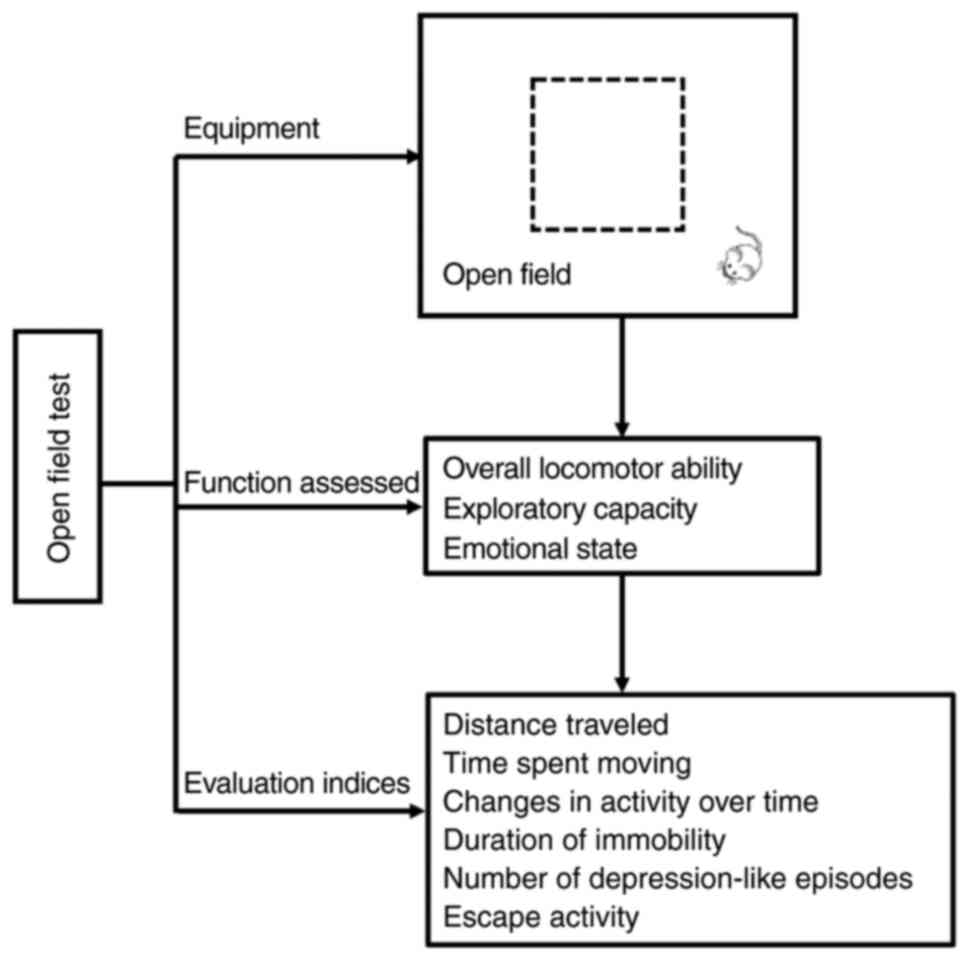
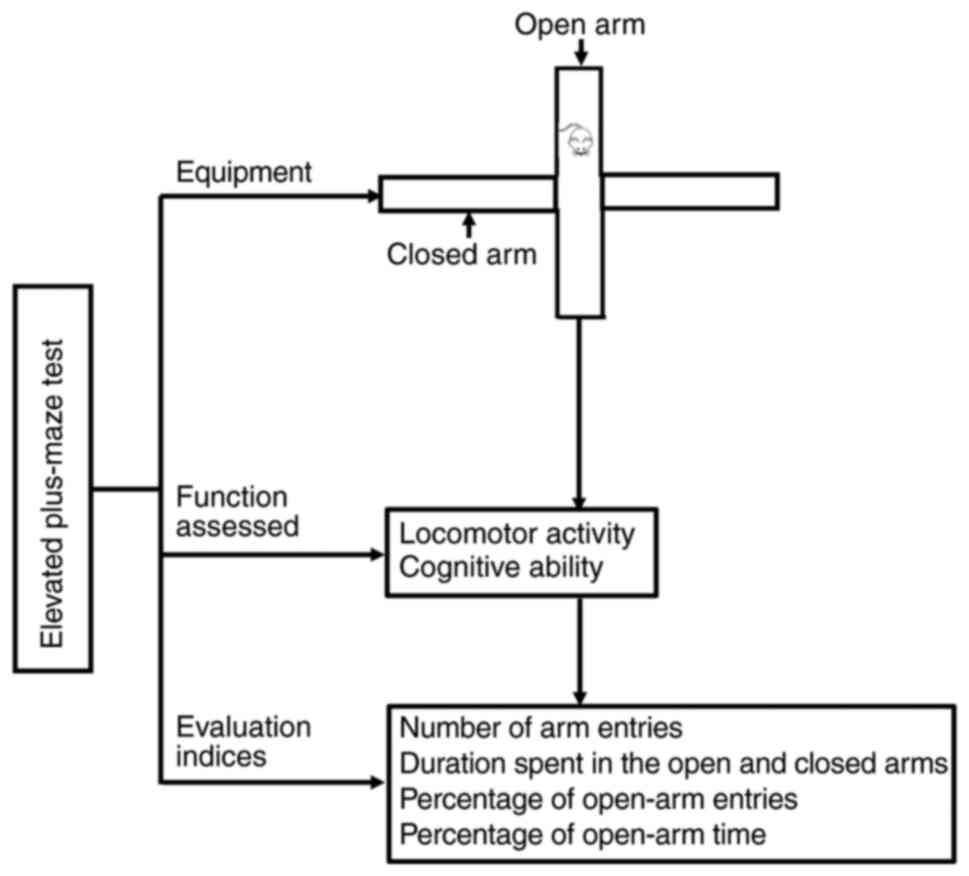
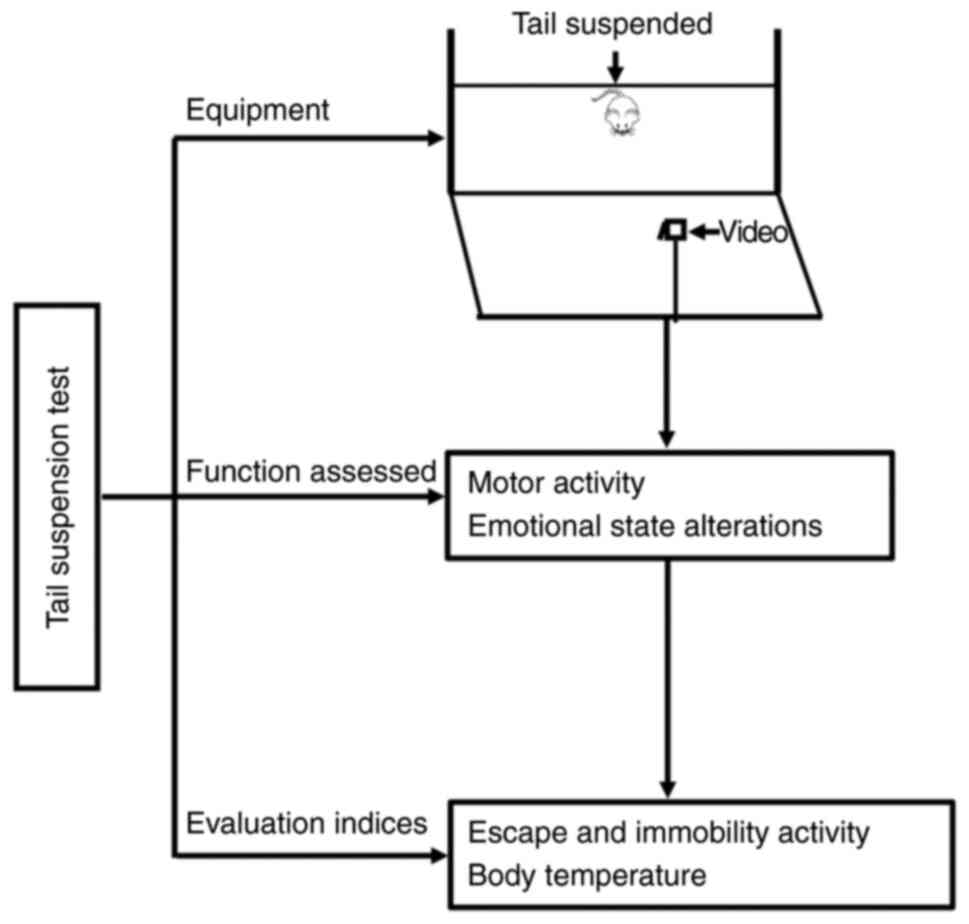
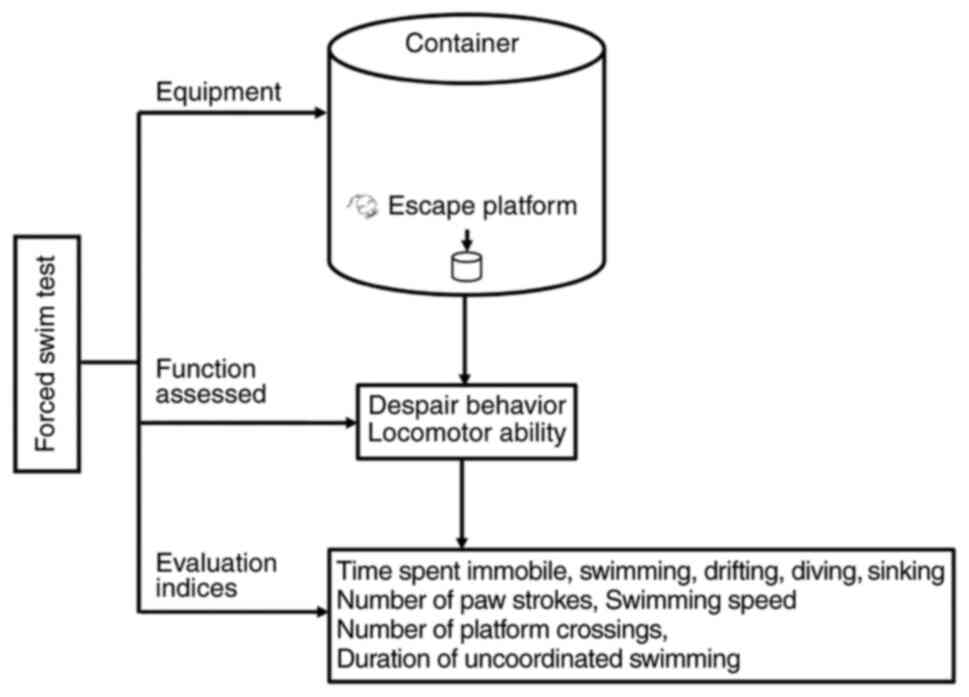
|
1
|
Sun P, Liu DZ, Jickling GC, Sharp FR and
Yin KJ: MicroRNA-based therapeutics in central nervous system
injuries. J Cereb Blood Flow Metab. 38:1125–1148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garofalo M, Pandini C, Bordoni M,
Pansarasa O, Rey F, Costa A, Minafra B, Diamanti L, Zucca S,
Carelli S, et al: Alzheimer's, Parkinson's disease and amyotrophic
lateral sclerosis gene expression patterns divergence reveals
different grade of RNA metabolism involvement. Int J Mol Sci.
21:95002020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bednarova A, Cizmarikova M, Habalova V and
Jarcuskova D: Evaluation of 5-HTTLPR (insertion/deletion) and BDNF
(rs6265) genetic variations in the Slovakian individuals suffering
from affective disorders. General Physiol Biophys. 40:365–376.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Velosky AG, Tucker LB, Fu AH, Liu J and
McCabe JT: Cognitive performance of male and female C57BL/6J mice
after repetitive concussive brain injuries. Behav Brain Res.
