Introduction
Sepsis, the leading cause of death in critically ill
patients worldwide, is a systemic inflammatory response to
infection (1). The annual incidence
of severe sepsis is increasing, with the mortality rate approaching
50% in the United States from 1993 to 2003 (2). Myocardial injury occurs in most
patients with sepsis and is associated with early mortality
(3). Moreover, septic
cardiovascular dysfunction is associated with a significantly
higher mortality rate than those who have sepsis without
cardiovascular dysfunction (4);
thus, infection management is key for the treatment of septic
myocardial injury (1). A previous
study has demonstrated that inflammatory mediators, hemostasis
dysregulation, immunosuppression, and tissue and organ dysfunction
are involved in the pathogenesis of septic myocardial injury
(5). Despite the importance of
myocardial depression in sepsis, its pathophysiology remains
unclear.
Solute carrier family 2 member 1 (SLC2A1) serves an
essential role in energy metabolism (6). Being present in nearly all mammalian
cells, SLC2A1 transports glucose (7). Glucose binding causes a conformational
change in SLC2A1 that results in the release of glucose into the
cytoplasm (7), which is a key step
in glycolysis (8). This process is
dysregulated in numerous diseases, including sepsis. SLC2A1 is
overexpressed in various types of cancer, including breast and lung
cancer (9,10). In a murine model of sepsis, SLC2A1
was also overexpressed, leading to increased glucose uptake
(11). Vary et al (12) demonstrated that sepsis caused a 67%
increase in glucose uptake compared with the control group, and
determined that sepsis enhances glucose uptake secondary to
increased SLC2A1 expression. However, the exact role of SLC2A1 and
glucose uptake in septic myocardial injury remains unclear.
Exosomes are small membrane microvesicles that have
attracted the attention of researchers for decades (13). Exosomes can deliver their cargo
(proteins, lipids and nucleic acids) to target cells, resulting in
metabolic reprogramming (14).
MicroRNAs (miRNAs/miRs/mis) are small, non-coding RNAs that
post-transcriptionally regulate gene expression (15). miRNAs, together with other types of
nucleic acids, including mRNAs and other non-coding RNAs, have been
identified in exosomes (16).
Target cells absorb these exosomal miRNAs, which results in cell
modulation (17). For example,
human serum albumin (hsa)-miR-1262 has been revealed to enhance the
anticancer effects of gefitinib on advanced non-small cell lung
cancer cells (18). Furthermore,
hsa-miR-1262 has been identified as one of the main differentially
expressed microRNAs in the pathogenesis of sepsis (19). However, the role of exosomal
hsa-miR-1262 in sepsis has not been fully elucidated.
Although previous studies have demonstrated that
SLC2A1 actively participates in glycolysis, and that exosomal
miRNAs are implicated in numerous diseases (20–22),
the roles of hsa-miR-1262 and SLC2A1 in septic myocardial injury
remain unclear. Therefore, the present study evaluated the role of
the hsa-miR-1262/SLC2A1 signaling pathway in septic myocardial
injury and explored the underlying mechanisms.
Materials and methods
Human serum samples
The present study was approved by the Ethics
Committee of The Seventh People's Hospital of Shanghai University
of Traditional Chinese Medicine (approval no. 2021-AR-006). A total
of 40 patients with sepsis (27 male patients and 13 female
patients; age range, 37–67 years; mean age, 52.1±6.0 years) and 88
healthy controls (50 male patients and 38 female patients; age
range, 37–67 years; mean age, 52.3±6.3 years). In all patients with
sepsis included in the present study, sepsis was caused by
bacterial infection. Patients with sepsis were diagnosed by blood
test. All participants were enrolled at The Seventh People's
Hospital of Shanghai University of Traditional Chinese Medicine
(Shanghai, China) between June 2017 and August 2019. Patients were
all newly diagnosed cases and recurrent cases were excluded.
Exclusion criteria included: i) Patients afflicted with other
diseases, such as cancer and metabolic diseases; and ii) patients
who were treated by any therapies prior to admission. Subsequently,
serum samples were collected from patients with sepsis and healthy
controls, which were then used for exosome isolation. Written
informed consent was obtained from all participants. The present
study was conducted in accordance with the Declaration of
Helsinki.
Cell culture
The human myocardial AC16 cell line was purchased
from The Cell Bank of Type Culture Collection of The Chinese
Academy of Sciences. The cells were cultured in DMEM (Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (cat. no. 16000-044;
Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (Beyotime Institute of Biotechnology).
Cells were maintained at 37°C with 5% CO2. Cells were
co-cultured with the Control-exo or sepsis-exosomes (50 µg/ml) at
37°C with 5% CO2 respectively.
Isolation and identification of
exosomes
Exosomes were isolated as previously described
(23). Briefly, the blood samples
from sepsis patients and healthy donator were collected and
centrifuged at 1,409 × g for 30 min at 4°C. The supernatants were
transferred to a fresh tube and centrifuged at 5,000 × g rpm for 30
min at 4°C. The supernatant was concentrated, added to 30%
sucrose/D2O and centrifuged at 100,000 × g for 1 h at
4°C. Pellets were diluted with PBS, filtered and maintained at
−70°C. Exosome concentration was measured using a bicinchoninic
acid kit (Pierce; Thermo Fisher Scientific, Inc.). The exosome
pellets were suspended in PBS and fixed in 4% paraformaldehyde and
4% glutaraldehyde at 4°C for 5 min. After adding a drop of the
exosomal sample, the carbon-coated copper grid was immersed in a
phosphotungstic acid solution (2%; pH 7.0) for 30 sec. The exosome
pellets were fixed with 2.5% glutaraldehyde and thencentrifuged at
100,000 × g 4°C for 5 min to remove the glutaraldehyde. Afterwards,
the pellets were stained by 3% aqueous phosphotungstic acid and
fixed on copper mesh formvar grids at 4°C for 30 min. A
transmission electron microscope (JEM-1200EX; Jeol, Ltd.;
magnification, ×100,000) was used to observe and assess the
morphology and size of the exosomes. Western blotting was performed
to examine the biomarkers of exosomes (CD9, CD63 and CD81).
Exosome endocytosis assay
AC16 cells (5×104 cells/well) were seeded
into 24-well plates. A total of 250 µg Sepsis-exo and Control-exo
were labeled using PKH Lipophilic Membrane Dyes (cat. no. PKH67GL;
Sigma-Aldrich; Merck KGaA) according to the manufacturer's
instructions. PKH67-labeled exosomes were centrifuged (40,000 × g
at 4°C for 70 min) and suspended in PBS (50 µl). Cells were
incubated with DMEM (Thermo Fisher Scientific, Inc.) or DMEM
containing PKH-67-labeled exosomes (20 µg/ml) at 37°C for 4 h.
Subsequently, DAPI was used to stain the nucleus at 37°C for 5 min.
Cells were observed under a fluorescent microscope (Olympus IX71;
Olympus Corporation; magnification, ×400).
