Introduction
A common disorder caused by the imbalance of
hormones, polycystic ovary syndrome (PCOS) can affect ≤20% of women
of reproductive age (1). PCOS is
often associated with adverse effects on reproductive function in
women, including infertility induced by irregular cycles of
anovulation and recurrent pregnancy loss (1–3).
Endometrial dysfunction has been attributed to the failure of
reproduction in patients with PCOS (4). Reduced glucose transporter type 4
insulin-responsive (GLUT4) expression in the endometrium has been
observed in women with PCOS (5–7).
Endometrial hyperplasia (EH) can be triggered by exposure to an
excessive level of estrogen in obese female patients as well as in
female patients receiving estrogen replacement therapy (8). At present, it is hypothesized that
the onset of cellular atypia increases the risk of uterine or
endometrial cancer (EC) (9). As a
premalignant disorder, EH can become invasive in ≤10% of patients
(10). If patients with PCOS are
not treated properly by utilizing contraceptive steroids, >30%
of patients with PCOS can eventually develop EH (11). In past research, nearly 2% of
patients with PCOS eventually suffered from EC (12).
A type of long RNA transcript with no protein-coding
ability, long non-coding (lnc)RNAs are speculated to have a diverse
range of biological roles, including the regulation of target gene
transcription in an epigenetic manner (13,14). Furthermore, lncRNAs can act as key
regulators in inflammatory reactions and the development of a wide
range of inflammatory disorders (15,16). On the other hand, microRNAs
(miRNAs/miRs), a type of short RNA transcript with no
protein-coding ability, participate in the control of a wide range
of biological activities, including cellular development, survival,
proliferation, tumorigenesis and inflammatory responses (17).
Metformin (MET) has clinical applications in the
alleviation of metabolic disorders. MET can alleviate the severity
of endometrial disorder by reducing the levels of androgen, thus
attenuating endometrial diseases, including EH, especially in
patients with PCOS who are also insulin resistant (18–22). Previous western blotting assays
have demonstrated that the expression of GLUT4 in the endometrium
is reduced in patients with PCOS. Furthermore, immunohistochemistry
results have suggested that the presence of PCOS rather than EH
acts as a key regulator of the expression level of GLUT4 in
epithelial cells (2,23). In addition, previous data
suggested that differences in GLUT4 expression levels may help to
distinguish between patients with EH with and without PCOS
(24). Downregulation of GLUT4 is
associated with the development of EH during PCOS, and it has been
reported that abnormal hormonal conditions, including PCOS, are
associated with endometrial GLUT4 expression (24). Changes in the insulin receptor, as
well as androgen-dependent alterations in androgen receptor
expression, are involved in changes in endometrial GLUT4 (25). In addition, MET has been reported
to alleviate uterine defects in PCOS animal models (26), and it is an effective treatment
for endothelial cell pyroptosis by regulating lncRNA-maternally
expressed gene 3 (MEG3) signaling in atherosclerosis (27). Furthermore, small nucleolar RNA
host gene 20 (SNHG20) has been reported to target the expression of
miR-4486 in the pathogenesis of glioma cell malignancy (28). In the present study, it was
hypothesized that administration of MET regulates the expression of
lncRNA-MEG3 and lncRNA-SNHG20, thereby influencing the expression
of their competing endogenous RNAs (ceRNAs), such as miR-223 and
miR-4486, which leads to upregulation of their target gene, GLUT4.
To test this hypothesis, cultured cells were treated with MET to
verify its effect on the lncRNA-MEG3/miR-223/GLUT4 and
lncRNA-SNHG20/miR-4486/GLUT4 signaling pathways to confirm the role
of MET in an animal model of PCOS.
Materials and methods
Animals and treatments
A PCOS rat model was established using a protocol
described previously to determine the effect of MET administration
on the endometrium (26). In
brief, female SD rats (mean weight of 295 g, average age of 70
days, n=80) were acquired from the experimental animal center of
Zhejiang Chinese Medical university laboratory animal research
center and housed in the animal facility for 7 days of adaptation.
In the experiment, all animals had unlimited access to drinking
water and food. The environment in the SPF animal facility was
controlled at a temperature of 23±1°C with a 12/12 h light/dark
cycle and 50-75% humidity. All SD rats had a normal estrous cycle,
which was confirmed by utilizing vaginal smear examinations
performed under a conventional light microscope prior to the study
treatment. Then, the rats were randomly assigned into four groups
with 20 rats in each group: i) SHAM (rats were treated with
saline); ii) SHAM + MET (rats were treated with saline + MET); iii)
PCOS [rats were treated with insulin + human chronic gonadotropin
(hCG) to trigger hyperandrogenism and hyperinsulinemia]; and iv)
PCOS + MET (PCOS rats were treated with MET). The establishment of
animal models, doses and drug treatment protocols were performed
according to a previous publication (29). In brief, treatment with insulin
was started at 0.5 IU per day and then gradually increased to 6.0
IU per day to trigger insulin resistance and hyperinsulinemia. At
the same time, 3.0 IU per day of hCG was used to trigger
hyperandrogenism. All doses were administered by subcutaneous
injections given twice a day until the experimental treatment was
finished. On day 23 of the experiment, the rats in each group were
further divided into two subgroups, with each subgroup containing
10 rats. For treatment with MET, the compound was mixed in saline
before it was orally administered at a dose of 500 mg/kg utilizing
a surgical cannula. Then, the trunk blood in each rat was
collected, while the uteri in the rats were collected for analysis.
