Introduction
Innate immune systems in the oral cavity constitute
the first line of the host defense during infection, during which
oral mucosal cells recognize microbial structures and
pathogen-associated molecular patterns via pattern recognition
receptors to defend against microorganism invasion of oral mucosa
(1–3). Retinoic acid-inducible gene I
(RIG-I) is a key cytosolic receptor that responds to viral nucleic
acids by activating downstream signaling for induction of
inflammatory mediators (4,5).
Our previous study reported that oral keratinocytes and
fibroblasts, which are major oral mucosal cells, express functional
RIG-I for recognizing transfected double-stranded (ds)RNA to
produce antiviral cytokine IFN-β expression (6). These systems have important roles in
host defense against viral invasion of oral mucosa.
Poly(ADP-ribose) polymerases (PARPs), a superfamily
with ≥17 members, are known to modulate cell division, cell cycle
and cell death programs triggered by DNA damage, as well as other
biological functions, such as inflammatory and degenerative
diseases (7–11). PARP13, a member of the PARP
superfamily [zinc-finger antiviral protein (ZAP)/zinc finger
CCCH-type containing, antiviral 1 (ZC3HAV1)], has four zinc finger
domains at the N terminus, each of which has a cysteine-histidine
repeat in a cys-cys-cys-his construction. Moreover, it was
originally found in rats as a host restriction factor and was
reported to prevent infection by the moloney murine leukemia virus
(7). PARP13 selectively bind to
specific RNA sequences and degrades target viral RNA (10,12), and can promote antiviral innate
immune responses via cytosolic receptor RIG-I (13,14). PARP13 has a strong antiviral
ability to suppress the replication of various viruses, including
alphaviruses, filoviruses and influenza A virus (15–17), and its expression has been
reported in a wide range of tissues (18). Therefore, it is considered that
oral mucosal cells may express functional PARP13.
PARP13 has two isoforms, the full-length proteins
ZAP and ZAPL, and a shorter protein ZAPS, arising from alternative
splicing, which differ in their individual C-terminal domains
(18). ZAPL consists of 902 amino
acids and has a PARP domain in the C-terminal, while ZAPS consists
of 699 amino acids and does not have a PARP domain (18). Both ZAPS and ZAPL have been
reported to possess the ability to reduce viral replication, such
as that of Japanese encephalitis virus, human cytomegalovirus and
sindbis virus (SINV) (14,19,20).
Furthermore, ZAPS has been shown to have an important role as a
potent stimulator of the RIG-I-mediated pathway in the innate
immune response to viral infection (21,22). Recently, it has been reported that
both ZAPL and ZAPS demonstrated differential IFN responses against
viral RNA (23). However, the
role, expression and function of these two PARP13 isoforms in oral
mucosa remain unknown.
The present study investigated the expression levels
of ZAPL and ZAPS induced by transfected dsRNA and dsDNA in
immortalized oral keratinocytes and fibroblasts (RT7 and GT1 cell
lines, respectively). Subsequently, the effects of ZAPL and ZAPS
knockdown on these ligand-induced antiviral factors were
examined.
Materials and methods
Reagents
Poly(I:C)-LMW, Poly(dA:dT) and LyoVec, a
transfection reagent, were purchased from InvivoGen. Antibodies
used for immunoblotting were anti-ZC3HAV1 (cat. no. GTX120134;
GeneTex, Inc.), anti-IFN regulatory factor 3 (IRF3; cat. no. 4302),
anti-phosphorylated (p)-IRF3 (cat. no. 4947; both Cell Signaling
Technology, Inc.), anti-GAPDH (cat. no. MAB374; MilliporeSigma) and
anti-RIG-I (cat. no. sc-376845; Santa Cruz Biotechnology, Inc.).
Secondary antibodies used for labeling were HRP-conjugated antibody
from Cytiva (cat. no. NA931) and Alexa 488-conjugated rabbit IgG
from Invitrogen (cat. no. A-11008; Thermo Fisher Scientific, Inc.).
The molecular weight marker for western blotting was purchased from
Invitrogen (MagicMark™ XP Western Protein Standard; cat. no.
LC5602; Thermo Fisher Scientific, Inc.).
Cell lines
RT7, an immortalized human oral keratinocyte cell
line, was previously established by transfection of hTERT and E7,
as described in a prior study (24), while GT1, a human oral fibroblast
cell line, was established by transfection of hTERT, as previously
reported (25). Briefly, RT7
cells were cultured in keratinocyte growth medium containing human
epithelial growth factors, insulin, hydrocortisone, Transferrin,
epinephrine, bovine pituitary extract and gentamicin sulfate
amphotericin-B (KGM-Gold Keratinocyte growth medium bulletkit;
Lonza Group, Ltd.), and GT1 cells were cultured in DMEM
(Sigma-Aldrich; Merck KGaA) containing 10% FBS (Biological
Industries), 100 U/ml penicillin and 100 µg/ml streptomycin.
Human gingival keratinocytes and fibroblasts were
obtained from a gingival mucosa excised at the extraction of an
impacted tooth of healthy volunteers (one male and two females,
age, 19 to 24) from April 2015 to March 2018 in Hiroshima
University Hospital (Hiroshima, Japan) and primary cultures were
prepared as previously reported (26). Informed consent for such
acquisition and use was obtained according to a protocol approved
by the Ethical Committee of Hiroshima University (approval no.
E-930).
Treatment of Poly(I:C) and
Poly(dA:dT)
For the experiments, Poly(I:C) and Poly(dA:dT) were
transfected by use of the cationic lipid-based transfection reagent
LyoVec, according to the manufacturer's instructions. Briefly, each
(1 mg/ml final concentration) was separately mixed with LyoVec and
incubated at 18–22°C (room temperature) for 15 min to allow the
formation of a lipid-RNA complex. The complex was then added to
cell cultures and incubated at 37°C for 12 h [RT-quantitative (q)
PCR] or 0, 6, 12 or 24 h (western blotting).
RNA extraction, RT-PCR and
RT-qPCR
Gene-specific oligonucleotide primers used for PCR
analysis are shown in Table I.
The primers were designed by using Primer3 software
(bioinfo.ut.ee/primer3-0.4.0/). Total RNA was prepared from the
cell lines using a RNeasy total RNA isolation kit (Qiagen GmbH) and
one-step RT-PCR was performed with an RT-PCR High Plus System
(Toyobo Life Sciences), according to the manufacturer's
instructions. Single-stranded cDNA for RT-PCR and a qPCR template
were synthesized using a First Strand cDNA Synthesis kit (Amersham;
Cytiva). The RT-PCR conditions for ZAP were 1 cycle (95°C, 15 min),
35 cycles (95°C, 2 min; 55°C, 30 sec; 72°C, 1 min) and 1 cycle
(72°C, 7 min), while those for β-actin were 1 cycle (95°C, 15 min),
25 cycles (95°C, 2 min; 55°C, 30 sec; 72°C, 1 min) and 1 cycle
(72°C, 7 min). The products were analyzed on 2% agarose gels
containing SYBR-Green (Invitrogen; Thermo Fisher Scientific, Inc.).
qPCR was performed using SYBR-Green Master mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.); Initial denaturation at 95°C for 2
min, for 40 cycles (denaturation at 95°C for 15 sec, annealing at
60°C for 60 sec, elongation at 72°C for 60 sec), final extension at
72°C for 5 min. qPCR analysis was performed using a CFX Connect
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.).
