Introduction
Sepsis is one of the serious complications of
critically ill patients with trauma, shock, infection and major
surgery. It is a primary cause of septic shock and multiple organ
dysfunction syndrome (MODS) (1,2).
The mortality rate of sepsis remains high, and sepsis with acute
kidney injury (AKI) is an independent risk factor for the poor
prognosis of critically ill patients (3–5).
Currently, AKI has become a major problem in critical care
medicine. Although early diagnosis and renal replacement therapy
continues to improve, the mortality rate of patients with AKI
caused by sepsis remains high, posing a huge threat to human health
and social economy (6).
Therefore, finding new methods for the treatment of septic AKI will
help to establish novel treatment strategies and reduce the high
incidence and fatality rate of septic AKI.
Hydrogen sulfide (H2S) is the third gas
signal molecule discovered after nitric oxide (NO) and carbon
monoxide (CO), and has a wide range of biological functions in the
body. H2S shows important pathophysiological
significance for the occurrence and development of various
diseases, and has great potential in therapeutic application
(7). An increased number of
studies have shown that low concentrations of H2S may
have a protective effect against AKI caused by factors such as
sepsis. For example, sodium hydrosulfide hydrate (NaHS) treatment
on rats can reduce cisplatin-induced nephrotoxicity (8). NaHS can reduce renal
ischemia-reperfusion injury through antioxidation,
anti-inflammatory and anti-apoptotic effects (9–11).
H2S can play a protective role in diabetic nephropathy
through a variety of mechanisms (12–14). NaHS can also prevent the renal
function damage caused by ureteral obstruction (15–17), and H2S reduces
LPS-induced AKI damage through anti-inflammatory and antioxidant
effects (18). Albeit these
findings, the mechanism of the protective effect of H2S
on AKI has not been fully elucidated.
Autophagy is ubiquitous in most eukaryotic cells. It
is a self-protection phenomenon that involves the formation of
autophagosomes, which are double-layer membrane structures that
wrap the damaged organelles, denatured proteins and various
macromolecular substances; the autophagosomes are transported to
lysosomes to form autophagolysosomes that degrades the contents,
thereby maintaining cell survival, renewal, material reuse and
internal environment stability (19,20). A number of studies have shown that
autophagy has a protective effect on AKI models (21–25). Therefore, proper induction of
autophagy might be able to improve the prognosis of AKI caused by
sepsis. Studies have shown that H2S can promote
autophagy levels (26,27), however, the relationship between
autophagy and apoptosis or inflammatory factors in LPS-induced AKI
has not yet been reported. In order to make up for this
shortcoming, the present study was performed.
In this study, the LPS-induced mouse AKI model and
HK-2 renal tubular epithelial cell injury model were used to
explore the effects of exogenous H2S on LPS-induced AKI.
Immunofluorescence, western blotting and other methods were used to
examine the effect of H2S on LPS-induced kidney cell
injury. Autophagy inhibitor was used to investigate whether the
protective effect of H2S was through regulating
autophagy.
Materials and methods
Animals
A total of 140 male C57BL/6 mice (8 weeks old) were
purchased from Hunan SJA Laboratory Animal Co., Ltd., with an
average weight of 20–25 g. Mice were fed in a clean and quiet
environment at a temperature of 25°C and humidity of 40–60% and
allowed free access to food and water. Mice were kept at 12/12 h
light and dark cycles. To observe the effect of NaHS on the
survival rate of LPS-induced AKI mice, 60 mice were randomly
divided into three groups (n=20): i) Control group; ii) LPS group;
and iii) LPS + NaHS (0.8 mg/kg) group. The mice in the LPS
intervention groups were intraperitoneally injected with LPS (10
mg/kg; Sigma-Aldrich; Merck KGaA), and the control group was
injected with the same amount of saline. The NaHS intervention
group was intraperitoneally injected with NaHS (Sigma-Aldrich;
Merck KGaA) 30 min before LPS injection, and the survival was
observed every 12 h until the 72-h point. To investigate the effect
of NaHS on LPS-induced kidney damage, 40 mice were randomly divided
into four groups (n=10): i) Control group; ii) NaHS group; iii) LPS
group; and iv) LPS + NaHS group. Mice in the LPS + NaHS group were
injected with NaHS (0.8 mg/kg) 30 min before LPS injection. Then,
12 h later, blood was collected from the mice, and the mice were
sacrificed to collect kidney tissue. To investigate the effect of
3-MA on the protective effect of NaHS, 40 mice were randomly
divided into four groups (n=10): i) Control group; ii) LPS group;
iii) LPS + NaHS group; and iv) LPS + NaHS + 3-MA group. Previous
studies have shown that 3-MA (30 mg/kg) injected intraperitoneally
for 30 min before NaHS or other drugs can effectively inhibit the
level of autophagy (28,29). Therefore, in the LPS + NaHS + 3-MA
group, 3-MA (30 mg/kg) was injected intraperitoneally at first; 30
min later, NaHS (0.8 mg/kg) was injected, followed by LPS injection
at 30 min later. Then, 12 h later, mice were anesthetized through
isoflurane (2%) inhalation and blood was collected, and then mice
were euthanized by cervical dislocation to collect kidney tissues.
All animal experiments were approved by the Institutional Animal
Ethics Committee of Central South University (approval no.
2021101119; Changsha, China). Both our preliminary experiments and
literature reports showed that 3-MA alone did not increase damage,
therefore our experiment did not set up a 3-MA group alone
(30).
Renal function test
After blood collection, the blood was allowed to
stand at room temperature for 2 h, centrifuged at 1,000 × g for 15
min at 4°C, and the serum was collected. An automatic biochemical
analyzer (Hitachi, Ltd.) was used to detect renal function
indicators, such as blood urea nitrogen (BUN) and creatinine
levels.
Hematoxylin and eosin (H&E)
staining
Fresh mouse kidney tissue was fixed in 4%
paraformaldehyde solution for 24 h at room temperature, embedded in
paraffin and sectioned at 4-µm thickness. H&E staining was
performed to observe the tissue damage condition. The sample was
incubated with hematoxylin for ~3-5 min at room temperature. After
washing, the sample was incubated with eosin for ~2 min at room
temperature. Kidney morphology was observed under an optical
microscope. Blind method (investigator-blinded) was used to score
the degree of injury: i) 0 points, normal; ii) 1 point, renal
tubular injury area <25%; iii) 2 points, 25~50%; iv) 3 points,
50~75%; and v) 4 points, 75~100%.
Transmission electron microscopy
After collection, the fresh kidney tissue was
completely fixed in the 2.5% glutaraldehyde solution at 4°C for 2–4
h. Then, the tissues were sent to Wuhan Servicebio Technology Co.,
Ltd., to prepare the specimens. Briefly, kidney tissue was
post-fixed in 1% osmium tetroxide, dehydrated in a graded series of
ethanol (50–100%) and acetone, embedded in Epon and sectioned at
60–80 nm, and then images were collected under a transmission
electron microscope.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
The fresh kidney tissue was fixed in 4%
paraformaldehyde solution for 24 h at room temperature, embedded in
paraffin and sectioned at 4-µm thickness. TUNEL apoptosis detection
kit (cat. no. 1684817910; Sigma-Aldrich; Merck KGaA) was used to
detect the level of apoptosis in paraffin-embedded sections of
kidney tissue. The specific procedures followed the manufacturer's
instructions. The sections were then incubated with DAB (cat. no.
DM827; Dako; Agilent Technologies, Inc.). When the cell nucleus was
brown under the light microscope, the section was washed to stop
the staining. Then, Harris hematoxylin was used to counterstain the
nucleus for 3 min at room temperature. Images were observed under
light microscopy (magnification, ×200). Positive cells were counted
at ×200 magnification. The percentage of apoptosis was calculated
as the number of apoptotic cells/total number of cells ×100% in
5–10 randomly selected fields.
