Introduction
Osteoporosis is defined as a progressive bone
disease, characterized by low bone mass and deterioration of bone
microstructure, resulting in increased brittleness and
susceptibility to fracture (1,2).
Osteoporosis is caused by an imbalance between bone resorption and
bone formation (3) and is a
ubiquitous disease that has gradually become a serious public
health problem affecting >200 million individuals worldwide
(4). Osteoclasts are the only
bone tissue cells that can resorb bones and excessive
differentiation of osteoclasts can lead to osteolytic diseases,
which serve an important role in bone loss in osteoporosis
(5). Currently, drugs used for
anti-resorption mainly include bisphosphates, calcitonin and
selective estrogen receptor modulators (6). However, these drugs have serious
side effects, such as osteonecrosis of the jaws and transverse
femur fractures, hypercalcemia, hearing loss and breast cancer
(7). Therefore, how to
effectively prevent and treat bone loss has attracted increasing
attention worldwide. Some novel plant-derived drugs deserve to be
evaluated in the prevention and treatment of osteoporosis due to
their fewer adverse reactions and anti-resorptive activity
(4,8,9).
Models of osteoporosis are mainly established
through ovariectomy, glucocorticoids induction, low calcium (Ca)
diet and disuse osteoporosis. These methods for modeling
osteoporosis in animals require complex and long-term effects
(10–13). As early as 1984, Lassila (14) reported that thioacetamide
(TAA)-induced liver injury is accompanied by changes in serum
protein and alveolar bone, mainly manifested in the reduction of
new bone formation. Our previous study established a stable model
of bone loss in New Zealand white rabbits via intraperitoneal
injection of TAA and it revealed that TAA-induced bone loss mainly
promoted osteoclast differentiation (15). Compared with other methods, TAA
has the advantages of a short modeling time, being a simple method
and high controllability in building a model of bone loss.
Icariin (ICA;
C33H40O15) is a flavonoid compound
extracted from Epimedium(16), which has anti-inflammatory,
antitumor, anti-depression, anti-oxidation and other biological
activities (17,18). Studies have reported that ICA
prevents bone loss in vitro and restores femoral strength
in vivo (19,20). ICA can inhibit the osteoclast
formation induced by receptor activator of nuclear factor κ-Β
ligand (RANKL) in RAW264.7 cells (21). Jing et al (22). demonstrated that oral ICA
treatment prevents bone loss in iron-overloaded mice and inhibited
the differentiation and function of osteoclasts. In addition, ICA
reduces bone loss induced by methotrexate chemotherapy in rats
(23). As these studies
demonstrate the osteoporosis-related activity of ICA, it was
hypothesized that ICA may exert a protective effect on TAA-induced
bone loss.
Although TAA is toxic and carcinogenic to numerous
organs, such as the liver, kidney and bone (24,25), it is still widely used in the
synthesis of a variety of clinical drugs, chemical materials,
pesticides and hair dyes (26,27). In the present study, a TAA-induced
bone loss model in rats was established, the protective effect of
ICA was explored and the potential mechanisms by which ICA may
inhibit osteoclast differentiation were evaluated.
Materials and methods
Reagents and antibodies
TAA (purity >98%) was obtained from Sangon
Biotech Co., Ltd. (cat. no. A600940). ICA (purity >98%) was
obtained from Xian kai lai Biological Engineering Co., Ltd. (cat.
no. I50081). The tartrate-resistant acid phosphatase (TRAP)
staining kit (cat. no. 294–67001) was obtained from FUJIFILM Wako
Pure Chemical Corporation. RIPA kit (cat. no. P0013B), ECL kit
(cat. no. P0018AS), hematoxylin and eosin (H&E; cat. no. C0105)
staining kit and acid alcohol slow differentiation solution (cat.
no. C0161M) were obtained from Beyotime Institute of Biotechnology.
The rat N-terminal telopeptide of type-I collagen (NTX–I) ELISA kit
(cat. no. YB-NTXI-Ra) was purchased from Shanghai Yubo
Biotechnology Co., Ltd. EDTA decalcified fluid (cat. no. G2520) and
the BCA kit (cat. no. PC0020) were obtained from Beijing Solarbio
Science & Technology Co., Ltd. The iScript cDNA Synthesis Kit
(cat. no. 1708890) and iTaq Universal SYBR Green Supermix (cat. no.
172–5122) were obtained from Bio-Rad Laboratories, Inc. The
antibodies against β-actin (cat. no. ab8226), osteopontin (OPN;
cat. no. ab73400), TRAP (cat. no. ab191406), cathepsin K (cat. no.
ab187647), peroxisome proliferator-activated receptor γ (PPAR-γ;
cat. no. ab272718), phosphorylated (p-)JNK (ab76572) and JNK
(ab17946) and the secondary antibody (cat. no. ab6721) were
purchased from Abcam. The antibodies against IκBα (bs-1287R),
p-IκBα (bs-18128R), p65 (bs-23217R), p-p65 (bs-20159R), p38 (cat.
no. bs-0637R), ERK (cat. no. bs-0022R), p-p38 (cat. no. bs-0636R)
and p-ERK (cat. no. bs-3016R) were obtained from BIOSS. The
antibodies against RANKL (cat. no. ABP52325), RANK (cat. no.
ABP60088) and c-Fos (cat. no. ABM0065) were purchased from Abbkine
Scientific Co., Ltd. The antibody against nuclear factor of
activated T cells (NFAT)c1 (cat. no. A1539) was purchased from
ABclonal Biotech Co., Ltd. TRIzol® reagent (cat. no.
15596026) was purchased from Thermo Fisher Scientific, Inc. All
primers were purchased from Zhejiang Shangya Biotechnology Co.,
Ltd.
Animals and treatment
A total of 32 specific-pathogen-free grade male
Sprague Dawley rats (SD rats; 230–280 g; 8 weeks) were provided by
the Shanghai B&K Co. Ltd. The animals were kept at 25°C and
50±10% humidity with free access to food and water and a 12-h
light/dark cycle. After a week of acclimation, all rats were
randomly divided into four groups: Rats in the control group were
intraperitoneally injected with normal saline as a negative
control, rats in the TAA group were intraperitoneally injected with
300 mg/kg TAA, rats in the ICA group were orally treated with 600
mg/kg ICA and rats in the TAA+ICA group were treated with TAA
combined with ICA. There were eight rats in each group. The dosages
of TAA and ICA were determined based on the results of previous
studies and other literature (15,28,29). All rats were treated once every
two days for 6 weeks. All animal experiments were performed in
accordance with the requirements of the Laboratory Animal-Guideline
for ethical review of animal welfare (GB/T 35892-2018) (30).
Blood sample analysis
Blood (0.5 ml) was drawn from the mandibular vein
every 2 weeks for blood tests and was collected three times. The
serum was obtained by centrifugation (1,500 × g for 15 min at 4°C).
The activity of alkaline phosphatase (ALP), the concentrations of
Ca, phosphorus (P) and magnesium (Mg) in the serum were analyzed
using a Roche analyzer (Roche Diagnostics). NTX- I levels were
detected using an ELISA kit.
H&E and TRAP staining
After fasting for 12 h, the rats were sacrificed by
exsanguination under pentobarbital sodium anesthesia (45 mg/kg
i.p.) followed by cervical dislocation. All rat femurs were removed
and parts of the femurs were placed in 10% neutral formaldehyde for
24 h at room temperature. After 6 weeks of decalcification in EDTA
decalcification solution at room temperature, the samples were
dehydrated using a gradient of different concentrations of ethanol
for 1 h each, washed with xylene and finally embedded in wax.
Paraffin sections with a thickness of 4 µm were obtained and
stained with H&E and TRAP according to the reagent instructions
and pathological changes were observed under an ordinary light
microscope (RX50; Ningbo Sunny Instruments Co., Ltd.). The
remaining femurs were preserved at −80°C for subsequent
experiments.
Micro computed tomography (CT)
analysis
The femur was scanned using a Skyscan1176 µCT
scanner (Bruker Belgium SA). The growth plate at the distal end of
the rat femur was used as a reference point to move along the
sagittal position towards the proximal end and 100 layers were
selected as the initial layer and then 100 layers were selected
along the sagittal position towards the proximal end as the region
of interest. The image was used to reconstruct the bone
microstructure and quantitatively analyze the bone parameters.