Relative quantification of mRNA levels noted for the samples was
performed according to User Bulletin #2 (Applied Biosystems; Thermo
Fisher Scientific, Inc.), with the results shown as the mean ± SD
from three independent experiments.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer
sequence |
|---|
| ZAPL | F:
5′-GCTGAGTTTCCAAGGGATGAT-3′ |
|
| R:
5′-AGTCCTCCTGAGGACGAAAGG-3′ |
| ZAPS | F:
5′-GCTGAGTTTCCAAGGGATGAT-3′ |
|
| R:
5′-AATGGAAACTGCAGAGTAATG-3′ |
| CXCL10 | F:
5′-TGCAAGCCAATTTTGTCCACGTG-3′ |
|
| R:
5′-GCAGCTGATTTGGTGACCATCAT-3′ |
| IFN-β | F:
5′-TGCTCTGGCACAACAGGTAG-3′ |
|
| R:
5′-GCTGCAGCTGCTTAATCTCC-3′ |
| β-actin | F:
5′-TCACCCACACTGTGCCCATCTACGA-3′ |
|
| R:
5′-CAGCGGAACCGCTCATTGCCAATGG-3′ |
Preparation of whole cell
extracts
Cell cultures were washed with ice-cold PBS, then
subjected to lysis with SDS sample buffer using a Mammalian Cell
Lysis kit (Sigma-Aldrich; Merck KGaA) to yield whole cell extracts,
according to the manufacturer's instructions.
Western blotting
Mammalian Cell Lysis kit (Sigma-Aldrich; Merck KGaA)
was used to extract protein, which was quantified using a Pierce
BCA Protein Assay kit (cat. no. 23227; Thermo Fisher Scientific,
Inc.). Proteins from each sample (50 mg) were separated on 10%
SDS-polyacrylamide gels and left for 1 h at 100 V, then transferred
to PVDF membranes (Amersham; Cytiva) and left for 1 h at 90 V.
After blocking for 1 h at room temperature with 5% BSA (cat. no.
01863-48; Nacalai Biochemicals Reagent) in PBS, the membrane was
incubated with the primary antibody (1:1,000) at 4°C overnight.
Immunoblots were labeled with an HRP-conjugated secondary antibody
(1:1,000) for 1 h at room temperature and developed using an ECL
Advance Western blotting Detection kit (Cytiva). Image data were
analyzed with an LAS 4000 mini-imaging system (FUJIFILM Wako Pure
Chemical Corporation). ImageJ version 1.47 (National Institutes of
Health), was used to analyze the intensities of the western
blotting bands. The ratio of the band density of each target
protein to that of GAPDH was calculated.
Immunocytochemistry
Cells were seeded into two-well chamber slides
(Matsunami Glass Ind., Ltd.) and fixed in 4% paraformaldehyde in
PBS at room temperature for 15 min, followed by permeabilization
with 0.2% Triton X-100 in PBS for 5 min. blocking for 30 min at
room temperature with 1% BSA (Nacalai Biochemicals Reagent) in PBS,
and incubation overnight at 4°C with the primary antibody (1:400)
in PBS containing 5% BSA. Next, cells were washed and incubated
with diluted Alexa Fluor secondary antibody (1:400) at room
temperature for 1 h. Vectashield anti-fade medium containing DAPI
(Vector Laboratories, Inc.) was used to mount the cells.
Fluorescent and phase contrast images were acquired with a BZ-9000
microscope (Keyence Corporation).
Small interfering RNA (siRNA)
A stealth siRNA for ZAPL and ZAPS was designed and
purchased from Japan Bio Services Co., Ltd. The siRNA sequences
were as follows: ZAPL, 5′-CAUGAAACUCAUGAAAACATT-3′ and
5′-UGUUUUCAUGAGUUUCAUGTA-3′; and ZAPS, 5′-CCUGUUUUCCUGAAAAGUUTT-3′
and 5′-AACUUUUCAGGAAAACAGGCT-3′. Negative control siRNA (2.5
mg/well) (Stealth RNAi™ siRNA Negative Control) was purchased from
Invitrogen. RT7 and GT1 cells were transiently transfected with
various combinations of the siRNAs (2.5 mg/well) using
Lipofectamine® 2000 transfection reagent (6 ml/well)
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 48 h in
6-well plates (Cellstar, Greiner Bio-one), according to the
manufacturer's recommendations. Thereafter, complex of Poly(I:C) or
Poly(dA:dT) and transfection reagent, Lyovec was added to cell
cultures and incubated at 37°C for 12 h (RT-qPCR) or 6 h (western
blotting).
Statistical analysis
SPSS software (version 24; IBM Corp., Japan, Inc.)
was employed for the statistical analysis. Statistical analysis was
performed using Student's t-test (paired or unpaired) and one-way
ANOVA followed by Dunnett's multiple comparison test and the values
are presented as the mean ± SD of three independent
experiments.
Results
Expression of two isoforms of PARP13
in oral mucosal cells
To investigate the effects of PARP13 on innate
antiviral response in oral mucosal cells, it was first examined
whether oral keratinocytes and fibroblasts expressed PARP13.
Immunocytochemistry analysis showed the expression of PARP13 in the
cytoplasm of both RT7 and GT1 cells, indicating that it was
functional in these cells (Fig.
1A). PARP13 has two isoforms (ZAPL, full length protein; ZAPS,
shorter protein) arising from alternative splicing (13). The RT-PCR results identified that
RT7 and GT1 cells, as well as primary oral keratinocytes and
fibroblasts, constitutively expressed ZAPL and ZAPS mRNA (Fig. 1B). However, since primary cells
show limited proliferative activity (24,27), RT7 and GT1 cells, immortalized
human oral keratinocytes and fibroblasts, respectively, were used
in the following experiments.