Enzyme-linked immunosorbent assay
(ELISA)
ELISA kit was used to detect the levels of
interleukin (IL)-6 (cat. no. EK0411), tumor necrosis factor-α
(TNF-α; cat. no. EK0527), IL-1β (cat. no. EK0394; Wuhan Boster
Biological Technology, Ltd.) and IL-18 (cat. no. CSB-E04609m;
Cusabio Technology LLC) in mouse kidney tissue. The specific
procedures followed the kit instructions.
Cell culture
The HK-2 cell line was purchased from American Type
Culture Collection and maintained in DMEM/F12 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C and 5%
CO2. The medium was renewed every 2–3 days. The HK-2
cells were plated in a 6-well plate at 1×105 cells/well
and divided into four groups: i) Control; ii) NaHS; iii) LPS; and
iv) LPS + NaHS group. The cells in the LPS + NaHS group were
pre-incubated with 0.1 mM NaHS for 30 min and then stimulated with
1,000 ng/ml LPS for 12 h at 37°C and 5% CO2. Following
which, the cells were collected for further analysis. For the 3-MA
experiment, the HK-2 cells were plated in a 6-well plate at
1×105 cells/well and divided into four groups: i)
Control; ii) LPS; iii) LPS + NaHS; and iv) LPS + NaHS + 3-MA group.
The cells in the LPS + NaHS + 3-MA group were incubated with 5 mM
3-MA for 30 min, and then 0.1 mM NaHS was added and incubated for
another 30 min, followed by stimulation with 1,000 ng/ml LPS for 12
h at 37°C and 5% CO2. The cells and supernatant were
collected for further analysis.
Western blotting
RIPA lysis buffer (plus phenylmethanesulfonyl
fluoride) (cat. no. WB-0072; Beijing Dingguo Changsheng
Biotechnology Co., Ltd.) was used to extract protein from mouse
kidney tissue and cells, and the protein concentration was
determined by the BCA (cat. no. BCA01; Beijing Dingguo Changsheng
Biotechnology Co., Ltd.) method. Protein samples (40 µl/lane) were
added with 1/4 volume of 5X SDS loading buffer and boiled at 95°C
for 10 min to denature the protein. After electrophoresis by 12%
gel and transfer to 0.2 µm PVDF membrane (cat. no. ISEQ00010;
MilliporeSigma), the membrane was blocked by 2% BSA (cat. no.
FA016; Genview) at room temperature for 1–2 h and incubated with
primary antibodies at 4°C overnight. The primary antibodies were
anti-LC-3B (1:1,000; cat. no. L7543; Sigma-Aldrich; Merck KGaA),
anti-p62 (1:1,000; cat. no. P0067; Sigma-Aldrich; Merck KGaA) and
anti-β-actin (1:2,000; cat. no. A1978; Sigma-Aldrich; Merck KGaA).
After incubation with the HRP-conjugated goat anti-rabbit IgG (H+L)
(1:5,000; cat. no. AS014; ABclonal Biotech Co., Ltd.) secondary
antibody at room temperature for 1 h, the membrane was developed
with enhanced chemiluminescence (ECL) (cat. no. GE2301; Genview).
ImageJ version 1.48 (National Institutes of Health) software was
used to analyze protein bands and calculate relative protein
expression.
Immunofluorescence
The slices were emersed in xylene I for 15 min,
xylene II for 15 min, anhydrous ethanol I for 5 min, anhydrous
ethanol II for 5 min, 85% alcohol for 5 min and 75% alcohol for 5
min, and then washed with distilled water. Then, the slices were
placed in a repair box filled with EDTA antigen retrieval buffer
(pH 8.0) in a microwave oven for antigen retrieval. The fire was
stopped for 8 min at medium heat for 8 min and switched to low fire
for 7 min. After natural cooling, the slides were placed in PBS (pH
7.4) and washed three times, and then the paraffin-embedded
sections (paraffin-embedded process and section thickness were the
same as aforementioned) were blocked with 1% BSA (cat. no. A8020;
Beijing Solarbio Science & Technology Co., Ltd.) for 30 min at
37°C, and incubated with a primary antibody against LC-3B (1:100;
cat. no. L7543; Sigma-Aldrich; Merck KGaA) overnight at 4°C. After
washing with PBS, the sections were incubated with a goat
anti-rabbit secondary antibody conjugated with fluoresceine
isothiocyanate (1:50; cat. no. AS011; ABclonal Biotech Co., Ltd.)
at room temperature in the dark for 1 h. DNA was counterstained
with DAPI (cat. no. C0065; Beijing Solarbio Science &
Technology Co., Ltd.) for 10 min at room temperature. Images were
acquired from a fluorescence microscope. For immunofluorescence
staining on cells, cells were plated on a cover glass in a 6-well
plate at 1×105 cells/well; and then following the
treatments outlined above, the cells were washed three times with
PBS, fixed with 4% paraformaldehyde for 10 min at room temperature,
and washed three times with PBS. The cells were blocked with 1% BSA
(cat. no. A8020; Beijing Solarbio Science & Technology Co.,
Ltd.) for 30 min at 37°C, and the subsequent steps were the same as
aforementioned for the paraffin-embedded sections.
Cell viability analysis
Cells were plated in a 96-well plate at
1×104 cells/well and given the treatments outlined
above. Next, the supernatant was discarded, and Cell Counting Kit-8
(CCK-8) reagent was added to detect cell viability. The specific
procedures followed the instructions of the CCK-8 kit (cat. no.
CK04; Dojindo Laboratories, Inc.).
Detection of lactic dehydrogenase
(LDH) level in cell culture medium
The supernatant of each well in the 96-well plate
was collected to detect the LDH levels. The specific procedures
followed the instructions of the LDH Cytotoxicity Detection Kit
(Nanjing Jiancheng Bioengineering Institute).
Detection of apoptosis via flow
cytometry
The cells were trypsinized, washed and tested for
apoptosis via flow cytometry (BD FACSCanto™ II flow cytometer; BD
Biosciences). The detailed procedures followed the instructions of
the Annexin V-FITC/PI Apoptosis Detection Kit (BD Pharmingen; BD
Biosciences). Data acquisition was performed using FlowJo version
7.6.1 analysis software (FlowJo, LLC). The apoptotic rate (%) was
calculated as the sum of Annexin V-FITC+/PI−
(early apoptosis, Q3) and Annexin-V-FITC+/PI+
(late apoptosis, Q2) cells.
