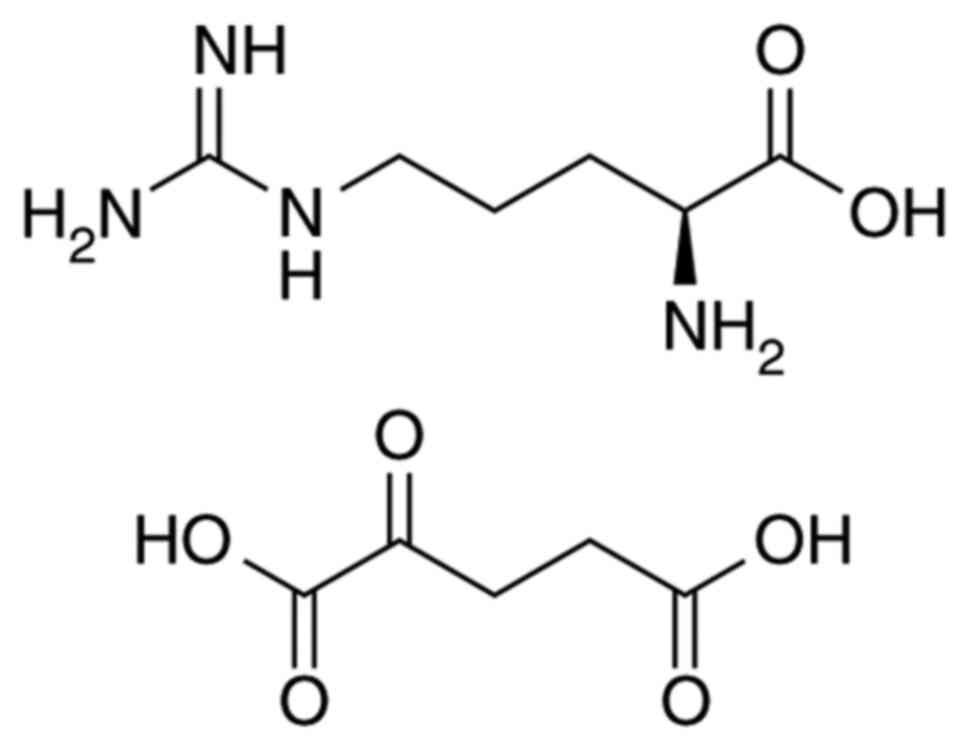
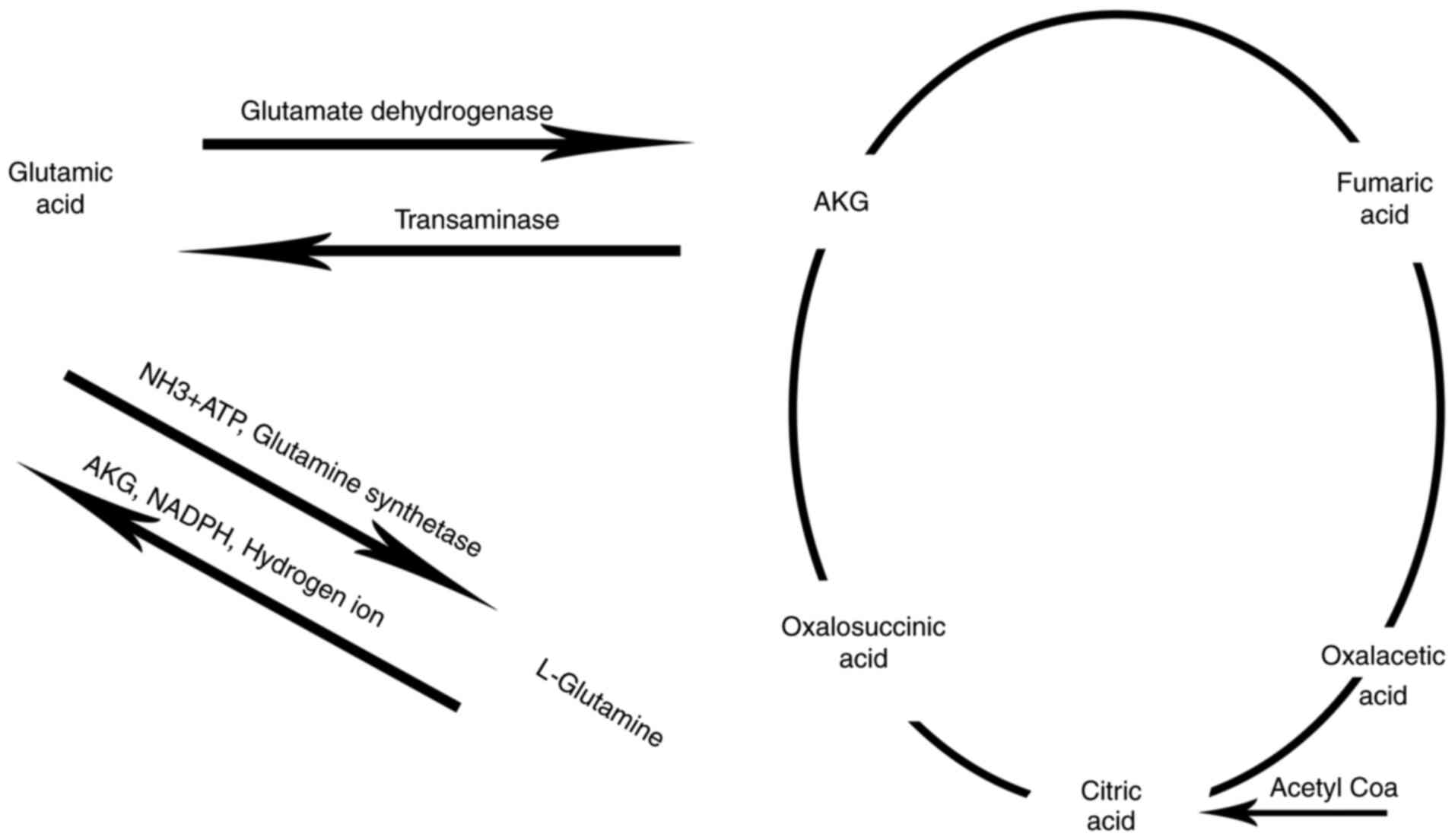
Introduction
α-Ketoglutaric acid (AKG) is involved in the
metabolism of carbohydrates, fatty acids and proteins via numerous
mechanisms (1). As an important
intermediate product in the tricarboxylic acid (TCA) cycle, AKG is
involved in both the source and pathway of carbon in the body.
