Introduction
Polycystic ovary syndrome (PCOS) is a prevalent and
heterogeneous endocrine and metabolic gynecological disorder, which
is also one of the most common causes of female infertility
(1). PCOS affects 5–10% women of
childbearing age globally and is characterized by hyperandrogenism,
polycystic ovaries, irregular menstruation or amenorrhea, hirsutism
and acne (2,3). In the absence of appropriate
therapeutic intervention, PCOS increases the risk of acne scars,
obesity, dyslipidemia, type 2 diabetes, cardiovascular disease and
endometrial cancer (4,5). At present, available PCOS treatment
strategies mainly focus on lifestyle changes, maintaining a healthy
diet and chemotherapeutic drugs, such as metformin, oral
contraceptive pills and spironolactone (6,7).
However, the mechanism of PCOS pathogenesis is complex and remains
to be fully elucidated (8).
Therefore, it is of importance to understand the mechanisms
underlying PCOS to provide novel insights into treatment
strategies.
Granulosa cells are somatic cells of the sex cord
and are closely associated with the development of oocytes
(9). There is accumulating
evidence that dysfunction of ovarian granulosa cells is an
important contributing factor of PCOS (10,11). In addition, previous studies have
also reported that high homocysteine (Hcy) levels, a
sulfur-containing amino acid that has also been reported to be
associated with insulin resistance and sex hormone level disorders
(12), may be a significant
triggering factor of PCOS (13,14).
Ferroptosis is a mechanism of cell death that is
regulated by iron and oxidative stress that is characterized by
increases in iron (Fe2+) content and lipid peroxidation
(15,16) 4. Increased ferroptosis levels
observed in granulosa cells is considered to be part of an
important signaling pathway and mechanism in PCOS pathogenesis
(17). High concentrations of Hcy
has been documented to induce oxidative stress and ferroptosis in
the nucleus pulposus by promoting methylase expression and
glutathione (GSH) peroxidase 4 (GPX4) methylation, which prevent
ferroptosis by converting lipid hydroperoxides into non-toxic lipid
alcohols (18,19). Ten-eleven translocation (TET) 1
and TET2 are two dioxygenases that serve an important role in
demethylating DNA sequences (20). However, their role in ovarian
granulosa cell injury during PCOS remain to be elucidated.
Therefore, the relationship among high Hcy levels, granulosa cell
ferroptosis, TET activity and DNA methylation in PCOS pathology
warrant further exploration.
Ferrostatin-1 (Fer-1) was the first aromatic amine
that can effectively suppress ferroptosis, inhibit lipid peroxide
accumulation and protect cells against numerous types of stress
and/or toxic chemicals, such as erastin-induced cancer cell death
and glutamate-induced neurotoxicity (21). Previous studies have reported the
beneficial effects of Fer-1 on a number of diseases, such as
dopaminergic neuroblastoma and cisplatin-induced acute kidney
injury (AKI) by protecting against reactive oxygen species (ROS),
apoptosis and ferroptosis (22,23). In the present study, ovarian
granulosa cells treated with Hcy were used to investigate the
effects of extracellular Fer-1 treatment on apoptosis, oxidative
stress and ferroptosis. The mechanisms of TET activity and DNA
methylation were also explored. The present study may provide a
basis for the use of Fer-1 as a therapeutic agent for PCOS.
Materials and methods
Cell culture and treatment
The human ovarian granulosa KGN cell line was
obtained from the American Type Culture Collection and maintained
in DMEM containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C with 5% CO2. It has been reported that Hcy
levels may be a significant triggering factor of PCOS, where high
concentrations of Hcy can induce oxidative stress and ferroptosis
(12,18). In the present study, KGN cells
were treated with various concentrations of Hcy (0.25, 0.5, 1, 2
and 4 mM, Shanghai Bang Jing Industrial Co., Ltd.; cat. no.
BJ-S964101) with or without 5, 10 and 20 µM Fer-1 (APExBIO
Technology LLC.; cat. no. A4371) for 24 h (24,25) at room temperature. KGN cells
treated with 1 mM Hcy only were used as the model group. The
HcY-induced cell models were divided into two groups, one group was
added with 10 µM fer-1, the other group was added sequentially with
both 10 µM Fer-1 and 40 nm final concentration of 10 µM TET1/2
inhibitor Bobcat339 hydrochloride (cat. no. T5198, TargetMol
Chemicals Inc.). Both groups were incubated at 37°C for 24 h.
Cell viability assay
Cell viability was evaluated using a Cell Counting
Kit-8 (CCK-8) assay. Cells were seeded into 96-well plates at a
density of 6×103 cells/well. Following treatment with
Hcy (0.25, 0.5, 1, 2 and 4 mM) and/or Fer-1 (5, 10 and 20 µM) for
6, 12, 24 and 48 h at 37°C, cells were incubated with CCK-8 reagent
(10 µl/well; cat. no. HY-K0301, MedChemExpress LLC.) for 2 h at
37°C with 5% CO2. Absorbance of samples was examined at
450 nm using a microliter plate reader (Thermomax Molecular
Devices).
Cell apoptosis assay
The TUNEL Staining kit (Beyotime Institute of
Biotechnology) was used to detect cell apoptosis. Briefly, cells at
a density of 2×106 were incubated with 4%
paraformaldehyde for 30 min at room temperature, followed by
treatment with 0.5% Triton X-100. Cells were subsequently incubated
with 50 µl TUNEL reaction buffer for 1 h at 37°C. Nuclei were
stained with DAPI (cat. no. C1005; Beyotime) at a concentration 5
µg/ml for 5 min at room temperature. The apoptosis-positive cells
were indicated by green staining. Cells were washed 3 times with
PBS for 3–5 min each and blocked with Antifade Mounting Medium
(cat. no. C1005, P0126-5 ml; Beyotime). The images from randomly
selected 5 fields were visualized and captured using a fluorescence
microscope (magnification, ×200; Olympus Corporation).
Oxidative stress-related chemical
quantification
Following cell treatment, the KGN cell culture
supernatant was collected to determine the ROS, malondialdehyde
(MDA), lactate dehydrogenase (LDH) and reduced GSH levels using
specific commercial assay kits of ROS (cat. no. E004-1-1), MDA
(cat. no. A003-1), LDH (cat. no. A020-2) and GSH (cat. no.
A006-2-1) purchased from Nanjing Jiancheng Bioengineering Institute
as per the procedures of manufacturer.
Fe2+ level analysis
Cellular Fe2+ levels were measured using
the Phen Green™ SK reagent (cat. no. P14313, Invitrogen;
Thermo Fisher Scientific, Inc.) at a final concentration of 50 µM
according to the manufacturer's protocol. Briefly, Phen
Green™ SK reagent was added to 2 ml cell-containing
medium at 37°C for 1 h. The fluorescence response to
Fe2+ ions was examined using a confocal laser scanning
microscope (magnification, ×400; Zeiss LSM510; Carl Zeiss AG).