The humane endpoints of this study were the point the uteri samples
were collected from the rats. The whole experiment lasted for 30
days. A total of 80 animals were used and sacrificed at the end of
this study. No rats were found dead during the study. The animal
health and behavior was observed at a frequency of 2 days. No
anesthetic agents were used for the collection of uteri samples,
instead the rats were sacrificed by the i.p. administration of 100
mg/kg of sodium pentobarbital. This study was performed in line
with the Guide for the Care and Use of Laboratory Animal by
National Research Council (US) Committee (8th edition) (30) and the protocols were approved by
the Animal Ethics Committee of Zhejiang Chinese Medical University
Laboratory animal research center (approval no. ZSLL-2016-42;
Hangzhou, China).
RNA isolation and reverse
transcription-quantitative (RT-q)PCR
In this study, RT-qPCR was performed on endometrial
tissues collected from rats in different experimental groups. In
brief, the total RNA was isolated using a miRNeasy Mini kit (Qiagen
GmbH) and evaluated using a Nanodrop 3000 spectrometer (Thermo
Fisher Scientific, Inc.). Next, cDNA was synthesized from isolated
total RNA (2 µg total RNA of each sample) utilizing a Transcriptor
First Strand assay kit (Roche Diagnostics) in accordance with the
manufacturer's instructions following a thermocycling protocol of
30 min at 16°C, 30 min at 42°C, 5 min at 85°C and kept at 4°C.
Then, 1 µl synthesized cDNA from each sample was utilized as a
template to perform qPCR with SYBR Green Master Mix (Toyobo) using
a LightCycler® 480 real-time PCR machine (Roche
Diagnostics) in accordance with the manufacturer's instructions to
assay the relative expression of MEG3 (forward,
5′-GGGAGCAGCTATGGATCACC-3′ and reverse,
5′-ATAGCGCCCCCTATTCATGC-3′), SNHG20 (forward,
5′-CCTGTGTGCCTGGAAAGGAAT-3′ and reverse,
5′-GGCACAGGAACCACAGAGTAT-3′), miR-223 (forward,
5′-CGTGTATTTGACAAGCTGA-3′ and reverse, 5′-GAACATGTCTGCGTATCTC-3′),
miR-4486 (forward, 5′-ACACTCCAGCTGGGGCTGCGCGA-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′), antisense non-coding RNA in the INK4 locus
(ANRIL; forward, 5′-CTGGGACTACAGATGCACCAC-3′ and reverse,
5′-GGAGGGAGCATGTCTGTTTCT-3′), down-regulated in hepatocellular
carcinoma (DREH; forward, 5′-GUGCCUGUCACAAACAGAUTT-3′ and reverse,
5′-AUCUGUUUGUGACAGGCACTT-3′), X inactive specific transcript
regulator (FTX; forward, 5′-TATGCCACCTAGCCTTTCTACA-3′ and reverse,
5′-ATCTCTTCAAAAGCGGCATAAT-3′), H19 imprinted maternally expressed
transcript (H19; forward, 5′-TACAACCACTGCACTACCTG-3′ and reverse,
5′-TGGAATGCTTGAAGGCTGCT-3′) and GLUT4 (forward,
5′-ATCCGGAACCTGGAGGGGCC-3′ and reverse, 5′-CGGCCAGGCCCAACAGATGG-3′)
in each sample. Relative expression was calculated using the
2−ΔΔCq method (31)
with U6 (forward, 5′-GCATGACGTCTGCTTTGGA-3′ and reverse,
5′-CCACAATCATTCTGCCATCA-3′) and GAPDH (forward,
5′-GGGAGCCAAAAGGGTCAT-3′ and reverse, 5′-GAGTCCTTCCACGATACCAA-3′)
used as the internal reference genes.
Cell culture and transfection
As EH is a medical condition characterized by
deregulation of endometrial cells proliferation (8), HCC-94 cells, a cervical carcinoma
cell line, were chosen for in vitro analysis. Other
candidate cell lines, such as HELA and 293T cells, were also
involved in the preliminary experiments, but the conditions were
not appropriate for the assays (data not shown). To further explore
the effect of MET treatment on expression levels of lncRNAs, the
expression levels of six candidate lncRNAs were analyzed in HCC-94
cells treated with or without 100 µM MET (Shanghai Squibb
Pharmaceutical Co., Ltd., Shanghai, China) at room temperature for
24 h. In brief, HCC-94 cells were maintained in modified DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 1 g/l insulin
(Gibco; Thermo Fisher Scientific, US), 2 mM glutamine (Gibco;
Thermo Fisher Scientific, Inc.), 0.67 mg/l selenium (Sijiqing,
Hangzhou, China), 10% FBS (Sijiqing, Hangzhou, China), 100 µg/ml
streptomycin, 100 U/ml penicillin and 0.55 g/l transferrin
(Invitrogen; Thermo Fisher Scientific, Inc.). The cells were
cultured in a 37°C humidified incubator supplied with 5%
CO2. After reaching confluence, cells were deprived of
serum for 24 h in a hypoxic environment consisting of 5%
CO2, 94% N2 and 1% O2. Next, the
cells were cultured in complete medium under reoxygenation with 20%
O2. To determine the effects of MET and siRNAs on gene
expression, the following six sets of experiments were performed
using HCC-94 cells at the density of 1×105/well.
