Introduction
Diabetes mellitus (DM) is a group of metabolic
diseases characterized by high blood sugar (1). Hyperglycemia occurs due to a defect
in insulin secretion or impaired biological effects, or both. The
long-term hyperglycemia during diabetes causes chronic damage and
dysfunction of various tissues, particularly the eyes, kidneys,
heart, blood vessels, muscle and nerves (2). The development of diabetes is a
complicated process, including lipid infiltration, glucose
metabolism disorder, autophagy imbalance and inflammatory response
(3). Therefore, targeting the
aforementioned mechanisms is an effective strategy to delay the
development of complications of diabetes.
Whole body vibration (WBV) is a new physical
exercise method to improve athletic ability (4,5).
Compared with traditional exercise methods, inactive patients need
less WBV and have higher compliance (6). WBV is widely used in warm-up of
athletes and rehabilitation training for patients. Previous study
has found that WBV can improve metabolic levels in diabetic rats
(7). The complex relationship
between autophagy and energy metabolism has attracted wide interest
and has been extensively studied. In the present study, the
relationships that enable autophagy to control or regulate energy
metabolism and allow metabolic pathways to regulate autophagy in a
diabetic state were examined. Specifically, the association between
autophagy and energy homeostasis from glycolysis, fatty acid
metabolism and amino acid metabolism was studied. However, its
further molecular mechanism has not yet been elucidated. Therefore,
it was hypothesized that WBV can attenuate the development of DM
and lead to lower level of low density lipoprotein (LDL) in the
blood.
In the present study, a mouse model of diabetes was
established and trained with WBV (15 Hz, 30 min) for 12 weeks to
explore the possible muscle protection mechanism of WBV for the
treatment of diabetes. The findings of the present study may
provide a new molecular basis for WBV to play a therapeutic role in
the treatment of diabetes and may have potential clinical
applications in the future.
Materials and methods
Animals
A total of 36 C57BL/6 mice were bred at the
Experimental Animal Center of Jinzhou Medical University (Jinzhou,
China). The present study was approved (approval no. 2020008) by
the Ethics Committee of Jinzhou Medical University (Jinzhou,
China). The mice were housed under controlled pathogenic (SPF)
conditions in a temperature-controlled (22°C) and
humidity-controlled (55–65%) light-dark cycle (12/12-h). Access to
food and water was provided ad libitum. A total of 12 male
mice (8 weeks of age, 20–23 g) were fed normally, and 24 male mice
of the same age were fed with high-fat diet (0.15% cholesterol) for
8 weeks and injected with streptozocin (200 mg/kg;
sigmaaldrich.cn/CN/zh/product/sigma/s0130.) under the
aforementioned conditions. After blood glucose levels reached 11.1
mmol/l, mice fed with high-fat diet were randomly divided into 2
groups. The WBV group was trained on a LD-P vertical vibration
testing machine (China Guangdong Central Vibration Machinery Co.,
Ltd., frequency 15 Hz, acceleration 0.68 g, amplitude 2 mm) for 30
min per day. Vibration intervention took place from Monday to
Saturday, with Sunday as a rest day. The training session started
at 9:00 AM and lasted 12 weeks. Then, the mice of each group were
weighed after 12 h of fasting, and blood samples were collected and
examined. At the end of the experiment, mice were euthanized by
intraperitoneal injection of sodium pentobarbital (100 mg/kg).
Death was confirmed from respiratory and cardiac arrest without
response to external stimuli.
Blood biochemical analysis
After WBV for 12 weeks, blood samples were collected
from the venous system, the serum was separated at 3,000 rpm (956
g) for 15 min at 4°C and stored at −80°C until analysis.
Triglycerides (TG; cat. no. A110-1-1), total cholesterol (TC; cat.
no. A111-1-1), high density lipoprotein cholesterol (HDL; cat. no.
A112-1-1) and LDL cholesterol (cat. no. A113-1-1) were detected by
commercially available kits (Nanjing Jiancheng Bioengineering
Institute).
HE staining
The 10-µm frozen (−80°C) gastrocnemius sections were
dried at room temperature for 30 min and immersed into hematoxylin
for 6 min; then the slides were rinsed with running water for 10
sec. The sections were incubated in HCl/95% alcohol (1:50) solution
for 5 sec. After washing with running water for 25 min, the slides
were stained with eosin with 1 min and then fixed with neutral
balsam after dehydration via 75, 95 and 100% alcohol with 3 min and
soaked in xylene for transparency for 5 min at room temperature.
The sections were observed under a light microscope.
Western blot analysis
Gastrocnemius tissues were chopped into small chunks
with fine scissors and then dissolved in RIPA lysis buffer
(Beyotime Institute of Biotechnology). The final protein
concentration was quantified by BCA Protein Kit (Beyotime Institute
of Biotechnology), with bovine serum albumin (BSA, Sigma) as
protein standard. The same amount (20 µg) of protein samples was
added into polyacrylamide gels (12%). The samples were subjected to
SDS-PAGE and transferred to a PVDF membrane, then blocked with 1%
BSA in TBST (0.05% Tween-20) at room temperature for 2 h. Then, the
membranes were incubated with the primary antibodies at 4°C
overnight. Following the primary incubation, membranes were
incubated with the secondary antibodies [Horseradish peroxidase
labeled goat anti rabbit IgG antibody (1:5,000; cat. no. SA00001-2)
and goat anti mouse IgG antibody (1:5,000, no. SA00001-1); both
ProteinTech Group, Inc; diluted with 0.5% BSA in TBST] at room
temperature for 2 h. The protein bands were visualized using
ChemiDoc-ItTMTS2Imager (Analytik Jena AG) and protein expression
was quantified using ImageJ software (National Institutes of
Health). Anti-G6P (1:1,000; cat. no. ab133964), anti-Beclin1
(1:1,000, ab207612), Atg7 (1:1,000, ab133528), AKT (1:1,000,
ab179463), P-AKT (1:1,000, ab192623), MTOR (1:1,000, ab134903),
P-MTOR (1:1,000, ab109268), PI3K (1:1,000, ab191606) and P-PI3K
(1:1,000, ab278545) were used as the primary antibodies, with
glyceraldehyde-3-phosphate dehydrogenase (cat. no. HC301; Beijing
Transgen Biotech Co., Ltd.) as the internal reference.
