Introduction
Lung cancer is one of the most common malignancies
worldwide, and associated with high rates of morbidity and
mortality (IARC, 2020). There are few early symptoms, and their
onset is relatively insidious. Therefore, most cases are confirmed
at mid and late stages of lung cancer. Currently, chemotherapy and
surgery are the most commonly used treatment modalities, which can
increase the survival rate of patients. However, long-term
chemotherapy also severely impacts important organs and induces
drug resistance in tumors (1).
The marine environment, characterized by high salt content, high
pressure, and low temperature, markedly differs from the
terrestrial environment. Numerous marine organisms can produce
massive active substances with novel structures that differ from
those synthesized by terrestrial organisms. Recent research on
marine drugs has found that numerous bioactive substances of marine
origin can significantly inhibit tumor growth in various ways
(2,3). Therefore, there is increasing
interest in discovering new anticancer drugs with low toxicity and
high efficacy from marine organisms. It is expected that a diverse
array of active substances of marine origin, such as peptides,
polysaccharides, alkaloids, terpenes, and macrolides, will be
developed into new antitumor drugs (4). For example, dolastatin-10 (obtained
from the sea hare) has been used in clinical trials (5).
Nereids are invertebrates of the polychaete family
(Nereidae). They are widely distributed in the Sea of Japan and the
Pacific coast, and endemic to Japan and China. In recent years, the
interest in the bioactive substances produced by the Nereids has
gradually increased. Japanese scholars have isolated biologically
active polypeptides from the Nereids (6). These polypeptides can strongly
shrink the esophagus of annelids. The first isolation and
purification of a protease from the Nereids were achieved at
Bethune School of Medicine, Jilin University (Jilin, China). This
protease is a serine protease with a molecular weight of 29 kDa, an
isoelectric point of 4.4, and an optimal temperature of 60°C. The
protease exerts a strong effect on the degradation of fibrin and
fibrinogen. The order of hydrolysis is as follows: Aα, Bβ, and γ
chains. Studies suggested that the protease isolated from the
Nereids can significantly inhibit various leukemic cells in
vitro (7). The protease
Nereis virens proteinase (ASP) also has strong antitumor
activity in leukemic cells and lung cancer SPC-A-1 cells (8–12).
However, there is limited knowledge on the enzymatic hydrolysis of
Nereid oligopeptides (NOP), and their inhibitory effects on
non-small cell lung cancer H1299 cells. The objective of the
present study was to investigate the preparation of an NOP and its
effects on the growth of H1299 cells. Moreover, the induction of
cell apoptosis and the mechanism of action are preliminarily
discussed, thereby providing an experimental basis for the clinical
application of NOP.
Materials and methods
Samples and reagents
The Nereid was purchased from Zhoushan Jusha
Aquaculture Co., Ltd., and was identified as Perinereis
aibuhitensis Grube by Professor Zhao Shenglong from Zhejiang
Ocean University (Zhoushan, China). Trypsin, papain, alkaline
protease, neutral protease, and pepsin were purchased from YTHX
Biotechnology Co., Ltd. Fetal bovine serum was obtained from
Hangzhou Sijiqing Biological Engineering Co., Ltd. RPMI-1640 powder
and medium were purchased from Gibco; Thermo Fisher Scientific,
Inc. Methyl thiazolyl tetrazolium (MTT) and dimethyl sulfoxide were
purchased from Sigma-Aldrich; Merck KGaA. The other reagents were
of analytical grade.
Cells and cell culture
Human lung cancer cell lines [A549 (cat. no.
SCSP-503), H1299 (cat. no. SCSP-589) and 95C (cat. no. SNL-168)]
and neuroblastoma [PC-12 (cat. no. SCSP-517) and SK-N-SH (cat. no.
SCSP-5029)] cell lines were purchased from the Shanghai Cell Bank
of the Chinese Academy of Sciences. The cells were passaged and
subcultured in RPMI-1640 nutrient solution containing 10% bovine
serum. All cells were static and incubated at 37°C in a humidified
atmosphere with 5% CO2 (Forma™ cat. no. 3111;
Thermo Fisher Scientific, Inc.); the culture medium was changed
once every 2–3 days.
Equipment and instruments
The equipment and instruments included the
following: refrigerated centrifuge (NUAIRE), carbon dioxide
incubator and microplate reader (both from Thermo Fisher
Scientific, Inc.), protein electrophoresis and western blotting
system (Bio-Rad Laboratories, Inc.), Odyssey Imager (LI-COR
Biosciences), horizontal shaker (Hangzhou Miu Instruments Co.,
Ltd.), clean bench (Suzhou Cleaning Equipment Co., Ltd.), −80°C
ultra-low temperature incubator (Haier Group), ice machine
(Changshu Xueke Electric Co., Ltd.), and electric-heated
thermostatic water bath (Changzhou Guohua Electric Appliance Co.,
Ltd.).
Preparation of Nereid enzymatic
hydrolysates and NOP separation and purification
Selection of enzyme species
The Nereid was rinsed and homogenized. Homogenized
samples (13,14) (10.0 g each) were obtained for
enzymatic hydrolysis with five enzymes (i.e., trypsin, alkaline
protease, neutral protease, pepsin, and papain) at certain
temperatures and pH values (Table
I). The optimal conditions for hydrolysis were as follows: 50°C
and pH 10 for 6 h, a material-to-liquid ratio of 1:1 (g/ml), and
addition of 400 U/g enzyme. Following hydrolysis, the samples were
inactivated in a water bath at 100°C for 10 min, and centrifuged at
10,000 × g, at 4°C for 20 min in the refrigerated centrifuge. The
supernatant was obtained and examined using the MTT method to
determine the inhibition rate (IR) on the proliferation of H1299
cells. This process permitted the recognition of the optimal enzyme
species.
 | Table I.Optimal temperature and pH value for
enzymatic hydrolysis. |
Table I.
Optimal temperature and pH value for
enzymatic hydrolysis.
| Enzyme | Temperature
(°C) | pH value |
|---|
| Trypsin | 50 | 8.0 |
| Alkaline
protease | 45 | 10.0 |
| Neutral
protease | 45 | 7.0 |
| Pepsin | 37 | 3.0 |
| Papain | 45 | 6.0 |
Optimization of enzymatic hydrolysis
conditions
The optimal protease was used for enzymatic
hydrolysis to determine the optimal temperature and pH. The
relevant literature detailing single factor experiments (15–17) involving temperature, pH,
material-to-liquid ratio, duration, and the amount of enzyme added
during the enzymatic hydrolysis, were reviewed. Subsequently, an
orthogonal test (Table II)
involving the aforementioned parameters was designed, using the IR
as an indicator to determine the optimal conditions for enzymatic
hydrolysis.
 | Table II.Orthogonal test design. |
Table II.
Orthogonal test design.
|
| Factor |
|---|
|
|
|
|---|
| Level | A | B | C | D | E |
|---|
| 1 | 35 | 7 | 1:1 | 2 | 400 |
| 2 | 40 | 8 | 1:2 | 4 | 600 |
| 3 | 45 | 9 | 1:3 | 6 | 800 |
| 4 | 50 | 10 | 1:4 | 8 | 1,000 |
NOP separation using
ultrafiltration
Enzymatic hydrolysate <3 kDa was separated using
the membrane ultrafiltration technique, cooled down, and dried. The
MTT method was used to detect the IR in A549, H1299 and 95C cells
after 24 h of intervention with different components at 4,000 mg/l.
Next, the cell lines demonstrating more pronounced anti-lung cancer
effects after treatment were identified for further separation.
NOP separation through
diethylaminoethanol (DEAE) Sepharose Fast Flow chromatography
The ultra-filtrated components at 0.25 g/ml were
further separated using DEAE Sepharose Fast Flow (Beijing
Asia-Pacific Hengxin Biological Technology Co., Ltd.)
chromatography. Following centrifugation (10,000 × g, 4°C, 20 min),
the supernatant was collected, filtered with a filter membrane
(pore size, 0.22 µm), and eluted. The separation conditions were as
follows: Column size, 3.6×20 cm; packing material, DEAE Sepharose
FF; column particle diameter, 45–165 µm; sample volume, 2 ml;
eluent, 0–0.4 mol/l NaCl solution; gradient elution, ~50 ml for
each column; flow rate, 1 ml/min; detection wavelength, 280 nm; and
collected volume, 5 ml per tube. The peaks were collected,
concentrated, and freeze-dried. The MTT method was applied to
select the peak components with the highest IR (inhibition rate)
for further separation.
NOP separation using Sephadex
G-25
The components with the highest IR collected in the
previous step were further separated and purified by chromatography
using the Sephadex G-25 column (Beijing Asia-Pacific Hengxin
Biological Technology Co., Ltd.). The mass concentration of the
sample was 0.25 g/ml. Following centrifugation (10,000 × g, 4°C, 20
min), the supernatant was filtered with a filter membrane (pore
size, 0.22 µm) and eluted. The conditions for separation were as
follows: Column size, 2.6×60 cm; packing material, Sephadex G-25;
column particle diameter, 50–150 µm; sample volume, 2 ml; eluent,
distilled water; flow rate, 1.0 ml/min; detection wavelength, 280
nm; and collected volume, 5 ml per tube. The peaks were collected,
concentrated, and freeze-dried. The MTT method was used to identify
the peak components with the highest IR (inhibition rate) for
further separation.
Separation and purification using
high-performance liquid chromatography (HPLC)
The peak components with the highest IR in H1299
cells collected in the previous step were further separated and
purified. The chromatographic conditions were as follows:
Chromatographic column, Luna 10u C18(2) analytical column (10×250
mm; 5 µm); detection wavelength, 280 nm; flow rate, 1.0 ml/min;
mobile phase A, acetonitrile; mobile phase B, ultrapure water
(containing 0.05% trifluoroacetic acid); gradient elution, 20–100%
elution A for 5–20 min; column temperature, 25°C; and automatic
sampling with a sample volume of 100 µl. The most active components
were collected, freeze-dried, and sequenced as described below.
Determination of molecular weight and
N-terminal sequencing of target peptides
The collected components with the highest activity
were freeze-dried for the measurement of the relative molecular
weight and N-terminal sequencing of target peptides at APTBIO.