324:115–124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zlokovic BV, Gottesman RF, Bernstein KE,
Seshadri S, McKee A, Snyder H, Greenberg SM, Yaffe K, Schaffer CB,
Yuan C, et al: Vascular contributions to cognitive impairment and
dementia (VCID): A report from the 2018 national heart, lung, and
blood institute and national institute of neurological disorders
and stroke workshop. Alzheimers Dement. 16:1714–1733. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yakovleva OV, Poluektov MG, Lyashenko EA
and Levin OS: Sleep and cognitive impairment in neurodegenerative
diseases. Zh Nevrol Psikhiatr Im SS Korsakova. 119:89–98. 2019.(In
Russian). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dehn LB and Beblo T: Depressed, biased,
forgetful: The interaction of emotional and cognitive dysfunctions
in depression. Neuropsychiatr. 33:123–130. 2019.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamdy N, Eide S, Sun HS and Feng ZP:
Animal models for neonatal brain injury induced by hypoxic ischemic
conditions in rodents. Exp Neurol. 334:1134572020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wintler T, Schoch H, Frank MG and Peixoto
L: Sleep, brain development, and autism spectrum disorders:
Insights from animal models. J Neurosci Res. 98:1137–1149. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leader RW and Padgett GA: The genesis and
validation of animal models. Am J Pathol. 101 (Suppl 3):S11–S16.
1980.PubMed/NCBI
|
|
11
|
Cox TC: Utility and limitations of animal
models for the functional validation of human sequence variants.
Mol Genet Genomic Med. 3:375–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morris RG, Garrud P, Rawlins JN and
O'Keefe J: Place navigation impaired in rats with hippocampal
lesions. Nature. 297:681–683. 1982. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chao OY, de Souza Silva MA, Yang YM and
Huston JP: The medial prefrontal cortex-hippocampus circuit that
integrates information of object, place and time to construct
episodic memory in rodents: Behavioral, anatomical and
neurochemical properties. Neurosci Biobehav Rev. 113:373–407. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sabariego M, Tabrizi NS, Marshall GJ,
McLagan AN, Jawad S and Hales JB: In the temporal organization of
episodic memory, the hippocampus supports the experience of elapsed
time. Hippocampus. 31:46–55. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
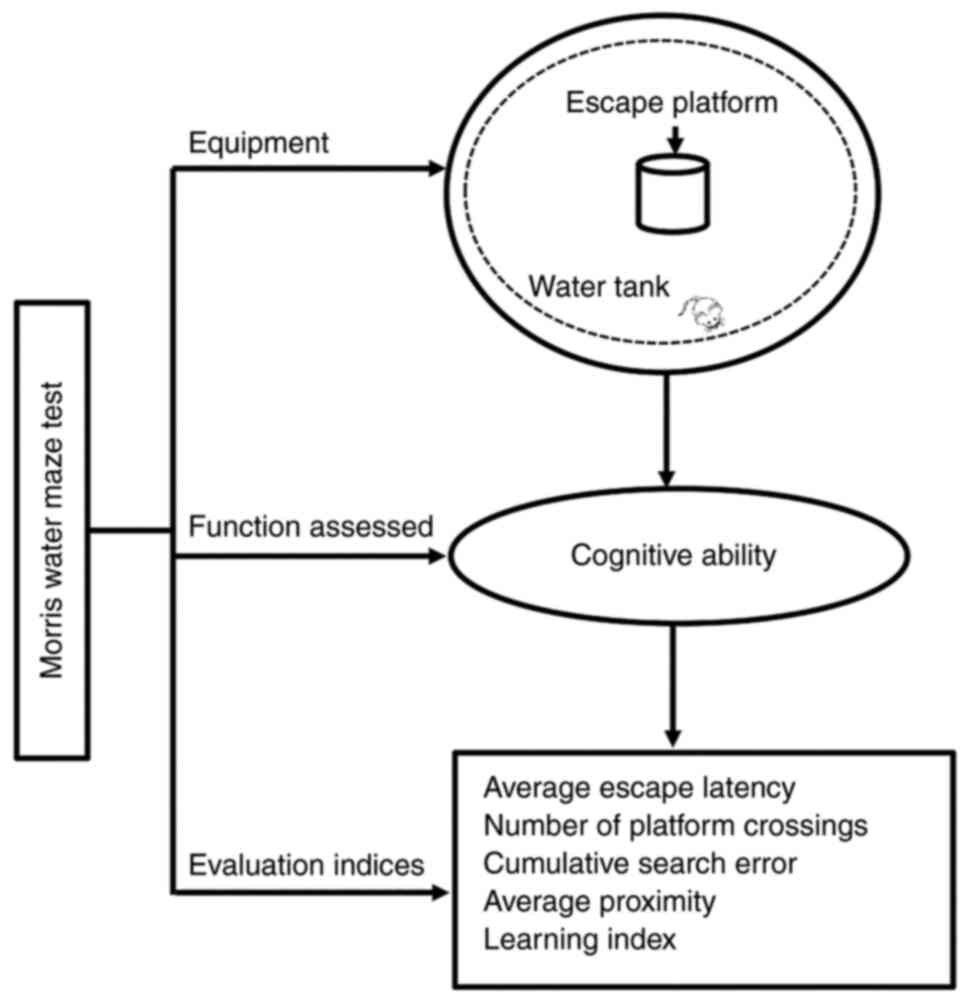
Pereira IT and Burwell RD: Using the
spatial learning index to evaluate performance on the water maze.
