Introduction
Ischemic stroke is a disorder of the blood supply in
local brain tissue caused by various factors and is a major cause
of death and disability in adults worldwide (1). Ischemic stroke leads to
ischemic-hypoxic brain tissue necrosis, which results in neuronal
dysfunction (2). A previous study
has suggested that the restoration of blood flow in the ischemic
hemispheric zone around the infarcted tissue constitutes the most
effective therapeutic strategy for reducing the clinical symptoms
of cerebral ischemia (3).
However, recanalization, reperfusion and reoxygenation therapy can
occasionally lead to the concomitant occurrence of brain damage,
namely cerebral ischemia/reperfusion (I/R) injury (CIRI) (4). CIRI consists of various deleterious
pathological changes, such as neuronal apoptosis, oxidative stress,
autophagy, inflammation and necrosis, escalating to neurological
dysfunction (5). Recent decades
have seen an increasing incidence of ischemic stroke, and as a
consequence, CIRI has become a focus of current research (6). At present, alternative treatment
strategies for this disease remain lacking, hence the imperative to
identify novel agents that can be used clinically (7).
Dihydromyricetin (DMY) is a natural flavonoid
isolated from Ampelopsis grossedentata that has strong
antioxidant effects (8). DMY was
reported to markedly improve
1-methyl4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced
behavioral impairment and dopaminergic neuronal loss in mice
(9). Furthermore, in MES23.5
cells, DMY attenuated MPP+ (a metabolite of
MPTP)-induced cellular damage and reactive oxygen species (ROS)
production in a dose-dependent manner (9). DMY has been shown to enhance
mitochondrial function and reduce oxidative stress by upregulating
sirtuin 3, thus improving the prognosis of patients with cardiac
I/R injury (10). A previous
study also reported that DMY significantly reduced serum
transaminase activity and inhibited hepatic I/R-induced cell
apoptosis (11). The
aforementioned studies collectively suggest that DMY serves a
neuroprotective function. It has also been revealed that DMY
treatment improved mitochondrial morphology and function, inhibited
the production of reactive oxygen species and reduced hippocampal
lipid peroxidation in hypoxia-treated HT22 cells (12). However, whether DMY can protect
HT22 from oxygen-glucose deprivation and reoxygenation
(OGD/R)-induced injury is yet to be fully elucidated.
It has been reported that DMY promotes the
osteogenic differentiation of human bone marrow mesenchymal stem
cells (BMSCs) in vitro, partly through Wnt/β-catenin
signaling (13). This suggests
that DMY may also activate the Wnt/β-catenin signaling pathway in
HT22 cells. Furthermore, it has been demonstrated that
DMY-activated Wnt/β-catenin signaling attenuates brain injury in
rats exposed to cerebral I/R (14). Thus, the aim of the present study
was to investigate whether DMY could ameliorate OGD/R-induced HT22
cell injury by activating the Wnt/β-catenin signaling pathway.
Materials and methods
Cell culture and treatment
The murine hippocampal neuronal cell line, HT22, was
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences. Cells were cultured in high-glucose
DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin and
streptomycin. All cells were grown at 37°C in a humidified
atmosphere of 95% air and 5% CO2. DMY was purchased from
Chengdu Kangbang Biological Technology, Co., Ltd. Cells
(1×106 cells/well) were pretreated with Wnt signaling
pathway inhibitor XAV939 (5 µM, cat. no. HY-15147, MedChemExpress)
for 2 h at 37°C.
In vitro induction of OGD/R injury in
HT22 neuronal cells
The induction of OGD/R injury in HT22 neuronal cells
was performed according to a previously described protocol
(15). HT22 neuronal cells were
seeded (1×106 cells/well) into glucose-free medium and
cultured under hypoxic conditions (3% O2 with 5%
CO2 and 92% N2). After incubation for 8 h at
37°C under OGD conditions, the medium was replaced with
glucose-containing normal medium, and cells were grown under
normoxic conditions (95% air with 5% CO2) at 37°C. After
reoxygenation for 24 h, cells were harvested for analysis. HT22
cells that were cultured in normal medium under normoxic conditions
were used as the control.
MTT assay
Cell viability was determined using the MTT Cell
Viability Assay kit (Beyotime Institute of Biotechnology) according
to the manufacturer's instructions (16). HT22 cells were seeded at a density
of 3×103 cells/well into 96-well-plates and cultured
with different concentrations of DMY (0, 10, 30, 100 or 300 µmol/l)
for 24 h at 37°C. Cells were exposed to OGD/R prior to a 4-h
incubation with 20 µl MTT solution administered to each well. DMSO
was then added to each well to dissolve the formazan particles. The
absorbance in each sample was then measured at a wavelength of 450
nm using a microplate reader (Thermo Fisher Scientific, Inc.). Each
assay was performed in triplicate.
Measurement of lactate dehydrogenase
(LDH) concentration
An LDH assay kit (C0016, Beyotime Institute of
Biotechnology) was used to detect the concentration of LDH in HT22
cells (17). According to the
manufacturer's protocol, the cell supernatant was collected via
centrifugation at 400 × g for 10 min at room temperature, after
which 20 µl of the supernatant was mixed with
2,4-dinitrophenylhydrazine and incubated at 37°C for 15 min. NaOH
(0.4 M) was added into the mixture and incubated for a further 15
min at 37°C. After the mixture was kept at room temperature for 5
min, the absorbance at 450 nm was measured using a microplate
reader (Thermo Fisher Scientific, Inc.).
Measurement of malondialdehyde (MDA)
and superoxide (SOD) levels
The levels of MDA and SOD were measured using a
Lipid Peroxidation MDA Assay kit (S0131S) and a Cu/Zn-SOD Mn-SOD
Assay kit (S0103; both Beyotime Institute of Biotechnology),
respectively. To determine MDA levels, the MDA test solution was
mixed with a crude enzyme solution at a ratio of 3:1, placed in a
water bath at 100°C for 30 min, then cooled on ice. After adding
the mixture into the cell supernatant, absorbance values at 532 nm
were measured using a microplate reader (Thermo Fisher Scientific,
Inc.). The SOD test solution was also prepared using components of
the commercial kit, and the absorbance was measured at 450 nm
(18).
