Introduction
Sepsis is a systemic inflammatory response syndrome
caused by infection, which is associated with high global morbidity
and mortality rates between 1979 and 2015 (1–3).
Although the application of antibiotics, vasoactive agents and
renal dialysis in the treatment of sepsis has greatly improved the
prognosis, the fatality rate of sepsis remains high (4). Therefore, it is crucial to develop
novel and effective treatments for sepsis in order to improve the
diagnostic accuracy and patient prognosis.
Acute kidney injury (AKI) is a common complication
in patients with severe sepsis. According to statistics, ~50% of
AKI cases are associated with sepsis and the fatality rate is as
high as 40% (5). It was previously
reported that sepsis was characterized by inflammation in the early
stage and by apoptosis-related immunosuppression in the late stage
(6). Therefore, inflammation and
apoptosis are the key mechanisms implicated in terminal organ
damage in sepsis.
The G protein-coupled receptor 55 (GPR55) is a newly
discovered G protein-coupled receptor, which is mainly located in
brain, gastrointestinal tract, spleen, endothelial cells and other
regions of the body, and is involved in the regulation of a series
of physiological activities, including nerve or inflammatory pain,
gastrointestinal inflammation, obesity, type 2 diabetes and cancer
(7). GPR55 is closely associated
with the occurrence and progression of inflammation. For instance,
Schicho et al (8) revealed
that the expression level of GPR55 in the gastrointestinal
epithelial cells of rats treated with lipopolysaccharide was
significantly increased, indicating that GPR55 may be involved in
intestinal inflammation. In addition, Staton et al (9) reported that GPR55 knockdown mice had a
high tolerance to inflammation induced by Freund's complete
adjuvant, and the levels of pro-inflammatory factors, such as IL-4,
were significantly increased in GPR55 knockdown mice. Through the
further study of GPR55, it was observed that the GPR55 antagonist
CID16020046 had significant anti-inflammatory properties; for
example, CID16020046 effectively inhibits intestinal inflammation
in mice by reducing myeloperoxidase activity and inflammation
(10). Of note, a previous study
revealed that inhibition of GPR55 effectively reduced the
production of the pro-inflammatory cytokines TNF-α and IL-6 in
sepsis models (11). However, to
the best of our knowledge, it has not been investigated whether
inhibition of GPR55 affects sepsis-induced AKI.
Therefore, the aim of the present study was to
systematically and comprehensively investigate the role and
potential underlying molecular mechanism of GPR55 inhibitors in a
septic mouse model by detecting the levels of related renal injury
indicators and inflammatory cytokines.
Materials and methods
Human serum samples
Peripheral blood (6 ml) was collected from 15
patients with sepsis and 15 healthy controls. The patients included
nine men and six women aged 18–55 years old, and the inclusion
criteria and the exclusion criteria for patients with sepsis
referred to a previous study (12).
The samples were collected from the Emergency Department of The
Second Affiliated Hospital of Guangzhou Medical University from
July 2015 to December 2016. The study protocol was approved by the
Ethics Committee of The Second Affiliated Hospital of Guangzhou
Medical University. All the subjects who participated in the
present study signed the informed consent form.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from patients' serum samples
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Subsequently, RNA was reverse-transcribed into
cDNA for 15 min at 42°C using a PrimeScript RT Reagent kit (Takara
Biotechnology Co., Ltd.). qPCR was performed using TB Green Fast
qPCR mix (Takara Biotechnology Co., Ltd.) according to the
manufacturer's instructions, using cDNA as the template. The primer
sequences were as follows: GPR55 forward,
5′-TCTACATGATCAACCTGGCAGTCT-3′ and reverse,
5′-CTGGGACAGGACCATCTTGAA-3′; and GAPDH forward,
5′-ATGGGGAAGGTGAAGGTCG-3′ and reverse,
5′-GGGGTCATTGATGGCAACAATA-3′. The reaction conditions were as
follows: 50°C for 2 min, initial denaturation at 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C
for 15 sec. The relative expression of GPR55 was calculated using
the 2−∆∆Cq method (13).
Cecal ligation/perforation (CLP) model
and experimental groups
In total, 36 male C57BL/6J mice (weight, 21–25 g;
age, 6 weeks) were purchased from the Qinglongshan Laboratory
Animal Farm (Nanjing, China). The mice were housed in a standard
environment including a 12-h light/dark cycle, controlled
temperature (22±2°C) and humidity (55±5%) with free access to water
and food. The GPR55 inhibitor (CID16020046) was purchased from
ChemDiv, Inc. Animals were adaptively fed for 1 week and fasted for
12 h before the start of the experiment.
The mice were randomly divided into four groups
(n=9/group) as follows: Control, sham, CLP and CID16020046 (20
mg/kg) groups. A sepsis model in mice was established via CLP. In
brief, the mice were intraperitoneally injected with 50 mg/kg
pentobarbital sodium. After the mice were anesthetized, a 2-cm
incision was made in the abdominal wall of the mice using a scalpel
under aseptic conditions, the cecum was ligated with no. 3 silk
thread and then the cecum was perforated twice with a no. 18
injection needle. Subsequently, the peritoneum and skin were
intermittently sutured with no. 4 silk thread. In addition, 50
mg/kg saline was injected subcutaneously to prevent the development
of shock in mice. No cecal ligation was performed in the sham
group. The CID16020046 group was injected intraperitoneally with
CID16020046 solution (20 mg/kg) immediately following CLP surgery
(10), while the other groups were
injected with an equal volume of saline. Urine samples were
collected from mice in each group 12 h after the operation. After
24 h, all mice were euthanized by cervical dislocation, and 2 ml
blood samples and renal tissue samples were collected for the
subsequent experiments. The animal experimental procedures were
approved by the Animal Committee of The Second Affiliated Hospital
of Guangzhou Medical University.