Reverse transcription-quantitative
(RT-q)PCR
After the mice were euthanized by excessive
anesthetic, a gastrocnemius tissue was received for RT-qPCR.
According to the manufacturer's protocol (Promega Corporation),
total RNA extracts were obtained using TRIzol® Reagent
(Ambion; Thermo Fisher Scientific, Inc.), and 5 µg of total RNA was
used to synthesize cDNA (Promega Corporation). RT-qPCR was
performed using SYBR-Green. cDNA samples were amplified on a 7500
fast RT-PCR system (Applied Biosystems) under the following
thermocycling conditions: 95°C for 3 min, followed by 40 cycles of
95°C for 15 sec and 60°C for 45 sec. The relative expression levels
of the target genes were normalized to those of the housekeeping
gene ribosomal protein S18 (RPS18) and the target genes from the
experimental group were compared with the corresponding target
genes from the control group using the 2−ΔΔCq method.
The following oligonucleotide primers were used: G6P forward,
5′-CCTTTGGGTAGCTGTGATTGGA-3′ and reverse,
5′-GGCACGGAAGTGTTGCTGTAGTAG-3′; FAS forward,
5′-GTGCTTGCTGGCTCACAGTTA-3′ and reverse,
5′-GGTTGGTGTACCCCCATTCA-3′; SREBP-1c forward,
5′-GCATCTTCTTGTGCAGTGCC-3′ and reverse, 5′-TACGGCCAAATCCGTTCACA-3′;
Beclin1 forward, 5′-AAAGAGTGGAAGATGTCCGGC-3′ and reverse,
5′-CAGCTGCTTCTCACCCTTGTA-3′; Atg7 forward,
5′-GCCAGGTACTCCTGAGCTGT-3′ and reverse, 5′-GGTCTTACCCTGCTCCATCA-3′;
and RPS18 forward, 5′-GCAATTATTCCCCATGAAG-3′ and reverse,
5′-GGCCTCACTAAACCATCCAA-3′. The amount of RPS18 in samples was used
to normalize the mRNA content (the RNA level was expressed relative
to that of the corresponding control).
Statistical analysis
Statistical analysis was performed using Prism 5
software (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference. Paired Student's
t-test and one-way ANOVA followed by post hoc Tukey's test were
used to compare 2 and >2 groups, respectively. Data are
