Introduction
Gestational diabetes mellitus (GDM) is one of the
most common complications to occur during pregnancy, posing a
serious or even fatal risk to the pregnant woman and their
offspring (1). It is estimated
that the incidence rate of GDM is ~14% worldwide (2). Maternal diabetes, through transfer
of glucose in the placenta, can lead to hyperglycemia in the fetus
(1). In addition, since the
pancreas of the fetus responds to the increased glucose
concentrations by producing and releasing more insulin, as a
newborn, they may exhibit hyperinsulinemia and respiratory distress
syndrome (1). It has also been
shown that these offspring are at greater risk of obesity and
diabetes when they grow up (3).
Additionally, GDM also has adverse effects on the mother; the
condition may lead to perinatal complications, with shoulder
dystocia being one of the more prominent perinatal risks. To avoid
this risk, the mother is required to have a cesarean section
without undergoing labor (4). In
addition, both pre-eclampsia (5)
and the incidence of cesarean sections (6) are increased in cases of undiagnosed,
untreated GDM. At present, the treatment for GDM is usually based
on self-monitoring, diet and medication. However, the use of oral
antidiabetic drugs in the absence of medical nutrition stimulates
the pancreas to produce and release insulin, leading to
hypoglycemia in newborns (7).
Therefore, there is an urgent need for more scientifically
effective diagnostic and therapeutic approaches to meet the
requirements for both maternal and neonatal health.
C1q/TNF-associated proteins (CTRPs), especially
CTRP9, have been identified as highly conserved homologs of
adiponectin (APN), pooling several regulatory functions of APN
(8). There has been considerable
interest in assessing the metabolic and cardiovascular roles of
CTRP9. An increasing number of studies have shown that CTRP9 exerts
protective effects on the cardiovascular system by attenuating
post-infarction cardiac fibrosis (9), inducing angiogenesis (10) and inhibiting vascular inflammation
(11). However, relatively few
studies have focused on the role of CTRP9 in GDM. Recently, it has
been shown that lipocalin expression is downregulated in GDM
(12), and this was suggested to
be an early predictor of GDM (13). In addition, it has been reported
that CTRP9 is also downregulated in GDM, and that this
downregulation of gene expression may have a protective effect
(14). Furthermore, CTRP9 is able
to interact with the endoplasmic reticulum (ER) molecular chaperone
calreticulin in cardiomyocytes to inhibit ER stress (15). Therefore, it is possible to
speculate that CTRP9 may be associated with the development of
GDM.
ER stress is provoked by a variety of endogenous and
exogenous processes that lead to cellular damage, including
environmental toxins, viral infections and inflammation (16). Cells experience ER stress when the
ability to fold ER proteins is overwhelmed (17). Moreover, ER stress is also a
central feature of peripheral insulin resistance, obesity and type
2 diabetes (18). It has also
been claimed that ER stress exacerbates the occurrence of GDM
(19). Furthermore, the chorionic
trophectoderm is an important component of the embryo and, if
impaired, this can lead to a variety of complications during
pregnancy, including GDM. Therefore, in the present study,
expression of CTRP9 was investigated in the HTR8/SVneo human
placental trophoblast cell line under conditions of high-glucose
(HG) induction, together with any subsequent effects on ER stress,
in an attempt to demonstrate whether CTPR9 inhibits HG-induced
trophoblast cell injury through the decrease in ER stress.
Materials and methods
Cell culture, treatment and
transfection
The human trophoblast cell line HTR8/SVneo was
obtained from Procell Life Science & Technology Co., Ltd.
HTR8/SVneo cells were grown in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(MilliporeSigma) and 1% penicillin-streptomycin (MilliporeSigma) in
a humidified incubator at 37°C in an atmosphere of 5%
CO2. The HTR8/SVneo cells were treated with 25 mM HG for
24 h to mimic the in vitro gestational diabetic environment
and treated with 78 ng/ml tunicamycin (Abcam) for 16 h. HTR8/SVneo
cells maintained in media containing 5 mM glucose were used as the
control group.
pcDNA3.1(+) CTRP9 overexpression vector (Oe-CTRP9)
and empty vector NC (Oe-NC; Shanghai GenePharma, Co., Ltd.) at a
concentration of 20 µM were transfected into HTR-8/SVneo cells
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), in accordance with the manufacturer's
instructions. Cells were transfected at 37°C for 8 h and were used
in subsequent experiments 48 h post-transfection.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
The extraction of total RNA from HTR8/SVneo cells
was performed using TRIzol reagent (Takara Biotechnology Co.,
Ltd.), following the manufacturer's instructions. The extracted RNA
was reverse-transcribed into cDNA using a PrimeScript reverse
transcriptase reagent kit (Takara Biotechnology Co., Ltd.)
according to the manufacturer's protocol. qPCR was performed using
Power SYBR® Green Master Mix (Thermo Fisher Scientific,
Inc.), following the manufacturer's instructions. The thermocycling
conditions were as follows: 95°C for 5 min, followed by 40 cycles
at 95°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec. The
samples were analyzed in triplicate. The data were quantified using
the 2−ΔΔCq method (20). The following primers were used:
CTRP9 forward, 5′-TAGGGTCCAGGTGATGTTTCC-3′ and reverse,
5′-CCACCAGATCCTCATGGTTCAG-3′; and GAPDH forward,
5′-TGGAAGGACTCATGACCACA-3′ reverse, 5′-AGGGGTCTACATGGCAACTG-3′.