Parameters for bone mineral density (BMD), ratio of bone volume
over tissue volume (BV/TV), trabecular pattern factor (Tb.Pf),
trabecular number (Tb.N), the trabecular thickness (Tb.Th) and
structure model index (SMI) were directly obtained.
Three-point bending test
The mechanical strength of the femur was measured
using a three-point bending test using the Electronic universal
testing machine 5569 (Instron) with a span of 15 mm and a loading
rate of 5 mm/min. The maximum load and elastic load were calculated
according to the load-deformation curve.
Reverse transcription-quantitative
PCR
Total RNA was extracted from the femoral head using
TRIzol® reagent and quantified by spectrophotometry at
260 nm. The gene amplification instrument 2720 (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used for reverse transcription
under the following conditions: 25°C for 5 min, 46°C for 20 min and
95°C for 1 min. A real-time quantitative fluorescence PCR
instrument 7500 system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used for quantitative PCR under the following
conditions: Initial denaturation at 95°C for 30 sec; followed by 40
cycles at 95°C for 15 sec and 60°C for 60 sec. qPCR were carried
out using iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories,
Inc.). RNA extraction, cDNA synthesis and qPCR were performed
according to the manufacturer's protocol. GAPDH was used as an
internal control. The 2−ΔΔCq method was used to analyze
relative gene expression data (31,32). Primer sequences for TRAP, PPAR-γ
and GAPDH are shown in Table
I.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR analysis. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR analysis.
| Gene | Sequence
(5′-3′) | Size, bp |
|---|
| GAPDH |
| 292 |
| Forward |
GTGCTGAGTATGTCGTGGAGTCT |
|
| Reverse |
ACAGTCTTCTGAGTGGCAGTGA |
|
| TRAP |
| 98 |
| Forward |
GTGCATGACGCCAATGACAAG |
|
| Reverse |
TTTCCAGCCAGCACGTACCA |
|
| PPAR-γ |
| 271 |
| Forward |
CCGAAGAACCATCCGATT |
|
| Reverse |
CGGGAAGGACTTTATGTA |
|
Western blot analysis
The femoral head samples were ground with liquid
nitrogen and then RIPA lysis buffer containing 1 mM PMSF and
phosphatase inhibitor was added. The samples were lysed on ice for
30 min, centrifuged at 12,000 × g at 4°C for 15 min and the
supernatant was collected. Protein samples were quantified using a
BCA kit. A 10% separation gel and a 5% concentration gel were
prepared and 20 µg protein per lane used for electrophoresis and
the proteins were transferred onto the PVDF membranes at 4°C and
200 mA for 2 h at room temperature. PVDF membranes were blocked
with 5% skimmed milk in TBS with 0.01% Tween-20 (TBS-T) for 2 h.
Subsequently, the PVDF membranes were incubated overnight at 4°C
with primary antibodies against β-actin (dilution, 1:5,000), OPN
(dilution, 1:2,000), TRAP (dilution, 1:5,000), cathepsin K
(dilution, 1:5,000), PPAR-γ (dilution, 1:1,000), RANK (dilution,
1:1,000), RANKL (dilution, 1:1,000), p38 (dilution, 1:1,000), p-p38
(dilution, 1:1,000), ERK (dilution, 1:1,000), p-ERK (dilution,
1:1,000), JNK (dilution, 1:1,000), p-JNK (dilution, 1:10,000), IκBα
(dilution, 1:1,000), p-IκBα (dilution, 1:1,000), p65 (dilution,
1:1,000), p-p65 (dilution, 1:1,000), c-Fos (dilution, 1:1,000) and
NFATc1 (dilution, 1:1,000). After three washes with TBS-T, the
membranes were incubated with the secondary antibody of IgG
(dilution, 1:5,000) for 2 h at room temperature. Finally, the
protein bands were visualized using an ECL kit and analyzed using
ImageJ 1.8.0 software (National Institutes of Health).
Statistical analysis
Experiments were performed at least three times.
Data were analyzed using SPSS 25.0 software (IBM Corp.). All data
are presented as the mean ± standard deviation. One-way ANOVA
followed by Tukey's post hoc test was performed to compare
differences among groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
ICA inhibits TAA-induced slow growth
of SD rats
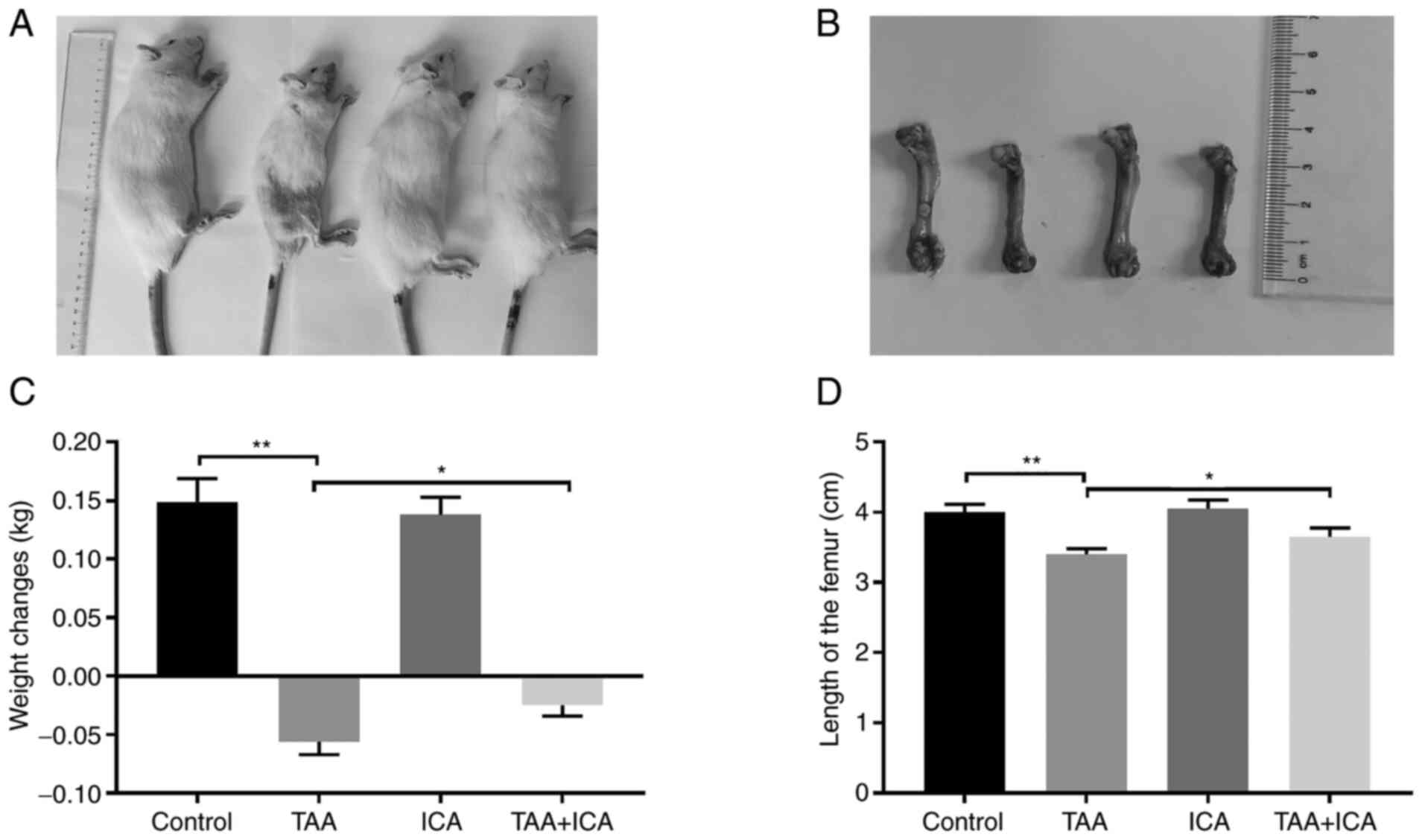
The growth of rats in each group was observed and
recorded. First, the body weight and body length of rats in the TAA
group were markedly reduced, the weight changes (weight
changes=final weight-initial weight) of the TAA group were markedly
reduced compared with the control group and although the weight
changes of the TAA + ICA group were also decreased, the weight
changes in the TAA + ICA group were increased compared with the TAA
group (Fig. 1A and C). The femur
of each group was dissected after euthanasia and it was revealed
that the femoral length in the TAA group was markedly decreased
compared with the control group and the changes were reversed in
the TAA + ICA group (Fig. 1B and
D).
