Introduction
The term ‘brain disease’ encompasses various
conditions, including brain injuries [e.g., stroke, white matter
injury (WMI), and traumatic brain injury] (1), neurodegenerative diseases (e.g.,
Alzheimer's disease, Parkinson's disease, and amyotrophic lateral
sclerosis) (2), and affective
disorders (e.g., depression and anxiety) (3), with the associated lesions mainly
localized in the cortex, hippocampus, corpus callosum, and nerve
nuclei (4). Patients with brain
injuries and neurodegenerative diseases typically exhibit movement
disorders and cognitive impairment (5,6),
whereas those with affective disorders typically exhibit working
memory deficits and impaired emotional processing (7). These conditions severely affect the
quality of life of patients; however, the pathogenetic mechanisms
underlying numerous brain diseases remain to be fully elucidated,
and effective strategies for clinical treatment of such diseases
are often lacking. Therefore, investigation of the pathogenesis and
treatment of human brain diseases is of considerable clinical
value. However, due to ethical and methodological limitations of
experimentation involving human participants, the dominant approach
for studying the nature, prevention, and treatment of human brain
diseases involves the use of animal models.
Human brain diseases are mainly modeled in mice and
rats, and considerable advancements have been made based on the
data derived using these models (8,9).
Despite such advancements, various testing methods with different
principles, operational procedures, and assessment criteria have
been used in animal research, and a specific optimal approach has
not been generally accepted, to date. Selecting the optimal methods
for investigating specific diseases will help in improving outcomes
in both research and clinical settings. The methods used to assess
brain disease in animal models can be generally categorized into
pathological observation, specific marker identification, and
behavioral performance assessment (10). Typically, behavioral tests are
used to determine whether movement, cognition, working memory, and
emotion have been affected, and such tests appear to be the most
effective approach for evaluating whether animal models mimic the
clinical characteristics of specific diseases (10,11).
The aim of the present review was to evaluate
behavioral assessment methods for investigating the effects of
brain diseases and relevant treatment strategies in animal models.
First, the typical behavioral tests used in rodent models of brain
diseases, including the Morris water maze (MWM) test, novel object
recognition test, balance beam walking test, rotarod test, open
field test (OFT), elevated plus-maze (EPM) test, tail suspension
test (TST), and forced swimming test (FST), were summarized and
reviewed. Then, the advantages and limitations of each approach
were compared and recommendations that can aid researchers in
selecting the optimal methods for their investigations were
provided.
The present study was designed as a narrative
review. It was performed by searching for the key words in
databases (PubMed and Web of science) including ‘behavioral tests’,
‘brain diseases’, ‘rodent models’, and ‘behavioral assessments’.
The studies searched were covered between 1947 and 2021. After
reading the abstract, the studies that met with the scope of the
present study were included and finally 104 studies were cited.
Principles, procedures, assessment, and
application of different behavioral tests
MWM test
The MWM test was originally developed in 1982 by
Morris et al, who sought to take advantage of the congenital
abilities for spatial navigation and swimming in rodents. This test
also relies on the innate drive of the animals to escape the water
by locating and reaching the standing platform, which has been
considered to reflect motivation for learning and memorization.
Following its development, this test was immediately adopted as the
standard method for investigating cognitive function based on the
spatial memory and navigation abilities of the animal (12). Morris et al developed the
test based on prior electrophysiological study showing that some
cells in the hippocampus responded during the spatial learning and
exploration phase, whereas other cells exhibited
electrophysiological activity only when rodents entered a familiar
environment (usually a specific and restricted area) (12). Moreover, damage to the hippocampus
or decreases in the number of hippocampal synapses can lead to
deficits in spatial learning and memory (12). Several recent studies have aimed
to verify the theory that the hippocampus functions as a dynamic
central hub for the hippocampal-cortical network, whose activation
is considered to occur during episodic memory acquisition and
retrieval in both humans and rodents (13,14). The capacity for episodic memory
acquisition and retrieval of an animal is usually considered to
reflect their ability to perceive spatial factors or cues, which
are processed and consolidated afterward and are finally used to
locate the standing platform in the MWM test (15). However, a previous study suggested
that the spatial learning and navigation aspects of the MWM test
performance do not solely rely on hippocampal activity but require
significant involvement of cortical and subcortical regions
(16). In addition, in another
previous study it was reported that focal injuries to the medial
thalamus impair the ability to adopt search strategies and swimming
behavior without impacting spatial mapping and navigation
performance (17). Furthermore,
another study examined several novel variables and measures
including a spatial learning index, which has greatly enhanced the
ability to assess subtle differences in the MWM test performance
(15). This index has
considerably facilitated comparisons among groups and has aided
correlation analyses with neurobiological markers or other
behavioral measures (15).
Moreover, one study demonstrated that the spatial learning index
was sensitive enough to detect delicate behavioral alterations
among aged individuals (15).
Therefore, findings of studies using this spatial learning index
have improved our understanding of age-related cognitive decline
and cognitive function maintenance in aged individuals.
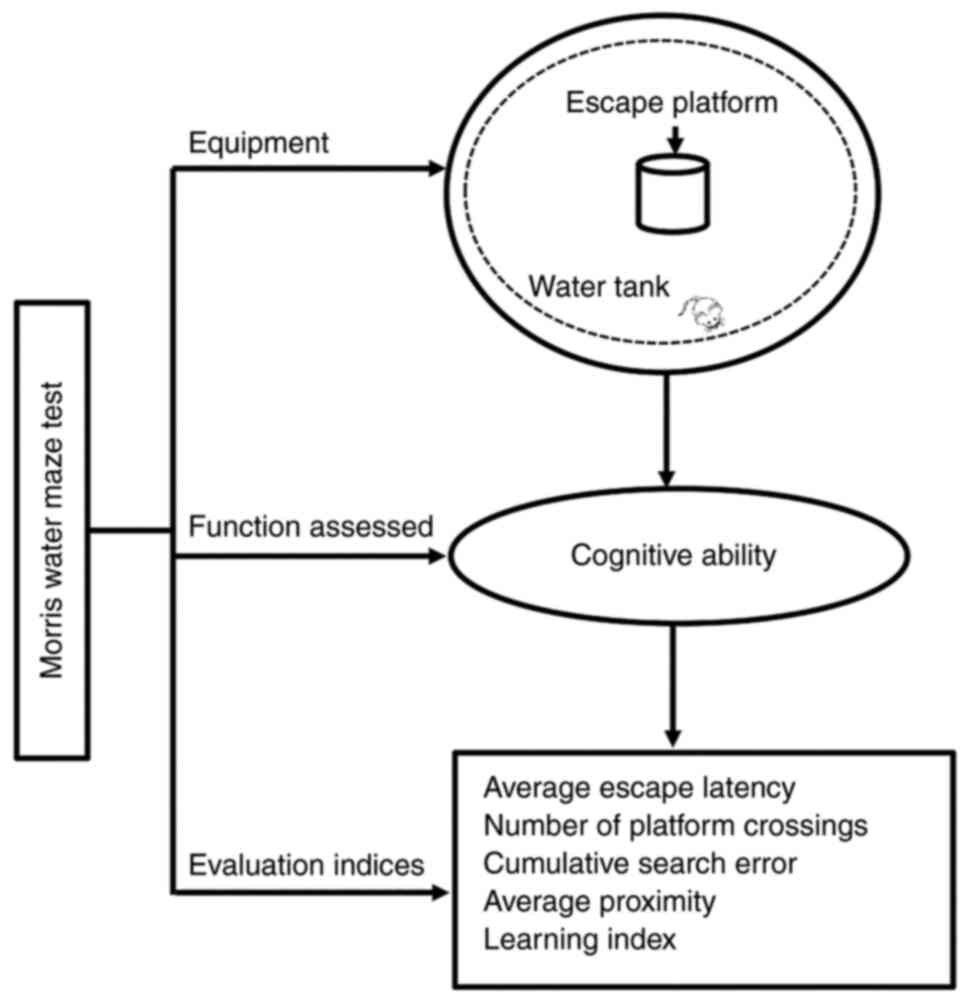
The equipment for the MWM test comprises three main
elements: A large water tank (150 cm in diameter), an escape
platform (15 cm in diameter), and a video monitor placed above the
tank. The MWM test involves a navigation training stage, followed
by a spatial exploration test to assess cognitive abilities
(18). Adult animals are trained
during the first 5–6 days. During training, the rodents are placed
in the tank and allowed to search for the platform (typically 2 min
for rats and 1 min for mice), and escape latency (i.e., the time
required to find the platform) is recorded. The mean escape latency
during the training stage is then used as a measure of the capacity
of the animal to understand spatial information. After 5–6 days of
training, animals undergo the spatial navigation test (18). First, the rodents are placed in
the third quadrant and allowed to swim freely in the tank (without
the platform) for 1 or 2 min, and the number of times they cross
the position of the removed platform is recorded for further
analysis (19) (Fig. 1).
In the MWM test, the average escape latency and the
number of platform crossings are used to evaluate learning and
memory ability (20). To improve
the assessment, researchers have developed a novel parameter known
as ‘proximity’, which is calculated as the frequency at which the
rodent comes near the platform in 1 sec. This measure generates two
additional variables, cumulative search error and average
proximity, which are more sensitive in detecting group differences
in behavior. In addition to their sensitivity, proximity measures
require a small number of experimental animals and can increase the
throughput of behavioral characterization facilities (21). Although proximity measures allow
for improved quantification of navigation ability in the MWM task,
other measures are still necessary. Accordingly, researchers have
proposed the ‘learning index’ that can be used to associate spatial
learning ability with other behavioral or neurobiological measures.