Western blot analysis
HTR8/SVneo cells were homogenized and lysed with
RIPA buffer (Beyotime Institute of Biotechnology) on ice, followed
by centrifugation at 12,000 × g for 10 min at 4°C. Subsequently,
the supernatant was collected, and the protein concentrations were
measured using a BCA protein assay kit (MilliporeSigma). The
proteins (20 µg/lane) were then separated using SDS-PAGE (10%
gels), after which they were transferred onto PVDF membranes
(MilliporeSigma). The membranes were blocked with 5% BSA for 1 h at
room temperature and incubated overnight at 4°C with primary
antibodies against CTRP9 (catalog no. LS-C373857; dilution, 1:400;
LifeSpan Biosciences, Inc.), glucose-regulated protein 78 (GRP78;
catalog no ab108615; dilution, 1:1,000; Abcam), cyclic
AMP-dependent transcription factor ATF-4 (ATF4; catalog no.
ab184909; dilution, 1:1,000; Abcam), C/EBP homologous protein
(CHOP; catalog no. ab194533; dilution, 1:1,000; Abcam), cleaved
caspase-3 (catalog no. ab32042; dilution, 1:500; Abcam), caspase 3
(catalog no. ab32351; dilution, 1:5,000; Abcam), Bcl-2 (catalog no.
ab32124; dilution, 1:1,000; Abcam), Bax (catalog no. ab32503;
dilution, 1:1,000; Abcam), p65 (catalog no. ab32536; dilution,
1:10,000; Abcam), phosphorylated (p)-p65 (catalog no. ab76302;
dilution, 1:1,000; Abcam), cyclo-oxygenase-2 (COX-2) (catalog no.
ab179800; dilution, 1:1,000; Abcam) and GAPDH (catalog no. ab9485;
dilution, 1:2,500; Abcam). After washing with TBS plus 0.1%
Tween-20 (Sigma-Aldrich; Merck KGaA) for 10 min, the membranes were
incubated with HRP-conjugated goat anti-rabbit IgG (catalog no.
ab6721; dilution, 1:2,000; Abcam) for 1 h at room temperature. The
protein blots were visualized using Pierce™ enhanced
chemiluminescence western blotting substrate (Thermo Fisher
Scientific, Inc.) and images were captured using a GE ImageQuant
LAS4000mini biomolecular imager (Cytiva). Protein expression was
evaluated by densitometry using ImageJ 1.8.0 software (National
Institutes of Health).
Cell viability assay
HTR8/SVneo cells were routinely cultured in 96-well
plates (4×104 cells/well) for 24 h. Subsequently, 10 µl
Cell Counting Kit-8 (CCK-8) solution (Dojindo Laboratories, Inc.)
was added to the medium, and the cells were incubated for 2 h at
37°C with 5% CO2. The optical density value was measured
at a wavelength of 450 nm with a Spectrafluor™ microreader plate
(Tecan Group, Ltd.). These experiments were repeated three
times.
TUNEL assay
A total of 1×106 cells were incubated for
24 h in serum-free medium and fixed with 4% paraformaldehyde on
slides at 4°C for 10 min. Subsequently, the cells were rinsed
briefly with PBS, and then permeabilized with 0.1% Triton X-100 for
2 min on ice. Cells were stained using the TUNEL Assay Kit (Abcam)
at 37°C in the dark for 1 h. The cells were subsequently rinsed
with PBS and counterstained with 10 µg/ml DAPI at 37°C for 2–3 min,
followed by being mounted in an anti-fade reagent (Beijing Solarbio
Science & Technology Co., Ltd.). Fluorescence in three random
fields of view was measured using a Zeiss LSM 880-Airyscan
(University College London) upright confocal multiphoton microscope
(Zeiss AG). The relative fluorescence intensity was determined by
measuring the ratio of green (TUNEL) to blue (DAPI) using MetaMorph
NX 2.5 software (Molecular Devices, LLC).
ELISA
Intracellular levels of TNF-α, interleukin (IL)-1β
and IL-6 in HTR8/SVneo cells were respectively detected by human
TNF-α (catalog no. PT518), IL-1β (catalog no. PI305) and IL-6
(catalog no. PI330) ELISA kits (all Beyotime Institute of
Biotechnology). HTR8/SVneo cells (1.0×104 cells/well)
were digested with trypsin and collected after centrifugation at
300 × g for 5 min at room temperature. After rinsing with washing
buffer, the cells were lysed and centrifuged again at 300 × g for
10 min at room temperature. The optical density values were
measured at 450 nm using a microplate reader.
Measurement of reaction oxygen species
(ROS), nitric oxide synthase (NOS) and NO
The level of ROS generation was assessed using the
2′,7′-dichlorofluorescein diacetate probe (MilliporeSigma)
according to the manufacturer's instructions. NOS activity was
assessed using the NOS Activity Assay kit (AmyJet Scientific, Inc.)
in accordance with the manufacturer's instructions. Finally, NO
content was measured using a Micro NO Content Assay kit (Beijing
Solarbio Science & Technology Co., Ltd.), following the
manufacturer's standard protocol.
Statistical analysis
The experimental data were analyzed using SPSS 21.0
software (IBM Corp.) and are expressed as the mean ± standard
deviation. An unpaired Student's t-test was used for comparisons
between two groups, whereas one-way analysis of variance (ANOVA)
followed by Tukey's post hoc test was applied for comparisons among
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
CTRP9 decreases ER stress in
trophoblast cell lines
The effects of CTRP9 on ER stress and the expression
levels ER stress-associated proteins were investigated in
HTR8/SVneo human trophoblast cells. The cells were treated with HG
to simulate the environment of GDM, which resulted in a significant
decrease in the protein expression levels of CTRP9 in comparison
with the control (Fig. 1A and B).
Subsequently, the cells were transfected with Oe-CTRP9, and the
transfection efficiency was confirmed using western blotting and
RT-qPCR (Fig. 1C and D,
respectively). In addition, the HG-induced downregulation of CTRP9
expression was reversed by the transfection of Oe-CTRP9. As shown
in Fig. 1E, CTRP9 overexpression
had no significant effect on the expression levels of ER
stress-associated proteins GRP78, ATF4 and CHOP under normal
glucose conditions. However, the expression levels of these
proteins were significantly increased under the induction of HG,
which were then significantly decreased in cells transfected with
Oe-CTRP9. This effect was reversed by the addition of the ER stress
inducer tunicamycin. Taken together, these results suggested that
CTRP9 had a significant effect on decreasing ER stress in cells
induced by HG.