RT-qPCR
Total RNA from AC16 cells was extracted using
TRIzol® reagent (cat. no. 1596-026; Thermo Fisher
Scientific, Inc.) and reverse transcribed into cDNA (cat. no.
K1622; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The SYBR Green fluorochrome (cat. no.
K0223; Thermo Fisher Scientific, Inc.) and following thermocycling
conditions were used for qPCR: Initial denaturation at 95°C for 10
min; followed by 38 cycles of denaturation at 95°C for 15 sec,
annealing at 60°C for 45 sec and extension at 72°C for 30 sec; and
final extension at 72°C for 3 min.
The following primers were used for qPCR: SLC2A1
forward (F), 5′-TGCAGGAGATGAAGGAAG-3′ and reverse (R),
5′-CAATGGTGGCATACACAG-3′; β-actin F, 5′-TGGCATCCACGAAACTAC-3′ and
R, 5′-CTTGATCTTCATGGTGCTG−3′; hsa-miR-1262 F,
5′-CGCGATGGGTGAATTTGTAG-3′ and R, 5′-AGTGCAGGGTCCGAGGTATT-3′; U6 F,
5′-CTCGGCTTCGGCAGCACA-3′ and R, 5′-AACGCTTCACGAATTTGCGT-3′. mRNA
and miRNA expression levels were quantified using the
2−∆∆Cq method (24) and
normalized to the internal reference genes β-actin or U6,
respectively. Each experiment was repeated three times.
Western blotting
Total protein from AC16 cells was extracted using
radioimmunoprecipitation assay buffer with protease inhibitors
(Beyotime Institute of Biotechnology). The protein levels were
quantified using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. Proteins (20 µg/lane) were separated via 10%
SDS-PAGE. The membranes were blocked using 5% skimmed milk at room
temperature for 2 h, followed by incubation overnight at 4°C with
the following primary antibodies: Anti-SLC2A1 (1:1,000; cat. no.
ab115730; Abcam), anti-SLC2A4 (1:1,000; cat. no. ab33780; Abcam),
anti-CD9 (1:1,000; cat. no. ab92726; Abcam), anti-CD63 (1:1,000;
cat. no. ab271286; Abcam), anti-CD81 (1:700; cat. no. ab109201;
Abcam), anti-β-actin (1:3,000; cat. no. 66009-1-Ig; ProteinTech
Group, Inc.). After washing with 0.1 M PBS, the membranes were
incubated with the secondary antibody (HRP-labeled goat anti-rabbit
IgG; cat. no. A16104; Thermo Fisher Scientific, Inc.) at 4°C for 2
h. The Enhanced Chemiluminescence Detection kit (cat. no.
WBKLS0100; MilliporeSigma) was used for signal detection.
Knockdown and overexpression of
SLC2A1
Short interfering RNAs (sis/siRNAs) targeting human
SLC2A1 (siSLC2A1-1, 5′-GCCCAUGUAUGUGGGUGAATT-3′; siSLC2A1-2,
5′-GCCUGUGUAUGCCACCAUUTT-3′; and siSLC2A1-3,
5′-GCUACCCUGGAUGUCCUAUTT-3′) and a scrambled siRNA negative control
(siNC; 5′-CAGUACUUUUGUGUAGUACAA-3′) were synthesized (Beyotime
Institute of Biotechnology) and inserted into the pLKO.1 vector
(Enable, Biotech). AC16 cells (5×105 cells/well) were
transfected with 100 nm SLC2A1 siRNAs or siNC using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. For
SCL2A1 overexpression, pLVX-puro lentiviral plasmid (5.94 µg)
containing SLC2A1 (NM_006516.4; NCBI database) cDNA or an empty
control plasmid were transfected into AC16 cells (5×105)
as indicated above. After incubation for 48 h at 37°C, cells were
used for subsequent experiments.
Glycolysis and mitochondrial
respiration assay
The extracellular acidification rate (ECAR) and
oxygen consumption rate (OCR) were determined every 7 min for 77
min using a Seahorse XFe96 analyzer (Agilent Technologies, Inc.).
Cells (1×104/well) were seeded into Seahorse XFe96
plates. After measuring the basal ECAR, the cells were incubated
with 10 mM glucose/well to test the capacity of glycolysis,
followed by the addition of 1 µm oligomycin for inhibition of
oxidative phosphorylation to inspect the maximum glycolytic ability
of the cells. Finally, the glycolysis inhibitor 2-DG (50 mM) was
added to determine acid production by non-glycolytic pathways. All
reagents were added at 0 min and the incubation temperature was
maintained at 38.5°C. Cells were detected every 7 min following
continuous administration of 10 mM glucose and inhibitors (1 µm
oligomycin and 50 mM 2-DG). For the OCR examination, the basal OCR
was first evaluated, after which the oxygen consumption for ATP
synthesis was assessed after exposure to 2 µm oligo, an ATP
synthase inhibitor. The maximum oxygen consumption capacity of the
cells was assessed after cells were given 2 µm mitochondrial
uncoupler (FCCP), and the cells were then treated with
mitochondrial respiratory chain inhibitors antimycin A (0.5 µm) and
oligomycin to prevent oxygen consumption by the mitochondria. All
reagents were added at 0 min and the incubation temperature was
maintained at 38.5°C. Cells were measured every 7 min following
continuous administration of 2.0 oligomycin, 2.0 FCCP and 0.5 µm
antimycin A. All reagents in this experiment were purchased from
Sigma-Aldrich (Merck KGaA).
Flow cytometry
Human AC16 cells (1×105 cells/well) were
harvested 48 h after transfection, stained with Annexin V-FITC
(Beyotime Institute of Biotechnology) and PI (Invitrogen; Thermo
Fisher Scientific, Inc.) for 20 min at 37°C in the dark and
analyzed by flow cytometry (Beckman Coulter, Inc.). CytExpert
software 2.0 (Beckman Coulter, Inc.) was used for analysis. Annexin
V-FITC+ and PI− populations indicated
apoptosis. Experiments were conducted in triplicate. Annexin
V− and PI− populations were healthy cells
that were considered negatively stained, Annexin V+ and
PI− cells indicated cells in early apoptosis, and
Annexin V+ and PI+ staining indicated cells
in necrosis (post-apoptotic necrosis or late apoptosis). Vehicle
cells were treated with PBS.