MTT assay for the detection of the
anti-proliferative activity of NOP
H1299 cells were seeded into 96-well culture plates
(density, 1×105 cells/ml) and cultured in RPMI-1640
medium (200 µl per well) under conventional conditions. After 24 h,
the culture medium was removed, and a blank control (no drug
treatment, RPMI-1640 medium used) group and a drug group were
prepared. The cells in the drug group were treated with NOP (250,
500, or 1,000 mg/l) for 12 and 24 h at 37°C in a humidified
atmosphere with 5% CO2. Subsequently, the culture
solution was discarded, and phosphate-buffered saline (PBS)
containing 10% MTT reagent was added. The cell culture was
continued for 4 h. The solution was removed again, dimethyl
sulfoxide (150 µl) was added, and the cells were thoroughly mixed.
The cell absorbance at 490 nm was detected using a microplate
reader. The cell proliferation IR (%) was calculated using the
following equation: IR (%)=[1-(A treated/A control)] ×100%.
Cell Counting Kit-8 (CCK-8) assay for
the detection of the anti-proliferative activity of NOP
Cell culture and drug administration were designed
as above. CCK-8 reagent (Shanghai Biyuntian Biotechnology Co.,
Ltd.) (10 µl) was added, and the cell culture was continued for 4
h. The cell absorbance at 450 nm was detected using a microplate
reader. The IC50 which is a measure of the potency of a
substance when the half maximal inhibitory concentration is
achieved was calculated as follows:
IC50=lg−1[Xm-i(ΣP-0.5)].
Observation of cell morphology using
hematoxylin and eosin (H&E) staining
Acid-treated coverslips were placed onto 96-well
plates, and A549 cells were routinely cultured (density,
1×105 cells/ml) for 24 h. The culture medium was
subsequently removed, and a blank control group and a drug group
were prepared. The cells in the drug group were treated with NOP
(250, 500, or 1,000 mg/l). After 24 h of culture, the coverslips
were removed, and the cells were fixed in 95% ethanol for 20 min at
room temperature. The samples were stained using hematoxylin for
3–5 min at room temperature, immersed in tap water, developed with
eosin for 1 min, dehydrated with ethanol, treated with xylene to
become transparent, mounted in neutral gum, and images were
captured under an optical microscope (BX2-FLB3 fluorescence
microscope; Olympus Corporation).
Morphology of tumor cells after
treatment with NOP using a transmission electron microscope
H1299 cells (1×107 cells/ml) cultured for
24 h were digested and transferred to a 10-ml centrifuge tube. This
was followed by centrifugation at 800 × g at 4°C for 6 min, washing
with PBS (800 × g, 4°C for 6 min), and removal of the supernatant.
Subsequently, 2.5% dialdehyde was added, and the cell suspension
was incubated overnight at 4°C. The cell suspension was treated at
the Biological Laboratory of Zhejiang University Medical College
(Hangzhou, China). The cells were fixed using 2.5% glutaraldehyde
at 4°C for 24 h. Sections were cut to a thickness of 100 nm and
uranyl acetate and lead citrate were used to stain cells at room
temperature for 15 min. The cells were embedded in resin at 37°C
for 4–6 h, and 60°C for 40 min. Finally, it was observed and images
were captured under a transmission electron microscope.
Cell apoptosis analysis
Apoptotic rates were measured by flow cytometry
using Annexin V-fluorescein isothiocyanate/propidium iodide
(FITC/PI) staining. H1299 cells (1×106 cells/ml) were
firstly seeded in six-well flat-bottomed plates and incubated for
24 h at 37°C. Cells were subsequently treated with NOP (250, 500,
or 1,000 mg/l) for another 24 h at 37°C and harvested. After
digestion with trypsin, the cells were suspended in PBS and
harvested by centrifugation at 800 × g at room temperature for 10
min. Next, the cells were incubated with Annexin V-FITC (5 µl) and
PI (10 µl) at room temperature for 15 min in the dark. After
staining, cells were immediately analyzed using flow cytometry
(Easy Cyte6 HT-2L Flow Cytometer; Luminex Corporation).
Western blot analysis
Total protein was extracted with
radioimmunoprecipitation assay (RIPA) lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd.), and protein
concentrations were determined using a bicinchoninic acid assay
kit. Proteins (20 µl) were subsequently separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to a 12
or 15% polyvinylidene difluoride membrane. The membranes were
rinsed once with PBS containing 0.05% Tween-20 (PBST) and blocked
in 5% skimmed milk powder at 4°C for 2 h. After two rinses in PBST
(10 ml) and one rinse in PBS (10 ml), the membranes were placed
into a hybridization bag containing the primary antibodies B-cell
lymphoma 2 (Bcl-2; 1:1,000; cat. no. A00040-2), Bcl-2 associated X
(Bax; 1:1,000; cat. no. BA0315-2), caspase-9 (CASP9; 1:1,000; cat.
no. BM4619), and caspase-3 (CASP3; 1:500; cat. no. BM4620) (all
from Wuhan Boster Biological Technology, Ltd.). The antibodies were
diluted with 1% bovine serum albumin solution according to the
instructions provided by the manufacturer. Following incubation
overnight at 4°C, the membranes were placed on a shaker at room
temperature. After 1 h, the membranes were rinsed thrice with PBST
(10 min per rinse). Thereafter, the membranes were incubated with
fluorescent secondary antibodies goat anti-rabbit (1:10,000; cat.
no. 925-68071) and goat anti-mouse (1:10,000; cat. no. 925-68070)
(both from LI-COR Biosciences) and incubated in the dark for 1 h at
room temperature. Following incubation, the membranes were washed
thrice with PBST (10 min per rinse). β-actin was used as an
internal reference, and protein bands were quantified using the
Odyssey Imager (LI-COR Biosciences).
Statistical analysis
Three parallel experiments were conducted for all
groups. Experimental data were analyzed and processed using SPSS
version 19.0 statistical software (IBM Corp.). One-way ANOVA with
Dunnett's post hoc test was used to determine the difference
between different groups. The data are expressed as the mean ±
standard deviation (n=3). P<0.05 was considered to indicate a
statistically significant difference.
Results
Optimization of the enzymolytic
process of NOP
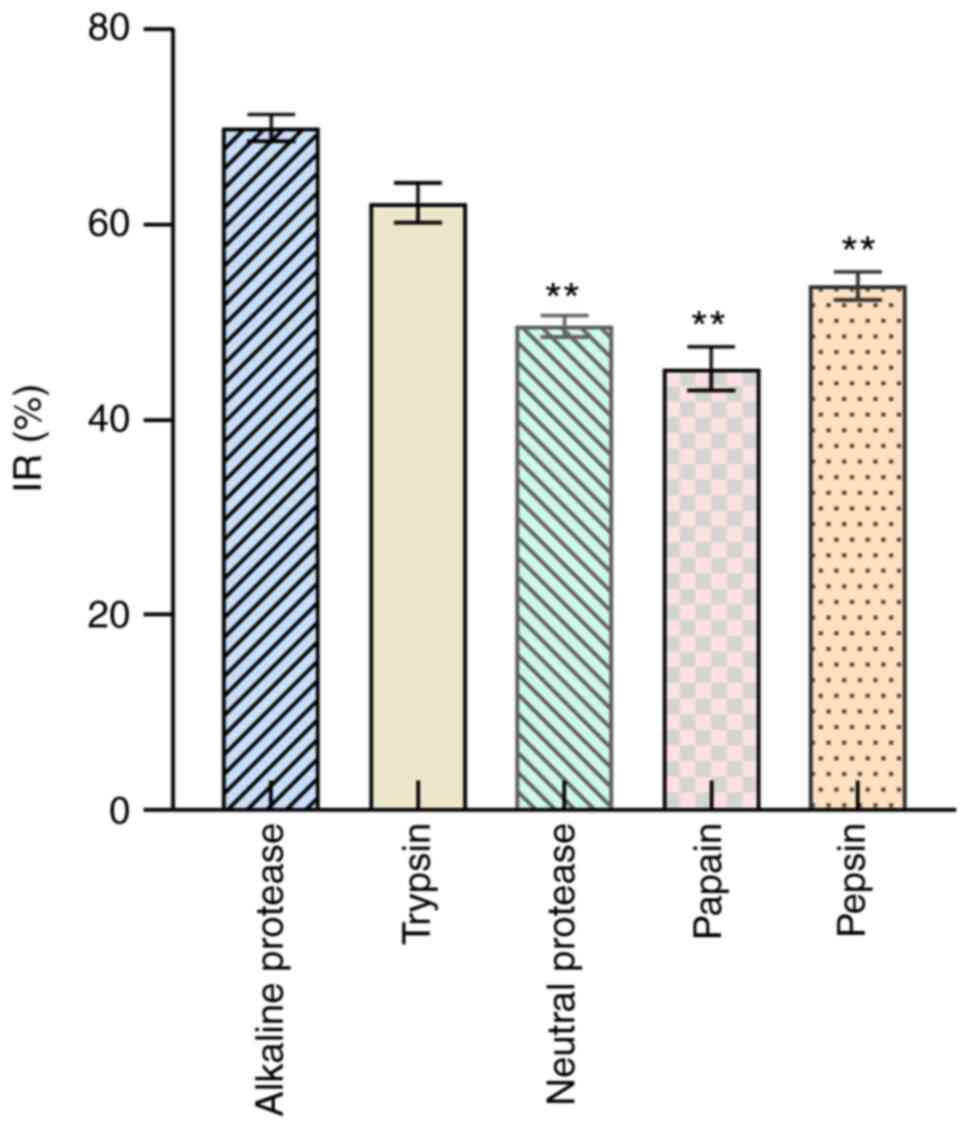
In H1299 cells, the IRs were 69.94±1.35% for
alkaline protease, 62.25±2.03% for trypsin, 53.76±1.43% for pepsin,
49.62±1.09% for neutral protease, and 45.27±2.25% for papain. In
addition, the order of activity was as follows: alkaline protease
> trypsin > pepsin > neutral protease > papain
(Fig. 1). According to the IR,
alkaline protease was selected for further screening.
Orthogonal test results
The IR was determined under different enzymolysis
conditions, such as temperature, pH value, material-to-liquid
ratio, duration, and the amount of enzyme added (Table III). The range method was used
to analyze the order of factors affecting the IR: C > E > A
> B > D. The factor with the greatest impact on IR was C
(material-to-liquid ratio). The optimal conditions for enzymatic
hydrolysis were A4B4C1D3E1, enzymatic hydrolysis at 50°C and pH 10
for 6 h, with a material-to-liquid ratio of 1:1 and the addition of
400 U/g enzyme.
 | Table III.Orthogonal experiment design and
results. |
Table III.
