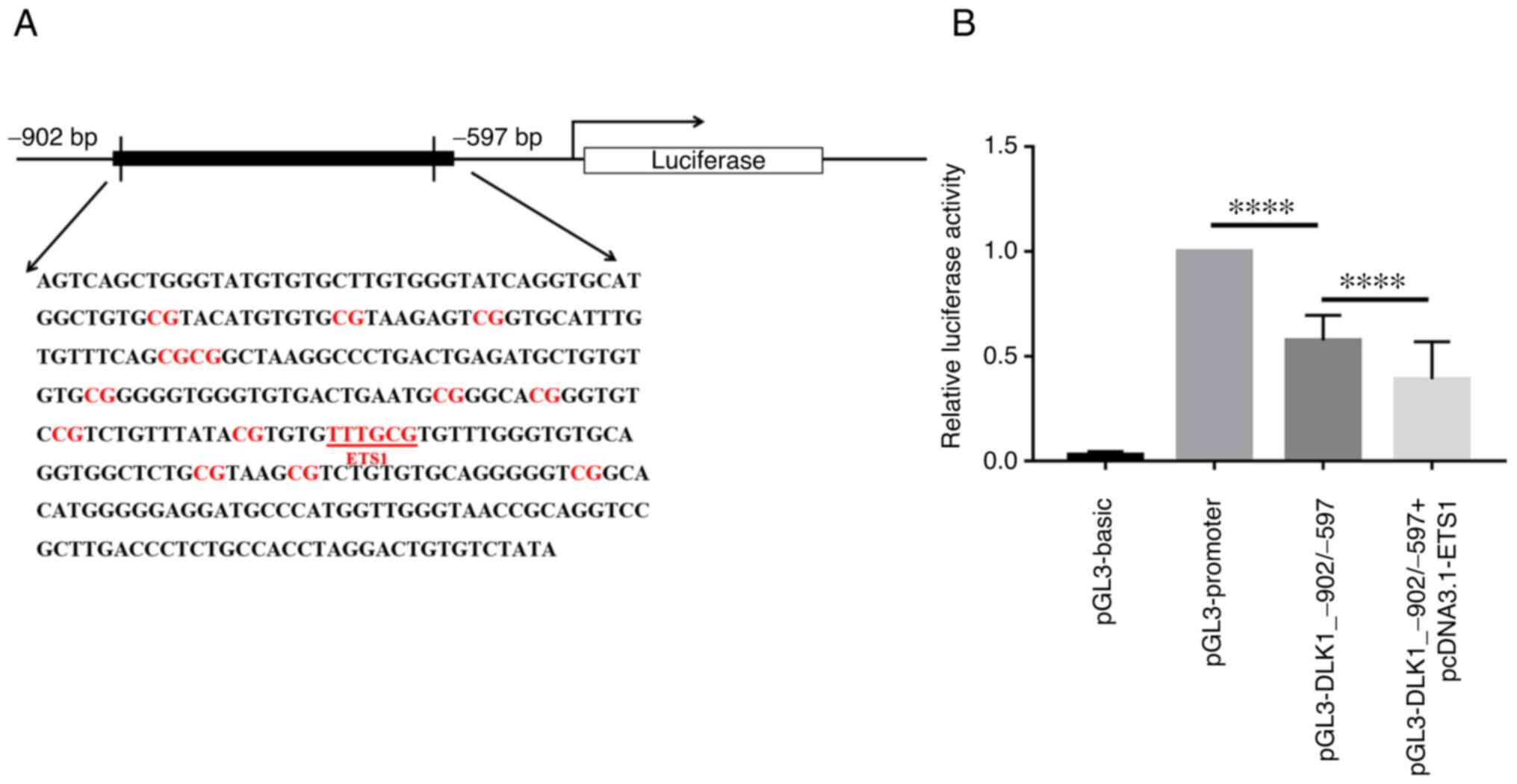
To explore the influence of the DLK1_R region on
gene transcription activity, a dual-luciferase assay was performed
using 293 cells. The pGL3-DLK1_-902/-597 plasmid was constructed by
placing the DLK1_-902/-597 fragment containing the DLK1_R region
(−899 bp to −641 bp) under pGL3-promoter (Fig. 4A). As shown in Fig. 4B, the luciferase activity in the
case of the pGL3-DLK1_-902/-597 vector was significantly lower than
in the case of the plasmid harboring the pGL3-promoter and lacking
the DLK1_-902/-597 fragment, which indicated that the examined
DLK1_-902/-597 region can inhibit gene transcription activity
(P<0.0001).