presented as the mean ± SD of at least three independent
experiments. Each data point in the figures represents one sample
from one mouse. The numbers of animals used in each experiment are
indicated in the figure legends.
Results
Effect of WBV on blood glucose and
blood lipid in DM mice
Previous studies have shown that in mouse models,
exercise training [such as swimming (8) and running wheels (9)] decreases as injuries develop. To
investigate the effects of WBV on gastrocnemius, mice were trained
with WBV for 12 weeks, and examined in 0, 2, 4, 6, 8, 10 and 12
weeks after feeding and blood sugar reached 11.1 mmol/l. In the
present study, the weight of diabetic mice was significantly higher
than that of normal mice, while there was no significant difference
in weight between the control and WBV groups (Fig. 1B). As revealed in Fig. 1C, a significant decrease in blood
glucose levels was observed after 12 weeks of WBV treatment
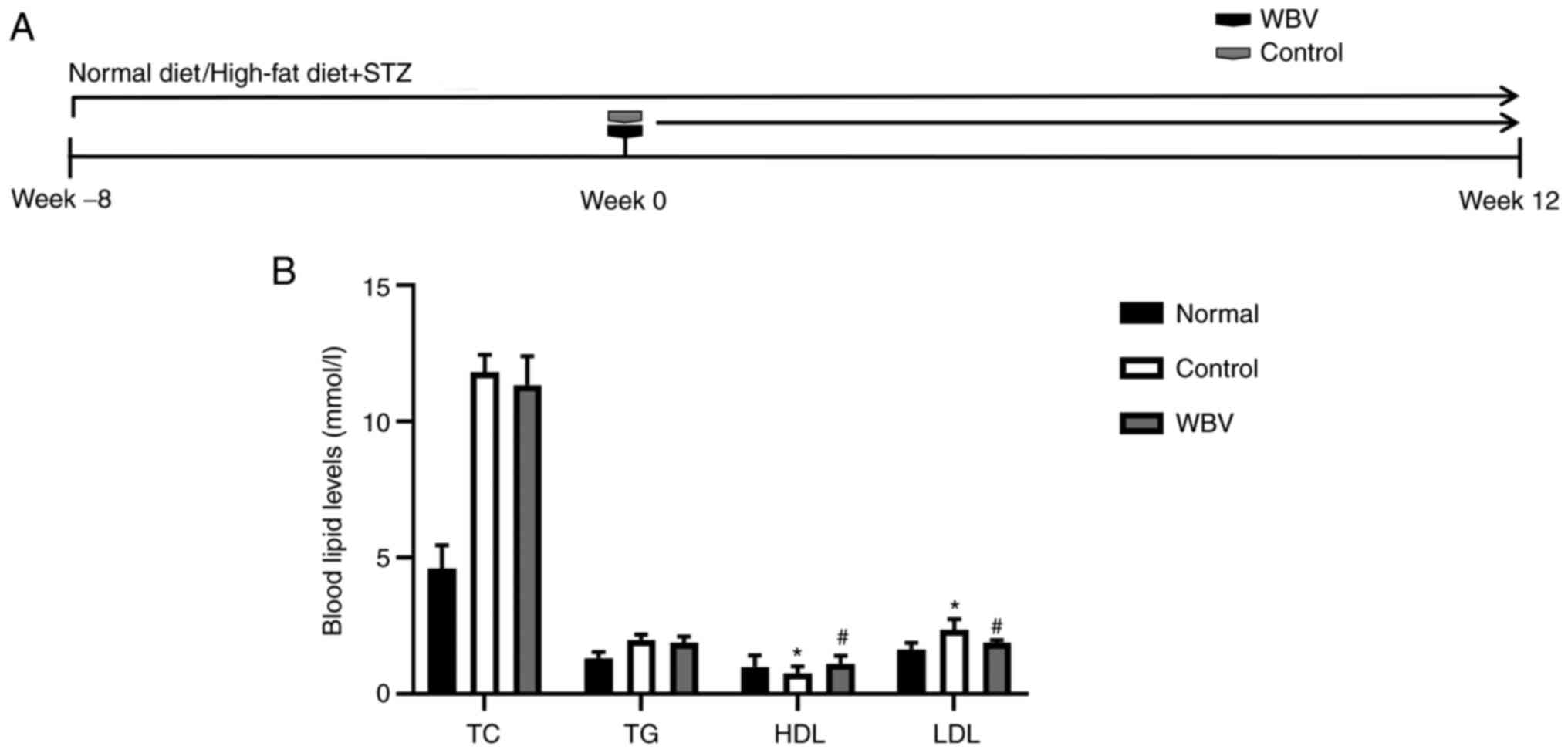
compared with the control group. Regarding blood lipids, WBV had no
significant effect on TC and TG concentrations in mice (Fig. 2B); however, the level of LDL was
significantly reduced in the WBV group, and the level of HDL was
significantly increased in the WBV group (Fig. 2B). Reductions in blood glucose and
lipoprotein indicated that WBV has an effect on diabetes in mice
after 12 weeks (Figs. 1A and
2A).
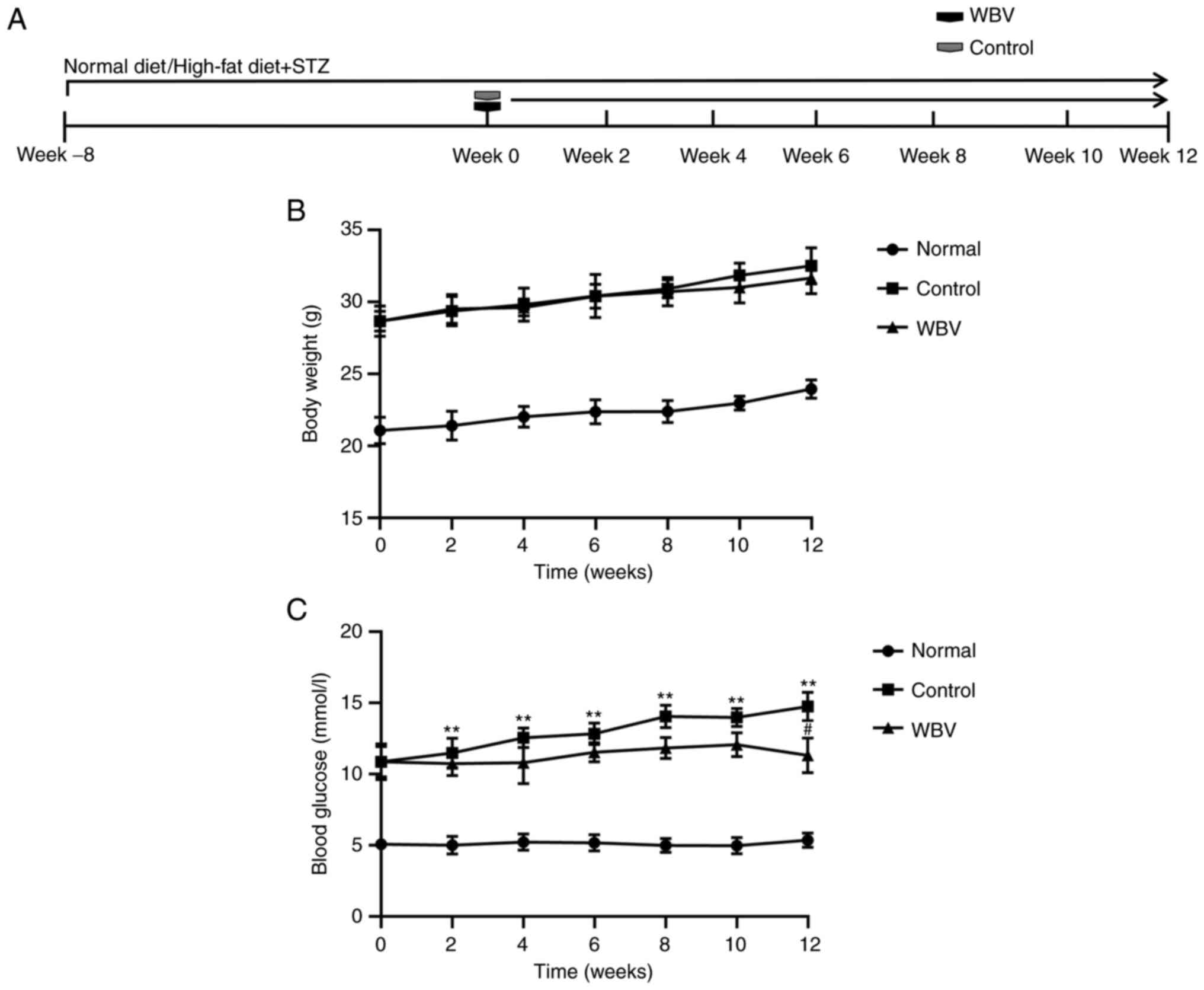 | Figure 1.Physiological conditions of the three
groups of mice. (A) Timing of body weight and blood glucose
measurements in different treatment mice. (B) Body weight was
measured before the intervention and at the 2, 4, 6, 8, 10 and 12
weeks after the intervention. (C) Levels of blood glucose were
evaluated before the intervention and at the 2, 4, 6, 8, 10 and 12
weeks after the intervention (n=5 animals/group). **P<0.01 vs.