Fluorescence randomly selected 5 fields was determined by an
Image-Pro Premier software (Version 9.2; Media Cybemetics) at
excitation and emission wavelengths of 488 and 520 nm,
respectively.
TET1/2 secretion levels and methylated
DNA quantification
ELISA was used to examine the secretion levels of
TET1 and TET2 in the cell culture supernatant according to the
manufacturer's protocols. These ELISA kits of TET1 (JC-E8295) and
TET2 (JLC-G4351) were purchased from Gelatins Biological Reagent
Co. A microplate reader was used to detect the optical density at
450 nm. DNA samples were isolated by PowerSoil DNA isolation kit
(cat. no. 12888-50; Anbiosci Tech, Ltd.) and the the level of DNA
methylation was detected by Methylight with a MethylFlash
Methylated DNA Quantification kit (cat. no. P-1035, EpiGentek Group
Inc.) according to the manufacturer's protocol. The results were
normalized with the control group.
Western blot analysis
Cells were harvested using RIPA buffer (Beyotime
Institute of Biotechnology) and protein concentrations were
quantified using a Bicinchoninic Acid Protein kit (Beyotime
Institute of Biotechnology). Protein separation (20 µg) was
performed by 10% SDS-PAGE followed by transfer onto PVDF membranes
(EMD Millipore). The membranes were blocked in 5% skim milk at 25°C
for 1 h, and then probed with the corresponding primary antibodies
overnight at 4°C. The HRP-conjugated secondary antibody (cat. no.
7074P2; 1:2,000; Cell Signaling Technology, Inc.) was added the
next day and incubated at room temperature for 1 h. Blot images
were captured using Pierce™ ECL Western Blotting
Substrate (cat. no. 32209; Thermo Fisher Scientific, Inc.). GAPDH
was the internal control and protein band images were analyzed
using the Image J software (Version 1.8.0.172; National Institutes
of Health). The relative intensity for cleaved caspase-3/caspase-3
was divided by the cleaved caspase-3 value by the value of
caspase-3. The relative intensity for other proteins was divided by
the corresponding target value by the value of GAPDH.
Anti-Bax (cat. no. 5023T; 1:1,000), anti-Bcl-2 (cat.
no. 4223T; 1:1,000), anti-cleaved caspase-3 (cat. no. 9661T;
1:1,000), anti-caspase-3 (cat. no. 14220T; 1:1,000), anti-solute
carrier family 7 member 11 (SLC7A11; cat. no. 12691S; 1:1,000) and
anti-GAPDH (cat. no. 5174T; 1:1,000) antibodies were provided by
Cell Signaling Technology, Inc. Anti-GPX4 (cat. no. sc-166570;
1:500) and anti-achaete-scute family BHLH transcription factor 4
(ASCL4; cat. no. sc-365230; 1:500) antibodies were purchased from
Santa Cruz Biotechnology, Inc. Anti-divalent metal transporter 1
(DMT1; cat. no. 20507-1-AP; 1:1,000) antibody was the product of
Proteintech Group, Inc.
Statistical analysis
All statistical analyzes were performed using the
GraphPad Prism 8.0 software (GraphPad Software, Inc.). Data from ≥
three independent experiments was used. Data are presented as the
mean ± SD. An unpaired t-test and one way ANOVA followed by Tukey's
post hoc test were performed to statistically compare two or ≥
three independent groups, respectively. P<0.05 was considered to
indicate a statistically significant difference.
Results
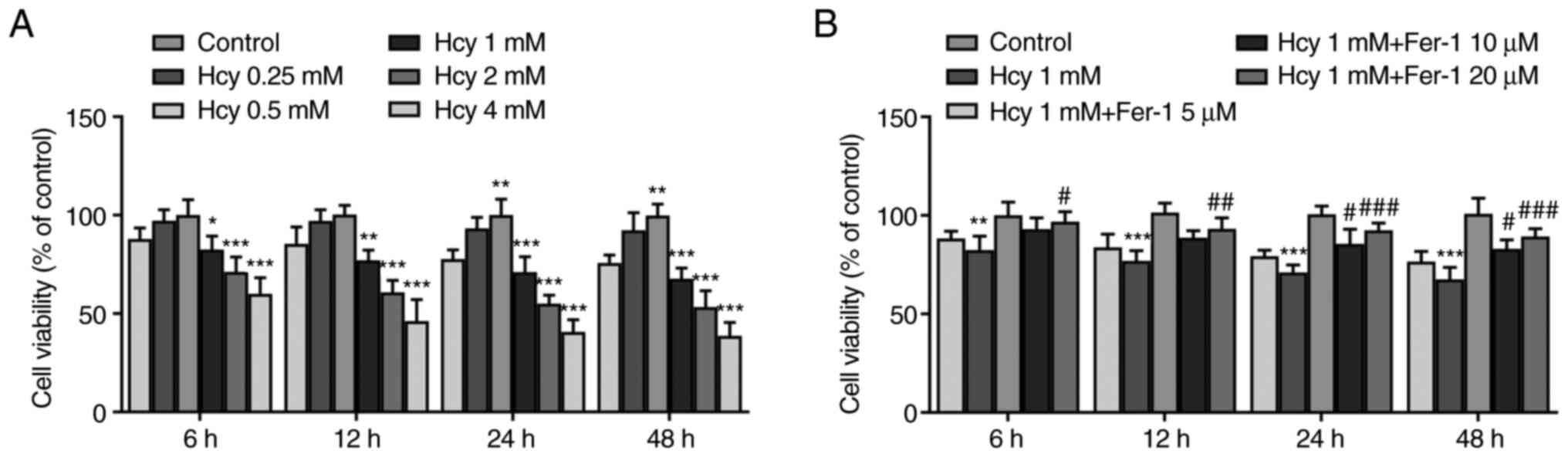
Fer-1 treatment restores viability in
Hcy-induced KGN cells
KGN cells were first treated with various
concentrations of Hcy (0.25, 0.5, 1, 2 and 4 mM) for 6, 12, 24 and
48 h. Results from the CCK-8 assay demonstrated that cell viability
was significantly reduced by Hcy treatment in a dose-dependent
manner compared with that in the control group (Fig. 1A). At concentrations of Hcy >1
mM, cell viability decreased more potently (Fig. 1A). Therefore, 1 mM Hcy was chosen
for subsequent experimentation. As presented in Fig. 1B, Fer-1 reversed the Hcy-induced
decrease in cell viability in a dose-dependent manner, reaching
significance at 10 and 20 µM. These results suggest that Fer-1
treatment can restore viability in Hcy-induced KGN cells.