Experiment 1, HCC-94 cells were divided into two groups: i)
Untreated (HCC-94 cells were cultured in normal medium); and ii)
MET (HCC-94 cells were treated with MET). Experiment 2, HCC-94
cells were divided into three groups: i) Negative control (NC)
small interfering (si)RNA (HCC-94 cells were transfected with
scrambled siRNA as the NC); ii) MEG3 siRNA (HCC-94 cells were
transfected with MEG3 siRNA); and iii) SNHG20 siRNA (HCC-94 cells
were transfected with SNHG20 siRNA). Experiment 3, HCC-94 cells
were divided into four groups: i) Untreated (HCC-94 cells were
cultured in normal medium); ii) MET + NC siRNA (HCC-94 cells were
treated with MET and scrambled siRNA as the NC); iii) MET + MEG3
siRNA (HCC-94 cells were treated with MET and MEG3 siRNA group);
and iv) MET + SNHG20 siRNA (HCC-94 cells were treated with MET and
SNHG20 siRNA). Experiment 4, HCC-94 cells were divided into two
groups: i) NC siRNA (HCC-94 cells transfected with scrambled siRNA
as the NC); and ii) GLUT4 siRNA (HCC-94 cells transfected with
GLUT4 siRNA). Experiment 5, HCC-94 cells were divided into four
groups: i) NC (HCC-94 cells were cultured in normal medium); ii)
lncRNA-MEG3 (HCC-94 cells transfected with lncRNA-MEG3); iii)
lncRNA-MEG3 + NC siRNA (HCC-94 cells transfected with lncRNA-MEG3
and scrambled siRNA as the NC); and iv) lncRNA-MEG3 + GLUT4 siRNA
(HCC-94 cells transfected with lncRNA-MEG3 and GLUT4 siRNA).
Experiment 6, HCC-94 cells were divided into four groups: i) NC
(HCC-94 cells were cultured in normal medium); ii) lncRNA-SNHG20
(HCC-94 cells transfected with lncRNA-SNHG20); iii) lncRNA-SNHG20 +
NC siRNA (HCC-94 cells transfected with lncRNA-SNHG20 and scrambled
siRNA as the NC); and iv) lncRNA-SNHG20 + GLUT4 siRNA (HCC-94 cells
transfected with lncRNA-SNHG20 and GLUT4 siRNA). The mass of
nucleic acid used was 50 nM, and the sequences (generated by Thermo
Fisher Scientific, Inc) used for transfection were as follows:
Scrambled siRNA (NC), 5′-UUGUACUACACAAAAGUACUG-3′; MEG3 siRNA,
5′-GAUCCCACCAACAUACAAATT-3′; SNHG20 siRNA,
5′-CCCUGUUUGUACCUCCAUUTT-3′; and GLUT4 siRNA,
5′-AGCTACAATGCAACTTGGCTGGGTA-3′. All transfections were performed
using 1×105 cells/well using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.) at 4°C overnight
in accordance with the manufacturer's instructions. The cells were
treated for 48 h before they were collected for subsequent
experiments.
Vector construction, mutagenesis and
luciferase assay
In this study, luciferase assays were performed to
confirm the regulatory relationships between SNHG20 and miR-4486,
miR-4486 and GLUT4, MEG3 and miR-223 and miR-223 and GLUT4. In
brief, the sequences of GLUT4, MEG3 and SNHG20 containing the
binding sites for miR-4486 or miR-223 were cloned into pcDNA 3.1
vectors (Promega Corporation) to generate wild-type (WT) vectors of
GLUT4, MEG3 and SNHG20. At the same time, site-directed mutagenesis
was performed using a Quick Change mutagenesis assay kit
(Stratagene; Agilent Technologies, Inc.) in accordance with the
manufacturer's instructions. Next, the mutated sequences of GLUT4,
MEG3 and SNHG20 containing the mutated binding sites for miR-4486
and miR-223 were also cloned into pcDNA 3.1 vectors to generate
mutant type (MUT) vectors of GLUT4, MEG3 and SNHG20. Then,
1×105/well HCC-94 cells were co-transfected with MUT/WT
GLUT4 vectors in conjunction with miR-4486 or miR-223 mimics
(Thermo Fisher Scientific, Inc.), MEG3 vectors in conjunction with
miR-223 mimics (Thermo Fisher Scientific, Inc.), as well as SNHG20
vectors in conjunction with miR-4486 mimics (Thermo Fisher
Scientific, Inc.) using Lipofectamine 3000. The sequences of
miR-4486 mimics were 5′-GCUGGGCGAGGCUGGCA-3′ and the sequences of
miR-223 mimics was 5′-ACCCCAUAAACUGUUUGACUGU-3′. The luciferase
activities in transfected cells were measured 48 h after
transfection using a Bright Glo assay kit (Promega Corporation) and
normalized to Renilla luciferase activity in accordance with the
manufacturer's instructions.
Western blot analysis
Protein expression of GLUT4 in HCC-94 cells was
measured using western blotting. In brief, the cells were first
lysed and the protein was extracted in RIPA buffer
(MilliporeSigma), and the concentration of GLUT4 protein was
determined using a BCA assay kit (Thermo Fisher Scientific, Inc.).
Subsequently, proteins in the supernatant were resolved via 10%
SDS-PAGE and subsequently blotted onto a PVDF membrane
(MilliporeSigma) in accordance with the manufacturer's instructions
(50 µg/lane). Next, the membrane was blocked with 5% non-fat milk
at room temperature for 1 h and incubated at 4°C overnight with
anti-GLUT4 primary antibody (1:1,000; catalogue number ab188317;
Abcam) and then further incubated for 1 h at room temperature with
HRP-tagged secondary antibody (dilution: 1:5,000; catalogue number
ab6721; Abcam) using β-actin as an internal reference protein
(1:1,000; cat. no. ab8226; Abcam). Finally, after treatment with
ECL reagent (Pierce; Thermo Fisher Scientific, Inc.) in accordance
with the manufacturer's instructions, the relative expression of
GLUT4 was calculated using Quantity One 1-D Analysis software
(V4.6.8, Bio-Rad Laboratories, Inc.).