Normal; #P<0.05 vs. Control. WBV, whole body
vibration; STZ, streptozocin. |
Effect of WBV on muscle reshaping in
DM mice
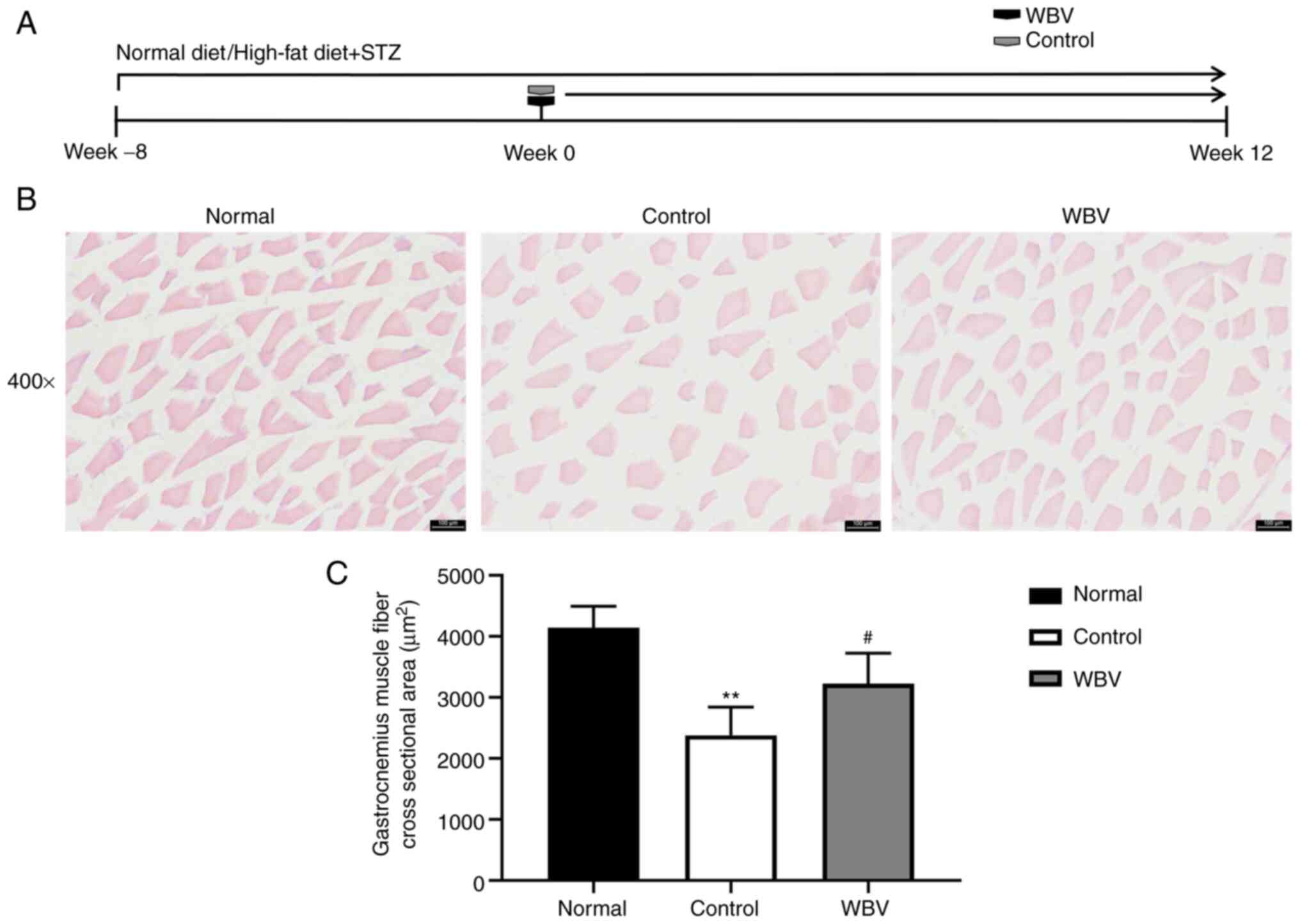
In order to explore whether chronic WBV training can
remodel skeletal muscle in diabetic mice, the cross-sectional area
of skeletal muscle was examined using H&E staining (Fig. 3A). The cross-sectional area of
skeletal muscle was measured to compare the difference between the
control and WBV groups. It was revealed that the skeletal muscle
area of the control group is smaller than that of the WBV group
(Fig. 3B). In addition, skeletal
muscle morphology showed that WBV training significantly reduced
the progression of DM complications (Fig. 3C). Therefore, long-term WBV
training can delay the development of complications in diabetic
mice.