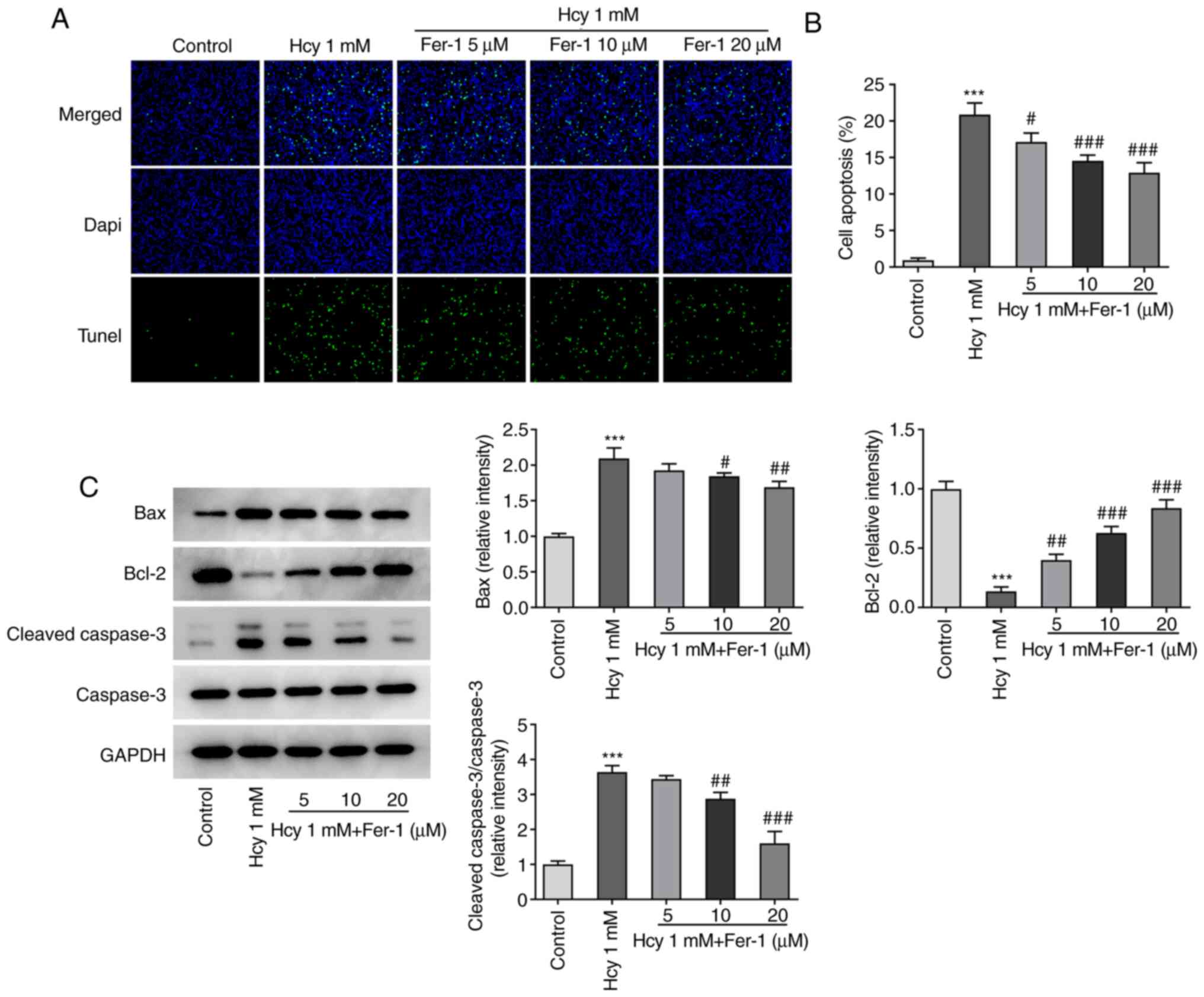
Fer-1 treatment attenuates apoptosis
in Hcy-induced KGN cells
Subsequently, cell apoptosis was assessed using
TUNEL staining. As demonstrated in Fig. 2A and B, the cell apoptotic rate
was significantly increased in the model group compared with that
in the control group. However, Fer-1 treatment significantly
inhibited Hcy-triggered cell apoptosis compared with that in the
model group in a dose-dependent manner. In addition, as
demonstrated in Fig. 2C, Hcy
treatment resulted in the significant downregulation of Bcl-2
expression and upregulation of Bax and cleaved caspase-3
expression, which was partially reversed following Fer-1 treatment
in a dose-dependent manner, reaching significance at 10 and 20 µM.
These results suggest that Fer-1 can potentially protect against
Hcy-induced apoptosis in KGN cells.
Fer-1 treatment inhibits oxidative
stress and ferroptosis in Hcy-induced KGN cells
The effects of Fer-1 on oxidative stress and
ferroptosis in Hcy-induced KGN cells were next investigated. As
demonstrated in Fig. 3A-D, KGN
cells treated with Hcy displayed significantly elevated ROS, MDA
and LDH levels and reduced GSH levels compared with those in the
untreated control group. However, Fer-1 treatment markedly reversed
these effects of Hcy addition on oxidative stress marker levels,
reaching significance at 10 and 20 µM. Furthermore, as shown in
Fig. 3E and F, intracellular
Fe2+ levels were significantly increased following Hcy
exposure, but Fer-1 treatment significantly reduced this
Hcy-induced increase in Fe2+ levels in a dose-dependent
manner. In addition to significantly decreasing GPX4 and SLC7A11
expression levels, significantly increased ASCL4 and DMT1
expression levels were also observed in the Hcy model group
compared with those in the control, which were significantly
reversed following 10 and 20 µM Fer-1 treatment (Fig. 3G). These results suggest that
Fer-1 treatment can alleviate Hcy-induced oxidative stress and
suppress ferroptosis in KGN cells.