Behav Neurosci. 129:533–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Urbach A, Baum E, Braun F and Witte OW:
Cortical spreading depolarization increases adult neurogenesis, and
alters behavior and hippocampus-dependent memory in mice. J Cereb
Blood Flow Metab. 37:1776–1790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holmberg P, Liljequist S and Wägner A:
Secondary brain injuries in thalamus and hippocampus after focal
ischemia caused by mild, transient extradural compression of the
somatosensori cortex in the rat. Curr Neurovasc Res. 6:1–11. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
D'Hooge R and De Deyn PP: Applications of
the Morris water maze in the study of learning and memory. Brain
Res Brain Res Rev. 36:60–90. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mulder GB and Pritchett K: The Morris
water maze. Contemp Top Lab Anim Sci. 42:49–50. 2003.
|
|
20
|
Barry DN and Commins S: A novel control
condition for spatial learning in the Morris water maze. J Neurosci
Methods. 318:1–5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shah D, Verhoye M, Van der Linden A and
D'Hooge R: Acquisition of spatial search strategies and reversal
learning in the Morris water maze depend on disparate brain
functional connectivity in mice. Cereb Cortex. 29:4519–4529. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan Z, Zhou H, Zhou N, Dong D, Chu Y,
Shen J, Han Y, Chu XP and Zhu K: Dynamic evaluation indices in
spatial learning and memory of rat vascular dementia in the Morris
water maze. Sci Rep. 9:72242019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim H, Seo JS, Lee SY, Ha KT, Choi BT,
Shin YI, Yun YJ and Shin HK: AIM2 inflammasome contributes to brain
injury and chronic post-stroke cognitive impairment in mice. Brain
Behav Immun. 87:765–776. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tucker LB, Velosky AG and McCabe JT:
Applications of the Morris water maze in translational traumatic
brain injury research. Neurosci Biobehav Rev. 88:187–200. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong JY, Magnusson KR, Swarts ME,
Clendinen CA, Reynolds NC and Moffat SD: The application of a
rodent-based morris water maze (MWM) protocol to an investigation
of age-related differences in human spatial learning. Behav
Neurosci. 131:470–482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schneider CB, Linse K, Schönfeld R, Brown
S, Koch R, Reichmann H, Leplow B and Storch A: Spatial learning
deficits in Parkinson's disease with and without mild cognitive
impairment. Parkinsonism Relat Disord. 36:83–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng-Bryant Y, Leung LY, Caudle K,
Tortella F and Shear D: Cognitive evaluation using Morris water
maze in neurotrauma. Methods Mol Biol. 1462:539–551. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Britten RA, Duncan VD, Fesshaye A,
Rudobeck E, Nelson GA and Vlkolinsky R: Altered cognitive
flexibility and synaptic plasticity in the rat prefrontal cortex
after exposure to low (≤15 cGy) doses of 28Si radiation.
Radiat Res. 193:223–235. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang R, Xue G, Wang S, Zhang L, Shi C and
Xie X: Novel object recognition as a facile behavior test for
evaluating drug effects in AβPP/PS1 Alzheimer's disease mouse
model. J Alzheimers Dis. 31:801–812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sadegzadeh F, Sakhaie N, Dehghany R, Adak
O and Saadati H: Effects of adolescent administration of fluoxetine
on novel object recognition memory, anxiety-like behaviors, and
hippocampal brain-derived neurotrophic factor level. Life Sci.
260:1183382020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen W, Xia M, Guo C, Jia Z, Wang J, Li C,
Li M, Tang X, Hu R, Chen Y, et al: Modified behavioural tests to
detect white matter injury-induced motor deficits after
intracerebral haemorrhage in mice. Sci Rep. 9:169582019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Uematsu A, Tsuchiya K, Suzuki S and
Hortobágyi T: Cognitive dual-tasking augments age-differences in
dynamic balance quantified by beam walking distance: A pilot study.
Exp Gerontol. 114:27–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gyengesi E, Rangel A, Ullah F, Liang H,
Niedermayer G, Asgarov R, Venigalla M, Gunawardena D, Karl T and
Münch G: Chronic microglial activation in the GFAP-IL6 mouse
contributes to age-dependent cerebellar volume loss and impairment
in motor function. Front Neurosci. 13:3032019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mychasiuk R, Farran A and Esser MJ:
Assessment of an experimental rodent model of pediatric mild
traumatic brain injury. J Neurotrauma. 31:749–757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El-Sahar AE, Rastanawi AA, El-Yamany MF
and Saad MA: Dapagliflozin improves behavioral dysfunction of
Huntington's disease in rats via inhibiting apoptosis-related
glycolysis. Life Sci. 257:1180762020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun J, Li H, Jin Y, Yu J, Mao S, Su KP,
Ling Z and Liu J: Probiotic Clostridium butyricum ameliorated motor
deficits in a mouse model of Parkinson's disease via gut
microbiota-GLP-1 pathway. Brain Behav Immun. 91:703–715. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marques-Carneiro JE, Faure JB, Cosquer B,
Koning E, Ferrandon A, de Vasconcelos AP, Cassel JC and Nehlig A:
Anxiety and locomotion in genetic absence epilepsy rats from
strasbourg (GAERS): Inclusion of Wistar rats as a second control.
Epilepsia. 55:1460–1468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bohr A, Schuhmann MK, Papp L, Volkmann J
and Fluri F: Deep brain stimulation for stroke: Continuous
stimulation of the pedunculopontine tegmental nucleus has no impact
on skilled walking in rats after photothrombotic stroke. Curr
Neurovasc Res. 17:636–643. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mitra NK, Xuan KY, Teo CC, Xian-Zhuang N,
Singh A and Chellian J: Evaluation of neuroprotective effects of
alpha-tocopherol in cuprizone-induced demyelination model of
multiple sclerosis. Res Pharm Sci. 15:602–611. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dong W and Zhang L, Sun C, Gao X, Guan F,
Li J, Chen W, Ma Y and Zhang L: Knock in of a hexanucleotide repeat
expansion in the C9orf72 gene induces ALS in rats. Animal Model Exp
Med. 3:237–244. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hayashi T, Onozato T, Wanajo I, Hayashi M,
Takeda H and Fujimori Y: Longitudinal analysis of motor symptoms
and histopathology in woozy mice, a model of cerebellar ataxia.
Neuroreport. 28:779–787. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Main SL and Kulesza RJ: Repeated prenatal
exposure to valproic acid results in cerebellar hypoplasia and
ataxia. Neuroscience. 340:34–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park G, Suh JH and Han SJ: Transcranial
direct current stimulation for balance and gait in repetitive mild
traumatic brain injury in rats. BMC Neurosci. 22:262021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Owfard M, Bigdeli MR, Safari A, Haghani M
and Namavar MR: Effect of dimethyl fumarate on the motor function
and spatial arrangement of primary motor cortical neurons in the
sub-acute phase of stroke in a rat model. J Stroke Cerebrovasc Dis.