Orthogonal experiment design and
results.
| Number | A | B | C | D | E | IR (%) |
|---|
| 1 | 1 (35) | 1 (7) | 1 (1:1) | 1 (2) | 1 (400) | 90.34±1.23 |
| 2 | 1 | 2 (8) | 2 (1:2) | 2 (4) | 2 (600) | 40.11±1.45 |
| 3 | 1 | 3 (9) | 3 (1:3) | 3 (6) | 3 (800) | 51.43±0.88 |
| 4 | 1 | 4 (10) | 4 (1:4) | 4 (8) | 4 (1,000) | 38.78±2.45 |
| 5 | 2 (40) | 1 | 2 | 3 | 4 | 56.41±3.02 |
| 6 | 2 | 2 | 1 | 4 | 3 | 58.47±2.41 |
| 7 | 2 | 3 | 4 | 1 | 2 | 42.12±0.88 |
| 8 | 2 | 4 | 3 | 2 | 1 | 88.71±1.69 |
| 9 | 3 (45) | 1 | 3 | 4 | 2 | 41.58±2.45 |
| 10 | 3 | 2 | 4 | 3 | 1 | 42.01±3.21 |
| 11 | 3 | 3 | 1 | 2 | 3 | 41.89±1.12 |
| 12 | 3 | 4 | 2 | 1 | 4 | 43.25±2.47 |
| 13 | 4 (50) | 1 | 4 | 2 | 3 | 49.64±0.89 |
| 14 | 4 | 2 | 3 | 1 | 4 | 54.11±2.11 |
| 15 | 4 | 3 | 2 | 4 | 1 | 55.54±2.55 |
| 16 | 4 | 4 | 1 | 3 | 2 | 89.32±1.76 |
| K1 | 55.165 | 59.492 | 70.005 | 57.455 | 69.150 |
|
| K2 | 61.427 | 48.675 | 48.827 | 55.087 | 53.282 |
|
| K3 | 42.183 | 47.745 | 58.957 | 59.792 | 50.698 |
|
| K4 | 62.152 | 65.015 | 43.138 | 48.592 | 47.797 |
|
| R | 19.969 | 17.270 | 26.867 | 11.200 | 21.353 |
|
NOP separation and purification
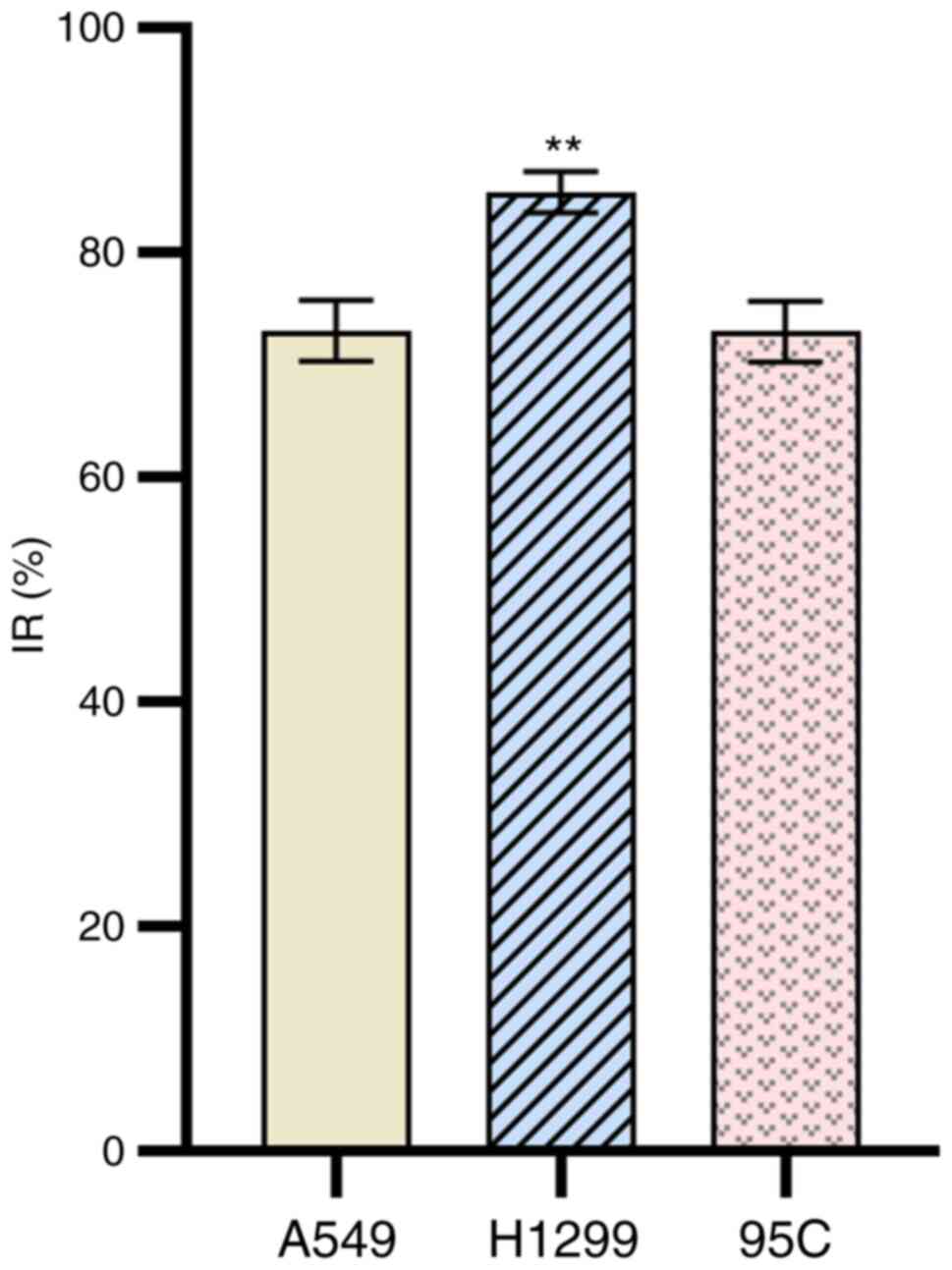
Ultrafiltration of NOP
After enzymatic hydrolysis, enzymatic hydrolysate
<3 kDa was separated from the supernatant using an
ultrafiltration membrane. The components were collected and
freeze-dried, and the proliferation of various cancer cells (A549,
H1299, 95C) was examined using MTT. After a 24-h intervention with
the components at 2,000 mg/l, the IR of the aforementioned cells
was 73.10±2.70%, 85.44±1.81%, and 73.02±2.71%, respectively
(Fig. 2). These findings revealed
that H1299 cells had the best IR, and could be selected for further
screening.
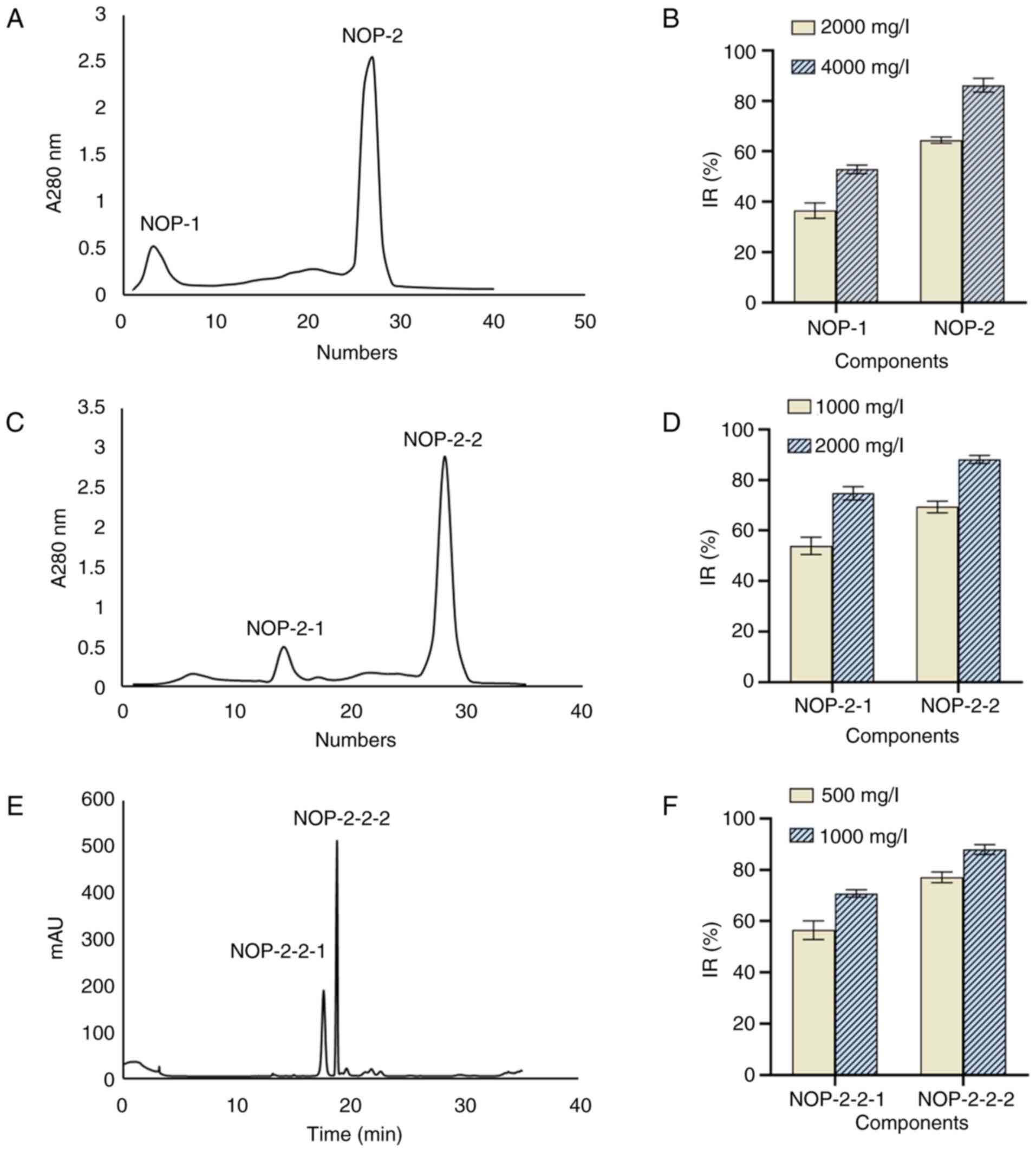
DEAE Sepharose Fast Flow
chromatography
Chromatography using a DEAE Sepharose Fast Flow
column yielded two elution peaks from the enzymatic hydrolysate
<3 kDa, namely NOP-1 and NOP-2 (Fig. 3A). After 24 h of intervention with
these two peptides at 2,000 mg/l, the IR of H1299 cells was
36.50±3.08% and 64.47±1.25%, respectively. At a concentration of
4,000 mg/l, these values were 52.88±1.64% and 86.23±2.71%,
respectively (Fig. 3B).