Western blotting
Following treatment, HT22 cells were lysed with RIPA
lysis buffer (Beyotime Institute of Biotechnology) supplemented
with protease inhibitors (Beyotime Institute of Biotechnology).
Protein concentrations were subsequently determined using a BCA
protein assay kit (Beyotime Institute of Biotechnology). Proteins
(40 µg) were separated by 10% SDS-PAGE and then transferred to PVDF
membranes (MilliporeSigma). The membranes were blocked with 5%
bovine serum albumin for 1.5 h at room temperature (BSA; Gibco;
Thermo Fisher Scientific, Inc.) and incubated with primary
antibodies overnight at 4°C. Samples were then washed with 5% BSA
in PBS/0.1% Tween-20 before incubation with HRP-conjugated
secondary antibodies for 1 h at room temperature. Protein bands
were visualized using an ECL kit (Beyotime Institute of
Biotechnology), and the gray value was analyzed using ImageJ
software (version 1.4.3.67; National Institutes of Health). The
antibodies used in this experiment were as follows: Anti-NADPH
oxidase 2 (NOX2; rabbit; 1:1,000; cat. no. ab129068; Abcam),
anti-NOX4 (rabbit; 1:1,000; cat. no. ab133303; Abcam), anti-Bcl-2
(mouse; 1:1,000; cat. no. 15071; Cell Signaling Technology, Inc.),
anti-Bax (mouse; dilution 1:1,000; cat. no. 5023; Cell Signaling
Technology, Inc.), anti-cleaved-caspase3 (mouse; 1:1,000; cat. no.
9661; Cell Signaling Technology, Inc.), anti-cleaved-caspase9
(mouse; 1:1,000; cat. no. 9509; Cell Signaling Technology, Inc.),
anti Wnt3 (rabbit; 1:1,000; cat. no. 2721; Cell Signaling
Technology, Inc.) anti-β-catenin (rabbit; 1:1,000; cat. no. 8480;
Cell Signaling Technology, Inc.), anti-GAPDH (rabbit; 1:1,000; cat.
no. 5174; Cell Signaling Technology, Inc.) and HRP-conjugated
anti-mouse IgG or anti-rabbit IgG (1:2,000; cat. nos. 7076 or 7074;
Cell Signaling Technology, Inc.).
TUNEL assay
Cell apoptosis was assessed using a TUNEL Apoptosis
Detection kit (Beyotime Institute of Biotechnology) (19). Cells were washed with PBS and then
fixed with 4% paraformaldehyde for 30 min at room temperature.
Subsequently, cells were incubated with permeabilization solution
for 5 min at room temperature followed by TUNEL solution for 1 h at
37°C. Then, 50 µl DAB (5 mg/ml) was added for 10 min at 15°C and
hematoxylin for 10 sec at room temperature. A total of 5 fields of
view were randomly selected and apoptotic cells were observed under
glass coverslip with PBS under a fluorescence microscope
(magnification ×200; Olympus Corporation).
Statistical analysis
Quantitative data are presented as the mean ± SD and