30:1056302021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chkhartishvili E, Maglakelidze N,
Babilodze M, Chijavadze E and Nachkebia N: Changes of open field
behavior in animal model of depression. Georgian Med News.
11:107–112. 2011.PubMed/NCBI
|
|
46
|
Lecorps B, Rödel HG and Féron C:
Assessment of anxiety in open field and elevated plus maze using
infrared thermography. Physiol Behav. 157:209–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Su X, Yuan H, Bai Y, Chen J, Sui M, Zhang
X, Liang Y, Feng W, Dou Z and Zhu H: Clobetasol attenuates white
matter injury by promoting oligodendrocyte precursor cell
differentiation. Pediatr Neurosurg. 55:188–196. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shoji H and Miyakawa T: Effects of test
experience, closed-arm wall color, and illumination level on
behavior and plasma corticosterone response in an elevated plus
maze in male C57BL/6J mice: A challenge against conventional
interpretation of the test. Mol Brain. 14:342021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ren C, Li LX, Dong AQ, Zhang YT, Hu H, Mao
CJ, Wang F and Liu CF: Depression induced by chronic unpredictable
mild stress increases susceptibility to Parkinson's disease in mice
via neuroinflammation mediated by P2X7 receptor. ACS Chem Neurosci.
12:1262–1272. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Castagné V, Moser P, Roux S and Porsolt
RD: Rodent models of depression: Forced swim and tail suspension
behavioral despair tests in rats and mice. Curr Protoc Neurosci.
8:Unit 8.10A. 2011.PubMed/NCBI
|
|
51
|
Wang W, Wang J, Tang Q, Zhu X, Zhu R, Cui
D, Wei C, Liu X, Liu X, Ran S, et al: CX3CR1 deficiency aggravates
brain white matter injury and affects expression of the
CD36/15LO/NR4A1 signal. Biochem Biophys Res Commun. 549:47–53.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ráez A, Oliveras I, Río-Álamos C,
Díaz-Morán S, Cañete T, Blázquez G, Tobeña A and Fernández-Teruel
A: A missing link between depression models: Forced swimming test,
helplessness and passive coping in genetically heterogeneous NIH-HS
rats. Behav Processes. 177:1041422020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Richler JJ, Wilmer JB and Gauthier I:
General object recognition is specific: Evidence from novel and
familiar objects. Cognition. 166:42–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ishikawa H, Yamada K, Pavlides C and
Ichitani Y: Sleep deprivation impairs spontaneous object-place but
not novel-object recognition in rats. Neurosci Lett. 580:114–118.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Miedel CJ, Patton JM, Miedel AN, Miedel ES
and Levenson JM: Assessment of spontaneous alternation, novel
object recognition and limb clasping in transgenic mouse models of
amyloid-β and tau neuropathology. J Vis Exp. 28:555232017.
|
|
56
|
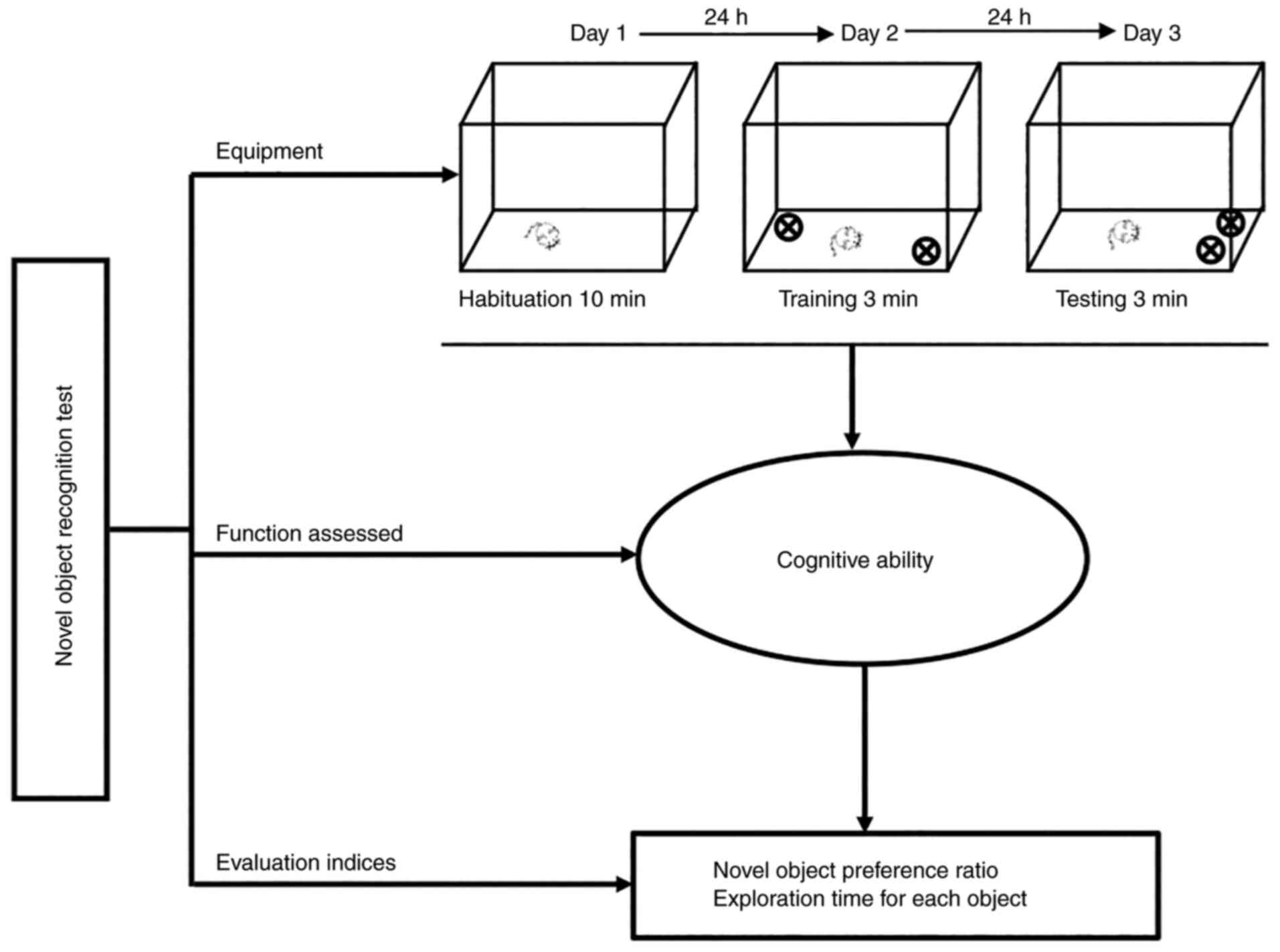
Antunes M and Biala G: The novel object
recognition memory: Neurobiology, test procedure, and its
modifications. Cogn Process. 13:93–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Grayson B, Leger M, Piercy C, Adamson L,
Harte M and Neill JC: Assessment of disease-related cognitive
impairments using the novel object recognition (NOR) task in
rodents. Behav Brain Res. 285:176–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cohen SJ and Stackman RW Jr: Assessing
rodent hippocampal involvement in the novel object recognition
task. A review. Behav Brain Res. 285:105–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Da Cruz JFO, Gomis-Gonzalez M, Maldonado
R, Marsicano G, Ozaita A and Busquets-Garcia A: An alternative maze
to assess novel object recognition in mice. Bio Protoc.