Effect of WBV to metabolism in DM
mice
Skeletal muscle remodeling in the WBV group
indicated that long-term intervention of WBV promotes the basic
physiological processes of skeletal muscle. Skeletal muscle glucose
metabolism (10,11) and lipid metabolism (12,13) play an important role in the
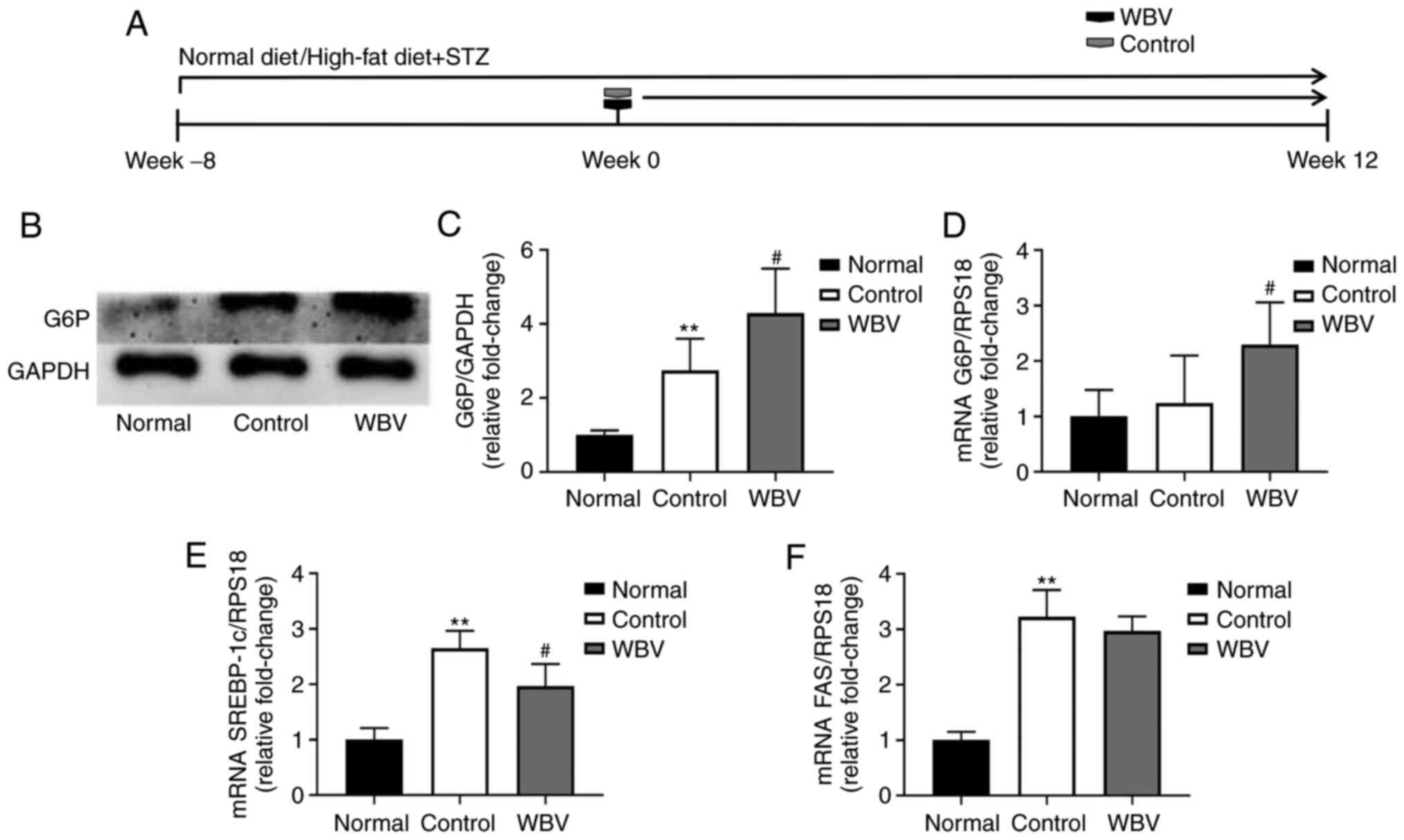
complications of DM. Among them, G6P is an important rate-limiting
enzyme of the glycolysis pathway (14) and Fas is an important
rate-limiting enzyme of fatty acid metabolism (15). Therefore, the mRNA and protein
expression of G6P was evaluated in three groups of mouse skeletal
muscle (Fig. 4A). As demonstrated
in Fig. 4B-D, G6P mRNA and
protein expression significantly increased after WBV treatment
compared with the control group. SREBP-1c mRNA significantly
decreased after WBV treatment in DM mice, FAS mRNA level had
indifferent in WBV and control groups (Fig. 4E and F). The aforementioned
results indicated that long-term WBV can influence glucose and
lipid metabolism of skeletal muscle.