Through the action of transaminase and the combined deamination,
AKG is converted into glutamic acid (2). In the presence of ammonia,
L-glutamate dehydrogenase also reverses the production of glutamate
from AKG to form other non-essential amino acids in the body.
Ammonia is produced in the amino acid catabolism process, and toxic
ammonia is eliminated via the urea cycle (3). AKG also plays a key role in the urea
cycle, and is metabolized in a similar manner as it is during the
purine nucleotide cycle (4).
During this cycle, AKG acts as an intermediate and participates in
the development, maturity and aging of the body. This cycle
demonstrates both the nutritional and physiological functions of
AKG, and provides a theoretical basis for the role of AKG in
clinical practice (5).
A previous study has demonstrated that AKG may act
as a dietary supplement, together with arginine and other amino
acids, to help athletes recover in a timely manner (6). In the cardiovascular system, AKG may
prevent or even reverse the aging of animals with arteriosclerosis
(7). During exercise, AKG acts as
a synthetic metabolic signal and promotes protein synthesis, which
in turn contributes to skeletal muscle development and increases
collagen metabolism, thus affecting bone structure through the
interaction between glutamate and glutamate receptors (8–10).
In the digestive system, AKG, as a glutamate family metabolite,
participates in the TCA cycle to provide energy for intestinal
epithelial cells, helps to maintain intestinal mucosal integrity,
promotes intestinal structural development and improves the
intestinal absorption of nutrients (11). Recent studies have suggested that
AKG may play a key role in promoting healthy aging (12,13). Following the increase of aging
populations worldwide, further investigations are required to focus
on improving the quality of life as the rates of life expectancy
increase. Therefore, current research has focused on the potential
role of AKG in age-related diseases, such as cancer, obesity and
diabetes. In recent years, increasing attention has been paid to
the metabolic and regulatory effects of AKG, but few reviews have
been carried out on the clinical application of AKG. Combining
these advances with the role of AKG in clinical practice may
provide a novel theoretical basis for the use of AKG in improving
long-term human health (Fig.
1).
Synthetic paths and methods
AKG is an intermediate metabolite of the TCA cycle.
It is involved in the synthesis and energy metabolism of amino
acids, vitamins and organic acids. It has a wide range of
application prospects and exhibits key applications in the field of
medicine, food, animal husbandry and agriculture (14–16). The method of synthesis mainly
involves the biosynthetic pathway and fermentation.
AKG is an intermediate product of the microbial TCA
cycle, and three important metabolic nodes are selected in the
biosynthetic pathway, namely, the
phosphoenolpyruvate-pyruvate-oxaloacetate node, the citric acid
node and the AKG node. Oxaloacetate is an important precursor for
the synthesis of AKG. In addition to originating from the TCA
cycle, it can also be obtained through the replenishment pathway,
which is catalyzed by pyruvate carboxylase and phosphoenolpyruvate
carboxylase. The former uses biotin as a coenzyme, and the latter
is usually feedback-inhibited by aspartate and AKG. Different
microorganisms contain either one or two of the aforementioned
carboxylases. For example, Escherichia coli (E. coli) only
contains phosphoenolpyruvate carboxylase, Bacillus subtilis
and Yarrowia lipolytica only contain pyruvate carboxylase,
and Corynebacterium glutamicum only contains pyruvate
carboxylase. The citrate node is an important metabolic node
controlling the metabolic flux of the TCA cycle, and citrate
synthase is the key enzyme of this node. Notably, its activity is
often feedback-inhibited by citric acid, adenosine triphosphate
(ATP) and NADP (17,18). The AKG node determines its
metabolic destination, and AKG synthesizes succinyl-CoA and
glutamate under the catalysis of α-ketoglutarate dehydrogenase and
glutamate dehydrogenase, respectively. Moreover, α-ketoglutarate
dehydrogenase uses thiamine as a coenzyme. Therefore, when thiamine
is insufficient in the medium, AKG will flow to glutamate due to
the weakened metabolic flux of the TCA cycle (18).
The production of AKG using fermentation was
initiated by Lockwood and Stodola (19) using Pseudomonas
fluorescens. Subsequently, various microorganisms were found to
be able to synthesize AKG in excess (20). Corynebacterium glutamicum,
Yarrowia lipolytica (21),
Saccharomyces glabrata and other yeasts are considered ideal
for the production of AKG using fermentation, due to their notable
physiological and genetic characteristics, and their rapid growth
(22). Therefore, current
research focusing on breeding AKG-producing strains mainly uses the
three aforementioned microorganisms, and the main strategies
include enhancing the supply of AKG precursors and weakening the
competitive metabolic branch (Fig.
2).
AKG, ketoglutaric acid, industrial synthesis method,
circulatory system, locomotion system, endocrine system, digestive
system and productive system were searched as keywords. Four major
electronic databases (PubMed, Springer, Wiley, and ScienceDirect)
were used to retrieve relevant literature in the past three
decades. After reading the articles, articles with relatively
complete experimental verification of the role of AKG in the body
were selected to be included in the references. The systemic
association between AKG and the development of diseases in the body
was summarized, and potential clinical application of AKG was
determined.
AKG in the circulatory system
At present, there is an increasing number of aging
populations in society, and cardiovascular disease attracts high
levels of attention as it is age-related (23). AKG exists as an important keto
acid in the blood. In previous clinical trials, blood samples of
participants were collected in test tubes containing
ethylenediaminetetraacetate. Following centrifugation, the plasma
samples were frozen at −80°C, until the sample was thawed and
centrifuged at 10,000 Hz for 10 min at 4°C. A total of 300 µl of
each sample was transferred to a 5-mm NMR tube, 200 µl of 0.2 mol/l
phosphate buffer and 50 µl D2O were added, and the
levels of AKG were determined using liquid chromatography-mass
spectrometry/mass spectrometry.
The content of AKG in the blood indicates the
conditions of vital organs and other systems. When hepatic
encephalopathy occurs, changes occur in both the mental state and
the blood components of the individual, and AKG levels in the blood
will rise. The concentration of AKG in the blood of patients with
diabetes significantly decreased (r=0.367; P<0.05) (24). AKG can be used as an antioxidant
in a multi-component solution to aid oxidative stress, and may be
used for the suppression of Kupffer cell activation during liver
transplantation and attenuates hepatic ischemia-reperfusion injury
(25). Results of a previous
study have demonstrated that AKG protects ischemic organs by
inhibiting dioxygenase Egln1, which may provide a novel method for
solving ischemic preconditioning in organ transplantation (26). The decrease of blood vessel
elasticity directly affects the function of the circulatory system
(27). Previous cellular
experiments have demonstrated that AKG can increase the activity of
glutathione peroxidase, while inhibiting the activity of
superoxidase, protecting the body from a stable redox state, and
avoiding free radical damage (28). The serum levels of AKG can reflect
the clinical severity of chronic Heart Failure(CHF) in non-diabetic
patients, but not in those with type 2 diabetes mellitus, and it
may also be used as a potential indicator of systolic dysfunction
of the left ventricle. These data are in accordance with
observations that support the role of certain metabolites in CHF
severity (29). In addition, AKG
improves myocardial hypertrophic remodeling and fibrosis in
stress-overloaded hearts, by promoting mitophagy to clear damaged
mitochondria and reduce reactive oxygen species production
(30).