Statistical analysis
The data were analyzed using GraphPad Prism 5
software (GraphPad Software, Inc.). All experiments were repeated
at least three times. Kaplan-Meier plots were used to illustrate
survival of mice between different groups, and statistical
assessment was performed by the log-rank test. Bonferroni
correction was used for statistical comparison between multiple
groups for the analysis of survival curves (P<0.0167 was
considered statistically significant). The median + interquartile
range were used to illustrate tubular injury score of mice between
different groups, and statistical assessment was performed by
Kruskal-Wallis followed by Dunn's post hoc test. One-way analysis
of variance (One-Way ANOVA) was used for comparison between
multiple groups, followed by the Tukey's post hoc test for pairwise
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
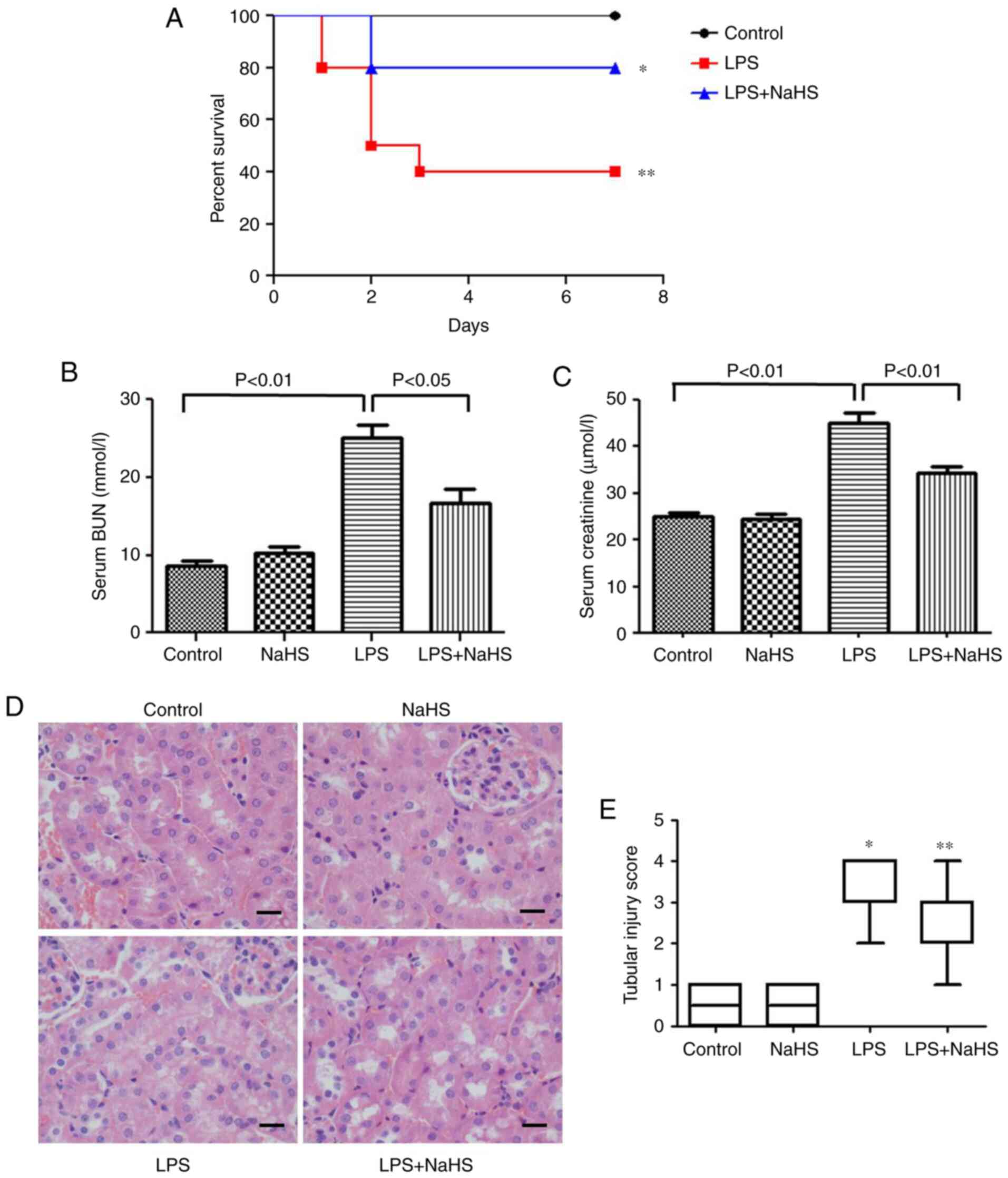
Effect of NaHS on the survival rate of
AKI mice induced by LPS
The effect of NaHS pretreatment on the survival rate
of mice injected with LPS was examined. The results showed that 0.8
mg/kg NaHS could increase the survival rate of LPS-induced mice
(Fig. 1A), however, the
statistical difference between the NaHS + LPS group and LPS group
was not significant. This may be related to the small sample size,
and thus the sample size will be increased for further verification
in subsequent experiments.
Pretreatment with NaHS reduces renal
function damage and histopathological damage in mice with
LPS-induced AKI
To clarify the effect of NaHS on renal function
damage, BUN and creatinine levels were measured in mice injected
with LPS. The results showed that LPS increased the BUN and
creatinine levels, which were significantly prevented by NaHS
pretreatment (Fig. 1B and C).
H&E staining was also performed to examine the changes in
kidney morphology, and the results showed that LPS treatment caused
kidney pathological changes, such as edema and granular
degeneration of tubular epithelial cells, and NaHS pretreatment
significantly reduced the tubular injury score induced by LPS
(Fig. 1D and E).
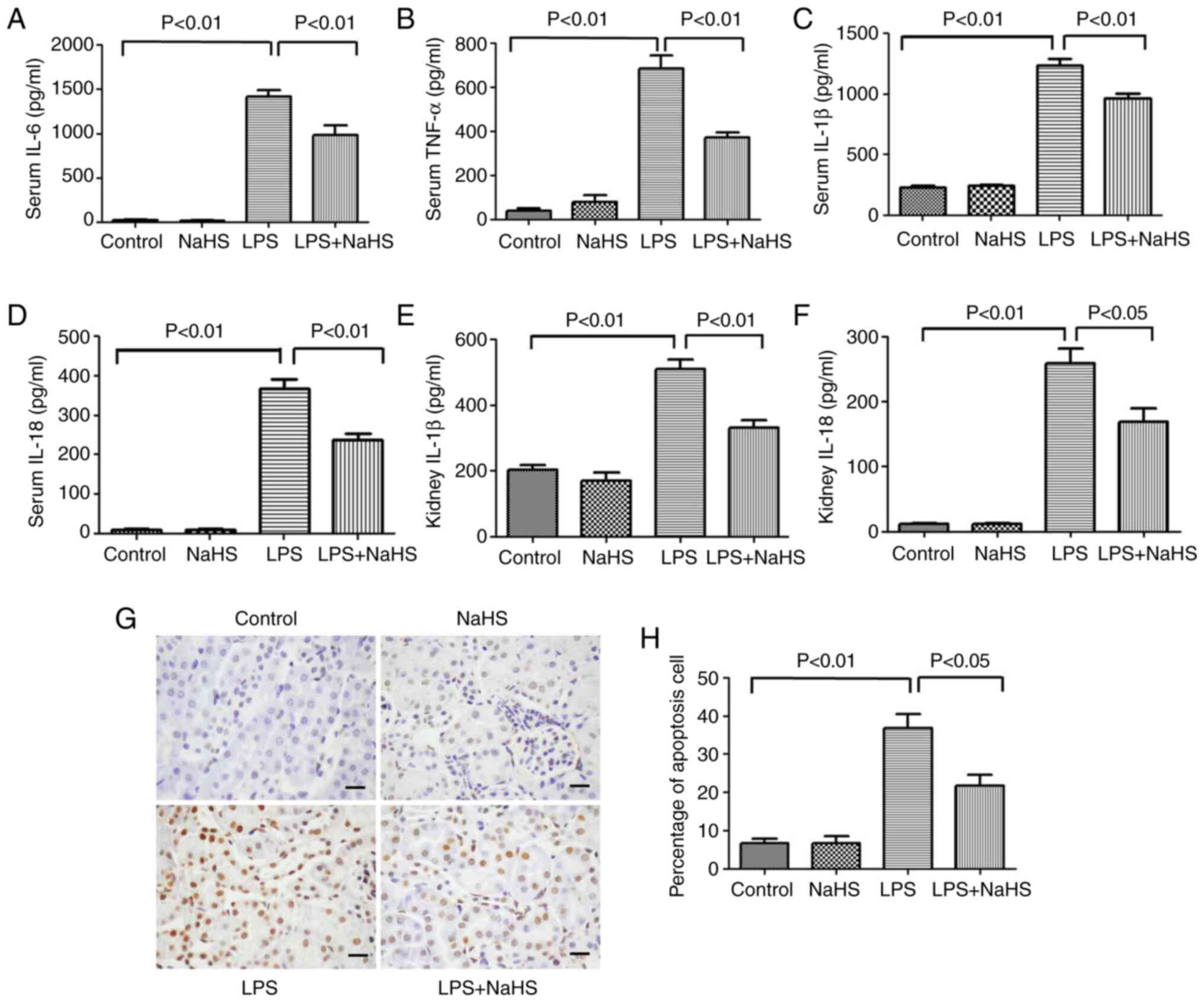
Pretreatment with NaHS reduces
inflammation in mice with LPS-induced AKI
To clarify the effect of NaHS on the release of
inflammatory factors in LPS-induced mice, the expression levels of
classic inflammatory factors, such as IL-6, TNF-α, IL-1β and IL-18,
were measured in mouse serum after 12 h of LPS stimulation. The
results showed that the serum levels of IL-6, TNF-α, IL-1β and
IL-18 significantly increased after LPS treatment, while NaHS
pretreatment could effectively reduce the elevation of these
inflammatory factors (Fig. 2A-D).