Immunohistochemistry
Expression of GLUT4 protein in collected endometrial
tissues was measured using routine immunohistochemistry assays. In
brief, the tissues were fixed at room temperature for 20 min in 4%
paraformaldehyde, dehydrated, embedded in paraffin and sliced into
5 µm sections, permeabilized using PBS containing 0.5% Triton
X-100, blocked at room temperature for 1 h in 10% FBS, and then
incubated in succession with anti-GLUT4 primary antibodies (1:500;
cat. no. ab216661; Abcam) at room temperature for 1 h and
HRP-tagged secondary antibodies (1:1,000; Cat. no. ab150077; Abcam)
at room temperature for 30 min. Finally, after counterstaining with
DAPI for 3 min at room temperature to stain the nuclei, the cells
were visualized under a fluorescence microscope to analyze GLUT4
expression. The quantification was performed with a fluorescence
microscope (IX71; Olympus Corporation) by using cellSens Software
(V1.16, Olympus Corporation).
H&E
The degree of EH was determined in endometrial
tissues (5 µm sections) by H&E staining using an H&E
Staining Kit (Abcam) in accordance with the manufacturer's
instructions. The tissues were fixed with 4% formalin for 24 h at
room temperature. The areas of endometrium were measured in three
sections from each rat at ×2 magnification under an Olympus light
microscope (Olympus Corporation), and the areas of endometrium were
calculated using Micro Image (V2.5, Olympus Corporation).
Cell viability assay
HCC-94 cells were collected 48 h after transfection
and subsequently seeded into a 96-well plate at a density of
1×104 cells per well in 100 µl DMEM. After the cells
reached 80% confluence, they were cultured with Cell Counting Kit-8
(CCK-8) assay reagent (Abcam) for 2 h in the dark. Finally, the
optical density value (450 nm) was measured using a microplate
reader.
Statistical analysis
All experimental results are presented as the mean ±
standard deviation (SD). Each experiment was repeated at least 3
times. One-way ANOVA was utilized for comparisons between groups,
and Tukey's test was used as the post hoc study. All statistical
analyses were performed using SPSS version 22.0 (IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
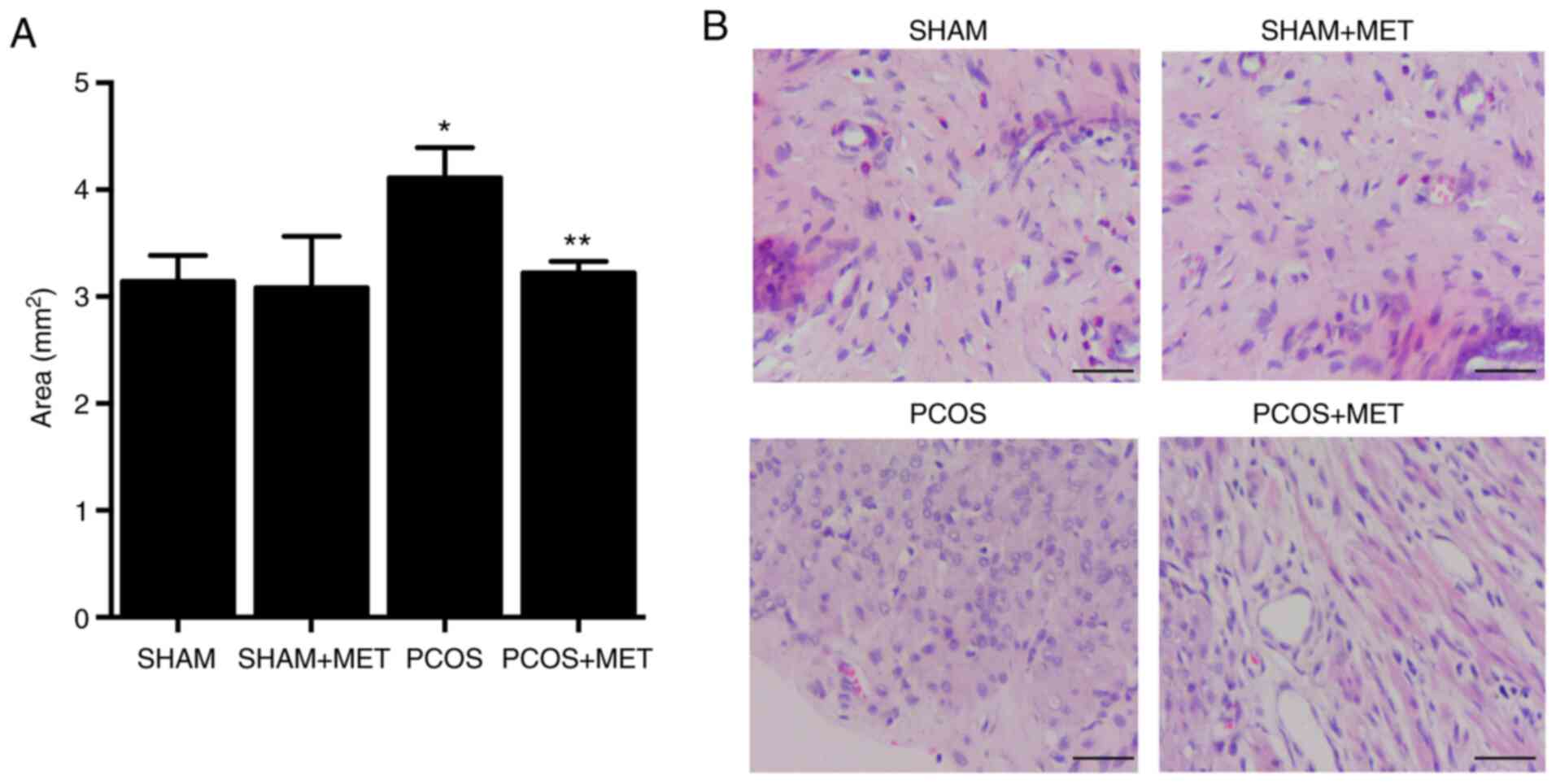
MET treatment prevents EH in PCOS
rats
The endometrial area was significantly increased in
PCOS rats compared with the SHAM rats. Administration of MET
significantly decreased the endometrial area in PCOS rats by 21.65%
compared with the PCOS group, but did not change the endometrial
area in the SHAM + MET rats (Fig.