statistical analysis was performed using GraphPad Prism 8 (GraphPad
Software, Inc.). Differences between groups were compared using
one-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference. Each
experiment was repeated three times.
Results
Effect of DMY treatment on the
viability of HT22 cells
The chemical structure of DMY is presented in
Fig. 1A. MTT assay revealed that
different concentrations (10, 30, 100 and 300 µmol/l) of DMY did
not affect the viability of HT22 cells (Fig. 1B). However, following OGD/R and
DMY treatment (Fig. 1C), the
viability of HT22 cells was reduced compared with the control
group. DMY administered at 10 and 30 µmol/l had no significant
effect on cell viability compared with the OGD/R group, whereas 100
and 300 µmol/l DMY significantly enhanced cell viability.
Subsequently, the effect of DMY on OGD/R-induced HT22 cytotoxicity
was examined using an LDH assay (Fig.
1D). Compared with the OGD/R group, DMY administered at a low
concentration (30 µmol/l) did not affect LDH levels, while a higher
concentration (100 or 300 µmol/l) of DMY significantly reduced LDH
levels. These results indicated that DMY treatment was not
cytotoxic to HT22 cells, and its propensity to improve cell
viability and alleviate OGD/R-induced cytotoxicity.
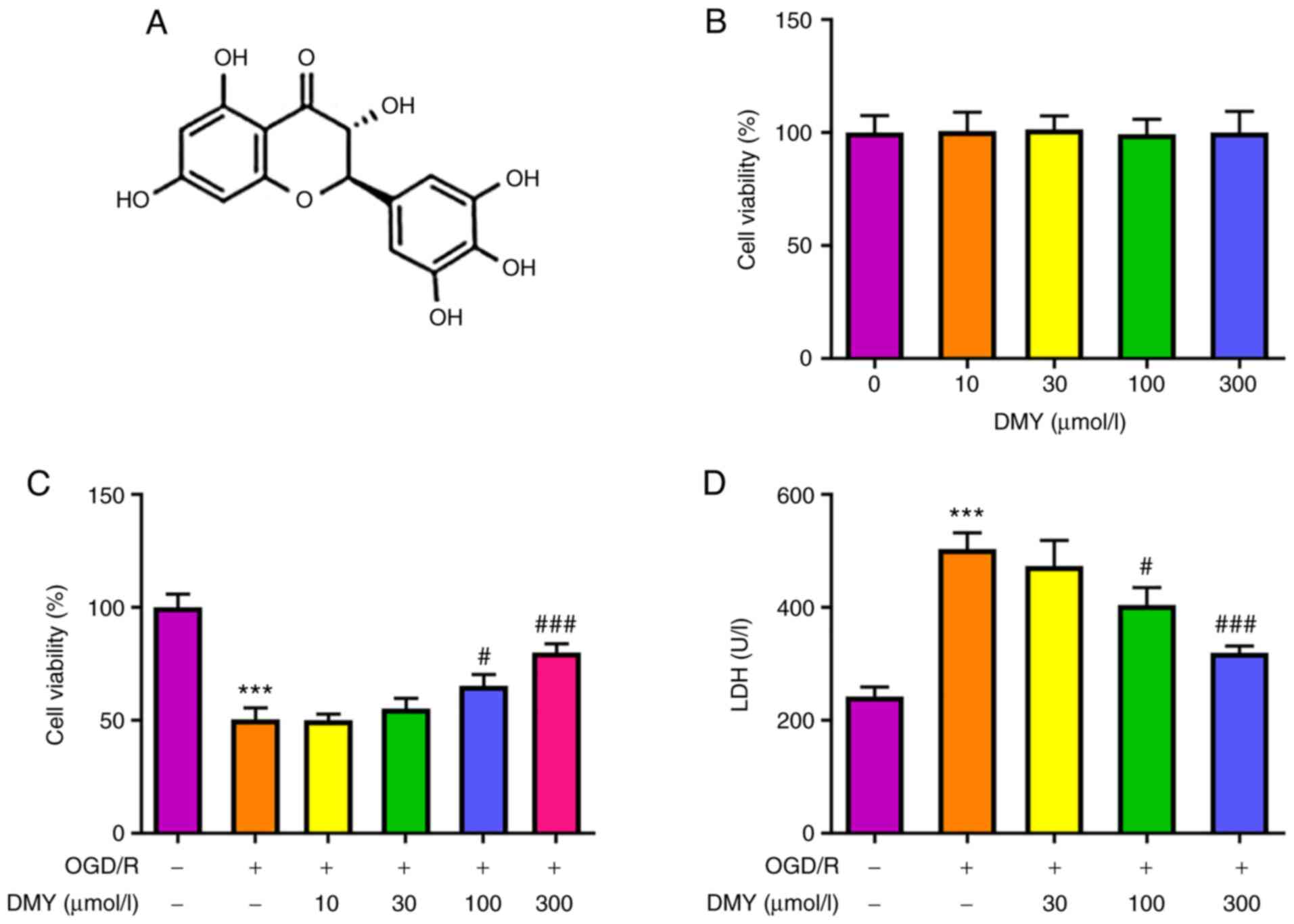 | Figure 1.Effects of DMY on HT22 cells following
OGD/R. (A) Chemical structure of DMY. (B) MTT assay of HT22 cell
viability after treatment with DMY (0, 10, 30, 100, 300 µmol/l).
(C) MTT assay of HT22 cell viability after OGD/R with or without
DMY (10, 30, 100, 300 µmol/l) treatment. (D) LDH release in HT22
cells after OGD/R with or without DMY (30, 100, 300 µmol/l)
treatment. Data are presented as the mean ± SD. n=3 per group.
***P<0.001 vs. Control; #P<0.05,
###P<0.001 vs. OGD/R group. DMY, dihydromyricetin;
OGD/R, oxygen-glucose deprivation and re-oxygenation. |
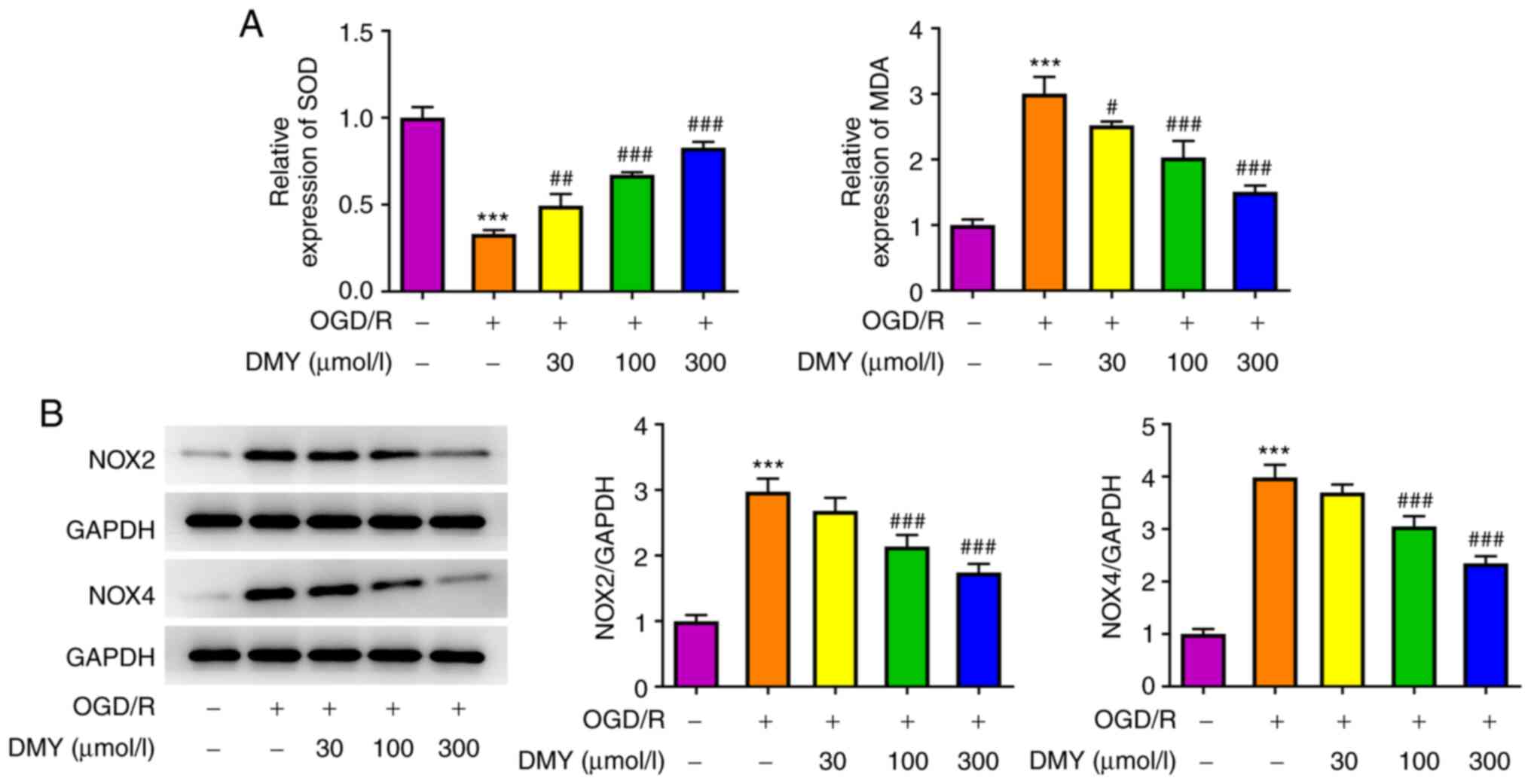
DMY treatment inhibits OGD/R-induced
oxidative stress
To investigate the effect of DMY treatment on
OGD/R-induced HT22 cell injury, the oxidative stress of HT22 cells
was examined by detecting the levels of SOD and MDA. Fig. 2A presents the changes in SOD and
MDA levels detected by the corresponding kits. OGD/R led to a
marked decrease in SOD levels in HT22 cells compared with the
control group. Following DMY treatment, the levels of SOD increased
in a concentration-dependent manner. Furthermore, the levels of MDA
significantly increased in OGD/R-stimulated HT22 cells, but
significantly decreased with increasing DMY concentration.