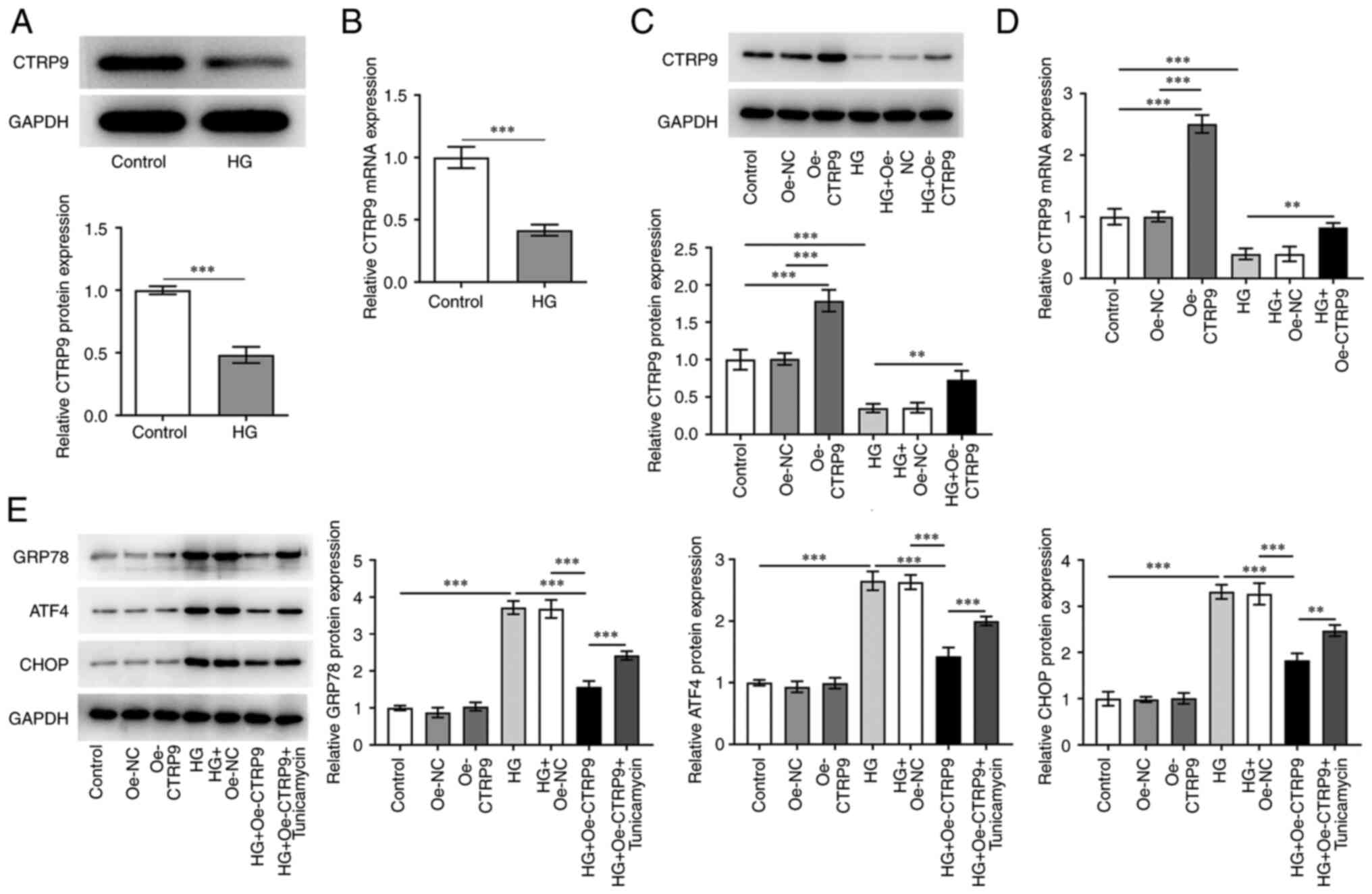 | Figure 1.CTRP9 decreases ER stress in a
trophoblast cell line. (A) Protein and (B) mRNA expression levels
of CTRP9 were detected in HTR8/SVneo human placental trophoblast
cells treated with HG using western blotting and RT-qPCR,
respectively. The transfection efficiency of Oe-CTRP9 and its
effects on HG-treated cells was detected using (C) western blotting
and (D) RT-qPCR. (E) Detection of ER stress-related protein
expression levels in cells were performed using western blotting.