Dual-luciferase reporter assay
The interaction between hsa-miR-1262 and SLC2A1 was
analyzed using TargetScan (http://www.targetscan.org/vert_72/) and starBase 2.0
(http://starbase.sysu.edu.cn/). Wild-type
(WT) and mutant (Mut) SLC2A1 3′ untranslated regions (UTRs) were
synthesized and ligated into pGL3 vectors. AC16 cells
(1×106) were transfected with the WT or Mut construct
(50 µg, cat. no. E1910; Promega Corporation), and co-transfected
with hsa-miR-1262 mimic (5′-AUGGGUGAAUUUGUAGAAGGAU-3′; Shanghai
Meiji Biomedical Technology Co., Ltd.), hsa-miR-1262 inhibitor
(5′-AUCCUUCUACAAAUUCACCCAU-3′; Shanghai Meiji Biomedical Technology
Co., Ltd.), miNC-mimic (5′-CAGUACUUUUGUGUAGUACAA-3′; Shanghai Meiji
Biomedical Technology Co., Ltd.) or miNC-inhibitor
(5′-UUCUCCGAACGUGUCACGUTT-3′; Shanghai Meiji Biomedical Technology
Co., Ltd.). Following incubation at 37°C for 48 h, the firefly and
Renilla luciferase activities were detected using a
Dual-Luciferase Reporter assay system (Promega Corporation)
according to the manufacturer's protocol. Firefly luciferase
activity was normalized to Renilla luciferase activity.
Statistical analysis
Statistical analyses were performed using Prism 7.0
(GraphPad Software, Inc.). Data are presented as the mean ±
standard deviation of three repeats. Comparisons among multiple
independent groups were analyzed using the Kruskal-Wallis test
followed by Dunn's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
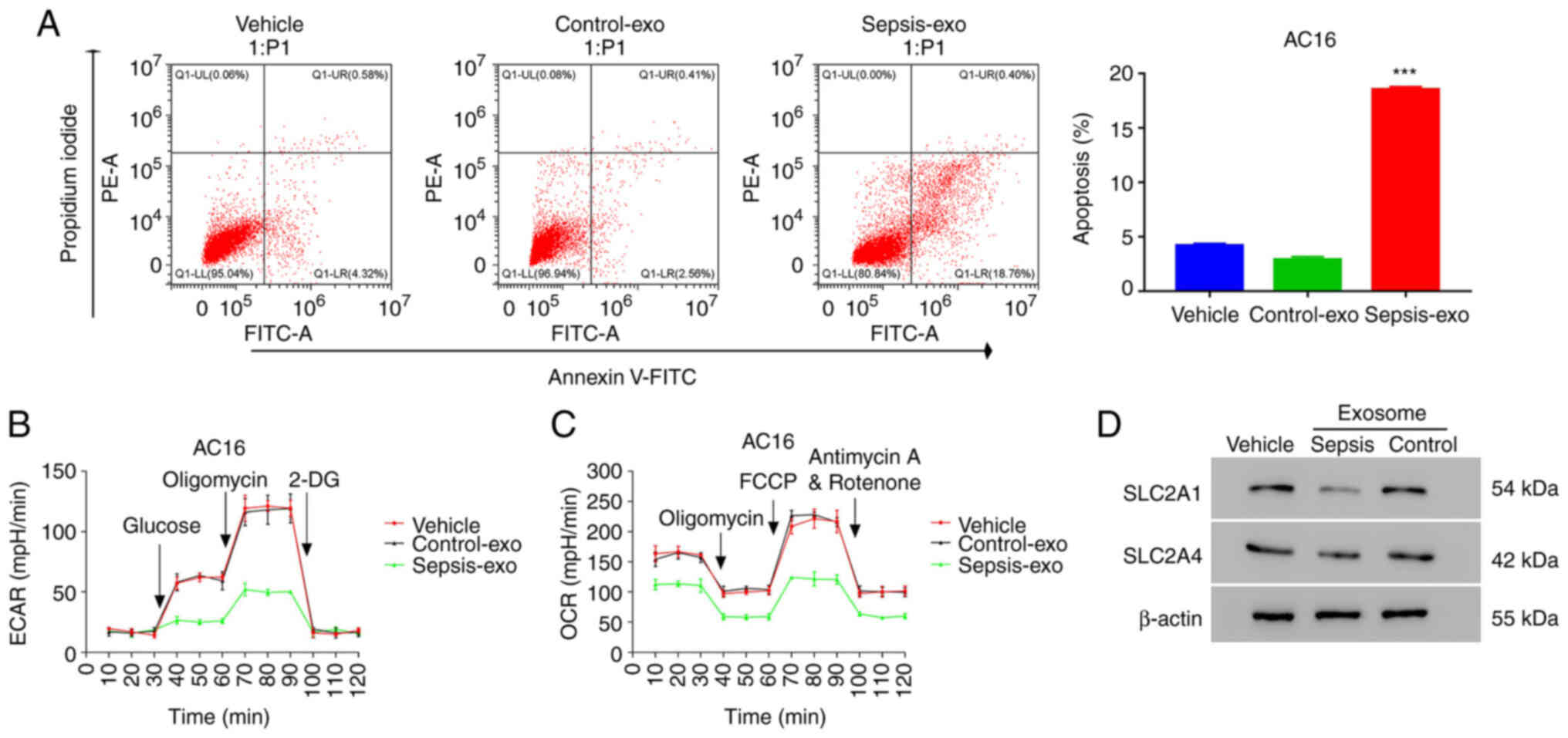
Exosomes isolated from the serum of
patients with sepsis reduce the aerobic glycolysis activity of AC16
cells
To study the role of serum exosomes, exosomes were
isolated from the serum of healthy individuals (Control-exo) and
patients with sepsis (Sepsis-exo; Fig.
S1A), and characterized by the exosomal markers CD9, CD63 and
CD81 (Fig. S1B). PKH-67 staining
revealed that exosomes were endocytosed by AC16 cells (Fig. S1C). These results indicated that
exosomes had been successfully isolated and could be used in
further experiments. Then, we examined the apoptosis of AC16 cells
co-cultured with Sepsis-exo. Co-culture results demonstrated that
Sepsis-exo treatment significantly increased the apoptosis of AC16
cells compared with that in the Control-exo treatment group
(Fig. 1A). Furthermore, Sepsis-exo
treatment resulted in marked suppression of ECAR (Fig. 1B) and OCR (Fig. 1C) compared with that in the
Control-exo group. The western blotting results demonstrated that
Sepsis-exo treatment notably decreased SLC2A1 protein expression
levels in AC16 cells compared with those in the Control-exo
treatment group (Fig. 1D). No
significant change in SLC2A4 protein expression levels was observed
in the Sepsis-exo treatment group compared with those in the
Control-exo treatment group. These data indicated that Sepsis-exo
treatment decreased aerobic glycolysis activity and increased
apoptosis in AC16 cells.
Sepsis-exo time-dependently promotes
apoptosis and inhibits aerobic glycolysis activity in AC16
cells
To further understand the effect of Sepsis-exo on
apoptosis and glycolysis, a time-course study was performed. The
flow cytometry results demonstrated that Sepsis-exo significantly
increased AC16 cell apoptosis in a time-dependent manner (Fig. 2A). Furthermore, Sepsis-exo treatment
markedly suppressed ECAR (Fig. 2B)
and OCR (Fig. 2C) in a
time-dependent manner. The RT-qPCR results revealed that Sepsis-exo
significantly increased hsa-miR-1262 expression levels, but
significantly decreased SLC2A1 mRNA expression levels in a
time-dependent manner (Fig. 2D).