Additionally, the protein expression levels of NOX2 and NOX4
increased following OGD/R, while DMY treatment decreased their
expression in a concentration-dependent manner (Fig. 2B). These data suggested that DMY
serves a protective effect against OGD/R-induced oxidative stress
in HT22 cells.
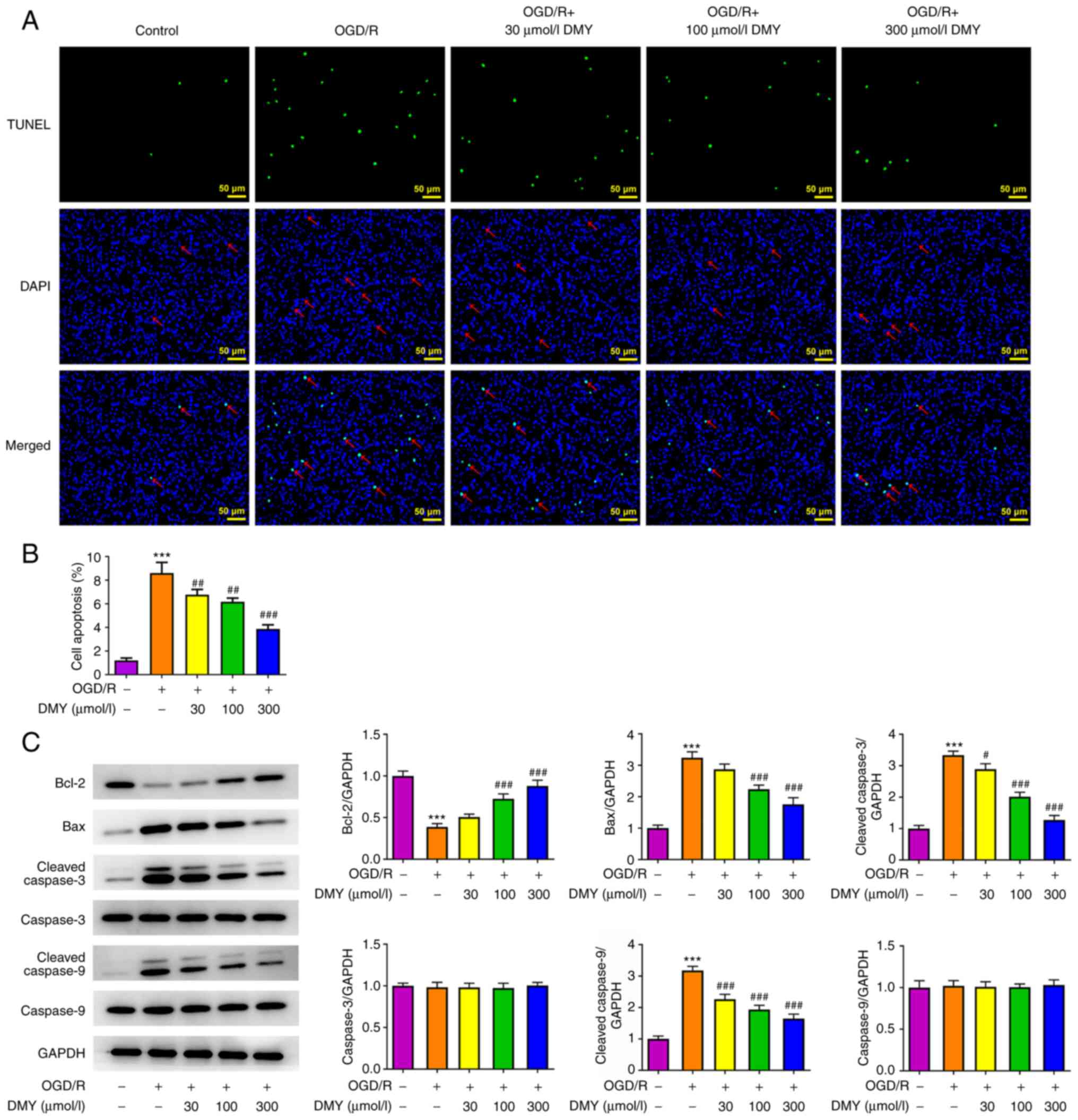
DMY treatment inhibits OGD/R-induced
cell apoptosis
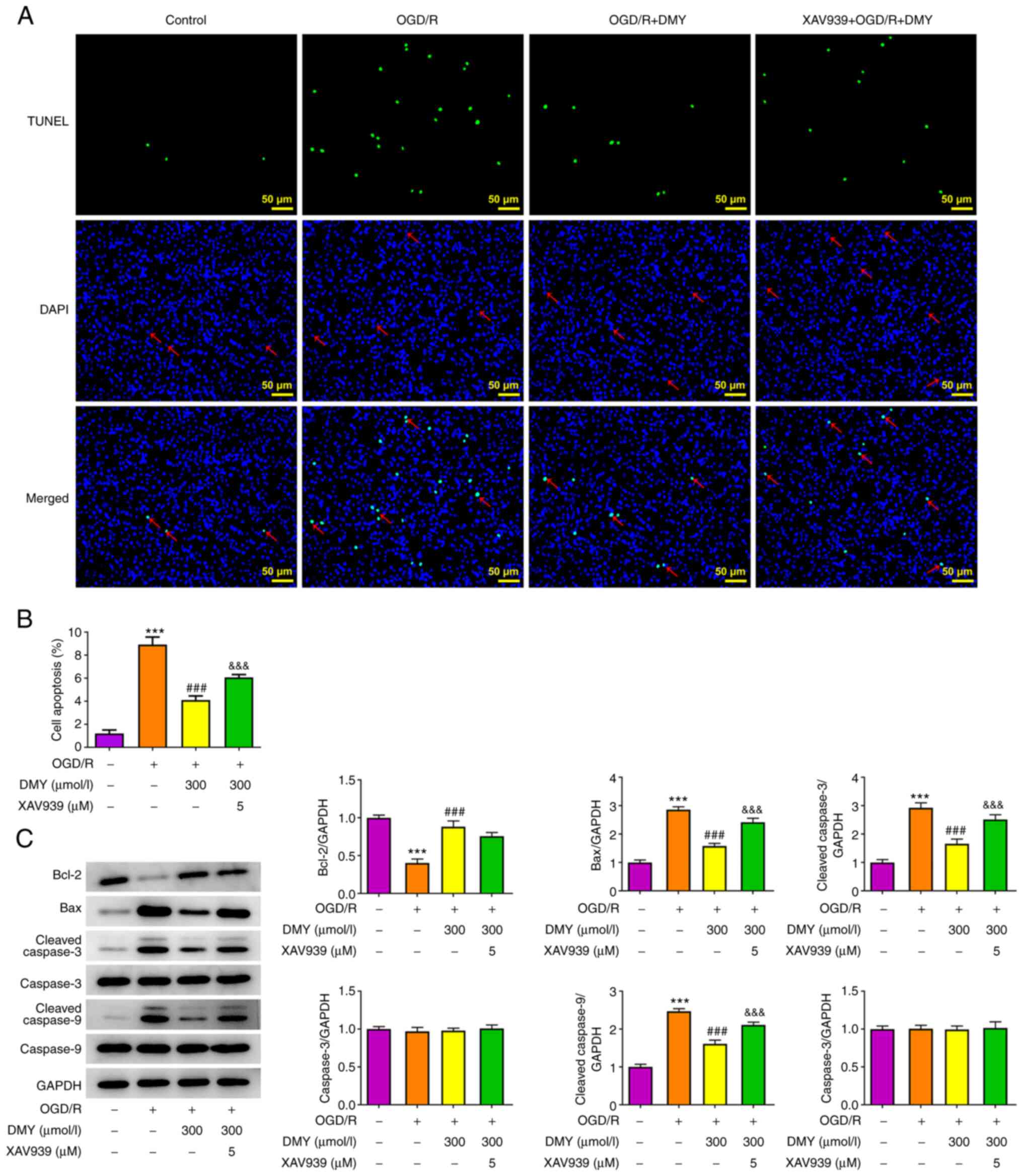
The effect of DMY on OGD/R-induced cell apoptosis
was assessed. As presented in Fig. 3A
and B, the results of the TUNEL assay revealed the presence of
significantly more apoptotic cells in the OGD/R model group.
However, DMY treatment significantly reduced the number of
apoptotic cells in a concentration-dependent manner. Moreover, DMY