Results of a previous study demonstrated that a
combined solution containing AKG and 5-hydroxymethylfurfural may
exhibit antitumor potential due to its antioxidant activity. These
substances exhibited both caspase-3 and apoptosis-activating
effects on cell proliferation in Jurkat and HF-SAR cells,
highlighting them as anti-leukemia cell/antitumor combinations
(31). Levels of AKG in the blood
indicate the state of various organs in the circulatory system.
Therefore, the AKG content of the circulatory system is mainly used
as a pathological detection index for various cardiovascular
diseases. Moreover, novel treatment options for ischemic
preconditioning and cardiovascular protection may be developed due
to the antioxidant properties of AKG.
AKG in the locomotion system
Previous research demonstrated that in humans and
primates, the cortical motor system includes a collection of brain
regions mainly associated with motor control (32). AKG also plays an important role in
the anabolism of skeletal muscle (33).
Using animal models, results of a previous study
demonstrated that adding AKG to a low-protein diet significantly
increased amino acid synthesis and improved protein metabolism in
skeletal muscle by activating the mammalian target of the rapamycin
(mTOR) pathway (34). AKG
activates mTOR complex 1 and promotes the expression of collagen
(35). Additionally, AKG notably
inhibits the degradation of collagen in fibroblasts, which is
associated with the mechanism mediated by AKG through PHD3/ADRB2.
These findings provided a molecular basis for the potential use of
AKG produced by exercise in the treatment of muscle wasting, and
identified PHD3 as a potential target for the development of
muscle-wasting therapy (33).
During bone formation, AKG activates the JNK and
mTOR/S6K1/S6 signaling pathways independently of GPR99 activation,
thereby promoting osteoblast differentiation (36). In addition, AKG increases serum
proline levels, and AKG-Ca causes beneficial changes in serum
C-terminal cross-linking telopeptide of type I collagen in women
with postmenopausal osteopenia, which is consistent with the
retention of lumbar bone mass (37). AKG can also be supplemented in the
postpartum diet for newborns and exhibits the potential to
improve/maintain the bone structure of animals treated with the
maximum therapeutic dose of dexamethasone (Dex). When a group of
children were exposed to synthetic glucocorticoid (GC), adverse
effects, such as GC-induced osteoporosis and growth retardation
were apparent (38). Using AKG as
a supplement may act as a novel treatment method for children
receiving GC therapy. AKG can also be used as an important
supplement following surgical trauma. It prevents the reduction of
free glutamine that is often produced following surgery (39). AKG can also maintain the amino
acid concentration and protein synthesis in muscles, and reduce the
adverse effects caused by surgery (40). As a synthetic precursor of
important amino acids such as glutamate, AKG also has the
physiological function of activating pathway targets (41). Therefore, the main clinical
application of AKG will focus on promoting protein synthesis to
improve muscle function and bone cell differentiation.
AKG in the endocrine system
In the present review, two common metabolic
endocrine diseases associated with AKG are explored, namely,
diabetes and obesity. Diabetes is a complex disease that is not yet
fully understood. It often manifests as hyperglycemia and exhibits
complications of abnormal metabolic conditions associated with
systemic damage to the vascular bed (42). Accumulating evidence has
demonstrated that the endocrine system of patients with diabetes is
not functioning properly, and the metabolic mechanisms require
further investigation. Most patients at risk of developing type 2
diabetes suffer from hyperinsulinemia, which may be a response to
insulin resistance (43).
AKG also causes insulin secretion in B6 and BTBR
pancreatic islets. Therefore, the formation of AKG is a necessary
step for the response of mouse pancreatic islets to
ketoisocaproate. Existing evidence demonstrated that AKG directly
stimulated insulin secretion (44). According to the analysis of
mitochondrial mechanisms, AKG may be converted into AKG
intermediates, such as citric acid or glutamic acid, for
mitochondrial synthesis. Moreover, the mitochondrial 2-oxoglutarate
carrier is part of the metabolic pathway that mediates insulin
secretion stimulated by glucose and glutamine (45,46). In addition, AKG regulates the
formation of insulin by inhibiting glutamate dehydrogenase
(47). These results demonstrated
that the causes of abnormal insulin secretion are complex; however,
these findings suggested that AKG affects hyperinsulinemia. During
the development of complications associated with diabetes, AKG also
plays a role in determining prognosis. It is associated with
delayed diabetic wound healing (48), and high levels of matrix
metalloproteinase-9 (MMP-9) can predict poor healing of diabetic
foot ulcers. Results of a previous study demonstrated that compared
with non-diabetic wounds, the levels of AKG, TET2 and MMP-9 in
diabetic wounds were significantly increased (P=0.01). This
increase in AKG was associated with a local hypoxic-ischemic state
and poor systemic blood sugar control (48).
Obesity is a nutritional disorder caused by a
long-term imbalance between energy intake and consumption (49). It may lead to premature death,
preventable disease and disability (50). Obesity affects a variety of
endocrine systems. It also affects the changes in sex steroids and
thyroid hormones, in addition to the dynamic balance of the
hypothalamic-pituitary axis and vitamin D. AKG also plays an
important regulatory role in the metabolism of obesity (51).
Results of previous study have demonstrated that AKG
regulates the differentiation and function of brown fat by
regulating histone methylation through IDH1. Ectopic expression of
IDH1 inhibits the formation of brown fat, while suppression of IDH1
levels promotes the differentiation of brown adipocytes. Moreover,
overexpression of IDH1 leads to increased levels of intracellular
AKG and inhibits the expression of genes involved in brown fat
formation. The IDH1-AKG axis plays an important role in regulating
the differentiation of brown adipocytes, and may therefore
represent a target for the treatment of metabolic diseases. Results
of previous studies have also demonstrated that in the early brown
fat formation process, the cellular level of AKG, a key metabolite
required for TET-mediated DNA demethylation, is greatly increased,
and it is the PRDM16 promoter required for active DNA demethylation
(52,53). AMPKα1 excision reduces isocitrate
dehydrogenase 2 activity and cellular AKG levels. Notably, the
activation of AMPK with AICAR or metformin after delivery reverses
inhibition of brown fat formation and thermogenesis caused by
obesity. In summary, AMPK is essential for the epigenetic control
of BAT development through AKG. Obesity is also associated with
macrophage infiltration and metabolic inflammation (54), both of which promote the
progression of metabolic diseases.