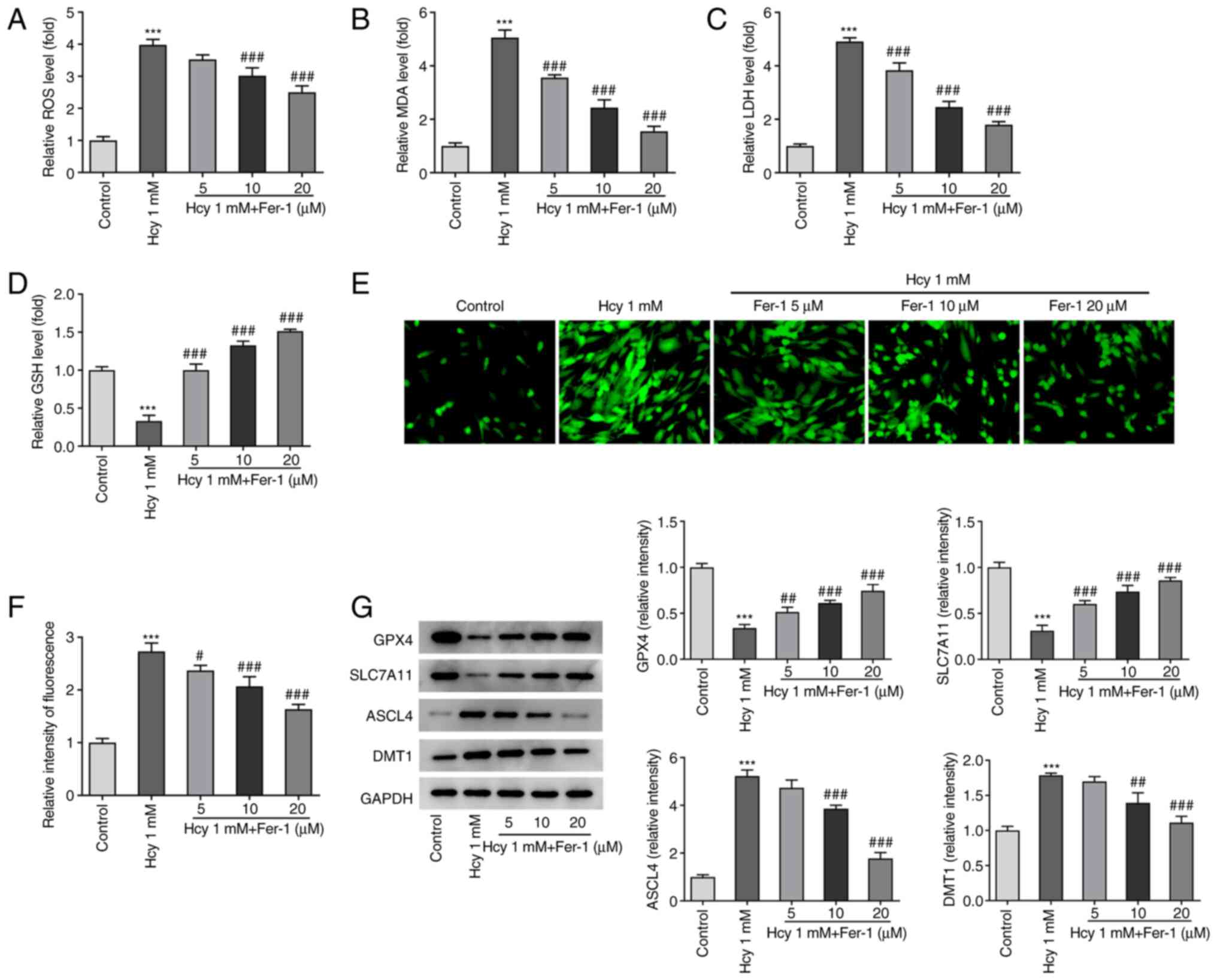 | Figure 3.Fer-1 treatment reduces oxidative
stress and ferroptosis in homocysteine-induced KGN cells. The
levels of (A) ROS, (B) MDA, (C) LDH and (D) GSH were measured using
their corresponding commercial kits. (E) Intracellular
Fe2+ levels were measured using Phen Green™ SK staining.
Magnification, ×400. (F) Quantification of relative fluorescence
intensity of Phen Green™ SK. (G) GPX4, SLC7A11, ASCL4 and DMT1
expression levels were measured using western blotting.
***P<0.001 vs. Control. #P<0.05,
##P<0.01 and ###P<0.001 vs. Model.
Fer-1, ferrostatin-1; ROS, reactive oxygen species; MDA,
malondialdehyde; LDH, lactate dehydrogenase; GSH, reduced
glutathione; GPX4, glutathione peroxidase 4; SLC7A11, solute
carrier family 7 member 11; ASCL4, achaete-scute family BHLH
transcription factor 4; DMT1, divalent metal transporter 1. |
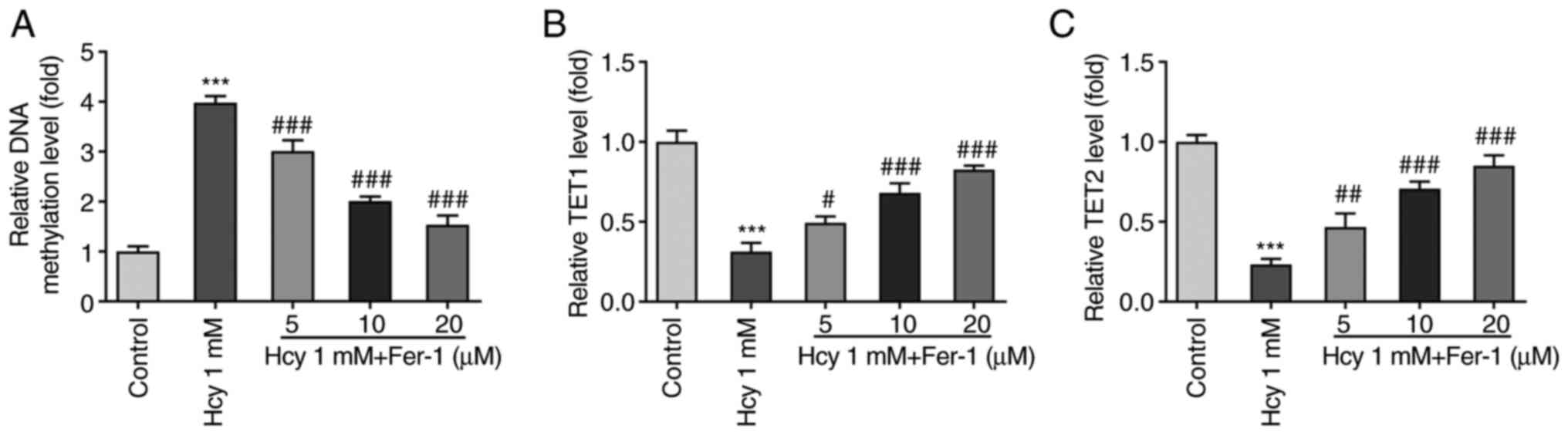
Fer-1 treatment suppresses DNA
methylation and elevates TET1/2 secretion levels in Hcy-induced KGN
cells
Overall DNA methylation levels in KGN cells were
subsequently measured following Hcy treatment. As displayed in
Fig. 4A, the DNA methylation
levels were significantly enhanced in the Hcy-induced group
compared with those in the control group. However, Fer-1 treatment
significantly reversed this Hcy-induced DNA methylation were in a
dose-dependent manner. Furthermore, TET1 and TET2 levels were
significantly decreased following Hcy induction, which were
subsequently restored following Fer-1 treatment in a dose-dependent
manner (Fig. 4B and C). These
results suggest that Fer-1 treatment can suppress DNA methylation
and increase TET1/2 levels in Hcy-induced KGN cells.