The rodents are subjected to four trials, and the average proximity
of the four probe trials is finally calculated as the learning
index (22). In summary, the
evaluation indices for the MWM test include the average escape
latency (sec), number of platform crossings, cumulative search
error, average proximity, and learning index (Fig. 1).
The MWM test is primarily designed to assess spatial
learning and memory function, as these processes are considered to
be similar in rodents and humans, particularly in terms of episodic
memory ability. Therefore, the MWM test has been widely used and is
well-recognized as a method for evaluating cognitive ability in
experimental models of brain injuries such as WMI, stroke, and
traumatic brain injury (23–25). Moreover, as the ‘visuospatial
navigation’ aspect of rodent performance is also reflected in
‘everyday cognitive’ processes in humans, the MWM test can be used
to study neurodegenerative diseases characterized by impaired
cognition, such as Alzheimer's disease or Parkinson's disease
(26,27). Additional studies have shown that
reversal learning and other aspects of cognitive flexibility rely
on the prefrontal cortex in both humans and rodents (21,28). Therefore, the MWM test has also
been used to assess the therapeutic effects of potential treatments
on cognitive deficits in experimental models, which can provide
critical information for clinical studies (Table I; 23-52).
 | Table I.Applicable conditions for behavioral
tests in rodent models of brain diseases. |
Table I.
Applicable conditions for behavioral
tests in rodent models of brain diseases.
| Authors, year | Behavioral
tests | Applicable
conditions | (Refs.) |
|---|
| Kim H et
al%, 2020 | Morris water maze
test | White matter
injury | (23) |
| Tucker LB et
al%, 2018 |
| Stroke | (24) |
| Zhong JY et
al%, 2017 |
| Traumatic brain
injury | (25) |
| Schneider CB et
al%, 2017 |
| Alzheimer's
disease | (26) |
| Deng-Bryant et
al%, 2016 |
| Parkinson's
disease | (27) |
| Zhang R et
al%, 2012 | Novel object
recognition test | Alzheimer's
disease, aging, traumatic brain injury, schizophrenia | (29,30) |
| Sadegzadeh F et
al%, 2020 |
|
|
|
| Chen W et
al%, 2019 | Balance beam
walking test | White matter
injury | (31) |
| Uematsu A et
al%, 2018 |
| Age-related motor
deficits | (32,33) |
| Gyengesi E et
al%, 2019 |
| Central nervous
system lesions |
|
| Mychasiuk R et
al%, 2014 |
| Huntington's
disease | (34) |
| El-Sahar AE et
al%, 2020 |
| Parkinson's
disease | (35) |
| Sun J et
al%, 2021 |
| Anxiety | (36) |
| Marques-Carneiro JE
et al%, 2014 |
| Stroke | (37) |
| Bohr A et
al%, 2020 |
| Multiple
sclerosis | (38) |
| Mitra NK et
al%, 2020 |
|
| (39) |
| Dong W et
al%, 2020 | Rotarod test | Amyotrophic lateral
sclerosis | (40) |
| Hayashi T et
al%, 2017 |
| Cerebellar
ataxia | (41,42) |
| Main SL et
al%, 2017 |
| Traumatic brain
injury |
|
| Park G et
al%, 2021 |
| Stroke | (43) |
| Owfard M et
al%, 2021 |
|
| (44) |
| Chkhartishvili E
et al%, 2011 | Open field
test | Depressive
disorder | (45) |
| Lecorps B et
al%, 2016 |
| Anxiety-like
behavior | (46) |
| Su X et al%,
2020 |
| White matter
injury | (47) |
| Shoi H et
al%, 2021 | Elevated plus-maze
test | Anxiety-like
behavior | (48) |
| Ren C et
al%, 2021 | Tail suspension
test | Anxiety | (49) |
| Castagné V et
al%, 2011 |
| Depression | (50) |
| Wang W et
al%, 2021 |
| White matter
injury | (51) |
| Ráez A et
al%, 2020 | Forced swim
test | Depression | (52) |
|
|
| Anxiety |
|
Novel object recognition test
The novel object recognition test is another
behavioral assessment method that is primarily associated with
cognitive ability. It was originally developed by examining the
natural tendency of rodents to explore novel objects (53). This test is unique in that it does
not follow strict rules: The rodents only need to be familiar with
the arena prior to testing, and the procedure is flexible and easy
to follow (54,55).
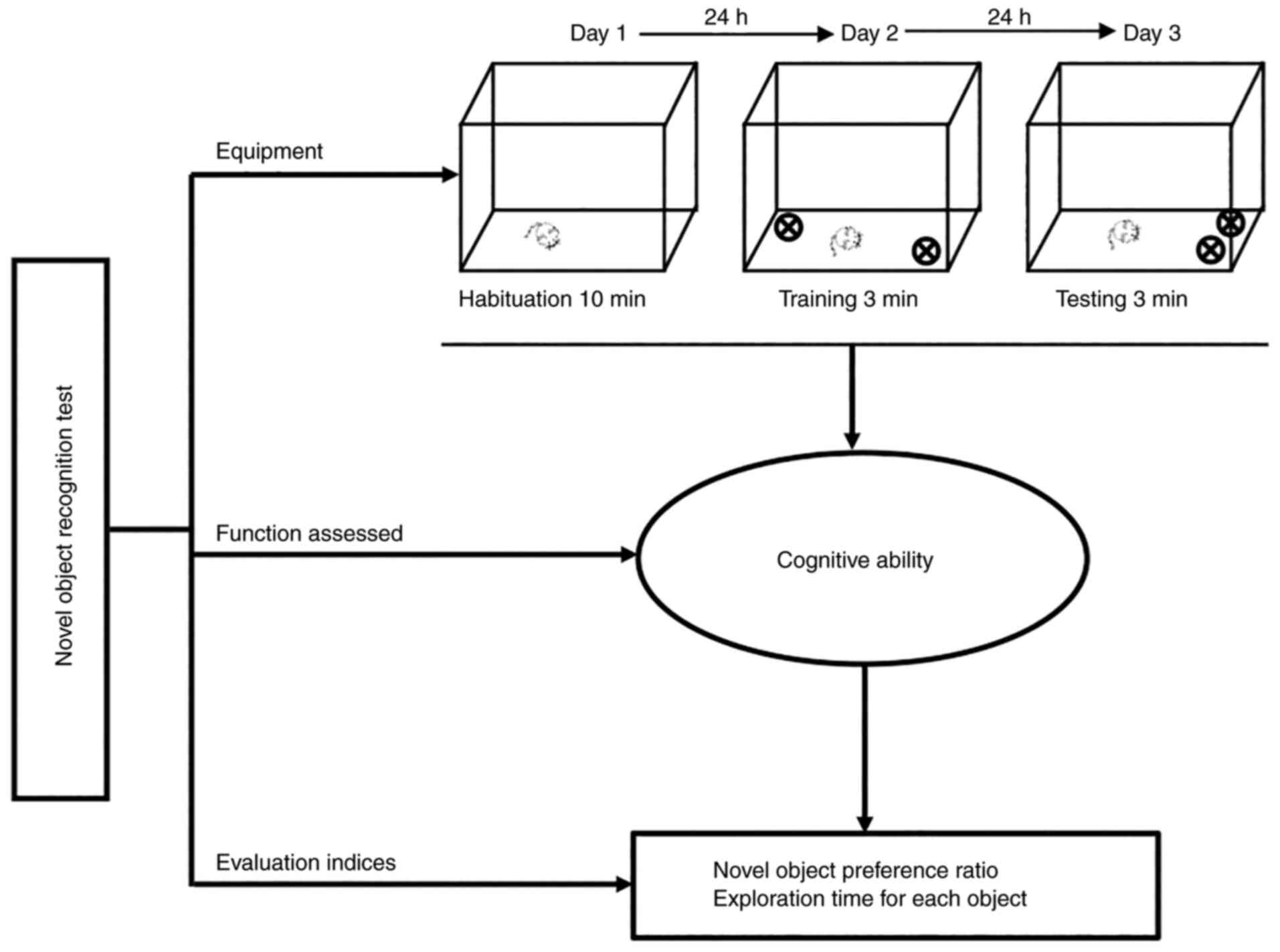
The novel object recognition test comprises three
stages: Habituation, training, and testing. On the 1st day
(habituation stage), rodents are placed in a plastic chamber (35 cm
in length ×35 cm in width ×35 cm in height) for 10 min for
familiarization with the arena. On the 2nd day (training stage),
two objects are placed symmetrically along the central line of the
arena, and the rodents are allowed to examine the objects for 3
min. The duration of exploration is recorded for each object as an
index of exploratory behavior. On the 3rd day (testing stage), the
rodents are returned to their home cages for 3 min, and one of the
objects is relocated to another adjacent quadrant, following which
the rodents are allowed to explore the objects again. The time
spent exploring the novel object is recorded and recorded as the
recognition index (56,57) (Fig.
2).
At present, there are two widely accepted indices
used to assess exploratory behavior during the test session. One is
the novel object preference ratio, which is calculated by dividing
the exploration time for the novel object by the exploration time
for the total objects. A value of >0.5 indicates preference for
the novel object, whereas a value of <0.5 indicates preference
for the familiar object. The exploration time for each object is
also used as an index of exploratory activity, such that the
duration for which the nose is within 1 cm of the object in the
novel location is recorded as the recognition index (58,59). Moreover, this test can be used to
evaluate memory ability based on the time required to identify the
novel objects (60) (Fig. 2).
Currently, this method is extensively used in
studies investigating conditions associated with memory deficits,
such as Alzheimer's disease, aging, traumatic brain injury, and
schizophrenia, as it can help in evaluating the neurobiology of
non-spatial memory in rodents (29,30) (Table
I).