1A). H&E staining analysis revealed that EH in PCOS rats
was efficiently inhibited by MET treatment (Fig. 1B). These results indicated that
MET treatment exerted a notable therapeutic effect on PCOS.
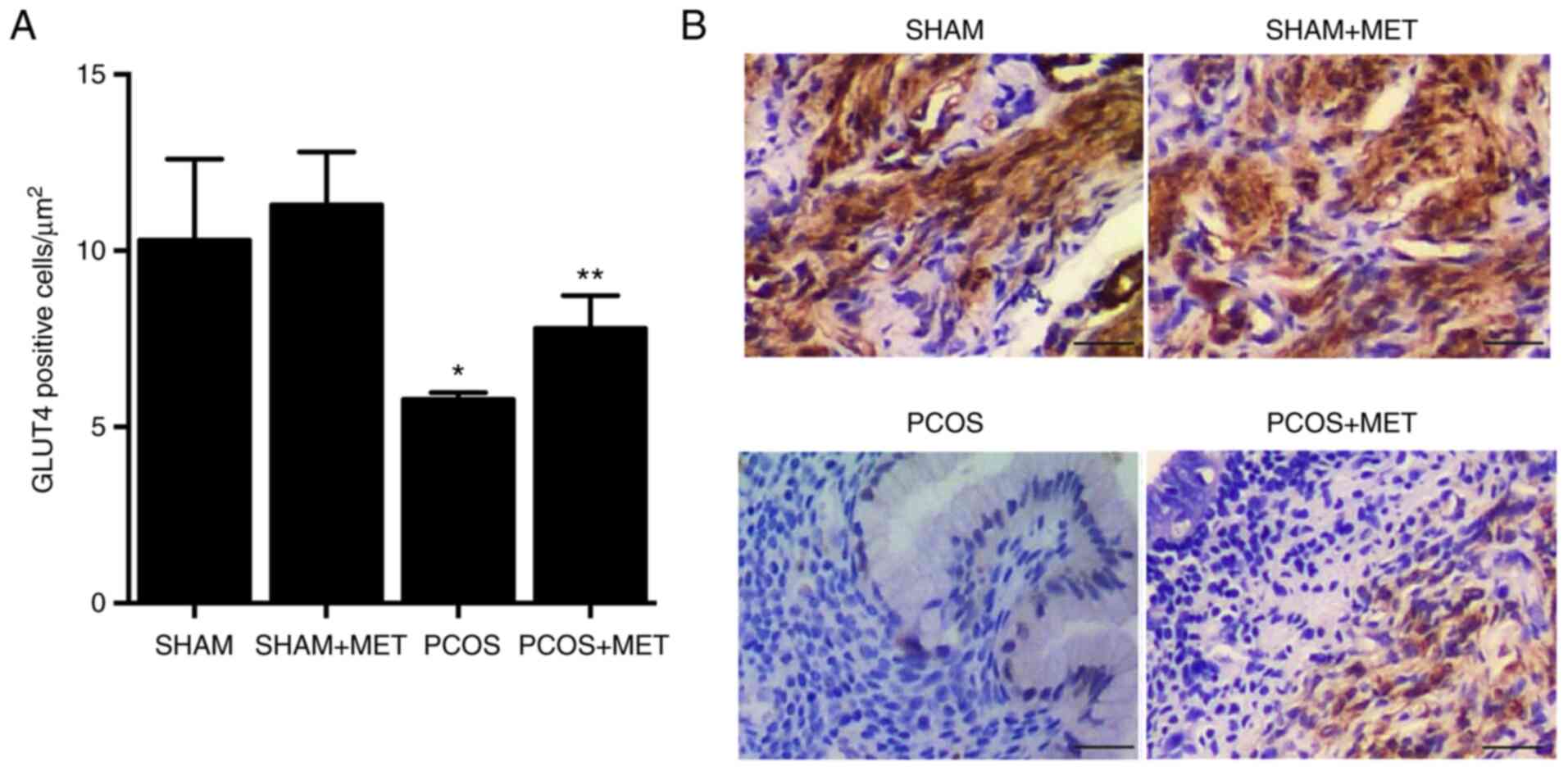
MET treatment rescues the repressed
expression of GLUT4 in the endometrium of PCOS rats
Immunohistochemistry was performed to evaluate the
expression of GLUT4 in the endometrium of PCOS rats in response to
different treatments. Expression of GLUT4 in the endometrium of
PCOS rats was significantly reduced by 43.89% compared with the
SHAM group. Treatment with MET in the PCOS + MET group
significantly rescued the expression of GLUT4 by 42.33% compared
with the PCOS group. Meanwhile, treatment of SHAM rats with MET had
no effect on the expression of GLUT4 in the endometrium (Fig. 2A and B).