10:e36512020.PubMed/NCBI
|
|
60
|
Lueptow LM: Novel object recognition test
for the investigation of learning and memory in mice. J Vis Exp.
126:557182017.PubMed/NCBI
|
|
61
|
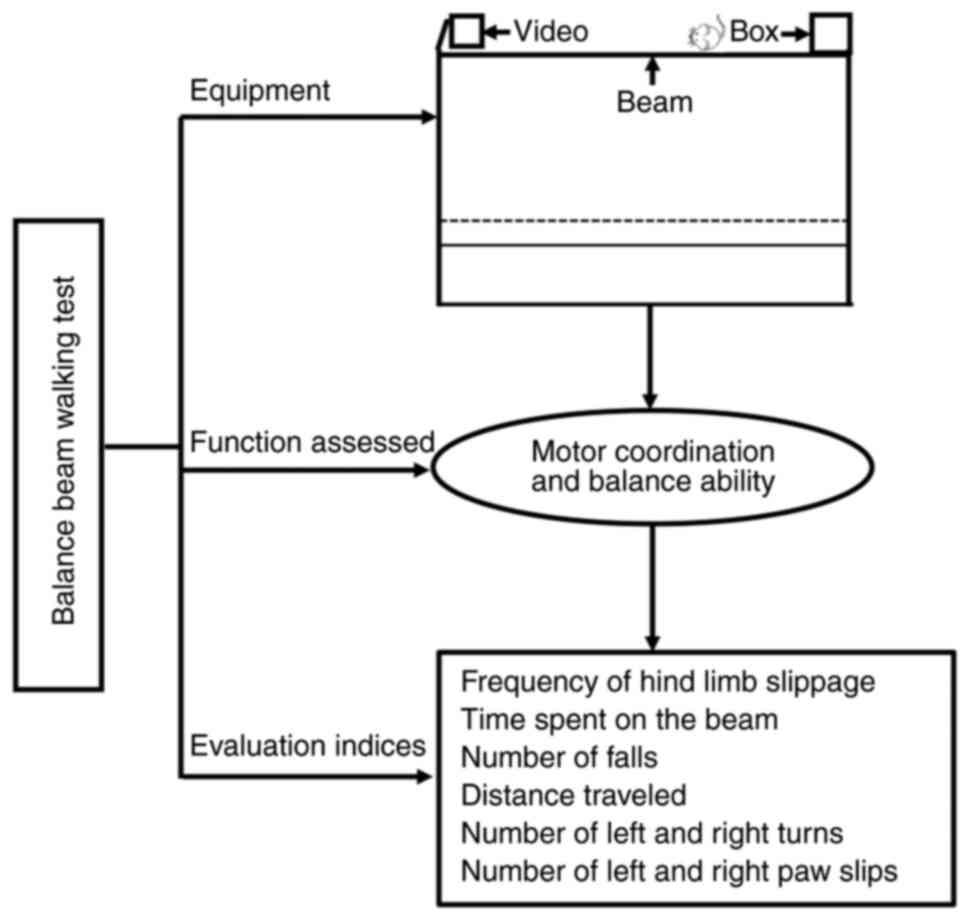
Luong TN, Carlisle HJ, Southwell A and
Patterson PH: Assessment of motor balance and coordination in mice
using the balance beam. J Vis Exp. 49:23762011.
|
|
62
|
Hortobágyi T, Uematsu A, Sanders L, Kliegl
R, Tollár J, Moraes R and Granacher U: Beam walking to assess
dynamic balance in health and disease: A protocol for the ‘BEAM’
multicenter observational study. Gerontology. 65:332–339. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Stanley JL, Lincoln RJ, Brown TA, McDonald
LM, Dawson GR and Reynolds DS: The mouse beam walking assay offers
improved sensitivity over the mouse rotarod in determining motor
coordination deficits induced by benzodiazepines. J
Psychopharmacol. 19:221–227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Seashore HG: The development of a
beam-walking test and its use in measuring development of balance
in children. Res Q. 18:246–259. 1947.PubMed/NCBI
|
|
65
|
Sawers A and Hafner B: Validation of the
narrowing beam walking test in lower limb prosthesis users. Arch
Phys Med Rehabil. 99:1491–1498.e1. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Castillo JT, Welch GW and Sarver CM:
Walking a high beam: The balance between employment stability,
workplace flexibility, and nonresident father involvement. Am J
Mens Health. 6:120–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sawers A and Ting LH: Beam walking can
detect differences in walking balance proficiency across a range of
sensorimotor abilities. Gait Posture. 41:619–623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kaur H, Kumar A, Jaggi AS and Singh N:
Pharmacologic investigations on the role of Sirt-1 in
neuroprotective mechanism of postconditioning in mice. J Surg Res.