**P<0.01 and ***P<0.001. ATF4, cyclic AMP-dependent
transcription factor ATF-4; CTRP9, C1q/TNF-α-related protein 9;
CHOP, C/EBP homologous protein; ER, endoplasmic reticulum; GRP78,
glucose-regulated protein 78; HG, high glucose; NC, negative
control; Oe, overexpression; RT-qPCR, reverse
transcription-quantitative PCR. |
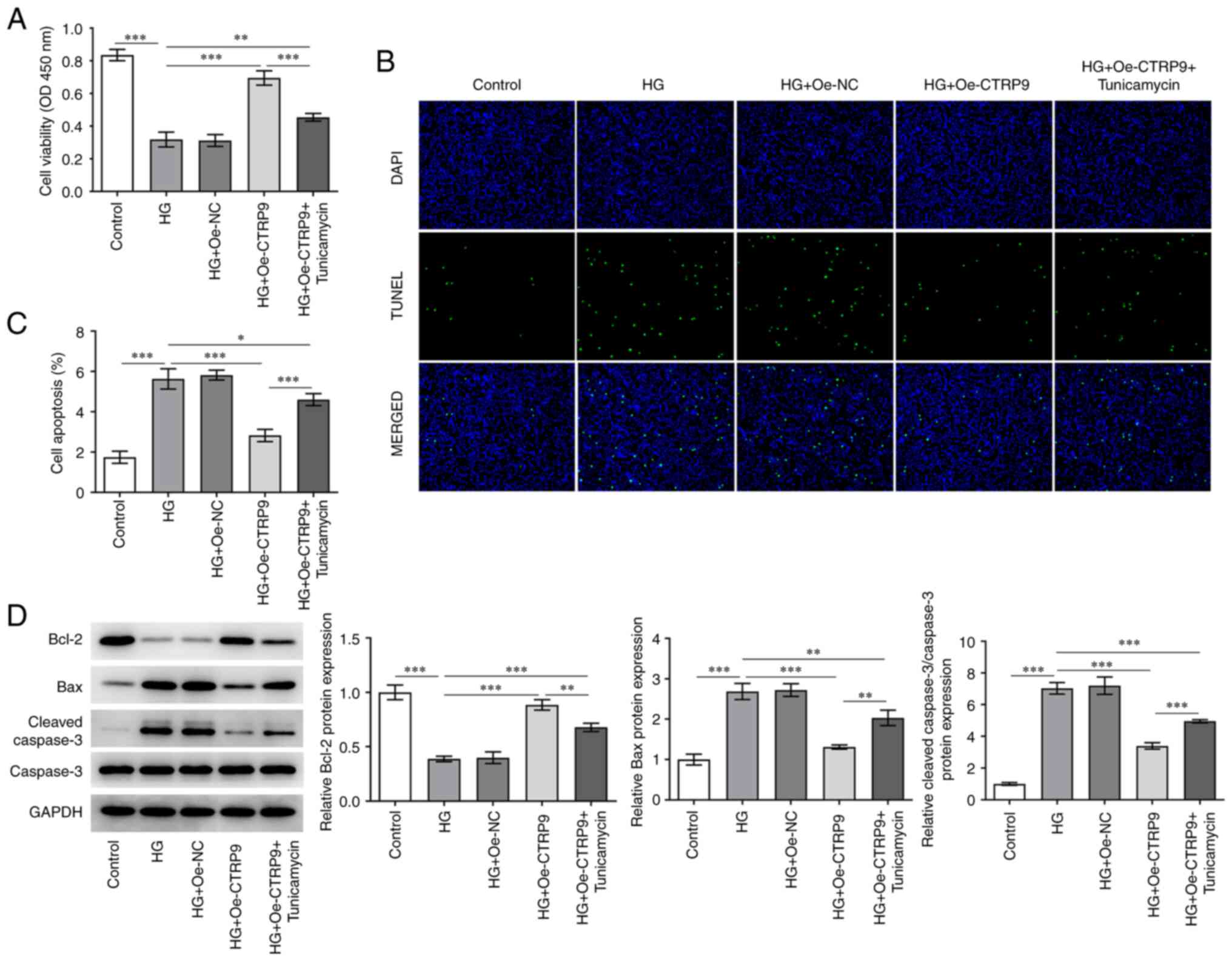
CTRP9 increases the viability of
HG-induced HTR8/SVneo cells by decreasing ER stress
To determine the effect of CTRP9 on the viability
and apoptosis of HTR8/SVneo cells under HG induction, CCK-8, TUNEL
and western blotting assays were performed. As shown in Fig. 2A, cell viability was significantly
decreased under HG induction relative to the control group, and was
restored to a certain extent following Oe-CTRP9 transfection, but
then decreased again after the addition of tunicamycin. Conversely,
HG treatment elevated the apoptotic rate of HTR8/SVneo cells in
comparison with the control group. In addition, the suppressed
apoptosis in HG-induced HTR8/SVneo cells transfected with Oe-CTRP9
was increased after the addition of tunicamycin (Fig. 2B and C). Additionally, Fig. 2D shows that HG treatment
downregulated Bcl-2 protein level while up-regulated Bax and
Cleaved caspase-3/Caspase-3 protein levels. After CTRP9 was
overexpressed, Bcl-2 protein level was elevated while Bax and
Cleaved caspase-3/Caspase-3 protein levels were decreased. There
was also a marked decrease in the protein level of Bcl-2 and
increase in the protein levels of Bax and Cleaved
caspase-3/Caspase-3 in HG + Oe-CTRP9 + Tunicamycin group.
Aforementioned results suggested that CTRP9 may increase the
viability and hinder the apoptosis of HG-treated HTR8/SVneo cells
by decreasing ER stress.