As a metabolic intermediate product of the TCA
cycle, AKG participates in regulation. Existing study have
confirmed that melatonin reduces fat inflammation by increasing AKG
and transferring fat-derived exosomes to mouse macrophages.
Melatonin reduces fat inflammation by increasing exosomal AKG. It
is transported to macrophages and promotes TET-mediated DNA
demethylation, reduces inflammation of fat cells, and increases the
ratio of M2 to M1 macrophages. In addition, exosomal AKG attenuates
signal transducers and activators of transduction-3 (STAT3)/NF-κB
signaling in adipocytes through its receptor oxoglutamate receptor
1. AKG can be used to determine the presence of non-alcoholic fatty
liver in morbidly obese patients, but it is not an effective
biomarker of steatohepatitis (55). Further research is required to
examine whether AKG can be used as an effective biomarker for other
obesity-related endocrine system diseases. In the endocrine system,
AKG mainly participates in the circulation and exerts a role
through its keto acid properties. Previous study have demonstrated
that AKG affects the production of insulin and the development of
hyperglycemia, but further experimental evidence is required for
the development of clinical treatment options for diabetes. AKG can
affect the differentiation of brown fat by regulating the
methylation of histones, and can also affect the inflammation of
adipose tissues, which may further guide clinical studies on AKG
intervention in obesity.
AKG in the digestive system
Certain functions of the digestive system
deteriorate as the body ages (55). The blood supply of the digestive
system is rich with both internal and external components.
Intramural blood vessels have plexiforms in different layers of the
intestinal wall, and function at the junction of the liver, small
intestine and gastro-esophagus to adapt to the functions of these
organs (56).
As an important intermediate product of the TCA
cycle, AKG in the blood is used as a biological indicator of the
health of the digestive system. Existing evidence has suggested
that compared with those in normal tissues, the levels of Krebs
cycle components, such as AKG, succinic acid, fumaric acid, malic
acid and oxaloacetic acid in gastric cancer tissues are
significantly increased (57). In
addition, compared with those in normal tissues, the levels of
glycolysis products, including pyruvate and lactic acid, and the
level of ketone bodies, such as 3-hydroxybutyrate in cancer tissues
are also significantly increased. The levels of ketone bodies in
cancer tissues with different histological characteristics are
notably increased. Similarly, trimethylamine oxide, hippurate,
3-indolyl sulfate, 2-oxoglutarate and citrate can be used as useful
urine biomarkers for gastric tumorigenesis in a mouse model
(58).
On one hand, this suggests the complexity of cancer
metabolism in the digestive system, and on the other hand, it
provides novel theories for the early diagnosis of digestive system
cancer. Cachexia occurs after tumors have formed in the digestive
system and is characterized by extreme weight loss, anemia,
weakness and inability of patients to take care of themselves.
Increased AKG and decreased glucose are observed in cachexia
gastrocnemius. In vitro experiments demonstrated that AKG
promotes the proliferation of myoblasts and reduces myotube atrophy
caused by glucose deficiency, providing a novel theoretical basis
for the development of novel treatment options for cachexia
(59).
The functioning of the digestive system is closely
associated with the growth and development of newborns. As a
prenatal preventive drug, AKG can also be used to treat the side
effects of Dex (60). Results of
further animal experiments have demonstrated that 2-oxoglutaric
acid increased cell proliferation in the duodenum and jejunum, as
well as the number and maturity of peripheral blood lymphocytes. It
supports the integrity of the epithelium and adapts the shape of
the nerve plexus. Administration of 2-oxoglutarate to piglets
during lactation completely eliminated intestinal damage caused by
Dex. Moreover, AKG reduced the incidence of diarrhea and reversed
the concentration of these serum parameters, as AKG upregulates the
expression of intestinal epithelial Water and ion absorption via
sensitive aquaporins and reduces the expression of ion transporters
(61). Lipopolysaccharide reduces
both gene and protein expression of components in the AMPK pathway,
including AMPKα1, AMPKα2, SIRT1, PGC-1α, ACC and TORC2, in the
jejunum and ileum. Notably, supplementing with AKG enhanced the
abundance of these proteins in piglets treated with
lipopolysaccharide. In addition, AKG plays an important role in
maintaining the homeostasis of water and ions by regulating the
AMPK pathway. Duodenal injection of AKG also reduced the growth
energy consumption of piglets (62). Furthermore, 1% AKG combined with
0.5% allicin in the diet improved the microbial composition and
diversity of the cecum, which may further promote the volatile
fatty acid metabolism of growing pigs.
AKG also altered the expression and energy status of
AMPK in the intestinal mucosa of piglets affected by E. coli
lipopolysaccharide (63). AKG
reversed the adverse effects of lipopolysaccharide and plays a key
role in the prevention and treatment of neonatal intestinal
dysfunction. AKG also prevented oxidative stress by activating
constitutive-androstane-receptor signals and regulating the
expression of key antioxidant-related targets, both in vivo
and in vitro, by improving cell respiration and antioxidant
capacity (64). The in
vivo and in vitro effects of AKG indicated that it may
be used to reduce oxidative stress and subsequently prevent
gastrointestinal diseases in both animals and humans. As an
intermediate product of glucose and lipid metabolism, AKG is
present in the gastrointestinal system, functioning as an indicator
of gastrointestinal diseases. Moreover, the nutritional function of
AKG may also reduce cachexia, thus improving intestinal development
of newborns, and preventing the occurrence of gastrointestinal
diseases. These factors demonstrate a novel direction for the
future clinical application of AKG.
AKG in the reproductive system
As the human body ages, the function of the
reproductive system gradually declines. Previous research
demonstrated that AKG is closely associated with the reproductive
aging of mammals, such as mice, pigs and humans (65).
In the female reproductive system, a potential
association was notable between the level of AKG in human
follicular fluid and the age of patients (66). In addition, AKG promotes porcine
oocyte maturation in vitro through an increase in the
excretion rate of polar bodies, and an increase in the rate of
parthenogenetic blastocysts and total cell number (67). Results of a previous study have
demonstrated that reducing the uptake of pyruvic acid into the
mitochondria in fetal mouse ovarian tissue culture resulted in the
inhibition of early follicular formation, a negative effect that
can be partially reversed by AKG (68). This highlighted the importance of
this metabolite.
