Introduction
Precocious puberty (PP) is one of the most common
pediatric endocrine diseases defined as the development of pubertal
changes before the age of 8 years in girls and 9 years in boys,
coupled with accelerated growth and elevated levels of sex hormones
(1). Reproduction and puberty
onset are complex biological processes that involve numerous
factors controlled by the hypothalamus-pituitary-gonadal (HPG) axis
(2,3). Hypothalamic cells can produce
gonadotropin-releasing hormone (GnRH), the final output of
neuroendocrine regulation that occurs during puberty, which is
released to stimulate the secretion of gonadotropins from the
pituitary to then act on the gonads (4). Due to the early activation of the
HPG axis in children, sex hormones reach puberty levels early,
which consumes the proliferative capacity of the epiphyseal
cartilage plate in advance, resulting in a reduced final height and
premature presentation of the secondary sexual characteristics,
which may cause psychological and mental problems (5). Therefore, PP has received
considerable attention within the medical field and from the
public.
Obesity is considered to be a crucial factor that
triggers idiopathic central PP (ICPP) through the excessive intake
of lipids and sugar, which leads to multiple metabolic disorders
and further affects the central nervous system (6). Compelling evidence indicates that
high levels of sugar and fat can regulate the expression of
estrogen receptor (ER) and PP-related genes in hypothalamic cells
(7). Live kinase B1 (LKB1), also
known as serine/threonine kinase 11 (STK11), is a serine-threonine
kinase that participates in several cellular functions, including
growth, cell energy metabolism, polarity and tumor formation
(8). In recent years, LKB1 has
been reported to be associated with obesity and LKB1 knockout in
the hypothalamus has been shown to intensify susceptibility to
obesity in mice administered with a high-fat (HF) diet, accompanied
by a deterioration of hypothalamic inflammation and downregulation
of neuronal expression (9). In
the endometrial glands, LKB1 can promote the phosphorylation of
AMP-activated protein kinase (AMPK), thereby increasing the
activity of forkhead box protein O1 (FOXO1) (10). A clearly reduced release of GnRH
is observed following the elevation of FOXO1 activity in the
hypothalamus (11). Therefore,
the effects of LKB1 on PP and whether LKB1 can regulate the
AMPK/FOXO1 pathway, prompted the current study.
In the present study, LKB1 level was detected in the
peripheral blood of children with PP. Then, GT1-7 mouse
hypothalamus cell line was exposed to high glucose (HG) and HF
conditions to stimulate a PP in vitro model, in order to
explore the roles of LKB1 in the progression of PP and its
regulatory effects on the AMPK/FOXO1 signaling pathway.
Materials and methods
Sample collection
The peripheral blood samples (5 ml)of healthy
children (n=25) and PP children with ICPP (n=25) were collected
from the Fujian Maternity and Child Health Hospital (Fuzhou,
China). All patients were female (age, 5-8 years) recruited between
April 2020 and August 2020. The diagnostic criteria used were
consistent with the previous study (12). The inclusion criteria were as
follows: Patients diagnosed with ICPP who had been treated with
GnRHa with a follow-up >3 months and complete clinical data.
Patients with precocious puberty due to tumor, organic or endocrine
disease, simple breast precocity, rare syndromes, contraceptive
pill abuse or other exogenous hormones were excluded. Patients with
poor quality ultrasound images or incomplete clinical information
were also excluded. Informed written consent was obtained from
parents or guardians. This study was approved by the Ethics
Committee of Fujian Maternity and Child Health Hospital (Fuzhou,
China).
Cell culture
The GT1-7 mouse hypothalamic cell line was purchased
from BLUEFBIO Life Sciences. Cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; MilliporeSigma) containing 10% fetal
bovine serum (FBS; Cytiva) under humidified conditions at 37°C in a
5% CO2-containing atmosphere. Cells in the control group
were maintained in complete DMEM (glucose concentration, 25 mM).
The HG and HF group cells were cultured in DMEM plus 45 mM glucose
+ 1 mM palmitate according to the previous study (7). Following incubation for 12 h, cells
were collected for subsequent experiments.
Cell transfection
PcDNA 3.1 plasmid containing LKB1 [overexpression
(Oe)-LKB1; 4 µg] or empty vectors [Oe-negative control (NC); 4 µg]
was synthesized by Shanghai GenePharma Co., Ltd. GT1-7 cells were
inoculated at a density of 2×105 cells/well in 6-well
plates and cultured at 37°C until they reached 80% confluence.
Transfection was then performed using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 48 h
in a 5% CO2-containing atmosphere, according to the
manufacturer's instructions. The effect of LKB1 overexpression was
validated by reverse transcription-quantitative (RT-q) PCR and
western blot analysis. The transfected cells were used for
subsequent experiments at 48 h after transfection.
RT-qPCR
Total RNA was extracted from 5×106 GT1-7
cells using TRIzol® reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Total RNA was
reverse-transcribed into complementary DNA (cDNA) using the
PrimeScript Strand cDNA synthesis kit (Takara Biotechnology Co.,
Ltd.) at 42°C for 30 min according to the manufacturer's protocol.