Balance beam walking test
It is widely accepted that rodents exhibit innate
abilities for coordination and balance. Researchers have taken
advantage of this characteristic to develop the balance beam
walking test, which is used to assess motor balance and
coordination ability in rodents with damage to the motor cortex
(61,62). This test is advantageous in that
it is easier to set up and is less expensive than the rotarod test
(63).
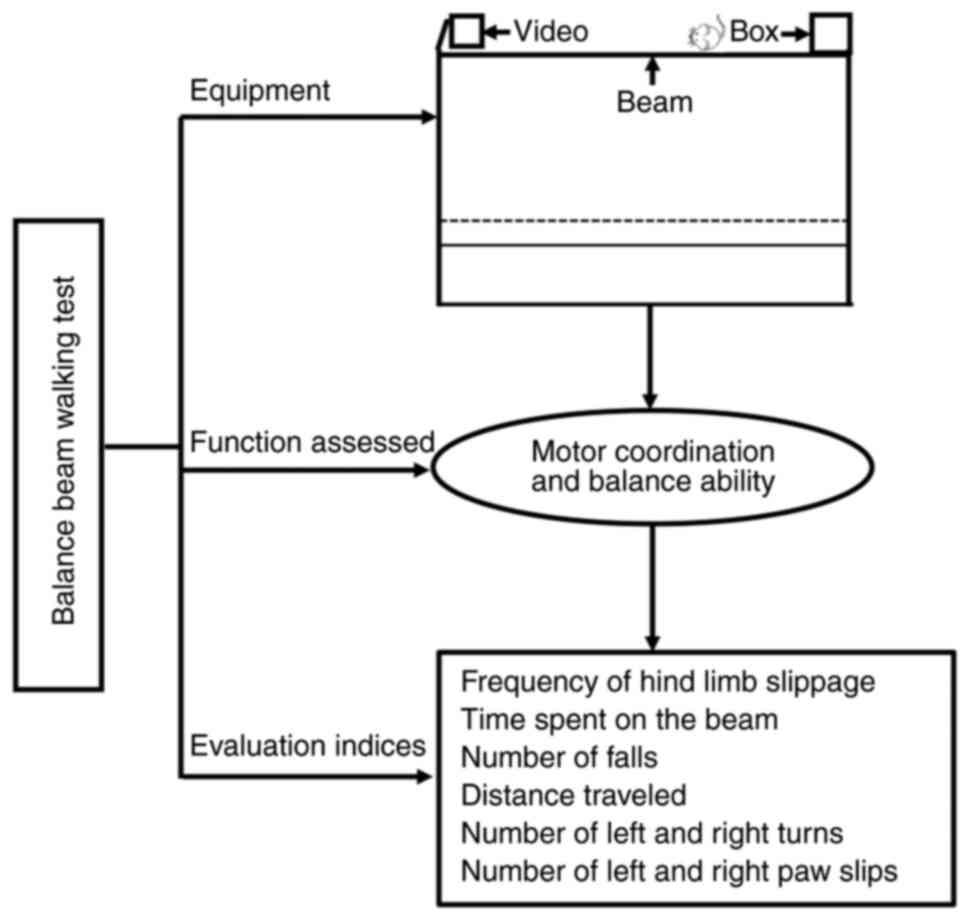
The modified beam walking test equipment comprises a
beam (80 cm in length, 0.5 cm in width, and 50 cm above the floor),
with a lamp on one end and a box (non-transparent) on the other end
and video-capturing equipment hanging above. First, for training,
the beam equipment is placed in a dark and enclosed room, and the
rodents are placed at the end of the beam containing the lamp.
During the training phase, the rodents are allowed to walk 30, 50,
and 70 cm for a maximum time of 60 sec. For each rodent, three
trials per day are performed for 3 consecutive days. Then, during
testing, the rodents are allowed to walk along the beam, similar to
that in the training phase, and the following three metrics are
used to evaluate performance: The frequency of hind limb slippage,
the time spent on the beam, and the number of falls when walking
the full distance (64,65) (Fig.
3).
The balance beam walking test performance is a
useful measure of fine coordination and balance (31). The results are typically used to
determine a beam walking performance index, which is calculated
using the frequency of hind limb slippage, the time spent walking
along the beam, the number of falls when walking the full distance
of the beam, the distance traveled within the set time, the number
of left and right turns, and the number of left and right paw slips
(66,67) (Fig.
3).
Since its development and widespread acceptance, the
balance beam walking test has been primarily used in studies of
age-related motor deficits (32,33), central nervous system lesions
(34), and genetic and
pharmacological manipulations (68). The test has also been used to
assess models of WMI (31),
Huntington's disease (35),
Parkinson's disease (36),
anxiety (37), stroke (38), and multiple sclerosis (39) (Table
I).
Rotarod test
The rotarod test represents another widely accepted
and utilized method for evaluating motor coordination and balance
in rodents, and both the balance beam walking test and the rotarod
test share nearly identical principles (69). The rotarod test is unique, in that
it is useful in evaluating endurance in rodents and is especially
sensitive to cerebellar disorders (70). Researchers have primarily taken
advantage of the ability of this test to assess motor coordination
to investigate the sedative properties of novel drugs and determine
their clinical value (71).
However, researchers have also begun to realize that the test is
associated with certain shortcomings. First, drug efficacy can
differ between animal experiments and clinical settings, with some
drugs exhibiting high sensitivity in rodents but insufficient
sensitivity in humans (72). For
example, administration of benzodiazepines or bretazenil exerts
nearly no effect on mouse rotarod performance, although it can lead
to excessive sedation in humans (63). Second, since most young adult mice
can maintain balance during the testing interval (60 sec) even at a
high speed (e.g., 44 rpm), researchers have argued that the rotarod
test should not be used to evaluate whether motor coordination or
balance ability has improved (73).
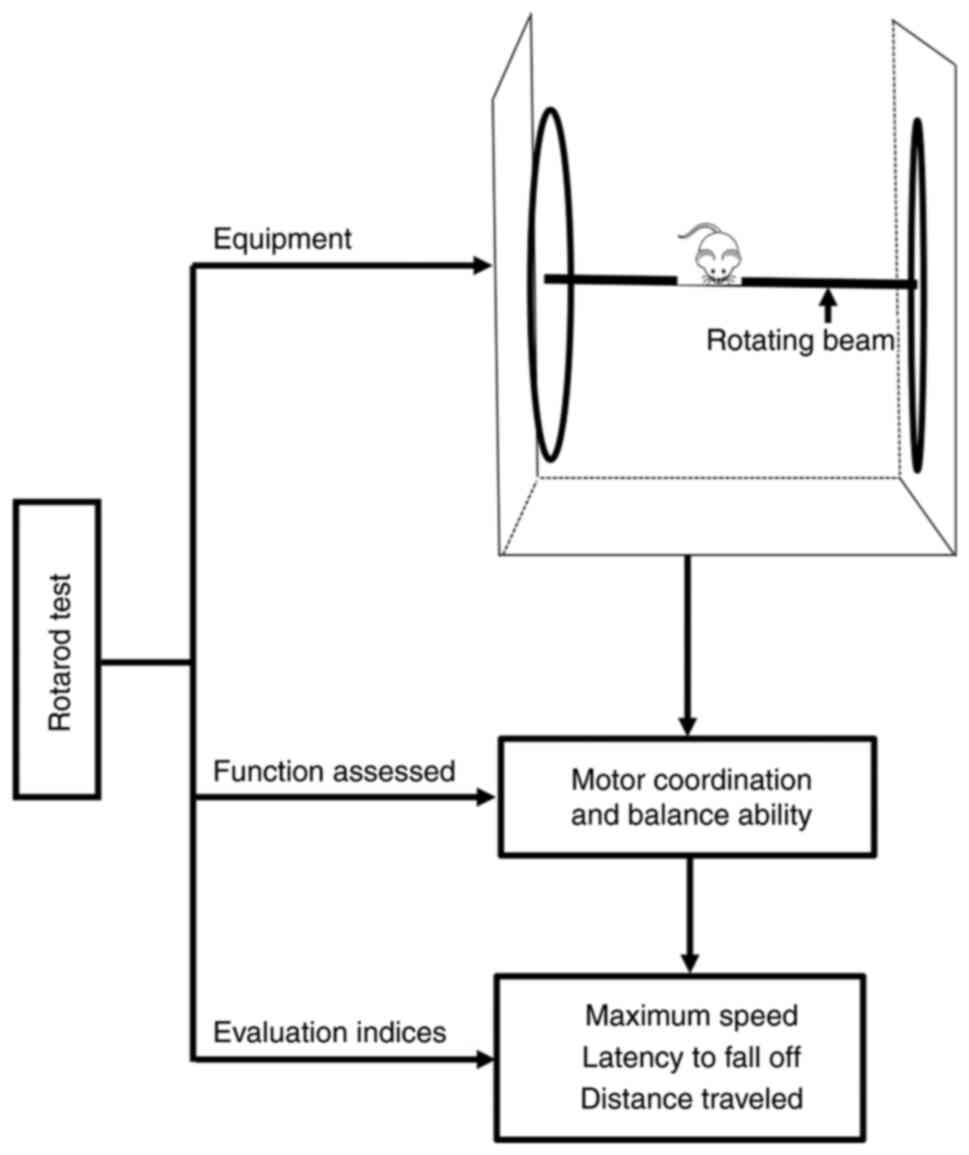
The rotarod is an automated apparatus comprising a
cylindrical rotating beam connected to a computer. Before the
trial, the rotarod is switched on with a starting rate of 4 rpm,
and the computer is checked to ensure that it is properly connected
for data recording. During the trial (generally lasting for 5 min),
rodents are allowed to walk on the rotating rod (4 rpm) so that
they can learn the motor coordination skills required for the
activity. The rotation speed is then slowly and incrementally
increased up to 40 rpm. The trial comes to an end when the tested
rodent touches the magnetized pressure sensor upon falling from the
rod. Three trials are conducted for each rodent, and the best trial
result of each rodent is recorded as the score for that day
(69,70) (Fig.