Therefore, the second peptide was selected for further separation
and purification.
Sephadex G-25 separation
The second peptide yielded in the above step was
freeze-dried and separated using a Sephadex G-25 column to obtain
two elution peaks, namely NOP-2-1 and NOP-2-2 (Fig. 3C). Following treatment of H1299
cells with these two peptides at a concentration of 1,000 mg/l, the
IR was 53.97±3.40% and 69.33±2.27%, respectively. At a
concentration of 2,000 mg/l, these values were 74.77±2.61% and
88.10±1.60%, respectively (Fig.
3D). Therefore, the second peptide with the highest activity
was used for further separation and purification using HPLC.
HPLC separation
After elution and purification through HPLC, two
elution peaks (NOP-2-2-1 and NOP-2-2-2) were noted (Fig. 3E). After treatment of H1299 cells
with these two peptides at a concentration of 500 mg/l, the IR was
56.50±3.64% and 77.10±2.09%, respectively. At a concentration of
1,000 mg/l, these values were 70.83±1.45% and 87.97±1.89%,
respectively (Fig. 3F). Thus, the
second peptide with the highest activity was termed NOP. The
molecular weight of the NOP was determined, and N-terminal amino
acid sequencing was performed.
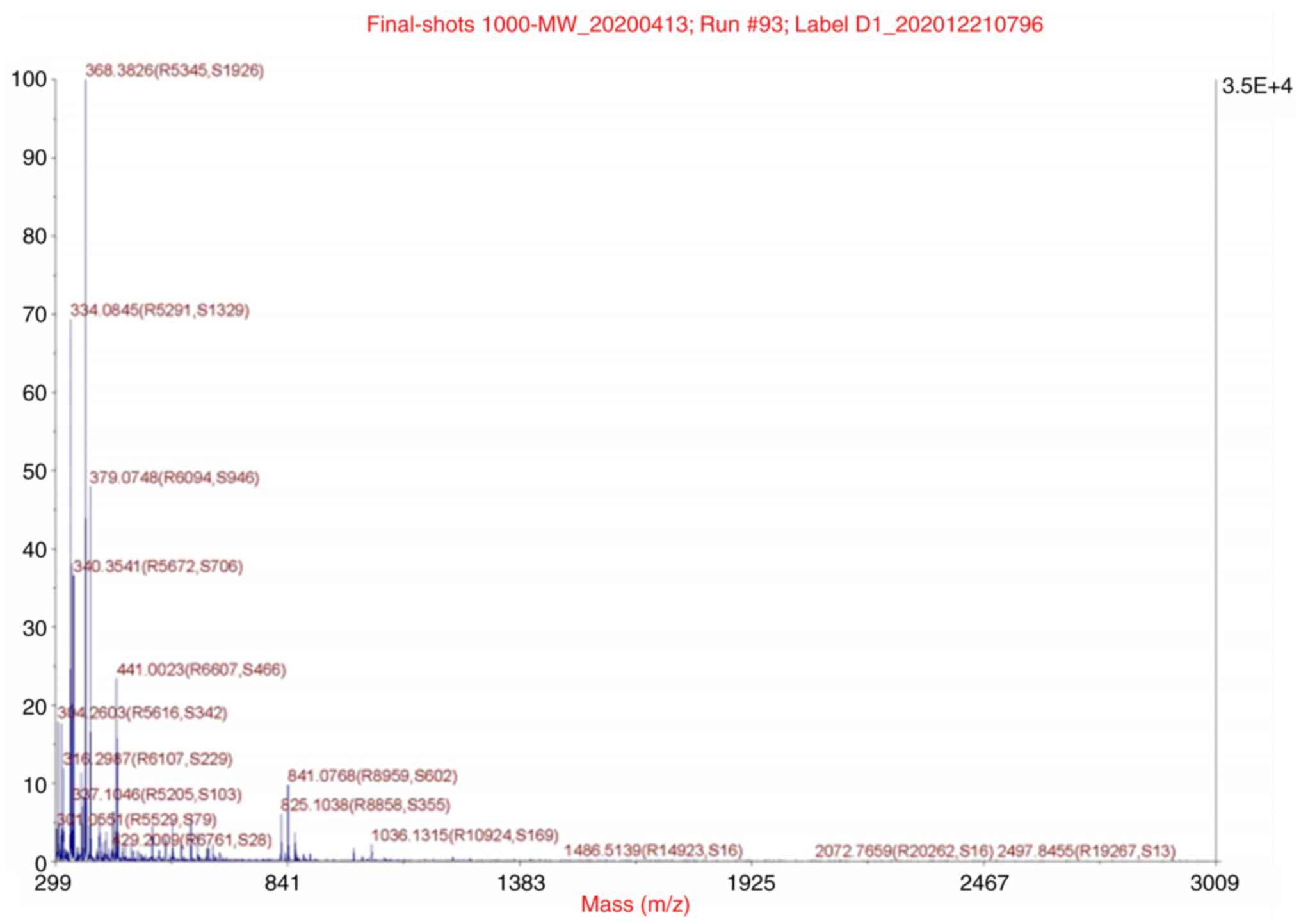
Molecular weight measurement and
N-terminal sequencing of target peptides
The molecular weight was 841.0768 kDa, as identified
by mass spectrometry at APTBIO (Fig.
4). The amino acid sequence was
glutamine-isoleucine-asparagine-glutamine-histidine-leucine
(Gln-Ile-Asn-Gln-His-Leu). The molecular weight of the peptide
obtained by amino acid sequencing was 841.91 kDa; this finding was
completely consistent with the results of molecular weight
determination.
Anti-lung cancer H1299 cell activity
Proliferation of H1299 cells
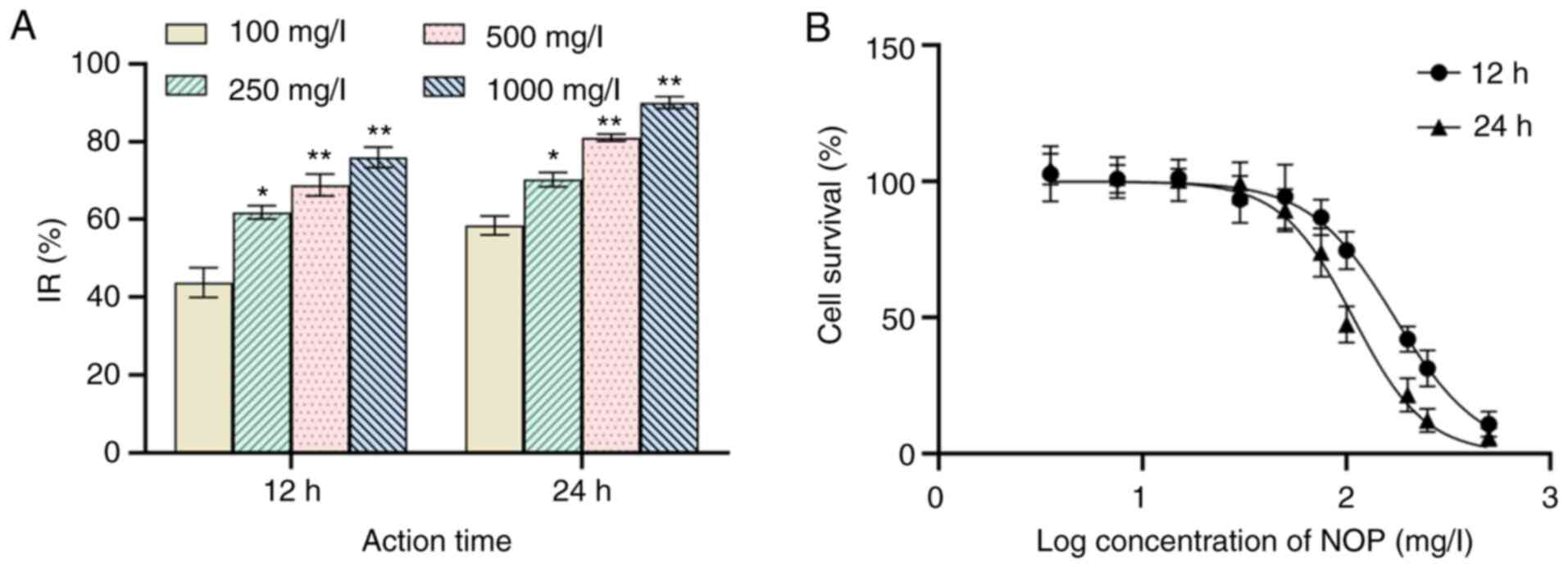
After 12 h of treatment with 100 mg/l NOP, the IR of
H1299 was 43.77±3.84%. At a concentration of 1,000 mg/l, this value
was 76.00±2.67%. After 24 h of intervention with 100 mg/l and 1,000
mg/l NOP, this value was 58.47±2.44% and 90.07±1.56%, respectively
(Fig. 5A). The 12- and 24-h
IC50 values were 172.5 and 107.8 mg/l, respectively
(Fig. 5B). Therefore, compared
with control, the IR was significantly elevated in parallel with
the increase in mass concentration and action time (P<0.01).
H&E staining results
H1299 cells grew well in the normal group and were
tightly arranged, exhibiting good morphology. The cytoplasm was
uniformly stained, the nucleus was in uniform size, and numerous
nucleoli were detected (Fig. 6A).
Following 24 h of treatment with NOP at low concentration (250
mg/l; Fig. 6B), the number of
H1299 cells per field of view decreased, and numerous vacuoles
appeared in the cytoplasm. The number of nucleoli decreased, and
the intercellular space appeared. The cell contour was blurred, the
area of plasma membrane was enlarged, the cytoplasm was vacuolated,
the nucleus appeared nuclear pyknosis and the number of nucleoli
was reduced (500 mg/l; Fig. 6C),
After 24 h of treatment with NOP at a high concentration (1,000
mg/l; Fig. 6D), the cell contour
was blurred, with irregular shape and numerous protrusions.
Morphological changes, such as chromatin condensation and pyknosis,
were also observed (Fig. 6D).