Subsequently, using cDNA as the template, the gene expression
levels were analyzed using RT-qPCR, which was conducted with an
iTaq Universal One-Step iTaq Universal SYBR® Green
Supermix (Bio-Rad Laboratories, Inc.) on an ABI 7500 instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The experiments were independently
replicated ≥3 times. The following thermocycling conditions were
used: Initial denaturation at 95°C for 7 min; and 40 cycles of 95°C
for 15 sec and 60°C for 30 sec; and a final extension at 72°C for
30 sec. The primers used in this study were designed and
synthesized by Sangon Biotech Co., Ltd. The sequences were as
follows: LKB1 (human) forward, 5′-GAAGTTGGGCTCTCCAGGT-3′ and
reverse, 5′-CGGACAAGTATGAACACGGC-3′; LKB1 (mouse) forward,
5′-GGGGACGAGGACAAAGAGTG-3′ and reverse, 5′-CTTGACGTTGGCCTCTCCAT-3′;
IL-6 forward, 5′-TCCGGAGAGGAGACTTCACA-3′ and reverse,
5′-TAACGCACTAGGTTTGCCGA-3′; TNF-α forward,
5′-CAGCCGATGGGTTGTACCTT-3′ and reverse, 5′-GGGGCTCTGAGGAGTAGACA-3′;
GnRH forward, 5′-AGCACTGGTCCTATGGGTTG-3′ and reverse,
5′-GGGGTTCTGCCATTTGATCCA-3′; GAPDH forward,
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse, 5′-GGGGTCGTTGATGGCAACA-3′.
GAPDH was used as a reference gene. Gene expression levels were
quantified according to the 2−ΔΔCq method (13).
Isolation of peripheral blood
mononuclear cells (PBMCs) from normal and prematurity groups
PBMCs were collected from the peripheral blood
samples of healthy children and PP children with ICPP. The blood
was treated with ethylenediaminetetraacetic acid anti-coagulant,
diluted with twice the volume of phosphate-buffered saline (PBS)
and mixed well (14). The cell
suspension was added with caution to the lymphocyte separation
liquid (Dakewe Biotech Co., Ltd.) equal in volume to the blood and
centrifuged horizontally at 500 × g at room temperature for 20 min.
The PBMCs at the junction of the plasma layer and the lymphocyte
separation liquid were aspirated, added with the equal amount of
PBS, mixed well and centrifuged at 500 × g for 10 min at 20°C.
After discarding the supernatant, the cells were washed twice to
remove the residual lymphocyte separation liquid.
Western blot analysis
For immunoblotting, cells were collected and lysed
with RIPA buffer (Wuhan Boster Biological Technology, Ltd.).
Protein concentration was detected using a bicinchoninic acid (BCA)
kit (Beyotime Institute of Biotechnology). Normalized volumes of
samples (40 µg protein per lane) were isolated using 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel
and transferred onto polyvinylidene fluoride (PVDF) membranes.
Membranes were subsequently blocked with 5% non-fat milk at room
temperature for 1.5 h, prior to incubation with primary antibodies
for the target proteins at 4°C overnight. The horseradish
peroxidase (HRP)-labeled secondary antibody (cat. no. 7074P2;
1:5,000; Cell Signaling Technology, Inc.) was added for 1 h at room
temperature. Protein bands were scanned and visualized using an
enhanced chemiluminescence detection system (MilliporeSigma). The
intensities of the protein bands were quantified using ImageJ
software v1.8.0 (National Institutes of Health) and the gray value
of the target protein was normalized to that of GAPDH. Anti-LKB1
(cat. no. 13031T; 1:1,000), anti-IL-6 (cat. no. 12912T; 1:1,000),
anti-TNF-α (cat. no. 11948T; 1:1,000), anti-CD36 (cat. no. 74002S;
1:1,000), anti-phosphorylated (p)-AMPK (cat. no. 2535T; 1:1,000),
anti-AMPK (cat. no. 5831T; 1:1,000), anti-p-FOXO1 (cat. no. 84192S;
1:1,000), anti-FOXO1 (cat. no. 2880T; 1:1,000) and anti-GAPDH (cat.
no. 5174S; 1:1,000) antibodies were obtained from Cell Signaling
Technology, Inc. Anti-estrogen receptor-β (ERβ; ab196787; 1:1,000)
and anti-G-protein-coupled receptor (GPR54; cat. no. ab100896:
1:1,000) antibodies were provided by Abcam.
Statistical analysis
All experiments were repeated independently in
triplicate. All data are expressed as the mean ± standard deviation
(SD) for each group. Statistical analysis was performed with
GraphPad Prism (version 8.0; GraphPad Software, Inc.). Contrastive
analysis of the measurement data in multiple groups was performed
applying one-away analysis of variance (ANOVA) followed by Turkey's
post hoc test, while the data in two groups was compared by
unpaired Student's t test. P<0.05 was considered to indicate a
statistically significant difference.