ICA inhibits the TAA-induced imbalance
in serum bone metabolism
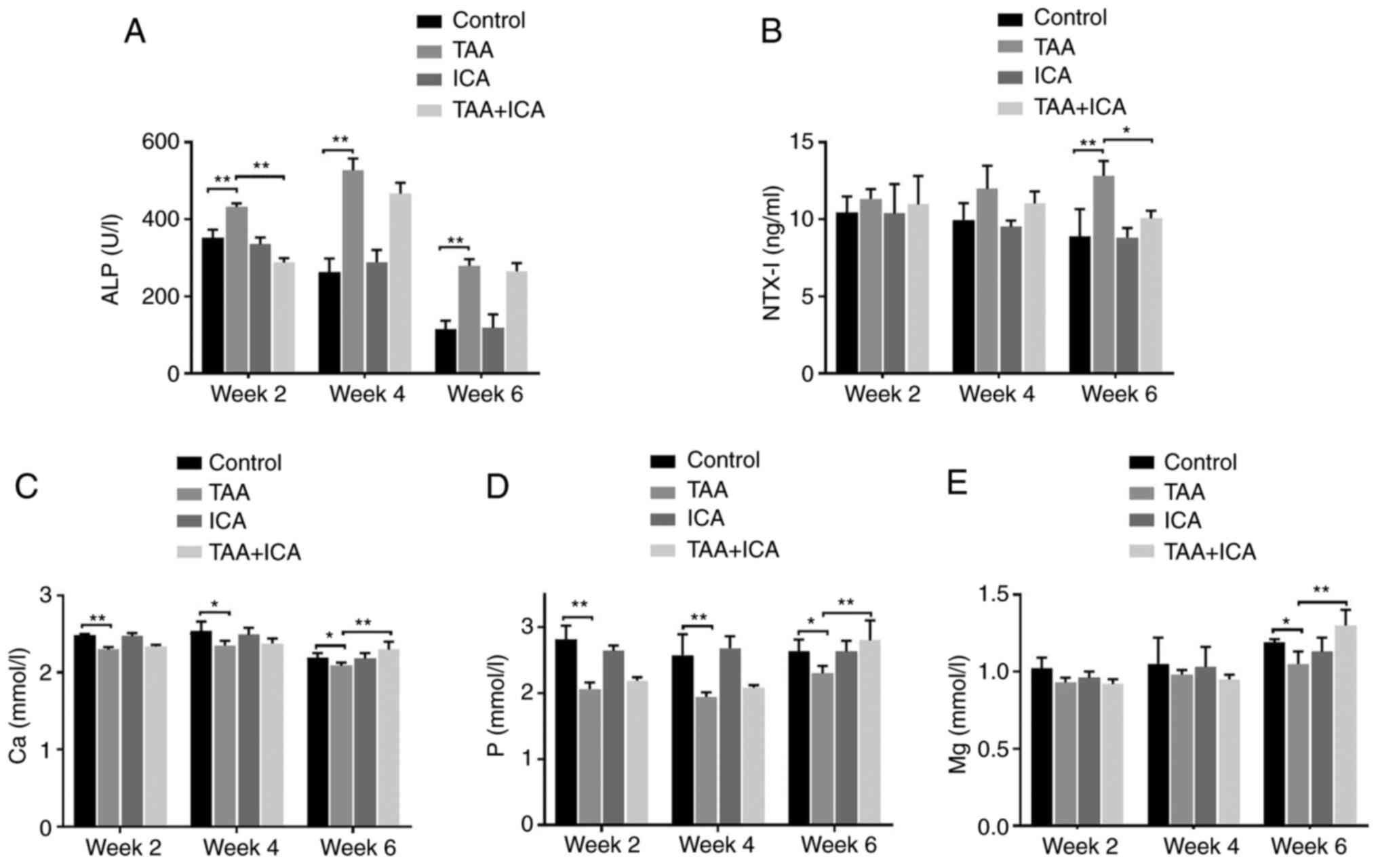
In order to observe the effects of ICA and TAA on
bone metabolism, the serum levels of ALP, NTX–I, Ca, P and Mg were
measured at weeks 2, 4 and 6. Compared with the control group, the
activity of ALP in the TAA group was markedly increased at weeks 2,
4 and 6 and these changes were reversed in the TAA + ICA group at
week 2 (Fig. 2A). Compared with
those of the control group, the levels of NTX–I were markedly
increased in the TAA group at week 6 and the NTX–I levels were
markedly reduced in the TAA + ICA group compared with the TAA group
at week 6 (Fig. 2B). In addition,
compared with those in the control group, the serum Ca, P and Mg
levels were decreased in the TAA group at weeks 2, 4 and 6 and the
TAA + ICA group exhibited a marked recovery at week 6 (Fig. 2C-E).
ICA protects against the TAA-induced
decrease of the bone trabecular area and osteoclastic
differentiation
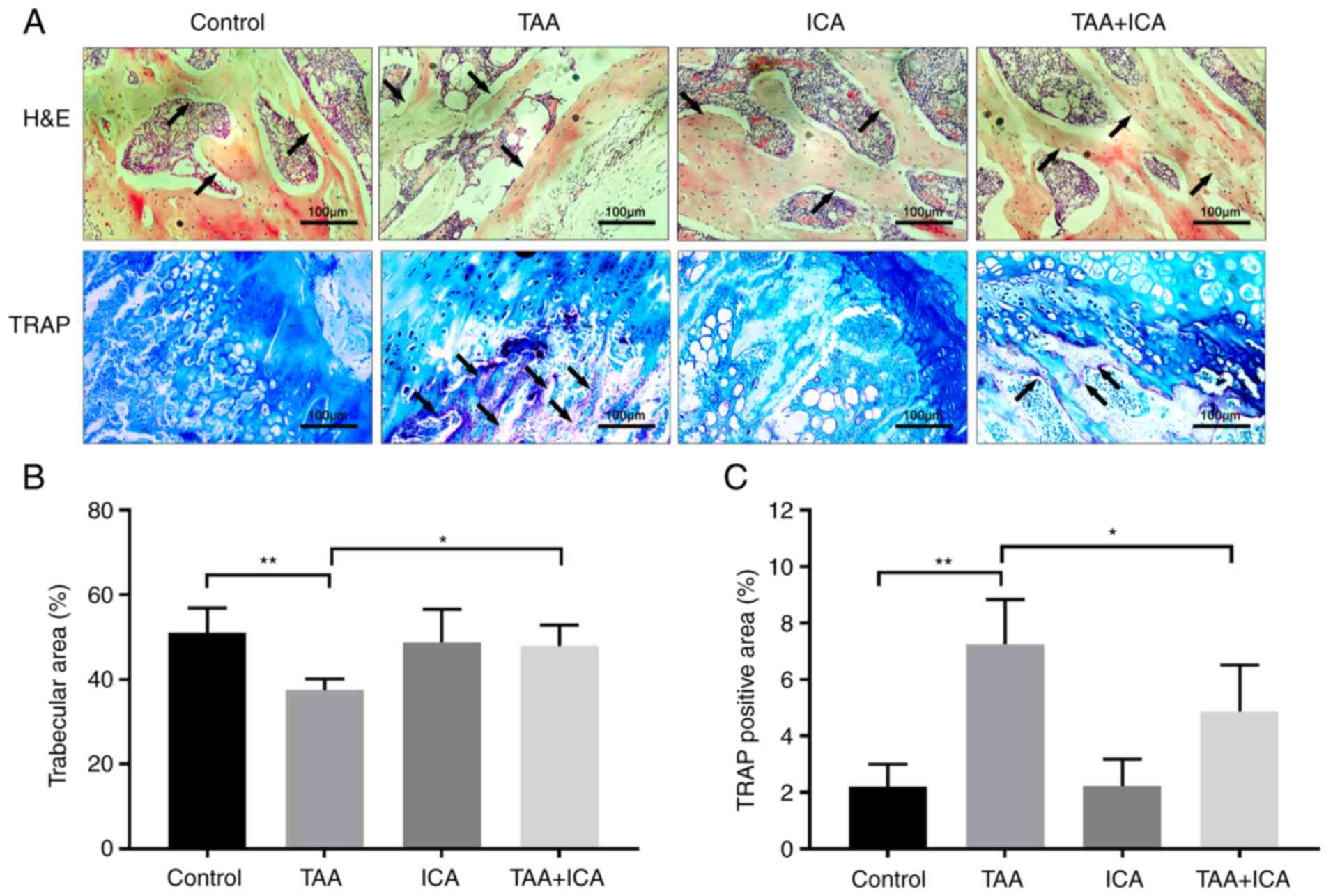
In order to further understand the effect of ICA on
bone tissue injury, pathological analysis of the bone tissue was
performed. H&E staining results demonstrated that the structure
of the bone trabeculae was disordered and the area of bone
trabecula was markedly reduced in the TAA group compared with the
control group and these changes were reversed in the TAA + ICA
group (Fig. 3A and B). TRAP
staining results revealed that there were obvious red deposits in
the TAA group. Compared with those in the TAA group, the positive
deposits in the TAA + ICA group were significantly reduced
(Fig. 3A and C).