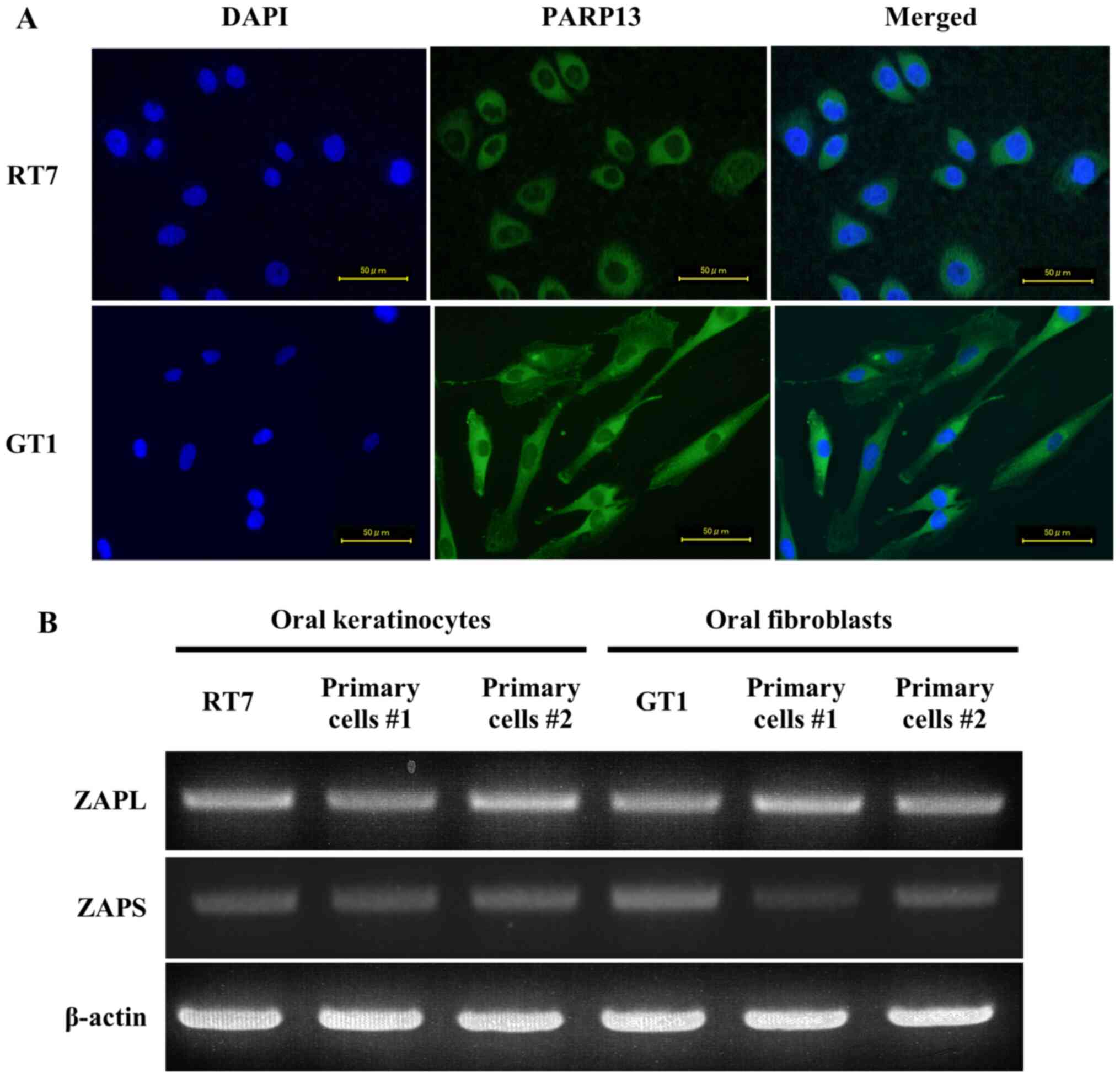 | Figure 1.Expression of two PARP13 isoforms in
oral keratinocytes and fibroblasts. (A) Localization of PARP13
expression in RT7 and GT1 cells. Cells were stained with
anti-ZC3HAV1 (PARP13) and Alexa Fluor® 488 conjugated
rabbit IgG, and nuclei were counter-stained with DAPI (blue). Green
staining indicates that PARP13 was observed in the cytoplasm of the
cells. Each experiment was performed ≥3 times, with representative
results shown. Scale bar, 50 µm. (B) mRNA expression level of two
PARP13 isoforms in RT7 cells, primary oral keratinocytes, GT1 cells
and primary oral fibroblasts cell lines. Total RNA was isolated
from each cell line after culturing to confluence, then reverse
transcription PCR assays of ZAPL, ZAPS and β-actin were performed.
ZAPL, long form of zinc-finger antiviral protein; ZAPS, short form
of zinc-finger antiviral protein; PARP13, poly(ADP-ribose)
polymerase 13. |
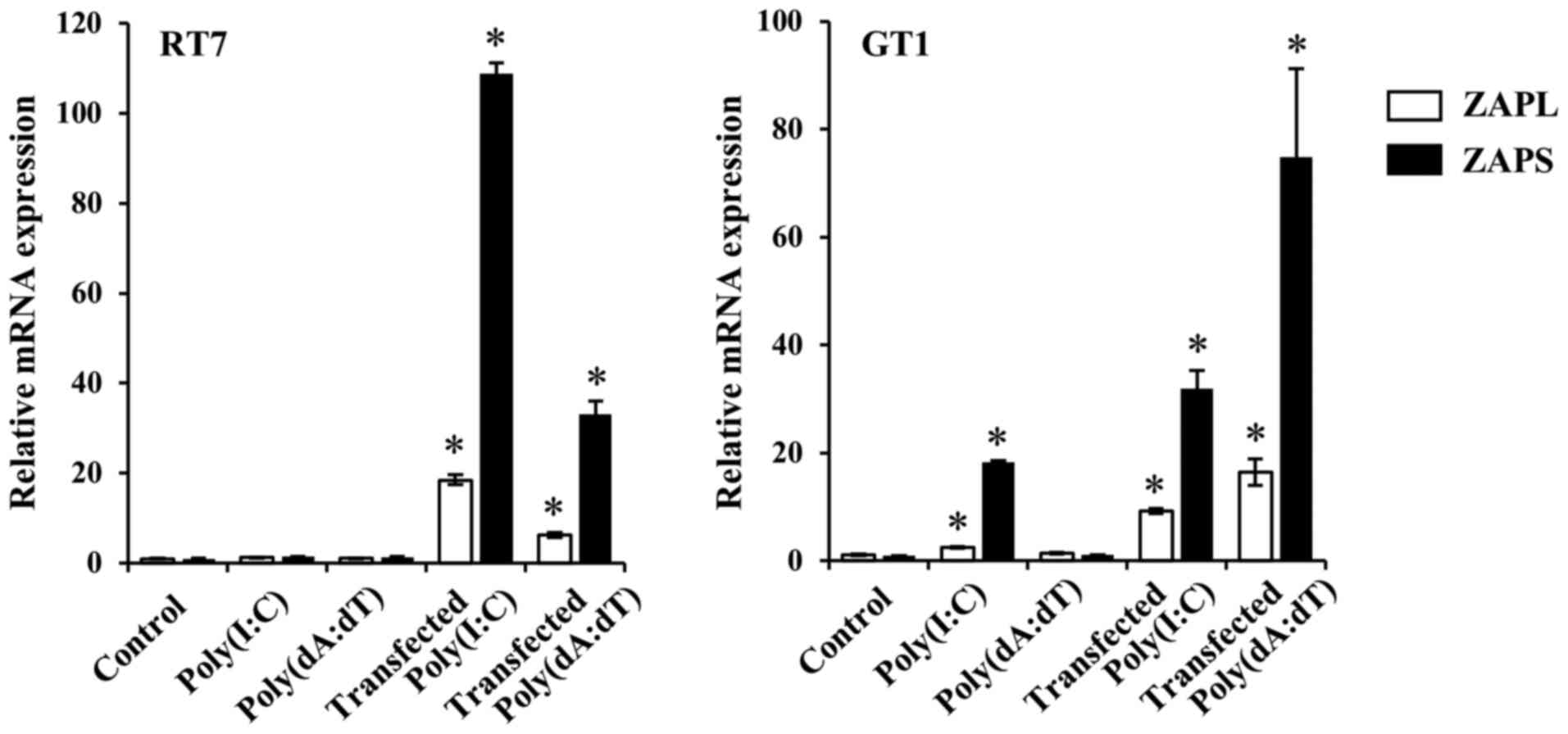
ZAPL and ZAPS expression induced by
transfected dsRNA and dsDNA in oral mucosal cells
Transfected nucleotides have been reported to
increase cytosolic nucleic acid sensor expression (28,29). Next, the effects of transfected
mimic dsRNA, Poly(I:C), and dsDNA, Poly(dA:dT), on ZAPL and ZAPS
expression in RT7 and GT1 cells were examined. Poly(I:C) alone
without the transfection reagent increased the mRNA expression
levels of ZAPL and ZAPS in GT1 cells, but not in RT7 cells, whereas