Results
LKB1 expression is markedly
downregulated in the peripheral blood of children with PP and HG-
and HF-induced GT1-7 cells
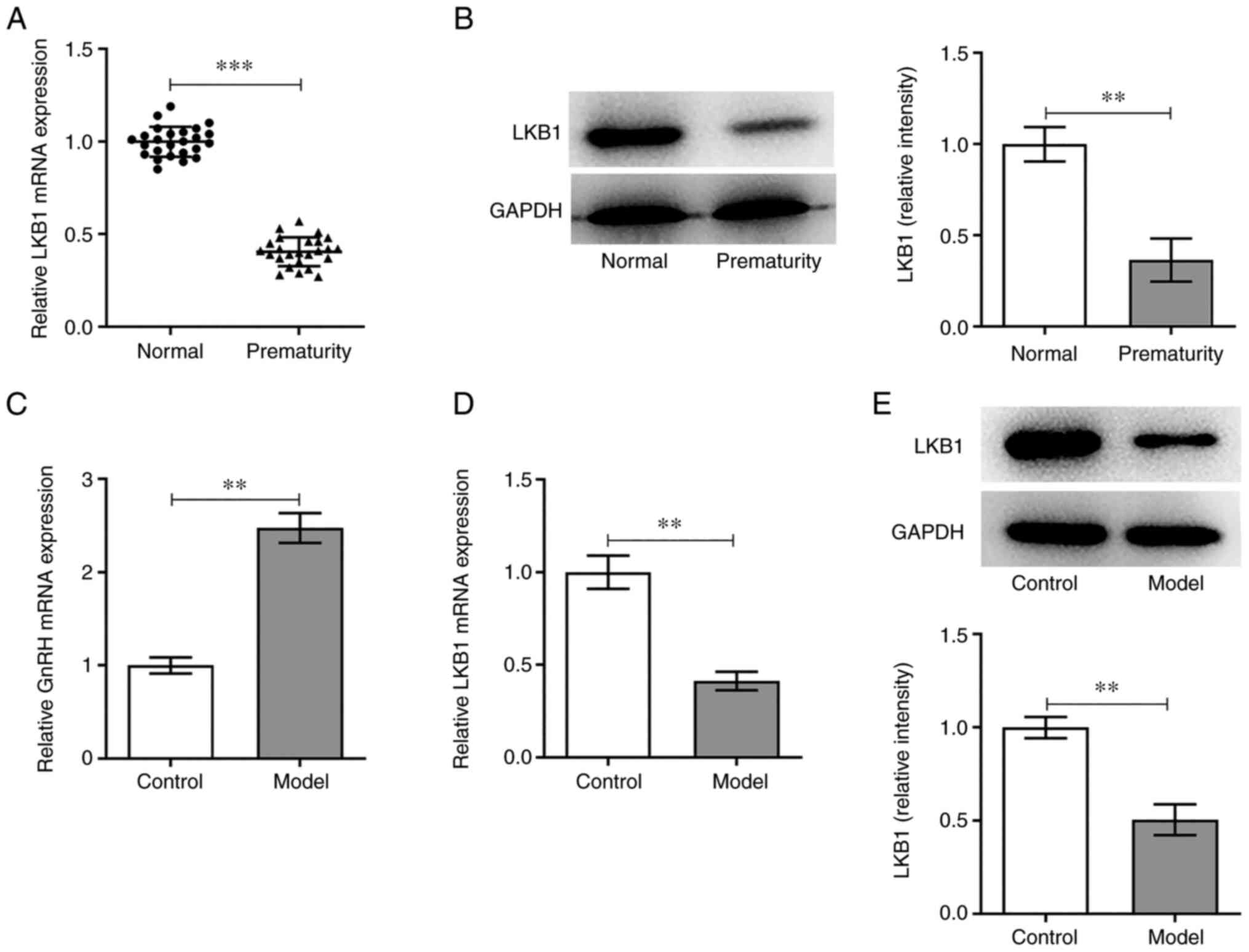
LKB1 expression in the peripheral blood of children
with PP was measured using RT-qPCR. As shown in Fig. 1A, LKB1 expression was
significantly reduced in the PP group compared with the normal
group. Additionally, LKB1 protein expression in PBMCs was tested by
western blot analysis and marked downregulation in LKB1 expression
was observed in the PP group compared with the normal group
(Fig. 1B). Next, GT1-7 cells were
exposed to HG and HF to simulate the PP model in vitro and
the expression of GnRH and LKB1 was examined. It was found that HF
and HG induction led to a marked increase in GnRH expression in the
PP group compared with the control group (Fig. 1C). Furthermore, the mRNA and
protein expression levels of LKB1 were markedly decreased in the
model group compared with the untreated control group (Fig. 1D and E). In conclusion, an
abnormal LKB1 expression may be associated with PP.
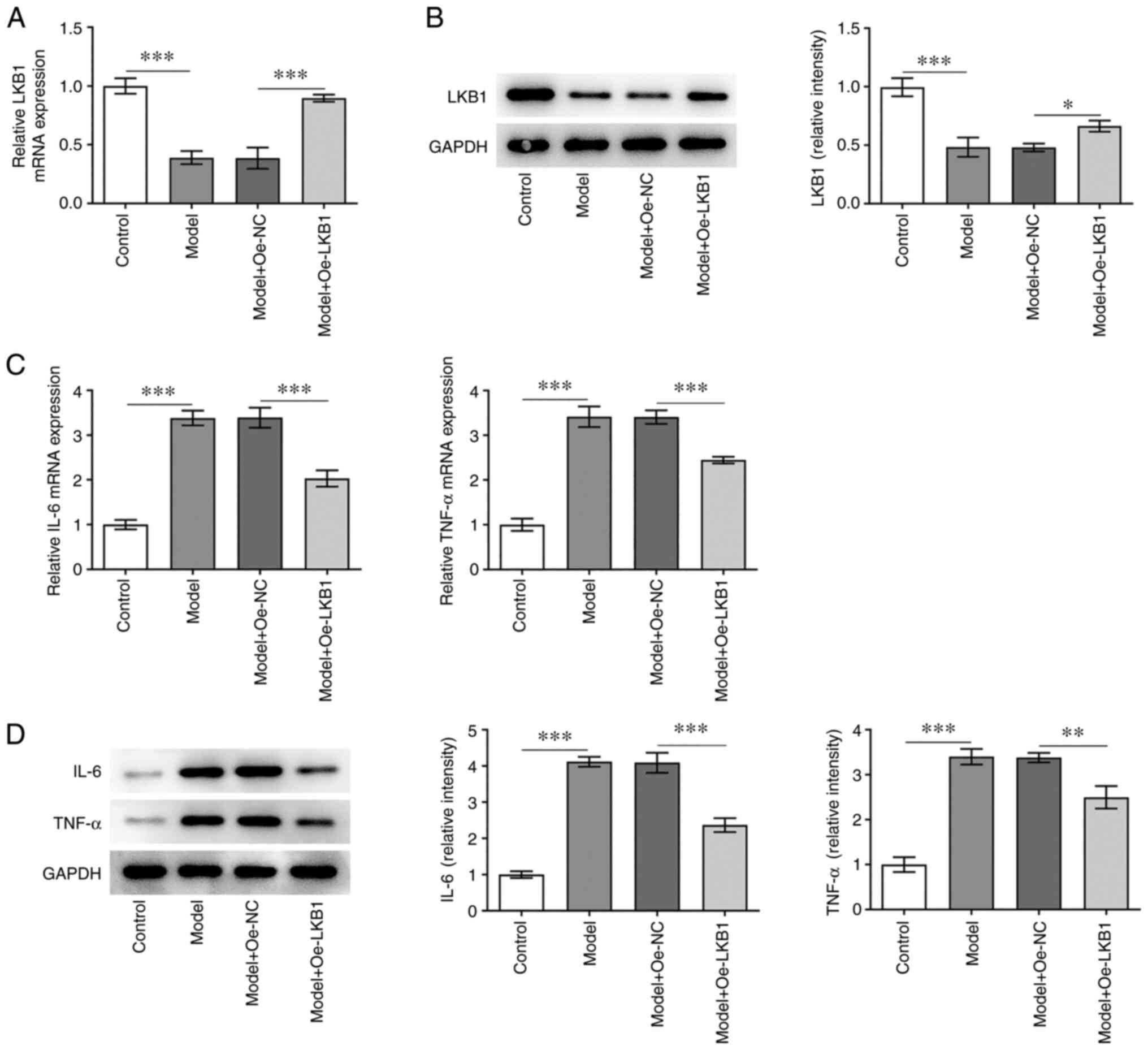
LKB1 overexpression alleviates
inflammatory response in HG- and HF-induced GT1-7 cells
To explore the effects of LKB1 on the inflammatory
response in HG combined with HF-induced GT1-7 cells, LKB1 was
overexpressed through LKB1-overexpressing vector transfection. It