Further examination of IL-1β and IL-18 levels in kidney tissues
showed that NaHS pretreatment significantly reduced the expression
of IL-1β and IL-18 in kidney tissues from the mice (Fig. 2E and F). The aforementioned
results indicated that NaHS pretreatment alleviated the
inflammatory response induced by LPS in both the serum and the
kidney tissues.
Pretreatment with NaHS reduces the
rate of apoptosis in kidney tissues of LPS-induced AKI mice
TUNEL staining was used to detect the effect of LPS
on kidney cell apoptosis in AKI mice. Cell apoptosis can occur in
both pathological and physiological conditions. As shown in
Fig. 2G and H, very few apoptotic
cells were detected in the normal control group or NaHS group.
However, 12 h after LPS injection, the number of apoptotic cells
with positive TUNEL staining in kidney tissues were significantly
increased, and NaHS pretreatment significantly prevented the
increase of apoptotic cells. These results indicated that NaHS
pretreatment alleviated LPS-induced apoptosis in mouse kidney
cells.
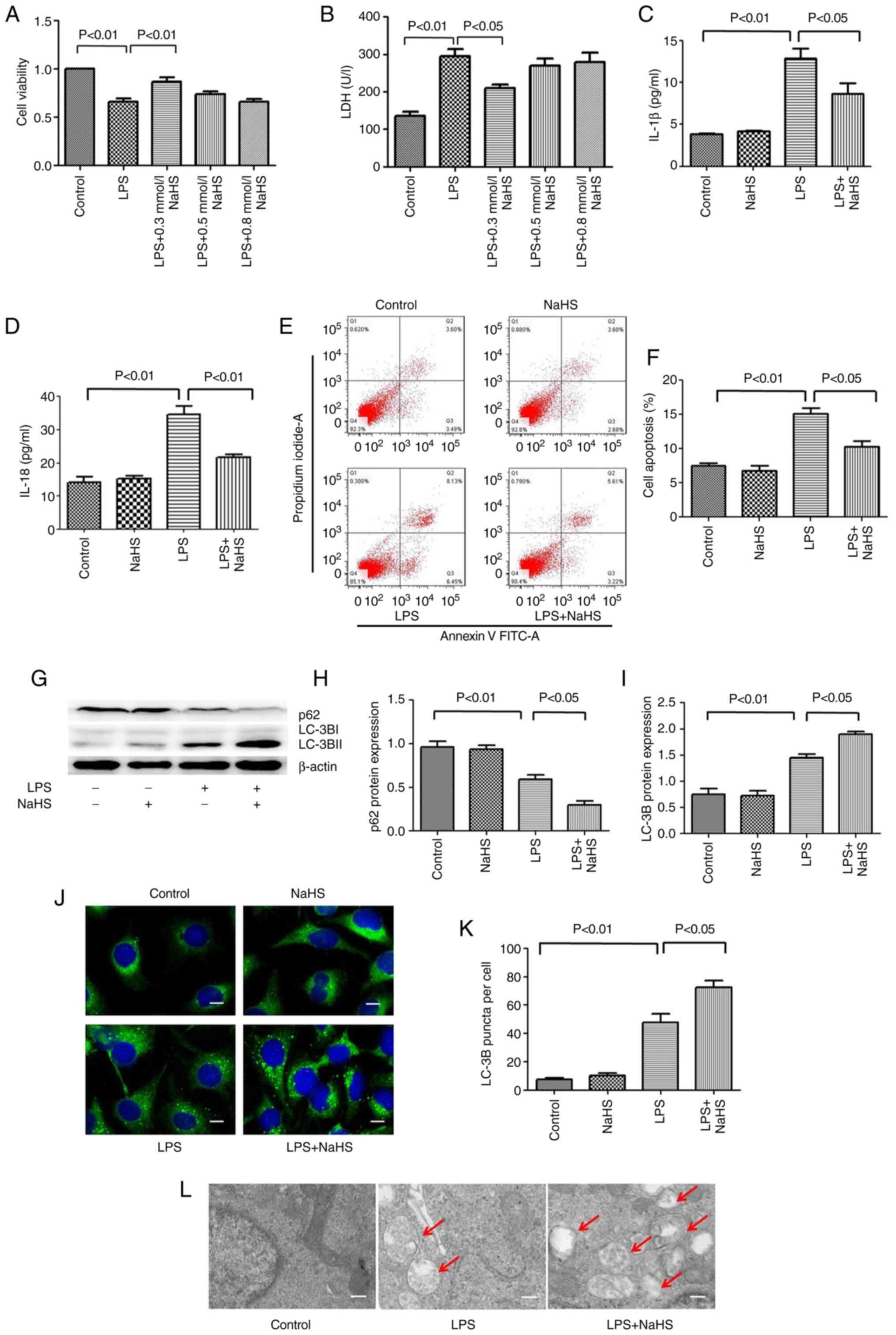
Pretreatment with NaHS reduces the LPS
induced damage on HK-2 cells
To further clarify the mechanism of NaHS in
LPS-induced AKI, assays at the cellular level were performed. The
HK-2 renal tubular epithelial cells were stimulated with LPS for 12
h, and the intervention groups were pretreated with different
concentrations of NaHS (0.1, 0.3 and 0.5 mM). To determine the
degree of cell damage, CCK-8 and LDH assays were used to detect
cell viability and LDH levels in the cell culture supernatant. The
results showed that LPS treatment decreased cell viability and
increased the release of LDH in the supernatant, while 0.1 mM NaHS
pretreatment could significantly reverse these effects. The effects
of 0.3 and 0.5 mM NaHS pretreatment were not obvious (Fig. 3A and B). Therefore, 0.1 mM NaHS
was chosen for pretreatment in all subsequent cell experiments.
Pretreatment with NaHS reduces the
LPS-induced release of inflammatory factors and apoptosis in HK-2
cells
To clarify the effect of NaHS pretreatment on the
LPS-induced release of inflammatory factors in HK-2 cells, ELISA
was used to detect the release of IL-1β and IL-18 in the cell
supernatant. The results showed that LPS treatment increased the
release of IL-1β and IL-18 in HK-2 cells, and NaHS pretreatment
significantly reduced these effects (Fig. 3C and D). Next, the present study
sought to investigate the effect of NaHS pretreatment on the
apoptosis of HK-2 cells induced by LPS using flow cytometry. The
results showed that LPS treatment increased the fraction of
apoptotic HK-2 cells, and NaHS pretreatment significantly reduced
LPS-induced apoptosis (Fig. 3E and
F). The aforementioned results suggested that the protective
effect of NaHS on LPS-induced AKI was partially mediated by its
anti-inflammatory and anti-apoptotic effects, but the underlying
mechanisms are still unclear.
Pretreatment with NaHS enhances the
autophagy level in HK-2 cells stimulated by LPS
To clarify the anti-inflammatory and anti-apoptotic
mechanism of NaHS, the effect of NaHS on autophagy was examined.