ICA inhibits TAA-induced changes in
bone stress
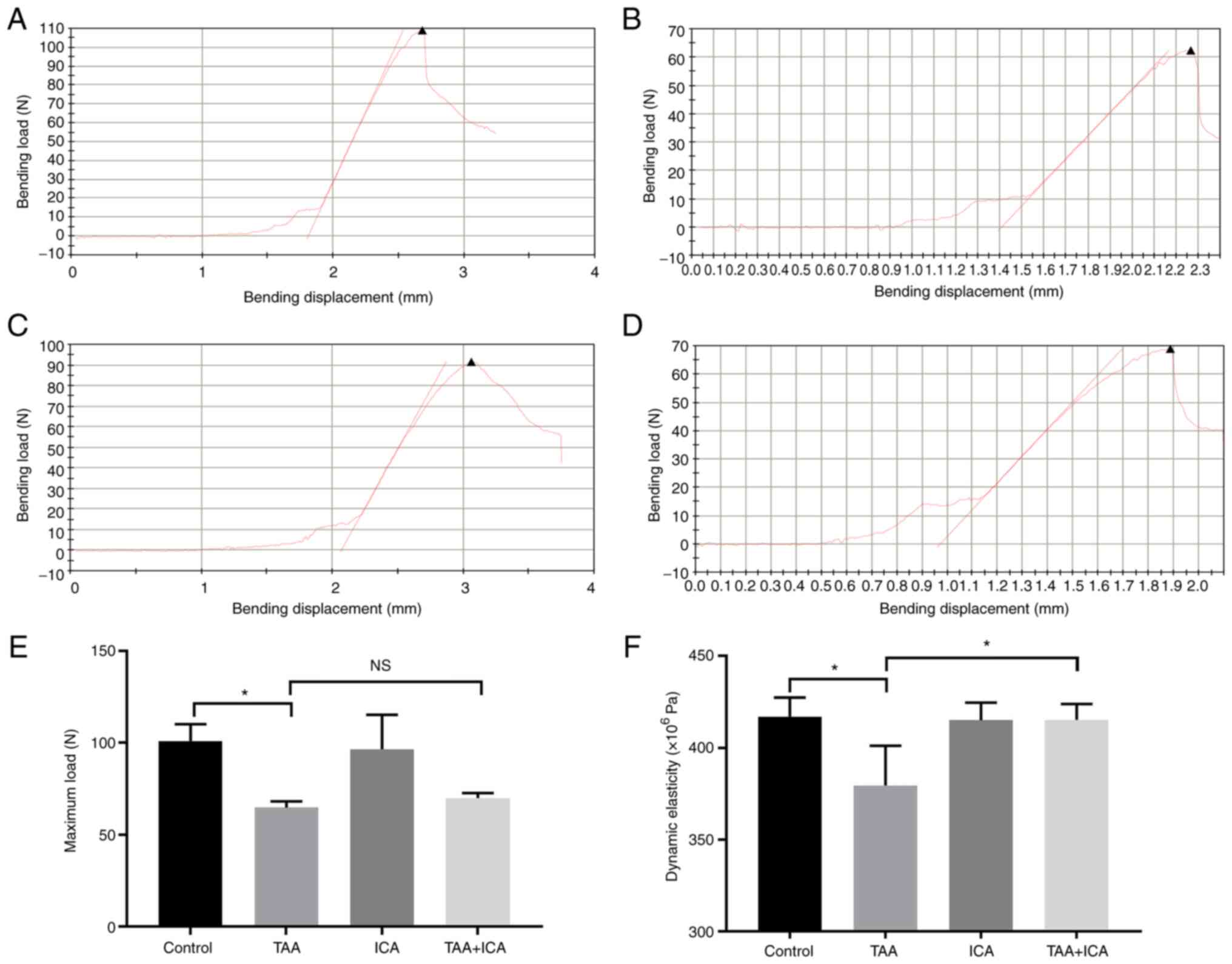
The effect of ICA on the biomechanical strength of
TAA rats was analyzed using a three-point bending experiment. The
load-deformation curves of each group were obtained using a
three-point bending test (Fig.
4A-D). Compared with those of the control group, the elastic
modulus and maximum load of the TAA group were markedly reduced
(Fig. 4E and F). In addition,
compared with that of the TAA group, the elastic modulus of the TAA
+ ICA group was markedly increased (Fig. 4F).
ICA protects against TAA-induced bone
loss
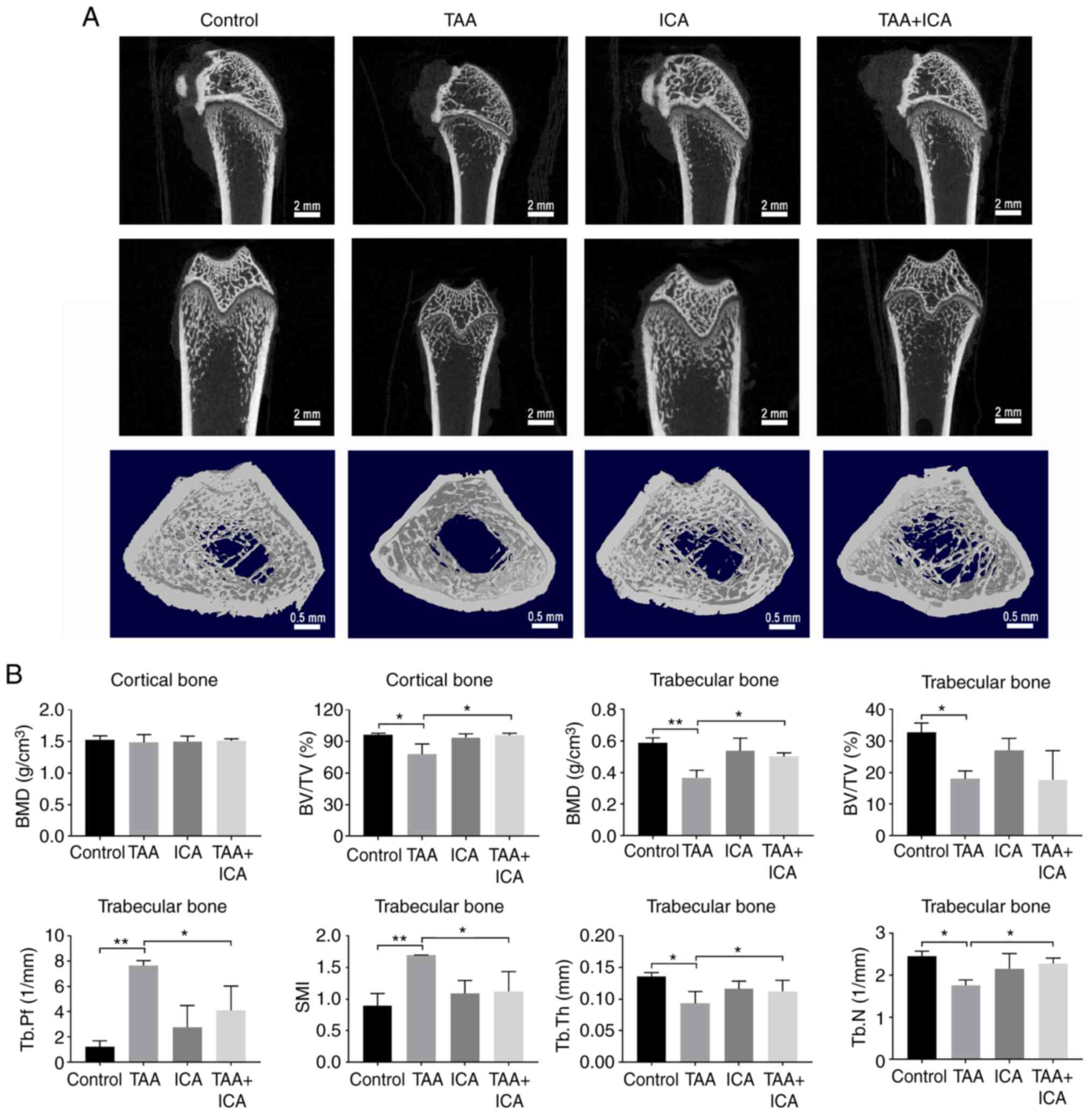
Micro-CT was used to detect the cortical and
trabecular microstructures of the femur. In the images obtained
using a Micro-CT scan, the number of bone trabeculae, cortical
thickness and bone circumference were reduced in the TAA group
compared with the control group and those in the TAA + ICA group
were improved compared with the TAA group (Fig. 5A). In the cortical bone, BV/TV was
markedly reduced in the TAA group compared with the control group
and this change was reversed in the TAA + ICA group. In the bone
trabeculae, the BMD, BV/TV, Tb.Th and Tb.N of the femur were
markedly decreased in the TAA group compared with that in the
control group, the Tb.Pf and SMI of the femur were markedly
increased in the TAA group compared with that in the control group,
and the BMD, Tb.Pf, SMI, Tb.Th and Tb.N were improved in the TAA +
ICA group (Fig. 5B).