was observed that LKB1 mRNA and protein levels were clearly
elevated in the Model + Oe-LKB1 group, when compared with the Model
+ Oe-NC group (Fig. 2A and B).
Subsequently, the RT-qPCR and western blot analysis (Fig. 2C and D) indicated that the IL-6
and TNF-α levels were markedly increased in GT1-7 cells compared
with cells in the control group, but were decreased following
further LKB1 overexpression. These findings suggested that LKB1
overexpression alleviates HG- and HF-induced inflammation in GT1-7
cells.
LKB1 overexpression attenuates GnRH
and PP-related protein expression in HG- and HF-induced GT1-7
cells
In order to assess the function of LKB1
overexpression on GnRH, RT-qPCR was used to determine GnRH
expression in GT1-7 cells following HG and HF exposure. As shown in
Fig. 3A, LKB1 upregulation
reduced GnRH levels compared with the Model + Oe-NC group. In
addition, ERβ, CD36 and GPR54 expression was clearly decreased
following LKB1 overexpression in GT1-7 cells under HG and HF
exposure, compared with cells in the negative control group
(Fig. 3B and C). These results
suggested that LKB1 overexpression inhibited GnRH and PP-related
protein expression in HG combined with HF-induced GT1-7 cells.
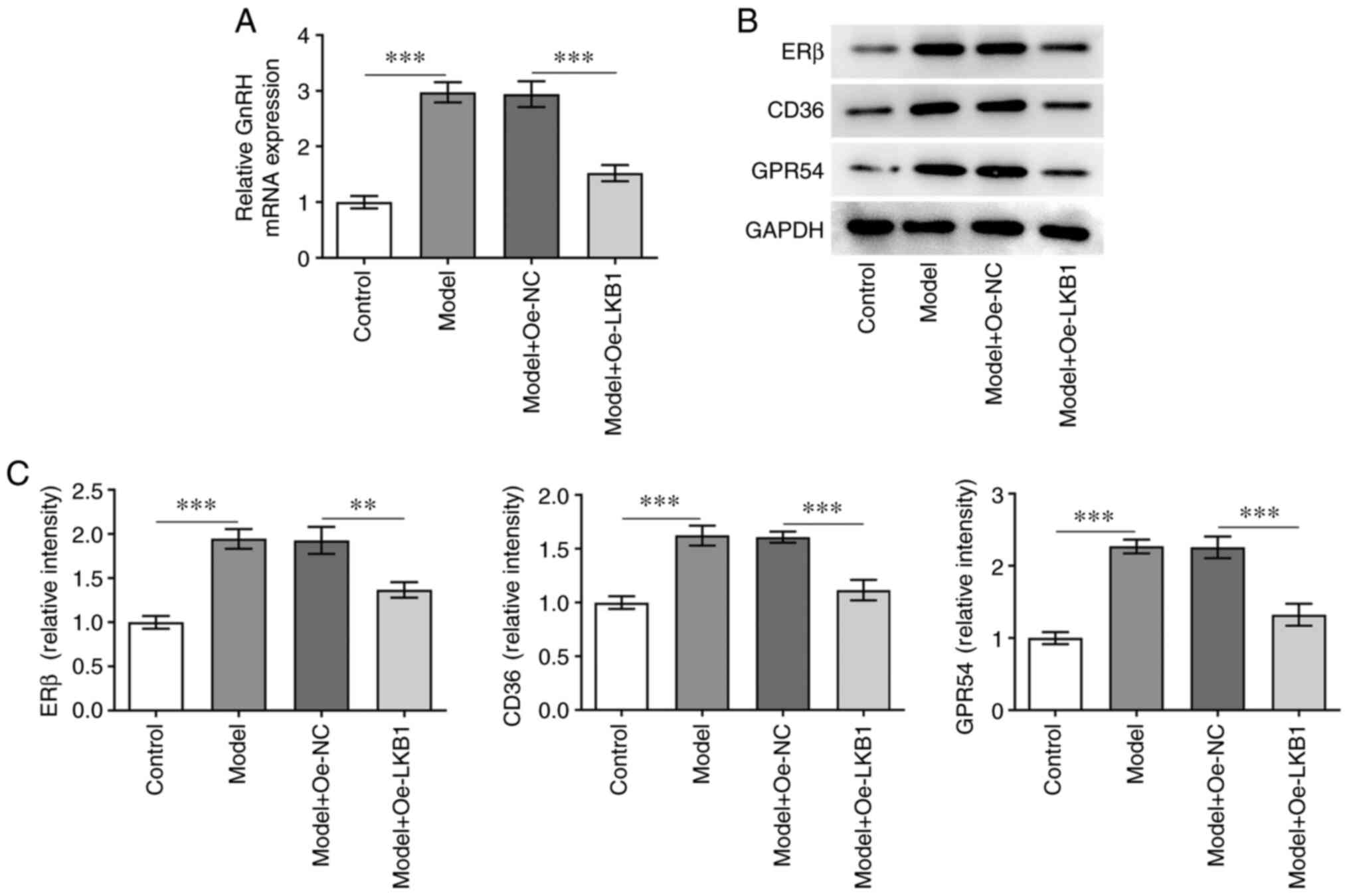 | Figure 3.LKB1 overexpression inhibits GnRH and
PP-related protein expression in HG- and HF-induced GT1-7 cells.