An ETS1-binding sequence (−728 bp to −723 bp)
containing the CpG site 11 (−724 bp) was predicted to be located in
the DLK1_R region of the DLK1 promoter using the JASPAR database
(Fig. 4A). To verify this
prediction, pcDNA3.1-ETS1 was co-transfected with
pGL3-DLK1_-902/-597 into 293 cells and the luciferase activity
assay was performed. The luciferase activity in the case of
pGL3-DLK1_-902/-597 + pcDNA3.1-ETS1 was significantly reduced
compared with the pGL3-DLK1_-902/-597 transfection alone
(P<0.0001; Fig. 4B).
These findings suggested that the ETS1-transcription
factor could bind to the promoter region of DLK1 and inhibit the
transcriptional activity of DLK1.
Validation of binding of ETS1 to the
DLK1 promoter in vitro
To determine whether the transcription factor ETS1
could directly bind to the CpG site 11 and whether the methylation
of DLK1_R could affect ETS1 binding affinity, EMSAs were performed
using the overexpressed ETS1 protein (Fig. 5A) and oligonucleotide probes that
contained ETS1 binding sequences. As shown in Fig. 5B (lane 2), a DNA-protein complex
was formed when the biotin-labeled probes were incubated with ETS1.
When unlabeled probes were added for competition, the ETS1 binding
was inhibited, which resulted in a lighter band (lane 3). However,
the bands in lane 4 and lane 5, where the mutation probes and
methylated probes were added, were even lighter than that in lane
3. The subsequent Shift-western blotting showed that the delayed
band detected in EMSA was the result of ETS1 protein binding
(Fig. 5B). These findings
demonstrated that the ETS1 transcription factor could bind to the
promoter of DLK1 and that the methylation status of DLK1_R was able
to influence ETS1 binding affinity.
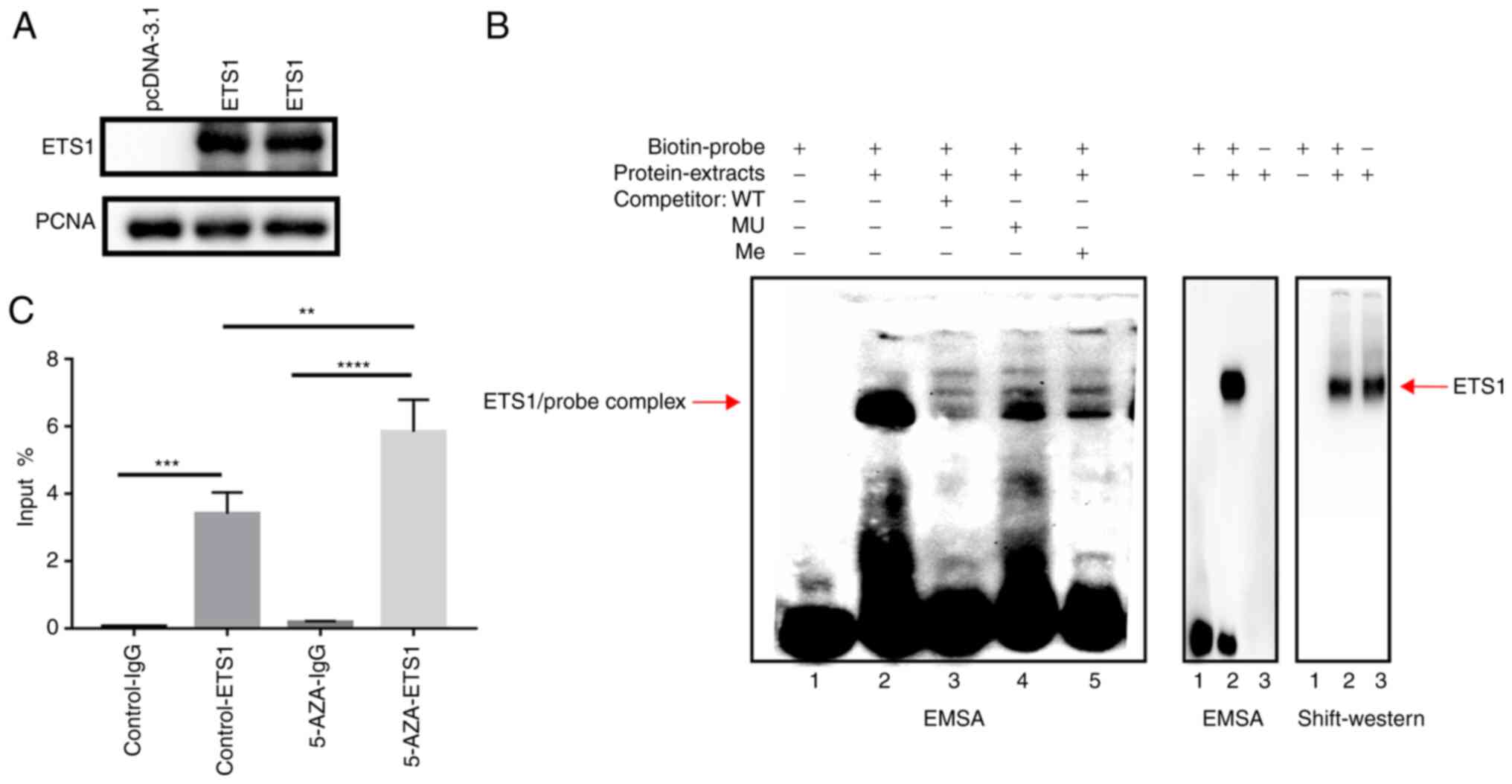 | Figure 5.Binding of ETS1 to the DLK1 promoter.
(A) Overexpression of ETS1 was verified via western blotting. (B)
EMSA: i) Lane 1, biotin-labeled probes alone; ii) lane 2,
biotin-labeled probes were incubated with ETS1 nuclear protein;
iii) lane 3, unlabeled probes as competitors were added based on
lane 2; iv) lane 4, mutation probes as competitors were added based
on lane 2; and v) lane 5, methylated probes as competitors were
added based on lane 2. Shift-western blotting confirmed ETS1
binding. (C) Chromatin immunoprecipitation assay was performed with
anti-ETS1 using 293 cells treated with or without 5-aza.
Quantitative PCR was used to verify the enrichment of DLK1_R.