Moreover, the western blotting results demonstrated that Sepsis-exo
markedly decreased SLC2A1 protein expression in a time-dependent
manner (Fig. 2D). These results
indicated that Sepsis-exo time-dependently inhibited glycolysis and
promoted apoptosis in AC16 cells.
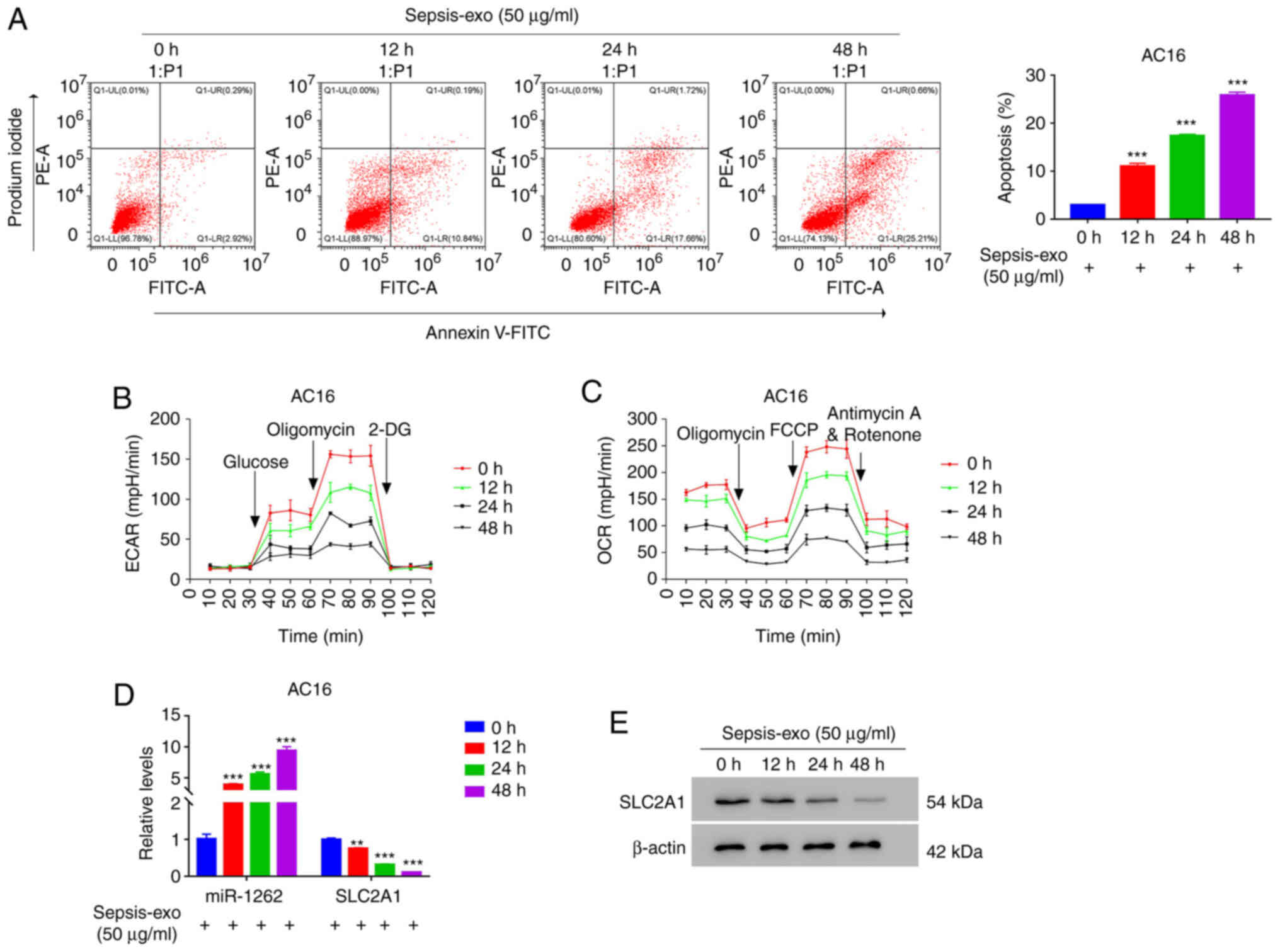 | Figure 2.Sepsis-exo promotes apoptosis and
inhibits glycolysis in AC16 cells in a time-dependent manner. AC16
cells were co-cultured with exosomes for 0, 12, 24 or 48 h. (A)
Flow cytometry analysis of AC16 cell apoptosis. Analysis of (B)
ECAR and (C) OCR in AC16 cells. (D) Reverse
transcription-quantitative PCR analysis of hsa-miR-1262 and SLC2A1
expression levels under Sepsis-exo treatment. (E) Western blotting
analysis of SLC2A1 in AC16 cells. **P<0.01 and ***P<0.001 vs.
0 h. ECAR, extracellular acidification rate; OCR, oxygen
consumption rate; SLC2A, solute carrier family 2 member; hsa, human
serum albumin; miR, microRNA; PE, phycoerythrin; 2-DG,
2-deoxy-D-glucose; FCCP, carbonyl
cyanide-4-(trifluoromethoxy)phenylhydrazone; exo, exosome. |
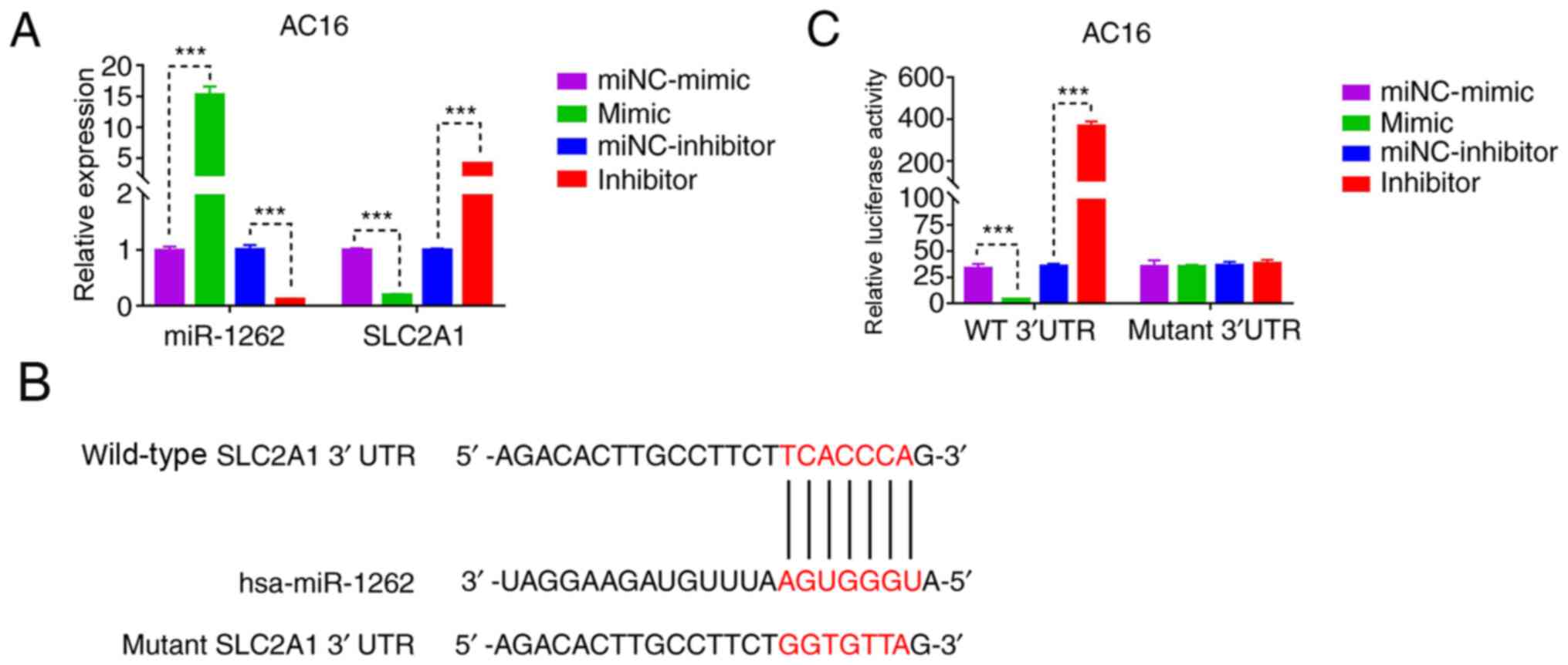
hsa-miR-1262 inhibits SLC2A1
transcription by binding to its 3′UTR
To understand how hsa-miR-1262 regulated SLC2A1
expression, hsa-miR-1262 was silenced or overexpressed in AC16
cells (Fig. 3A). Bioinformatics
analysis indicated a potential binding site of hsa-miR-1262 in the
3′UTR of SLC2A1 (Fig. 3B).