MET treatment alleviates the
dysregulation of lncRNA-MEG3, lncRNA-SNHG20, miR-223 and miR-4486
in the endometrium of PCOS rats
In the present study, qPCR was performed to analyze
the expression of lncRNA-MEG3 and lncRNA-SNHG20 in the endometrium
of PCOS rats in response to different treatments. Compared with the
SHAM group, the expression levels of lncRNA-MEG3 (Fig. 3A) and lncRNA-SNHG20 (Fig. 3B) were significantly inhibited in
the endometrium of PCOS rats by 67.65 and 85.57%, respectively.
Administration of MET significantly upregulated the expression of
lncRNA-MEG3 (Fig. 3A) and
lncRNA-SNHG20 (Fig. 3B) by 109.09
and 343.62%, respectively, in the endometrium of PCOS rats.
Expression of lncRNA-MEG3 and lncRNA-SNHG20 in SHAM + MET rats
remained unchanged after MET administration. Moreover, the
expression of miR-223 and miR-4486 exhibited the opposite pattern.
Compared with the SHAM group, expression levels of miR-223 and
miR-4486 were significantly elevated in the endometrium of PCOS
rats by 360.88 and 308.55%, respectively, while treatment with MET
significantly reduced the expression of miR-223 (Fig. 3C) and miR-4486 (Fig. 3D) by 53.34 and 87.00%,
respectively, in the endometrium of PCOS rats.
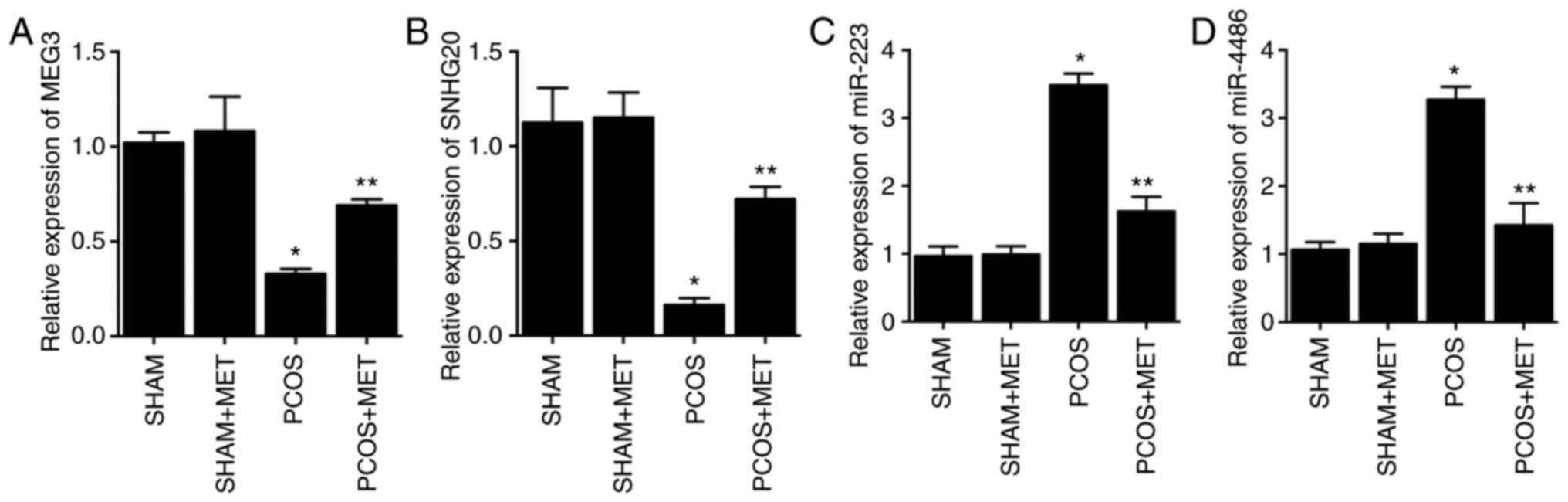 | Figure 3.MET treatment partially restores the
normal expression of lncRNA-MEG3, lncRNA-SNHG20, miR-223 and
miR-4486 in the endometrium of PCOS rats as determined via reverse
transcription-quantitative PCR. (A) The expression of lncRNA-MEG3