4).
The rotarod test is the dominant method for
evaluating balancing ability in rodents (70). The test includes two stages: A
constant speed stage and an accelerating speed stage. The constant
speed stage is used to estimate muscle strength, whereas the
accelerating speed stage is used to assess coordination, endurance,
and muscular power (74). The
rotarod test not only measures the maximum rotation speed at which
the animal can maintain balance for a given running duration (e.g.,
30 sec) but also calculates the latency to fall from the rod at
different speeds and distances traveled. These are recorded as
indices of motor coordination and balance performance, respectively
(70). However, the surface and
diameter of the rod as well as the rodents' body weight and
physiological (e.g., fatigue) and biochemical parameters should be
considered because they may influence the test results (69) (Fig.
4).
Given the extensive evidence accumulated thus far,
researchers generally agree that the test can be used to assess
sensorimotor abilities in several animal models, including those of
amyotrophic lateral sclerosis (40), cerebellar ataxia disorders
(41,42), traumatic brain injury (43), and stroke (44) (Table
I).
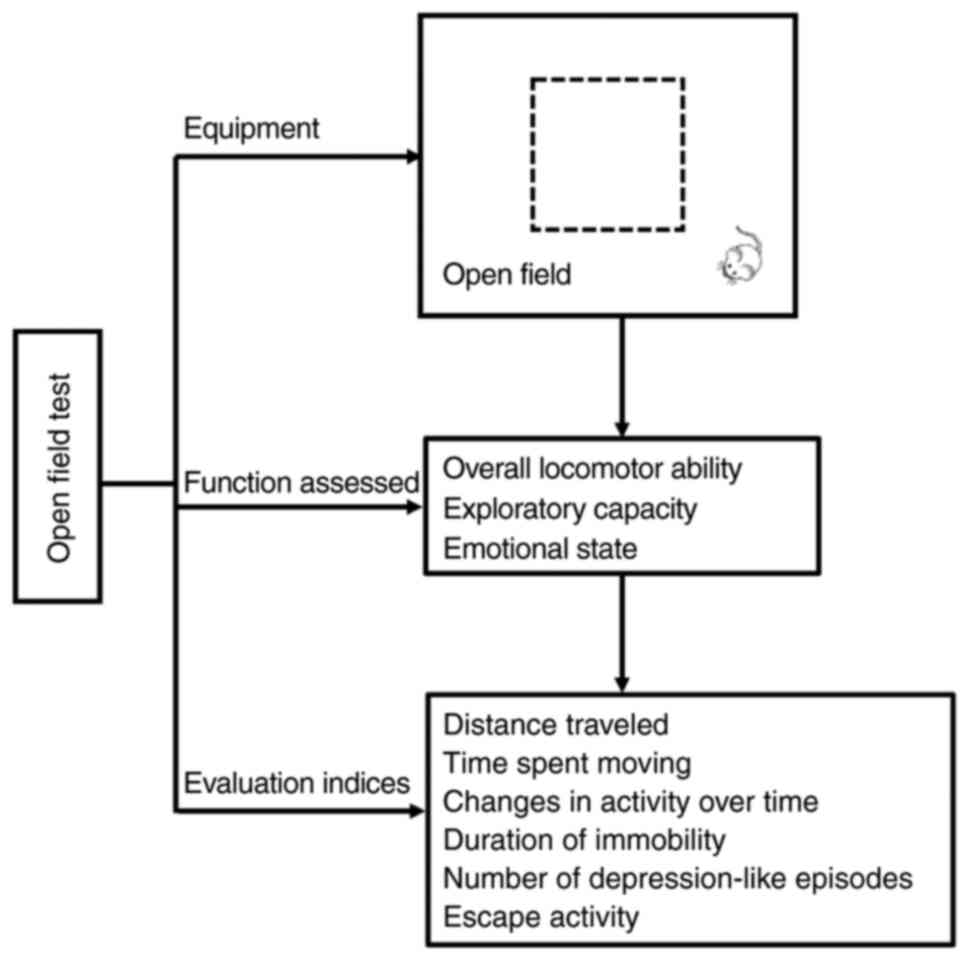
OFT
While the balance beam walking test and rotarod test
are widely used to evaluate motor coordination, the OFT is
specifically used to assess overall locomotor function in rodents.
Although it was initially developed to assess ‘timidity’ in mice
based on defecation (75,76), the test takes advantage of the
ability of the rodent to perceive new surroundings. The test may
seem contradictory, given that rodents can exhibit two types of
behaviors when entering a new setting (i.e., exploration of new
settings and escaping from the bright/exposed area due to fear)
(77). However, rodent responses
are assessed by monitoring movement parameters when the rodent
enters a new open field, which can help in determining the general
pattern of locomotor activity (78), the exploratory ability (79), and the level of fear (80).
The OFT requires an open field and a video computer
system. The field is a box (70 cm in width ×70 cm in length ×46 cm
in height), wherein rodents are allowed to stay for 15 min for
familiarization with the surroundings before starting the test.
During the test, the rodents are placed in the center of the field
and are allowed to move for 5 min. The distance traveled, the time
spent in the center of the field, the level of spontaneous
activity, and the number of entries into the central area are
recorded. This test can be conducted either in dark or light
settings (81,82) (Fig.
5).
Performance indices for the OFT include the distance
traveled, the time spent moving, and the alterations in activity
over time, which are integrated to determine the exploratory
capacity of the animal (83).
Additionally, the OFT can be used to assess the emotional state of
a rodent by measuring the duration for which the animal remains
stationary, the number of ‘depression-like’ episodes, and the
escape activity. These variables are then integrated to determine
the level of anxiety (84)
(Fig. 5).
At present, the OFT is widely used to evaluate
animal models of depressive disorders (45) and anxiety-like behavior (46). The test can also be used to assess
animal models of WMI by measuring the distance traveled and the
amount of time spent moving or being immobile in the central area
or the periphery (47) (Table I).
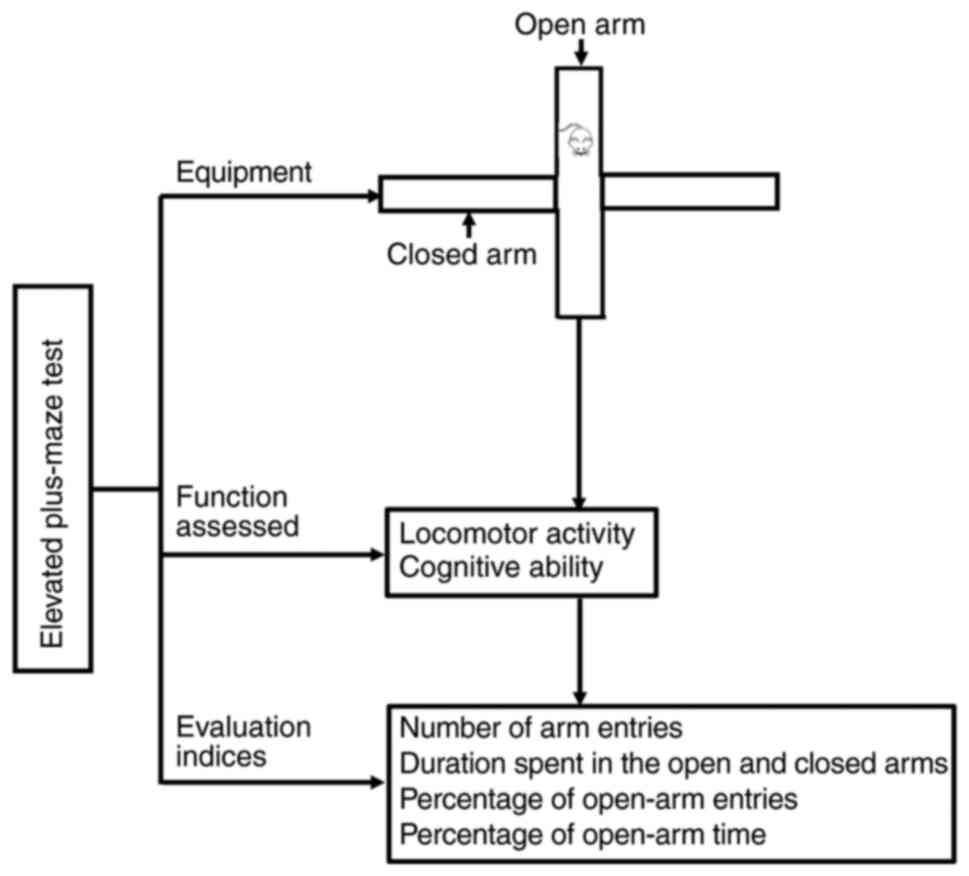
EPM test
Although both the EPM test and the OFT are used to
assess anxiety-like behavior, the principle of the EPM test differs
from that of the OFT to some degree. The EPM test was originally
devised based on the natural fondness of the rodents for dark and
enclosed spaces, their fear of open areas and heights, and their
desire to explore unfamiliar environments (48).
The EPM apparatus comprises two open arms (40×8 cm)
positioned at right angles and two closed arms of the same size
that are surrounded by black walls with a height of 30 cm. Before
the test, rodents are subjected to single-frequency ultrasonic
stimulation for 6 min to induce anxiety-like conditions. Following
stimulation, each rodent is placed in the central platform of the
maze facing an open arm and is allowed to freely explore the EPM
for 5 min (85,86) (Fig.