Poly(dA:dT) alone without the transfection reagent did not affect
those isoforms mRNA expressions in either cell line. On the other
hand, transfected Poly(I:C) and Poly(dA:dT) significantly increased
the mRNA expression levels of ZAPL and ZAPS in both cell lines as
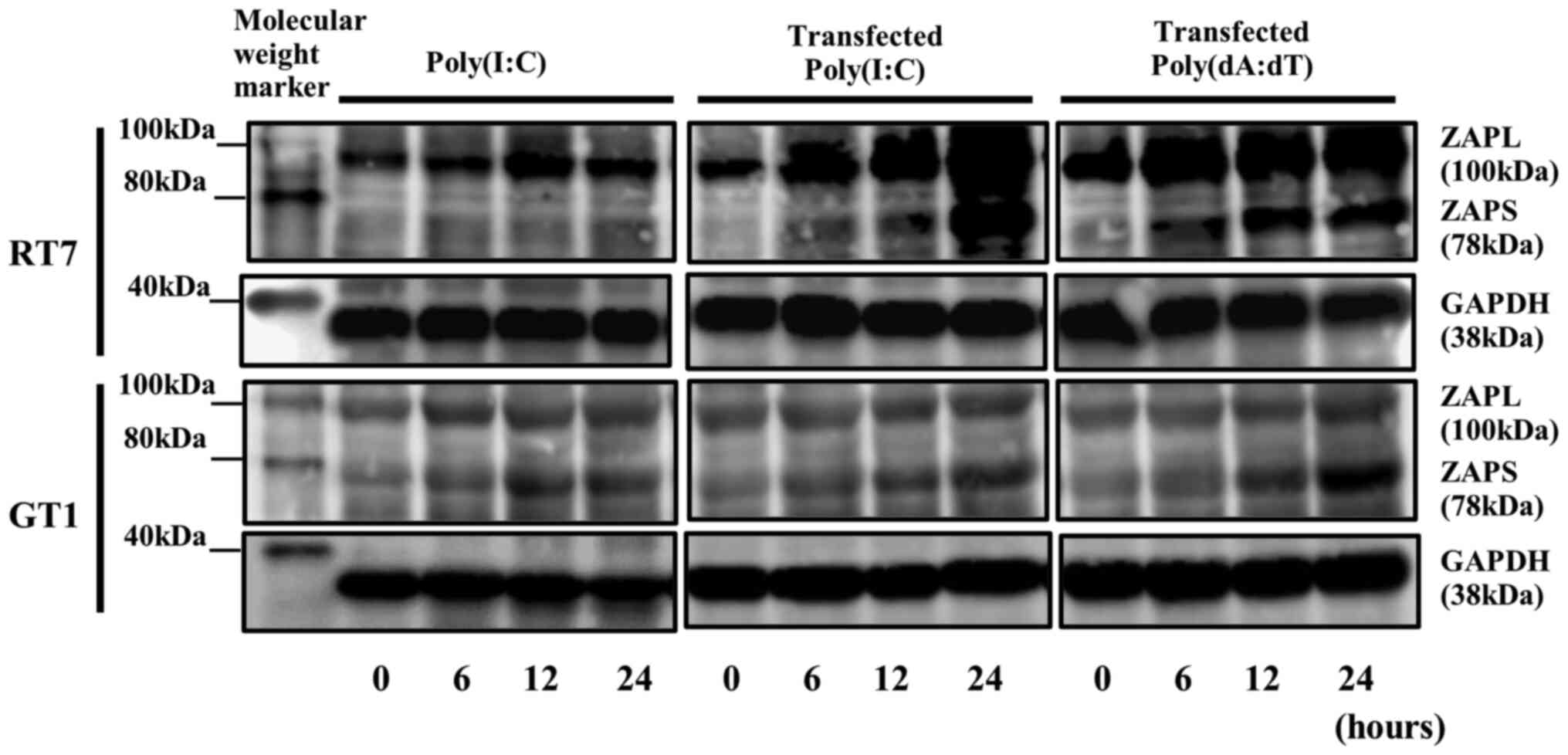
compared with each nucleotide alone (Fig. 2). Western blotting results also
demonstrated a high constitutive level of ZAPL protein expression
with a molecular size of 100 kDa, indicating 0 h without
nucleotides, in both cell lines, whereas a low constitutive level
of expression of ZAPS protein with a molecular size of 78 kDa,
indicating 0 h without nucleotides, was shown in both cell lines.
Furthermore, transfected Poly(I:C) and Poly(dA:dT) enhanced the
expression levels of ZAPL and ZAPS proteins, similar to their mRNA
expression results, in both cell lines (Fig. 3).
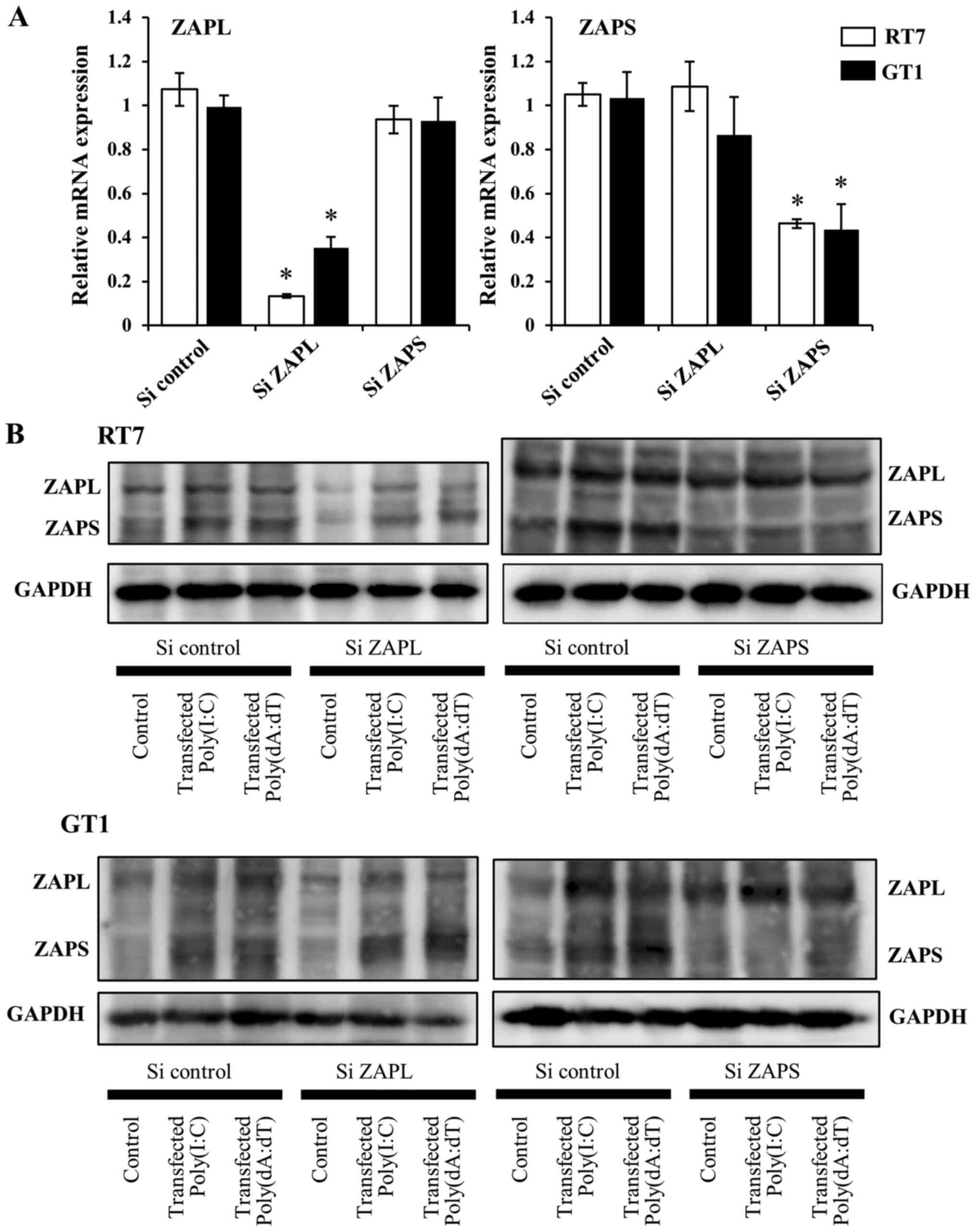
Knockdown of both ZAPL and ZAPS using
specific siRNA
To examine the effects of ZAPL and ZAPS on
transfected dsRNA and dsDNA-induced antiviral factor,
siRNA-mediated knockdown of each was performed and their expression
was subsequently examined. Each specific siRNA decreased the mRNA
expression levels of ZAPL or ZAPS (Fig. 4A). Furthermore, knockdown of ZAPL
or ZAPS protein expression using each specific siRNA was also
confirmed following stimulation after transfection with Poly(I:C)
and Poly(dA:dT)(Figs. 4B,
S1 and S2).