(A) RT-qPCR was performed to examine GnRH expression. (B-C)
Measurement of ERβ, CD36 and GPR54 expression using western blot
analysis. **P<0.01 and ***P<0.001. LKB1, live kinase B1;
GnRH, gonadotropin-releasing hormone; PP, precocious puberty; HF,
high fat; HG, high glucose; RT-qPCR, reverse
transcription-quantitative qPCR; ERβ, estrogen receptor-β; CD36,
cluster of differentiation 36; GPR54, G-protein-coupled receptor;
Oe, overexpression; NC, negative control. |
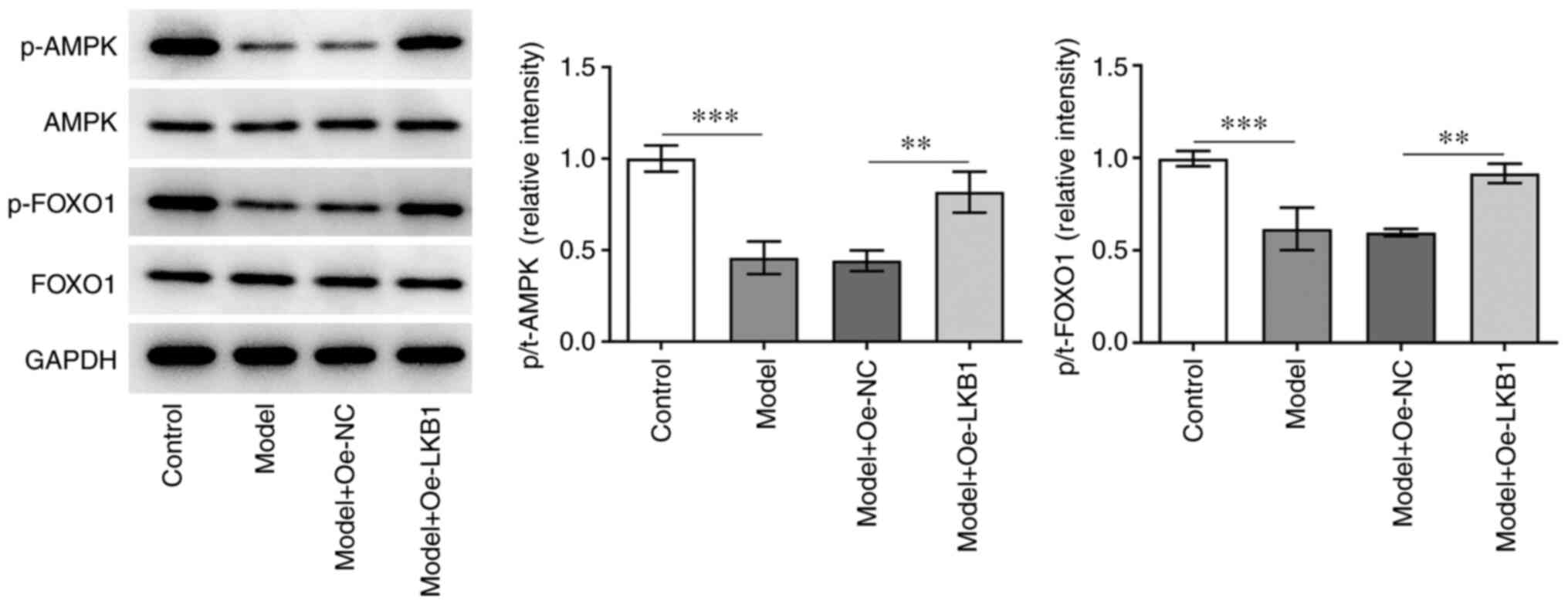
LKB1 overexpression suppresses the
AMPK/FOXO1 signaling pathway in HG- and HF-induced GT1-7 cells
To investigate the potential mechanism of LKB1 in
the regulation of HG- and HF-induced GT1-7 cells, the expression of
AMPK/FOXO1 signaling proteins was examined using western blot
analysis. A significant decrease in p-AMPK and p-FOXO1 expression
was observed in the model group compared with the control group,
while further LKB1 overexpression elevated both the p-AMPK and
p-FOXO1 expression compared with the Model + Oe-NC group (Fig. 4). Collectively, these data
provided evidence that LKB1 overexpression restrained the
AMPK/FOXO1 signaling pathway in HG- and HF-induced GT1-7 cells.