was repressed in the endometrium of PCOS rats, while MET treatment
recovered the repressed lncRNA-MEG3 expression in PCOS rats. (B)
The expression of lncRNA-SNHG20 was repressed in the endometrium of
PCOS rats, while MET treatment recovered the repressed lncRNA-MEG3
expression in PCOS rats. (C) The expression of miR-223 was
increased in the endometrium of PCOS rats, and it was restored by
MET treatment in PCOS rats. (D) The expression of miR-4486 was
promoted in the endometrium of PCOS rats, and it was restored by
MET treatment in PCOS rats. *P<0.05 vs. SHAM rats; **P<0.05
vs. PCOS rats. PCOS, polycystic ovary syndrome; MET, metformin;
lnc, long non-coding; MEG3, maternally expressed gene 3; SNHG20,
small nucleolar RNA host gene 20; miR, microRNA. |
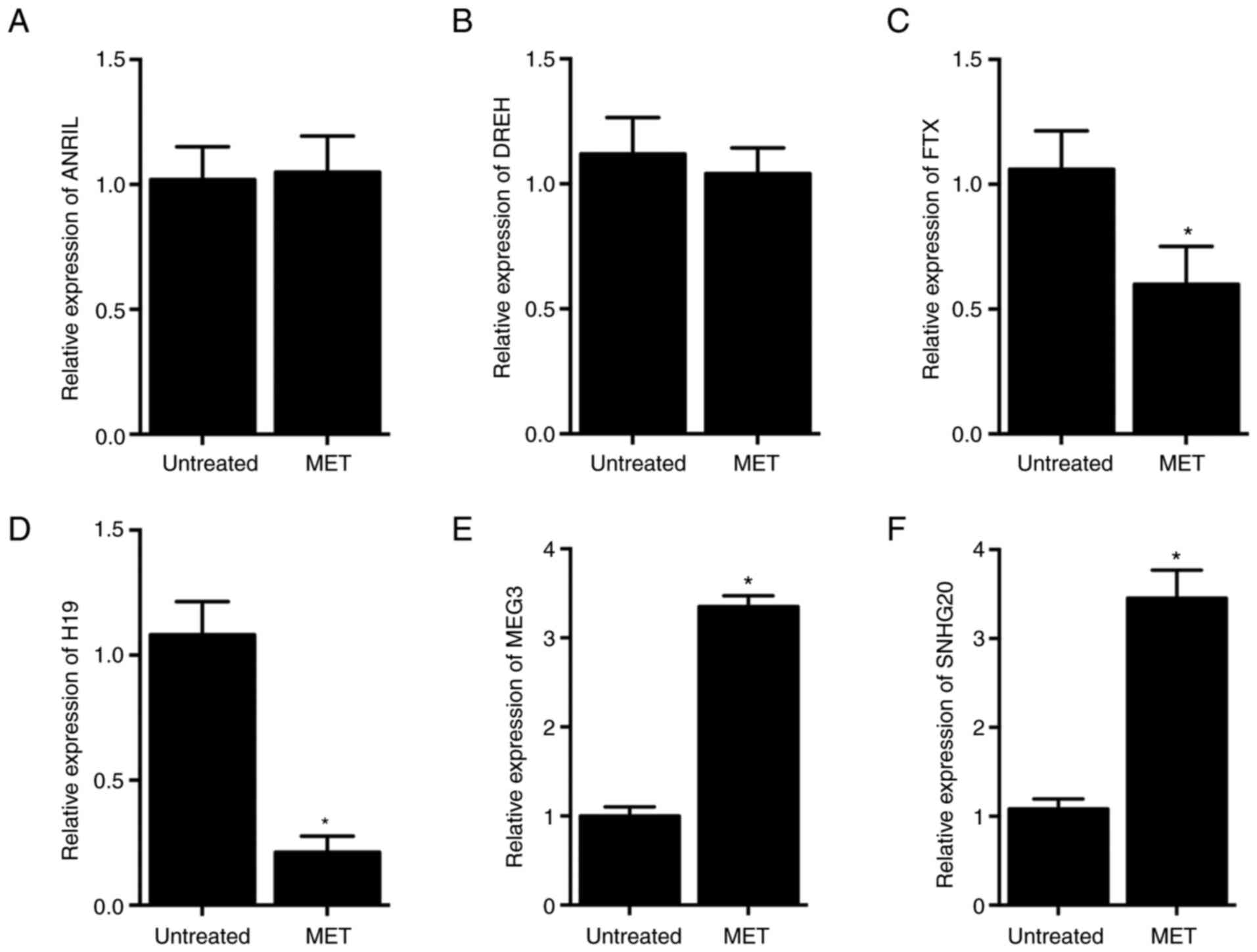
Differential effects of MET treatment
on the expression of lncRNAs in HCC-94 cells
In this study, the effect of MET treatment on the
expression of six lncRNAs was analyzed in HCC-94 cells. No
significant difference was observed in the expression of
lncRNA-ANRIL (Fig. 4A) or
lncRNA-DREH (Fig. 4B) in HCC-94
cells in response to MET treatment. By contrast, expression of
lncRNA-FTX (Fig. 4C) and
lncRNA-H19 (Fig. 4D) was
significantly inhibited by 43.40 and 80.46%, respectively, while
expression of lncRNA-MEG3 (Fig.
4E) and lncRNA-SNHG20 (Fig.
4F) was significantly increased by 235.15 and 219.10%,
respectively, in HCC-94 cells undergoing MET treatment.