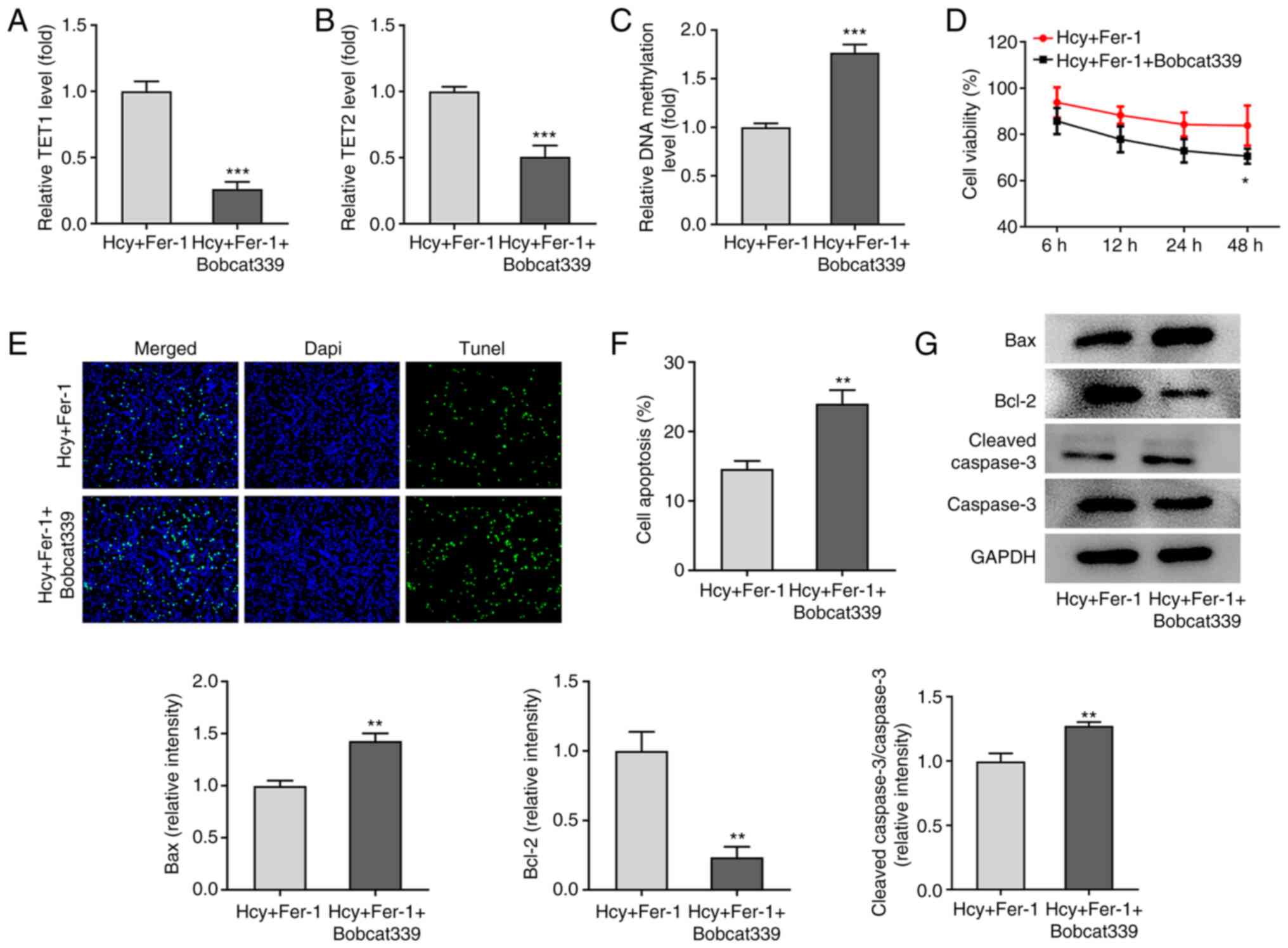
TET1/2 inhibitor Bobcat339
hydrochloride reverses the effects of Fer-1 on Hcy-induced
apoptosis, oxidative stress and ferroptosis in KGN cells
To further explore the regulatory mechanism of Fer-1
on Hcy-induced apoptosis, oxidative stress and ferroptosis, the
TET1/2 inhibitor Bobcat339 hydrochloride was used to treat
Hcy-induced KGN cells. As shown in Fig. 5A-C, the results demonstrated that
in the presence of Fer-1, Bobcat339 hydrochloride treatment
significantly reduced TET1 and TET2 levels whilst significantly
elevating DNA methylation levels. In addition, as presented in
Fig. 5D, cell viability was
significantly decreased following 48 h treatment with Bobcat339
hydrochloride in Hcy and Fer-1 treated KGN cells. TUNEL and western
blotting assays demonstrated that compared with that in the Hcy and
Fer-1 treated group, a significant increase in cell apoptosis in
the Fer-1 + Bobcat339 hydrochloride group was observed, which is
accompanied with significantly downregulated Bcl-2 and upregulated
Bax and cleaved caspase-3 protein expression levels (Fig. 5E-G).
Furthermore, as demonstrated in Fig. 6A-D, Bobcat339 hydrochloride
significantly enhanced ROS, MDA and LDH levels but reduced GSH
levels compared with those in the Fer-1 group in Hcy-stimulated KGN
cells. Fe2+ levels were also significantly decreased
following Bobcat339 hydrochloride treatment, whilst GPX4 and
SLC7A11 protein expression levels were significantly decreased
(Fig. 6E-G). By contrast, ASCL4
and DMT1 protein expression levels were significantly increased by
Bobcat339 hydrochloride treatment compared with those in the Fer-1
group in Hcy-stimulated KGN cells (Fig. 6E-G). These results suggest that
Bobcat339 hydrochloride can reverse the effects of Fer-1 on
Hcy-induced apoptosis, oxidative stress and ferroptosis in KGN
cells.