Histopathological examination
The tissue samples of mice were examined following
H&E and periodic acid-Schiff (PAS) staining. For H&E
staining, at 24 h after dissection, the heart, liver, spleen, lung
and kidney tissues of each group were fixed with 4% formaldehyde
for 24 h at room temperature and embedded in paraffin after
dehydration. The tissues were cut into 4-µm sections and stained
with hematoxylin for 15 min and eosin for 5 min both at room
temperature, and the histopathological changes of each organ were
observed under a light microscope (Leica DM 500; Leica Microsystems
GmbH) at a magnification of ×400.
For PAS staining, 1-mm3 of kidney tissue
was fixed with 10% formaldehyde 4°C for 24 h, dehydrated using an
ethanol gradient through 30, 50, 70, 80, 95 and 100% ethanol
successively, embedded in paraffin and cut into 5-µm sections.
These sections were then stained with PAS at room temperature for
10 min and observed and imaged under a light microscope
(magnification, ×200).
Renal function assessment
After 24 h of modeling, the abdominal aortic blood
(2 ml) of mice in each group was collected and centrifuged at 3,000
× g for 5 min at 4°C. According to the manufacturer's instructions,
blood urea nitrogen (BUN; cat. no. SG7106B) and creatinine (Cre;
GG7107A) ELISA kits (Beijing Wantai Drd Co., Ltd.) were used to
determine the levels of Cre and BUN in the supernatant. In
addition, the urine (~1 ml) from the rat cage was collected after
12 h of modeling and centrifuged at 1,000 × g at 4°C for 15 min.
The supernatant was collected, and the levels of kidney injury
molecule 1 (KIM1, PK683) and neutrophil gelatinase-associated
lipocalin (NGAL, PN758) in the urine were detected via ELISA kits
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions.
Detection of inflammatory factors
TNF-α (PT512), IL-6 (cat. no. PI326) and IL-1β
(PI301) levels in the serum of septic mice were detected using
respective ELISA kits according to the manufacturer's instructions
(Beyotime Institute of Biotechnology). In brief, 2 ml blood was
collected from mice at room temperature and centrifuged at 4°C for
5 min at 3,000 × g. The absorbance of the serum sample was measured
at 450 nm using an Automatic Microplate reader (Syngene).
TUNEL staining assay
The TUNEL Apoptosis Assay kit (cat. no. C1088;
Beyotime Institute of Biotechnology) was used to detect the
apoptosis of renal tissue of mice. Briefly, the paraffin-embedded
tissues were deparaffinized with xylene and rehydrated with graded
ethanol at room temperature. The renal tissue sections were washed
with PBS, fixed with 1% paraformaldehyde at room temperature for 15
min and treated with proteinase K working solution for 10 min at
37°C, followed by incubation with the TUNEL reaction mixture at
37°C for 60 min in the dark. The nucleus was stained with DAPI for
5 min at room temperature and mounted in an anti-fade reagent
(Beijing Solarbio Science & Technology Co., Ltd.). In total,
five random fields (magnification, ×200) were imaged using an
inverted fluorescence microscope (Olympus IX71; Olympus
Corporation).
Western blot analysis
Total proteins were extracted from cells and renal
tissues using RIPA lysis buffer containing protease inhibitors
(Beyotime Institute of Biotechnology). The protein concentration
was detected using a BCA assay kit (cat. no. BCA-02; Beijing
Dingguo Changsheng Biotechnology Co., Ltd.). The proteins (30 µg)
were separated by 12% SDS-PAGE and transferred to PVDF membranes.
Next, these membranes were blocked with 5% skimmed milk powder for
2 h at room temperature, and then incubated overnight at 4°C with
the primary antibodies (diluted 1:1,000) against GPR55 (cat. no.
ab203663; Abcam), TNF-α (cat. no. ab215188; Abcam), IL-6 (cat. no.
ab233706; Abcam), IL-1β (cat. no. ab234437; Abcam), Bax (cat. no.
ab32503; Abcam), Bcl-2 (cat. no. ab182858; Abcam), caspase3 (cat.
no. ab13847; Abcam), cleaved caspase3 (cat. no. ab2302; Abcam),
caspase9 (cat. no. ab32539; Abcam), cleaved caspase9 (cat. no.
ab2324; Abcam), Ras homolog family member A (RhOA; cat. no.
ab187027; Abcam), Rho-associated protein kinase (ROCK) 1 (cat. no.
ab134181; Abcam), ROCK2 (cat. no. ab125025; Abcam) and GAPDH (cat.
no. ab9485; Abcam). Subsequently, the membranes were washed with
TBS-0.1% Tween 20 and then co-incubated with secondary antibodies
conjugated to HRP (1:5,000; cat. no. sc-2357, Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature. The protein bands
were detected using an ECL kit (Amersham Biosciences; Cytiva) and
analyzed with ImageJ version 1.46 software (National Institutes of
Health). GAPDH was used as an internal reference.
Statistical analysis
All data were analyzed using GraphPad Prism Software
8 (GraphPad Software, Inc.). All experimental data are expressed as
the mean ± SEM, and each experiment was repeated ≥3 times. An
unpaired Student's t-test was performed to compare two groups, and
one-way ANOVA followed by Tukey's post hoc test was used for the
comparison among multiple groups. For clinical analysis, the
expression level of GRP55 between patients and healthy controls was
analyzed using the Wilcoxon-Mann-Whitney test. P<0.05 was
considered to indicate statistically significant differences.