reversed the OGD/R-induced downregulation of Bcl-2 and upregulation
of cleaved caspase-3 and cleaved caspase-9 (Fig. 3C). These results indicated that
DMY treatment could suppress OGD/R-induced apoptosis.
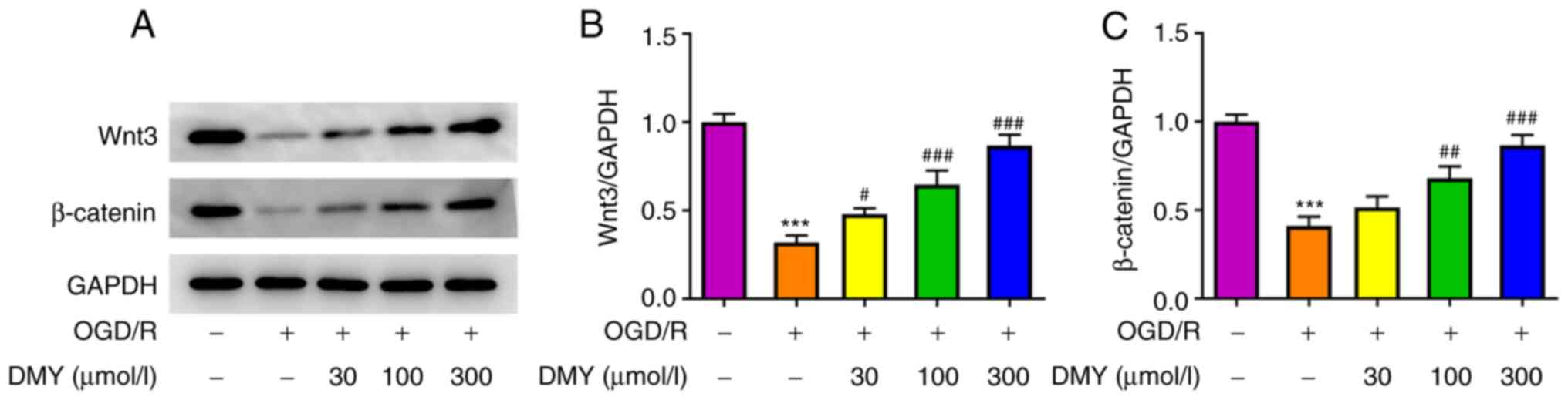
DMY activates the Wnt/β-catenin
signaling pathway in HT22 cells
To determine the mechanism underlying the
amelioration of OGD/R cell injury following DMY treatment, proteins
related to the Wnt/β-catenin signaling pathway were analyzed. The
protein expression levels of Wnt and β-catenin significantly
declined in the OGD/R model group but increased after DMY treatment
in a dose-dependent manner (Fig.
4). Therefore, a positive association may exist between DMY and
the expression of the Wnt/β-catenin signaling pathway in HT22
cells.