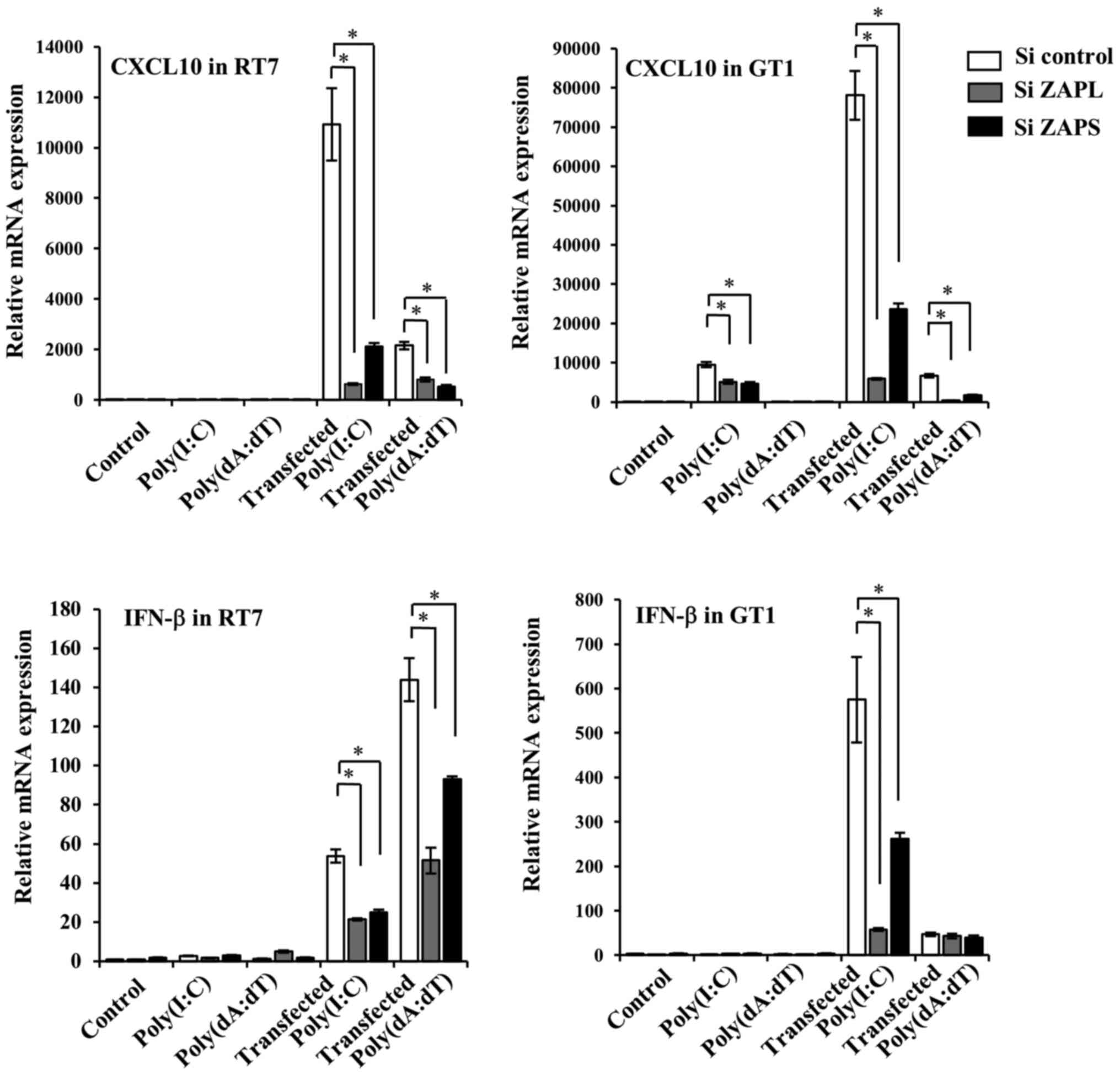
Effects of knockdown of ZAPL and ZAPS
on transfected dsRNA and dsDNA-induced antiviral factor
expression
In our previous study, transfected dsRNA and dsDNA
increased the expression of antiviral cytokines and chemokines,
IFN-β and CXCL10 (6,30). Therefore, the current study
examined the effects knockdown of ZAPL and ZAPS on transfected
dsRNA and dsDNA-induced IFN-β and CXCL10. Specific knockdown of
ZAPL and ZAPS in RT7 cells caused decreased IFN-β and CXCL10
expression levels induced by transfected Poly(I:C) and Poly(dA:dT)
(Fig. 5). On the other hand,
knockdown of ZAPL and ZAPS in GT1 cells decreased transfected
Poly(I:C) and Poly(dA:dT)-induced CXCL10 expression. Although
transfected Poly(I:C)-induced IFN-β expression was also inhibited
by knockdown of ZAPL and ZAPS, knockdown of each in GT1 cells had
no effects on transfected Poly(dA:dT)-induced IFN-β expression
(Fig. 5).