Results
CID16020046 attenuates sepsis-induced
renal injury
In order to examine the effect of GPR55 on
sepsis-induced renal injury, the expression level of GPR55 was
first determined in the serum of healthy subjects and patients with
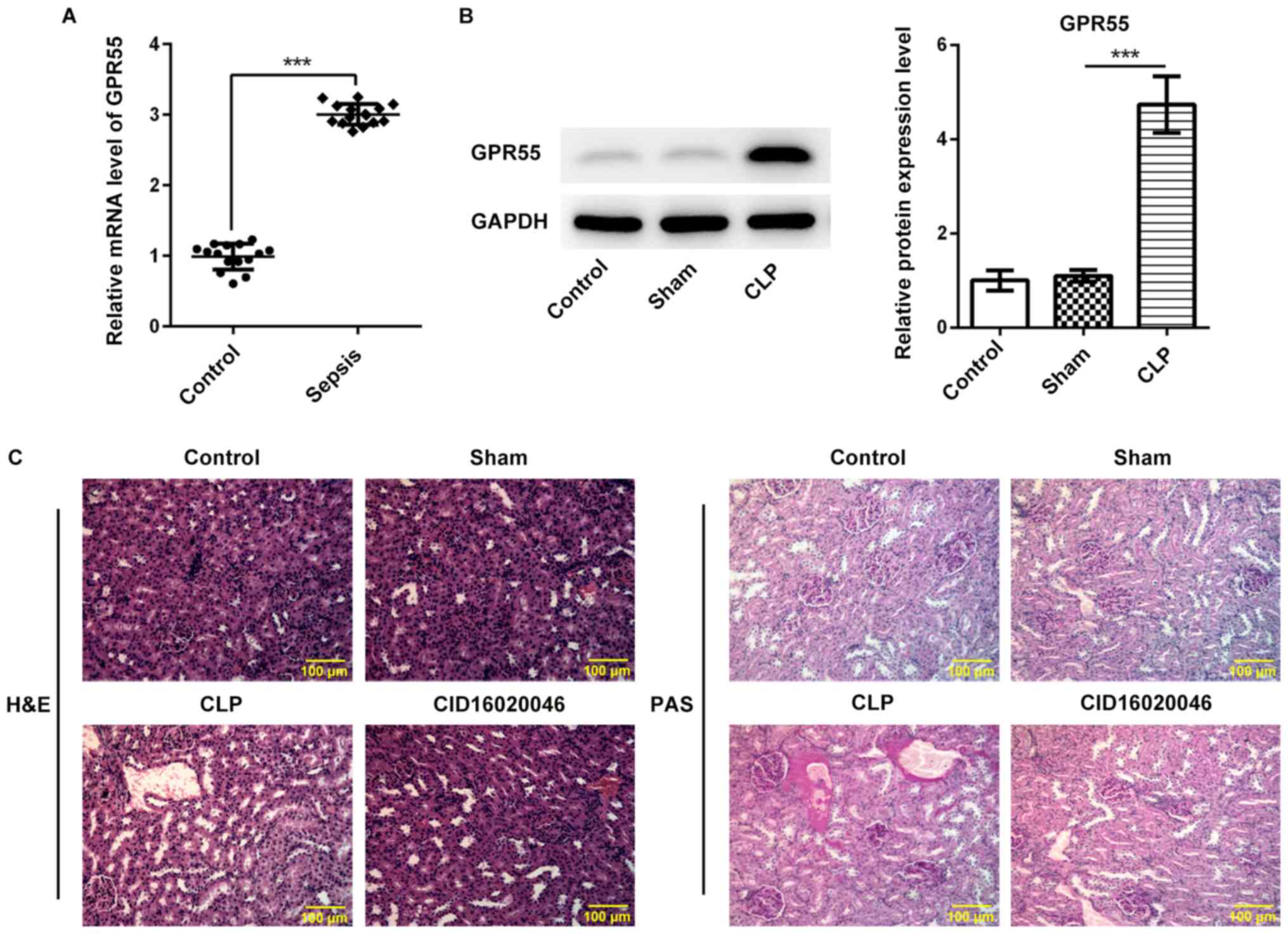
sepsis. As shown in Fig. 1A, the
results of RT-qPCR revealed that the expression level of GPR55 in
the serum of patients with sepsis was significantly higher compared
with that in the control group. Next, the role of GPR55 in
sepsis-induced renal injury was further evaluated using CLP to
construct a murine model of polymicrobial sepsis. As presented in
Fig. 1B, the expression level of
GPR55 in the CLP group was significantly higher compared with that
in the sham group.
Renal pathological injury was analyzed via H&E
and PAS staining. The results of H&E and PAS staining
demonstrated that the complete renal glomerulus and tubule
morphology was destroyed after CLP operation, indicating a more
prominent renal injury in the CLP group compared with that in the
sham group, whereas the GPR55 inhibitor CID16020046 (20 mg/kg)
notably decreased the thickening of the basement membrane of renal
tubules and glomerular hypertrophy, thereby alleviating the
severity of renal pathological injury induced by sepsis (Fig. 1C).
CID16020046 reduces the levels of
renal injury biomarkers induced by sepsis
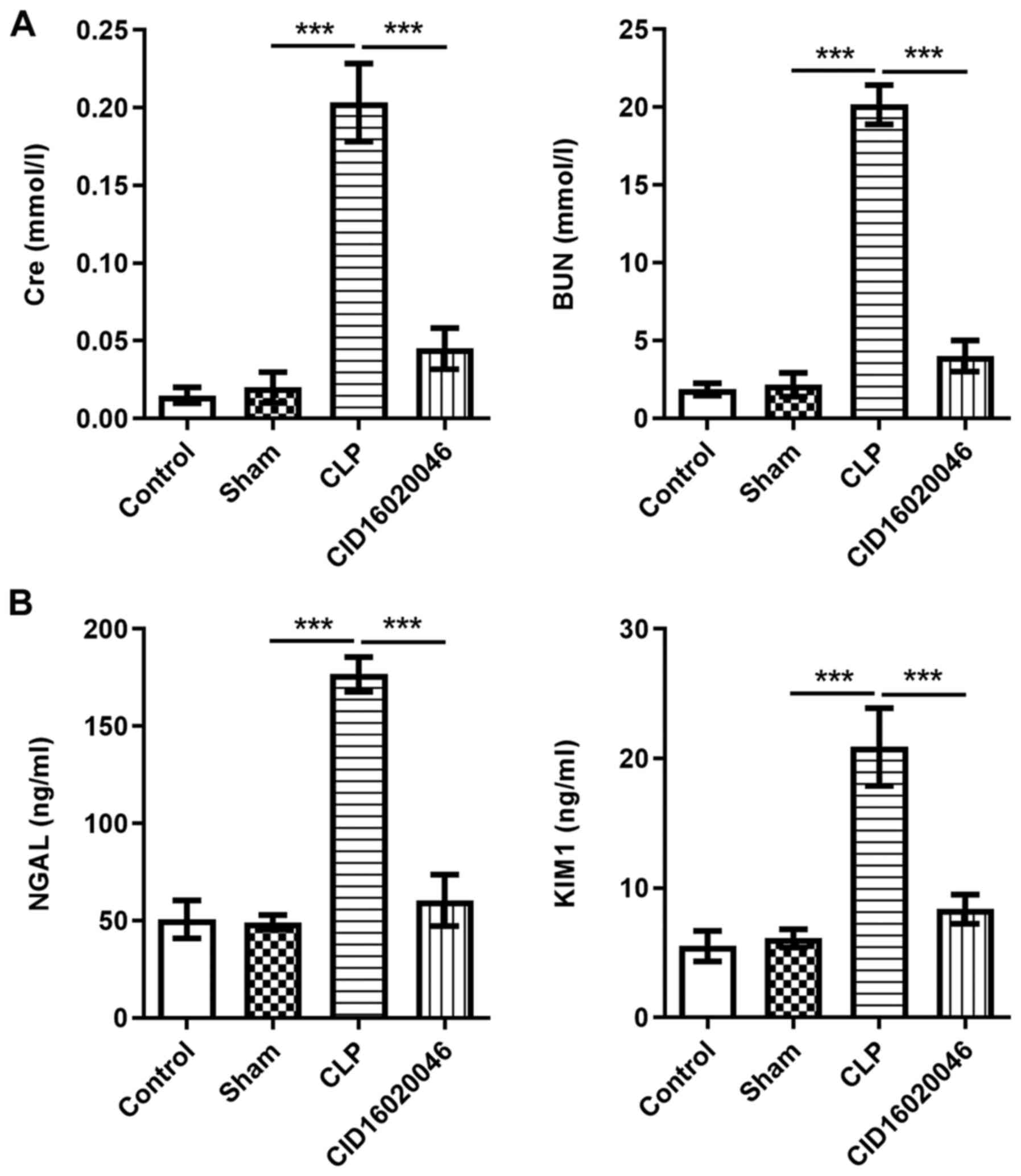
To further verify the protective effect of