First, western blotting was performed to detect the expression of
autophagy-related proteins microtubule associated protein 1 light
chain 3 (LC3B) and sequestosome 1 (p62) in HK-2 cells. It was found
that the expression of LC3B and p62 protein did not change when
NaHS was administered alone. However, after LPS stimulation, the
ratio of LC3B-II/LC3B-I increased and the expression of p62 protein
decreased. NaHS pretreatment further increased the ratio of
LC3B-II/LC3B-I and decreased the expression of p62 protein
(Fig. 3G-I), suggesting that NaHS
could promote autophagy. Immunofluorescence and electron microscopy
were then used to further verify the effect of NaHS on autophagy.
Immunofluorescence results showed that the punctate autophagosomes
in the renal tubules increased significantly after LPS stimulation,
and NaHS pretreatment further increased the number of
autophagosomes (Fig. 3J and K).
Electron microscopy results showed that the number of autophagic
vesicles increased notably in HK-2 cells after LPS stimulation, and
NaHS pretreatment further augmented this change (Fig. 3L). Altogether, these results
indicated that NaHS pretreatment could enhance the autophagy level
in the HK-2 cells stimulated with LPS.
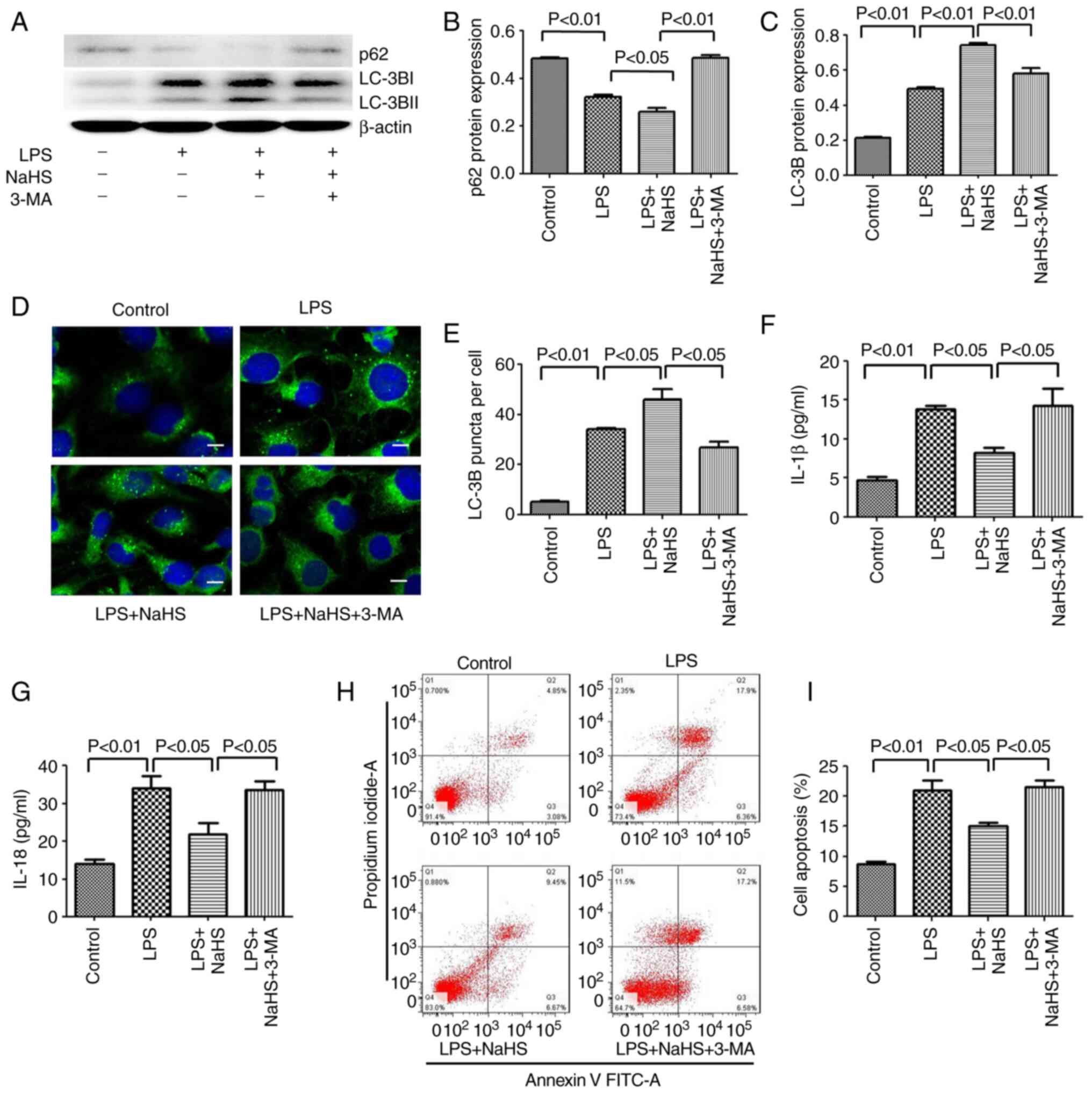
Inhibition of autophagy by 3-MA
reverses the protective effect of NaHS on LPS-induced HK-2 cell
apoptosis and the release of inflammatory factors
Since NaHS pretreatment can further enhance the
autophagy induced by LPS, the anti-inflammatory and anti-apoptotic
effects of NaHS may be related to autophagy. To test this
hypothesis, the autophagy inhibitor 3-MA was used to block
autophagy and it was examined whether the protective effect of NaHS
on HK-2 cells could be reversed. First, western blotting was used
to detect the inhibitory effect of 3-MA on autophagy, and the
results showed that 3-MA significantly reduced the ratio of
LC3B-II/LC3B-I in the LPS + NaHS + 3-MA group, and the expression
of p62 protein was increased (Fig.
4A-C). Secondly, the immunofluorescence results showed that the
number of punctate fluorescent autophagosomes induced in the LPS +
NaHS group was significantly reduced in the LPS + NaHS + 3-MA group
(Fig. 4D and E). These results
demonstrated that 3-MA effectively inhibited the level of
autophagy. Next, the effects of 3-MA on HK-2 cell apoptosis and the
release of inflammatory factors were examined. The apoptosis rate
of HK-2 cells and the levels of released IL-1β and IL-18 were
significantly increased in the LPS + NaHS + 3-MA group compared
with the LPS + NaHS group (Fig.
4F-I), indicating that the autophagy block by 3-MA partially
reversed the protective effect of NaHS on LPS-induced apoptosis and
the release of inflammatory factors in HK-2 cell.