**P<0.01, ***P<0.001, ****P<0.0001. DLK1, δ like
non-canonical Notch ligand 1; ETS1, ETS proto-oncogene 1; EMSA,
electrophoretic mobility shift assay; 5-AZA, 5-azacytidine; WT,
wild-type; MU, mutant; Me, methylated; PCNA, proliferating cell
nuclear antigen. |
Validation of binding of ETS1 to the
DLK1 promoter in vivo
ChIP DNA samples and input DNA samples were
quantified via qPCR. The fragments containing the predicted ETS1
transcription factor binding sequences were significantly enriched
in the anti-ETS1 group, when compared with the anti-IgG group
(P<0.001; Fig. 5C). When 293
cells were treated with DNA-demethylating agent 5-AZA-dC, the
enrichment of the fragment was higher than that in the control
group without 5-AZA-dC (P<0.01; Fig. 5C).
Discussion
The Notch pathway is a highly conserved cell
signaling pathway required for cell fate specification and tissue
patterning in the embryo (19). A
consistent set of data in animal models and humans has demonstrated
that the Notch signaling pathway plays an important role in
cardiogenesis (20–22). DLK1, one of the Notch ligands, is
commonly expressed during fetal development and highly expressed in
cardiac development (9). In the
current study, by using immunohistochemistry, it was found that
DLK1 protein expression in RVOT tissues of patients with TOF was
significantly lower than that in controls. Considering the very low
frequency of DLK1 in patients with TOF and the importance of
epigenetics on the pathogenesis of TOF, it was deduced that the
epigenetic changes may be closely associated with the abnormal
expression of the DLK1 gene.
Several studies have demonstrated that epigenetic
mechanisms play an important role in cardiac development by
altering the expression of cardiac-related genes (23,24). One of the epigenetic modifications
regulating gene transcription is DNA methylation status (25). Aberrant methylation of promoter
CpG islands is known to contribute to the phenotypic expression of
CHDs, including TOF and VSD (3).
Our previous studies identified that the altered expression of some
genes involved in cardiac development, such as Notch4 and COUP
transcription factor 2 in TOF, may be associated with the abnormal
methylation of their promoters (26,27). In the current study, no
significant difference was observed in the integral methylation
level of DLK1 promoter between the patients with TOF and controls.