CID16020046 against sepsis-induced renal injury, the levels of Cre
and BUN in the serum, and the levels of KIM1 and NGAL in the urine
of septic mice were measured by ELISA. As presented in Fig. 2A, the levels of Cre and BUN in the
CLP group were significantly higher compared with those in the sham
group. Moreover, the levels of KIM1 and NGAL in the CLP group were
significantly increased compared with those in the sham group
(Fig. 2B). However, these elevated
levels of Cre, BUN, KIM1 and NGAL in mice receiving CLP operation
were significantly reduced after the administration of CID16020046
(20 mg/kg). These results indicated that CID16020046 could
effectively improve renal tissue injury induced by sepsis.
CID16020046 alleviates inflammation in
the serum and renal tissue induced by sepsis
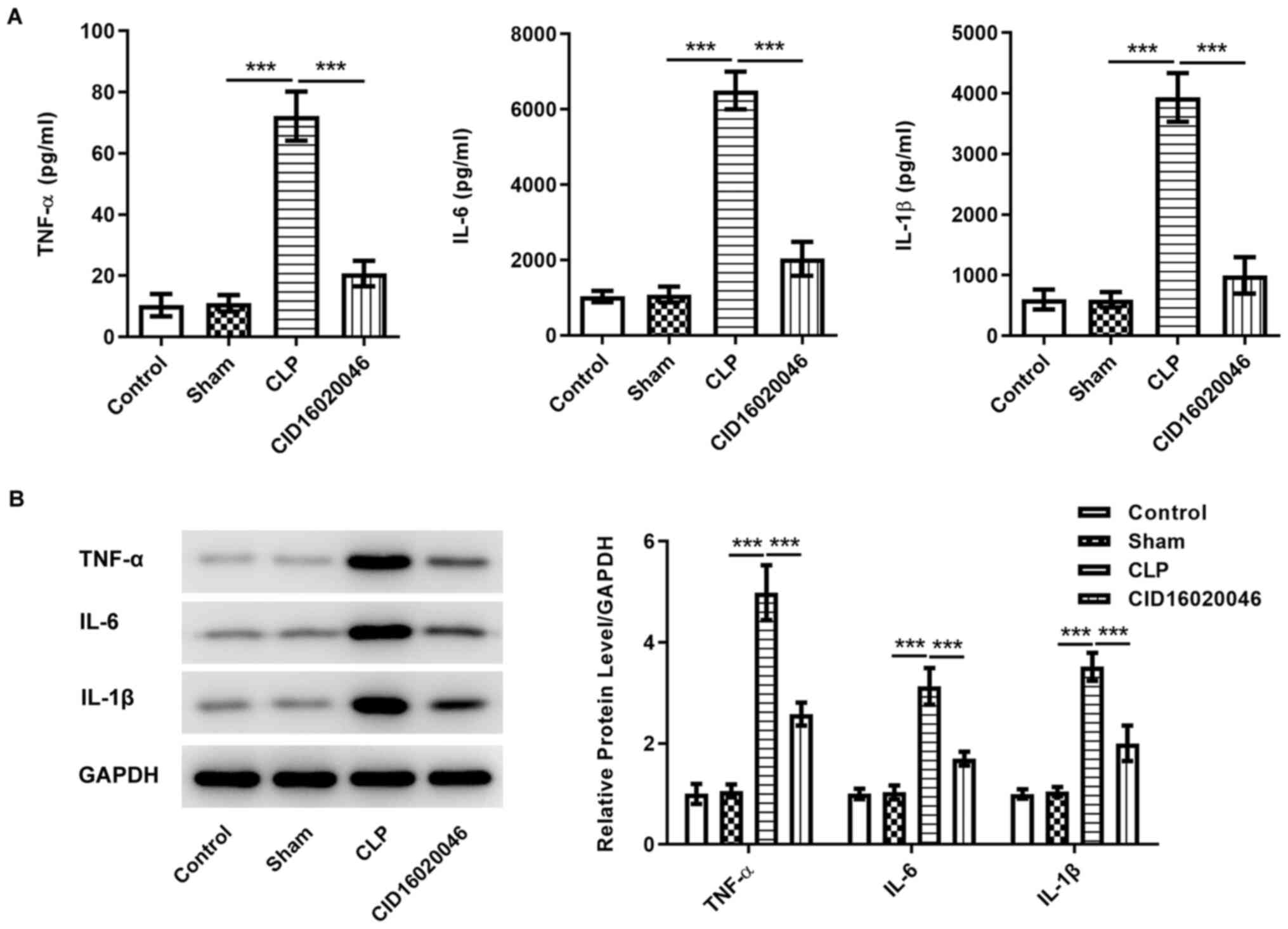
ELISA and western blotting were performed to examine
the sepsis-induced levels of the pro-inflammatory factors TNF-α,
IL-6 and IL-1β. ELISA results demonstrated that the levels of
TNF-α, IL-6 and IL-1 β were significantly increased in the CLP
group compared with those in the sham group, while CID16020046
effectively suppressed the levels of TNF-α, IL-6 and IL-1β
(Fig. 3A). The western blotting
results also identified that CID16020046 partially abolished the
upregulation of TNF-α, IL-6 and IL-1β expression induced by sepsis
(Fig. 3B), suggesting that
CID16020046 alleviates inflammation in sepsis-induced AKI.