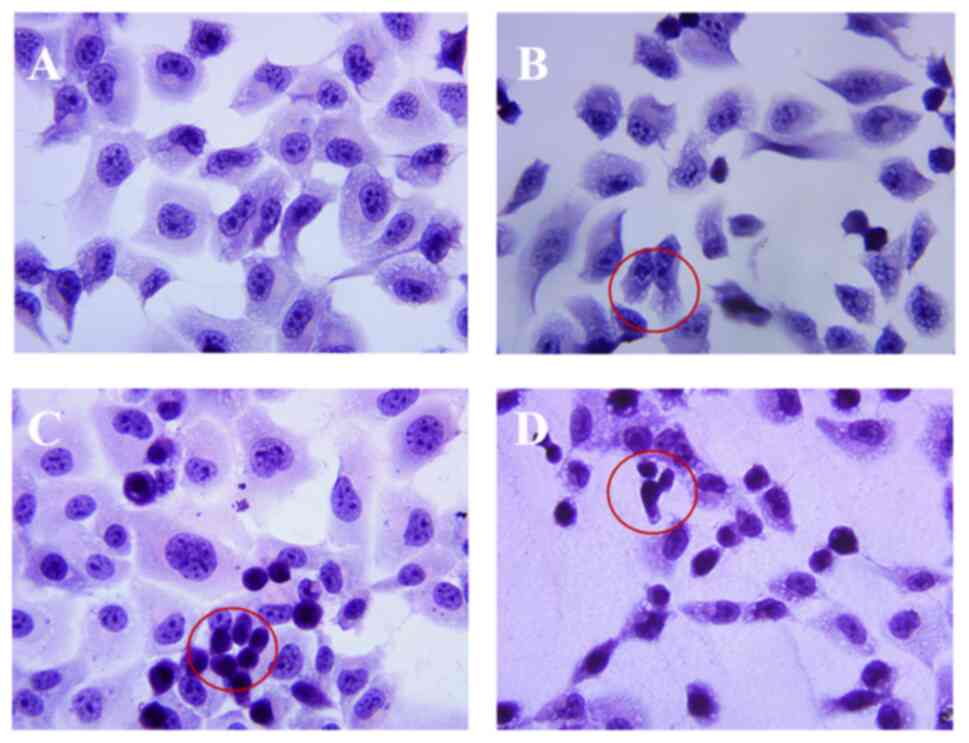 | Figure 6.H&E staining images of H1299
cells after treatment with NOP (×400). (A) The negative control
group (normal H1299 cells) grew well, had full morphology, clear
borders, and a large number of nucleoli. (B) Treatment of H1299
cells with 250 mg/l NOP. The cell morphology was irregular, the
intercellular space was enlarged, and vacuoles were detected in the
cytoplasm (red circles). (C) Treatment of H1299 cells with 500 mg/l
NOP. The cell morphology was irregular, the cell outline was
blurred, the plasma membrane area was enlarged, vacuoles were
detected in the cytoplasm, pyknosis was observed in the nucleus,
and the number of nucleoli was decreased (red circles). (D)
Treatment of H1299 cells with 1,000 mg/l NOP. The cell morphology
was irregular, the cells shrank and became rounder and smaller, the
volume was decreased, and pyknosis was observed in the nucleus (red
circles). H&E, hematoxylin and eosin; NOP, Nereid
oligopeptide. |
Cell ultrastructural changes
Under the transmission electron microscope, H1299
cells in the control group showed dense microvilli on the surface
and had rich cytoplasm without swelling. Moreover, the mitochondria
were relatively intact, there were no obvious effects on the
endoplasmic reticulum, and the plasma and nuclear membranes were
relatively intact (Fig. 7A and
B). After intervention with 1,000 mg/l NOP, the cells were
almost round in shape without microvilli, but with obvious
cytoplasmic swelling. In addition, the cell membrane was disrupted,
swelling of the nuclear membrane was observed, the endoplasmic
reticulum was severely vacuolated, and a large number of vacuoles
appeared in the cytoplasm (Fig.
7C). After treatment with NOP at a high concentration (1,000
mg/l; Fig. 7D), H1299 cells had
broken plasma and nuclear membranes, a shrunken nucleus,
heterochromatin aggregation, extended smooth endoplasmic reticulum,
and swollen mitochondria. More obvious changes were observed as the
concentration of NOP increased. Under the transmission electron
microscope, H1299 cells exhibited apoptotic characteristics after
24 h of exposure to NOP.
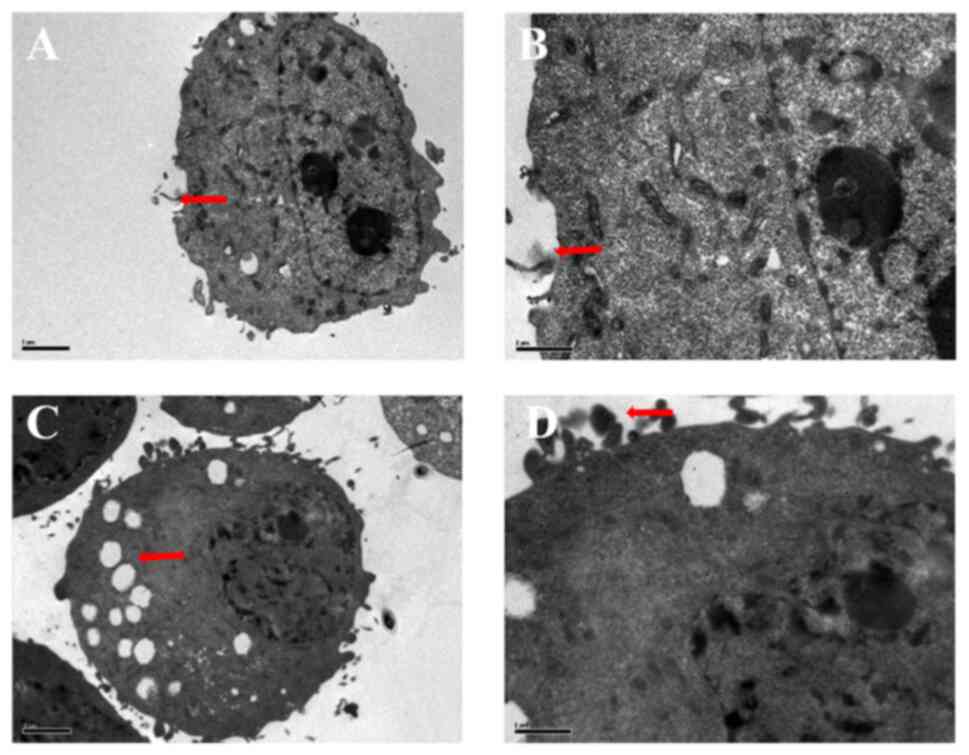 | Figure 7.Ultrastructure changes in H1299
cells. (A) Magnification of the ultrastructure of normal H1299
cells (×2,500), revealing microvilli around them, less
heterochromatin, and one nucleolus (red arrow). (B) Magnification
of the ultrastructure of normal H1299 cells (×5,900), demonstrating
more obvious surrounding slender microvilli (red arrow). (C)
Following treatment of H1299 cells with 1,000 mg/l NOP for 24 h,
their ultrastructure was visualized through magnification (×2,500).
The nuclear volume was reduced, the morphology was abnormal,
heterochromatin edge aggregation was obvious, numerous vacuoles
were detected in the cells, and the cell microvilli disappeared
(red arrow). (D) Magnification of the cell ultrastructure (×5,900)
after treatment with 1,000 mg/l NOP for 24 h showed an apparent
increase in apoptotic bodies (red arrow). NOP, Nereid
oligopeptide. |
Detection of the apoptotic rate of
cells by flow cytometry
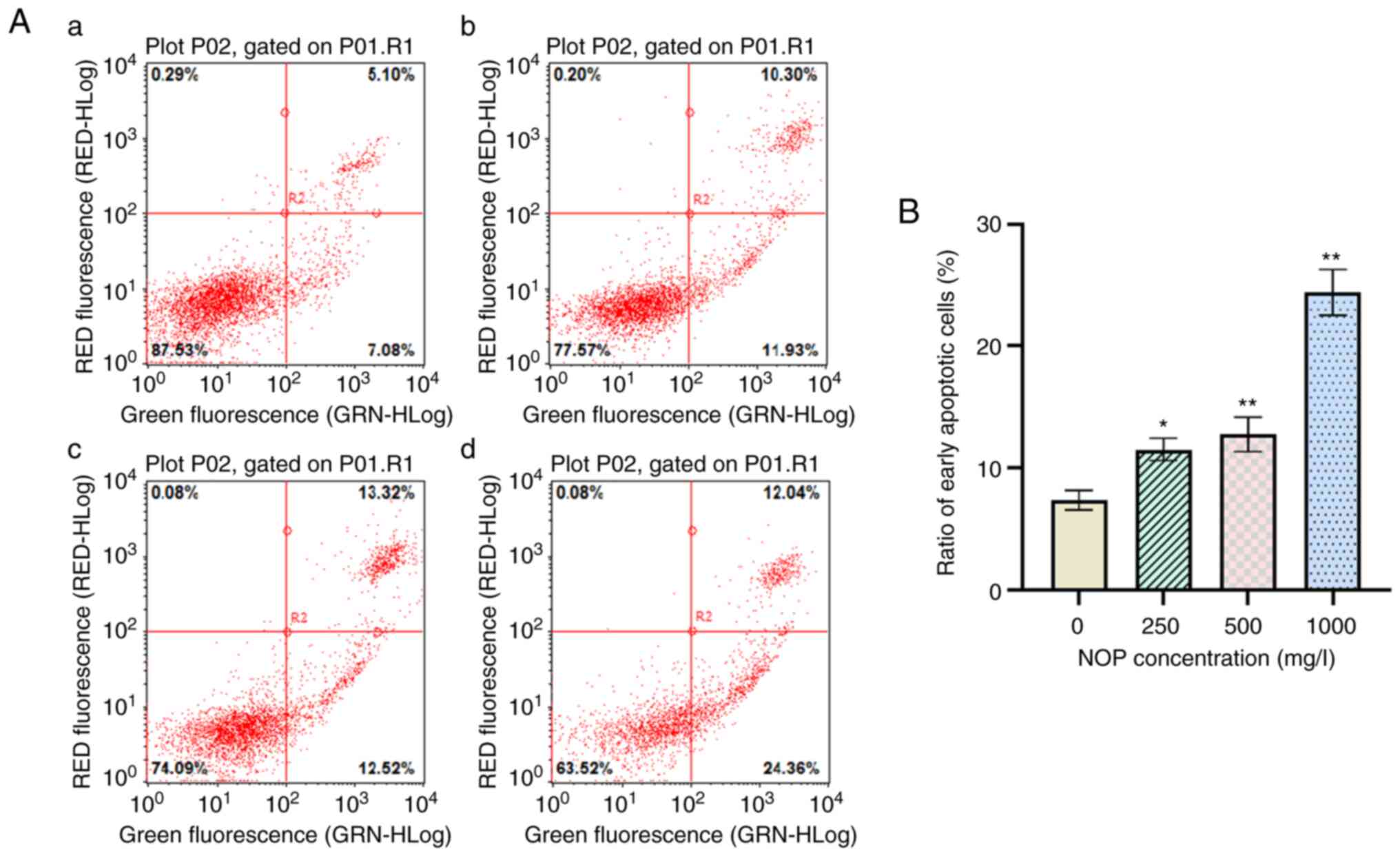
In order to quantitatively measure the NOP-induced
apoptosis in H1299 cells, Annexin V-FITC and PI double staining was
used to determine the number of apoptotic cells. As revealed in
Fig. 8, for the control, the
percentage of Annexin V-FITC-stained H1299 cells was 7.08±0.65%.