Therefore, SLC2A1 WT 3′UTR + hsa-miR-1262 inhibitor, WT 3′UTR +
hsa-miR-1262 mimic, Mut 3′UTR + hsa-miR-1262 inhibitor and Mut
3′UTR + hsa-miR-1262 mimic were co-transfected into AC16 cells. The
dual-luciferase reporter assay results demonstrated that
hsa-miR-1262 silencing significantly increased SLC2A1 promoter
activity compared with that of the miNC-inhibitor group, whereas
SLC2A1 promoter activity was significantly inhibited by
hsa-miR-1262 overexpression compared with that of the miNC-mimic
group. Furthermore, a mutation in the hsa-miR-1262 binding site of
SLC2A1 blocked the effect of hsa-miR-1262 on the SLC2A1 promoter
(Fig. 3C). These results indicated
that hsa-miR-1262 bound to the 3′UTR of SLC2A1 to negatively
regulate its expression.
hsa-miR-1262 mimic and Sepsis-exo
exhibit similar effects on AC16 cells
Both Sepsis-exo and hsa-miR-1262 mimic were used to
treat overexpression (oe)SLC2A1-AC16 cells (Fig. S2C and D) to further investigate
their roles. Sepsis-exo and hsa-miR-1262 mimic significantly
increased the apoptosis of oeNC-transfected AC16 cells compared
with that in vehicle cells (Fig.
4A). Sepsis-exo and hsa-miR-1262 mimic-induced increases in
apoptosis were significantly attenuated by SLC2A1 overexpression.
Furthermore, SLC2A1 overexpression markedly diminished Sepsis-exo
and hsa-miR-1262 mimic-mediated effects on ECAR (Fig. 4B) and OCR (Fig. 4C). The western blotting results
demonstrated that both Sepsis-exo and hsa-miR-1262 mimic markedly
decreased SLC2A1 protein expression levels compared with those in
the vehicle group; however, these effects were abolished by SLC2A1
overexpression (Fig. 4D). Overall,
these findings suggested that hsa-miR-1262 mimic and Sepsis-exo
displayed similar effects on AC16 cells.
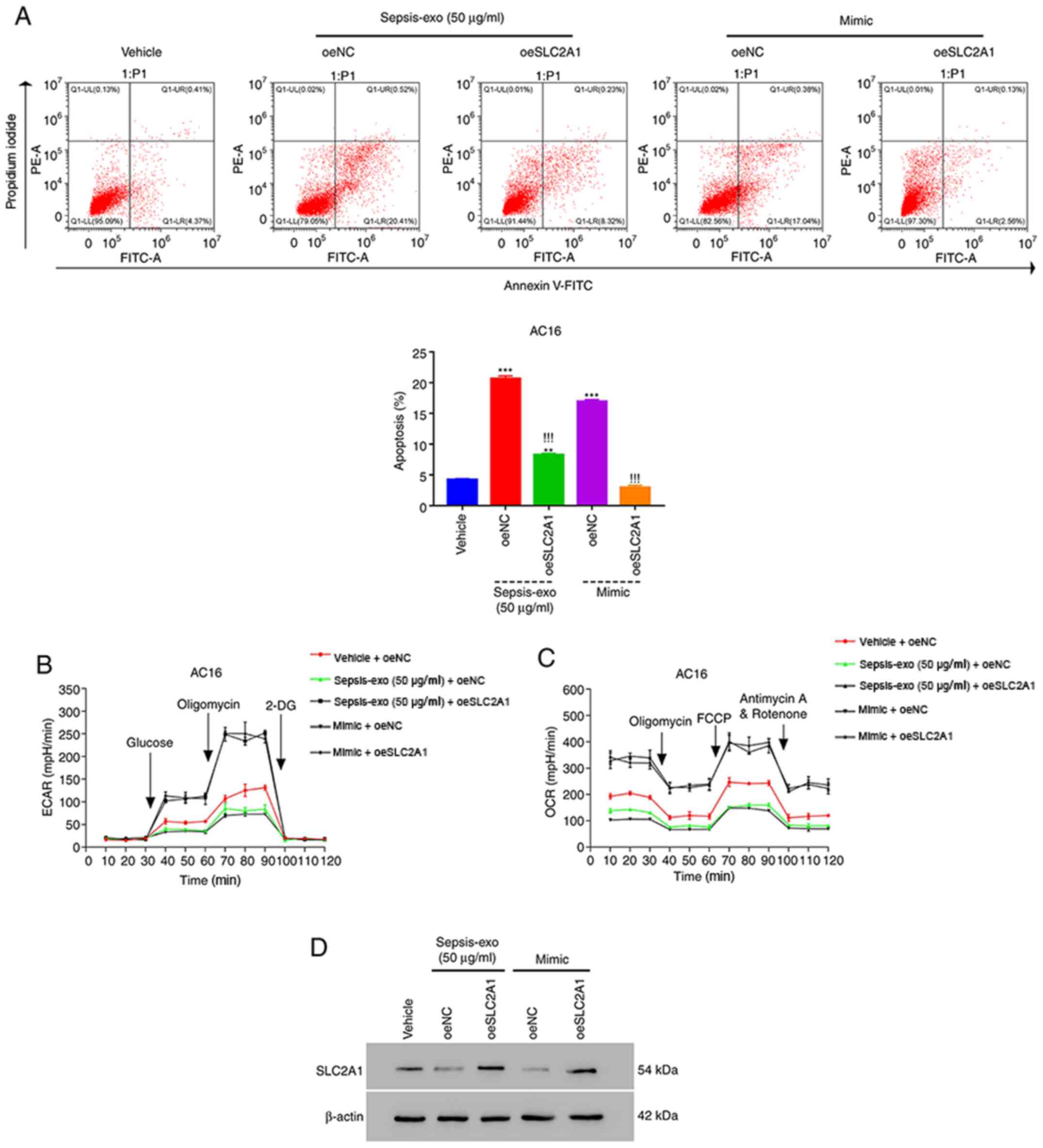 | Figure 4.hsa-miR-1262 mimic and Sepsis-exo
exhibit similar effects on AC16 cells. (A) Sepsis-exo and
hsa-miR-1262 mimic significantly suppressed the apoptosis of
oeSLC2A1-transfected AC16 cells. (B) ECAR and (C) OCR in
oeSLC2A1-transfected AC16 cells were significantly suppressed by
Sepsis-exo or hsa-miR-1262 mimic. (D) Western blotting analysis of
SLC2A1. ***P<0.001 vs. vehicle; !!!P<0.001 vs.