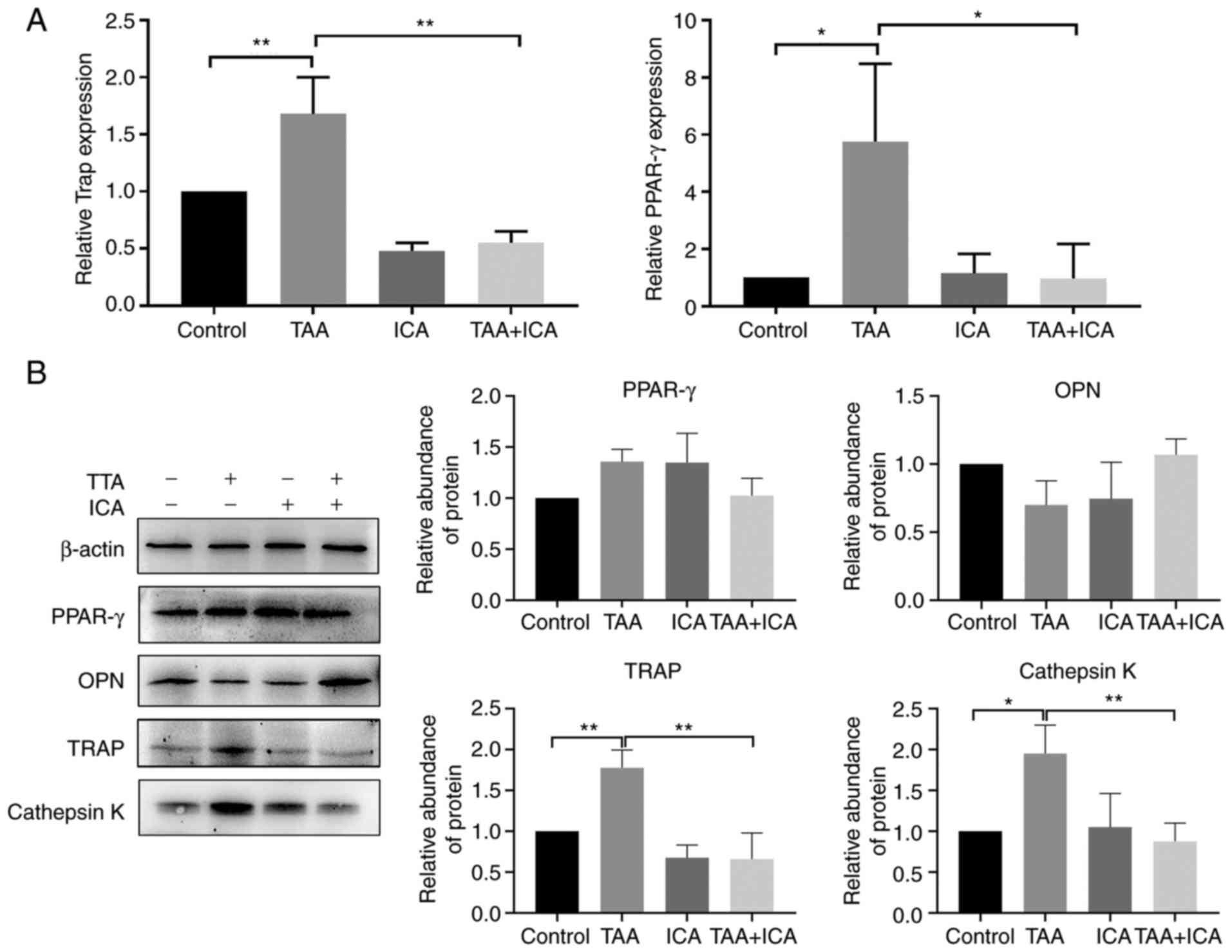
ICA inhibits TAA-induced expression of
osteoclast differentiation-related proteins and genes
To further investigate how ICA affects TAA-induced
bone loss, the effects of ICA on bone resorption-related gene and
protein expression were examined. The results demonstrated that TAA
markedly increased the expression levels of TRAP and PPAR-γ genes,
whereas ICA markedly inhibited the expression of these genes
(Fig. 6A). The results of western
blotting revealed that only the expression levels of
osteoclast-related proteins, TRAP and cathepsin K, were
significantly different in the TAA group compared with the control
group and were decreased in the TAA + ICA group compared with the
TAA group, even returning to the levels in the control group.
Adipocyte-related protein PPAR-γ and osteoblast-related protein OPN
showed no statistical difference among all groups (Fig. 6B).
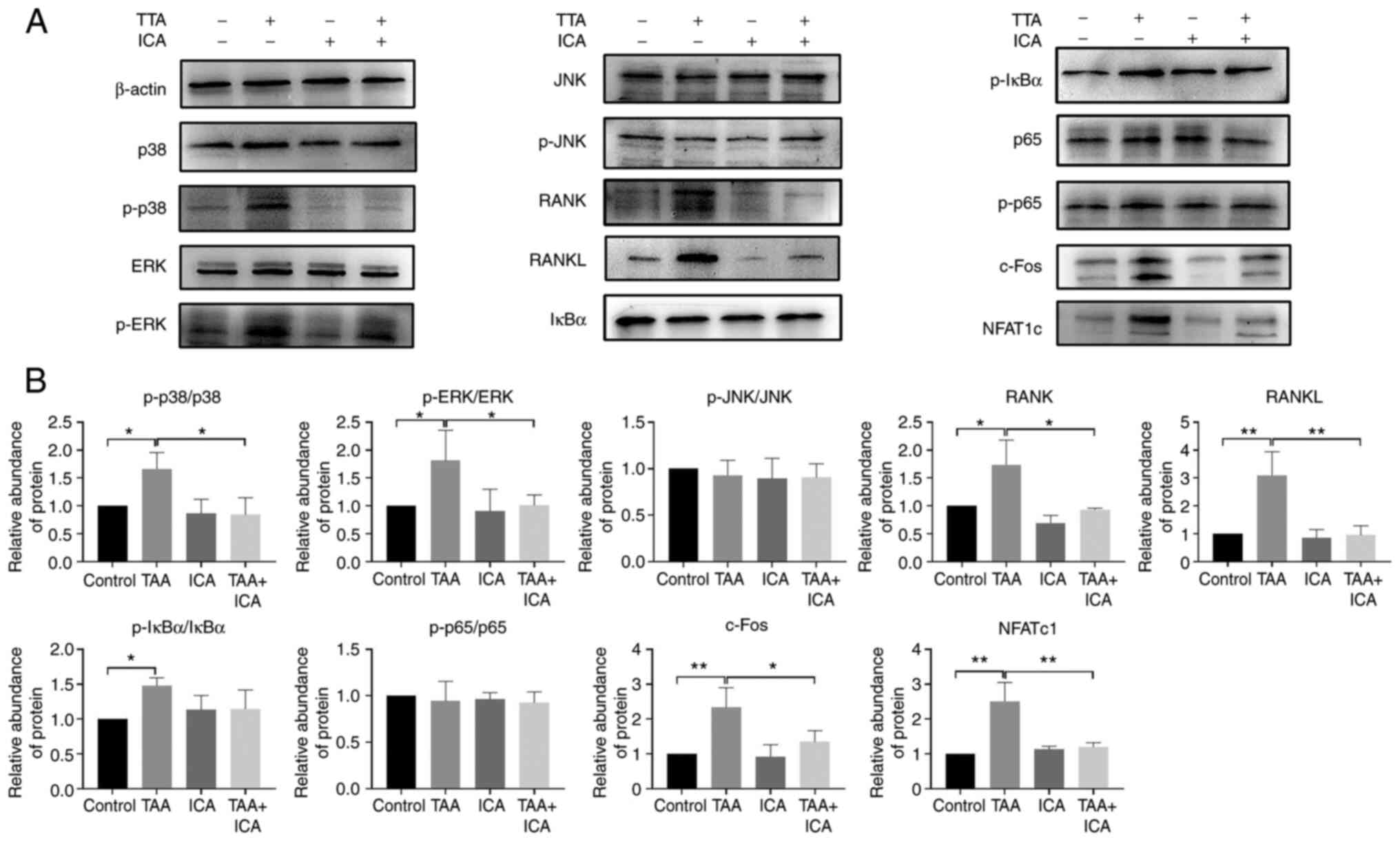
ICA inhibits TAA-induced expression of
RANKL-p38/ERK-NFAT pathway-associated proteins
To explore how ICA affects TAA-induced osteoclast
differentiation, the proteins in osteoclast differentiation-related
signaling pathways were further examined. Compared with the control
group, there was no significant difference in p-JNK and p65 levels
in the TAA and TAA + ICA groups and the levels of p-IκBα were
increased in the TAA group only. The protein expression levels of
RANK, RANKL, p38, ERK, c-Fos and NFATc1 in the TAA group were
increased and the protein expression in the TAA + ICA group was
inhibited to different degrees compared with the TAA group
(Fig. 7).