AMPK/FOXO1 signaling inactivation
reverses the impact of LKB1 overexpression on inflammation and GnRH
expression in HG- and HF-induced GT1-7 cells
AMPK/FOXO1 signaling was inhibited by treatment with
AMPK inhibitor compound C to examine the regulatory effect of LKB1
on this pathway in HG- and HF-induced GT1-7 cells. As shown in
Fig. 5A and B, Compound C
addition elevated the mRNA and protein expression level of IL-6 and
TNF-α in HG- and HF-induced GT1-7 cells compared with the Model +
Oe-LKB1 group. In addition, a partial increase in GnRH expression
was observed in the Model + Oe-LKB1 + compound C group compared
with the corresponding control group (Fig. 5C). In addition, ERβ, CD36 and
GPR54 expression was notably upregulated in HG- and HF-induced
GT1-7 cells with LKB1 overexpression and compound C treatment,
compared with the LKB1 overexpression-only group (Fig. 5D). These findings revealed that
LKB1 overexpression suppresses HG- and HF-induced inflammation and
GnRH expression in GT1-7 cells by activating AMPK/FOXO1
signaling.
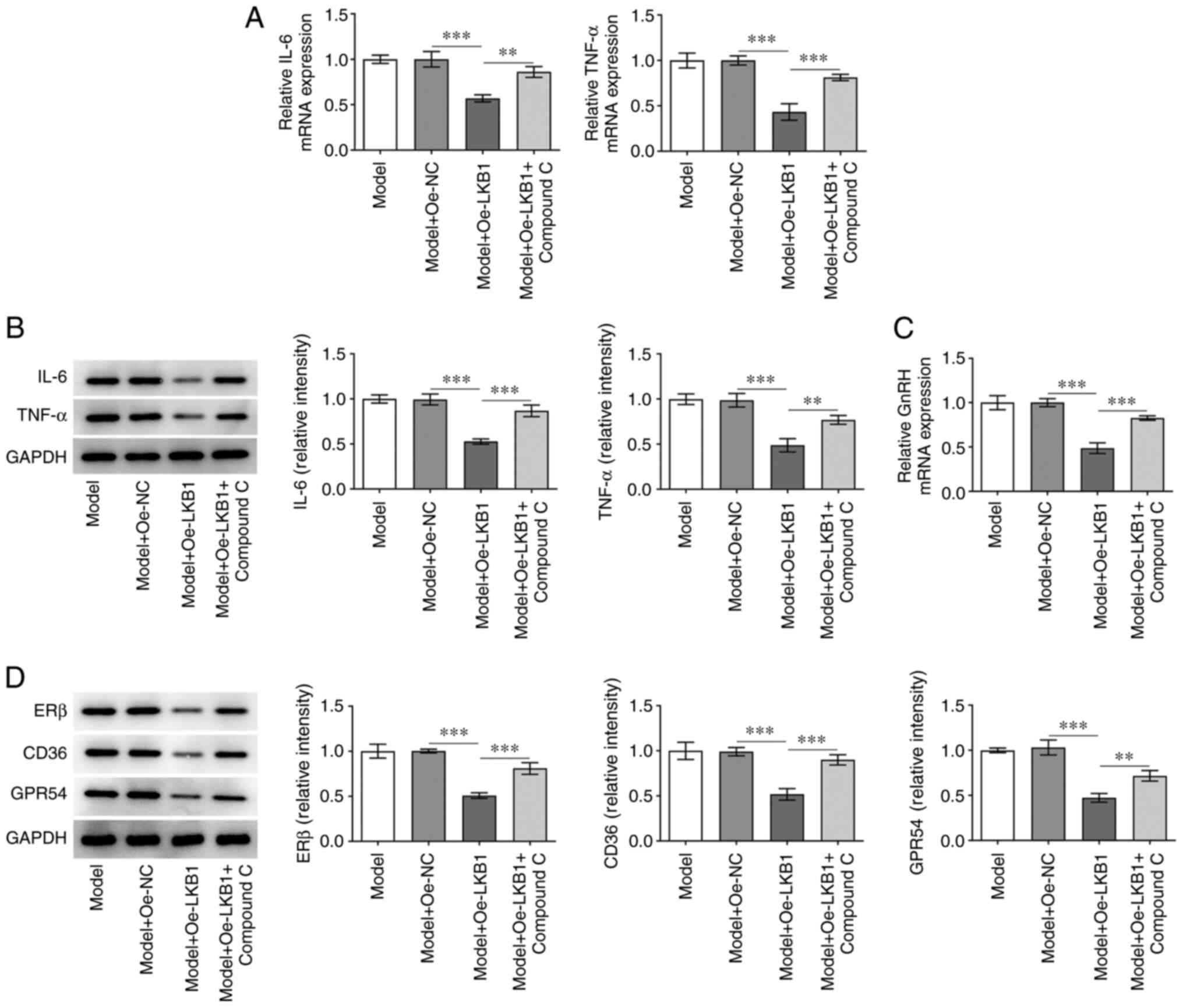 | Figure 5.Inactivation of AMPK/FOXO1 signaling
reversed the impacts of LKB1 overexpression on inflammation and
GnRH expression in HG- and HF-induced GT1-7 cells. Detection of
LKB1 (A) mRNA and (B) protein expression using RT-qPCR and western
blot analysis. (C) RT-qPCR was performed to examine GnRH
expression. (D) Measurement of ERβ, CD36 and GPR54 expression using
western blot analysis. **P<0.01 and ***P<0.001. AMPK,
AMP-activated protein kinase; FOXO1, forkhead box protein O1; LKB1,
live kinase B1; GnRH, gonadotropin-releasing hormone; HF, high fat;
HG, high glucose; RT-qPCR, reverse transcription-quantitative qPCR;
ERβ, estrogen receptor-β; CD36, cluster of differentiation 36;
GPR54, G-protein-coupled receptor; Oe, overexpression; NC, negative
control. |
Discussion
PP is one of the most common endocrine diseases in
children; it is characterized by an early onset of puberty and has
a far-reaching influence on children's growth, development and
mental health. ICPP is caused by the premature activation of the
HPG axis (15). Obesity caused by
excessive intake of lipids and sugars is considered to play an
important role in the occurrence of ICPP (6). ICPP can lead to metabolic
abnormalities and affect the central nervous system. It has been
shown that a HG and HF diet can affect the expression of ER- and
PP-related genes in hypothalamic cells (7). In the present study, a HG- and
HF-induced GT1-7 mouse hypothalamic cell line was used as the PP
in vitro model to simulate the physiological environment of
PP in the body, as previously described (7). The role of LKB1 in the progression
of PP in this in vitro model was also explored. It was
demonstrated that LKB1 could alleviate HG- and HF-induced
inflammation and GnRH expression in mouse hypothalamic cells
through the activation of the AMPK/FOXO1 signaling pathway.