In the male reproductive system, the lactate
hydrogenase isoenzyme LDHC in the testis converts pyruvate into
lactic acid, and AKG into S-2HG, thereby altering histones or DNA
methylation, and controlling epigenetics (69). hGDH2 (rather than hGDH1) is
densely expressed in Sertoli cells, which are known to provide
sperm with lactic acid and other nutrients. AKG, as a product of
glutamate dehydrogenase, catalyzes the reversible deamination of
L-glutamate (70). In addition,
results of previous study have demonstrated that the addition of
AKG when culturing sperm in vitro significantly enhances the
levels of tyrosine phosphorylation protein and provides sufficient
ATP for sperm movement. Moreover, AKG can be used as an effective
antioxidant to protect rat sperm from hydrogen peroxide attack,
which may improve the antioxidant capacity of Biggers, Whitten and
Whittingham medium (71).
Results of a previous study also demonstrated that
an AKG-based formula received by 42 individuals reduced biological
aging by an average of eight years (P=6.538×10−12).
Thus, such interventions may demonstrate potential in slowing the
process of biological aging (72).
Conclusion
AKG is an essential metabolite of almost every
organism and plays an important role in numerous biological systems
(73). Not only is it a central
metabolite, but also an important ketoacid. It exerts numerous
functions, including energy metabolism, antioxidant defense, signal
transduction and genetic regulation (64,74,75).
Further investigations into AKG are required. For
example, systematic clinical data demonstrating its presence in
healthy human serum is lacking, which highlights that the role of
AKG in different disease states must be further elucidated. In
addition, the numerous therapeutic and preventive effects of AKG
supplementation on the body are almost all based on the results of
animal experiments. A lack of clinical experimental data indicates
that AKG cannot yet be directly used as a dietary supplement. Thus,
further animal experiments are required and additional clinical
sample data must be obtained.
Results of the present study demonstrated the role
of AKG in aging, and further highlighted AKG as one of the most
effective solutions for age-related diseases, such as diabetes,
hyperlipidemia and cancer. AKG may be involved in aging-related
chronic diseases through the regulation of oxidative stress and
other pathways, and therefore exhibits potential as an adjuvant
therapy drug (Table I) (76).
 | Table I.Clinical application of
α-ketoglutaric acid in disease. |
Table I.
Clinical application of
α-ketoglutaric acid in disease.
| Application of
AKG | Test subject | Dose | Supplementary
method | Author, year | (Refs.) |
|---|
| Continuous infusion
of AKG protects ischemic organs | Lewis male rat | 10 mg/kg/min | Perfusion | Olenchock et
al, 2016 | (26) |
| As an indicator of
the severity of CHF in diabetic patients | Clinical patient
serum | Measure
content |
| Chen et al,
2018 | (29) |
| As an indicator of
left ventricular systolic dysfunction | Clinical patient
serum | Measure
content |
| An et al,
2021 | (30) |
|
|
|
|
| Greilberger et
al, 2021 | (31) |
| AKG supplementation
reduces protein degradation caused by corticosterone | C57BL/6J male
mice | 2% Drinking
water | Drinking water | Ge et al,
2018 | (34) |
|
|
|
|
| Xiong et al,
2018 | (35) |
|
|
|
|
| Zurek et al,
2019 | (36) |
| AKG supplementation
to reduce bone loss after menopause | Clinical
patient | 6 g AKG and 1.68 g
Ca daily | Oral | Sliwa et al,
2006 | (38) |
| AKG supplementation
reduces osteoporosis and growth retardation caused by
dexamethasone | Large polish white
breed sows | 0.4 g/kg/day | Oral | Hammarqvist et
al, 1991 | (39) |
| AKG supplementation
causes increased insulin secretion | B6 and BTBR mouse
cells | 15 mM |
| Macdonald,
2003 | (45) |
| Increasing AKG
affects slow healing of diabetic wounds | Clinical patient
serum, urine, wound fluid | Measure
content |
| Ren et al,
2019 | (49) |
| AKG supplementation
is beneficial to early brown fat recruitment and activation | C57BL/6J male
mouse/C3H 10T1R cells | 1 mM |
| Yang et al,
2016 | (53) |
| AKG supplementation
helps reduce fat inflammation | C57BL/6J male
mice | 2 g/kg/day | Oral | Liu et al,
2016 | (54) |
| As an indicator of
non-alcoholic fatty liver in morbidly obese women | Clinical patient
serum | Measure
content |
| Shamburek and
Farrar, 1990 | (55) |
| As a biomarker for
gastric cancer | Clinical patient
gastric cancer tissue/mouse | Organic acid mass
spectroscopy/NMR spectroscopy |
| Hur et al,
2014 | (57) |
|
|
|
|
| Kim et al,
2010 | (58) |
| Supplementation of
AKG reduces intestinal injury induced by dexamethasone | Piglet | 0.4 g/kg/day | Oral | He et al,
2017 | (61) |
| AKG supplementation
helps regulate intestinal water and ion homeostasis | Piglet | 1% diet | Diet | Junghans et
al, 2006 | (62) |
| AKG supplementation
is beneficial to reverse the intestinal damage caused by
lipopolysaccharide | Piglet | 1% diet | Diet | He et al,
2018 | (64) |
| AKG supplementation
is beneficial to slow down the decline of mammalian fertility | Clinical
patient/pig/mouse/cell etc. |
|
| Tanaka et
al, 2021 | (67) |
| AKG supplementation
is beneficial to in vitro maturation of porcine oocytes | Pig oocyte | 5–20 mM |
| Plaitakis et
al, 2017 | (69) |
| A potential
life-extending compound formulation | A group of 42
self-reported healthy individuals (14 females and 28 males) who had
submitted saliva samples | Each dose contained
1 g of calcium α-ketoglutarate | Oral | Demidenko et
al, 2021 | (72) |
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific Research Key
Funding Project (grant no. 19A428) of the Ministry of Education of
China Hunan Foundation, the Natural Science Foundation of Hunan
Province (grant no. 2021JJ30593), the College Student Innovation
and Entrepreneurship Training Program of the University of South
China (grant no. X202110555543) and the Postgraduate Research and
Innovation Project (grant no. CX2020961) of China Hunan
Foundation.
Availability of data and materials
Not applicable.