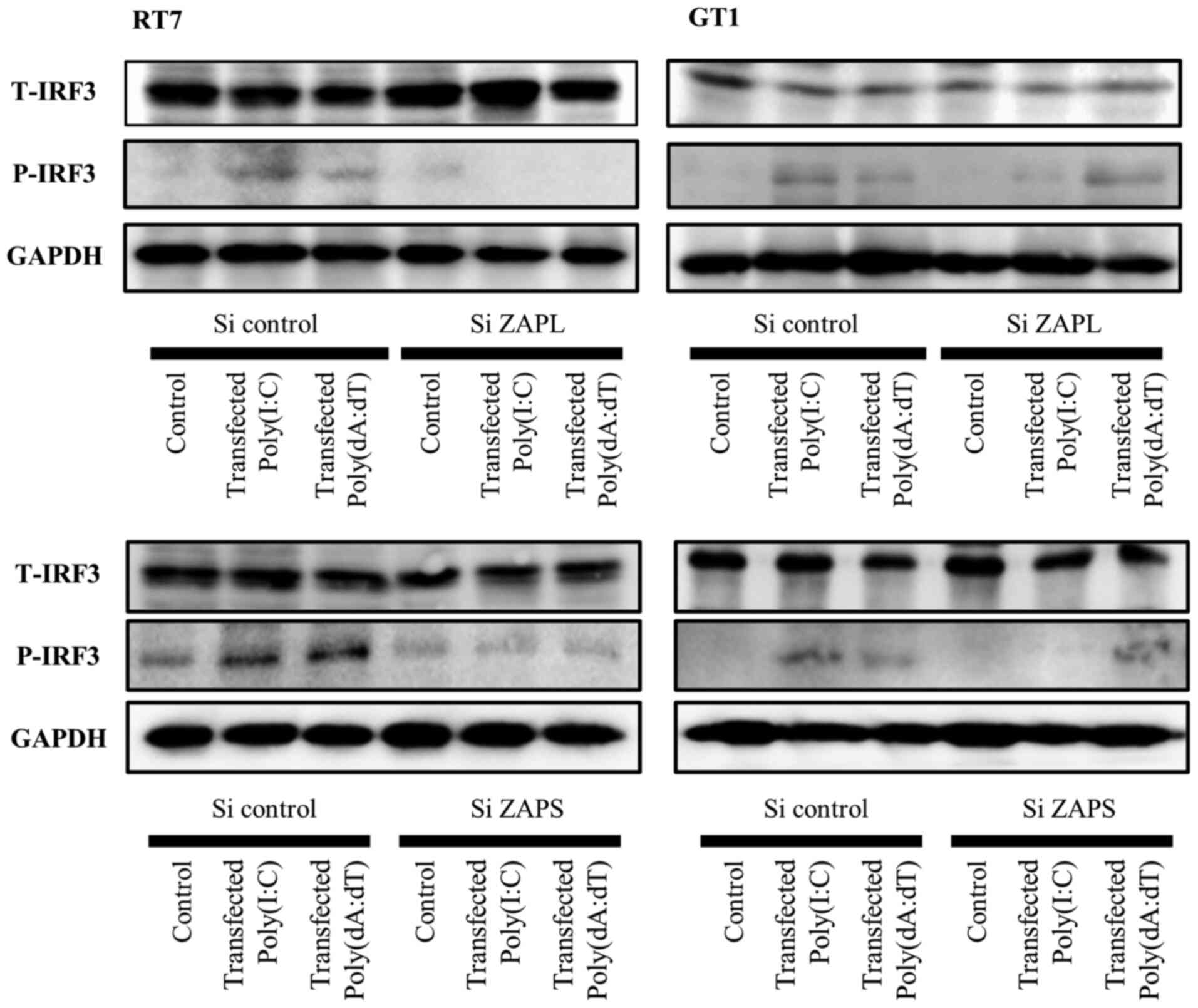
Effects of the knockdown of ZAPL and
ZAPS on transfected dsRNA and dsDNA-induced IRF3 activation
IRF3 is a key transcriptional factor involved in the
signaling pathway response to viral infection (31). Our previous study revealed that
transfected Poly(I:C)-induced IFN-β was associated with IRF3
activation in RT7 and GT1 cells (6). Therefore, the current study examined
the effects of knockdown of ZAPL and ZAPS on transfected Poly(I:C)
and Poly(dA:dT)-induced IRF3 phosphorylation in both cell types.
The results demonstrated that knockdown of ZAPL and ZAPS decreased
transfected Poly(I:C) and Poly(dA:dT)-induced IRF3 phosphorylation
in RT7 cells (Fig. 6).
Furthermore, knockdown of both in GT1 cells decreased transfected
Poly(I:C)-induced IRF3 phosphorylation, though it did not affect
transfected Poly(dA:dT)-induced IRF3 phosphorylation (Fig. 6). These findings indicate that
these two PARP isoforms in oral keratinocytes and fibroblasts are
associated with activation of IRF3 signaling to increase antiviral
factor expression.
Discussion
The PARP superfamily is known to have important
roles in several different biological and pathological processes,
and some members directly regulate the replication of certain
viruses (7–9). Notably, PARP13 (ZAP/ZC3HAV1) has
been reported to inhibit the replication of a wide variety of
viruses, including several RNA viruses, such as murine leukemia
virus, SINV, HIV and Epstein-Barr virus, as well as the RNA
intermediate of the hepatitis B DNA virus (7,16,32–36). PARP13 has two isoforms,
full-length PARP13.1 (ZAPL) and the C-terminal truncated isoform
PARP13.2 (ZAPS), both of which are expressed in a wide range of
tissues, including those of the lung, colon and salivary gland;
though ZAPS has a broader expression pattern compared with ZAPL in
some human tissues, such as those of the kidney and liver (18). ZAPS expression has also been
observed in immune cells, including human monocytes, as well as in
non-immune cells, such as human embryonic kidney and human fetal
lung cells (21,34). In the present study, PARP13
protein was found in cytoplasm of oral keratinocytes and
fibroblasts, with constitutive mRNA expressions of both ZAPL and
ZAPS also noted in both types of cells, indicating that these two
isoforms have functions for defense against cytosolic viral
nucleotide invasion.
Hayakawa et al (21) reported that ZAPL and ZAPS are
constitutively expressed in human embryonic kidney cells and
CD14+ monocytes. In their experiments, ZAPS mRNA was
shown to be markedly induced by stimulation with a 5′-triphosphate
modification (3pRNA), essential for RIG-I recognition and
activation in both cell types, whereas the level of ZAPL mRNA
expression induced by 3pRNA was very low (21). In the present study, high levels
of ZAPL protein expression were noted, whereas the level of
constitutive expression of ZAPS protein was low (indicating
proteins at 0 h) in oral keratinocytes and fibroblasts.
Furthermore, ZAPL and ZAPS expression levels were induced by
transfection of those nucleotides in both RT7 and GT1 cells.
Compared with ZAPS, ZAPL is more active against alphaviruses, such
as SINV and Semliki Forest virus, and carries signatures of
positive selection (18), whereas
ZAPS is upregulated to a greater level than ZAPL by viral infection
(21,37). These two PARP13 isoforms have also
been reported to be important components of cellular response to
stress, and are associated with cell survival and apoptosis
(34,38). Therefore, it is considered that
ZAPL functions in cellular homeostasis and antiviral defense,
whereas ZAPS mainly acts as an intrinsic antiviral factor in oral
mucosa.
IRF3 has been shown essential for induction of
antiviral response. Various type of viral infection trigger IRF3
phosphorylation, and result in induction of antiviral cytokine,
such as IFN-β (31). Our previous
study revealed that transfected Poly(I:C) increased IRF3 activation
in oral keratinocytes and fibroblasts (6). On the other hand, ZAPS in 239T cells
was shown to promote oligomerization and ATPase activity of RIG-I,
thereby increasing downstream RIG-I signaling via IRF3 signaling,
when stimulated with 3pRNA (21).