After 24 h of exposure to 250, 500, and 1,000 mg/l NOP, the
percentage of apoptotic cells increased to 11.93±0.75%,
12.52±1.16%, and 24.36±1.53%, respectively (Fig. 8). The apoptotic effect on H1299
cells was significantly enhanced with the increasing concentration
of NOP compared with that observed in the control group. Therefore,
NOP was able to effectively induce apoptosis in H1299 cells.
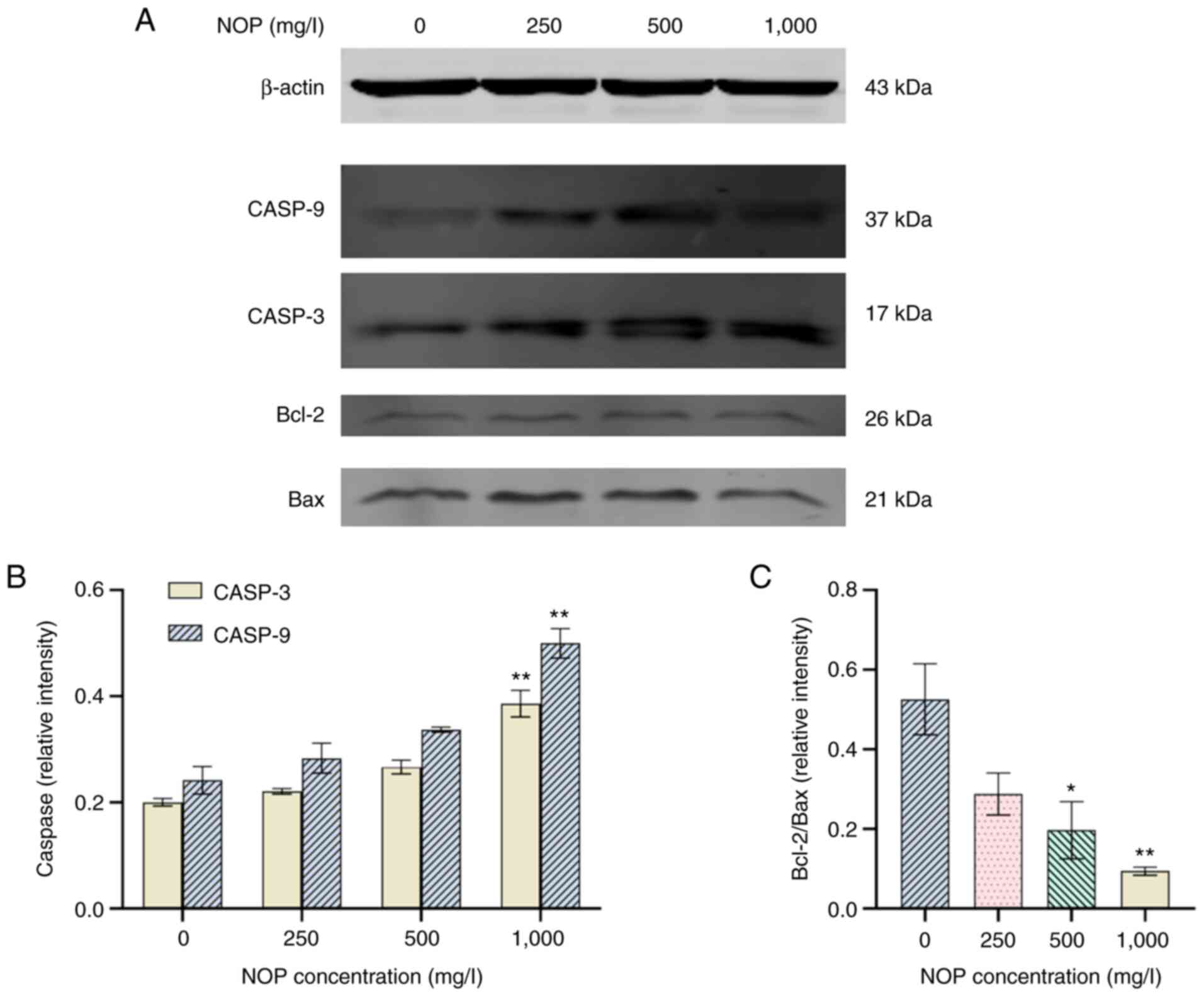
Western blotting
The protein bands of Bcl-2, Bax, cleaved-CASP9, and
cleaved-CASP3 in samples obtained from H1299 cells treated with
different concentrations of NOP for 24 h are revealed in Fig. 9A. The protein expression levels of
each gene are presented in Fig. 9B
and C. The results showed that the levels of Bcl-2 protein were
decreased, and the ratio of Bcl-2/Bax was significantly decreased
in the 1,000 mg/l NOP group compared with the control group
(P<0.01). Moreover, the levels of cleaved-CASP9 and
cleaved-CASP3 were significantly upregulated, while the ratio of
Bcl-2/Bax was significantly reduced by 24.72% compared with the
control group. The relative expression of cleaved-CASP9 and
cleaved-CASP3 was 2.55- and 1.71-fold higher than that noted in the
control group, respectively. Therefore, at a certain concentration
range, NOP can significantly upregulate the protein levels of
cleaved-CASP9, cleaved-CASP3, and Bax, and significantly
downregulate those of Bcl-2.
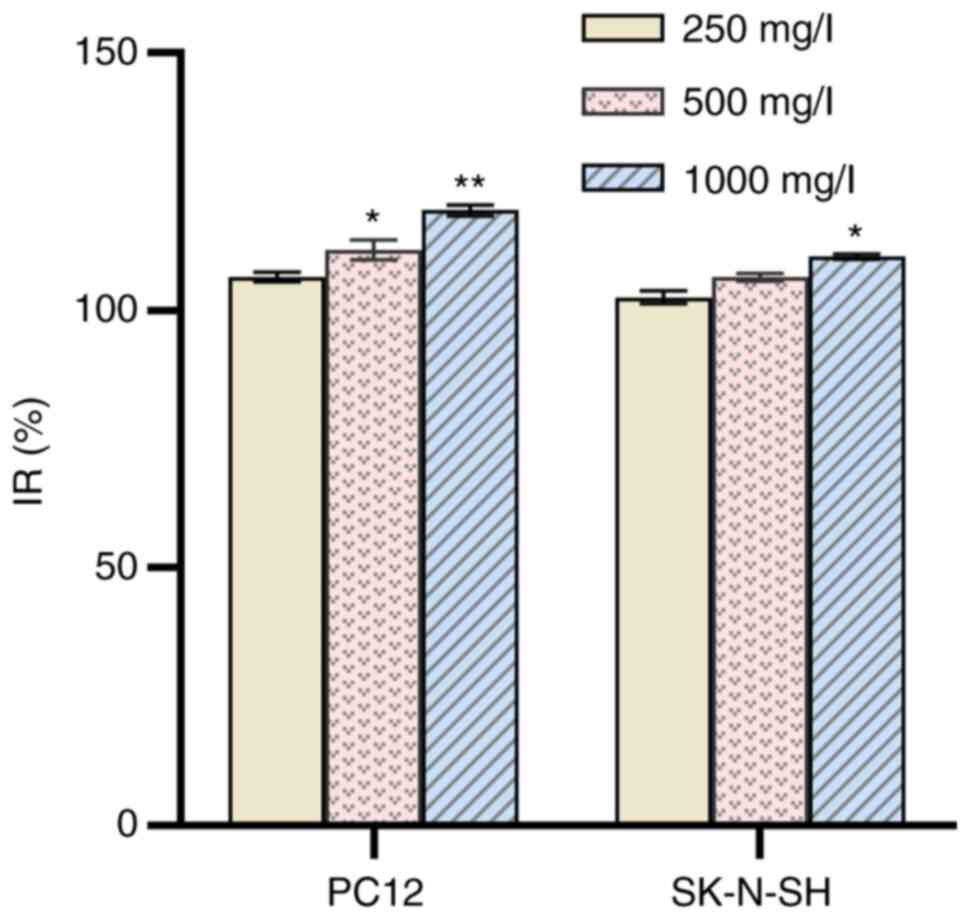
Activity on the proliferation of PC12
and SK-N-SH cells
Following treatment of PC12 and SK-N-SH cells with
250 mg/l NOP for 24 h, the IR was 106.52±0.92% and 102.53±1.24%,
respectively. At a concentration of 1,000 mg/l, these values were
119.38±1.10% and 110.41±0.52%, respectively (Fig. 10). Higher mass concentration
indicated higher IR. The differences between the control and NOP
groups were statistically significant (P<0.05). These findings
indicate that NOP did not exert a toxic effect on PC12 and SK-N-SH
cells; in contrast, it promoted cell proliferation.
Discussion
Bioactive peptides are composed of multiple amino
acids linked by peptide bonds (18). They exert several beneficial
effects on the human body (e.g., anti-swelling, analgesic,
immune-modulatory, anti-viral, and anti-hypertensive). Currently,
there are three main methods utilized for the extraction of
bioactive peptides, namely direct extraction, synthesis, and
hydrolysis. The direct extraction method is only suitable for
laboratory extraction of some natural bioactive peptides with low
content and complex structure in animals and plants. Although it is
easy to obtain pure bioactive peptides using the synthetic method,
its large-scale use is limited by the high cost and numerous
adverse reactions. Hydrolysis can be divided into two types, namely
chemical and enzymatic. The latter is a controllable process, in
which the proteins are hydrolyzed using appropriate proteases
(19,20). It is characterized by mild
conditions, a controllable process, high yield, and good safety.