Authors' contributions
SL and WH designed the outline for this review. XM,
HL and LP wrote the manuscript The final version of the manuscript
has been read and approved by all authors. Data authentication is
not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xiao D, Zeng L, Yao K, Kong X, Wu G and
Yin Y: The glutamine-alpha-ketoglutarate (AKG) metabolism and its
nutritional implications. Amino Acids. 48:2067–2080. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunha RA: How does adenosine control
neuronal dysfunction and neurodegeneration? J Neurochem.
139:1019–1055. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Medeiros HC, Constantin J,
Ishii-Iwamoto EL and Mingatto FE: Effect of fipronil on energy
metabolism in the perfused rat liver. Toxicol Lett. 236:34–42.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeevanandam M, Begay CK, Holaday NJ and
Petersen SR: Nutritional and metabolic effects and significance of
mild orotic aciduria during dietary supplementation with arginine
or its organic salts after trauma injury in rats. Metabolism.
46:785–792. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Legendre F, MacLean A, Appanna VP and
Appanna VD: Biochemical pathways to alpha-ketoglutarate, a
multi-faceted metabolite. World J Microbiol Biotechnol. 36:1232020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valenzuela PL, Morales JS, Emanuele E,
Pareja-Galeano H and Lucia A: Supplements with purported effects on
muscle mass and strength. Eur J Nutr. 58:2983–3008. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niemiec T, Sikorska J, Harrison A, Szmidt
M, Sawosz E, Wirth-Dzieciolowska E, Wilczak J and Pierzynowski S:
Alpha-ketoglutarate stabilizes redox homeostasis and improves
arterial elasticity in aged mice. J Physiol Pharmacol. 62:37–43.
2011.PubMed/NCBI
|
|
8
|
Radzki RP, Bienko M, Filip R and
Pierzynowski SG: The protective and therapeutic effect of exclusive
and combined treatment with alpha-ketoglutarate sodium salt and
ipriflavone on bone loss in orchidectomized rats. J Nutr Health
Aging. 20:628–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tomaszewska E, Swiatkiewicz S,
Arczewska-Wlosek A, Wojtysiak D, Dobrowolski P, Domaradzki P,
Świetlicka I, Donaldson J, Hułas-Stasiak M and Muszyński S:
Alpha-Ketoglutarate: An effective feed supplement in improving bone
metabolism and muscle quality of laying hens: A preliminary study.
Animals (Basel). 10:24202020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang R, Wang X, Liu S, Zhang W, Wang P,
Liu X, Ren Y, Tan X and Chi B: Bioinspired poly (ү-glutamic acid)
hydrogels for enhanced chondrogenesis of bone marrow-derived
mesenchymal stem cells. Int J Biol Macromol. 142:332–344. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian Q, Bravo Iniguez A, Sun Q, Wang H, Du
M and Zhu MJ: Dietary alpha-ketoglutarate promotes epithelial
metabolic transition and protects against DSS-induced colitis. Mol
Nutr Food Res. 65:e20009362021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asadi Shahmirzadi A, Edgar D, Liao CY, Hsu
YM, Lucanic M, Asadi Shahmirzadi A, Wiley CD, Gan G, Kim DE, Kasler
HG, et al: Alpha-Ketoglutarate, an endogenous metabolite, extends
lifespan and compresses morbidity in aging mice. Cell Metab.
32:447–456, e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Deng P, Liu Y, Wu Y, Chen Y, Guo
Y, Zhang S, Zheng X, Zhou L, Liu W, et al: Alpha-ketoglutarate
ameliorates age-related osteoporosis via regulating histone
methylations. Nat Commun. 11:55962020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gyanwali B, Lim ZX, Soh J, Lim C, Guan SP,
Goh J, Maier AB and Kennedy BK: Alpha-Ketoglutarate dietary
supplementation to improve health in humans. Trends Endocrinol
Metab. 33:136–146. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto T, Sato K, Yamaguchi M, Mitamura
K and Taga A: Development of simultaneous quantitative analysis of
tricarboxylic acid cycle metabolites to identify specific
metabolites in cancer cells by targeted metabolomic approach.
Biochem Biophys Res Commun. 584:53–59. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Woyke S, Mair N, Ortner A, Haller T,
Ronzani M, Rugg C, Ströhle M, Wintersteiger R and Gatterer H: Dose-
and sex-dependent changes in hemoglobin oxygen affinity by the
micronutrient 5-hydroxymethylfurfural and α-ketoglutaric acid.
Nutrients. 13:34482021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niebisch A, Kabus A, Schultz C, Weil B and
Bott M: Corynebacterial protein kinase G controls 2-oxoglutarate
dehydrogenase activity via the phosphorylation status of the OdhI
protein. J Biol Chem. 281:12300–12307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krawczyk S, Raasch K, Schultz C, Hoffelder
M, Eggeling L and Bott M: The FHA domain of OdhI interacts with the
carboxyterminal 2-oxoglutarate dehydrogenase domain of OdhA in
Corynebacterium glutamicum. FEBS Lett. 584:1463–1468. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lockwood LB and Stodola FH: Preliminary
studies on the production of alpha-ketoglutaric acid by Pseudomonas
fluorescens. J Biol Chem. 164:81–83. 1946. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Otto C, Yovkova V and Barth G:
Overproduction and secretion of alpha-ketoglutaric acid by
microorganisms. Appl Microbiol Biotechnol. 92:689–695. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo H, Su S, Madzak C, Zhou J, Chen H and
Chen G: Applying pathway engineering to enhance production of
alpha-ketoglutarate in Yarrowia lipolytica. Appl Microbiol
Biotechnol. 100:9875–9884. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo H, Du G, Zhou J and Chen J: Progress
in microbial production of alpha-ketoglutarate. Sheng Wu Gong Cheng
Xue Bao. 29:141–152. 2013.(In Chinese). PubMed/NCBI
|
|
23
|
Bartlett DE, Miller RB, Thiesfeldt S,
Lakhani HV, Shapiro JI and Sodhi K: The role of Na/K-ATPase
signaling in oxidative stress related to aging: Implications in
obesity and cardiovascular disease. Int J Mol Sci. 19:21392018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang WD, Huang PJ, Xiong LH, Zhou S, Ye
RY, Liu JR, Wei H and Lai RY: Metabolomics and its application in
the mechanism analysis on diabetic bone metabolic abnormality. Eur
Rev Med Pharmacol Sci. 24:9591–9600. 2020.PubMed/NCBI
|
|
25
|
Cheng MX, Cao D, Chen Y, Li JZ, Tu B and
Gong JP: α-ketoglutarate attenuates ischemia-reperfusion injury of
liver graft in rats. Biomed Pharmacother. 111:1141–1146. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olenchock BA, Moslehi J, Baik AH, Davidson
SM, Williams J, Gibson WJ, Chakraborty AA, Pierce KA, Miller CM,
Hanse EA, et al: EGLN1 inhibition and rerouting of
alpha-ketoglutarate suffice for remote ischemic protection. Cell.