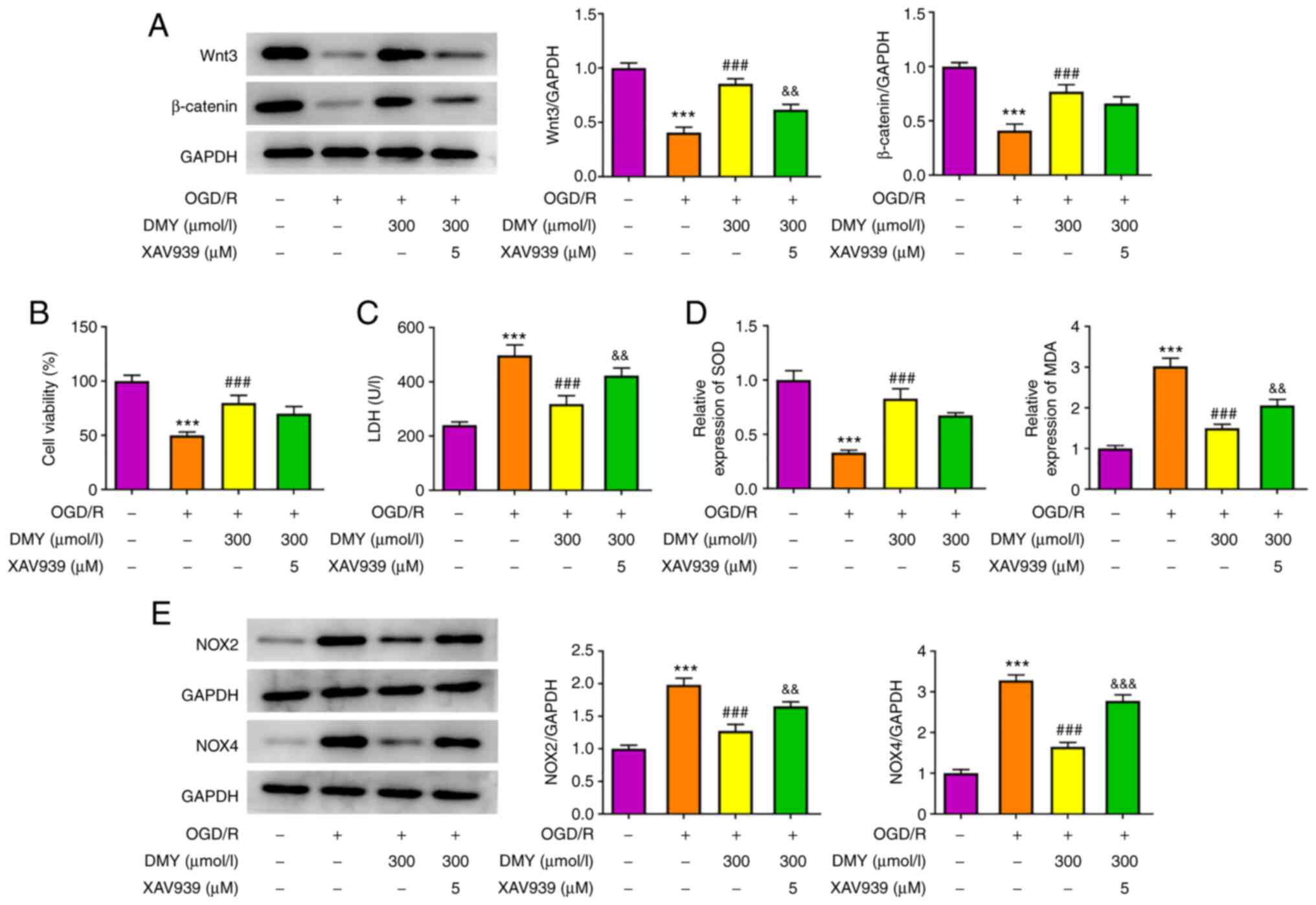
DMY inhibits OGD/R-induced HT22 cell
injury by activating the Wnt/β-catenin signaling pathway
The effects of DMY were investigated in
OGD/R-injured HT22 cells after inhibiting the Wnt/β-catenin
signaling pathway using XAV939. In the aforementioned experimental
results, DMY exerted the most marked protective effects at a
concentration of 300 µmol/l; therefore, this concentration was used
in the following experiments. The results of western blotting
revealed that XAV939 (5 µM) pretreatment inhibited the protein
expression of Wnt, but not β-catenin, (Fig. 5A). In MTT assays, XAV039 did not
affect cell viability following DMY treatment (Fig. 5B). By contrast, LDH assays
revealed a significant LDH release in OGD/R-stimulated HT22 cells
treated with both DMY and XAV939, when compared with DMY treatment
alone (Fig. 5C). Similarly, MDA,
NOX2 and NOX4 levels significantly increased, although SOD
expression remained unchanged, in the XAV939 and DMY co-treatment
group compared with the DMY group (Fig. 5D and E).
Furthermore, the XAV939 pretreatment group exhibited
significantly higher apoptosis rates than the DMY group (Fig. 6A and B). Increased levels of Bax,
cleaved caspase-3 and cleaved caspase-9 were also observed,
although Bcl-2 expression remained unaltered (Fig. 6C). These results suggested that
XAV939 pretreatment attenuated the effect of DMY on OGD/R-induced
oxidative stress and apoptosis in HT22 cells.
Discussion
Ischemic stroke, also known as cerebral infarction,
refers to the localized ischemic necrosis and softening of brain
tissue caused by the disturbance of cerebral blood circulation,
ischemia and hypoxia; it is a disease that is primarily diagnosed
in middle-aged and elderly individuals (20–22). Ischemic stroke has various
clinical manifestations, most of which are symptoms of focal
neurological deficits, such as hemiplegia and the disturbance of
consciousness (23,24). After treatment, certain patients
may be left with varying degrees of nerve and limb damage seriously
affecting their quality of life (25,26). Therefore, preventing nerve cell
injury, restoring nerve function and suppressing cytotoxicity may
be key to the treatment of this condition (27).