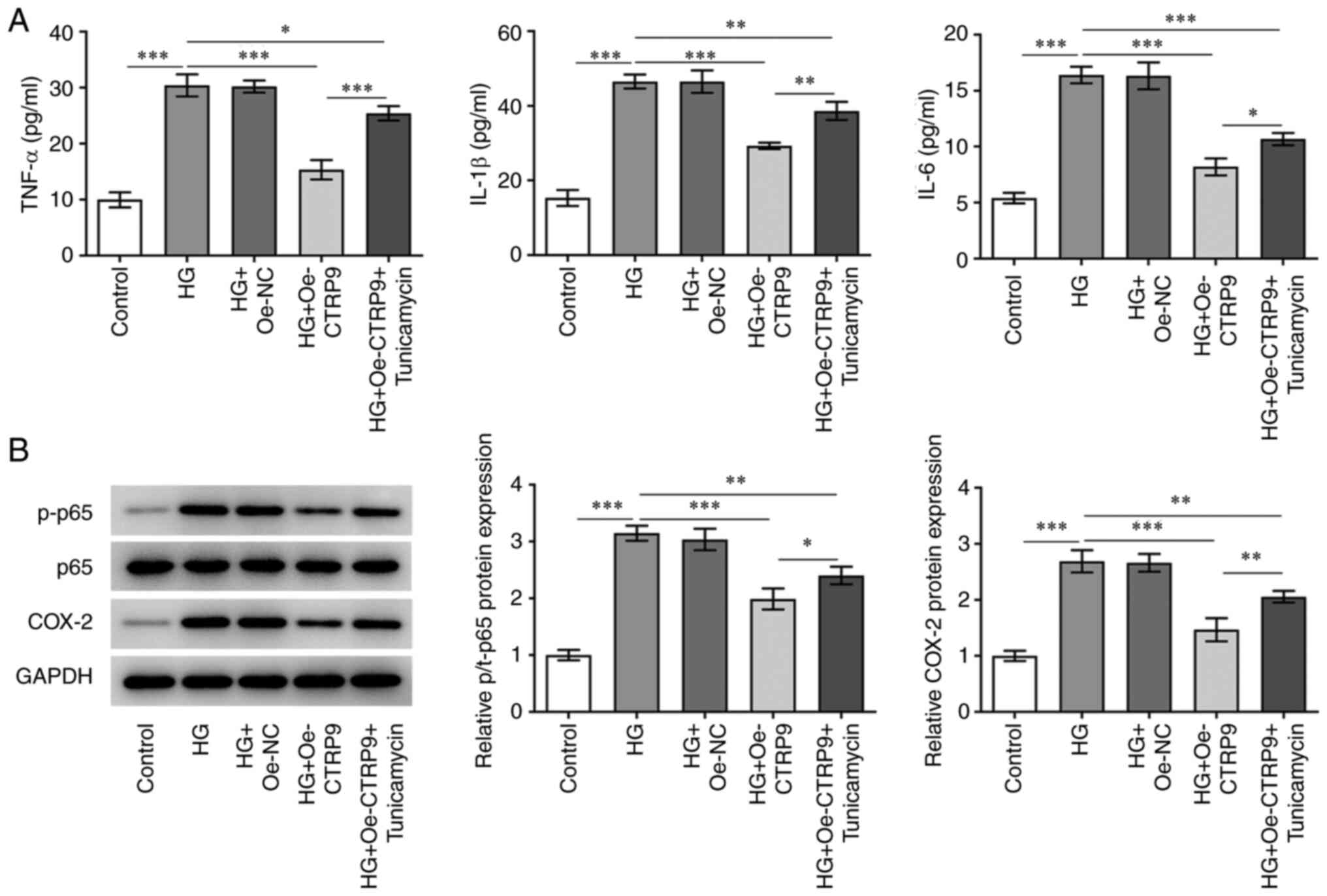
CTRP9 inhibits the inflammatory
response in HG-induced HTR8/SVneo cells by decreasing ER
stress
To determine whether CTRP9 has a role in the
inflammatory response of HTR8/SVneo cells, the intracellular levels
of the inflammatory factors TNF-α, IL-1β and IL-6, and the protein
expression levels of the inflammation-associated factors, p-p65 and
COX-2, were detected by ELISA and western blotting assays,
respectively. HG treatment significantly enhanced levels of TNF-α,
IL-1β, IL-6 and increased the protein levels of p-p65 and COX-2.
After transfection of Oe-CTRP9 into HG-induced HTR8/SVneo cells, a
decrease was observed in the concentration levels of TNF-α, IL-1β
and IL-6 (Fig. 3A), and a
decrease was also observed in the protein expression levels of
p-p65 and COX-2 (Fig. 3B)
compared with the HG + Oe-NC group. However, the changes caused by
Oe-CTRP9 were partially reversed by tunicamycin. Taken together,
these results indicated that CTRP9 exerted an inhibitory effect on
the inflammatory response in HG-induced HTR8/SVneo cells by
decreasing ER stress.
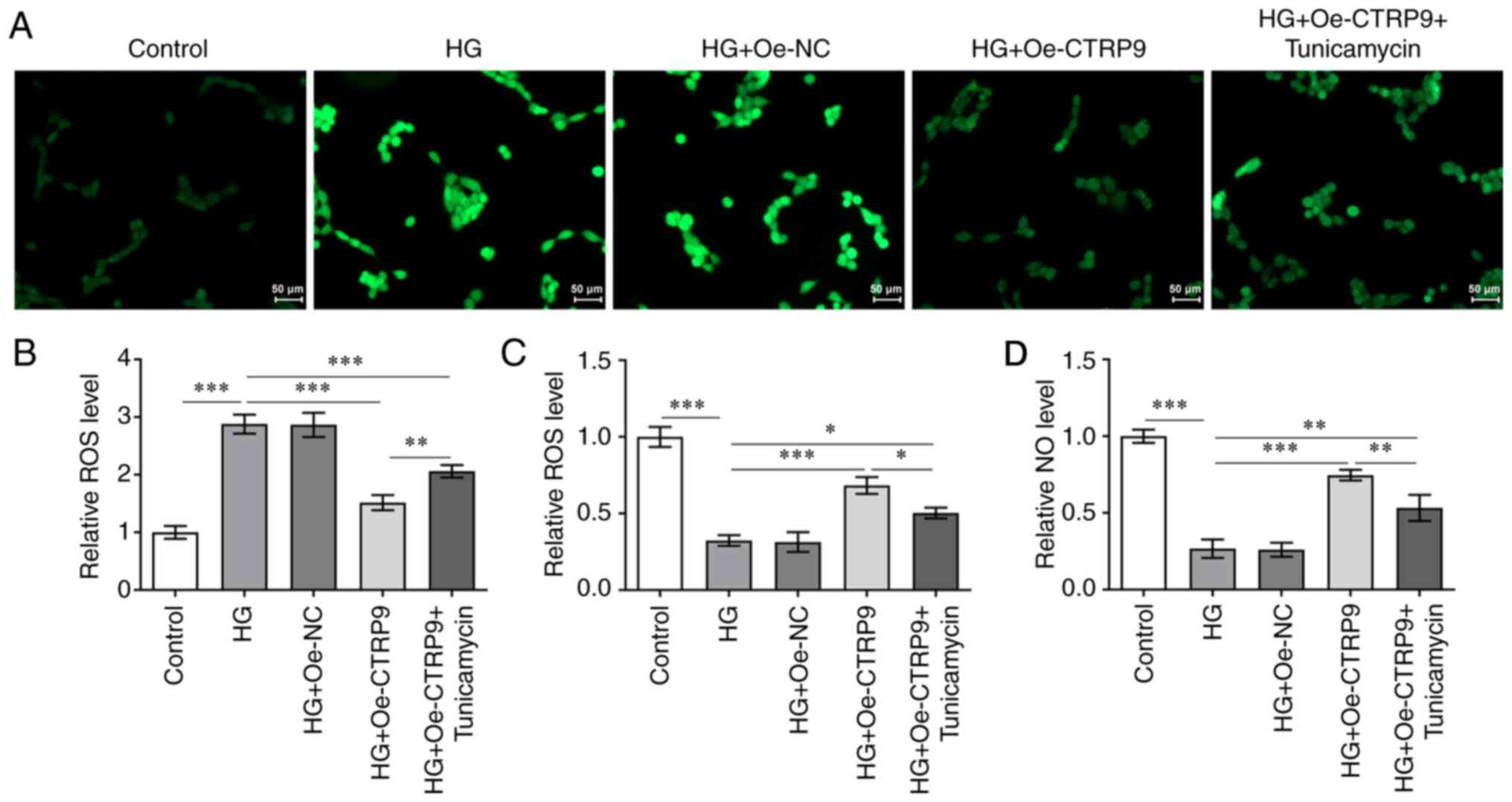
CTRP9 inhibits oxidative stress in
HG-induced HTR8/SVneo cells by decreasing ER stress
To study in the effect of CTRP9 on oxidative stress
in HG-induced HTR8/SVneo cells, the levels of ROS, NOS and NO
associated with oxidative stress in HG-induced HTR8/SVneo cells
were examined. Fig. 4A and B show
that the HG-induced ROS level was decreased after transfection with
Oe-CTRP9 compared with that in HG + Oe-NC group, but were
significantly increased after the addition of tunicamycin.
Moreover, as shown in Fig. 4C and
D, the level of NOS was increased in HG-treated cells
transfected with Oe-CTRP9, and this was decreased following the
addition of tunicamycin. A similar trend was observed for the NO
level. Taken together, these results have provided important
insights into the participatory role of CTRP9 in oxidative stress
in HG-induced cells.
Collectively, the experiments performed in the
present study suggested that CTRP9 inhibited oxidative stress in
HG-induced HTR8/SVneo cells through the decrease in ER stress.
Discussion
GDM is a medical complication that may be
encountered during pregnancy; it is associated with adverse
maternal and neonatal outcomes (1). At present, uniform screening and
diagnostic strategies for GDM are lacking globally (21). CTRP9 expression has been reported
to be downregulated in GDM, and may have a protective effect
against GDM (14). Serum CTRP9 is
an independent risk factor for the progression of GDM in pregnant
women (22). The present study
demonstrated that CTRP9 expression was downregulated in the human