However, previous studies have demonstrated that the methylation of
certain CpG sites can affect gene expression (26,28,29). Therefore, the methylation status
of each CpG site in the DLK1_R region was analyzed in the present
study. CpG site 10 and CpG site 11 had lower methylation levels in
patients with TOF compared with controls. Moreover, a significant
positive correlation between the DLK1 methylation level and DLK1
expression in patients with TOF could be observed in the CpG site
11. It was deduced that the hypomethylation level of the CpG site
11 may be associated with lower expression of DLK1 in patients with
TOF compared with controls.
The present findings differ from previous studies
that have reported a repressive role of hypermethylation in gene
expression, which acts by preventing transcription factors from
binding to CGI promoters (30,31). However, several publications have
reported similar findings that promoter hypermethylation can
facilitate gene transcription, mainly in tumor occurrence,
development and metastasis (32–34). The most commonly hypothesized
mechanism is that the increased methylation of the promoter can
prevent the binding of a repressive transcription factor, which
facilitates active gene transcription (35). To verify this hypothesis, the
JASPAR database was used in the current study and it was found that
ETS1 is a potential transcription factor that can bind to the
DLK1_R region containing the CpG site 11. ETS1, as a member of the
ETS family required for normal vascular development, can mediate
early cardiomyocyte development in the embryo (36,37). The current research determined
that ETS1 could bind to the DLK1 promoter and inhibit gene
activity. This finding was consistent with the aforementioned
previous studies.
To further investigate whether the methylation
status of the DLK1_R region could affect the binding of ETS1, EMSA
Shift-western blotting and ChIP-qPCR were performed. EMSA
demonstrated that ETS1 could directly bind to the promoter of DLK1,
and its binding could be blocked by methylation. Moreover,
Shift-western blotting confirmed that the band detected as a result
of EMSA was indeed the ETS1 protein. Furthermore, ChIP-qPCR was
performed and it was determined that DNA-demethylating agent
5-AZA-dC increased the enrichment of the DLK1_R fragment in ETS1.
Taken together, these findings suggested that ETS1 could bind to
the DLK1 promoter, with its affinity affected by the methylation
status of the DLK1 promoter. This dependence proved to influence
DLK1 protein expression. Although these results clarified that ETS1
could regulate gene expression by directly binding to the DLK1
promoter, the specific mechanism of demethylation and repression
needs further exploration.
There are several limitations of the present study.
The first limitation of this study was the insufficient number of
samples due to the difficulty in obtaining cardiac tissues,
especially from healthy controls. In addition, it could not be
determined whether TOF caused abnormal methylation of DLK1 or
whether this abnormality contributed to TOF. Therefore, the
aforementioned issues need to be further explored in animal models
or a prospective cohort. The second limitation was that 293 cells
were used rather than cardiomyocytes, 293 cells were used in order
to improve transfection efficiency as the transfection efficiency
using cardiomyocytes was very low and not suitable for
experiments.
In summary, this study demonstrated that the
hypomethylation of specific sites in the DLK1_R region may
influence DLK1 gene expression in patients with TOF. ETS1 proved to
be a repressive transcription factor binding to the DLK1 promoter.
Therefore, hypomethylation of CpG site 10 and CpG site 11 may
increase the binding affinity of ETS1 and reduce DLK1 expression
levels in patients with TOF. Given the aforementioned information,
this study has the potential to provide novel epigenetic insights
into the pathogenesis of TOF and contribute to further research on
this subject.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Key Research and
Development Program of China (grant no. 2021YFC2701000), the
National Natural Science Foundation of China (grant nos. 81873482
and 81873483), Shanghai Basic Research Project of Science and
Technology Innovation Action Plan (grant no. 20JC1418300), Youth
Program of National Natural Science Foundation of China (grant no.
81800282), CAMS Innovation Fund for Medical Sciences (grant no.
2019-I2M-5-002) and the Shanghai Sailing Program (grant no.
18YF1402600).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS and GH made major contributions to the design of
this study. GT, LH and RG performed the experiments and wrote the
manuscript. JS, WC and YQ collected the samples and patient
information, and analyzed the clinical data. ZZ, XM and WY
participated in analyzing the experimental data. ZX and MS prepared
the tables and figures, and made contributions to the
interpretation of data. GT and WS confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Children's Hospital of
Fudan University [Shanghai, China; approval no. 2015(26)] and Soochow University (Suzhou,
China) approved the collection of cardiac tissues. Written informed
consent of all study participants was obtained from their parents
or relatives.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Apitz C, Webb GD and Redington AN:
Tetralogy of Fallot. Lancet. 374:1462–1471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Page DJ, Miossec MJ, Williams SG, Monaghan
RM, Fotiou E, Cordell HJ, Sutcliffe L, Topf A, Bourgey M, Bourque
G, et al: Whole exome sequencing reveals the major genetic
contributors to Nonsyndromic Tetralogy of Fallot. Circ Res.