DMY has demonstrated promising potential in neural
protection in addition to its antioxidant qualities, as evidenced
by previous research. For example, in Parkinson's disease, DMY has
been found to exert a neuroprotective effect by reducing the
disturbance of behavior and dopaminergic neuron loss in vivo
and ameliorating neuronal injury and ROS production in vitro
(9,28). It has also been reported that DMY
treatment alleviated adriamycin-induced cardiotoxicity,
cardiomyocyte apoptosis and oxidative stress in an imprinting
control region (ICR) mouse model (29). The results of the current study
are consistent with these, in that treatment with DMY increased the
survival rate of HT22 neurons under OGD/R and effectively prevented
OGD/R-induced cytotoxicity in a concentration-dependent manner.
DMY is a known scavenger of ROS (which are
considered as the mediators of oxidative stress) with
pharmacological value in cardiovascular and other diseases
(8,30,31). DMY also improves mitochondrial
function and reduces oxidative stress by upregulating sirtuin 3
signaling, which may represent a promising therapeutic approach and
improve the prognosis of patients with cardiac I/R injury (10). In the present study, DMY
alleviated OGD/R-induced oxidative stress in HT22 cells by
enhancing the activity of the antioxidant enzyme SOD and inhibiting
that of the oxidant MDA, as well as the levels of oxidative stress
indicators NOX2 and NOX4.
Chen et al (11) determined that DMY diminished the
activity of serum aminotransferase and inhibited cell apoptosis in
a mouse model of liver I/R. This suggested that while DMY promotes
the apoptosis of cancer cells, it also prevents cell injury induced
by different stimulants through the inhibition of unfavorable
apoptosis. Similar results were also observed in the present study;
OGD/R-induced HT22 cell apoptosis was largely improved in a
concentration-dependent manner by DMY treatment. Additionally, DMY
increased the expression of the anti-apoptotic protein Bcl2 and
reduced that of the apoptosis markers Bax, cleaved caspase-3 and
cleaved caspase-9.
The Wnt/β-catenin signal transduction pathway is a
key participant in several cellular processes such as cell
survival, apoptosis, cellular metabolism and the mediation of
oxidative stress (32–34). It has been demonstrated that
Wnt/β-catenin signaling has pleiotropic functions in the neuronal
activities of patients with Alzheimer's disease, regulating
neuronal survival and neurogenesis (35). Furthermore, DMY can strengthen
BMSC osteogenic differentiation in vitro by activating
Wnt/β-catenin (13). In a rat
model of cerebral I/R injury, treatment with DMY demonstrated a
protective effect on brain injury by activating PI3K/AKT/GSK3β and
its downstream Wnt/β-catenin signaling pathway (14). In the present study, downregulated
expression of Wnt3 and β-catenin was detected in HT22 cells
following OGD/R, with additional DMY treatment restoring their
expression levels. Furthermore, the Wnt inhibitor XAV939 reduced
the protective effects of DMY against the OGD/R-induced
cytotoxicity, oxidative stress and apoptosis of HT22 cells. Thus,
these results verify the authors' hypothesis that DMY could improve
OGD/R-induced HT22 cell injury by activating the Wnt/β-catenin
pathway.
To the best of our knowledge, the present study was
the first to elucidate the role of DMY in the treatment or
prevention of ischemic stroke. The findings of the current study
revealed that DMY could ameliorate OGD/R-induced injury of HT22
neurons in part by activating the Wnt/β-catenin signaling pathway.
However, the neuroprotective effect of DMY should be further
verified in animal models of ischemic stroke.
Acknowledgements
Not applicable.
Funding
This work was supported by The Taizhou Science and Technology
Plan Project (grant no. 1701KY47) and The Medical and Health
Science and Technology Program of Zhejiang Province (grant no.
2020KY1043).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
XT and FP wrote the manuscript and analyzed the
data. YJ, XJ and XZ performed the experiments and supervised the
study. XJ searched the literature and revised the manuscript for
important intellectual content. XT and FP confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Katan M and Luft A: Global burden of
stroke. Semin Neurol. 38:208–211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Diener HC and Hankey GJ: Primary and
secondary prevention of ischemic stroke and cerebral hemorrhage:
JACC focus seminar. J Am Coll Cardiol. 75:1804–1818. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suda S, Nito C, Yokobori S, Sakamoto Y,
Nakajima M, Sowa K, Obinata H, Sasaki K, Savitz SI and Kimura K:
Recent advances in cell-based therapies for ischemic stroke. Int J
Mol Sci. 21:67182020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun MS, Jin H, Sun X, Huang S, Zhang FL,
Guo ZN and Yang Y: Free radical damage in ischemia-reperfusion
injury: An obstacle in acute ischemic stroke after
revascularization therapy. Oxid Med Cell Longev. 2018:38049792018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ulamek-Koziol M, Czuczwar SJ, Januszewski
S and Pluta R: Proteomic and genomic changes in tau protein, which
are associated with Alzheimer's disease after ischemia-reperfusion
brain injury. Int J Mol Sci. 21:8922020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng Z, Sun Q, Chen W, Bai Y, Hu D and Xie
X: The neuroprotective mechanisms of ginkgolides and bilobalide in
cerebral ischemic injury: A literature review. Mol Med. 25:572019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhaskar S, Stanwell P, Cordato D, Attia J
and Levi C: Reperfusion therapy in acute ischemic stroke: Dawn of a
new era? BMC Neurol. 18:82018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Chen Y, Luo H, Sun L, Xu M, Yu J,
Zhou Q, Meng G and Yang S: Recent update on the pharmacological
effects and mechanisms of dihydromyricetin. Front Pharmacol.