placental trophoblast cell line HTR8/SVneo. Furthermore, CTRP9
overexpression was shown to increase cell viability and suppress
the inflammatory response as well as oxidative stress in HG-induced
HTR8/SVneo cells through the decrease in ER stress.
CTRP9 has been associated with the prevention of
atherosclerosis and the development of cardiovascular disease
(23). Appari et al
(24) suggested that upregulation
of CTRP9 promotes the poor cardiac remodeling and left ventricular
dysfunction associated with hypertrophic heart disease. CTRP3 and
CTRP9 were shown to be downregulated in patients with heart failure
(25). CTRP9 is also closely
associated with the development of ER stress (15). According to Bai et al
(26), CTRP9 exerts
cardioprotective effects by increasing the levels of disulfide-bond
A oxidoreductase-like protein to decrease ER stress in the diabetic
heart. Cardiac-derived CTRP9 was shown to activate the PKA-CREB
signaling pathway and to inhibit ER stress-associated apoptosis
signaling during myocardial ischemia-reperfusion injury (15). The present study, to the best of
our knowledge, was the first to show that CTRP9 expression was
downregulated in HG-induced HTR8/SVneo cells. Moreover, after
transfection with Oe-CTRP9, ER stress in HG-induced HTR8/SVneo
cells was notably lower.
In addition, CTRP9 is also associated with
apoptosis. For example, CTRP9 regulates human keratinocyte growth,
differentiation and apoptosis through a TGFβ1-p38-dependent
signaling pathway (27). CTRP9
has also been shown to regulate the hypoxia-mediated proliferation,
apoptosis and migration of human pulmonary artery smooth muscle
cells through the TGF-β1/ERK1/2 signaling pathway (28). In another previous study, CTRP9
also significantly attenuated palmitic acid-induced oxidative
stress and ER stress-induced apoptosis in neonatal rat cardiac
myocytes (29). Furthermore,
CTRP9 was shown to inhibit HG-induced oxidative stress and
apoptosis in retinal pigment epithelial cells through the
activation of the AMPK/Nrf2 signaling pathway (30). It is well established that the
upregulation of Bax, caspase 3 and the downregulation of Bcl-2 are
observed in apoptosis (31). In
the present study, after CTRP9 had been overexpressed in the
HG-treated cells, cell viability was increased and apoptosis was
decreased. In addition, the expression levels of cleaved caspase-3
and Bax were decreased, whereas the expression level of Bcl-2 was
increased. However, after addition of the ER-stress inducer
tunicamycin, the trends observed for the aforementioned genes were
reversed. Together, these findings suggested that CTRP9 increases
HG-induced HTR8/SVneo cell viability by decreasing ER stress.