124:553–563. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grunert M, Dorn C, Cui H, Dunkel I, Schulz
K, Schoenhals S, Sun W, Berger F, Chen W and Sperling SR:
Comparative DNA methylation and gene expression analysis identifies
novel genes for structural congenital heart diseases. Cardiovasc
Res. 112:464–477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finn J, Sottoriva K, Pajcini KV,
Kitajewski JK, Chen C, Zhang W, Malik AB and Liu Y: Dlk1-Mediated
Temporal Regulation of Notch Signaling is required for
differentiation of Alveolar Type II to Type I cells during repair.
Cell Rep. 26:2942–2954.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de la Pompa JL and Epstein JA:
Coordinating tissue interactions: Notch signaling in cardiac
development and disease. Dev Cell. 22:244–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCright B, Lozier J and Gridley T: A
mouse model of Alagille syndrome: Notch2 as a genetic modifier of
Jag1 haploinsufficiency. Development. 129:1075–1082. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Digilio MC, Luca AD, Lepri F, Guida V,
Ferese R, Dentici ML, Angioni A, Marino B and Dallapiccola B: JAG1
mutation in a patient with deletion 22q11.2 syndrome and tetralogy
of Fallot. Am J Med Genet A. 161A:3133–3136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garg V: Notch signaling in aortic valve
development and disease, Etiology and Morphogenesis of Congenital
Heart Disease: From Gene Function and Cellular Interaction to
Morphology. Nakanishi T, Markwald RR, Baldwin HS, Keller BB,
Srivastava D and Yamagishi H: Springer; Tokyo: pp. 371–376.
2016
|
|
9
|
Huang CC, Kuo HM, Wu PC, Cheng SH, Chang
TT, Chang YC, Kung ML, Wu DC, Chuang JH and Tai MH: Soluble
delta-like 1 homolog (DLK1) stimulates angiogenesis through
Notch1/Akt/eNOS signaling in endothelial cells. Angiogenesis.
21:299–312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shamis Y, Cullen DE, Liu L, Yang G, Ng SF,
Xiao L, Bell FT, Ray C, Takikawa S, Moskowitz IP, et al: Maternal
and zygotic Zfp57 modulate NOTCH signaling in cardiac development.
Proc Natl Acad Sci USA. 112:E2020–2029. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodriguez P, Sassi Y, Troncone L, Benard
L, Ishikawa K, Gordon RE, Lamas S, Laborda J, Hajjar RJ and Lebeche
D: Deletion of delta-like 1 homologue accelerates
fibroblast-myofibroblast differentiation and induces myocardial
fibrosis. Eur Heart J. 40:967–978. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu H, Wang G and Qian J: Transcription
factors as readers and effectors of DNA methylation. Nat Rev Genet.
17:551–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moore-Morris T, van Vliet PP, Andelfinger
G and Puceat M: Role of epigenetics in cardiac development and
congenital diseases. Physiol Rev. 98:2453–2475. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Serra-Juhé C, Cuscó I, Homs A, Flores R,
Torán N and Pérez-Jurado LA: DNA methylation abnormalities in
congenital heart disease. Epigenetics. 10:167–177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao J, Wu Q, Huang Y, Wang L, Su Z and Ye
H: The role of DNA methylation in syndromic and non-syndromic
congenital heart disease. Clin Epigenetics. 13:932021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sheng W, Qian Y, Wang H, Ma X, Zhang P,
Diao L, An Q, Chen L, Ma D and Huang G: DNA methylation status of
NKX2-5, GATA4 and HAND1 in patients with tetralogy of fallot. BMC
Med Genomics. 6:462013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fornes O, Castro-Mondragon JA, Khan A, van
der Lee R, Zhang X, Richmond PA, Modi BP, Correard S, Gheorghe M,
Baranasic D, et al: JASPAR 2020: update of the open-access database
of transcription factor binding profiles. Nucleic Acids Res.