LKB1, also known as STK11, is a serine/threonine
kinase that is widely expressed in mammalian tissues (16,17). A recent study demonstrated that
LKB1 is closely associated with obesity and LKB1 deletion in the
hypothalamus intensifies susceptibility to obesity in mice
administered with a HF diet, accompanied by a deterioration of
hypothalamic inflammation and decreased neuronal expression
(9). Wu et al (18) also report LKB1 as a novel
potential therapeutic target, due to its significant suppressing
effects on hypothalamic inflammation and alleviating effects on
diet-induced obesity in mice. As a key regulator of energy
metabolism, an intraventricular injection of LKB1 in rats induced
by diet is found to suppress the occurrence of obesity through the
activation of the AMPK-proopiomelanocortin neuronal axis (19). The present study found that LKB1
expression was significantly decreased in the peripheral blood and
PBMCs of children with PP, as well as HG- and HF-induced mouse
hypothalamic cells, suggesting that the abnormal LKB1 expression
may be associated with PP, an obesity-related disease.
A number of studies have confirmed that LKB1 serves
a crucial anti-inflammatory role in multiple diseases. For
instance, activating LKB1 relieves thioacetamide-induced hepatic
fibrosis and inflammation in mice (20). Chen et al (20) demonstrate that LKB1 contributes to
a decreased inflammatory state in skeletal muscle by suppressing
the expression of inflammation-related genes, including IL-6 and
TNF-α. Another previous study suggests that LKB1 upregulation
reduces the production of inflammatory cytokines in macrophages
infected with mycobacterium tuberculosis (21). Notably, LKB1 elevation ameliorates
hypothalamic inflammation and relieves diet-induced obesity in mice
(18). In the present study, LKB1
overexpression markedly decreased the HG- and HF-induced elevation
of IL-6 and TNF-α expression. Furthermore, ERs exert strong effects
on the maintenance of female secondary sexual characteristics and
reproductive cycles and affect fertility (22). Estrogen and its receptors adjust
the synthesis and release of GnRH by acting on GnRH neurons in the
hypothalamus, thereby regulating the entire reproductive system
(23). ERβ, CD36 and GPR54 are
important ER- and sexual precocity-related genes, which have been
shown to be expressed in GT1-7 cells (7). The present study showed that the
expression of ERβ, CD36 and GPR54 was clearly decreased following
LKB1 overexpression in GT1-7 cells under HG and HF conditions,
compared with the control group, which was in line with a previous
study performed by Wang et al (7).
The cellular functions of LKB1 are considered to be
achieved through the phosphorylation of AMPK (19). As the upstream kinase capable of
AMPK, LKB1 can activate AMPK through the phosphorylation of p-AMPKα
of its catalytic α subunit (24).
In the endometrial glands, LKB1 can promote the phosphorylation of
AMPK, thereby increasing the activity of FOXO1 (10). A clear reduction in GnRH release
is observed following the elevation in the FOXO1 activity in the
hypothalamus (11). The results
of the present study suggested that LKB1 gain-of-function increased
the expression of p-AMPK and p-FOXO1 in HG- and HF-induced GT1-7
cells. In order to explore whether LKB1 could regulate
inflammation, as well as the expression of GnRH and sexual
precocity-related genes by activating AMPK/FOXO1 signaling,
compound C, an inhibitor of AMPK/FOXO1 signaling, was used to treat
GT1-7 cells. It was found that the use of compound C suppressed the
effect of LKB1 overexpression on inflammation, GnRH and sexual
precocity-related gene expression.
In conclusion, LKB1 suppressed the inflammation,
GnRH and sexual precocity-related gene expression by inactivating
AMPK/FOXO1 signaling in HG- and HF-induced GT1-7 cells. LKB1 may
serve as an effective biomarker for PP and therefore a novel target
for PP treatment. The lack of the upstream mechanism of LKB1 and
the animal study are limitations of the present study, therefore,
comprehensive analysis is required in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by Scientific Research
Foundation of Fujian Maternal and Child Health Hospital (grant no.