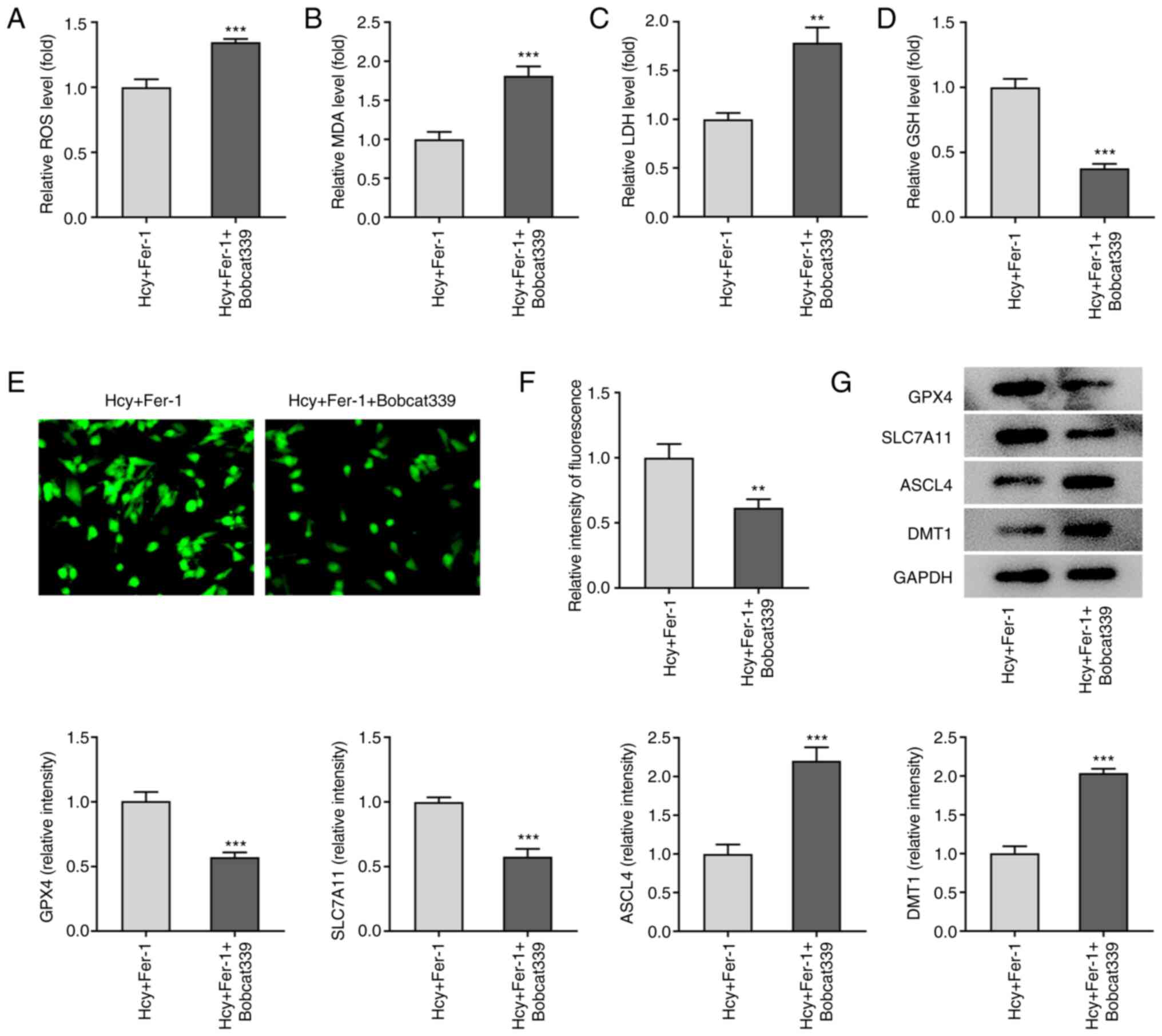 | Figure 6.Bobcat339 hydrochloride reverses the
inhibitory effects of Fer-1 on ferroptosis in homocysteine-induced
KGN cells. The levels of (A) ROS, (B) MDA, (C) LDH and (D) GSH were
determined using their corresponding commercial kits. (E)
Intracellular Fe2+ levels were determined using Phen
Green™ SK staining. Magnification, ×400. (F) Quantification of the
relative Phen Green™ SK fluorescence intensity. (G) Protein
expression levels of GPX4, SLC7A11, ASCL4 and DMT1 were measured
using western blotting, which was quantified. **P<0.01 and
***P<0.001 vs. Fer-1. Fer-1, ferrostatin-1; ROS, reactive oxygen
species; MDA, malondialdehyde; LDH, lactate dehydrogenase; GSH,
reduced glutathione; GPX4, glutathione peroxidase 4; SLC7A11,
solute carrier family 7 member 11; ASCL4, achaete-scute family BHLH
transcription factor 4; DMT1, divalent metal transporter 1. |
Discussion
PCOS is a heterogeneous disease that affects the
female endocrine and reproductive system (26). The present study demonstrated that
treatment with the ferroptosis inhibitor, Fer-1, ameliorated the
Hcy-induced decrease in KGN cell viability and induction of cell
apoptosis, in addition to inhibiting oxidative stress and
ferroptosis in Hcy-induced cells. Furthermore, the results
demonstrated that Fer-1 treatment suppressed DNA methylation whilst
elevating TET1/2 levels in Hcy-induced KGN cells. Treatment with
the TET1/2 inhibitor, Bobcat339 hydrochloride, reversed the
protective effects of Fer-1 on Hcy-induced KGN cell damage. These
findings suggest that the protective properties of Fer-1 against
KGN cell Hcy-induced injury were mediated by TET activity and DNA
demethylation.
Granulosa cells are specifically located around
oocytes and serve an important role in oocyte maturation and
ovulation (27). Dysfunction of
ovarian granulosa cells is considered to be an important
contributing factor of PCOS pathogenesis (28,29). The ovarian granulosa cell line KGN
has been widely applied to explore the pathogenesis and regulatory
mechanism of PCOS in previous studies (30–32). Iron metabolism serves a key role
in endocrine disorders such as hypogonadotropic hypogonadism,
hypothyroidism, and hypoparathyroidism (17). Compared with those in healthy
individuals, patients with PCOS were previously found to have
elevated serum iron concentrations (33). Ferroptosis is a unique type of
programmed necrosis that is characterized by lipid
peroxidation-induced cell death, which depends on the availability
of iron and ROS (34).
Ferroptosis has been associated with a number of pathophysiological
states, including cancer, neurodegeneration and cardiovascular and
endocrine diseases (35–38). Lipid peroxidation is a key process
that occurs during ferroptosis, whereas ROS accumulation is
considered to be an indicator of ferroptosis (39). GPX4, SLC7A11, ASCL4 and DMT1 are
to be important components in ferroptosis regulation (40,41). GPX4 inhibits ferroptosis by using
GSH as a reductase to catalyze the reduction of lipid peroxides
(16). SLC7A11, a component of
System Xc, can inhibit ferroptosis by importing the extracellular
oxidized form of cysteine into the cell, reducing it to the
cysteine that synthesizes the major antioxidant GSH (40). Doll et al (42) previously suggested that ACSL4
downregulation can induce ferroptosis and can be used to predict
sensitivity to ferroptosis in GPX4-knockdown Pfa1 cells. As an
important component of ferroptosis, the divalent metal transporter
DMT1 can regulate intracellular iron levels and has been found to
be essential for maintaining iron homeostasis (43). In a recent study, increased
ferroptosis levels have been reported in granulosa cells during
PCOS (17). In addition, high
concentrations of Hcy can induce oxidative stress and ferroptosis
in the nucleus pulposus by promoting the expression of methylases
and enhancing methylation of the GPX4 gene (18). Fer-1 is a lipophilic radical
scavenger that is a potent inhibitor of ferroptosis (34). Fer-1 can inhibit peroxidation
induced by traces of lipid hydroperoxides in iron and liposomes
(35). Several studies previously
reported the beneficial effects of Fer-1 in dopaminergic
neuroblastoma and cisplatin-induced AKI as a result of inhibiting
ROS accumulation, apoptosis and ferroptosis (22,23). The present study, to the best of
our knowledge, was the first to explore the effects of Fer-1 on
PCOS by using a Hcy-induced KGN cell model. The results
demonstrated that Fer-1 treatment inhibited apoptosis, oxidative
stress and ferroptosis in Hcy-induced KGN cells.