Although IRF3 selectively binds the ZAPL promoter following viral
infection, it is unclear whether the effect of ZAPL is associated
with IRF3 activation (39). In
the present study, knockdown of ZAPL and ZAPS in both cell types
decreased transfected Poly(I:C)-induced IRF3 activation. While
transfected Poly(dA:dT)-induced IRF3 activation in RT7 cells was
shown to be decreased by knockdown of ZAPL and ZAPS, knockdown of
these in GT1 cells did not affect transfected Poly(dA:dT)-induced
IRF3 phosphorylation. Taken together, these results indicate that
ZAPL and ZAPS in oral mucosal cells are important for activated
antiviral response against viral infection of oral mucosa.
IFN-β plays an important role in antiviral immunity
by directly inhibiting viral replication in infected cells
(40,41). However, the response of ZAPS for
induction of IFN-β was different in two prior studies. For
instance, Hayakawa et al (21) revealed that knockdown of ZAPS
decreased 3pRNA-induced IFN-β expression and activation of IRF3 in
239T cells. Conversely, Schwerk et al (23) reported that knockdown of ZAPS
increased IFN-β expression induced by polyU/UC RNA, whereas
knockdown of ZAPL did not have an effect on IFN-β expression in Hu7
or 239T cells. This authors also showed that IRF3 phosphorylation
was not affected by ZAPL knockdown in those cell types (23). In the present study, knockdown of
ZAPL and ZAPS decreased Poly(I:C)-induced IFN-β in RT7 and GT1
cells, while knockdown of those decreased transfected
Poly(dA:dT)-induced IFN-β expression in RT7 cells but not GT1
cells. These results are in accordance with the notion of
nucleotide-induced IRF3 phosphorylation in those cell types. The
differential regulation of IFN-β via ZAP and ZAPS shown previously
and also in the present report may be associated with differences
related to the intracellular signaling pathways, including IRF3,
among the examined cell types. Therefore, a future study is
required to investigate the contributions of ZAPL and ZAPS to the
regulation of IFN-β expression induced by various nucleotides in
assorted cell types.
CXCL10 is an inflammatory chemokine that mainly
recruits activated T and natural killer cells to sites of infection
or inflammation, and plays a critical role in host defense towards
a variety of viral infections when its expression is significantly
induced (42,43). Our previous study reported that
transfected dsRNA increased IFN-β expression via RIG-I-mediated
IRF3 activation, after which IFN-β enhanced CXCL10 expression via
the IFN-β/α receptor in oral keratinocytes and fibroblasts
(6). In the present study, though
knockdown of ZAPL and ZAPS did not affect IFN-β expression induced
by transfected Poly(dA:dT) in GT1 cells, their knockdown decreased
CXCL10 expression induced by transfected Poly(dA:dT) and Poly(I:C).
Therefore, transfected-Poly(dA:dT)-induced CXCL10 induction in oral
fibroblasts via ZAPL and ZAPS may be independent of
IRF3/IFN-β-mediated signaling.
The present study has certain limitation. First, we
examined immune responses via ZAPL and ZAPS using immortalized
human oral keratinocytes and fibroblasts. Because primary cells
show limited proliferative activity, they are unsuitable for
long-term experiments and do not guarantee reproducibility
(24,27). Secondly, transfected mimic ds
nucleic acids Poly(I:C) and Poly(dA:dT) were used instead of actual
viruses. Therefore, function of ZAPS and ZAPL in oral keratinocytes
and fibroblast against viral invasion need to be investigated in
future.
In conclusion, the present findings are the first
known to demonstrate that oral keratinocytes as well as oral
fibroblasts express two PARP13 isoforms, ZAPL and ZAPS.
Furthermore, the expression of each was increased by transfected
dsRNA and dsDNA. In addition, ZAPL and ZAPS were found to be
associated with antiviral chemokine and cytokine expressions via
IRF3 activation. Taken together, these results suggest that ZAPL
and ZAPS in oral keratinocytes and fibroblasts may participate in
host defense against viral infection of oral mucosa.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by Grant-in-Aids for Scientific Research
from the Japan Society for Scientific Research (C) (grant no.
2646301) and the Ministry of Education, Culture, Sports, Science
and Technology of Japan (grant no. 17K11840).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KO and HK designed the study, analyzed data and
wrote the manuscript. MS, SF, TN, HS and HN performed the
experiments, analyzed the data and drafted the manuscript. MT
contributed to the conception and design, as well as drafting of
the manuscript. All authors have read and approved the final
version of the manuscript. KO and HK confirm the authenticity of
the raw data.
Ethics approval and consent for
participation
Informed consent for such acquisition and use was
obtained according to a protocol approved by the Ethical Committee
of Hiroshima University (approval no. E-930).
Patient consent for participation
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fukui A, Ohta K, Nishi H, Shigeishi H,
Tobiume K, Takechi M and Kamata N: Interleukin-8 and CXCL10
expression in oral keratinocytes and fibroblasts via Toll-like
receptors. Microbiol Immunol. 57:198–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beklen A, Hukkanen M, Richardson R and
Konttinen YT: Immunohistochemical localization of Toll-like
receptors 1–10 in periodontitis. Oral Microbiol Immunol.
23:425–431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mahanonda R, Sa-Ard-Iam N, Montreekachon
P, Pimkhaokham A, Yongvanichit K, Fukuda MM and Pichyangkul S: IL-8
and IDO expression by human gingival fibroblasts via TLRs. J
Immunol. 178:1151–1157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Desmet CJ and Ishii KJ: Nucleic acid
sensing at the interface between innate and adaptive immunity in
vaccination. Nat Rev Immunol. 12:479–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoneyama M, Kikuchi M, Natsukawa T,
Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S and Fujita T:
The RNA helicase RIG-I has an essential function in double-stranded
RNA-induced innate antiviral responses. Nat Immunol. 5:730–737.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohta K, Fukui A, Shigeishi H, Ishida Y,
Nishi H, Tobiume K, Takechi M and Kamata N: Expression and function
of RIG-I in oral keratinocytes and fibroblasts. Cell Physiol
Biochem. 34:1556–1565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao G, Guo X and Goff SP: Inhibition of
retroviral RNA production by ZAP, a CCCH-type zinc finger protein.
Science. 297:1703–1706. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schreiber V, Dantzer F, Ame JC and de
Murcia G: Poly(ADP-ribose): Novel functions for an old molecule.
Nat Rev Mol Cell Biol. 7:517–528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hakmé A, Wong HK, Dantzer F and Schreiber
V: The expanding field of poly(ADP-ribosyl)ation reactions.
‘Protein Modifications: Beyond the Usual Suspects’ Review Series.
EMBO Rep. 9:1094–1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu H, Tang YD, Zhan G, Su C and Zheng C:
The critical role of PARPs in regulating innate immune responses.
Front Immunol. 12:7125562021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malgras M, Garcia M, Jousselin C, Bodet C
and Lévêque N: The antiviral activities of Poly-ADP-ribose
polymerases. Viruses. 13:5822021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo X, Wang X, Gao Y, Zhu J, Liu S, Gao G
and Gao P: molecular mechanism of RNA recognition by zinc-finger
antiviral protein. Cell Rep. 30:46–52.e4. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu M, Zhou J, Liang Y, Nair V, Yao Y and
Cheng Z: CCCH-type zinc finger antiviral protein mediates antiviral
immune response by activating T cells. J Leukoc Biol. 107:299–307.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chiu HP, Chiu H, Yang CF, Lee YL, Chiu FL,
Kuo HC, Lin RJ and Lin YL: Inhibition of Japanese encephalitis
virus infection by the host zinc-finger antiviral protein. PLoS
Pathog. 14:e10071662018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Burke CW, Ryman KD and Klimstra
WB: Identification and characterization of interferon-induced
proteins that inhibit alphavirus replication. J Virol.