Effect of WBV to autophagy in DM
mice
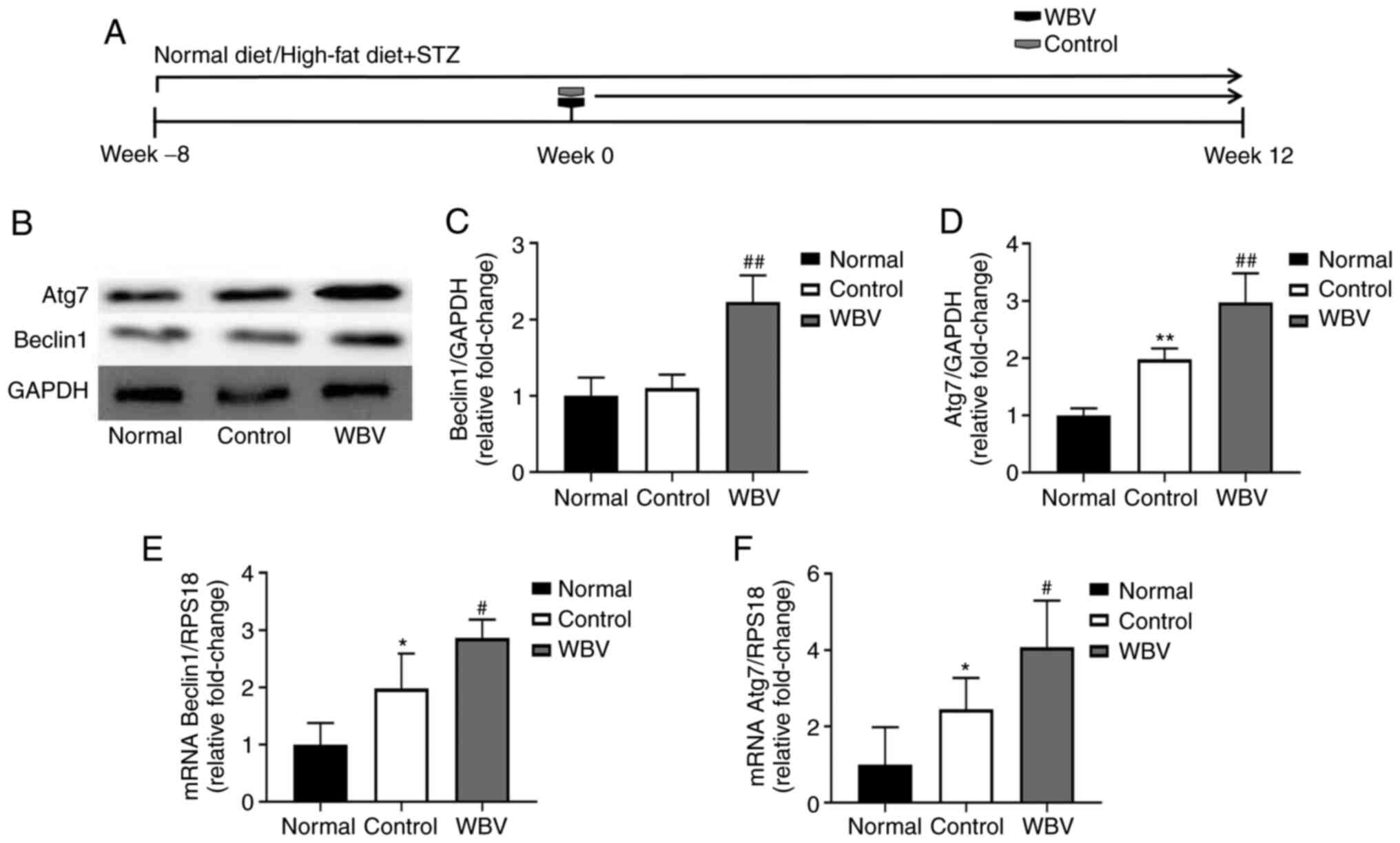
Previous studies showed that autophagy can promote
skeletal muscle recovery in disease (16,17). In order to confirm whether WBV
promotes skeletal muscle remodeling through autophagy, western blot
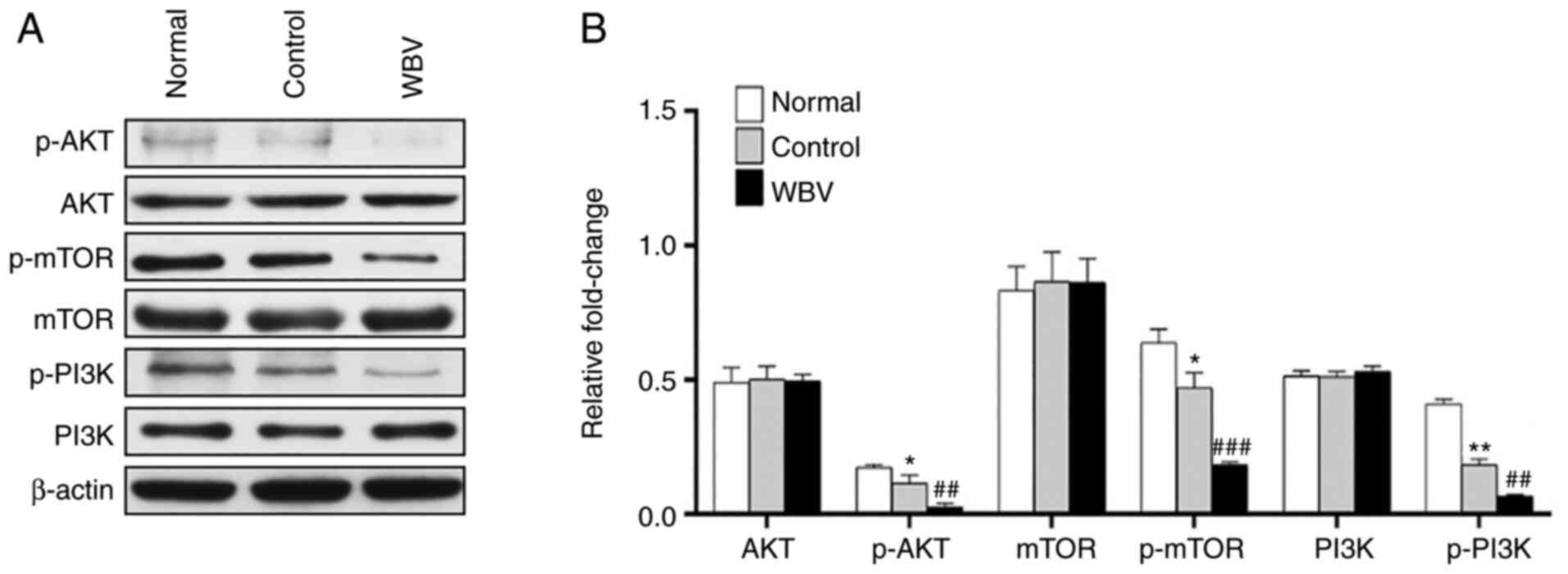
analysis was performed (Fig. 5A).