9:12042018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren ZX, Zhao YF, Cao T and Zhen XC:
Dihydromyricetin protects neurons in an MPTP-induced model of
Parkinson's disease by suppressing glycogen synthase kinase-3 beta
activity. Acta Pharmacol Sin. 37:1315–1324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei L, Sun X, Qi X, Zhang Y, Li Y and Xu
Y: Dihydromyricetin ameliorates cardiac ischemia/reperfusion injury
through Sirt3 activation. Biomed Res Int. 2019:68039432019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Y, Lv L, Pi H, Qin W, Chen J, Guo D,
Lin J, Chi X, Jiang Z, Yang H and Jiang Y: Dihydromyricetin
protects against liver ischemia/reperfusion induced apoptosis via
activation of FOXO3a-mediated autophagy. Oncotarget. 7:76508–76522.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu P, Zou D, Chen K, Zhou Q, Gao Y, Huang
Y, Zhu J, Zhang Q and Mi M: Dihydromyricetin improves hypobaric
hypoxia-induced memory impairment via modulation of SIRT3
signaling. Mol Neurobiol. 53:7200–7212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Wang S, Yin H, Chen E, Xue D,
Zheng Q, Gao X and Pan Z: Dihydromyricetin enhances the osteogenic
differentiation of human bone marrow mesenchymal stem cells in
vitro partially via the activation of Wnt/β-catenin signaling
pathway. Fundam Clin Pharmacol. 30:596–606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li P, Zhang Y and Liu H: The role of
Wnt/β-catenin pathway in the protection process by dexmedetomidine
against cerebral ischemia/reperfusion injury in rats. Life Sci.
236:1169212019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du Y, Ma X, Ma L, Li S, Zheng J and Lv J,
Cui L and Lv J: Inhibition of microRNA-148b-3p alleviates
oxygen-glucose deprivation/reoxygenation-induced apoptosis and
oxidative stress in HT22 hippocampal neuron via reinforcing
Sestrin2/Nrf2 signalling. Clin Exp Pharmacol Physiol. 47:561–570.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du L, Li X, Zhen L, Chen W, Mu L, Zhang Y
and Song A: Everolimus inhibits breast cancer cell growth through
PI3K/AKT/mTOR signaling pathway. Mol Med Rep. 17:7163–7169.
2018.PubMed/NCBI
|
|
17
|
Guo Y and Pei X: Tetrandrine-induced
autophagy in MDA-MB-231 triple-negative breast cancer cell through
the inhibition of PI3K/AKT/mTOR signaling. Evid Based Complement
Alternat Med. 2019:75174312019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kangari P, Zarnoosheh Farahany T, Golchin
A, Ebadollahzadeh S, Salmaninejad A, Mahboob SA and Nourazarian A:
Enzymatic antioxidant and lipid peroxidation evaluation in the
newly diagnosed breast cancer patients in Iran. Asian Pac J Cancer
Prev. 19:3511–3515. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao W, Geng D, Li S, Chen Z and Sun M:
LncRNA HOTAIR influences cell growth, migration, invasion, and
apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer
Med. 7:842–855. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang HL, Wang N, Zhou H and Yu CY: Study
on influence of transient ischemic attack on subsequent cerebral
infarction. Eur Rev Med Pharmacol Sci. 20:5164–5167.
2016.PubMed/NCBI
|
|
21
|
Cipolla MJ, Liebeskind DS and Chan SL: The
importance of comorbidities in ischemic stroke: Impact of
hypertension on the cerebral circulation. J Cereb Blood Flow Metab.
38:2129–2149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paul S and Candelario-Jalil E: Emerging
neuroprotective strategies for the treatment of ischemic stroke: An
overview of clinical and preclinical studies. Exp Neurol.
335:1135182021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koh SH and Park HH: Neurogenesis in stroke
recovery. Transl Stroke Res. 8:3–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paci M: Physiotherapy based on the Bobath
concept for adults with post-stroke hemiplegia: A review of
effectiveness studies. J Rehabil Med. 35:2–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gad H, Khan A, Akhtar N, Kamran S,
El-Sotouhy A, Dargham SR, Petropoulos IN, Ponirakis G, Shuaib A,
Streletz LJ and Malik RA: Corneal nerve and endothelial cell damage
in patients with transient ischemic attack and minor ischemic
stroke. PLoS One. 14:e02133192019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pertoldi S and Di Benedetto P:
Shoulder-hand syndrome after stroke. A complex regional pain
syndrome. Eura Medicophys. 41:283–292. 2005.PubMed/NCBI
|
|
27
|
Che F, Du H, Wei J, Zhang W, Cheng Z and
Tong Y: MicroRNA-323 suppresses nerve cell toxicity in cerebral
infarction via the transforming growth factor-β1/SMAD3 signaling
pathway. Int J Mol Med. 43:993–1002. 2019.PubMed/NCBI
|
|
28
|
Guo CH, Cao T, Zheng LT, Waddington JL and
Zhen XC: Development and characterization of an inducible Dicer
conditional knockout mouse model of Parkinson's disease: Validation
of the antiparkinsonian effects of a sigma-1 receptor agonist and
dihydromyricetin. Acta Pharmacol Sin. 41:499–507. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu H, Luo P, Fu Y, Wang J, Dai J, Shao J,
Yang X, Chang L, Weng Q, Yang B and He Q: Dihydromyricetin prevents
cardiotoxicity and enhances anticancer activity induced by
adriamycin. Oncotarget. 6:3254–3267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li H, Li Q, Liu Z, Yang K, Chen Z, Cheng Q
and Wu L: The versatile effects of dihydromyricetin in health. Evid
Based Complement Alternat Med. 2017:10536172017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan TF, Wu TF, Bu LL, Ma SR, Li YC, Mao L,
Sun ZJ and Zhang WF: Dihydromyricetin promotes autophagy and
apoptosis through ROS-STAT3 signaling in head and neck squamous
cell carcinoma. Oncotarget. 7:59691–59703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen N and Wang J: Wnt/β-catenin signaling
and obesity. Front Physiol. 9:7922018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou L, Chen X, Lu M, Wu Q, Yuan Q, Hu C,
Miao J, Zhang Y, Li H, Hou FF, et al: Wnt/β-catenin links oxidative
stress to podocyte injury and proteinuria. Kidney Int. 95:830–845.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li P, Wang Y, Liu X, Zhou Z, Wang J, Zhou
H, Zheng L and Yang L: Atypical antipsychotics induce human
osteoblasts apoptosis via Wnt/β-catenin signaling. BMC Pharmacol
Toxicol. 20:102019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jia L, Piña-Crespo J and Li Y: Restoring
Wnt/β-catenin signaling is a promising therapeutic strategy for
Alzheimer's disease. Mol Brain. 12:1042019. View Article : Google Scholar : PubMed/NCBI
|