81:11246–11255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mao R, Nie H, Cai D, Zhang J, Liu H, Yan
R, Cuconati A, Block TM, Guo JT and Guo H: Inhibition of hepatitis
B virus replication by the host zinc finger antiviral protein. PLoS
Pathog. 9:e10034942013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang Q, Wang X and Gao G: The short form
of the zinc finger antiviral protein inhibits influenza a virus
protein expression and is antagonized by the virus-encoded NS1. J
Virol. 91:e01909–e01916. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kerns JA, Emerman M and Malik HS: Positive
selection and increased antiviral activity associated with the
PARP-containing isoform of human zinc-finger antiviral protein.
PLoS Genet. 4:e212008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li MM, Lau Z, Cheung P, Aguilar EG,
Schneider WM, Bozzacco L, Molina H, Buehler E, Takaoka A, Rice CM,
et al: TRIM25 enhances the antiviral action of zinc-finger
antiviral protein (ZAP). PLoS Pathog. 13:e10061452017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gonzalez-Perez AC, Stempel M, Wyler E,
Urban C, Piras A, Hennig T, Ganskih S, Wei Y, Heim A, Landthaler M,
et al: The zinc finger antiviral protein ZAP restricts human
cytomegalovirus and selectively binds and destabilizes viral
UL4/UL5 transcripts. MBio. 12:e02683–e20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayakawa S, Shiratori S, Yamato H,
Kameyama T, Kitatsuji C, Kashigi F, Goto S, Kameoka S, Fujikura D,
Yamada T, et al: ZAPS is a potent stimulator of signaling mediated
by the RNA helicase RIG-I during antiviral responses. Nat Immunol.
12:37–44. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu HM and Gale M Jr: ZAPS electrifies
RIG-I signaling. Nat Immunol. 12:11–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schwerk J, Soveg FW, Ryan AP, Thomas KR,
Hatfield LD, Ozarkar S, Forero A, Kell AM, Roby JA, So L, et al:
RNA-binding protein isoforms ZAP-S and ZAP-L have distinct
antiviral and immune resolution functions. Nat Immunol.
20:1610–1620. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujimoto R, Kamata N, Yokoyama K, Taki M,
Tomonari M, Tsutsumi S, Yamanouchi K and Nagayama M: Establishment
of immortalized human oral keratinocytes by gene transfer of a
telomerase component. J Jpn Oral Muco Membr. 8:1–8. 2002.
View Article : Google Scholar
|
|
25
|
Kamata N, Fujimoto R, Tomonari M, Taki M,
Nagayama M and Yasumoto S: Immortalization of human dental papilla,
dental pulp, periodontal ligament cells and gingival fibroblasts by
telomerase reverse transcriptase. J Oral Pathol Med. 33:417–423.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohta K, Shigeishi H, Taki M, Nishi H,
Higashikawa K, Takechi M and Kamata N: Regulation of CXCL9/10/11 in
oral keratinocytes and fibroblasts. J Dent Res. 87:1160–1165. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gröger S, Michel J and Meyle J:
Establishment and characterization of immortalized human gingival
keratinocyte cell lines. J Periodontal Res. 43:604–614. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kato H, Sato S, Yoneyama M, Yamamoto M,
Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O,
et al: Cell type-specific involvement of RIG-I in antiviral
response. Immunity. 23:19–28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ablasser A, Bauernfeind F, Hartmann G,
Latz E, Fitzgerald KA and Hornung V: RIG-I-dependent sensing of
poly(dA:dT) through the induction of an RNA polymerase
III-transcribed RNA intermediate. Nat Immunol. 10:1065–1072. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Naruse T, Ohta K, Kato H, Ishida Y,
Shigeishi H, Sakuma M, Fukui A, Nakagawa T, Tobiume K, Nishi H, et
al: Immune response to cytosolic DNA via intercellular receptor
modulation in oral keratinocytes and fibroblasts. Oral Dis. Nov
17–2020.(Epub ahead of print). doi: 10.1111/odi.13725. PubMed/NCBI
|
|
31
|
Tsuchida T, Kawai T, Akira S and Tsuchida
T: Inhibition of IRF3-dependent antiviral responses by cellular and
viral proteins. Cell Res. 19:3–4. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bick MJ, Carroll JW, Gao G, Goff SP, Rice
CM and MacDonald MR: Expression of the zinc-finger antiviral
protein inhibits alphavirus replication. J Virol. 77:11555–11562.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Müller S, Möller P, Bick MJ, Wurr S,
Becker S, Günther S and Kümmerer BM: Inhibition of filovirus
replication by the zinc finger antiviral protein. J Virol.
81:2391–2400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu M, Ma X, Cui X, Zhou J, Li C, Huang L,
Shang Y and Cheng Z: Inhibition of avian tumor virus replication by
CCCH-type zinc finger antiviral protein. Oncotarget. 8:58865–58871.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Todorova T, Bock FJ and Chang P: PARP13
regulates cellular mRNA post-transcriptionally and functions as a
pro-apoptotic factor by destabilizing TRAILR4 transcript. Nat
Commun. 5:53622014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee H, Komano J, Saitoh Y, Yamaoka S,
Kozaki T, Misawa T, Takahama M, Satoh T, Takeuchi O, Yamamoto N, et
al: Zinc-finger antiviral protein mediates retinoic acid inducible
gene I-like receptor-independent antiviral response to murine
leukemia virus. Proc Natl Acad Sci USA. 110:12379–12384. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vyas S, Chesarone-Cataldo M, Todorova T,
Huang YH and Chang P: A systematic analysis of the PARP protein
family identifies new functions critical for cell physiology. Nat
Commun. 4:22402013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Todorova T, Bock FJ and Chang P:
Poly(ADP-ribose) polymerase-13 and RNA regulation in immunity and
cancer. Trends Mol Med. 21:373–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang N, Dong Q, Li J, Jangra RK, Fan M,
Brasier AR, Lemon SM, Pfeffer LM and Li K: Viral induction of the
zinc finger antiviral protein is IRF3-dependent but
NF-kappaB-independent. J Biol Chem. 285:6080–6090. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Müller U, Steinhoff U, Reis LF, Hemmi S,
Pavlovic J, Zinkernagel RM and Aguet M: Functional role of type I
and type II interferons in antiviral defense. Science.
264:1918–1921. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kadowaki N, Antonenko S, Lau JY and Liu
YJ: Natural interferon alpha/beta-producing cells link innate and
adaptive immunity. J Exp Med. 192:219–226. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qin S, Rottman JB, Myers P, Kassam N,
Weinblatt M, Loetscher M, Koch AE, Moser B and Mackay CR: The
chemokine receptors CXCR3 and CCR5 mark subsets of T cells
associated with certain inflammatory reactions. J Clin Invest.
101:746–754. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dufour JH, Dziejman M, Liu MT, Leung JH,
Lane TE and Luster AD: IFN-gamma-inducible protein 10 (IP-10;
CXCL10)-deficient mice reveal a role for IP-10 in effector T cell
generation and trafficking. J Immunol. 168:3195–3204. 2002.
View Article : Google Scholar : PubMed/NCBI
|