The results revealed that the expression of autophagy-specific
proteins Beclin1 and Atg7 was increased in the WBV group compared
with the control group (Fig.
5B-D). In addition, the aforementioned results were validated
using RT-qPCR (Fig. 5E and F).
Next, to further investigate whether or not WBV could affect
autophagy-specific signaling pathways in vivo. We found that
the administration of WBV decreased the level of p-AKT, P-MTOR and
P-PI3K via using western blot (Fig.
6A-6B). In summary, long-term WBV can increase autophagy,
thereby promoting skeletal muscle remodeling.
Discussion
In the present study, it was evaluated whether WBV
can promote skeletal muscle remodeling in diabetes. Mice
administered high-fat diet for 8 weeks showed skeletal muscle
contraction. The 12-week vibration training significantly increased
the cross-sectional area, autophagy and glucose metabolism of
skeletal muscle in DM mice, while significantly reducing serum LDL.
These results indicated that long-term WBV can delay DM
concurrency. The disease progresses with low levels of lipoproteins
and high levels of autophagy.
DM is a chronic inflammatory disease (2). Oxidative stress not only plays a key
role in the formation of initial lesions, but also plays a key role
in the progression and instability of lesions (2,18,19). In the complication stage, abnormal
blood glucose and accumulation of blood glucose in skeletal muscle
lead to oxidative stress (20).
In the present study, vibrational motion did not significantly
reduce TC in DM mice, although previous study has shown that WBV
significantly reduced elevated levels of TC and TG in obese mice
(4). In addition, lipoprotein
levels were examined and it was found that WBV significantly
decreased LDL in DM mice. Numerous studies have confirmed that
prolonged or high-intensity exercise causes oxidative damage to
large molecules in blood and skeletal muscle (19,20).
Skeletal muscle is the main tissue for glucose
uptake by insulin in the body and plays an important role in
glucose metabolism balance, particularly in patients with diabetes
(21,22). Skeletal muscle contraction
involves multiple regulatory steps in glucose metabolism, of which
glucose glycolysis is considered a rate-limiting step (3). G6P is a carrier of rate-limiting
enzymes in the glucose glycolysis pathway of the cell. G6P exists
in various tissues and cells of the human body. It stimulates
glucose metabolism by regulating the rate of glucose breakdown
under the signal of insulin (14). Previous research has found that
exercise training may affect glucose metabolism by increasing the
glycoprotein content of myoblasts and increasing the transport and
use of glucose by myoblasts (21). Moreover, a previous study
demonstrated that the activation of autophagy during exercise may
increase the positive regulation of skeletal muscle utilization
(23). A previous study
demonstrated that the effects of exercise on glucose transport
depend on autophagy through studies of lateral femoral muscles of
mice, cultured rat toe extensors and L6 muscle tubules (24).
Autophagy is a conservative catabolic process. When
cells are in a hungry environment, autophagy can reuse long-lived
proteins and organelles as nutrients (16). Genes and proteins involved in
autophagy have been identified in yeast. These genes are called
ATGs, which control autophagy formation through two ubiquitin like
systems Atg12/5 and Atg7/Atg8 (17). There are similar genes and
proteins in human. The transformation of LC3 (Atg8) from lc3i to
lc3ii represents autophagy (17).
The signal of autophagy is regulated by the mTOR pathway (25). Through the PI3K/Akt pathway
(25), p70S6 kinase and eIF2α
kinase activate the mTOR pathway, while beclin-1 (Atg6) regulates
autophagy process through the class III PI3K pathway (26). Recent studies (16,17) have revealed that autophagy plays
an important role in maintaining the metabolic balance of skeletal
muscle cells under certain pathological conditions (atrophy of
skeletal muscle caused by heart failure and hyperglycemia), but the
specific mechanism remains to be explored. Autophagy is a
lysosome-dependent catabolic process. Both extracellular and
intracellular components are phagocytosed by autophagosomes and
degraded into simple molecules such as monosaccharides, fatty acids
and amino acids. These molecules can then be further used to
produce ATP through catabolic reactions and/or provide the basis
for the synthesis of essential proteins. Therefore, it is
considered that autophagy is a key and fine-tuned process for
maintaining energy homeostasis, particularly in diseases (25). The complex relationship between
autophagy and energy metabolism has attracted wide interest and has
been extensively studied. In the present study, the relationships
that enable autophagy to control or regulate energy metabolism and
allow metabolic pathways to regulate autophagy in a diabetic state
were investigated. Specifically, the association between autophagy
and energy homeostasis from glycolysis, fatty acid metabolism, and
amino acid metabolism was studied. Understanding the role of
autophagy in energy homeostasis can help in improved understanding
of how autophagy determines skeletal muscle fate through energy
metabolism in diabetic mice.
The present study confirmed that WBV can attenuate
the development of DM and lead to lower level of LDL in the blood.
In addition, G6P level plays an important role in WBV-treated DM
model and may be used to monitor the effect of WBV in patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SA designed and performed the experiments, analyzed
the data and wrote the manuscript. DW performed the staining and
western blot experiments and analyzed data. XM performed the PCR
experiments. CL supervised the study, designed the experiments,
interpreted data and wrote the manuscript. SA, DW, XM and CL
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
2020008) by the Ethics Committee of Jinzhou Medical University
(Jinzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wei H, Hu Q, Wu J, Yao C, Xu L, Xing F,
Zhao X, Yu S, Wang X and Chen G: Molecular mechanism of the
increased tissue uptake of trivalent inorganic arsenic in mice with
type 1 diabetes mellitus. Biochem Biophys Res Commun. 504:393–399.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rampogu S and Lemuel M: Network based
approach in the establishment of the relationship between type 2
diabetes mellitus and its complications at the molecular level
coupled with molecular docking mechanism. Biomed Res Int.