165:4972016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Rourke MF and Hashimoto J: Mechanical
factors in arterial aging: A clinical perspective. J Am Coll
Cardiol. 50:1–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Satpute RM, Hariharakrishnan J and
Bhattacharya R: Effect of alpha-ketoglutarate and N-acetyl cysteine
on cyanide-induced oxidative stress mediated cell death in PC12
cells. Toxicol Ind Health. 26:297–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen P, Hou L, Luo Y, Chen L, Li S, Lei X,
Huang J and Wu D: Effect of diabetes on the assessment role of
2-oxoglutarate to the severity of chronic heart failure. Exp Clin
Endocrinol Diabetes. 126:478–486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
An D, Zeng Q, Zhang P, Ma Z, Zhang H, Liu
Z, Li J, Ren H and Xu D: Alpha-ketoglutarate ameliorates pressure
overload-induced chronic cardiac dysfunction in mice. Redox Biol.
46:1020882021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Greilberger J, Herwig R, Greilberger M,
Stiegler P and Wintersteiger R: Alpha-Ketoglutarate and 5-HMF: A
potential anti-tumoral combination against leukemia cells.
Antioxidants (Basel). 10:18042021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mendoza G and Merchant H: Motor system
evolution and the emergence of high cognitive functions. Prog
Neurobiol. 122:73–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai X, Yuan Y, Liao Z, Xing K, Zhu C, Xu
Y, Yu L, Wang L, Wang S, Zhu X, et al: alpha-Ketoglutarate prevents
skeletal muscle protein degradation and muscle atrophy through
PHD3/ADRB2 pathway. FASEB J. 32:488–499. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ge J, Cui H, Xie N, Banerjee S, Guo S,
Dubey S, Barnes S and Liu G: Glutaminolysis promotes collagen
translation and stability via alpha-ketoglutarate-mediated mTOR
activation and proline hydroxylation. Am J Respir Cell Mol Biol.
58:378–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiong G, Stewart RL, Chen J, Gao T, Scott
TL, Samayoa LM, O'Connor K, Lane AN and Xu R: Collagen prolyl
4-hydroxylase 1 is essential for HIF-1α stabilization and TNBC
chemoresistance. Nat Commun. 9:44562018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zurek A, Mizerska-Kowalska M,
Slawinska-Brych A, Kaławaj K, Bojarska-Junak A, Kandefer-Szerszeń M
and Zdzisińska B: Alpha ketoglutarate exerts a pro-osteogenic
effect in osteoblast cell lines through activation of JNK and
mTOR/S6K1/S6 signaling pathways. Toxicol Appl Pharmacol. 374:53–64.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Filip RS, Pierzynowski SG, Lindegard B,
Wernerman J, Haratym-Maj A and Podgurniak M: Alpha-ketoglutarate
decreases serum levels of C-terminal cross-linking telopeptide of
type I collagen (CTX) in postmenopausal women with osteopenia:
Six-month study. Int J Vitam Nutr Res. 77:89–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sliwa E, Tatara MR, Nowakowski H,
Pierzynowski SG and Studzinski T: Effect of maternal dexamethasone
and alpha-ketoglutarate administration on skeletal development
during the last three weeks of prenatal life in pigs. J Matern
Fetal Neonatal Med. 19:489–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hammarqvist F, Wernerman J, von der Decken
A and Vinnars E: Alpha-ketoglutarate preserves protein synthesis
and free glutamine in skeletal muscle after surgery. Surgery.
109:28–36. 1991.PubMed/NCBI
|
|
40
|
Radzki RP, Bienko M and Pierzynowski SG:
Anti-osteopenic effect of alpha-ketoglutarate sodium salt in
ovariectomized rats. J Bone Miner Metab. 30:651–659. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Godsora BKJ, Prakash P, Punekar NS and
Bhaumik P: Molecular insights into the inhibition of glutamate
dehydrogenase by the dicarboxylic acid metabolites. Proteins.
90:810–823. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alrefai H, Allababidi H, Levy S and Levy
J: The endocrine system in diabetes mellitus. Endocrine.
18:105–119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Temneanu OR, Trandafir LM and Purcarea MR:
Type 2 diabetes mellitus in children and adolescents: A relatively
new clinical problem within pediatric practice. J Med Life.
9:235–239. 2016.PubMed/NCBI
|
|
44
|
Rabaglia ME, Gray-Keller MP, Frey BL,
Shortreed MR, Smith LM and Attie AD: Alpha-Ketoisocaproate-induced
hypersecretion of insulin by islets from diabetes-susceptible mice.
Am J Physiol Endocrinol Metab. 289:E218–E224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Macdonald MJ: Export of metabolites from
pancreatic islet mitochondria as a means to study anaplerosis in
insulin secretion. Metabolism. 52:993–998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Odegaard ML, Joseph JW, Jensen MV, Lu D,
Ilkayeva O, Ronnebaum SM, Becker TC and Newgard CB: The
mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway
that mediates glucose- and glutamine-stimulated insulin secretion.
J Biol Chem. 285:16530–16537. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fahien LA, MacDonald MJ, Kmiotek EH, Mertz
RJ and Fahien CM: Regulation of insulin release by factors that
also modify glutamate dehydrogenase. J Biol Chem. 263:13610–13614.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tan Q, Wang W, Yang C, Zhang J, Sun K, Luo
HC, Mai LF, Lao Y, Yan L and Ren M: α-ketoglutarate is associated
with delayed wound healing in diabetes. Clin Endocrinol (Oxf).
85:54–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ren W, Xia Y, Chen S, Wu G, Bazer FW, Zhou
B, Tan B, Zhu G, Deng J and Yin Y: Glutamine metabolism in
macrophages: A novel target for obesity/type 2 diabetes. Adv Nutr.
10:321–330. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Poddar M, Chetty Y and Chetty VT: How does
obesity affect the endocrine system? A narrative review. Clin Obes.