Discussion
Bone homeostasis is maintained by a balance between
osteoclast-induced bone resorption and osteoblast-induced bone
formation (33). Destruction of
bone remodeling is usually caused by an increase in bone
resorption, or a decrease in both bone formation and resorption,
resulting in an imbalance of bone homeostasis that leads to bone
loss (34). Virtanen et al
(35) reported that after daily
injection of TAA in female rats, the osteoclastic resorption in the
alveolar ridge was increased and there was a persistent Ca
deficiency in horizontal bone. Furthermore, Nakano et al
(25) observed that TAA caused
hepatic osteodystrophy, which was demonstrated by the reduction of
bone volume and bone mass. Therefore, TAA mainly induces
osteoporosis by enhancing the activity of osteoclasts and promoting
bone resorption. Previous studies have demonstrated that ICA
possesses a strong anti-osteoporosis effect and inhibits the
differentiation and formation of osteoclasts (36,37). Therefore, in the present study,
ICA was used as a drug to inhibit TAA-induced bone loss.
Long-term and short-term weight loss is associated
with decreased cortical density, increased cortical porosity and
decreased trabecular density and number (38). ICA is beneficial to bone health
and can improve bone weight loss, bone length and bone diameter
induced by retinoic acid (39).
In the present study, ICA inhibited TAA-induced weight loss and the
reduction of femoral length in rats. In other words, ICA is
beneficial to the normal growth of the body and bone. In addition,
when bone loss occurs, the serum ALP concentration increases and
the Ca, P and Mg concentrations decrease (40,41). This is consistent with the
biochemical results observed following TAA treatment in the present
study, where ICA inhibited the TAA-induced increase of serum ALP
activity and the decrease of the Ca, P and Mg concentrations. The
increased serum ALP activity may be associated with increased bone
turnover status (42).
Osteoclasts release type I collagen and degrade into NTX–I, which
is a representative biochemical marker of bone absorption (43). ICA decreased the levels of NTX–I,
which were upregulated by TAA and alleviated bone resorption.
Bone mass is low and bone microstructure is damaged
in bone loss. BMD or bone mineral content is an important indicator
for clinical diagnosis and evaluation of bone loss (44). As a specific marker enzyme for
osteoclasts, TRAP can be used as an indicator of the
differentiation degree of osteoclasts. There are a large number of
TRAP-positive cells in the femur of osteoporotic animals (45). In the present study, the staining
results demonstrated that ICA inhibited TAA-induced bone structure
disorders, trabecular thinning and the increase of TRAP. At the
same time, the three-point bending test and Micro-CT results also
revealed that the femur endurance was decreased after TAA treatment
and the bone density and other indicators indicated the reduction
of bone mass and bone deterioration, especially in the trabecular
bone, and ICA could inhibit the TAA-induced bone loss. Similarly,
Xu et al (46) demonstrate
that ICA prevents the loss of alveolar bone and microstructural
deterioration caused by estrogen deficiency. Huang et al
(47) revealed that ICA treatment
increased trabecular bone density during glucocorticoid exposure.
This demonstrates that ICA serves an important role in the
improvement of bone structure.
Excessive bone resorption caused by increased
osteoclasts is the key to bone loss. In addition, the increase of
adipogenic differentiation and the decrease of osteogenic
differentiation also lead to the relative increase of osteoclasts,
which leads to the increase of bone resorption (36). In order to explore what type of
osteocyte differentiation is affected by ICA treatment in bone
injury induced by TAA, osteoblast, osteoclast and
adipogenesis-related proteins and mRNAs in the femur were detected
in the present study. It was revealed that TAA caused bone loss
mainly by promoting the expression of osteoclast-related proteins,
namely TRAP and cathepsin K, indicating that TAA caused bone loss
by promoting osteoclast differentiation.
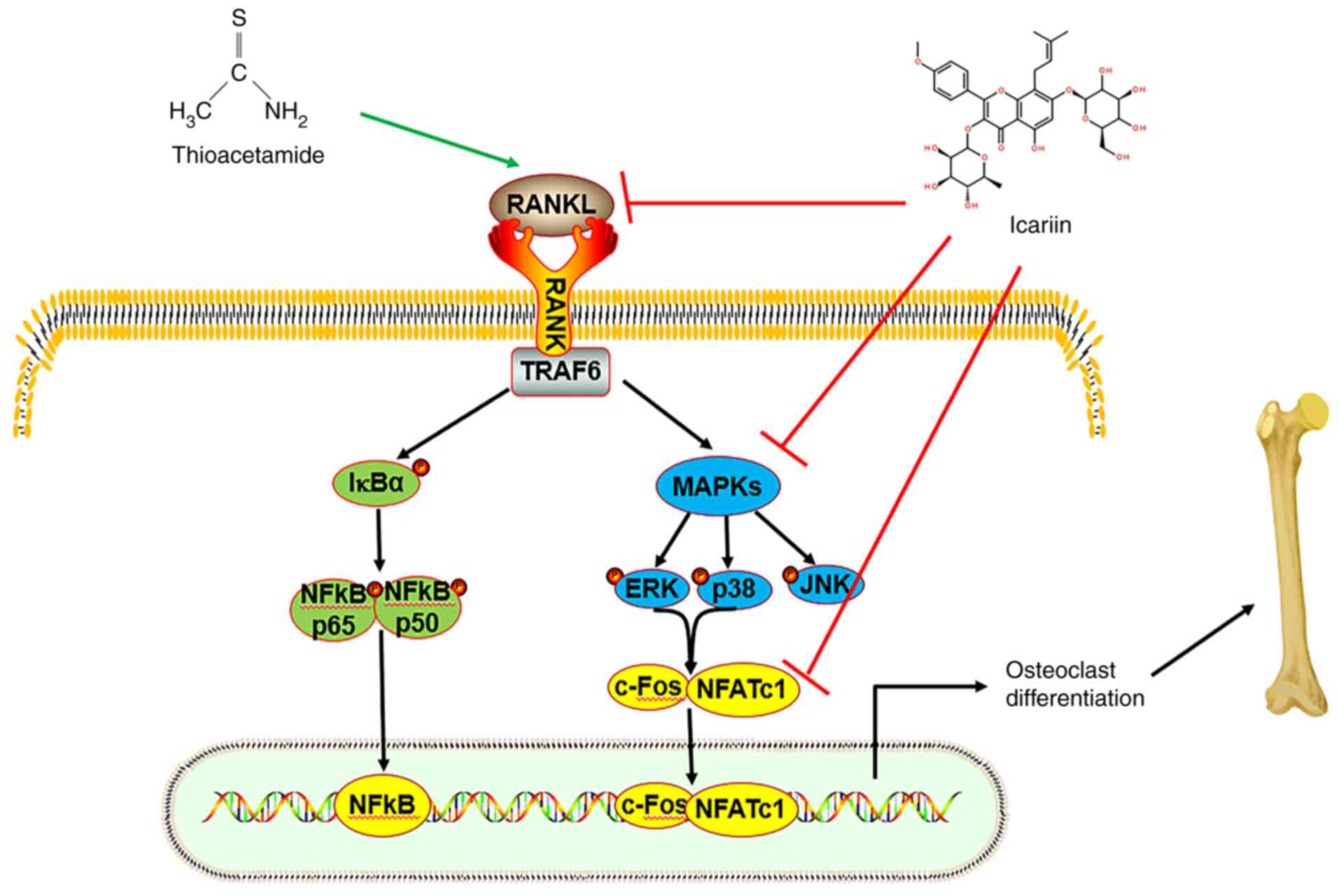
RANKL binds to RANK on the surface of osteoclast
precursor cells to recruit TNF receptor associated factor 6 (TRAF6)
and the RANKL-RANK-TRAF6 complex forms and activates NF-κB, MAPKs
(ERK, JNK, p38) and NFATc1 signaling pathways to jointly promote
osteoclast differentiation and function (48). In the present study, ICA inhibited
TAA-induced phosphorylation of p38 and ERK but had no effect on JNK
in MAPK kinase. Additionally, ICA inhibited the expression of
RANK/RANKL protein induced by TAA and RANKL is a key stimulator of
osteoclasts (49), suggesting
that ICA and TAA may have antagonistic effects on the binding of
RANKL protein. In addition, the activity of p65 was not affected by
TAA and ICA did not inhibit the effect of TAA through NF-κB
signaling, although the phosphorylation of IκBα was activated. ICA
markedly inhibited the expression of c-Fos and NFATc1 induced by
TAA. Therefore, ICA downregulated the inhibitory activity of NFATc1
and its anti-TAA-induced osteoclast activity might be mediated via
inhibition of the activation of RANKL, P38/ERK and NFATc1 signaling
pathways. It has been reported that ICA, the main active component
of Epimedium, has an anti-osteoporosis effect (50). Feng et al (51) demonstrated that ICA activates ERK
signaling through estrogen receptor and inhibited
glucocorticoid-induced apoptosis of bone cells, demonstrating that
ICA may inhibit osteoclast differentiation induced by TAA via the
RANKL-p38/ERK-NFAT signaling pathway.
To the best of the authors' knowledge, the bone loss
model induced by TAA has not so far been reported. Identifying ICA
for the treatment of this model provides ideas for studying the
mechanism of bone loss. However, the present study also has some