CID16020046 reduces renal cell
apoptosis induced by sepsis
Cell apoptosis serves an important role in the
occurrence and development of sepsis (14). Next, a TUNEL staining assay and
western blotting were used to determine the extent of cell
apoptosis in renal tissue. As shown in Fig. 4A, the results of TUNEL staining
demonstrated that the fluorescence intensity of renal tissue cells
in the CID16020046 group was weaker compared with that in the CLP
group, indicating that CID16020046 markedly alleviated the
apoptosis of renal tissue cells induced by sepsis. In addition, the
western blotting results revealed that, compared with the CLP
group, CID16020046 significantly inhibited the downregulation of
the anti-apoptotic protein Bcl-2 and the upregulation of the
pro-apoptotic proteins Bax, cleaved caspase3 and cleaved caspase9
induced by sepsis (Fig. 4B).
CID16020046 ameliorates sepsis-induced
renal injury by inhibiting the RhOA/ROCK pathway
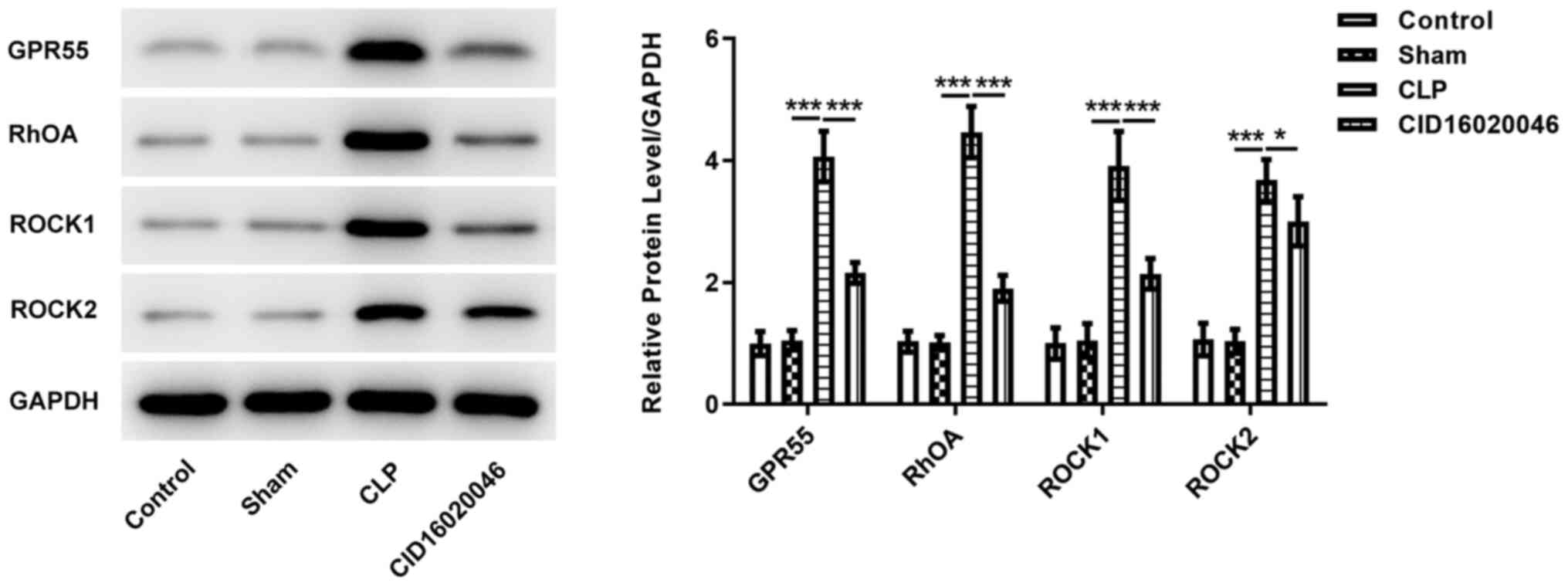
The effect of GPR55 on the expression levels of
RhOA/ROCK pathway-related proteins were determined via western
blotting. As shown in Fig. 5, the
expression levels of RhOA, ROCK1 and ROCK2 in the CLP group were
significantly higher compared with those in the sham group, whereas
CD16020046 significantly reduced the increased expression levels of
RhOA, ROCK1 and ROCK2 induced by sepsis. These results indicated
that CID16020046 could effectively alleviate sepsis-induced renal
injury by suppressing the RhOA/ROCK pathway.