6).
Performance indices in the EPM test include the
number of arm entries and the duration spent in the open and closed
arms. Entry into an arm is defined as the animal placing its two
front paws inside an arm. Two additional measures can be derived as
indices of anxiety: The percentage of open-arm entries and the
percentage of the open-arm time. The percentage of time spent is
calculated as follows: (Time spent in an arm/300 sec) × 100
(48) (Fig. 6). A higher percentage of open-arm
time or open-arm entries indicates a lower level of anxiety
(78).
Currently, the EPM test is primarily used to assess
anxiety-like behaviors and anxiolytic drug properties in rodents
(78,87) (Table
I).
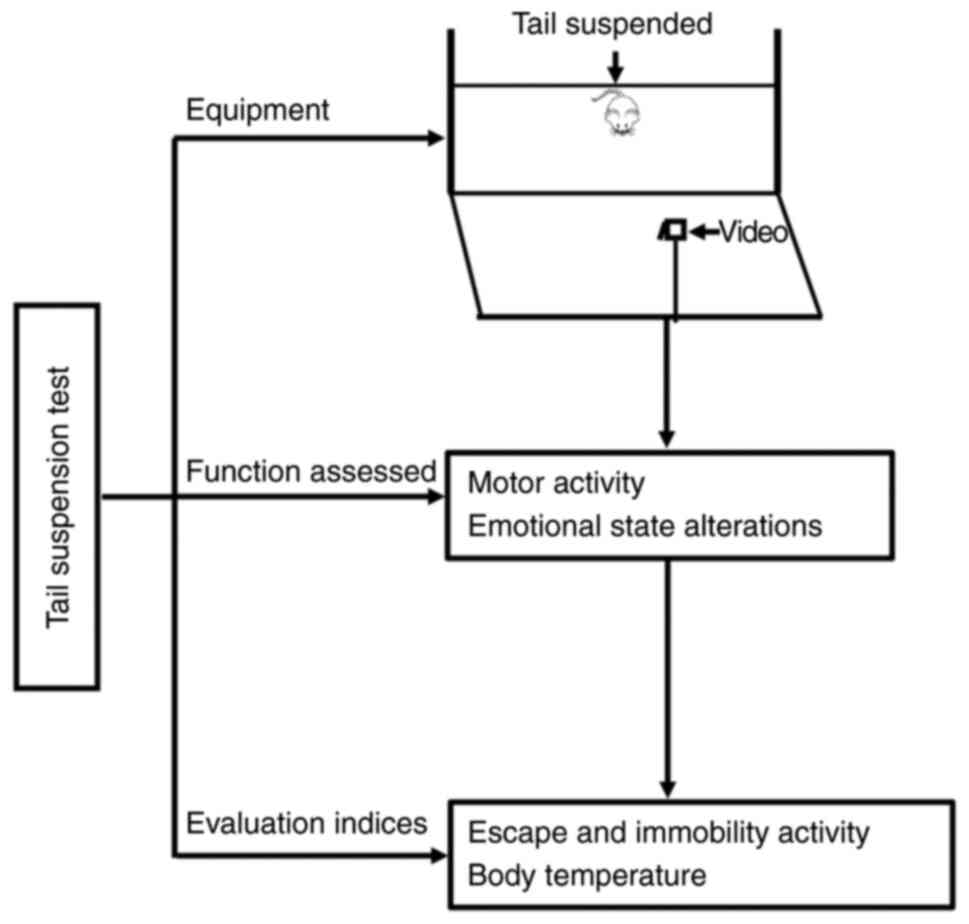
TST
Apart from the OFT and the EPM test, dominant
methods for assessment of affective disorders include the TST and
FST. The TST was developed based on the innate ability of rodents
to respond to an external stimulus via a set of affective
alterations; it is usually used to investigate depression-like
behaviors in rodents and screen the effects of psychotropic drugs
(e.g., antidepressants) (88–91). Moreover, the TST has been used to
investigate motor changes (i.e., motor coordination) in models of
Parkinson's disease (92) and
other metrics such as stress responses (93), helplessness (94), and anxiety (49).
The tail suspension apparatus mainly comprises a
rectangular box (60 cm in total length × 40–55 cm in height × 15 cm
in width) without external cues. During the test, rodents are
suspended by their tails in each compartment for ~5 min, and the
duration of immobility is calculated (95) (Fig.
7).
The TST performance is usually assessed over two
periods: An escape stage and a stationary stage. Escape activity
includes movements of the hind/forelimbs, movements of the head,
and the number of attempts made per minute to reach the tail by
bending the body and crawling upward. Immobility activity is
defined as a lack of attempts made to rescue oneself. Both escape
and immobility behaviors are recorded as the typical indices of the
TST performance. Measurements of body temperature, including
hyperthermia assessment, are also used as an index of emotional
stress (96,97) (Fig.
7).
The TST was originally developed to investigate
depression-like behavior (50).
Currently, the TST is extensively used to assess animal models of
depression and anxiety (49), and
it has recently been applied to assess behavioral performance in
models of WMI (51) (Table I).
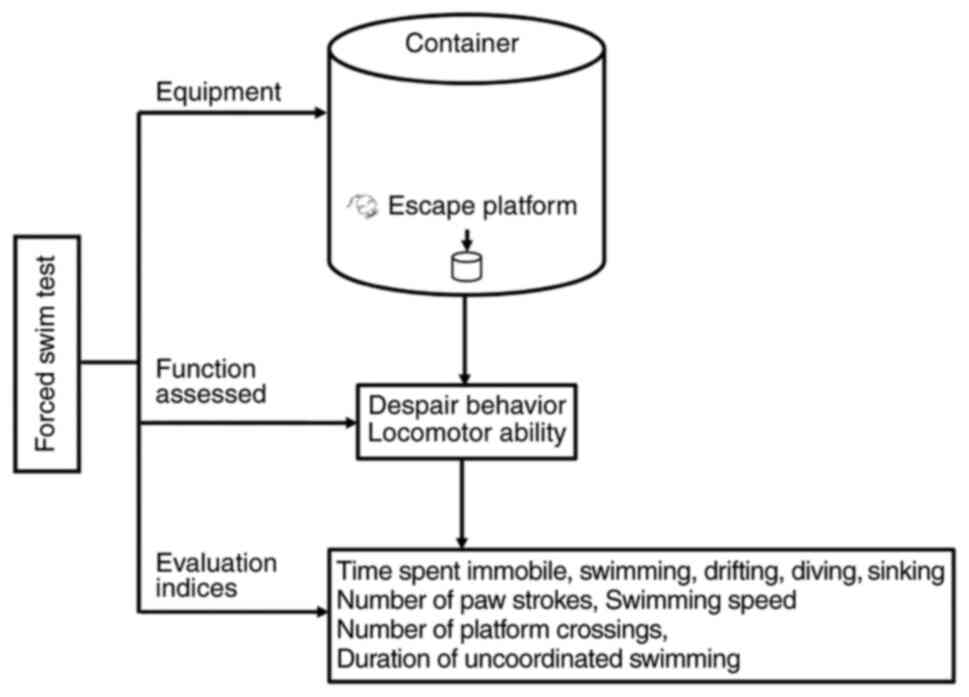
FST
The FST is a dominant approach for investigating
affective disorders and was originally developed based on the
immobility response of rodents to external stimuli, which is
considered to indicate ‘behavioral despair’ and a state of
‘depression’ (98). Researchers
have demonstrated that immobility in the FST is achieved gradually
through repeated failures, indicating that memory consolidation has
occurred, which is associated with the role of the left
dorsolateral striatum (99).
The FST apparatus comprises a small container with a
visible escape platform and a video capture system, and the
procedure includes two forced swimming sessions. On day 1, all the
rodents are exposed to a 15-min pretest. On day 2, the rodents are
placed in containers filled with water and are forced to swim for 6
min; the first 2 min represent the adaptation period (excluded from
analysis), whereas the remaining 4 min are used to calculate the
immobility time. Finally, behaviors (such as clawing at the edges
of the container, aggressive swimming, and diving) and the number
of escape attempts are recorded in the immobility condition
(100) (Fig. 8).
The FST performance is typically determined based on
the amount of time the animal spends in being immobile, swimming,
drifting, diving, and sinking. Other measurements, such as the
number of paw strokes, the swimming speed, the number of platform
crossings, and uncoordinated swimming movements, are also used to
evaluate locomotor ability (52,101) (Fig. 8).
Initially, this test was primarily used to assess
depression-like behavior based on behavioral despair and motor
behavior (52). However, the
results can be influenced by changes in motor activity, thus
producing false-positive results in drug screenings (102). Therefore, the original paradigm
and its analysis have been modified for the screening of potential
antidepressant drugs, and researchers have reached an agreement
that the FST can be used in animal models of depression and anxiety
(102) (Table I).
Discussion
In this review, the principles, procedures,
evaluation, and applications of representative methods for
behavioral assessment in rodent models of brain diseases were
comprehensively analyzed. As illustrated above, all these methods
were developed by taking advantage of the innate features of
rodents, with each method based on different principles and
performance indices. For example, the MWM test, novel object
recognition test, balance beam walking test, and rotarod test are
preferred for assessing brain injuries and neurodegenerative
diseases, whereas the OFT, EPM test, TST, and FST are preferred for
assessing affective disorders. These preferences are based on the
expected alterations in cognitive function, motor coordination, and
emotional states associated with the disease being modeled. For
example, the MWM test is usually preferred when assessing models of
WMI because the pathological changes associated with WMI occur in
brain areas related to the principles of the MWM test (Fig. S1) (Table II).
 | Table II.Behavioral tests used in rodent
models of brain diseases. |
Table II.