limitations; the molecular pattern of ICA and TAA on the femur and
its effect on other bones other than the femur remain to be
elucidated. The model of bone injury induced by TAA cannot
completely simulate clinical osteoporosis caused by long-term
effects. It can mainly be used to quickly establish a bone loss
model and provide a warning regarding bone damage in humans exposed
to TAA for a long time. In order to further understand the
molecular mechanism of TAA-induced bone injury, further cytological
experiments will be conducted in the future.
In conclusion, excessive osteoclast differentiation
led to an imbalance of bone metabolism, resulting in bone loss and
even osteoporosis. Finding effective drugs for bone injury and
studying the mechanism of action are of great significance for the
prevention, treatment and basic research of diseases. In the
current study, the results demonstrated that ICA could improve the
bone injury induced by TAA by inhibiting osteoclast
differentiation. ICA inhibited TAA-induced osteoclast
differentiation by downregulating the RANKL-p38/ERK-NFAT signaling
pathway (Fig. 8). The
RANKL-p38/ERK-NFAT signaling pathway is a crucial pathway affecting
osteoclasts and its alteration demonstrated the importance of ICA
in TAA-induced osteoclast differentiation. These results are
helpful for understanding the anti-resorption activity and
molecular mechanism of ICA and provide novel ideas for the
treatment of destructive bone diseases caused by osteolysis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Zhejiang Province (grant no. LY19H060001), the
Traditional Chinese Medicine Science and Technology Plan of
Zhejiang Province (grant no. 2022ZB093) and the Zhejiang University
Student Science and Technology Innovation Activity Plan (grant no.
2020R410056).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LC, XJ and HS performed the experiments. LC and HS
wrote the manuscript. BX, LC and XC analyzed and interpreted the
data. JC and JX contributed to the study design. BX and JX
supervised the study and revised the manuscript. LC and JX confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Animal Ethical
and Welfare Committee of Zhejiang Chinese Medical University
(approval no. IACUC-20191223-07; Zhejiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Genant HK, Cooper C, Poor G, Reid I,
Ehrlich G, Kanis J, Nordin BE, Barrett-Connor E, Black D, Bonjour
JP, et al: Interim report and recommendations of the world health
organization task-force for osteoporosis. Osteoporosis Int.
10:259–264. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bawa S: The significance of soy protein
and soy bioactive compounds in the prophylaxis and treatment of
osteoporosis. J Osteoporosis. 2010:8910582010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kenkre JS and Bassett J: The bone
remodelling cycle. Ann Clin Biochem. 55:308–327. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang YY, Wang ZH, Deng LH, Wang H and
Zheng Q: Oral administration of quercetin or its derivatives
inhibit bone loss in animal model of osteoporosis. Oxid Med Cell
Longev. 2020:1–21. 2020. View Article : Google Scholar
|
|
5
|
Tanaka S: RANKL-Independent
osteoclastogenesis: A long-standing controversy. J Bone Miner Res.
32:431–433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Z, Zhang M, Shen Z, Ke J, Zhang D and
Yin F: Efficacy and safety of 18 anti-osteoporotic drugs in the
treatment of patients with osteoporosis caused by glucocorticoid: A
network meta-analysis of randomized controlled trials. PLoS One.
15:e02438512020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Looker AC, Johnston CC Jr, Wahner HW, Dunn
WL, Calvo MS, Harris TB, Heyse SP and Lindsay RL: Prevalence of low
femoral bone density in older U.S. adults from NHANES III. J Bone
Miner Res. 10:796–802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma XQ, Han T, Zhang X, Wu JZ, Rahman K,
Qin LP and Zheng CJ: Kaempferitrin prevents bone lost in
ovariectomized rats. Phytomedicine. 22:1159–1162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hwang YH, Ha H, Kim R, Cho CW, Song YR,
Hong HD and Kim T: Anti-osteoporotic effects of polysaccharides
isolated from persimmon leaves via osteoclastogenesis inhibition.
Nutrients. 10:9012018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Z, Zhao Q, Liu T, Zhao H, Wang R, Li
H, Zhang Y, Shan L, He B, Wang X, et al: Effect of Vicenin-2 on
ovariectomy-induced osteoporosis in rats. Biomed Pharmacother.
129:1104742020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Topolska K, Radzki RP,
Filipiak-Florkiewicz A, Florkiewicz A, Leszczyńska T and Cieślik E:
Fructan-enriched diet increases bone quality in female growing rats
at calcium deficiency. Plant Foods Hum Nutr. 73:172–179. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Chen J, Chen J, Dong C, Yan X, Zhu
Z, Lu P, Song Z, Liu H and Chen S: Daphnetin ameliorates
glucocorticoid-induced osteoporosis via activation of
Wnt/GSK-3β/β-catenin signaling. Toxicol Appl Pharm. 409:1153332020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Komori T: Animal models for osteoporosis.
Eur J Pharmacol. 759:287–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lassila V and Virtanen P: Influence of
experimental liver injury on rat blood and alveolar bone under
stress. Acta Anat (Basel). 118:116–121. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao YT, Lu XR, Li Y, Zhou YK, Ren J and Xu
J: The new toxic effect of cortical bone osteoporosis induced by
thioacetamide. J Toxicol. 33:370–374. 2019.
|
|
16
|
Liang X, Hou Z, Xie Y, Yan F, Li S, Zhu X
and Cai L: Icariin promotes osteogenic differentiation of bone
marrow stromal cells and prevents bone loss in OVX mice via
activating autophagy. J Cell Biochem. 120:13121–13132. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang GQ, Li DD, Huang C, Lu DS, Zhang C,
Zhou S, Liu J and Zhang F: Icariin reduces dopaminergic neuronal
loss and microglia-mediated inflammation in vivo and in vitro.