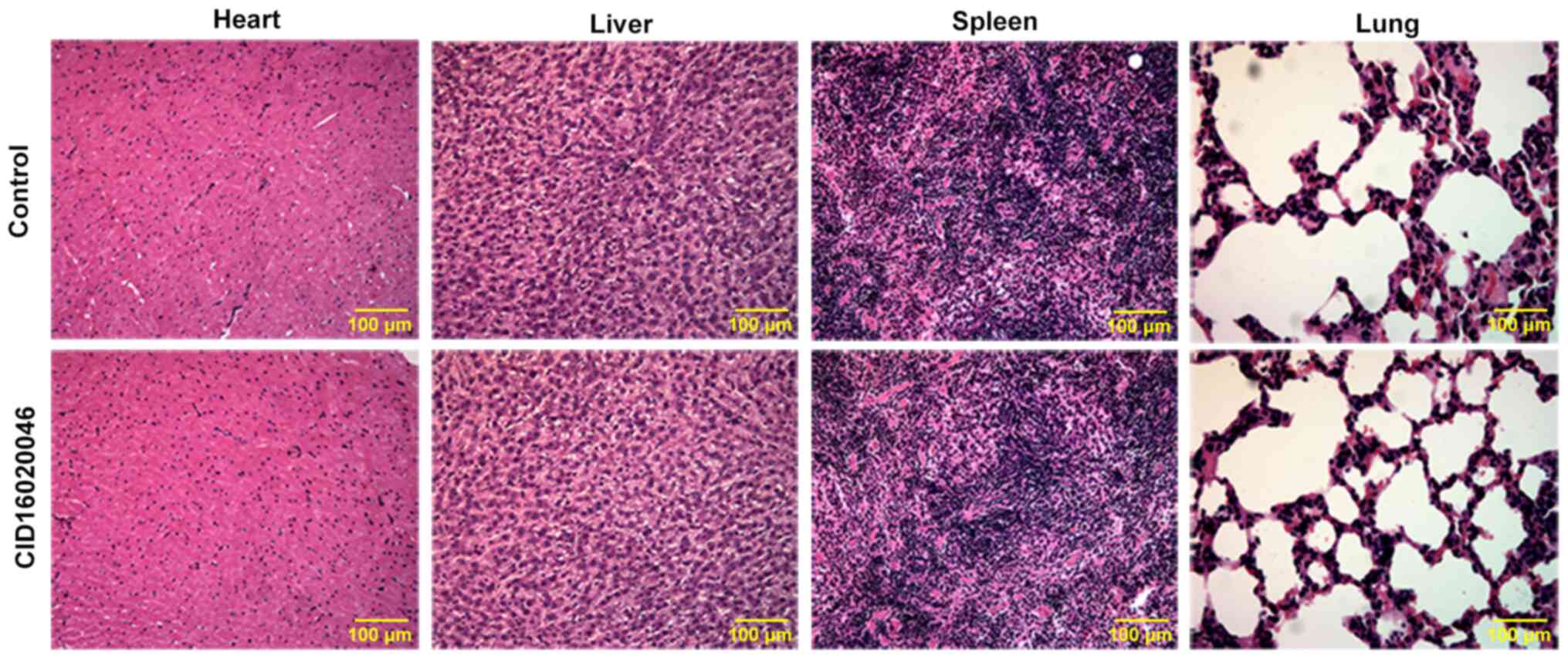
CID16020046 does not adversely affect
the heart, liver, spleen or lung
Finally, the toxicity of CID16020046 (20 mg/kg) on
the heart, liver, spleen and lung in normal mice was analyzed by
H&E staining. The results demonstrated that there were no
apparent pathological changes in the heart, liver, spleen or lung
between normal mice treated with 20 mg/kg CID16020046 or normal
mice without treatment (Fig.
6).
Discussion
In the present study, a CLP-induced sepsis mouse
model was constructed to analyze the effect of CID16020046 on renal
injury induced by sepsis. The results demonstrated that GPR55
expression was upregulated in the renal tissue of septic mice. It
was found that the GPR55 inhibitor CID16020046 could improve the
sepsis-induced renal injury, including inflammation and apoptosis,
by inhibiting the activation of the RhOA/ROCK signaling pathway. In
addition, CID16020046 at 20 mg/kg was proven to exert no toxic side
effects in normal mice.
As it was determined in a previous study that
inhibition of GPR55 could attenuate experimental sepsis, the role
of GPR55 in sepsis-induced AKI was further investigated. Cre and
BUN levels in the serum mainly reflect glomerular filtration
function, and so, these two indices are often used to determine the
integrity of renal function (15,16).
KIM1 is a transmembrane glycoprotein that can be cleaved into
soluble fragments and is eventually excreted into urine to
participate in the process of immune tolerance (17,18).
Moreover, NGAL is a type of injury-induced transferrin that may
improve the activation of intracellular coenzyme iron and regulate
various important proteins involved in cellular activities
(19). For example, Guo et
al (20) revealed that NGAL
modulates cell apoptosis and epithelial-mesenchymal transition in
nasopharyngeal carcinoma. Han et al (21) demonstrated that NGAL participates in
LPS-mediated apoptosis of renal tubular epithelial cells.
Therefore, KIM1 and NGAL are often used as markers of acute renal
tubular injury. In the present study, GPR55 expression was
upregulated in the serum of patients with sepsis and in the renal
tissue of mice with CLP-induced sepsis. Thus, CID16020046 was
applied to reduce GPR55, and the results demonstrated that
CID16020046 significantly lowered the level renal injury biomarkers
(Cre, BUN, KIM1 and NGAL), directly suggesting that CID16020046
possessed a potential protective function in sepsis-induced
AKI.
Subsequently, how CID16020046 exerted its protective
effects on sepsis-induced AKI was further evaluated. Previous
studies have reported that the GPR55 antagonist CID16020046
exhibited notable anti-inflammatory properties in different disease
processes. For example, CID16020046 could effectively suppress
intestinal inflammation and endothelial cell inflammation induced
by the oxidized low-density lipoprotein (10,22).
These studies indicated that CID16020046 exerted notable
anti-inflammatory effects and may hold promise in clinical
application. In agreement with these previous reports, CID16020046
also exhibited significant inhibitory effects on the production of
TNF-α, IL-6 and IL-1β both in serum and kidney tissues in septic
mice in the present study. Therefore, it was indicated that
CID16020046 may have a positive protective effect against
sepsis-induced renal injury by inhibiting inflammation.