Enzymatic hydrolysis has been used to extract peptides from marine
organisms, which have demonstrated favorable antitumor activity.
For example, Zhou (21) used the
compound enzymatic hydrolysis method to extract fucoidan that was
deproteinized using the Sevag method. In their study, homogeneous
components with reducing ability and quenching lipid peroxidation
ability were obtained through chromatography using the DEAE
Sepharose Fast Flow ion exchange column and Sephadex-G150 column.
Zhu et al (22) used a
two-step enzymatic hydrolysis with papain and trypsin to obtain
polysaccharides from Urechis unicinctus, reporting a yield
of 6.2%. Visceral polysaccharides from Urechis unicinctus
are glycosaminoglycan-like, with a molecular weight of
approximately 4.1×103 kDa. A preliminary study
investigating in vitro antioxidant activity revealed that
visceral polysaccharides possess significant lipid peroxide
scavenging activity, with a half scavenging mass concentration of
2.47 mg/ml (22). Huang et
al (23) and Ding et
al (24) used enzymatic
hydrolysis to extract a polypeptide from cuttlefish ink. They found
that this cuttlefish polypeptide could inhibit prostate cancer
cells DU-145, PC-3, and LNCaP in a time- and dose-dependent manner.
Therefore, in the present study, trypsin, papain, alkaline
protease, neutral protease, and pepsin were used. Considering the
IR as an indicator, alkaline protease was determined to be the most
effective protease for NOP. According to the results of the single
factor test, the enzymatic hydrolysis process was optimized through
an L16 (45) orthogonal test. The optimal conditions for
the extraction of NOP were as follows: Enzymatic hydrolysis at 50°C
and pH 10 for 6 h, a material-to-liquid ratio of 1:1, and addition
of 400 U/g enzyme. The purified oligopeptide was termed NOP, and
its amino acid sequence is Gln-Ile-Asn-Gln-His-Leu.
The MTT assay is a colorimetric assay for measuring
cell survival and proliferation, involving the conversion of
yellow-dyed MTT to bluish-purple formazan by the action of
mitochondrial reductase. However, this conversion does not occur in
dead cells (25). The MTT method
is often used to detect inhibitory effects on cell proliferation.
Ma et al (26) applied the
MTT method to detect the proliferation of human glomerular
mesangial cells treated with different concentrations of
Lumbricus's active ingredients. They found that the middle dose (40
µg/ml) exerted the best effect. Using the MTT assay, Chen et
al (27) reported that
phycoerythrin had an inhibitory effect on human cervical cancer
HeLa cells in a dose-effect manner. The results of flow cytometry
showed that phycoerythrin arrested the cell cycle of HeLa cells
from the G2/M to the S phase, thereby playing an inhibitory role.
In the present study, the MTT method was used to detect the
inhibitory effect of NOP on the proliferation of H1299 cells. It
was determined that NOP could inhibit the activity of H1299 cells
in a time- and dose-dependent manner. In other words, the IR of
cells was significantly increased in parallel with the increase in
the mass concentration of the NOP and time extension. The discovery
of new anticancer drugs from marine organisms, including peptides,
glycosaminoglycans, and macrolides, is a new goal of anticancer
drug research. Marine peptides are characterized by low molecular
weight, high activity, and low toxicity, and have become a research
hotspot worldwide. In 1987, Pettit et al (28) and Cao and Song (29) isolated dolastatin-10, a small
linear peptide from the sea hare. In a Phase II clinical trial, the
combination of dolastatin-10 and other anticancer drugs exerted an
obvious anticancer effect. Its derivatives, such as TZT-1027
(auristatin PE) and auristatin PYE, also showed significant
clinical therapeutic effects (30). Chi et al (31) isolated two polypeptides, namely
BCP-A (Trp-Pro-Pro) and BCP-B (GlnPro), from Tegillarca
granosa Linnaeus. BCP-A exhibited a favorable effect on the IR
of PC-3, DU-145, H-1299, and HeLa cells in a time- and
dose-dependent manner. Following treatment with 15 mg/ml BCP-A, the
early apoptotic rate of PC-3 cells increased from 11.22 to 22.78%.
Yao et al (32) extracted
a type of polypeptide from Tegillarca granosa Linnaeus,
which induced cell cycle arrest at the G2/M and G0/G1 phases in
A549 and Ketr-3 cells, respectively; these polypeptides could
significantly inhibit transplanted tumors in mice. Wang et
al (33) extracted and
isolated the polypeptide Mere15 from clams through ion exchange and
gel filtration. Mere15 could inhibit the growth of A549 cells in a
dose- and time-dependent manner. With the increase in treatment
time, cell cycle arrest at the G2/M phase was induced in A549
cells; this effect was related to the inhibition of tubulin
polymerization. In the present study, the MTT method was used to
detect the IR of H1299 cells treated with NOP. It was determined
that H1299 cells treated with 250 mg/l NOP for 12 h exhibited
apoptotic characteristics. After treatment with 1,000 mg/l NOP for
24 h, the IR of H1299 cells was increased to 90.07%.
The morphological changes strongly indicated the
occurrence of apoptosis, which corresponded with a previous study
(34). The characteristic
morphology of early apoptotic cells could be directly detected
using H&E staining under a transmission electron microscope.
H1299 cells exhibited obvious cell morphological changes after
intervention with NOP, such as increased intercellular space,
irregular shape, decreased size, vacuoles, and apoptotic bodies in
some cells. These changes became more detectable as the
concentration increased. Overall, NOP extracted by alkaline
protease can effectively inhibit the proliferation of H1299 cells
in vitro. In addition, H1299 cells showed obvious apoptotic
characteristics after 24 h of intervention with NOP. Thus, Annexin
V-FITC was used to detect cells undergoing apoptosis, and PI was
used to determine the number of necrotic cells. Flow cytometry
revealed that the percentage of apoptotic cells was significantly
increased in parallel with the increasing concentration of NOP. The
results of the present study indicated that NOP exhibits anti-lung
cancer activity through the induction of apoptosis.
Apoptosis refers to programmed cell death that is
controlled by genes; it includes two classical pathways, namely the
exogenous and endogenous (35,36). The endogenous apoptotic pathway is
the main process of apoptosis. In the present study, the expression
of related proteins, including intracellular Bax and Bcl-2, was
detected using western blotting assays. The aim of this analysis
was to determine whether NOP induces apoptosis in H1299 cells
through the endogenous pathway. The Bcl-2 family plays a vital role
in regulating the initiation of the endogenous pathway. During
apoptosis, Bax and other pro-apoptotic proteins are translocated
from the cytoplasm to the outer mitochondrial membrane, altering
the permeability of the mitochondrial membrane. Bcl-2 and Bax
regulate the release of cytochrome c (Cyt-C) by forming
homodimers or heterodimers (37–39), and the ratio of Bcl-2/Bax is used
to indicate the regulatory effect of apoptosis. Specifically, an
increase in the Bcl-2/Bax ratio denotes inhibition of apoptosis. In
contrast, a decrease in the Bcl-2/Bax ratio indicates promotion of
apoptosis. The findings of the present study revealed that
treatment with 250, 500, and 1,000 mg/l NOP significantly increased
the protein expression of Bax and decreased that of Bcl-2.
Moreover, the Bcl-2/Bax ratio was decreased. These effects promote
the release of Cyt-C and initiate the endogenous apoptotic pathway
from the upstream. Changes in the intracellular levels of activated
CASP9 and CASP3 were also detected. In the mid and late stages of
endogenous apoptosis, Cyt-C that enters the cytoplasm is combined
with the WD repeat sequence at the carboxyl end of apoptotic
protease-activating factor 1 (Apaf-1) in a 2:1 ratio to form an
Apaf-1/Cyt-C complex. This complex can change the conformation of
Apaf-1, inducing the formation of apoptotic bodies, triggering
caspase cascade reactions, and eventually resulting in cell
apoptosis (40,41). The present study showed that the
protein content of activated CASP9 and CASP3 was significantly
increased in parallel with the increase in the concentration of
NOP. This observation indicated that activated CASP9 induces the
downstream activated CASP3. CASP3 is the key executor in the
downstream process of apoptosis. Activated CASP3 can degrade poly
ADP-ribose polymerase, activate endonuclease, and cleave DNA
strands between nucleosomes by hydrolyzation; these effects lead to
DNA segmentation that is unique to apoptosis (42). The results of the present study
showed that the protein levels of cleaved-CASP9 and cleaved-CASP3
were significantly increased. This indicates that NOP can activate
the key downstream caspases via the endogenous apoptotic pathway,
thereby inducing apoptosis in H1299 cells.
In summary, in the present study, alkaline protease
was selected to enzymatically hydrolyze a Nereid homogenate at 50°C
and pH 10 for 6 h. The material-to-liquid ratio was 1:1 and the
amount of enzyme added was 400 U/g. An NOP with a molecular weight
of 847 kDa was obtained after separation and purification through
ultrafiltration, ion exchange chromatography, gel filtration
chromatography, and HPLC. The amino acid sequence of the NOP was
Gln-Ile-Asn-Gln-His-Leu. Collectively, the present findings
indicated that NOP could inhibit the proliferation of H1299 cells
in a dose- and time-dependent manner. Apoptosis in H1299 cells may
be triggered by downregulation of Bcl-2 protein, upregulation of
Bax protein, and stimulation of the cascade reaction of the caspase
family. NOP did not exert a toxic effect on PC12 and SK-N-SH cells;
in contrast, it promoted cell proliferation. The limitation of the
present study is that the yield of separated and purified samples
was relatively low, and it is hoped to improve the yield of samples
in the future. In subsequent studies, the precise action pathway
and specific action compounds will be further studied.
Acknowledgements
Not applicable.
Funding
This work was supported by the Youth Fund of Zhejiang Academy of
Medical Sciences (grant no. C51912Q-04).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS conceived and designed the experiments. GZ, HL,
SL, ML and LT performed the experiments and statistical analysis of
the data. GZ and HL contributed equally to this study and share
first authorship. All authors contributed to the writing of the
manuscript and read and approved the final manuscript. LS and GZ
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NOP
|
Nereid oligopeptide
|
|
CASP3
|
caspase-3
|
|
CASP9
|
caspase-9
|
|
IR
|
inhibition rate
|
References
|
1
|
Guo Q: Current situation of medical
treatment of non-small cell lung cancer. Chin J Cancer Prev Treat.