YCXM20-18).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL, LG and QZ designed the study and performed the
experiments. HL and QZ drafted and revised the manuscript. LG, HH
and LX analyzed the data. HH and LX performed the literature
search. All authors have read and approved the final manuscript. HL
and LX confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Fujian Maternity and Child Health Hospital (approval number is
2020KY043.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neeman B, Bello R, Lazar L, Phillip M and
de Vries L: Central precocious puberty as a presenting sign of
nonclassical congenital adrenal hyperplasia: Clinical
characteristics. J Clin Endocrinol Metab. 104:2695–2700. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bai GL, Hu KL, Huan Y, Wang X, Lei L,
Zhang M, Guo CY, Chang HS, Zhao LB, Liu J, et al: The traditional
chinese medicine fuyou formula alleviates precocious puberty by
inhibiting GPR54/GnRH in the hypothalamus. Front Pharmacol.
11:5965252021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ullah R, Su Y, Shen Y, Li C, Xu X, Zhang
J, Huang K, Rauf N, He Y, Cheng J, et al: Postnatal feeding with
high-fat diet induces obesity and precocious puberty in C57BL/6J
mouse pups: A novel model of obesity and puberty. Front Med.
11:266–276. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plant TM: Neuroendocrine control of the
onset of puberty. Front Neuroendocrinol. 38:73–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berberoglu M: Precocious puberty and
normal variant puberty: Definition, etiology, diagnosis and current
management. J Clin Res Pediatr Endocrinol. 1:164–174. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sinthuprasith P, Dejkhamron P, Wejaphikul
K and Unachak K: Near final adult height, and body mass index in
overweight/obese and normal-weight children with idiopathic central
precocious puberty and treated with gonadotropin-releasing hormone
analogs. J Pediatr Endocrinol Metab. 32:1369–1375. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang S, Yao H, Ding L, Gao Y, Wang P and
Xue Y: Effects of high-glucose and high-fat condition on estrogen
receptor- and sexual precocity-related genes in GT1-7 cells. Med
Sci Monit. 26:e9228602020.PubMed/NCBI
|
|
8
|
Shukuya T, Yamada T, Koenig MJ, Xu J,
Okimoto T, Li F, Amann JM and Carbone DP: The effect of LKB1
activity on the sensitivity to PI3K/mTOR inhibition in non-small
cell lung cancer. J Thorac Oncol. 14:1061–1076. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Z, Han J, Xue J, Xi P, Wang H, He L,
Wang Q, Liang H, Sun X and Tian D: Deletion of liver kinase B1 in
POMC neurons predisposes to diet-induced obesity. Life Sci.
258:1182042020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li SY, Song Z, Yan YP, Li B, Song MJ, Liu
YF, Yang ZS, Li MY, Liu AX, Quan S and Yang ZM: Aldosterone from
endometrial glands is benefit for human decidualization. Cell Death
Dis. 11:6792020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi C, Shi R and Guo H: Tumor necrosis
factor α reduces gonadotropin-releasing hormone release through
increase of forkhead box protein O1 activity. Neuroreport.
31:473–477. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pagani S, Calcaterra V, Acquafredda G,
Montalbano C, Bozzola E, Ferrara P, Gasparri M, Villani A and
Bozzola M: MKRN3 and KISS1R mutations in precocious and early
puberty. Ital J Pediatr. 46:392020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao J, Niu G, Yin S, Xie S, Li Y, Nie D,
Ma L, Wang X and Wu Y: The role of AMP-activated protein kinase in
quercetin-induced apoptosis of HL-60 cells. Acta Biochim Biophys
Sin (Shanghai). 46:394–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eugster EA: Treatment of central
precocious puberty. J Endocr Soc. 3:965–972. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Zhang W, Zhang M, Zhu H, Moriasi C
and Zou MH: Liver kinase B1 suppresses lipopolysaccharide-induced
nuclear factor κB (NF-κB) activation in macrophages. J Biol Chem.
290:2312–2320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shan T, Xu Z, Liu J, Wu W and Wang Y: Lkb1
regulation of skeletal muscle development, metabolism and muscle
progenitor cell homeostasis. J Cell Physiol. 232:2653–2656. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Z, Xi P, Zhang Y, Wang H, Xue J, Sun X
and Tian D: LKB1 up-regulation inhibits hypothalamic inflammation
and attenuates diet-induced obesity in mice. Metabolism.
116:1546942021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xi P, Du J, Liang H, Han J, Wu Z, Wang H,
He L, Wang Q, Ge H, Li Y, et al: Intraventricular injection of LKB1
inhibits the formation of diet-induced obesity in rats by
activating the AMPK-POMC neurons-sympathetic nervous system axis.
Cell Physiol Biochem. 47:54–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen T, Hill JT, Moore TM, Cheung ECK,
Olsen ZE, Piorczynski TB, Marriott TD, Tessem JS, Walton CM, Bikman
BT, et al: Lack of skeletal muscle liver kinase B1 alters gene
expression, mitochondrial content, inflammation and oxidative
stress without affecting high-fat diet-induced obesity or insulin
resistance. Biochim Biophys Acta Mol Basis Dis. 1866:1658052020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui J, Li M, Liu W, Zhang B, Sun B, Niu W
and Wang Y: Liver kinase B1 overexpression controls mycobacterial
infection in macrophages via FOXO1/Wnt5a signaling. J Cell Biochem.
120:224–231. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alejandro EU: Males require estrogen
signaling too: Sexual dimorphism in the regulation of glucose
homeostasis by nuclear ERα. Diabetes. 68:471–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Izzi-Engbeaya C, Hill TG and Bowe JE:
Kisspeptin and glucose homeostasis. Semin Reprod Med. 37:141–146.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Woods A, Johnstone SR, Dickerson K, Leiper
FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M and
Carling D: LKB1 is the upstream kinase in the AMP-activated protein
kinase cascade. Curr Biol. 13:2004–2008. 2003. View Article : Google Scholar : PubMed/NCBI
|