48(D1): D87–D92. 2020.PubMed/NCBI
|
|
18
|
Lacazette E: A laboratory practical
illustrating the use of the ChIP-qPCR method in a robust model:
Estrogen receptor alpha immunoprecipitation using Mcf-7 culture
cells. Biochem Mol Biol Educ. 45:152–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuhnert F, Kirshner JR and Thurston G:
Dll4-Notch signaling as a therapeutic target in tumor angiogenesis.
Vasc Cell. 3:202011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
MacGrogan D, Münch J and de la Pompa JL:
Notch and interacting signalling pathways in cardiac development,
disease, and regeneration. Nat Rev Cardiol. 15:685–704. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luxan G, D'Amato G, MacGrogan D and de la
Pompa JL: Endocardial Notch signaling in cardiac development and
disease. Circ Res. 118:e1–e18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen H, Zhang W, Sun X, Yoshimoto M, Chen
Z, Zhu W, Liu J, Shen Y, Yong W, Li D, et al: Fkbp1a controls
ventricular myocardium trabeculation and compaction by regulating
endocardial Notch1 activity. Development. 140:1946–1957. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Weerd JH, Koshiba-Takeuchi K, Kwon C
and Takeuchi JK: Epigenetic factors and cardiac development.
Cardiovasc Res. 91:203–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Zhai W, Richardson JA, Olson EN,
Meneses JJ, Firpo MT, Kang C, Skarnes WC and Tjian R: Polybromo
protein BAF180 functions in mammalian cardiac chamber maturation.
Genes Dev. 18:3106–3116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jones PA: Functions of DNA methylation:
Islands, start sites, gene bodies and beyond. Nat Rev Genet.
13:484–492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu Y, Ye M, Xu H, Gu R, Ma X, Chen M, Li
X, Sheng W and Huang G: Methylation status of CpG sites in the
NOTCH4 promoter region regulates NOTCH4 expression in patients with
tetralogy of Fallot. Mol Med Rep. 22:4412–4422. 2020.PubMed/NCBI
|
|
27
|
Xiaodi L, Ming Y, Hongfei X, Yanjie Z,
Ruoyi G, Ma X, Wei S and Guoying H: DNA methylation at CpG island
shore and RXRα regulate NR2F2 in heart tissues of tetralogy of
Fallot patients. Biochem Biophys Res Commun. 529:1209–1215. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vohra M, Adhikari P, Souza SC, Nagri SK,
Umakanth S, Satyamoorthy K and Rai PS: CpG-SNP site methylation
regulates allele-specific expression of MTHFD1 gene in type 2
diabetes. Lab Invest. 100:1090–1101. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dayeh TA, Olsson AH, Volkov P, Almgren P,
Ronn T and Ling C: Identification of CpG-SNPs associated with type
2 diabetes and differential DNA methylation in human pancreatic
islets. Diabetologia. 56:1036–1046. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bogdanović O and Lister R: DNA methylation
and the preservation of cell identity. Curr Opin Genet Dev.
46:9–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Greenberg MVC and Bourc'his D: The diverse
roles of DNA methylation in mammalian development and disease. Nat
Rev Mol Cell Biol. 20:590–607. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bahar Halpern K, Vana T and Walker MD:
Paradoxical role of DNA methylation in activation of FoxA2 gene
expression during endoderm development. J Biol Chem.
289:23882–23892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nabilsi NH, Broaddus RR and Loose DS: DNA
methylation inhibits p53-mediated survivin repression. Oncogene.
28:2046–2050. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jia N, Wang J, Li Q, Tao X, Chang K, Hua
K, Yu Y, Wong KK and Feng W: DNA methylation promotes paired box 2
expression via myeloid zinc finger 1 in endometrial cancer.
Oncotarget. 7:84785–84797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smith J, Sen S, Weeks RJ, Eccles MR and
Chatterjee A: Promoter DNA Hypermethylation and Paradoxical Gene
Activation. Trends Cancer. 6:392–406. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ruan H, Liao Y, Ren Z, Mao L, Yao F, Yu P,
Ye Y, Zhang Z, Li S, Xu H, et al: Single-cell reconstruction of
differentiation trajectory reveals a critical role of ETS1 in human
cardiac lineage commitment. BMC Biol. 17:892019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nie S and Bronner ME: Dual developmental
role of transcriptional regulator Ets1 in Xenopus cardiac neural
crest vs. heart mesoderm. Cardiovasc Res. 106:67–75. 2015.
View Article : Google Scholar : PubMed/NCBI
|