Behavioral tests used in rodent
models of brain diseases.
| Author, year | Rodent models | Behavioral
tests | (Refs.) |
|---|
| Kim H et
al%, 2020 | White matter
injury | Morris water maze
test | (23) |
| Chen W et
al%, 2019 |
| Balance beam
walking test | (31) |
| Su X et al%,
2020 |
| Open field
test | (47) |
| Wang W et
al%, 2021 |
| Tail suspension
test | (51) |
| Tucker LB et
al%, 2018 | Stroke | Morris water maze
test | (24) |
| Bohr A et
al%, 2020 |
| Balance beam
walking test | (38) |
| Owfard M et
al%, 2021 |
| Rotarod test | (44) |
| Zhong JY et
al%, 2017 | Traumatic brain
injury | Morris water maze
test | (25) |
| Zhang R et
al%, 2012 |
| Novel object
recognition test | (29,30) |
| Sadegzadeh F et
al%, 2020 |
| Rotarod test |
|
| Park G et
al%, 2021 |
| | (43) |
| Schneider CB et
al%, 2017 | Alzheimer's
disease | Morris water maze
test | (26) |
| Zhang R et
al%, 2012 |
| Novel object
recognition test | (29,30) |
| Sadegzadeh F et
al%, 2020 |
|
|
|
| Deng-Bryant et
al%, 2016 | Parkinson's
disease | Morris water maze
test | (27) |
| Sun J et
al%, 2021 |
| Balance beam
walking test | (36) |
| Zhang R et
al%, 2012 | Schizophrenia | Novel object
recognition test | (29,30) |
| Sadegzadeh F et
al%, 2020 |
|
|
|
| Marques-Carneiro JE
et al, 2014 | Anxiety | Balance beam
walking test | (37) |
| Lecorps B et
al%, 2016 |
| Open field
test | (46) |
| Shoi H et
al%, 2021 |
| Elevated plus-maze
test | (48) |
| Ren C et
al%, 2021 |
| Tail suspension
test | (49) |
| Ráez A et
al%, 2020 |
| Forced swim
test | (52) |
| Mitra NK et
al%, 2020 | Multiple
sclerosis | Balance beam
walking test | (39) |
| Dong W et
al%, 2020 |
| Rotarod test | (40) |
| Chkhartishvili E
et al%, 2011 | Depressive
disorder | Open field
test | (45) |
| Castagné V et
al%, 2011 |
| Tail suspension
test | (50) |
| Ráez A et
al%, 2020 |
| Forced swim
test | (52) |
Importantly, each test has both advantages and
limitations. For example, the MWM test is a versatile tool that can
be used to evaluate cognitive deficits associated with brain
injuries and neurodegenerative diseases; however, it requires
extensive setup, strict procedures, and long experimental duration
(7 days) compared with the novel object recognition test, which is
more flexible and easier to follow. Moreover, although both these
tests are used to assess cognitive ability, the MWM test is
considered to be more reflective of spatial learning and memory
(e.g., WMI), whereas the novel object recognition test is
considered to be more reflective of non-spatial memory (e.g.,
Alzheimer's disease). Furthermore, while the balance beam walking
test is easier to set up and lower in cost than the rotarod test,
it requires a longer training period (2 days). Moreover, although
both these tests are used to evaluate motor coordination and
balance ability, the balance beam walking test exhibits improved
sensitivity for detecting motor coordination deficits compared with
the rotarod test. Nonetheless, the rotarod test is more effective
in evaluating endurance and disorders that affect the cerebellum.
In terms of affective disorders, although both the OFT and EPM test
are used to assess anxiety-like behavior, a previous study has
indicated that the walls in the EPM test form visual barriers that
may affect the performance results (103). Furthermore, while the OFT, TST,
and FST are used for screening depression-like behavior, the OFT is
more reflective of ‘exploratory fear’ behavior, whereas the TST is
more reflective of depression induced by ‘stress reactivity’. The
TST is also simpler, more drug sensitive, and more reliable than
the FST, particularly in response to selective serotonin reuptake
inhibitors (104). Importantly,
the FST cannot be used for antidepressant drug screening, given
that it can be influenced by changes in motor activity that lead to
false-positive results (102)
(Fig. S1) (Table II).
Conclusion
After making a comprehensive comparison of these
behavioral tests, it was found that each test could be used to
evaluate more than one kind of disease animal models. However,
using only a single test might not precisely reflect the
characteristic of a specific disease. Therefore, at present, lack
of the specific behavioral approaches for assessing specific
disease animal models is a key problem that needs to be solved.
Thus, developing new behavioral tests or modifying the available
tests which will concisely reflect the specific animal models would
be a future research direction.
In summary, our review of the preferred settings,
advantages, and limitations of various behavioral assessment
methods may aid researchers in selecting the optimal strategies
based on their research aims, which will in turn help in improving
the reliability of their experimental results.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant nos. 81971428 and 81771634).
Availability of data and materials
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
Authors' contributions
XS and LH contributed to the conception and design
of the review and drafted the manuscript. DX and HS critically
reviewed the article for important intellectual content. YQ gave
important suggestions for the writing of the review. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun P, Liu DZ, Jickling GC, Sharp FR and
Yin KJ: MicroRNA-based therapeutics in central nervous system
injuries. J Cereb Blood Flow Metab. 38:1125–1148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garofalo M, Pandini C, Bordoni M,
Pansarasa O, Rey F, Costa A, Minafra B, Diamanti L, Zucca S,
Carelli S, et al: Alzheimer's, Parkinson's disease and amyotrophic
lateral sclerosis gene expression patterns divergence reveals
different grade of RNA metabolism involvement. Int J Mol Sci.
21:95002020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bednarova A, Cizmarikova M, Habalova V and
Jarcuskova D: Evaluation of 5-HTTLPR (insertion/deletion) and BDNF
(rs6265) genetic variations in the Slovakian individuals suffering
from affective disorders. General Physiol Biophys. 40:365–376.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Velosky AG, Tucker LB, Fu AH, Liu J and
McCabe JT: Cognitive performance of male and female C57BL/6J mice
after repetitive concussive brain injuries. Behav Brain Res.
324:115–124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zlokovic BV, Gottesman RF, Bernstein KE,
Seshadri S, McKee A, Snyder H, Greenberg SM, Yaffe K, Schaffer CB,
Yuan C, et al: Vascular contributions to cognitive impairment and
dementia (VCID): A report from the 2018 national heart, lung, and
blood institute and national institute of neurological disorders
and stroke workshop. Alzheimers Dement. 16:1714–1733. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yakovleva OV, Poluektov MG, Lyashenko EA
and Levin OS: Sleep and cognitive impairment in neurodegenerative
diseases. Zh Nevrol Psikhiatr Im SS Korsakova. 119:89–98. 2019.(In
Russian). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dehn LB and Beblo T: Depressed, biased,
forgetful: The interaction of emotional and cognitive dysfunctions
in depression. Neuropsychiatr. 33:123–130. 2019.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamdy N, Eide S, Sun HS and Feng ZP:
Animal models for neonatal brain injury induced by hypoxic ischemic
conditions in rodents. Exp Neurol. 334:1134572020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wintler T, Schoch H, Frank MG and Peixoto
L: Sleep, brain development, and autism spectrum disorders:
Insights from animal models. J Neurosci Res. 98:1137–1149. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leader RW and Padgett GA: The genesis and
validation of animal models. Am J Pathol. 101 (Suppl 3):S11–S16.
1980.PubMed/NCBI
|
|
11
|
Cox TC: Utility and limitations of animal
models for the functional validation of human sequence variants.
Mol Genet Genomic Med. 3:375–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morris RG, Garrud P, Rawlins JN and
O'Keefe J: Place navigation impaired in rats with hippocampal
lesions. Nature. 297:681–683. 1982. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chao OY, de Souza Silva MA, Yang YM and
Huston JP: The medial prefrontal cortex-hippocampus circuit that
integrates information of object, place and time to construct
episodic memory in rodents: Behavioral, anatomical and
neurochemical properties. Neurosci Biobehav Rev. 113:373–407. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sabariego M, Tabrizi NS, Marshall GJ,
McLagan AN, Jawad S and Hales JB: In the temporal organization of
episodic memory, the hippocampus supports the experience of elapsed
time. Hippocampus. 31:46–55. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pereira IT and Burwell RD: Using the
spatial learning index to evaluate performance on the water maze.