oeNC + Sepsis-exo; **P<0.01 vs. vehicle. hsa, human serum
albumin; exo, exosome; miR, microRNA; SLC2A, solute carrier family
2 member; ECAR, extracellular acidification rate; OCR, oxygen
consumption rate; oe, overexpression; NC, negative control; PE,
phycoerythrin; 2-DG, 2-deoxy-D-glucose; FCCP, carbonyl
cyanide-4-(trifluoromethoxy)phenylhydrazone. |
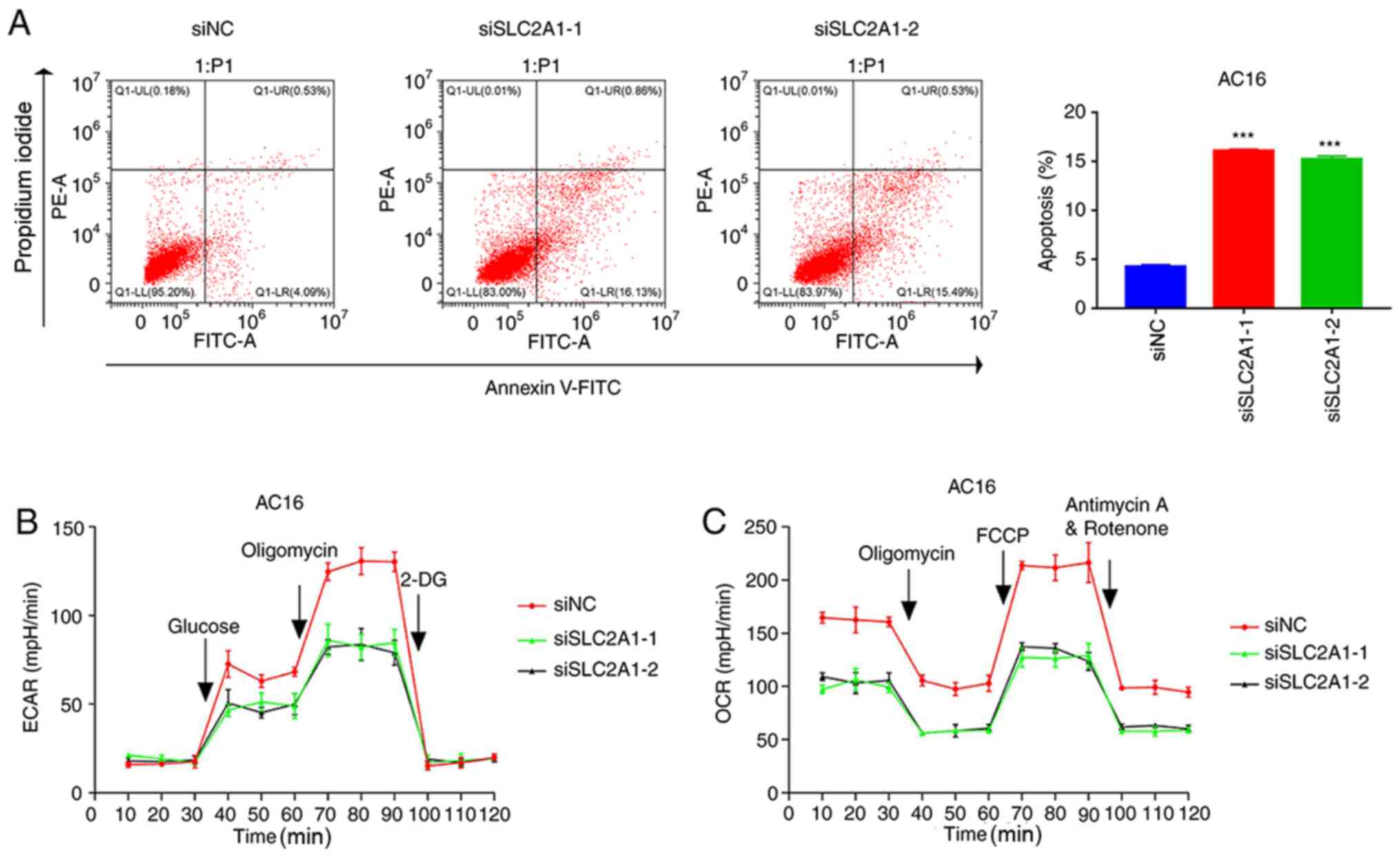
SLC2A1 silencing promotes apoptosis
and suppresses glycolysis in AC16 cells
To further confirm the role of SLC2A1, SLC2A1 was
silenced in AC16 cells (Fig. S2A and
B), and then apoptosis and glycolysis were quantified. As shown
in Fig. S2B, siSLC2A1-1 and
siSLC2A1-2 were more effective compared with siSLC2A1-3. Therefore,
SLC2A1-3 was not used in subsequent experiments. The flow cytometry
results revealed that the silencing of SLC2A1 significantly
promoted the apoptosis of AC16 cells compared with that in the siNC
group (Fig. 5A). Furthermore,
SLC2A1 silencing markedly inhibited ECAR (Fig. 5B) and OCR (Fig. 5C) in AC16 cells compared with that
in siNC group. These data suggested that SLC2A1 silencing promoted
apoptosis and suppressed glycolysis in AC16 cells. A graphical
abstract summarizing the results of the present study is presented
in Fig. S2E.
Discussion
To the best of our knowledge, the present study was
the first to report that exosomes isolated from the blood of
patients with sepsis reduced aerobic glycolysis activity and
promoted apoptosis in human AC16 cells in a time-dependent manner.