Accumulating evidence supports the notion that
aberrant gene methylation is a key contributing factor in the
pathogenesis of PCOS (44,45).
Methylation of numerous genes regulating vital ovarian functions
has been previously found to be altered according to the DNA
methylome profiling data of granulosa cells (46). Therefore, DNA methylation was
analyzed in the present study. The results of the present study
demonstrated elevated DNA methylation levels in Hcy-induced KGN
cells. However, Fer-1 treatment dose-dependently reduced this
methylation induced by Hcy. Furthermore, TET1 and TET2 are two
dioxygenases that serve significant roles in decreasing DNA
methylation (47). The present
study demonstrated that Fer-1 notably increased both TET1 and TET2
levels, which suggested that Fer-1 may exert an inhibitory effect
on DNA methylation in Hcy-induced ovarian granulosa cells. To
verify the mechanism of Fer-1 in DNA methylation further, Bobcat339
hydrochloride, an inhibitor of TET1 and TET2, was used to treat KGN
cells under Hcy-treated conditions (48). The results demonstrated that
Bobcat339 hydrochloride reversed the protective effects of Fer-1
against Hcy-induced apoptosis, oxidative stress and ferroptosis in
KGN cells.
In conclusion, results of the present study
demonstrated that the ferroptosis inhibitor Fer-1 may alleviate
Hcy-induced ovarian granulosa cell injury. This protective effect
may be due to the enhancement of TET levels and DNA methylation.
The present study provided an experimental basis for the
application of Fer-1 as a potential therapeutic agent for the
clinical treatment of PCOS. However, the present study is a
preliminary investigation on the possible regulatory effects of
Fer-1 on DNA methylation in Hcy-induced ovarian granulosa cells.
In-depth study of the DNA methylation profile in granulosa cells
after Hcy and Fer-1 treatment must be performed in future studies.
Additionally, subsequent studies will need to focus on the
collection of clinical data and the usage of granulosa cells from
the follicular fluid of the ovary in patients with PCOS.
Acknowledgements
Not applicable.
Funding
The present study was supported by Ningxia Natural Science
Foundation (grant no. 2021AAC03353), Ningxia Science and technology
benefit people project (grant no. 2020CMG03006) and First-Class
Discipline Construction Founded Project of NingXia Medical
University and the School of Clinical Medicine (grant no.
NXYLXK2017A05).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QS and LC designed the study and performed the
experiments. LC and RL drafted and revised the manuscript. QS
analyzed the data. RL performed the literature search and analyzed
the data. QS and LC confirm the authenticity of the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cena H, Chiovato L and Nappi RE: Obesity,
polycystic ovary syndrome, and infertility: A new avenue for GLP-1
receptor agonists. J Clin Endocrinol Metab. 105:e2695–e2709. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goodarzi MO, Dumesic DA, Chazenbalk G and
Azziz R: Polycystic ovary syndrome: Etiology, pathogenesis and
diagnosis. Nat Rev Endocrinol. 7:219–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Azziz R, Woods KS, Reyna R, Key TJ,
Knochenhauer ES and Yildiz BO: The prevalence and features of the
polycystic ovary syndrome in an unselected population. J Clin
Endocrinol Metab. 89:2745–2749. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ignatov A and Ortmann O: Endocrine risk
factors of endometrial cancer: Polycystic ovary syndrome, oral
contraceptives, infertility, tamoxifen. Cancers (Basel).
12:17662020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Franks S: Polycystic ovary syndrome. N
Engl J Med. 333:853–861. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Witchel SF, Oberfield SE and Peña AS:
Polycystic ovary syndrome: Pathophysiology, presentation, and
treatment with emphasis on adolescent girls. J Endocr Soc.
3:1545–1573. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Legro RS, Arslanian SA, Ehrmann DA, Hoeger
KM, Murad MH, Pasquali R and Welt CK; Endocrine Society, :
Diagnosis and treatment of polycystic ovary syndrome: An endocrine
society clinical practice guideline. J Clin Endocrinol Metab.
98:4565–4592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bednarska S and Siejka A: The pathogenesis
and treatment of polycystic ovary syndrome: What's new? Adv Clin
Exp Med. 26:359–367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eppig JJ: Reproduction: Oocytes call,
granulosa cells connect. Curr Biol. 28:R354–R356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Das M, Djahanbakhch O, Hacihanefioglu B,
Saridogan E, Ikram M, Ghali L, Raveendran M and Storey A: Granulosa
cell survival and proliferation are altered in polycystic ovary
syndrome. J Clin Endocrinol Metab. 93:881–887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dumesic DA and Richards JS: Ontogeny of
the ovary in polycystic ovary syndrome. Fertil Steril. 100:23–38.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kondapaneni V, Gutlapalli SD, Poudel S,
Zeb M, Toulassi IA and Cancarevic I: Significance of homocysteine
levels in the management of polycystic ovarian syndrome: A
literature review. Cureus. 12:e111102020.PubMed/NCBI
|
|
13
|
Kelly CJ, Speirs A, Gould GW, Petrie JR,
Lyall H and Connell JM: Altered vascular function in young women
with polycystic ovary syndrome. J Clin Endocrinol Metab.
87:742–746. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Legro RS, Kunselman AR and Dunaif A:
Prevalence and predictors of dyslipidemia in women with polycystic
ovary syndrome. Am J Med. 111:607–613. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv Q, Niu H, Yue L, Liu J, Yang L, Liu C,
Jiang H, Dong S, Shao Z, Xing L and Wang H: Abnormal ferroptosis in
myelodysplastic syndrome. Front Oncol. 10:16562020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Wang F, Li D, Yan Y and Wang H:
Transferrin receptor-mediated reactive oxygen species promotes
ferroptosis of KGN cells via regulating NADPH oxidase 1/PTEN
induced kinase 1/acyl-CoA synthetase long chain family member 4
signaling. Bioengineered. 12:4983–4994. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Huang Z, Xie Z, Chen Y, Zheng Z,
Wei X, Huang B, Shan Z, Liu J, Fan S, et al: Homocysteine induces
oxidative stress and ferroptosis of nucleus pulposus via enhancing
methylation of GPX4. Free Radic Biol Med. 160:552–565. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Collignon E, Canale A, Al Wardi C, Bizet
M, Calonne E, Dedeurwaerder S, Garaud S, Naveaux C, Barham W,
Wilson A, et al: Immunity drives TET1 regulation in cancer through
NF-κB. Sci Adv. 4:eaap73092018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kabiraj P, Valenzuela CA, Marin JE,
Ramirez DA, Mendez L, Hwang MS, Varela-Ramirez A, Fenelon K,
Narayan M and Skouta R: The neuroprotective role of ferrostatin-1
under rotenone-induced oxidative stress in dopaminergic
neuroblastoma cells. Protein J. 34:349–358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deng F, Sharma I, Dai Y, Yang M and Kanwar
YS: Myo-inositol oxygenase expression profile modulates pathogenic
ferroptosis in the renal proximal tubule. J Clin Invest.