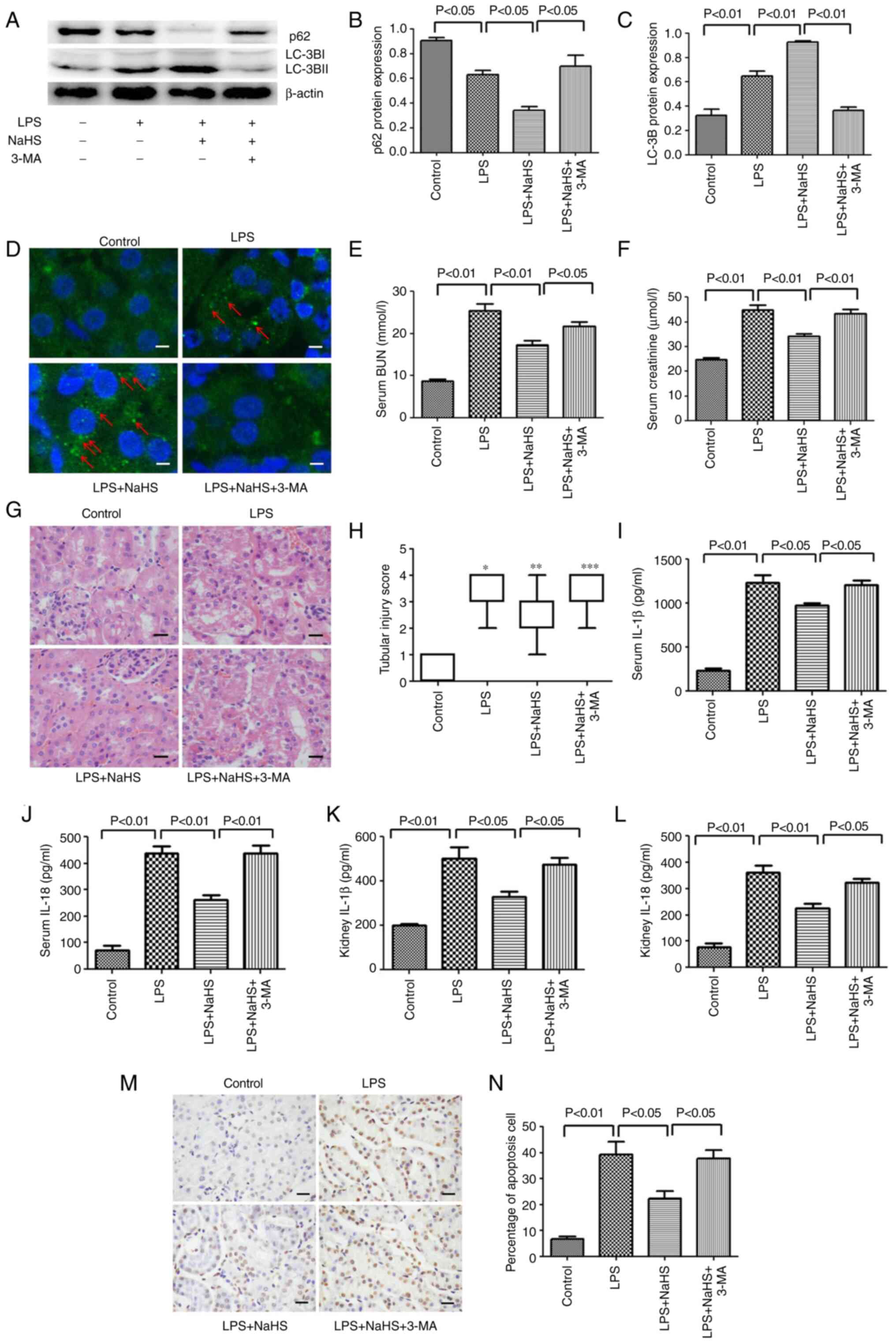
Inhibition of autophagy by 3-MA
reverses the protective effect of NaHS on LPS-induced AKI in
mice
To further verify the aforementioned protective
effect of NaHS in animals, 3-MA was intraperitoneally injected into
mice to inhibit autophagy and it was examined whether the
protective effect of NaHS still existed in mice. As shown in
Fig. 5, western blotting and
immunofluorescence results on mouse kidney tissue showed that the
ratio of LC3B-II/LC3B-I was significantly reduced in the LPS + NaHS
+ 3-MA group, and the expression of p62 protein was increased
compared with the LPS + NaHS group (Fig. 5A-C). Furthermore, the
immunofluorescence results showed that the number of punctate
fluorescent autophagosomes induced in the LPS + NaHS group was
notably reduced in the LPS + NaHS + 3-MA group (Fig. 5D). Thus, these results suggested
that 3-MA effectively blocked the autophagy induced by NaHS. Based
on these findings, the renal function, kidney morphological
changes, release of inflammatory factors and level of apoptosis
were examined in mice. Compared with the LPS + NaHS group, the BUN
and creatinine levels in the LPS + NaHS + 3-MA group were
significantly increased (Fig. 5E and
F). H&E staining showed that the tissue structure damage,
such as edema of tubular epithelial cells, in the LPS + NaHS + 3-MA
group was aggravated (Fig. 5G and
H). Consistently, ELISA results showed that the release of
IL-1β and IL-18 in serum and kidney tissues was significantly
higher in the LPS + NaHS + 3-MA group than that in the LPS + NaHS
group (Fig. 5I-L). TUNEL staining
also showed that the apoptosis rate of renal tubular cells in the
LPS + NaHS + 3-MA group was significantly higher than that in the
LPS + NaHS group (Fig. 5M and
N).
Discussion
The pathogenesis of AKI is very complicated, it
involves hemodynamic changes, apoptosis, inflammation, coagulation
activation and oxidative stress (5). Renal tubular epithelial cells are
the main target cell type of AKI. In the environment with ischemia
and/or toxins, renal tubular epithelial cells undergo necrosis,
apoptosis and shedding, which is an important cause of AKI
(31). In addition, the
inflammatory response of kidney is also an important factor
involved in the occurrence and development of AKI (31). It is known that LPS and other
pathogen-related molecular patterns (PAMP) can directly interact
with toll-like receptor (TLR)-2 and TLR-4 on renal tubular
epithelial cells (TEC), and induce the release of IL-6, TNF-α and
other cytokines (32–34). This pro-inflammatory effect on TEC
eventually leads to infiltration of white blood cells, which
promotes tissue damage (5). In
the present study, LPS was used to construct septic a AKI mouse
model and cell injury model. The kidney tissue and cells showed
obvious damage, and the apoptosis rate and the release of
inflammatory factors were significantly increased.
As a gas signal molecule, H2S plays an
important role in the human body. 1/3 of H2S exists as
gas molecules in the body and 2/3 is in the form of NaHS, which not
only ensures the stability of H2S in the body, but also
does not change the pH level of the internal environment (35). Thus, the current study chose NaHS
as the H2S donor. Studies shows that different
concentrations of H2S have different effects, such as
low concentrations have protection (12,36), and high concentrations have
damaging effects (37,38). Therefore, our study choose a low
concentration dose. In the future, we will verify the optimal
dosage of H2S in follow-up experiments. The protective
mechanisms of H2S include anti-inflammatory and
antioxidant effects, and regulation of cell proliferation and
apoptosis (12–18,39). The present results also showed
that NaHS can reduce LPS-induced kidney damage through
anti-inflammatory and anti-apoptotic effects. The anti-inflammatory
and anti-apoptotic mechanism of NaHS is still unclear. In previous
years, studies have shown that H2S also has a regulatory
effect on autophagy. For example, H2S can promote liver
autophagy, reduce serum triglyceride levels and improve
non-alcoholic fatty liver disease (40). Yang et al (26) found that H2S can
prevent diabetic cardiomyopathy by activating autophagy. The
present study also found that pretreatment with NaHS further
promoted the level of autophagy in HK-2 cells stimulated by LPS,
suggesting that the protective effect of NaHS may be through
regulating autophagy.