The results demonstrated that Sepsis-exo significantly increased
hsa-miR-1262 expression levels, but significantly decreased SLC2A1
mRNA expression levels in a time-dependent manner. Moreover, the
results indicated that Sepsis-exo exerted its effects via exosomal
hsa-miR-1262. hsa-miR-1262 was also demonstrated to bind to SLC2A1
and negatively regulate its expression. Furthermore, SLC2A1
silencing promoted apoptosis and suppressed glycolysis in AC16
cells. The present study revealed that hsa-miR-1262 from Sepsis-exo
inhibited glycolysis and promoted apoptosis in AC16 cells by
targeting SLC2A1.
hsa-miR-1262 serves a role in numerous different
diseases. For example, it has been reported that hsa-miR-1262
overexpression suppresses lung cancer cell proliferation by
targeting Unc-51-like kinase 1 and Ras-related protein RAB3D
(25). A recent study demonstrated
that hsa-miR-1262 regulates tumor progression via low-density
lipoprotein receptor-related protein 8 in breast cancer (26). hsa-miR-1262 has also been shown to
serve a role in sepsis pathogenesis (19). The present study demonstrated that
hsa-miR-1262 inhibited glycolysis and induced apoptosis, leading to
suppression of AC16 cell proliferation. These results revealed a
potential novel role for hsa-miR-1262 in sepsis and septic
myocardial injury.
Among the 14 glucose transporter (GLUT) family
members (27), SLC2A1 is the most
extensively studied and has been shown to be overexpressed during
oncogenesis to increase glucose uptake and glycolysis in tumor
cells (28). Furthermore, it has
been demonstrated that glucose uptake is dysregulated in sepsis
(29). In a previous study,
lipopolysaccharide, the cause of serious sequelae in patients with
gram-negative bacterial sepsis, was attributed to the dysregulation
of glucose uptake via SLC2A1, but not to other GLUT family members
(30). It has also been
demonstrated that dysregulated glucose uptake is associated with
dysregulated SLC2A1 expression during septic shock (12). The present study reported that
exosomal hsa-miR-1262 bound directly to the 3′UTR of the SLC2A1
promoter to negatively regulate its expression. The protection of
AC16 cells by SLC2A1 overexpression against hsa-miR-1262 mimic- or
Sepsis-exo-induced proliferation suppression further demonstrated
that SLC2A1 was a target of hsa-miR-1262. These results not only
indicated a role of hsa-miR-1262/SLC2A1 in septic cardiomyocyte
injury, but may also improve the understanding of sepsis
pathogenesis.
Glycolysis dysregulation has been demonstrated to
serve a role in numerous diseases, including rheumatoid arthritis
and cancer (31). A previous study
indicated that the dysregulation of glycolysis by inflammatory
cytokines might be the molecular mechanism underlying severe sepsis
(32). Zheng et al (33) reported that glycolytic metabolism
serves a crucial role in septic cardiomyopathy, most likely by
regulating inflammation and apoptosis. Yang et al (34) indicated that the inhibition of
glycolysis protected against sepsis by partially suppressing
lactate production and high mobility group box 1 release. Moreover,
MacFarlane et al (35)
reported that inhibiting glycolysis using 2-deoxyglucose
potentiates TNF-related apoptosis-inducing ligand-induced cell
apoptosis. Furthermore, Mason et al (36) determined that decreased glycolysis
promoted cell apoptosis via the activation of p53 and induction of
the proapoptotic protein p53 upregulated modulator of apoptosis. In
the present study, SLC2A1 overexpression significantly diminished
Sepsis-exo- and hsa-miR-1262 mimic-induced decreases ECAR and OCR,
suggesting that glycolysis may serve an important role in
Sepsis-exo-induced cardiomyocyte injury. Furthermore, in addition
to glycolysis suppression, miR-1262 overexpression or Sepsis-exo
administration significantly promoted the apoptosis of AC16 cells.
Overall, these results suggested a role of
hsa-miR-1262/SLC2A1/glycolysis in the pathogenesis of septic
cardiomyocyte injury and sepsis. Although further studies are
needed, the present study reported a potential mechanism underlying
septic myocardial injury.
In conclusion, the present study indicated a novel
role of the hsa-miR-1262/SLC2A1 signaling pathway, suggesting that
serum exosomes isolated from patients with sepsis may cause
cardiomyocyte proliferation suppression via hsa-miR-1262 and its
target SLC2A1. These results may provide the foundations for the
future development of novel therapeutic strategies for septic
myocardial injury. A key limitation of the present study was that
the results were not verified in vivo; thus, an animal model should
be used in future studies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81973649 and 82174189), the Youth
Project of Scientific Research Project of Shanghai Health Committee
(grant no. 20214Y0281), the Science and Technology Development Fund
of Shanghai Pudong (grant no. PKJ2019-Y16), the Leading Medical
Talent Training Program of Pudong Health Bureau of Shanghai (grant
nos. PWR12019-02 and PWRd2021-05), the Outstanding Clinical
Discipline Project of Shanghai Pudong (grant nos. PWYgy2018-01 and
PWZy2020-07), the Postgraduate Innovation Training Project of
Shanghai University of Traditional Chinese Medicine (grant no.
Y2021022), the Training Plan for the Inheritor of Famous
Traditional Chinese Medicine of Shanghai Pudong (grant no.
PWRzj2020-02) and the Talents Training Program of Seventh People's
Hospital of Shanghai University of Traditional Chinese Medicine
(grant no. BDX2021-02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML designed the project and revised the manuscript.
FS and HG performed the experiments and drafted the manuscript. YS
and WF analyzed the data and edited the figures. TT and LY analyzed
the data and confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Seventh People's Hospital of Shanghai University
of Traditional Chinese Medicine, Shanghai, China (approval no.
2021-AR-006). Written informed consent was obtained for all
patients. The present study was performed in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kakihana Y, Ito T, Nakahara M, Yamaguchi K
and Yasuda T: Sepsis-induced myocardial dysfunction:
Pathophysiology and management. J Intensive Care. 4:222016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dombrovskiy VY, Martin AA, Sunderram J and
Paz HL: Rapid increase in hospitalization and mortality rates for
severe sepsis in the United States: A trend analysis from 1993 to
2003. Crit Care Med. 35:1244–1250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frencken JF, Donker DW, Spitoni C,
Koster-Brouwer ME, Soliman IW, Ong DS, Horn J, van der Poll T, van
Klei WA, Bonten MJ and Cremer OL: Myocardial injury in patients
with sepsis and its association with long-term outcome. Circ
Cardiovasc Qual Outcomes. 11:e0040402018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parrillo JE, Parker MM, Natanson C,
Suffredini AF, Danner RL, Cunnion RE and Ognibene FP: Septic shock
in humans. Advances in the understanding of pathogenesis,
cardiovascular dysfunction, and therapy. Ann Intern Med.