Behav Neurosci. 129:533–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Urbach A, Baum E, Braun F and Witte OW:
Cortical spreading depolarization increases adult neurogenesis, and
alters behavior and hippocampus-dependent memory in mice. J Cereb
Blood Flow Metab. 37:1776–1790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holmberg P, Liljequist S and Wägner A:
Secondary brain injuries in thalamus and hippocampus after focal
ischemia caused by mild, transient extradural compression of the
somatosensori cortex in the rat. Curr Neurovasc Res. 6:1–11. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
D'Hooge R and De Deyn PP: Applications of
the Morris water maze in the study of learning and memory. Brain
Res Brain Res Rev. 36:60–90. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mulder GB and Pritchett K: The Morris
water maze. Contemp Top Lab Anim Sci. 42:49–50. 2003.
|
|
20
|
Barry DN and Commins S: A novel control
condition for spatial learning in the Morris water maze. J Neurosci
Methods. 318:1–5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shah D, Verhoye M, Van der Linden A and
D'Hooge R: Acquisition of spatial search strategies and reversal
learning in the Morris water maze depend on disparate brain
functional connectivity in mice. Cereb Cortex. 29:4519–4529. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan Z, Zhou H, Zhou N, Dong D, Chu Y,
Shen J, Han Y, Chu XP and Zhu K: Dynamic evaluation indices in
spatial learning and memory of rat vascular dementia in the Morris
water maze. Sci Rep. 9:72242019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim H, Seo JS, Lee SY, Ha KT, Choi BT,
Shin YI, Yun YJ and Shin HK: AIM2 inflammasome contributes to brain
injury and chronic post-stroke cognitive impairment in mice. Brain
Behav Immun. 87:765–776. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tucker LB, Velosky AG and McCabe JT:
Applications of the Morris water maze in translational traumatic
brain injury research. Neurosci Biobehav Rev. 88:187–200. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong JY, Magnusson KR, Swarts ME,
Clendinen CA, Reynolds NC and Moffat SD: The application of a
rodent-based morris water maze (MWM) protocol to an investigation
of age-related differences in human spatial learning. Behav
Neurosci. 131:470–482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schneider CB, Linse K, Schönfeld R, Brown
S, Koch R, Reichmann H, Leplow B and Storch A: Spatial learning
deficits in Parkinson's disease with and without mild cognitive
impairment. Parkinsonism Relat Disord. 36:83–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng-Bryant Y, Leung LY, Caudle K,
Tortella F and Shear D: Cognitive evaluation using Morris water
maze in neurotrauma. Methods Mol Biol. 1462:539–551. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Britten RA, Duncan VD, Fesshaye A,
Rudobeck E, Nelson GA and Vlkolinsky R: Altered cognitive
flexibility and synaptic plasticity in the rat prefrontal cortex
after exposure to low (≤15 cGy) doses of 28Si radiation.
Radiat Res. 193:223–235. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang R, Xue G, Wang S, Zhang L, Shi C and
Xie X: Novel object recognition as a facile behavior test for
evaluating drug effects in AβPP/PS1 Alzheimer's disease mouse
model. J Alzheimers Dis. 31:801–812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sadegzadeh F, Sakhaie N, Dehghany R, Adak
O and Saadati H: Effects of adolescent administration of fluoxetine
on novel object recognition memory, anxiety-like behaviors, and
hippocampal brain-derived neurotrophic factor level. Life Sci.
260:1183382020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen W, Xia M, Guo C, Jia Z, Wang J, Li C,
Li M, Tang X, Hu R, Chen Y, et al: Modified behavioural tests to
detect white matter injury-induced motor deficits after
intracerebral haemorrhage in mice. Sci Rep. 9:169582019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Uematsu A, Tsuchiya K, Suzuki S and
Hortobágyi T: Cognitive dual-tasking augments age-differences in
dynamic balance quantified by beam walking distance: A pilot study.
Exp Gerontol. 114:27–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gyengesi E, Rangel A, Ullah F, Liang H,
Niedermayer G, Asgarov R, Venigalla M, Gunawardena D, Karl T and
Münch G: Chronic microglial activation in the GFAP-IL6 mouse
contributes to age-dependent cerebellar volume loss and impairment
in motor function. Front Neurosci. 13:3032019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mychasiuk R, Farran A and Esser MJ:
Assessment of an experimental rodent model of pediatric mild
traumatic brain injury. J Neurotrauma. 31:749–757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El-Sahar AE, Rastanawi AA, El-Yamany MF
and Saad MA: Dapagliflozin improves behavioral dysfunction of
Huntington's disease in rats via inhibiting apoptosis-related
glycolysis. Life Sci. 257:1180762020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun J, Li H, Jin Y, Yu J, Mao S, Su KP,
Ling Z and Liu J: Probiotic Clostridium butyricum ameliorated motor
deficits in a mouse model of Parkinson's disease via gut
microbiota-GLP-1 pathway. Brain Behav Immun. 91:703–715. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marques-Carneiro JE, Faure JB, Cosquer B,
Koning E, Ferrandon A, de Vasconcelos AP, Cassel JC and Nehlig A:
Anxiety and locomotion in genetic absence epilepsy rats from
strasbourg (GAERS): Inclusion of Wistar rats as a second control.
Epilepsia. 55:1460–1468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bohr A, Schuhmann MK, Papp L, Volkmann J
and Fluri F: Deep brain stimulation for stroke: Continuous
stimulation of the pedunculopontine tegmental nucleus has no impact
on skilled walking in rats after photothrombotic stroke. Curr
Neurovasc Res. 17:636–643. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mitra NK, Xuan KY, Teo CC, Xian-Zhuang N,
Singh A and Chellian J: Evaluation of neuroprotective effects of
alpha-tocopherol in cuprizone-induced demyelination model of
multiple sclerosis. Res Pharm Sci. 15:602–611. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dong W and Zhang L, Sun C, Gao X, Guan F,
Li J, Chen W, Ma Y and Zhang L: Knock in of a hexanucleotide repeat
expansion in the C9orf72 gene induces ALS in rats. Animal Model Exp
Med. 3:237–244. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hayashi T, Onozato T, Wanajo I, Hayashi M,
Takeda H and Fujimori Y: Longitudinal analysis of motor symptoms
and histopathology in woozy mice, a model of cerebellar ataxia.
Neuroreport. 28:779–787. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Main SL and Kulesza RJ: Repeated prenatal
exposure to valproic acid results in cerebellar hypoplasia and
ataxia. Neuroscience. 340:34–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park G, Suh JH and Han SJ: Transcranial
direct current stimulation for balance and gait in repetitive mild
traumatic brain injury in rats. BMC Neurosci. 22:262021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Owfard M, Bigdeli MR, Safari A, Haghani M
and Namavar MR: Effect of dimethyl fumarate on the motor function
and spatial arrangement of primary motor cortical neurons in the
sub-acute phase of stroke in a rat model. J Stroke Cerebrovasc Dis.
30:1056302021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chkhartishvili E, Maglakelidze N,
Babilodze M, Chijavadze E and Nachkebia N: Changes of open field
behavior in animal model of depression. Georgian Med News.
11:107–112. 2011.PubMed/NCBI
|
|
46
|
Lecorps B, Rödel HG and Féron C:
Assessment of anxiety in open field and elevated plus maze using
infrared thermography. Physiol Behav. 157:209–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Su X, Yuan H, Bai Y, Chen J, Sui M, Zhang
X, Liang Y, Feng W, Dou Z and Zhu H: Clobetasol attenuates white
matter injury by promoting oligodendrocyte precursor cell
differentiation. Pediatr Neurosurg. 55:188–196. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shoji H and Miyakawa T: Effects of test
experience, closed-arm wall color, and illumination level on
behavior and plasma corticosterone response in an elevated plus
maze in male C57BL/6J mice: A challenge against conventional
interpretation of the test. Mol Brain. 14:342021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ren C, Li LX, Dong AQ, Zhang YT, Hu H, Mao
CJ, Wang F and Liu CF: Depression induced by chronic unpredictable
mild stress increases susceptibility to Parkinson's disease in mice
via neuroinflammation mediated by P2X7 receptor. ACS Chem Neurosci.
12:1262–1272. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Castagné V, Moser P, Roux S and Porsolt
RD: Rodent models of depression: Forced swim and tail suspension
behavioral despair tests in rats and mice. Curr Protoc Neurosci.
8:Unit 8.10A. 2011.PubMed/NCBI
|
|
51
|
Wang W, Wang J, Tang Q, Zhu X, Zhu R, Cui
D, Wei C, Liu X, Liu X, Ran S, et al: CX3CR1 deficiency aggravates
brain white matter injury and affects expression of the
CD36/15LO/NR4A1 signal. Biochem Biophys Res Commun. 549:47–53.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ráez A, Oliveras I, Río-Álamos C,
Díaz-Morán S, Cañete T, Blázquez G, Tobeña A and Fernández-Teruel
A: A missing link between depression models: Forced swimming test,
helplessness and passive coping in genetically heterogeneous NIH-HS
rats. Behav Processes. 177:1041422020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Richler JJ, Wilmer JB and Gauthier I:
General object recognition is specific: Evidence from novel and
familiar objects. Cognition. 166:42–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ishikawa H, Yamada K, Pavlides C and
Ichitani Y: Sleep deprivation impairs spontaneous object-place but
not novel-object recognition in rats. Neurosci Lett. 580:114–118.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Miedel CJ, Patton JM, Miedel AN, Miedel ES
and Levenson JM: Assessment of spontaneous alternation, novel
object recognition and limb clasping in transgenic mouse models of
amyloid-β and tau neuropathology. J Vis Exp. 28:555232017.
|
|
56
|
Antunes M and Biala G: The novel object
recognition memory: Neurobiology, test procedure, and its
modifications. Cogn Process. 13:93–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Grayson B, Leger M, Piercy C, Adamson L,
Harte M and Neill JC: Assessment of disease-related cognitive
impairments using the novel object recognition (NOR) task in
rodents. Behav Brain Res. 285:176–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cohen SJ and Stackman RW Jr: Assessing
rodent hippocampal involvement in the novel object recognition
task. A review. Behav Brain Res. 285:105–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Da Cruz JFO, Gomis-Gonzalez M, Maldonado
R, Marsicano G, Ozaita A and Busquets-Garcia A: An alternative maze
to assess novel object recognition in mice. Bio Protoc.
10:e36512020.PubMed/NCBI
|
|
60
|
Lueptow LM: Novel object recognition test
for the investigation of learning and memory in mice. J Vis Exp.