197:191–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
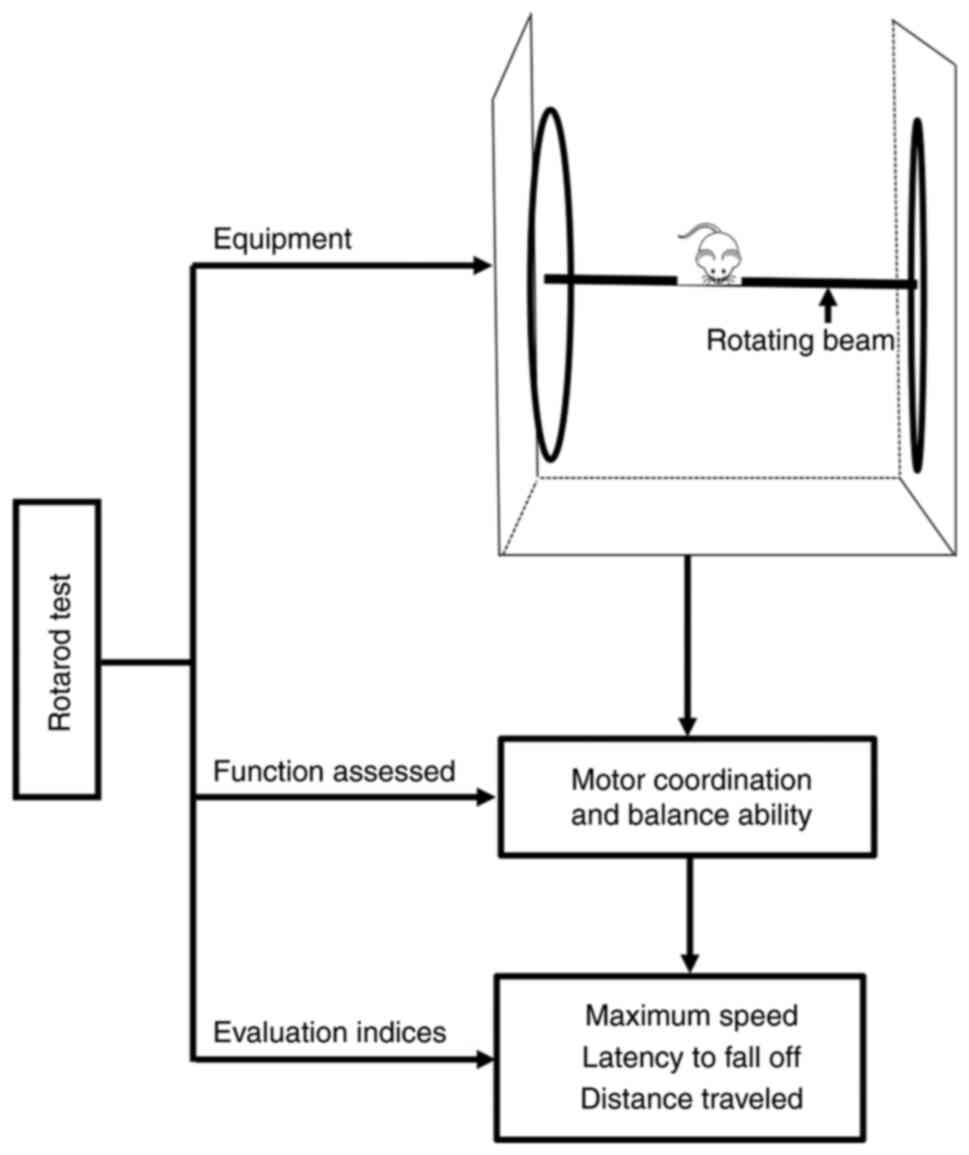
Monville C, Torres EM and Dunnett SB:
Comparison of incremental and accelerating protocols of the rotarod
test for the assessment of motor deficits in the 6-OHDA model. J
Neurosci Methods. 158:219–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shiotsuki H, Yoshimi K, Shimo Y, Funayama
M, Takamatsu Y, Ikeda K, Takahashi R, Kitazawa S and Hattori N: A
rotarod test for evaluation of motor skill learning. J Neurosci
Methods. 189:180–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Matias M, Silvestre S, Falcão A and Alves
G: Considerations and pitfalls in selecting the drug vehicles for
evaluation of new drug candidates: Focus on in vivo
pharmaco-toxicological assays based on the rotarod performance
test. J Pharm Pharm Sci. 21:110–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kirschbaum KM, Hiemke C and Schmitt U:
Rotarod impairment: Catalepsy-like screening test for antipsychotic
side effects. Int J Neurosci. 119:1509–1522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tkáč I, Benneyworth MA, Nichols-Meade T,
Steuer EL, Larson SN, Metzger GJ and Uğurbil K: Long-term
behavioral effects observed in mice chronically exposed to static
ultra-high magnetic fields. Magn Reson Med. 86:1544–1559. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Toklu HZ, Yang Z, Ersahin M and Wang KKW:
Neurological exam in rats following stroke and traumatic brain
injury. Methods Mol Biol. 2011:371–381. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jeffery ND, Brakel K, Aceves M, Hook MA
and Jeffery UB: Variability in open-field locomotor scoring
following force-defined spinal cord injury in rats: Quantification
and implications. Front Neurol. 11:6502020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Miller CK, Halbing AA, Patisaul HB and
Meitzen J: Interactions of the estrous cycle, novelty, and light on
female and male rat open field locomotor and anxiety-related
behaviors. Physiol Behav. 228:1132032021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sturman O, Germain PL and Bohacek J:
Exploratory rearing: A context- and stress-sensitive behavior
recorded in the open-field test. Stress. 21:443–452. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kraeuter AK, Guest PC and Sarnyai Z: The
open field test for measuring locomotor activity and anxiety-like
behavior. Methods Mol Biol. 1916:99–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tartar JL, Ward CP, Cordeira JW, Legare
SL, Blanchette AJ, McCarley RW and Strecker RE: Experimental sleep
fragmentation and sleep deprivation in rats increases exploration
in an open field test of anxiety while increasing plasma
corticosterone levels. Behav Brain Res. 197:450–453. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Walz N, Mühlberger A and Pauli P: A human
open field test reveals thigmotaxis related to agoraphobic fear.