16:721–725. 2009.
|
|
2
|
Ahmed S, Mirzaei H, Aschner M, Khan A,
Al-Harrasi A and Khan H: Marine peptides in breast cancer:
Therapeutic and mechanistic understanding. Biomed Pharmacother.
142:1120382021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi F, Yan X, Cheong KL and Liu Y:
Extraction, purification, and characterization of polysaccharides
from marine algae gracilaria lemaneiformis with anti-tumor
activity. Process Biochem. 73:197–203. 2018. View Article : Google Scholar
|
|
4
|
Huang ZZ, Du X, Ma CD, Zhang RR, Gong WL
and Liu F: Identification of antitumor active constituents in
polygonatum sibiricum flower by UPLC-Q-TOF-MSE and
network pharmacology. ACS Omega. 5:29755–29764. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Margolin K, Longmate J, Synold TW, Gandara
DR, Weber J, Gonzalez R, Johansen MJ, Newman R, Baratta T and
Doroshow JH: Dolastatin-10 in metastatic melanoma: A phase II and
pharmokinetic trial of the California cancer consortium. Invest New
Drugs. 19:335–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takahashi T, Furukawa Y, Muneoka Y,
Matsushima O, Ikeda T, Fujita T, Minakata H and Nomoto K: Isolation
and characterization of four novel bioactive peptides from a
polychaete annelid, Perinereis vancaurica. Comp Biochem
Physiol C Pharmacol Toxicol Endocrinol. 110:297–304. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
LI Q: Purification, characterization and
pharmacodynamic activity of proteases and its isoenzyme from
Nereis virens (unpublished PhD thesis). Jilin University;
2008
|
|
8
|
Bai R, Li Q, Liu J, Jiang X and Hong M:
Effects of Nereis virens proteinase on platelet aggregation
and hemorheology. Chin J New Drugs. 18:930–933. 2009.(In
Chinese).
|
|
9
|
Bo Q, Ge X, Cui J, Jiang X, Liu J and Hong
M: Effects of acidic serine protease ASPNJ on inhibition and injury
of leukemia cell K562. Chin J Biochem Pharm. 33:736–739. 2012.(In
Chinese).
|
|
10
|
Ge X, Bo Q, Hong X, Cui J, Jiang X, Hong M
and Liu J: A novel acidic serine protease, ASPNJ inhibits
proliferation, induces apoptosis and enhances chemo-susceptibility
of acute promyelocytic leukemia cell. Leuk Res. 37:1697–1703. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McNair K, Forrest CM, Vincenten M,
Darlington LG and Stone TW: Serine protease modulation of
dependence receptors and EMT protein expression. Cancer Biol Ther.
20:349–367. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang G, Yang M, Ding G, Huang F and Zhao
Y: Study on the mechanism of apoptosis induced by SPC-A-1 cells in
human lung cancer. Mod Food Technol. 31:6–11. 2015.
|
|
13
|
Yoon SK, Sung SK, Lee DH and Kim HW:
Tissue inhibitor of metalloproteinase-1 (TIMP-1) and IL-23 induced
by polysaccharide of the black hoof medicinal mushroom, phellinus
linteus (Agaricomycetes). Int J Med Mushrooms. 19:213–223. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan W, Liu X, Ge F, Han J and Zheng T:
Perinerin, a novel antimicrobial peptide purified from the clamworm
Perinereis aibuhitensis grube and its partial
characterization. J Biochem. 135:297–304. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Luo Y, Qi B, Luo J and Wan Y:
Improving the hydrolysis efficiency of soy sauce residue using
ultrasonic probe-assisted enzymolysis technology. Ultrason
Sonochem. 35:351–358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang Z, Xu Y and Su Y: Preparation
process of active enzymolysis polypeptides from seahorse bone meal.
Food Sci Nutr. 2:490–499. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qu W, Ma H, Jia J, He R, Luo L and Pan Z:
Enzymolysis kinetics and activities of ACE inhibitory peptides from
wheat germ protein prepared with SFP ultrasound-assisted
processing. Ultrason Sonochem. 19:1021–1026. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitts DD and Weiler K: Bioactive proteins
and peptides from food sources. Applications of bioprocesses used
in isolation and recovery. Curr Pharm Des. 9:1309–1023. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian B, Zhao X, Yang Y and Tian C:
Antioxidant and anti-inflammatory peptide fraction from oyster soft
tissue by enzymatic hydrolysis. Food Sci Nutr. 8:3947–3956. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maqsoudlou A, Sadeghi MA, Mora L,
Mohebodini H, Ghorbani M and Toldrá F: Controlled enzymatic
hydrolysis of pollen protein as promising tool for production of
potential bioactive peptides. J Food Biochem. 43:e128192019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou J: Fucoidan extraction, purification,
structure analysis and antioxidant research (unpublished PhD
thesis). Hangzhou: Zhejiang University; 2006
|
|
22
|
Zhu S, Chen M, Niu Q, Li T, Qu Y and Chen
Y: Physico-chemical properties and structure analysis of viscera
polysaccharide of urechis unicinctus and its lipid peroxidation
inhibition activity. Food Sci. 36:67–71. 2015.(In Chinese).
|
|
23
|
Huang F, Yang Z, Yu D, Wang J, Li R and
Ding G: Sepia ink oligopeptide induces apoptosis in prostate cancer
cell lines via caspase-3 activation and elevation of Bax/Bcl-2
ratio. Mar Drugs. 10:2153–2165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding G, Huang F, Yang Z, Yu D and Yang Y:
Anticancer activity of an oligopeptide isolated from hydrolysates
of sepia ink. Chin J Nat Med. 9:151–155. 2011.
|
|
25
|
Grela E, Kozłowska J and Grabowiecka A:
Current methodology of MTT assay in bacteria-a review. Acta
Histochem. 120:303–311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma Y, Zhou B, Sun X, Zhang L, Song L and
Xiao H: The effect of diosone active component on the proliferation
of glomerular mesangial cells was determined by MTT assay. Tradit
Chin Med Inf. 27:34–36. 2010.
|
|
27
|
Chen M, Ge A, Cui P and Liao Z: Study on
inhibition effects of phycoerythrin from Gracilaria
lemaneiformis on Hela cells and its mechanism. Food Sci.
28:549–552. 2007.(In Chinese).
|
|
28
|
Pettit GR, Kamano Y, Herald CL, Tuinman
AA, Boettner FE, Kizu H, Schmidt JM, Baczynskyj L, Tomer KB and
Bontems RJ: The isolation and structure of a remarkable marine
animal antineoplastic constituent: Dolastatin 10. J Am Chem Soc.
109:6883–6885. 1987. View Article : Google Scholar
|
|
29
|
Cao W and Song J: Progress in the
antitumor polypeptide Aplysia toxin 10 and its derivatives of
marine organisms. Grad Med J. 24:1208–1211. 2011.(In Chinese).
|
|
30
|
Shnyder SD, Cooper PA, Millington NJ,
Pettit GR and Bibby MC: Auristatin PYE, a novel synthetic
derivative of dolastatin 10, is highly effective in human colon
tumour models. Int J Oncol. 31:353–360. 2007.PubMed/NCBI
|
|
31
|
Chi CF, Hu FY, Wang B, Li T and Ding GF:
Antioxidant and anticancer peptides from the protein hydrolysate of
blood clam (Tegillarcagranosa) muscle. J Funct Foods. 15:301–313.
2015. View Article : Google Scholar
|
|
32
|
Yao R Chu X, Chen S, Wang C and Liu W:
Experimental study on the antitumor effects of polypeptides. Chin
Pharm J. 41:868–870. 2006.
|
|
33
|
Wang C, Liu M, Wang F and Lin X:
Growth-inhibition effects of a novel anti-tumor polypeptide from
Meretrix meretrix Linnaeus associated with tubulin
polymerization. Chin J Biochem Pharm. 33:225–228. 2012.(In
Chinese).
|
|
34
|
Zhu X: Progress in studying the
morphological characteristics and molecular mechanisms of cell
apoptosis. Chin J Gerontol. 31:2595–2597. 2011.(In Chinese).
|
|
35
|
Chio S, Lew KL, Xiao H, Herman-Antosiewicz
A, Xiao D, Brown CK and Singh SV: D,L-Sulforaphane-induced cell
death in human prostate cancer cells is regulated by inhibitor of
apoptosis family proteins and Apaf-1. Carcinogenesis. 28:151–162.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Clark CS and Maurelli AT: Shigella
flexneri inhibits staurosporine-induced apoptosis in epithelial
cells. Infect Immun. 75:2531–2539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen ZQ, Jie X and Mo ZN: Curcumin
inhibits growth, induces G1 arrest and apoptosis on human prostatic
stromal cells by regulating Bcl-2/Bax. Zhongguo Zhong Yao Za Zhi.
33:2022–2025. 2008.(In Chinese). PubMed/NCBI
|
|
38
|
Liu W, Hμang XF, Qi Q, Dai QS, Yang L, Nie
FF, Lu N, Gong DD, Kong LY and Guo QL: Asparanin A induces G(2)/M
cell cycle arrest and apoptosis in human hepatocellular carcinoma
HepG2 cells. Biochem Biophys Res Commun. 381:700–705. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu X, Liu Y, Wang L, He J, Zhang H, Chen
X, Li Y, Yang J and Tao J: Gambogic acid induces apoptosis by
regulating the expression of Bax and Bcl-2 and enhancing caspase-3
activity in human malignant melanoma A375 cells. Int J Dermatol.
48:186–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bhattacharjee M, Acharya S, Ghosh A,
Sarkar P, Chatterjee S, Kumar P and Chaudhuri S: Bax and Bid act in
synergy to bring about T11TS-mediated glioma apoptosis via the
release of mitochondrial cytochrome c and subsequent caspase
activation. Int Immunol. 20:1489–1505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu HR, Peng XD, He HB, Wang YH, Li Y, He
GX, Liu YL, Li YL and Zeng CJ: Antiproliferative activity of the
total saponin of solanum lyratum thunb in Hela cells by inducing
apoptosis. Pharmazie. 63:836–842. 2008.PubMed/NCBI
|
|
42
|
Bressenot A, Marchal S, Bezdetnaya L,
Garrier J, Guillemin F and Plénat F: Assessment of apoptosis by
immunohistochemistry to active caspase-3, active caspase-7, or
cleaved PARP in monolayer cells and spheroid and subcutaneous
xenografts of human carcinoma. J Histochem Cytochem. 57:289–300.
2009. View Article : Google Scholar : PubMed/NCBI
|