126:557182017.PubMed/NCBI
|
|
61
|
Luong TN, Carlisle HJ, Southwell A and
Patterson PH: Assessment of motor balance and coordination in mice
using the balance beam. J Vis Exp. 49:23762011.
|
|
62
|
Hortobágyi T, Uematsu A, Sanders L, Kliegl
R, Tollár J, Moraes R and Granacher U: Beam walking to assess
dynamic balance in health and disease: A protocol for the ‘BEAM’
multicenter observational study. Gerontology. 65:332–339. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Stanley JL, Lincoln RJ, Brown TA, McDonald
LM, Dawson GR and Reynolds DS: The mouse beam walking assay offers
improved sensitivity over the mouse rotarod in determining motor
coordination deficits induced by benzodiazepines. J
Psychopharmacol. 19:221–227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Seashore HG: The development of a
beam-walking test and its use in measuring development of balance
in children. Res Q. 18:246–259. 1947.PubMed/NCBI
|
|
65
|
Sawers A and Hafner B: Validation of the
narrowing beam walking test in lower limb prosthesis users. Arch
Phys Med Rehabil. 99:1491–1498.e1. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Castillo JT, Welch GW and Sarver CM:
Walking a high beam: The balance between employment stability,
workplace flexibility, and nonresident father involvement. Am J
Mens Health. 6:120–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sawers A and Ting LH: Beam walking can
detect differences in walking balance proficiency across a range of
sensorimotor abilities. Gait Posture. 41:619–623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kaur H, Kumar A, Jaggi AS and Singh N:
Pharmacologic investigations on the role of Sirt-1 in
neuroprotective mechanism of postconditioning in mice. J Surg Res.
197:191–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Monville C, Torres EM and Dunnett SB:
Comparison of incremental and accelerating protocols of the rotarod
test for the assessment of motor deficits in the 6-OHDA model. J
Neurosci Methods. 158:219–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shiotsuki H, Yoshimi K, Shimo Y, Funayama
M, Takamatsu Y, Ikeda K, Takahashi R, Kitazawa S and Hattori N: A
rotarod test for evaluation of motor skill learning. J Neurosci
Methods. 189:180–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Matias M, Silvestre S, Falcão A and Alves
G: Considerations and pitfalls in selecting the drug vehicles for
evaluation of new drug candidates: Focus on in vivo
pharmaco-toxicological assays based on the rotarod performance
test. J Pharm Pharm Sci. 21:110–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kirschbaum KM, Hiemke C and Schmitt U:
Rotarod impairment: Catalepsy-like screening test for antipsychotic
side effects. Int J Neurosci. 119:1509–1522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tkáč I, Benneyworth MA, Nichols-Meade T,
Steuer EL, Larson SN, Metzger GJ and Uğurbil K: Long-term
behavioral effects observed in mice chronically exposed to static
ultra-high magnetic fields. Magn Reson Med. 86:1544–1559. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Toklu HZ, Yang Z, Ersahin M and Wang KKW:
Neurological exam in rats following stroke and traumatic brain
injury. Methods Mol Biol. 2011:371–381. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jeffery ND, Brakel K, Aceves M, Hook MA
and Jeffery UB: Variability in open-field locomotor scoring
following force-defined spinal cord injury in rats: Quantification
and implications. Front Neurol. 11:6502020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Miller CK, Halbing AA, Patisaul HB and
Meitzen J: Interactions of the estrous cycle, novelty, and light on
female and male rat open field locomotor and anxiety-related
behaviors. Physiol Behav. 228:1132032021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sturman O, Germain PL and Bohacek J:
Exploratory rearing: A context- and stress-sensitive behavior
recorded in the open-field test. Stress. 21:443–452. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kraeuter AK, Guest PC and Sarnyai Z: The
open field test for measuring locomotor activity and anxiety-like
behavior. Methods Mol Biol. 1916:99–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tartar JL, Ward CP, Cordeira JW, Legare
SL, Blanchette AJ, McCarley RW and Strecker RE: Experimental sleep
fragmentation and sleep deprivation in rats increases exploration
in an open field test of anxiety while increasing plasma
corticosterone levels. Behav Brain Res. 197:450–453. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Walz N, Mühlberger A and Pauli P: A human
open field test reveals thigmotaxis related to agoraphobic fear.
Biol Psychiatry. 80:390–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Walsh RN and Cummins RA: The open-field
test: A critical review. Psychol Bull. 83:482–504. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Knight P, Chellian R, Wilson R,
Behnood-Rod A, Panunzio S and Bruijnzeel AW: Sex differences in the
elevated plus-maze test and large open field test in adult Wistar
rats. Pharmacol Biochem Behav. 204:1731682021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Justel N, Salguero A, Marengo L,
Psyrdellis M and Pautassi RM: Open field exposure facilitates the
expression of a spatial, recognition memory. Neurosci Lett.
757:1359972021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Snyder CN, Brown AR and Buffalari D:
Similar tests of anxiety-like behavior yield different results:
Comparison of the open field and free exploratory rodent
procedures. Physiol Behav. 230:1132462021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hogg S: A review of the validity and
variability of the elevated plus-maze as an animal model of
anxiety. Pharmacol Biochem Behav. 54:21–30. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Carobrez AP and Bertoglio LJ: Ethological
and temporal analyses of anxiety-like behavior: The elevated
plus-maze model 20 years on. Neurosci Biobehav Rev. 29:1193–1205.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bespalov A and Steckler T: Pharmacology of
anxiety or pharmacology of elevated plus maze? Biol Psychiatry.
89:e732021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Holmes A, Li Q, Koenig EA, Gold E,
Stephenson D, Yang RJ, Dreiling J, Sullivan T and Crawley JN:
Phenotypic assessment of galanin overexpressing and galanin
receptor R1 knockout mice in the tail suspension test for
depression-related behavior. Psychopharmacology (Berl).
178:276–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shao S, Cui Y, Chen ZB, Zhang B, Huang SM
and Liu XW: Androgen deficit changes the response to antidepressant
drugs in tail suspension test in mice. Aging Male. 23:1259–1265.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Iyer KA, Alix K, Eltit JM, Solis E Jr, Pan
X, Argade MD, Khatri S, Felice LJD, Sweet DH, Schulte MK and Dukat
M: Multi-modal antidepressant-like action of 6- and
7-chloro-2-aminodihydroquinazolines in the mouse tail suspension
test. Psychopharmacology (Berl). 236:2093–2104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Poleszak E, Szopa A, Bogatko K, Wyska E,
Wośko S, Świader K, Doboszewska U, Wlaź A, Wróbel A, Wlaź P and
Serefko A: Antidepressant-like activity of typical antidepressant
drugs in the forced swim test and tail suspension test in mice is
augmented by DMPX, an adenosine A2A receptor antagonist. Neurotox
Res. 35:344–352. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Pelloux Y, Hagues G, Costentin J and
Duterte-Boucher D: Helplessness in the tail suspension test is
associated with an increase in ethanol intake and its rewarding
effect in female mice. Alcohol Clin Exp Res. 29:378–388. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kale PP, Addepalli V and Ghadawale SR:
Impact of pre-exposure of tail suspension on behavioural parameters
like locomotion, exploration, and anxiety in mice. Indian J Exp
Biol. 51:732–738. 2013.PubMed/NCBI
|
|
94
|
Reis-Silva TM, Sandini TM, Calefi AS,
Orlando BCG, Moreira N, Lima APN, Florio JC, Queiroz-Hazarbassanov
NGT and Bernardi MM: Stress resilience evidenced by grooming
behaviour and dopamine levels in male mice selected for high and
low immobility using the tail suspension test. Eur J Neurosci.
50:2942–2954. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Nomura S, Naruse R and Okada H: The tail
suspension test: Its theory and practical application. Yakubutsu
Seishin Kodo. 12:207–213. 1992.(In Japanese). PubMed/NCBI
|
|
96
|
Rosa I, Di Censo D, Ranieri B, Di Giovanni
G, Scarnati E, Alecci M, Galante A and Florio TM: Comparison
between tail suspension swing test and standard rotation test in
revealing early motor behavioral changes and neurodegeneration in
6-OHDA hemiparkinsonian rats. Int J Mol Sci. 21:28742020.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Stukalin Y, Lan A and Einat H: Revisiting
the validity of the mouse tail suspension test: Systematic review
and meta-analysis of the effects of prototypic antidepressants.
Neurosci Biobehav Rev. 112:39–47. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Vieira C, De Lima TC, de Pádua Carobrez A
and Lino-de-Oliveira C: Frequency of climbing behavior as a
predictor of altered motor activity in rat forced swimming test.
Neurosci Lett. 445:170–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Flores-Serrano AG, Zaldivar-Rae J, Salgado
H and Pineda JC: Immobility time during the forced swimming test
predicts sensitivity to amitriptyline, whereas traveled distance in
the circular corridor indicates resistance to treatment in female
Wistar rats. Neuroreport. 26:233–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Petit-Demouliere B, Chenu F and Bourin M:
Forced swimming test in mice: A review of antidepressant activity.
Psychopharmacology (Berl). 177:245–255. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Herbst LS, Gaigher T, Siqueira AA, Joca
SRL, Sampaio KN and Beijamini V: New evidence for refinement of
anesthetic choice in procedures preceding the forced swimming test
and the elevated plus-maze. Behav Brain Res. 368:1118972019.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Dang H, Chen Y, Liu X, Wang Q, Wang L, Jia
W and Wang Y: Antidepressant effects of ginseng total saponins in
the forced swimming test and chronic mild stress models of
depression. Prog Neuropsychopharmacol Biol Psychiatry.
33:1417–1424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rebolledo-Solleiro D, Crespo-Ramírez M,
Roldán-Roldán G, Hiriart M and de la Mora M: Role of thirst and
visual barriers in the differential behavior displayed by
streptozotocin-treated rats in the elevated plus-maze and the open
field test. Physiol Behav. 120:130–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lucki I, Dalvi A and Mayorga AJ:
Sensitivity to the effects of pharmacologically selective
antidepressants in different strains of mice. Psychopharmacology
(Berl). 155:315–322. 2001. View Article : Google Scholar : PubMed/NCBI
|