113:227–242. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gyawali B, Ramakrishna K and Dhamoon AS:
Sepsis: The evolution in definition, pathophysiology, and
management. SAGE Open Med. 7:20503121198350432019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ooi AT and Gomperts BN: Molecular
pathways: Targeting cellular energy metabolism in cancer via
inhibition of SLC2A1 and LDHA. Clin Cancer Res. 21:2440–2444. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Dedda C, Vignali D, Piemonti L and
Monti P: Pharmacological targeting of GLUT1 to control autoreactive
T cell responses. Int J Mol Sci. 20:49622019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang B: Aerobic glycolysis and high level
of lactate in cancer metabolism and microenvironment. Genes Dis.
4:25–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krzeslak A, Wojcik-Krowiranda K, Forma E,
Jozwiak P, Romanowicz H, Bienkiewicz A and Brys M: Expression of
GLUT1 and GLUT3 glucose transporters in endometrial and breast
cancers. Pathol Oncol Res. 18:721–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo W, Sun S, Guo L, Song P, Xue X, Zhang
H, Zhang G, Li R, Gao Y, Qiu B, et al: Elevated SLC2A1 expression
correlates with poor prognosis in patients with surgically resected
lung adenocarcinoma: A study based on immunohistochemical analysis
and bioinformatics. DNA Cell Biol. 39:631–644. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palmer CS, Cherry CL, Sada-Ovalle I, Singh
A and Crowe SM: Glucose metabolism in T cells and monocytes: New
perspectives in HIV pathogenesis. EBioMedicine. 6:31–41. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vary TC, Drnevich D, Jurasinski C and
Brennan WA Jr: Mechanisms regulating skeletal muscle glucose
metabolism in sepsis. Shock. 3:403–410. 1995.PubMed/NCBI
|
|
13
|
Zhang Y, Liu Y, Liu H and Tang WH:
Exosomes: Biogenesis, biologic function and clinical potential.
Cell Biosci. 9:192019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pegtel DM and Gould SJ: Exosomes. Annu Rev
Biochem. 88:487–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai Y, Yu X, Hu S and Yu J: A brief review
on the mechanisms of miRNA regulation. Genomics Proteomics
Bioinformatics. 7:147–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato-Kuwabara Y, Melo SA, Soares FA and
Calin GA: The fusion of two worlds: Non-coding RNAs and
extracellular vesicles-diagnostic and therapeutic implications
(Review). Int J Oncol. 46:17–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Li S, Li L, Li M, Guo C, Yao J
and Mi S: Exosome and exosomal microRNA: Trafficking, sorting, and
function. Genomics Proteomics Bioinformatics. 13:17–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lei T, Zhang L, Song Y, Wang B, Shen Y,
Zhang N and Yang M: miR-1262 transcriptionally modulated by an
enhancer genetic variant improves efficiency of epidermal growth
factor receptor-tyrosine kinase inhibitors in advanced lung
adenocarcinoma. DNA Cell Biol. 39:1111–1118. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahmad S, Ahmed MM, Hasan PMZ, Sharma A,
Bilgrami AL, Manda K, Ishrat R and Syed MA: Identification and
validation of potential miRNAs, as biomarkers for sepsis and
associated lung injury: A network-based approach. Genes.
11:13272020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding X, Liu J, Liu T, Ma Z, Wen D and Zhu
J: miR-148b inhibits glycolysis in gastric cancer through targeting
SLC2A1. Cancer Med. 6:1301–1310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia X, Wang Y, Huang Y, Zhang H, Lu H and
Zheng JC: Exosomal miRNAs in central nervous system diseases:
Biomarkers, pathological mediators, protective factors and
therapeutic agents. Prog Neurobiol. 183:1016942019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu C, Meiners S, Lukas C, Stathopoulos GT
and Chen J: Role of exosomal microRNAs in lung cancer biology and
clinical applications. Cell Prolif. 53:e128282020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh A: Exosome-mediated transfer of
integrins promotes cell-cell communication in prostate cancer.
(2017). ETD Collection for Thomas Jefferson University.
AAI10617823. https://jdc.jefferson.edu/dissertations/AAI10617823/February
24–2021
|
|
24
|
Kenneth JL and Thomas DS: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie K, Chen M, Zhu M, Wang C, Qin N, Liang
C, Song C, Dai J, Jin G, Shen H, et al: A polymorphism in miR-1262
regulatory region confers the risk of lung cancer in Chinese
population. Int J Cancer. 141:958–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Qu WH, Ma HP, Wang LL, Zhang YB and
Ma Y: LRP8, modulated by miR-1262, promotes tumour progression and
forecasts the prognosis of patients in breast cancer. Arch Physiol
Biochem. 29:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Macintyre AN, Gerriets VA, Nichols AG,
Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen
BJ, Hale LP and Rathmell JC: The glucose transporter Glut1 is
selectively essential for CD4 T cell activation and effector
function. Cell Metab. 20:61–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szablewski L: Expression of glucose
transporters in cancers. Biochim Biophys Acta. 1835:164–169.
2013.PubMed/NCBI
|
|
29
|
Lang CH and Dobrescu C: Sepsis-induced
increases in glucose uptake by macrophage-rich tissues persist
during hypoglycemia. Metabolism. 40:585–593. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fukuzumi M, Shinomiya H, Shimizu Y, Ohishi
K and Utsumi S: Endotoxin-induced enhancement of glucose influx
into murine peritoneal macrophages via GLUT1. Infect Immun.
64:108–112. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tan Q, Huang Q, Ma YL, Mao K, Yang G, Luo
P, Ma G, Mei P and Jin Y: Potential roles of IL-1 subfamily members
in glycolysis in disease. Cytokine Growth Factor Rev. 44:18–27.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taylor DJ: Interleukin-1 stimulation of
fibroblast glycolysis is accompanied by reduced glucose oxidation
in the tricarboxylic acid cycle. Biochem Soc Trans. 18:982–983.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng Z, Ma H, Zhang X, Tu F, Wang X, Ha
T, Fan M, Liu L, Xu J, Yu K, et al: Enhanced glycolytic metabolism
contributes to cardiac dysfunction in polymicrobial sepsis. J
Infect Dis. 215:1396–1406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W,
Kang R, Lotze MT, Billiar TR, Wang H, et al: PKM2 regulates the
Warburg effect and promotes HMGB1 release in sepsis. Nat Commun.
5:44362014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
MacFarlane M, Robinson GL and Cain K:
Glucose-a sweet way to die: Metabolic switching modulates tumor
cell death. Cell Cycle. 11:3919–3925. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mason EF, Zhao Y, Goraksha-Hicks P, Coloff
JL, Gannon H, Jones SN and Rathmell JC: Aerobic glycolysis
suppresses p53 activity to provide selective protection from
apoptosis upon loss of growth signals or inhibition of BCR-Abl.
Cancer Res. 70:8066–8076. 2010. View Article : Google Scholar : PubMed/NCBI
|