Chronic inflammatory processes are associated with
an increase in the levels of inflammatory cytokines, including
IL-1β, TNF-α and IL-6 (32). The
levels of the proinflammatory cytokine IL-1β have been shown to be
high in the adipose tissue of women with GDM, which significantly
inhibits insulin signaling in the adipose tissue of pregnant women
(33). In the present study, it
was found that the concentrations of the proinflammatory cytokines
TNF-α, IL-1β and IL-6 were elevated in HTR8/SVneo cells upon
induction by HG. Additionally, the role of CTRP9 in decreasing ER
stress in cells has been demonstrated: A previous study showed that
ER stress induced inflammation and insulin resistance associated
with obesity and type 2 diabetes (34). In the present study, Oe-CTRP9 was
transfected into HG-induced cells, which resulted in decreased
expression levels of inflammatory cytokines, indicating that CTRP9
did exert an inhibitory effect on inflammation. Tunicamycin is an
ER-stress inducer, which can effectively trigger ER stress by
inhibiting protein glycosylation (35). In the present study, elevated
expression levels of the proinflammatory cytokines TNF-α, IL-1β and
IL-6 were found after adding tunicamycin to the cell culture
experiments, as were the protein expression levels of p-p65 and
COX-2. Collectively, these experiments confirmed that CTRP9 may
inhibit the inflammatory response in HG-induced HTR8/SVneo cells by
decreasing ER stress.
ER stress is an important local factor that is not
only related to inflammation, but is also closely associated with
oxidative stress (36). Oxidative
stress is caused by an imbalance between the oxidative and
antioxidant systems of cells and tissues as a consequence of the
overproduction of oxidative free radicals and associated ROS
(37). Oxidative stress is
associated with the development of metabolic diseases, including
diabetes (38). Additionally,
CTRP9 has been shown to inhibit glomerular and tubule glycogen
accumulation and fibrosis, and to relieve oxidative stress mediated
by hyperglycemia (39). In the
present study, it was observed that the levels of ROS were elevated
in HTR8/SVneo cells under HG induction, but decreased after
overexpression of CTRP9, and the levels then increased again
following the addition of tunicamycin, whereas the levels of NOS
and NO changed in the opposite direction. These findings
corroborated those of the other experiments, suggesting that CTRP9
inhibited oxidative stress in HG-induced HTR8/SVneo cells by
decreasing ER stress.
In conclusion, the present study showed that CTRP9
was expressed at a low level in the HTR8/SVneo trophoblast cell
line and may decrease ER stress. In addition, CTRP9 overexpression
enhanced cell viability and inhibited the inflammatory response as
well as oxidative stress in HG-induced HTR8/SVneo cells through the
decrease in ER stress. In summary, CTRP9 decreases trophoblast cell
damage induced by HG by decreasing ER stress, and CTRP9 is
therefore a promising target for the therapeutic treatment of GDM
in the future. However, the present study was limited to a single
cell line, and loss of function experiments, as well as in
vivo experiments, should be undertaken in the future to clarify
the precise role played by CTRP9 in GDM.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LZ and HD designed the study and drafted and revised
the manuscript. YS, DZ and XY analyzed the data and searched the
literature. LZ, YS and DZ performed the experiments. All authors
have read and approved the manuscript. LZ and YS confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coustan DR: Gestational diabetes mellitus.
Clin Chem. 59:1310–1321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laredo-Aguilera JA, Gallardo-Bravo M,
Rabanales-Sotos JA, Cobo-Cuenca AI and Carmona-Torres JM: Physical
activity programs during pregnancy are effective for the control of
gestational diabetes mellitus. Int J Environ Res Public Health.
17:61512020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Sullivan JB, Gellis SS, Dandrow RV and
Tenney BO: The potential diabetic and her treatment in pregnancy.
Obstet Gynecol. 27:683–689. 1966.PubMed/NCBI
|
|
4
|
Hill MG and Cohen WR: Shoulder dystocia:
Prediction and management. Womens Health (Lond). 12:251–261. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crowther CA, Hiller JE, Moss JR, McPhee
AJ, Jeffries WS and Robinson JS; Australian Carbohydrate
Intolerance Study in Pregnant Women (ACHOIS) Trial Group, : Effect
of treatment of gestational diabetes mellitus on pregnancy
outcomes. N Engl J Med. 352:2477–2486. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Landon MB, Spong CY, Thom E, Carpenter MW,
Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM Jr, et
al: A multicenter, randomized trial of treatment for mild
gestational diabetes. N Engl J Med. 361:1339–1348. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lengyel Z, Boér K, Halászlaki C and Németh
Z: Diabetes in patients with malignant tumors. Magy Onkol.
57:177–181. 2013.(In Hu). PubMed/NCBI
|
|
8
|
Rezabakhsh A, Sadeghpour Y, Ghaderi S,
Rahbarghazi R and Geranmayeh MH: CTRP9: An emerging potential
anti-aging molecule in brain. Cell Signal. 73:1096942020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu D, Lei H, Wang JY, Zhang CL, Feng H, Fu
FY, Li L and Wu LL: CTRP3 attenuates post-infarct cardiac fibrosis
by targeting Smad3 activation and inhibiting myofibroblast
differentiation. J Mol Med (Berl). 93:1311–1325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bossi F, Tripodo C, Rizzi L, Bulla R,
Agostinis C, Guarnotta C, Munaut C, Baldassarre G, Papa G, Zorzet
S, et al: C1q as a unique player in angiogenesis with therapeutic
implication in wound healing. Proc Natl Acad Sci USA.
111:4209–4214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin C, Ackermann S, Ma Z, Mohanta SK,
Zhang C, Li Y, Nietzsche S, Westermann M, Peng L, Hu D, et al: ApoE
attenuates unresolvable inflammation by complex formation with
activated C1q. Nat Med. 25:496–506. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balachandiran M, Bobby Z, Dorairajan G,
Gladwin V, Vinayagam V and Packirisamy RM: Decreased maternal serum
adiponectin and increased insulin-like growth factor-1 levels along
with increased placental glucose transporter-1 expression in
gestational diabetes mellitus: Possible role in fetal overgrowth.
Placenta. 104:71–80. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sweeting AN, Wong J, Appelblom H, Ross GP,
Kouru H, Williams PF, Sairanen M and Hyett JA: A Novel early
pregnancy risk prediction model for gestational diabetes mellitus.