Biol Psychiatry. 80:390–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Walsh RN and Cummins RA: The open-field
test: A critical review. Psychol Bull. 83:482–504. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Knight P, Chellian R, Wilson R,
Behnood-Rod A, Panunzio S and Bruijnzeel AW: Sex differences in the
elevated plus-maze test and large open field test in adult Wistar
rats. Pharmacol Biochem Behav. 204:1731682021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Justel N, Salguero A, Marengo L,
Psyrdellis M and Pautassi RM: Open field exposure facilitates the
expression of a spatial, recognition memory. Neurosci Lett.
757:1359972021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Snyder CN, Brown AR and Buffalari D:
Similar tests of anxiety-like behavior yield different results:
Comparison of the open field and free exploratory rodent
procedures. Physiol Behav. 230:1132462021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hogg S: A review of the validity and
variability of the elevated plus-maze as an animal model of
anxiety. Pharmacol Biochem Behav. 54:21–30. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Carobrez AP and Bertoglio LJ: Ethological
and temporal analyses of anxiety-like behavior: The elevated
plus-maze model 20 years on. Neurosci Biobehav Rev. 29:1193–1205.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bespalov A and Steckler T: Pharmacology of
anxiety or pharmacology of elevated plus maze? Biol Psychiatry.
89:e732021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Holmes A, Li Q, Koenig EA, Gold E,
Stephenson D, Yang RJ, Dreiling J, Sullivan T and Crawley JN:
Phenotypic assessment of galanin overexpressing and galanin
receptor R1 knockout mice in the tail suspension test for
depression-related behavior. Psychopharmacology (Berl).
178:276–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shao S, Cui Y, Chen ZB, Zhang B, Huang SM
and Liu XW: Androgen deficit changes the response to antidepressant
drugs in tail suspension test in mice. Aging Male. 23:1259–1265.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Iyer KA, Alix K, Eltit JM, Solis E Jr, Pan
X, Argade MD, Khatri S, Felice LJD, Sweet DH, Schulte MK and Dukat
M: Multi-modal antidepressant-like action of 6- and
7-chloro-2-aminodihydroquinazolines in the mouse tail suspension
test. Psychopharmacology (Berl). 236:2093–2104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Poleszak E, Szopa A, Bogatko K, Wyska E,
Wośko S, Świader K, Doboszewska U, Wlaź A, Wróbel A, Wlaź P and
Serefko A: Antidepressant-like activity of typical antidepressant
drugs in the forced swim test and tail suspension test in mice is
augmented by DMPX, an adenosine A2A receptor antagonist. Neurotox
Res. 35:344–352. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Pelloux Y, Hagues G, Costentin J and
Duterte-Boucher D: Helplessness in the tail suspension test is
associated with an increase in ethanol intake and its rewarding
effect in female mice. Alcohol Clin Exp Res. 29:378–388. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kale PP, Addepalli V and Ghadawale SR:
Impact of pre-exposure of tail suspension on behavioural parameters
like locomotion, exploration, and anxiety in mice. Indian J Exp
Biol. 51:732–738. 2013.PubMed/NCBI
|
|
94
|
Reis-Silva TM, Sandini TM, Calefi AS,
Orlando BCG, Moreira N, Lima APN, Florio JC, Queiroz-Hazarbassanov
NGT and Bernardi MM: Stress resilience evidenced by grooming
behaviour and dopamine levels in male mice selected for high and
low immobility using the tail suspension test. Eur J Neurosci.
50:2942–2954. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Nomura S, Naruse R and Okada H: The tail
suspension test: Its theory and practical application. Yakubutsu
Seishin Kodo. 12:207–213. 1992.(In Japanese). PubMed/NCBI
|
|
96
|
Rosa I, Di Censo D, Ranieri B, Di Giovanni
G, Scarnati E, Alecci M, Galante A and Florio TM: Comparison
between tail suspension swing test and standard rotation test in
revealing early motor behavioral changes and neurodegeneration in
6-OHDA hemiparkinsonian rats. Int J Mol Sci. 21:28742020.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Stukalin Y, Lan A and Einat H: Revisiting
the validity of the mouse tail suspension test: Systematic review
and meta-analysis of the effects of prototypic antidepressants.
Neurosci Biobehav Rev. 112:39–47. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Vieira C, De Lima TC, de Pádua Carobrez A
and Lino-de-Oliveira C: Frequency of climbing behavior as a
predictor of altered motor activity in rat forced swimming test.
Neurosci Lett. 445:170–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Flores-Serrano AG, Zaldivar-Rae J, Salgado
H and Pineda JC: Immobility time during the forced swimming test
predicts sensitivity to amitriptyline, whereas traveled distance in
the circular corridor indicates resistance to treatment in female
Wistar rats. Neuroreport. 26:233–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Petit-Demouliere B, Chenu F and Bourin M:
Forced swimming test in mice: A review of antidepressant activity.
Psychopharmacology (Berl). 177:245–255. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Herbst LS, Gaigher T, Siqueira AA, Joca
SRL, Sampaio KN and Beijamini V: New evidence for refinement of
anesthetic choice in procedures preceding the forced swimming test
and the elevated plus-maze. Behav Brain Res. 368:1118972019.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Dang H, Chen Y, Liu X, Wang Q, Wang L, Jia
W and Wang Y: Antidepressant effects of ginseng total saponins in
the forced swimming test and chronic mild stress models of
depression. Prog Neuropsychopharmacol Biol Psychiatry.
33:1417–1424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rebolledo-Solleiro D, Crespo-Ramírez M,
Roldán-Roldán G, Hiriart M and de la Mora M: Role of thirst and
visual barriers in the differential behavior displayed by
streptozotocin-treated rats in the elevated plus-maze and the open
field test. Physiol Behav. 120:130–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lucki I, Dalvi A and Mayorga AJ:
Sensitivity to the effects of pharmacologically selective
antidepressants in different strains of mice. Psychopharmacology
(Berl). 155:315–322. 2001. View Article : Google Scholar : PubMed/NCBI
|