129:5033–5049. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu B, Liu Y, Chen X, Zhao J, Han J, Dong
H, Zheng Q and Nie G: Ferrostatin-1 protects auditory hair cells
from cisplatin-induced ototoxicity in vitro and in vivo. Biochem
Biophys Res Commun. 533:1442–1448. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu L, Jia F, Wei J, Yu Y, Yu T, Wang Y,
Sun J and Luo G: Salidroside protects against homocysteine-induced
injury in human umbilical vein endothelial cells via the regulation
of endoplasmic reticulum stress. Cardiovasc Ther. 35:33–39. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ajmal N, Khan SZ and Shaikh R: Polycystic
ovary syndrome (PCOS) and genetic predisposition: A review article.
Eur J Obstet Gynecol Reprod Biol X. 3:1000602019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Knochenhauer ES, Key TJ, Kahsar-Miller M,
Waggoner W, Boots LR and Azziz R: Prevalence of the polycystic
ovary syndrome in unselected black and white women of the
southeastern United States: A prospective study. J Clin Endocrinol
Metab. 83:3078–3082. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He M, Mao G, Xiang Y, Li P, Wu Y, Zhao D
and Li T: MicroRNA-664a-3p inhibits the proliferation of ovarian
granulosa cells in polycystic ovary syndrome and promotes apoptosis
by targeting BCL2A1. Ann Transl Med. 9:8522021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Wang H, Zhou D, Shuang T, Zhao H and
Chen B: Up-regulation of long noncoding RNA SRA promotes cell
growth, inhibits cell apoptosis, and induces secretion of estradiol
and progesterone in ovarian granular cells of mice. Med Sci Monit.
24:2384–2390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Zheng Q, Sun D, Cui X, Chen S,
Bulbul A, Liu S and Yan Q: Dehydroepiandrosterone stimulates
inflammation and impairs ovarian functions of polycystic ovary
syndrome. J Cell Physiol. 234:7435–7447. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu G, Xia J, Yang Z, Chen Y, Jiang W, Yin
T and Yang J: CircASPH promotes KGN cells proliferation through
miR-375/MAP2K6 axis in polycystic ovary syndrome. J Cell Mol Med.
Dec 28–2020.(Epub ahead of print). View Article : Google Scholar
|
|
32
|
Zheng Q, Li Y, Zhang D, Cui X, Dai K, Yang
Y, Liu S, Tan J and Yan Q: ANP promotes proliferation and inhibits
apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex
and improves ovary functions of PCOS rats. Cell Death Dis.
8:e31452017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JW, Kang KM, Yoon TK, Shim SH and Lee
WS: Study of circulating hepcidin in association with iron excess,
metabolic syndrome, and BMP-6 expression in granulosa cells in
women with polycystic ovary syndrome. Fertil Steril.
102:548–554.e2. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao JY and Dixon SJ: Mechanisms of
ferroptosis. Cell Mol Life Sci. 73:2195–2209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu J, Xiong Y, Zhang Y, Wen J, Cai N,
Cheng K, Liang H and Zhang W: The molecular mechanisms of
regulating oxidative stress-induced ferroptosis and therapeutic
strategy in tumors. Oxid Med Cell Longev. 2020:88107852020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Do Van B, Gouel F, Jonneaux A, Timmerman
K, Gelé P, Pétrault M, Bastide M, Laloux C, Moreau C, Bordet R, et
al: Ferroptosis, a newly characterized form of cell death in
Parkinson's disease that is regulated by PKC. Neurobiol Dis.
94:169–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Peng X, Zhang M, Jia Y, Yu B and
Tian J: Revisiting tumors and the cardiovascular system:
Mechanistic intersections and divergences in ferroptosis. Oxid Med
Cell Longev. 2020:97381432020.PubMed/NCBI
|
|
38
|
Li D, Jiang C, Mei G, Zhao Y, Chen L, Liu
J, Tang Y, Gao C and Yao P: Quercetin alleviates ferroptosis of
pancreatic β cells in type 2 diabetes. Nutrients. 12:29542020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang WS and Stockwell BR: Ferroptosis:
Death by lipid peroxidation. Trends Cell Biol. 26:165–176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song Q, Peng S, Sun Z, Heng X and Zhu X:
Temozolomide drives ferroptosis via a DMT1-dependent pathway in
glioblastoma cells. Yonsei Med J. 62:843–849. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xue X, Ramakrishnan SK, Weisz K, Triner D,
Xie L, Attili D, Pant A, Győrffy B, Zhan M, Carter-Su C, et al:
Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling
to promote colorectal tumorigenesis. Cell Metab. 24:447–461. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cao P, Yang W, Wang P, Li X and Nashun B:
Characterization of DNA methylation and screening of epigenetic
markers in polycystic ovary syndrome. Front Cell Dev Biol.
9:6648432021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pan JX, Tan YJ, Wang FF, Hou NN, Xiang YQ,
Zhang JY, Liu Y, Qu F, Meng Q, Xu J, et al: Aberrant expression and
DNA methylation of lipid metabolism genes in PCOS: A new insight
into its pathogenesis. Clin Epigenetics. 10:62018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sagvekar P, Kumar P, Mangoli V, Desai S
and Mukherjee S: DNA methylome profiling of granulosa cells reveals
altered methylation in genes regulating vital ovarian functions in
polycystic ovary syndrome. Clin Epigenetics. 11:612019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Poole CJ, Lodh A, Choi JH and van Riggelen
J: MYC deregulates TET1 and TET2 expression to control global DNA
(hydroxy)methylation and gene expression to maintain a neoplastic
phenotype in T-ALL. Epigenetics Chromatin. 12:412019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chua GNL, Wassarman KL, Sun H, Alp JA,
Jarczyk EI, Kuzio NJ, Bennett MJ, Malachowsky BG, Kruse M and
Kennedy AJ: Cytosine-based TET enzyme inhibitors. ACS Med Chem
Lett. 10:180–185. 2019. View Article : Google Scholar : PubMed/NCBI
|