7:136–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kang HS, Lee JH, Oh KJ, Lee EW, Han BS,
Park KY, Suh JM, Min JK, Chi SW, Lee SC, et al: IDH1-dependent
alpha-KG regulates brown fat differentiation and function by
modulating histone methylation. Metabolism. 105:1541732020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu L, Xia M, Duan Y, Zhang L, Jiang H, Hu
X, Yan H, Zhang Y, Gu Y, Shi H, et al: Berberine promotes the
recruitment and activation of brown adipose tissue in mice and
humans. Cell Death Dis. 10:4682019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang Q, Liang X, Sun X, Zhang L, Fu X,
Rogers CJ, Berim A, Zhang S, Wang S, Wang B, et al:
AMPK/α-ketoglutarate axis dynamically mediates DNA demethylation in
the Prdm16 promoter and brown adipogenesis. Cell Metab. 24:542–554.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Z, Gan L, Zhang T, Ren Q and Sun C:
Melatonin alleviates adipose inflammation through elevating
α-ketoglutarate and diverting adipose-derived exosomes to
macrophages in mice. J Pineal Res. 64:2018. View Article : Google Scholar
|
|
55
|
Shamburek RD and Farrar JT: Disorders of
the digestive system in the elderly. N Engl J Med. 322:438–443.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Geboes K, Geboes KP and Maleux G: Vascular
anatomy of the gastrointestinal tract. Best Pract Res Clin
Gastroenterol. 15:1–14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hur H, Paik MJ, Xuan Y, Nguyen DT, Ham IH,
Yun J, Cho YK, Lee G and Han SU: Quantitative measurement of
organic acids in tissues from gastric cancer patients indicates
increased glucose metabolism in gastric cancer. PLoS One.
9:e985812014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim KB, Yang JY, Kwack SJ, Park KL, Kim
HS, Ryu DH, Kim YJ, Hwang GS and Lee BM: Toxicometabolomics of
urinary biomarkers for human gastric cancer in a mouse model. J
Toxicol Environ Health A. 73:1420–1430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cui P, Huang C, Guo J, Wang Q, Liu Z, Zhuo
H and Lin D: Metabolic profiling of tumors, sera, and skeletal
muscles from an orthotopic murine model of gastric cancer
associated-cachexia. J Proteome Res. 18:1880–1892. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tomaszewska E, Dobrowolski P and Puzio I:
Postnatal administration of 2-oxoglutaric acid improves the
intestinal barrier affected by the prenatal action of dexamethasone
in pigs. Nutrition. 28:190–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
He L, Huang N, Li H, Tian J, Zhou X, Li T,
Yao K, Wu G and Yin Y: AMPK/α-ketoglutarate axis regulates
intestinal water and ion homeostasis in young pigs. J Agric Food
Chem. 65:2287–2298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Junghans P, Derno M, Pierzynowski S,
Hennig U, Eberhard Rudolph P and Souffrant WB: Intraduodenal
infusion of alpha-ketoglutarate decreases whole body energy
expenditure in growing pigs. Clin Nutr. 25:489–496. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hou Y, Yao K, Wang L, Ding B, Fu D, Liu Y,
Zhu H, Liu J, Li Y, Kang P, et al: Effects of α-ketoglutarate on
energy status in the intestinal mucosa of weaned piglets
chronically challenged with lipopolysaccharide. Br J Nutr.
106:357–363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
He L, Wu J, Tang W, Zhou X, Lin Q, Luo F,
Yin Y and Li T: Prevention of oxidative stress by
alpha-ketoglutarate via activation of CAR signaling and modulation
of the expression of key antioxidant-associated targets in vivo and
in vitro. J Agric Food Chem. 66:11273–11283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang Z, He C, Zhang L, Zhu T, Lv D, Li G,
Song Y, Wang J, Wu H, Ji P and Liu G: Alpha-ketoglutarate affects
murine embryo development through metabolic and epigenetic
modulations. Reproduction. 158:123–133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang Z, He C, Gao Y, Zhang L, Song Y, Zhu
T, Zhu K, Lv D, Wang J, Tian X, et al: α-ketoglutarate delays
age-related fertility decline in mammals. Aging Cell.
20:e132912021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tanaka K, Hayashi Y, Takehara A,
Ito-Matsuoka Y, Tachibana M, Yaegashi N and Matsui Y: Abnormal
early folliculogenesis due to impeded pyruvate metabolism in mouse
oocytesdagger. Biol Reprod. 105:64–75. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Teng X, Emmett MJ, Lazar MA, Goldberg E
and Rabinowitz JD: Lactate dehydrogenase C produces
S-2-hydroxyglutarate in mouse testis. ACS Chem Biol. 11:2420–2427.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Plaitakis A, Kalef-Ezra E, Kotzamani D,
Zaganas I and Spanaki C: The glutamate dehydrogenase pathway and
its roles in cell and tissue biology in health and disease. Biology
(Basel). 6:112017.PubMed/NCBI
|
|
70
|
Li SF, Liu HX, Zhang YB, Yan YC and Li YP:
The protective effects of alpha-ketoacids against oxidative stress
on rat spermatozoa in vitro. Asian J Androl. 12:247–256. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kalawaj K, Slawinska-Brych A,
Mizerska-Kowalska M, Żurek A, Bojarska-Junak A, Kandefer-Szerszeń M
and Zdzisińska B: Alpha ketoglutarate exerts in vitro
anti-osteosarcoma effects through inhibition of cell proliferation,
induction of apoptosis via the JNK and caspase 9-dependent
mechanism, and suppression of TGF-β and VEGF production and
metastatic potential of cells. Int J Mol Sci. 21:94062020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Demidenko O, Barardo D, Budovskii V,
Finnemore R, Palmer FR, Kennedy BK and Budovskaya YV: Rejuvant(R),
a potential life-extending compound formulation with
alpha-ketoglutarate and vitamins, conferred an average 8 year
reduction in biological aging, after an average of 7 months of use,
in the TruAge DNA methylation test. Aging (Albany NY).
13:24485–24499. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gou L, Lee J, Yang JM, Park YD, Zhou HM,
Zhan Y and Lü ZR: The effect of alpha-ketoglutaric acid on
tyrosinase activity and conformation: Kinetics and molecular
dynamics simulation study. Int J Biol Macromol. 105:1654–1662.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bayliak MM, Hrynkiv OV, Knyhynytska RV and
Lushchak VI: Alpha-ketoglutarate enhances freeze-thaw tolerance and
prevents carbohydrate-induced cell death of the yeast Saccharomyces
cerevisiae. Arch Microbiol. 200:33–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chen J, Zhang H, Gao H, Kang B, Chen F, Li
Y, Fu C and Yao K: Effects of dietary supplementation of
alpha-ketoglutarate in a low-protein diet on fatty acid composition
and lipid metabolism related gene expression in muscles of growing
pigs. Animals (Basel). 9:8382019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bayliak MM and Lushchak VI: Pleiotropic
effects of alpha-ketoglutarate as a potential anti-ageing agent.
Ageing Res Rev. 66:1012372021. View Article : Google Scholar : PubMed/NCBI
|