So far, a large number of studies have shown that
autophagy has a regulatory effect on apoptosis and the release of
inflammatory factors. For example, in cisplatin-induced renal
tubular cell injury, both autophagy and caspases are activated, and
the activation of autophagy is earlier than the activation of
caspases (41–43). In renal tubular epithelial cells
cultured in vitro, inhibition of cisplatin-induced autophagy
by 3-MA and knockdown of autophagy gene Atg5 or Beclin1 via siRNA
can enhance the activation of caspase-3, 7, 6 (41,43) and apoptosis (41–43); and overexpression of Atg5 and
Beclin-1 protein can prevent the cisplatin-induced activation of
caspase and apoptosis (44). The
specific knockout of Atg5 or Atg7 in mouse proximal tubular cells
has been found to inhibit autophagy and enhance cisplatin-induced
apoptosis and caspase activity (23,44). In addition, Han et al
(46) showed that the autophagy
induced by AXL receptor tyrosine kinase could reduce acute liver
injury by inhibiting the activation of NLRP3 inflammasome. Tong
et al (47) found that
heat shock factor 1 could inhibit the release of inflammatory
factors by inducing autophagy, thus exerting a protective effect on
septic mice. Since the activation of autophagy can inhibit
apoptosis and the release of inflammatory factors, the protective
effect of NaHS observed in this study could also be mediated by
regulating autophagy. To test this hypothesis, the autophagy
inhibitor 3-MA was used to block autophagy both in vivo and
in vitro. The results showed that 3-MA pretreatment
significantly inhibited the protective effect of NaHS, suggesting
that NaHS inhibited renal tubular cell apoptosis and renal
interstitial inflammation by promoting autophagy, thereby
protecting from the AKI induced by LPS. The aforementioned results
indicated that the regulation of autophagy by NaHS may be a key
protective mechanism to alleviate AKI. Recently, studies have
reported that H2S can activate autophagy by inhibiting
the MAPK pathway, thereby exerting anti-inflammatory and
anti-apoptotic effects (48,49). H2S can also induce
autophagy by activating AMPK (26,50). In addition, H2S can
induce autophagy by activating the Nrf2/Keap1 signaling pathway and
play a protective role (51–53). However, how NaHS regulates
autophagy in LPS-induced AKI is not yet fully elucidated and
further investigation is needed.
In summary, this study found that NaHS can prevent
AKI in mice with endotoxemia, and its protective effect is
partially mediated by promoting autophagy to inhibit renal tubular
epithelial cell apoptosis and the release of inflammatory
factors.
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation
of China (grant nos. 81902020 and 82172147), Natural Science
Foundation of Shanxi Province (grant no. 201801D221444), Scientific
and Technological Innovation Programs of Higher Education
Institutions in Shanxi (grant no. 2019L0662), Scientific Research
Programs of Health Commission of Shanxi Province (grant no.
2018126), Project of Academic Technology Leader in Changzhi Medical
College (grant no. XSQ201903), Innovation Team Project of Changzhi
Medical College (grant no. CX201501) and Natural Science Foundation
of Hunan Province (grant no. 2021JJ30900).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TL, JZ and YL conceived and designed the
experiments. TL, JZ, SM and YC performed the experiments and
analyzed the samples. TL and YX analyzed the data. TL wrote the
manuscript. All authors interpreted the data and critically revised
the manuscript for important intellectual contents. All authors
have read and approved the final manuscript. TL and YL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Ethics Committee of Central South University
(Changsha, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alberti C, Brun-Buisson C, Burchardi H,
Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R,
Boulmé R, et al: Epidemiology of sepsis and infection in ICU
patients from an international multicentre cohort study. Intensive
Care Med. 28:108–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hotchkiss RS and Karl IE: The
pathophysiology and treatment of sepsis. N Engl J Med. 348:138–50.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bagshaw SM, George C and Bellomo R; ANZICS
Database Management Committee, : Early acute kidney injury and
sepsis: A multicentre evaluation. Crit Care. 12:R472008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mehta RL, Bouchard J, Soroko SB, Ikizler
TA, Paganini EP, Chertow GM and Himmelfarb J; Program to Improve
Care in Acute Renal Disease (PICARD) Study Group, : Sepsis as a
cause and consequence of acute kidney injury: Program to improve
care in Acute Renal Disease. Intensive Care Med. 37:241–248. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gomez H, Ince C, De Backer D, Pickkers P,
Payen D, Hotchkiss J and Kellum JA: A unified theory of
sepsis-induced acute kidney injury: Inflammation, microcirculatory
dysfunction, bioenergetics, and the tubular cell adaptation to
injury. Shock. 41:3–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peerapornratana S, Manrique-Caballero CL,
Gómez H and Kellum JA: Acute kidney injury from sepsis: Current
concepts, epidemiology, pathophysiology, prevention and treatment.
Kidney Int. 96:1083–1099. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szabo C: Hydrogen sulphide and its
therapeutic potential. Nat Rev Drug Discov. 6:917–935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahangarpour A, Abdollahzade Fard A,
Gharibnaseri MK, Jalali T and Rashidi I: Hydrogen sulfide
ameliorates the kidney dysfunction and damage in cisplatin-induced
nephrotoxicity in rat. Vet Res Forum. 5:121–127. 2014.PubMed/NCBI
|
|
9
|
Bos EM, Leuvenink HG, Snijder PM,
Kloosterhuis NJ, Hillebrands JL, Leemans JC, Florquin S and van
Goor H: Hydrogen sulfide-induced hypometabolism prevents renal
ischemia/reperfusion injury. J Am Soc Nephrol. 20:1901–1905. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han SJ, Kim JI, Park JW and Park KM:
Hydrogen sulfide accelerates the recovery of kidney tubules after
renal ischemia/reperfusion injury. Nephrol Dial Transplant.
30:1497–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu JX, Kalbfleisch M, Yang YX, Bihari R,
Lobb I, Davison M, Mok A, Cepinskas G, Lawendy AR and Sener A:
Detrimental effects of prolonged warm renal ischaemia-reperfusion
injury are abrogated by supplemental hydrogen sulphide: An analysis
using real-time intravital microscopy and polymerase chain
reaction. BJU Int. 110:E1218–E1227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou X, Feng Y, Zhan Z and Chen J:
Hydrogen sulfide alleviates diabetic nephropathy in a
streptozotocin-induced diabetic rat model. J Biol Chem.
289:28827–28834. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HJ, Mariappan MM, Feliers D,
Cavaglieri RC, Sataranatarajan K, Abboud HE, Choudhury GG and
Kasinath BS: Hydrogen sulfide inhibits high glucose-induced matrix
protein synthesis by activating AMP-activated protein kinase in
renal epithelial cells. J Biol Chem. 287:4451–4461. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Safar MM and Abdelsalam RM: H2S donors
attenuate diabetic nephropathy in rats: Modulation of oxidant
status and polyol pathway. Pharmacol Rep. 67:17–23. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang D, Zhang Y, Yang M, Wang S, Jiang Z
and Li Z: Exogenous hydrogen sulfide prevents kidney damage
following unilateral ureteral obstruction. Neurourol Urodyn.
33:538–543. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song K, Wang F, Li Q, Shi YB, Zheng HF,
Peng H, Shen HY, Liu CF and Hu LF: Hydrogen sulfide inhibits the
renal fibrosis of obstructive nephropathy. Kidney Int.
85:1318–1329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dursun M, Otunctemur A, Ozbek E, Sahin S,
Besiroglu H, Ozsoy OD, Cekmen M, Somay A and Ozbay N: Protective
effect of hydrogen sulfide on renal injury in the experimental
unilateral ureteral obstruction. Int Braz J Urol. 41:1185–1193.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Jin S, Teng X, Hu Z, Zhang Z, Qiu
X, Tian D and Wu Y: Hydrogen sulfide attenuates LPS-Induced acute
kidney injury by inhibiting inflammation and oxidative stress. Oxid
Med Cell Longev. 2018:67172122018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levine B and Klionsky DJ: Development by
self-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizushima N, Yoshimori T and Ohsumi Y: The
role of Atg proteins in autophagosome formation. Annu Rev Cell Dev
Biol. 27:107–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsiao HW, Tsai KL, Wang LF, Chen YH,
Chiang PC, Chuang SM and Hsu C: The decline of autophagy
contributes to proximal tubular dysfunction during sepsis. Shock.