In addition to inflammation, tubular cell apoptosis
has been recognized as another main histopathologic characteristic
in sepsis-induced AKI (23). A
recent study revealed that GPR55 was involved in apoptosis
processing (24). The antagonists
of GPR55 were able to block apoptosis in PC12 cells, whereas GPR55
agonists protected against endoplasmic reticulum-induced apoptosis
in pancreatic β cells (25,26). In the present study, apoptotic cells
were more likely to occur in the septic mice, accompanied with the
highly expressed pro-apoptotic proteins and the lowly expressed
anti-apoptotic proteins. The administration of CID16020046 markedly
attenuated this condition, suggesting that CID16020046 may have a
positive protective effect against sepsis-induced renal injury by
inhibiting apoptosis.
To further study the effect of CID16020046 on renal
injury induced by sepsis, its effect on the potential downstream
regulatory pathway RhOA/ROCK was investigated. It was previously
reported that RhOA may be the molecular initiator of intracellular
signal transduction (27). ROCK, a
downstream protein of RhOA kinase, can phosphorylate a variety of
substrates, and is closely associated with tumor invasion and
metastasis, tissue and organ fibrosis, nerve regeneration and
remodeling, and the occurrence and development of
cardio-cerebrovascular diseases (28). In addition, ROCK serves a key role
in inflammation and autoimmune diseases. For example, a previous
study revealed that ROCK may participate in the activation of the
NF-κB pathway and induce the production of TNF-α and other
inflammatory factors (29). In
addition, ROCK inhibitors were found to attenuate the
nephrotoxicity of chemicals, inhibit inflammatory and apoptotic
factors, and reduce renal fibrosis (30,31).
Of note, inhibition of the RhOA/ROCK signaling pathway markedly
decreases sepsis-induced AKI in rats (32). Furthermore, GPR55 can directly
regulate the expression levels of downstream effectors of ROCK
signaling (33,34). Therefore, it may be hypothesized
that CID16020046 improves sepsis-induced renal injury by inhibiting
ROCK signaling. In the present study, CID16020046 significantly
inhibited RhOA/ROCK signaling and participated in the regulation of
renal injury induced by sepsis.
However, some limitations existed in the current
study. This study preliminarily suggested that CID16020046 exerted
effects in sepsis-induced AKI by regulating the RhOA/ROCK pathway,
but an inhibition assay should be conducted to validate the
protective mechanism mediated by the ROCK pathway. These
experiments will be conducted in future work.
In conclusion, the GPR55 antagonist CID16020046 was
shown to significantly reduce renal injury, including inflammation
and apoptosis, induced by sepsis. Moreover, it was identified that
the protective effect of CID16020046 may be mediated via inhibition
of the RhOA/ROCK pathway. These results may provide the theoretical
basis for the treatment of sepsis-induced AKI by CID16020046, but
further research is required to fully elucidate the function and
underlying mechanism of action of CID16020046.
Acknowledgements
Not applicable.
Funding
This study was supported by the Guangdong Provincial Natural
Science Foundation (grant no. 2018A030313434), the Guangdong
Provincial Medical Science Research Foundation (grant no. A2018259)
and the Guangzhou Municipal Science and Technology Program (grant
no. 201904010006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RC and ZC designed the experimental study. RC, HX,
ZG, PZ and JC performed the experiments, analyzed and interpreted
the data. RC and HX wrote the manuscript. ZC revised the
manuscript. All authors confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This present study was performed based on the
principles expressed in the Declaration of Helsinki. The present
study was approved by the Ethics Committee of The Second Affiliated
Hospital of Guangzhou Medical University and conducted in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals. Each participant provided
signed informed consent prior to participate in the present study.
Patients or their legal surrogates provided signed informed consent
for the surgical procedures.
The animal experimental procedures were approved by
the Animal Committee of The Second Affiliated Hospital of Guangzhou
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fleischmann C, Scherag A, Adhikari NK,
Hartog CS, Tsaganos T, Schlattmann P, Angus DC and Reinhart K;
International Forum of Acute Care Trialists, : Assessment of global
incidence and mortality of hospital-treated sepsis. Current
estimates and limitations. Am J Respir Crit Care Med. 193:259–272.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shankar-Hari M, Phillips GS, Levy ML,
Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD and
Singer M; Sepsis Definitions Task Force, : Developing a new
definition and assessing new clinical criteria for septic shock:
For the third international consensus definitions for sepsis and
septic shock (sepsis-3). JAMA. 315:775–787. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Poll T, van de Veerdonk FL,
Scicluna BP and Netea MG: The immunopathology of sepsis and
potential therapeutic targets. Nat Rev Immunol. 17:407–420. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vincent JL, Jones G, David S, Olariu E and
Cadwell KK: Frequency and mortality of septic shock in Europe and
North America: A systematic review and meta-analysis. Crit Care.
23:1962019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uchino S, Kellum JA, Bellomo R, Doig GS,
Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, et al:
Acute renal failure in critically ill patients: A multinational,
multicenter study. JAMA. 294:813–818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma S, Evans RG, Iguchi N, Tare M,
Parkington HC, Bellomo R, May CN and Lankadeva YR: Sepsis-induced
acute kidney injury: A disease of the microcirculation.
Microcirculation. 26:e124832019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharir H and Abood ME: Pharmacological
characterization of GPR55, a putative cannabinoid receptor.
Pharmacol Ther. 126:301–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schicho R, Bashashati M, Bawa M, McHugh D,
Saur D, Hu HM, Zimmer A, Lutz B, Mackie K, Bradshaw HB, et al: The
atypical cannabinoid O-1602 protects against experimental colitis
and inhibits neutrophil recruitment. Inflamm Bowel Dis.