Fetal Diagn Ther. 45:76–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia L, Zhang H, Shi Q, Zhang X, Wang C and
Lin G: Protective role of CTRP3 and CTRP9 in the development of
gestational diabetes mellitus. Clin Lab:. 66:2002472020. View Article : Google Scholar
|
|
15
|
Zhao D, Feng P, Sun Y, Qin Z, Zhang Z, Tan
Y, Gao E, Lau WB, Ma X, Yang J, et al: Cardiac-derived CTRP9
protects against myocardial ischemia/reperfusion injury via
calreticulin-dependent inhibition of apoptosis. Cell Death Dis.
9:7232018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen X, Zhang K and Kaufman RJ: The
unfolded protein response-a stress signaling pathway of the
endoplasmic reticulum. J Chem Neuroanat. 28:79–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi
NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH and Hotamisligil
GS: Endoplasmic reticulum stress links obesity, insulin action, and
type 2 diabetes. Science. 306:457–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liong S and Lappas M: Endoplasmic
reticulum stress is increased in adipose tissue of women with
gestational diabetes. PLoS One. 10:e01226332015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alfadhli EM: Gestational diabetes
mellitus. Saudi Med J. 36:399–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Na N and Ji M: Role of first-trimester
serum C1q/TNF-related protein 9 in gestational diabetes mellitus.
Clin Lab:. 66:2004342020. View Article : Google Scholar
|
|
23
|
Miyatake N, Adachi H, Nomura-Nakayama K,
Okada K, Okino K, Hayashi N, Fujimoto K, Furuichi K and Yokoyama H:
Circulating CTRP9 correlates with the prevention of aortic
calcification in renal allograft recipients. PLoS One.
15:e02265262020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Appari M, Breitbart A, Brandes F,
Szaroszyk M, Froese N, Korf-Klingebiel M, Mohammadi MM, Grund A,
Scharf GM, Wang H, et al: C1q-TNF-related protein-9 promotes
cardiac hypertrophy and failure. Circ Res. 120:66–77. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao C, Zhao S, Lian K, Mi B, Si R, Tan Z,
Fu F, Wang S, Wang R, Ma X and Tao L: C1q/TNF-related protein 3
(CTRP3) and 9 (CTRP9) concentrations are decreased in patients with
heart failure and are associated with increased morbidity and
mortality. BMC Cardiovasc Disord. 19:1392019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai S, Cheng L, Yang Y, Fan C, Zhao D, Qin
Z, Feng X, Zhao L, Ma J, Wang X, et al: C1q/TNF-related protein 9
protects diabetic rat heart against ischemia reperfusion injury:
Role of endoplasmic reticulum stress. Oxid Med Cell Longev.
2016:19020252016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jung TW, Park HS, Choi GH, Kim D and Lee
T: CTRP9 regulates growth, differentiation, and apoptosis in human
keratinocytes through TGFβ1-p38-dependent pathway. Mol Cells.
40:906–915. 2017.PubMed/NCBI
|
|
28
|
Li YX, Run L, Shi T and Zhang YJ: CTRP9
regulates hypoxia-mediated human pulmonary artery smooth muscle
cell proliferation, apoptosis and migration via TGF-beta1/ERK1/2
signaling pathway. Biochem Biophys Res Commun. 490:1319–1325. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zuo A, Zhao X, Li T, Li J, Lei S, Chen J,
Xu D, Song C, Liu T, Li C and Guo Y: CTRP9 knockout exaggerates
lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac
hypertrophy through inhibiting the LKB1/AMPK pathway. J Cell Mol
Med. 24:2635–2647. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng Y, Qi Y, Liu S, Di R, Shi Q, Li J
and Pei C: C1q/TNF-related Protein 9 inhibits high glucose-induced
oxidative stress and apoptosis in retinal pigment epithelial cells
through the activation of AMPK/Nrf2 signaling pathway. Cell
Transplant. 29:9636897209620522020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L and Zhang S: Modulating Bcl-2
family proteins and caspase-3 in induction of apoptosis by
paeoniflorin in human cervical cancer cells. Phytother Res.
25:1551–1557. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duleba AJ and Dokras A: Is PCOS an
inflammatory process? Fertil Steril. 97:7–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lappas M: Activation of inflammasomes in
adipose tissue of women with gestational diabetes. Mol Cell
Endocrinol. 382:74–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liong S and Lappas M: Endoplasmic
reticulum stress regulates inflammation and insulin resistance in
skeletal muscle from pregnant women. Mol Cell Endocrinol.
425:11–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu J, Chen S, Liu H, Zhang Z, Ni Z, Chen
J, Yang Z, Nie Y and Fan D: Tunicamycin specifically aggravates ER
stress and overcomes chemoresistance in multidrug-resistant gastric
cancer cells by inhibiting N-glycosylation. J Exp Clin Cancer Res.
37:2722018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brenjian S, Moini A, Yamini N, Kashani L,
Faridmojtahedi M, Bahramrezaie M, Khodarahmian M and Amidi F:
Resveratrol treatment in patients with polycystic ovary syndrome
decreased pro-inflammatory and endoplasmic reticulum stress
markers. Am J Reprod Immunol. 83:e131862020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ali SS, Ahsan H, Zia MK, Siddiqui T and
Khan FH: Understanding oxidants and antioxidants: Classical team
with new players. J Food Biochem. 44:e131452020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang P, Li T, Wu X, Nice EC, Huang C and
Zhang Y: Oxidative stress and diabetes: Antioxidative strategies.
Front Med. 14:583–600. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu H, Li W, Liu M, Xiong J, Li Y, Wei Y,
Huang C and Tang Y: C1q/Tumor necrosis factor-related protein-9
attenuates diabetic nephropathy and kidney fibrosis in db/db mice.
DNA Cell Biol. 39:938–948. 2020. View Article : Google Scholar : PubMed/NCBI
|