2016:60684372016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maegawa H and Kashiwagi A: Molecular
mechanism and clinical impact of insulin resistance in type 2
diabetes mellitus. Nihon Rinsho. 57:539–544. 1999.PubMed/NCBI
|
|
4
|
Lai CC, Tu YK, Wang TG, Huang YT and Chien
KL: Effects of resistance training, endurance training and
whole-body vibration on lean body mass, muscle strength and
physical performance in older people: A systematic review and
network meta-analysis. Age Ageing. 47:367–373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sierra-Guzmán R, Jiménez-Diaz F, Ramírez
C, Esteban P and Abián-Vicén J: Whole-body-vibration training and
balance in recreational athletes with chronic ankle instability. J
Athl Train. 53:355–363. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sierra-Guzmán R, Jiménez JF, Ramírez C,
Esteban P and Abián-Vicén J: Effects of synchronous whole body
vibration training on a soft, unstable surface in athletes with
chronic ankle instability. Int J Sports Med. 38:447–455. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Liu C, Lu ML, Tang FT, Hou XW, Yang
J and Liu T: Vibration exercise decreases insulin resistance and
modulates the insulin signaling pathway in a type 2 diabetic rat
model. Int J Clin Exp Med. 8:13136–13144. 2015.PubMed/NCBI
|
|
8
|
Szostak J, Miguet-Alfonsi C, Berthelot A
and Laurant P: Training-induced anti-atherosclerotic effects are
associated with increased vascular PPARgamma expression in
apolipoprotein E-deficient mice. Acta Physiol (Oxf). 216:221–230.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shing CM, Fassett RG, Peake JM and Coombes
JS: Voluntary exercise decreases atherosclerosis in nephrectomised
ApoE knockout mice. PLoS One. 10:e01202872015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao C, Yang C, Wai STC, Zhang Y, P
Portillo M, Paoli P, Wu Y, San Cheang W, Liu B, Carpéné C, et al:
Regulation of glucose metabolism by bioactive phytochemicals for
the management of type 2 diabetes mellitus. Crit Rev Food Sci Nutr.
59:830–847. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu H, Deng X, Shi Y, Su Y, Wei J and Duan
H: PGC-1α, glucose metabolism and type 2 diabetes mellitus. J
Endocrinol. 229:R99–R115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoo JS and Lee SJ: A meta-analysis of the
effects of exercise programs on glucose and lipid metabolism and
cardiac function in patients with type II diabetes mellitus. Taehan
Kanho Hakhoe Chi. 35:546–554. 2005.(In Korean). PubMed/NCBI
|
|
13
|
Iizuka Y, Murase T and Iizuka Y:
Abnormalities in lipid metabolism associated with diabetes
mellitus. Nihon Rinsho. 55 (Suppl):S603–S608. 1997.PubMed/NCBI
|
|
14
|
Kundu BK, Zhong M, Sen S, Davogustto G,
Keller SR and Taegtmeyer H: Remodeling of glucose metabolism
precedes pressure overload-induced left ventricular hypertrophy:
review of a hypothesis. Cardiology. 130:211–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herrera E and Desoye G: Maternal and fetal
lipid metabolism under normal and gestational diabetic conditions.
Horm Mol Biol Clin Investig. 26:109–127. 2016.PubMed/NCBI
|
|
16
|
Jiao J and Demontis F: Skeletal muscle
autophagy and its role in sarcopenia and organismal aging. Curr
Opin Pharmacol. 34:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee DE, Bareja A, Bartlett DB and White
JP: Autophagy as a therapeutic target to enhance aged muscle
regeneration. Cells. 8:1832019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oguntibeju OO: Type 2 diabetes mellitus,
oxidative stress and inflammation: Examining the links. Int J
Physiol Pathophysiol Pharmacol. 11:45–63. 2019.PubMed/NCBI
|
|
19
|
Annunziata G, Barrea L, Ciampaglia R,
Cicala C, Arnone A, Savastano S, Nabavi SM, Tenore GC and Novellino
E: Arctium lappa contributes to the management of type 2 diabetes
mellitus by regulating glucose homeostasis and improving oxidative
stress: A critical review of in vitro and in vivo animal-based
studies. Phytother Res. 33:2213–2220. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ighodaro OM: Molecular pathways associated
with oxidative stress in diabetes mellitus. Biomed Pharmacother.
108:656–662. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teng S and Huang P: The effect of type 2
diabetes mellitus and obesity on muscle progenitor cell function.
Stem Cell Res Ther. 10:1032019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sala D and Zorzano A: Differential control
of muscle mass in type 1 and type 2 diabetes mellitus. Cell Mol
Life Sci. 72:3803–3817. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tam BT and Siu PM: Autophagic cellular
responses to physical exercise in skeletal muscle. Sports Med.
44:625–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen HC, Bandyopadhyay G, Sajan MP, Kanoh
Y, Standaert M, Farese RV Jr and Farese RV: Activation of the ERK
pathway and atypical protein kinase C isoforms in exercise- and
aminoimidazole-4-carboxamide-1-beta-D-riboside (AICAR)-stimulated
glucose transport. J Biol Chem. 277:23554–23562. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu YT, Tan HL, Huang Q, Ong CN and Shen
HM: Activation of the PI3K-Akt-mTOR signaling pathway promotes
necrotic cell death via suppression of autophagy. Autophagy.
5:824–834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furuya N, Yu J, Byfield M, Pattingre S and
Levine B: The evolutionarily conserved domain of Beclin 1 is
required for Vps34 binding, autophagy and tumor suppressor
function. Autophagy. 1:46–52. 2005. View Article : Google Scholar : PubMed/NCBI
|