17:1651–1664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Staton PC, Hatcher JP, Walker DJ, Morrison
AD, Shapland EM, Hughes JP, Chong E, Mander PK, Green PJ, Billinton
A, et al: The putative cannabinoid receptor GPR55 plays a role in
mechanical hyperalgesia associated with inflammatory and
neuropathic pain. Pain. 139:225–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stančić A, Jandl K, Hasenöhrl C, Reichmann
F, Marsche G, Schuligoi R, Heinemann A, Storr M and Schicho R: The
GPR55 antagonist CID16020046 protects against intestinal
inflammation. Neurogastroenterol Motil. 27:1432–1445. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou J, Yang H and Lehmann C: Inhibition
of GPR 55 improves dysregulated immune response in experimental
sepsis. Clin Hemorheol Microcirc. 70:553–561. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng Q, Wu J and Yang S: Circulating
lncRNA ITSN1-2 is upregulated, and its high expression correlates
with increased disease severity, elevated inflammation, and poor
survival in sepsis patients. J Clin Lab Anal. 33:e228362019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harjai M, Bogra J, Kohli M and Pant AB: Is
suppression of apoptosis a new therapeutic target in sepsis?
Anaesth Intensive Care. 41:175–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song S, Meyer M, Türk TR, Wilde B,
Feldkamp T, Assert R, Wu K, Kribben A and Witzke O: Serum cystatin
C in mouse models: A reliable and precise marker for renal function
and superior to serum creatinine. Nephrol Dial Transplant.
24:1157–1161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han WK, Waikar SS, Johnson A, Betensky RA,
Dent CL, Devarajan P and Bonventre JV: Urinary biomarkers in the
early diagnosis of acute kidney injury. Kidney Int. 73:863–869.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuchroo VK, Umetsu DT, DeKruyff RH and
Freeman GJ: The TIM gene family: Emerging roles in immunity and
disease. Nat Rev Immunol. 3:454–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuwabara T, Mori K, Mukoyama M, Kasahara
M, Yokoi H, Saito Y, Yoshioka T, Ogawa Y, Imamaki H, Kusakabe T, et
al: Urinary neutrophil gelatinase-associated lipocalin levels
reflect damage to glomeruli, proximal tubules, and distal nephrons.
Kidney Int. 75:285–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo Y, Zhai J, Zhang J and Zhou H: NGAL
protects in nasopharyngeal carcinoma by inducing apoptosis and
blocking epithelial-mesenchymal transition. Oncol Lett.
19:3711–3718. 2020.PubMed/NCBI
|
|
21
|
Han M, Pan Y, Gao M, Zhang J and Wang F:
JNK signaling pathway suppresses LPS-mediated apoptosis of HK-2
cells by upregulating NGAL. Int J Inflam.
2020:39805072020.PubMed/NCBI
|
|
22
|
Wang Y, Pan W, Wang Y and Yin Y: The GPR55
antagonist CID16020046 protects against ox-LDL-induced inflammation
in human aortic endothelial cells (HAECs). Arch Biochem Biophys.
681:1082542020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garofalo AM, Lorente-Ros M, Goncalvez G,
Carriedo D, Ballén-Barragán A, Villar-Fernández A, Peñuelas Ó,
Herrero R, Granados-Carreño R and Lorente JA: Histopathological
changes of organ dysfunction in sepsis. Intensive Care Med Exp. 7
(Suppl 1):S452019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vong CT, Tseng HHL, Kwan YW, Lee SM and
Hoi MPM: Novel protective effect of O-1602 and abnormal
cannabidiol, GPR55 agonists, on ER stress-induced apoptosis in
pancreatic β-cells. Biomed Pharmacother. 111:1176–1186. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akimov MG, Ashba AM, Gretskaya NM and
Bezuglov VV: N-acyl dopamines induce apoptosis in PC12 cell line
via the GPR55 receptor activation. Dokl Biochem Biophys.
474:155–158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vong CT, Tseng HHL, Kwan YW, Lee SM and
Hoi MPM: Novel protective effect of O-1602 and abnormal
cannabidiol, GPR55 agonists, on ER stress-induced apoptosis in
pancreatic β-cells. Biomed Pharmacother. 111:1176–1186. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wan L, Bagshaw SM, Langenberg C, Saotome
T, May C and Bellomo R: Pathophysiology of septic acute kidney
injury: What do we really know? Crit Care Med. 36 (4
Suppl):S198–S203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lochhead PA, Wickman G, Mezna M and Olson
MF: Activating ROCK1 somatic mutations in human cancer. Oncogene.
29:2591–2598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimizu S, Tahara M, Ogata S, Hashimoto K,
Morishige K, Tasaka K and Murata Y: Involvement of nuclear
factor-kB activation through RhoA/Rho-kinase pathway in LPS-induced
IL-8 production in human cervical stromal cells. Mol Hum Reprod.
13:181–187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park JW, Park CH, Kim IJ, Bae EH, Ma SK,
Lee JU and Kim SW: Rho kinase inhibition by fasudil attenuates
cyclosporine-induced kidney injury. J Pharmacol Exp Ther.
338:271–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu H, Chen W, Ding J, Jia M, Yin J and Guo
Z: Fasudil prevents calcium oxalate crystal deposit and renal
fibrogenesis in glyoxylate-induced nephrolithic mice. Exp Mol
Pathol. 98:277–285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan XX, Zheng AD, Zhang ZE, Pan GC and
Zhou W: Protective effect of pantoprazole against sepsis-induced
acute lung and kidney injury in rats. Am J Transl Res.
11:5197–5211. 2019.PubMed/NCBI
|
|
33
|
Vong CT, Tseng HHL, Kwan YW, Lee SM and
Hoi MPM: G-protein coupled receptor 55 agonists increase insulin
secretion through inositol trisphosphate-mediated calcium release
in pancreatic β-cells. Eur J Pharmacol. 854:372–379. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robertson-Gray OJ, Walsh SK, Ryberg E,
Jönsson-Rylander AC, Lipina C and Wainwright CL:
l-α-Lysophosphatidylinositol (LPI) aggravates myocardial
ischemia/reperfusion injury via a GPR55/ROCK-dependent pathway.
Pharmacol Res Perspect. 7:e004872019. View
Article : Google Scholar : PubMed/NCBI
|