37:289–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leventhal JS, Ni J, Osmond M, Lee K,
Gusella GL, Salem F and Ross MJ: Autophagy limits endotoxemic acute
kidney injury and alters renal tubular epithelial cell cytokine
expression. PLoS One. 11:e1500012016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang M, Wei Q, Dong G, Komatsu M, Su Y
and Dong Z: Autophagy in proximal tubules protects against acute
kidney injury. Kidney Int. 82:1271–1283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu S, Hartleben B, Kretz O, Wiech T,
Igarashi P, Mizushima N, Walz G and Huber TB: Autophagy plays a
critical role in kidney tubule maintenance, aging and
ischemia-reperfusion injury. Autophagy. 8:826–837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li T, Liu Y, Zhao J, Miao S, Xu Y, Liu K,
Liu M, Wang G and Xiao X: Aggravation of acute kidney injury by
mPGES-2 down regulation is associated with autophagy inhibition and
enhanced apoptosis. Sci Rep. 7:102472017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang F, Zhang L, Gao Z, Sun X, Yu M, Dong
S, Wu J, Zhao Y, Xu C, Zhang W and Lu F: Exogenous H2S protects
against diabetic cardiomyopathy by activating autophagy via the
AMPK/mTOR Pathway. Cell Physiol Biochem. 43:1168–1187. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Liao R, Qiang Z, Yang W, Cao J and
Zeng H: Exogenous H2S protects colon cells in ulcerative
colitis by inhibiting NLRP3 and activating autophagy. DNA Cell
Biol. 40:748–756. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang S, Yang G, Guan W, Li B, Feng X and
Fan H: Autophagy plays a protective role in sodium
hydrosulfide-induced acute lung injury by attenuating oxidative
stress and inflammation in rats. Chem Res Toxicol. 34:857–864.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Feng X, Li B, Sha J, Wang C, Yang
T, Cui H and Fan H: Dexmedetomidine protects against
lipopolysaccharide-induced acute kidney injury by enhancing
autophagy through inhibition of the PI3K/AKT/mTOR pathway. Front
Pharmacol. 11:1282020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu L, Guo J, Zhou L, Zhu S, Wang C, Liu J,
Hu S, Yang M and Lin C: Hydrogen sulfide protects retinal pigment
epithelial cells from oxidative stress-induced apoptosis and
affects autophagy. Oxid Med Cell Longev. 2020:88685642020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lerolle N, Nochy D, Guérot E, Bruneval P,
Fagon JY, Diehl JL and Hill G: Histopathology of septic shock
induced acute kidney injury: Apoptosis and leukocytic infiltration.
Intensive Care Med. 36:471–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang B, Ramesh G, Uematsu S, Akira S and
Reeves WB: TLR4 signaling mediates inflammation and tissue injury
in nephrotoxicity. J Am Soc Nephrol. 19:923–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ho AW, Wong CK and Lam CW: Tumor necrosis
factor-alpha up-regulates the expression of CCL2 and adhesion
molecules of human proximal tubular epithelial cells through MAPK
signaling pathways. Immunobiology. 213:533–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Allam R, Scherbaum CR, Darisipudi MN,
Mulay SR, Hägele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu
M, Schwarzenberger C, et al: Histones from dying renal cells
aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol.
23:1375–1388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hosoki R, Matsuki N and Kimura H: The
possible role of hydrogen sulfide as an endogenous smooth muscle
relaxant in synergy with nitric oxide. Biochem Biophys Res Commun.
237:527–531. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin F, Liao C, Sun Y, Zhang J, Lu W, Bai
Y, Liao Y, Li M, Ni X, Hou Y, et al: Hydrogen sulfide inhibits
cigarette smoke-induced endoplasmic reticulum stress and apoptosis
in bronchial epithelial cells. Front Pharmacol. 8:6752017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng X, Zhang H, Shi M, Chen Y, Yang T and
Fan H: Toxic effects of hydrogen sulfide donor NaHS induced liver
apoptosis is regulated by complex IV subunits and reactive oxygen
species generation in rats. Environ Toxicol. 35:322–332. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahmad A, Druzhyna N and Szabo C: Delayed
treatment with sodium hydrosulfide improves regional blood flow and
alleviates cecal ligation and puncture (CLP)-Induced septic shock.
Shock. 46:183–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen YH, Teng X, Hu ZJ, Tain DY, Jin S and
Wu YM: Hydrogen sulfide attenuated sepsis-induced myocardial
dysfunction through TLR4 pathway and endoplasmic reticulum stress.
Front Physiol. 12:6536012021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun L, Zhang S, Yu C, Pan Z, Liu Y, Zhao
J, Wang X, Yun F, Zhao H, Yan S, et al: Hydrogen sulfide reduces
serum triglyceride by activating liver autophagy via the AMPK-mTOR
pathway. Am J Physiol Endocrinol Metab. 309:E925–E935. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang C, Kaushal V, Shah SV and Kaushal GP:
Autophagy is associated with apoptosis in cisplatin injury to renal
tubular epithelial cells. Am J Physiol Renal Physiol.
294:F777–F787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Periyasamy-Thandavan S, Jiang M, Wei Q,
Smith R, Yin XM and Dong Z: Autophagy is cytoprotective during
cisplatin injury of renal proximal tubular cells. Kidney Int.
74:631–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kaushal GP, Kaushal V, Herzog C and Yang
C: Autophagy delays apoptosis in renal tubular epithelial cells in
cisplatin cytotoxicity. Autophagy. 4:710–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Herzog C, Yang C, Holmes A and Kaushal GP:
zVAD-fmk prevents cisplatin-induced cleavage of autophagy proteins
but impairs autophagic flux and worsens renal function. Am J
Physiol Renal Physiol. 303:F1239–F1250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang Z and Klionsky DJ: Mammalian
autophagy: Core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Han J, Bae J, Choi CY, Choi SP, Kang HS,
Jo EK, Park J, Lee YS, Moon HS, Park CG, et al: Autophagy induced
by AXL receptor tyrosine kinase alleviates acute liver injury via
inhibition of NLRP3 inflammasome activation in mice. Autophagy.
12:2326–2343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tong Z, Jiang B, Zhang L, Liu Y, Gao M,
Jiang Y, Li Y, Lu Q, Yao Y and Xiao X: HSF-1 is involved in
attenuating the release of inflammatory cytokines induced by LPS
through regulating autophagy. Shock. 41:449–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang GY, Lu D, Duan SF, Gao YR, Liu SY,
Hong Y, Dong PZ, Chen YG, Li T, Wang DY, et al: Hydrogen sulfide
alleviates lipopolysaccharide-induced diaphragm dysfunction in rats
by reducing apoptosis and inflammation through ROS/MAPK and
TLR4/NF-κB signaling pathways. Oxid Med Cell Longev.
2018:96478092018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Han X, Mao Z, Wang S, Xin Y, Li P,
Maharjan S and Zhang B: GYY4137 protects against MCAO via p38 MAPK
mediated anti-apoptotic signaling pathways in rats. Brain Res Bull.
158:59–65. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang H, Zhong P and Sun L: Exogenous
hydrogen sulfide mitigates NLRP3 inflammasome-mediated inflammation
through promoting autophagy via the AMPK-mTOR pathway. Biol Open.
8:bio0436532019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao S, Song T, Gu Y, Zhang Y, Cao S, Miao
Q, Zhang X, Chen H, Gao Y, Zhang L, et al: Hydrogen sulfide
alleviates liver injury Through the S-Sulfhydrated-Kelch-Like
ECH-Associated Protein 1/Nuclear Erythroid 2-Related Factor
2/Low-Density Lipoprotein Receptor-Related Protein 1 pathway.
Hepatology. 73:282–302. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu J, Tian Z, Sun Y, Lu C, Liu N, Gao Z,
Zhang L, Dong S, Yang F, Zhong X, et al: Exogenous H2S
facilitating ubiquitin aggregates clearance via autophagy
attenuates type 2 diabetes-induced cardiomyopathy. Cell Death Dis.
8:e29922017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhao S, Yang L, Li L and Fan Z: NaHS
alleviated cell apoptosis and mitochondrial dysfunction in remote
lung tissue after renal ischemia and reperfusion via Nrf2
activation-mediated NLRP3 pathway inhibition. Biomed Res Int.
2021:55988692021. View Article : Google Scholar : PubMed/NCBI
|