Front Mol Neurosci. 10:4412018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song L, Chen X, Mi L, Liu C, Zhu S, Yang
T, Luo X, Zhang Q, Lu H and Liang X: Icariin-induced inhibition of
SIRT6/NF-κB triggers redox mediated apoptosis and enhances
anti-tumor immunity in triple-negative breast cancer. Cancer Sci.
111:4242–4256. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nian H, Ma MH, Nian SS and Xu LL:
Antiosteoporotic activity of icariin in ovariectomized rats.
Phytomedicine. 16:320–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Tao Y, Ping Z, Zhang W, Hu X, Wang
Y, Wang L, Shi J, Wu X, Yang X, et al: Icariin attenuates
titanium-particle inhibition of bone formation by activating the
Wnt/β-catenin signaling pathway in vivo and in vitro. Sci Rep.
6:238272016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim B, Lee KY and Park B: Icariin
abrogates osteoclast formation through the regulation of the
RANKL-mediated TRAF6/NF-κB/ERK signaling pathway in Raw264.7 cells.
Phytomedicine. 51:181–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jing X, Du T, Chen K, Guo J, Xiang W, Yao
X, Sun K, Ye Y and Guo F: Icariin protects against iron
overload-induced bone loss via suppressing oxidative stress. J Cell
Physiol. 234:10123–10137. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hassanshahi M, Su YW, Khabbazi S, Fan CM,
Tang Q, Wen X, Fan J, Chen KM and Xian CJ: Icariin attenuates
methotrexate chemotherapy-induced bone marrow microvascular damage
and bone loss in rats. J Cell Physiol. 234:1–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schyman P, Printz RL, Estes SK, Boyd KL,
Shiota M and Wallqvist A: Identification of the toxicity pathways
associated with thioacetamide-induced injuries in rat liver and
kidney. Front Pharmacol. 9:12722018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakano A, Kanda T and Abe H: Bone changes
and mineral metabolism disorders in rats with experimental liver
cirrhosis. J Gastroenterol Hepatol. 11:1143–1154. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Shwiniy WH and Sadeek SA: Synthesis and
characterization of new 2-cyano-2-(p-tolyl-hydrazono)-thioacetamide
metal complexes and a study on their antimicrobial activities.
Spectrochimica Acta A Mol Biomol Spectrosc. 137:535–546. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao M, Wang M and Zheng QH: Synthesis of
carbon-11-labeled imidazopyridine- and purine-thioacetamide
derivatives as new potential PET tracers for imaging of nucleotide
pyrophosphatase/phosphodiesterase 1 (NPP1). Bioorg Med Chem Lett.
26:1371–1375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khattab A, Hassanin L and Zaki N:
Self-nanoemulsifying drug delivery system of coenzyme (Q10) with
improved dissolution, bioavailability, and protective efficiency on
liver fibrosis. AAPS PharmSciTech. 18:1657–1672. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Han B, Wei Y, Jing J and Li J:
Icariin promotes fracture healing in ovariectomized rats. Med Sci
Monit. 26:e9245542020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clark JA and Sun D: Guidelines for the
ethical review of laboratory animal welfare People's Republic of
China National Standard GB/T 35892-2018 [Issued 6 February 2018
Effective from 1 September 2018]. Animal Model Exp Med. 3:103–113.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng Q, Lu W, Deng Z, Wu J and Xu X:
Tablysin-15 inhibits osteoclastogenesis and LPS-induced bone loss
via attenuating the integrin αvβ3 pathway. Chem Biol Interact.
327:1091792020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Z, Irie N, Takada Y, Shimoda K,
Miyamoto T, Nishiwaki T, Suda T and Matsuo K: Bidirectional
ephrinB2-EphB4 signaling controls bone homeostasis. Cell
Metabolism. 4:111–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Armas LA and Recker RR: Pathophysiology of
osteoporosis: New mechanistic insights. Endocrinol Metab Clin North
Am. 41:475–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Virtanen P and Lassila V: Influence of
thioacetamide-provoked liver injury on female rat blood and
alveolar bone under stress. Acta Anat (Basel). 127:285–289. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qi S, He J, Zheng H, Chen C and Lan S:
Icariin prevents diabetes-induced bone loss in rats by reducing
blood glucose and suppressing bone turnover. Molecules.
24:18712019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cui J, Zhu M, Zhu S, Wang G, Xu Y and Geng
D: Inhibitory effect of icariin on Ti-induced inflammatory
osteoclastogenesis. J Surg Res. 192:447–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu CT, Shivani S, Xu H, McLean RR, Broe
KE, Hannan MT, Boyd SK, Bouxsein ML, Kiel DP and Samelson EJ:
Long-term and recent weight change are associated with reduced
peripheral bone density, deficits in bone microarchitecture, and
decreased bone strength: The framingham osteoporosis study. J Bone
Miner Res. 33:1851–1858. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oršolić N, Nemrava J, Jeleč Ž, Kukolj M,
Odeh D, Terzić S, Fureš R, Bagatin T and Bagatin D: The beneficial
effect of proanthocyanidins and icariin on biochemical markers of
bone turnover in rats. Int J Mol Sci. 19:27462018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang J, Fu Q, Ren Z, Wang Y, Wang C, Shen
T, Wang G and Wu L: Changes of serum cytokines-related Th1/Th2/Th17
concentration in patients with postmenopausal osteoporosis. Gynecol
Endocrinol. 31:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang W, Zhang Y, Yang J, Tan L and Yang K:
Potential antiosteoporosis effect of biodegradable magnesium
implanted in STZ-induced diabetic rats. J Biomed Mater Res A.
99:386–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jing Z, Wang C, Yang Q, Wei X, Jin Y, Meng
Q, Liu Q, Liu Z, Ma X, Liu K, et al: Luteolin attenuates
glucocorticoid-induced osteoporosis by regulatingERK/Lrp-5/GSK-3β
signaling pathway in vivo and in vitro. J Cell Physiol.
234:4472–4490. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dénarié D, Constant E, Thomas T and
Marotte H: Could biomarkers of bone, cartilage or synovium turnover
be used for relapse prediction in rheumatoid arthritis patients?
Mediators Inflamm. 2014:5373242014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Feng M, Zhang RR, Gong F, Yang P, Fan L,
Ni J, Bi W, Zhang Y, Wang C and Wang K: Protective effects of
necrostatin-1 on glucocorticoid-induced osteoporosis in rats. J
Steroid Biochem Mol Biol. 144:455–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu X, Fan JB, Hu J, Li F, Yi R, Tan F and
Zhao X: Lactobacillus Fermentum ZS40 prevents secondary
osteoporosis in wistar rat. Food Sci Nutr. 8:5182–5191. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu H, Zhou S, Qu R, Yang Y, Gong X, Hong
Y, Jin A, Huang X, Dai Q and Jiang L: Icariin prevents oestrogen
deficiency-induced alveolar bone loss through promoting
osteogenesis via STAT3. Cell Prolif. 53:e127432020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang M, Wang Y and Peng R: Icariin
alleviates glucocorticoid-induced osteoporosis through
EphB4/Ephrin-B2 axis. Evid Based Complement Alternat Med.
2020:29824802020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guo J, Ren R, Sun K, Yao X, Lin J, Wang G,
Guo Z, Xu T and Guo F: PERK controls bone homeostasis through the
regulation of osteoclast differentiation and function. Cell Death
Dis. 11:8472020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Boyce BF: Advances in the regulation of
osteoclasts and osteoclast functions. J Dent Res. 92:860–867. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen KM, Ge BF, Liu XY, Ma PH, Lu MB, Bai
MH and Wang Y: Icariin inhibits the osteoclast formation induced by
RANKL and macrophage-colony stimulating factor in mouse bone marrow
culture. Pharmazie. 62:388–391. 2007.PubMed/NCBI
|
|
51
|
Feng R, Feng L, Yuan Z, Wang D, Wang F,
Tan B, Han S, Li T, Li D and Han Y: Icariin protects against
glucocorticoid-induced osteoporosis in vitro and prevents
glucocorticoid-induced osteocyte apoptosis in vivo. Cell Biochem
Biophys. 67:189–197. 2013. View Article : Google Scholar : PubMed/NCBI
|