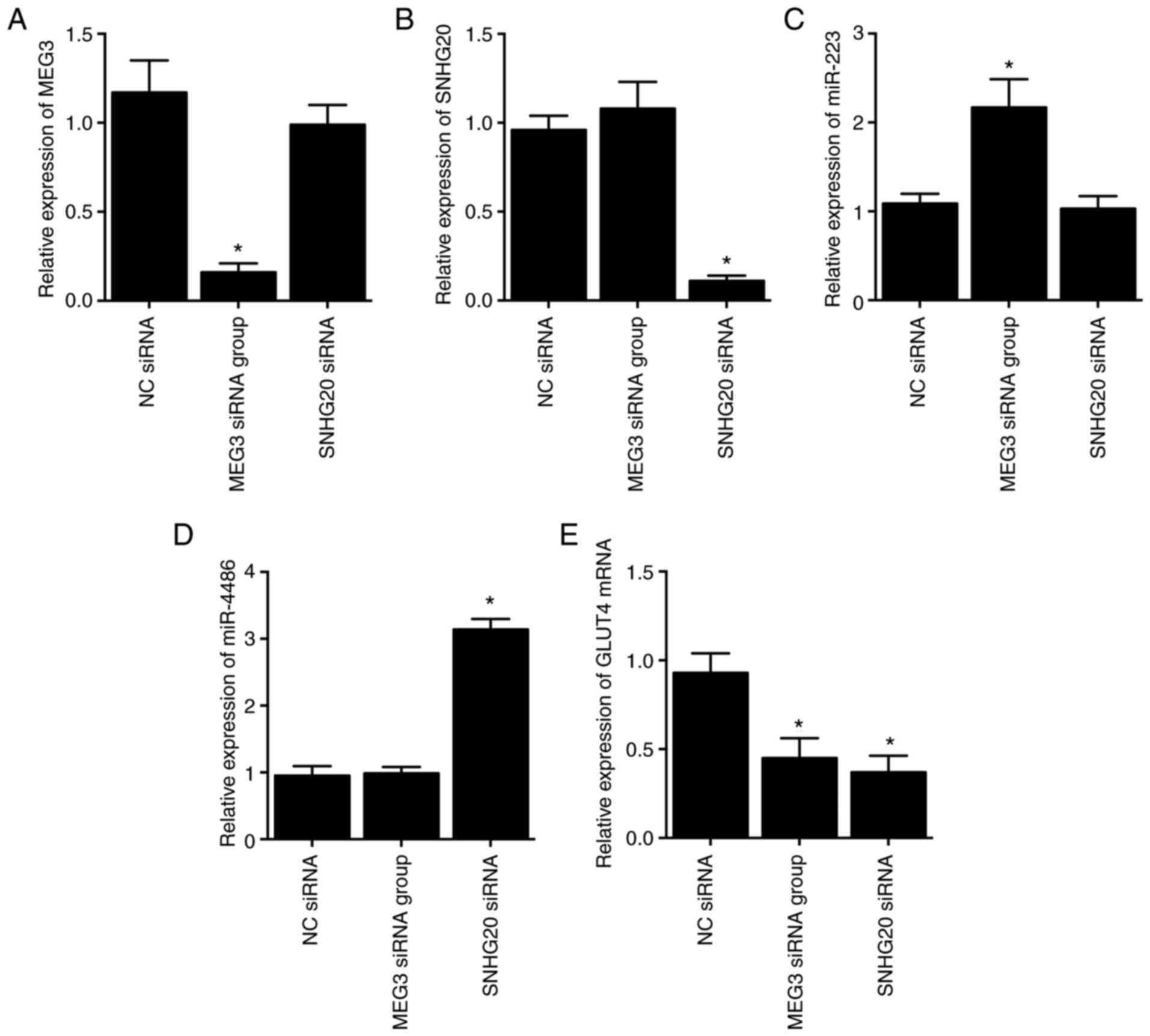
Knockdown of MEG3 and SNHG20 inhibits
GLUT4 mRNA expression via upregulating miR-223 and miR-4486
expression, respectively, in HCC-94 cells
As shown by the results, compared with the NC siRNA
group, MEG3 expression was significantly suppressed by the
transfection of MEG3 siRNA (Fig.
5A), while the expression of SNHG20 was decreased by the
transfection of SNHG20 siRNA (Fig.
5B), thus verifying the successful transfection of MEG3 siRNA
and SNHG20 siRNA. Moreover, the expression of miR-223 (Fig. 5C) and miR-4486 (Fig. 5D) was promoted by transfection
with MEG3 siRNA and SNHG20 siRNA, respectively. However, the gene
expression of GLUT4 was both significantly inhibited in HCC-94
cells transfected with MEG3 siRNA or SNHG20 siRNA compared with the
NC siRNA group (Fig. 5E).
Knockdown of MEG3 and SNHG20 alters
the effects of MET on the expression of their competing miRNAs and
GLUT mRNA and protein expression in HCC-94 cells
Expression of lncRNA-MEG3 was significantly
upregulated by 205.10% in response to MET treatment alone. Compared
with the MET + NC siRNA group, MET + lncRNA-MEG3 siRNA
significantly reduced the upregulated expression of lncRNA-MEG3 by
59.67%, while MET + lncRNA-SNHG20 siRNA into HCC-94 cells had no
effect on the expression of lncRNA-MEG3 induced by MET treatment
(Fig. 6A). Moreover, the
expression of miR-223 was significantly repressed by 76.85% upon
MET treatment, and lncRNA-MEG3 siRNA rather than lncRNA-SNHG20
showed considerable effects in restoring the expression of miR-223,
which was upregulated by 190.44% in the MET + lncRNA-MEG3 siRNA
group (Fig. 6B). Similarly, the
expression of lncRNA-SNHG20 and miR-4486 was also restored by the
transfection of lncRNA-SNHG20 siRNA in HCC-94 cells treated with
MET (Fig. 6C and D).
Subsequently, the expression levels of GLUT4 mRNA and protein were
analyzed via qPCR and western blotting, respectively. Expression of
GLUT4 mRNA (Fig. 6E) and protein
(Fig. 6F) was notably increased
by 225.10 and 221.26%, respectively, in HCC-94 cells treated with
MET. The transfection of lncRNA-MEG3 and lncRNA-SNHG20 siRNAs
restored the normal expression of GLUT4 mRNA and protein increased
by MET. These results indicated that expression of GLUT4 was
associated with that of lncRNA-MEG3 and lncRNA-SNHG20.
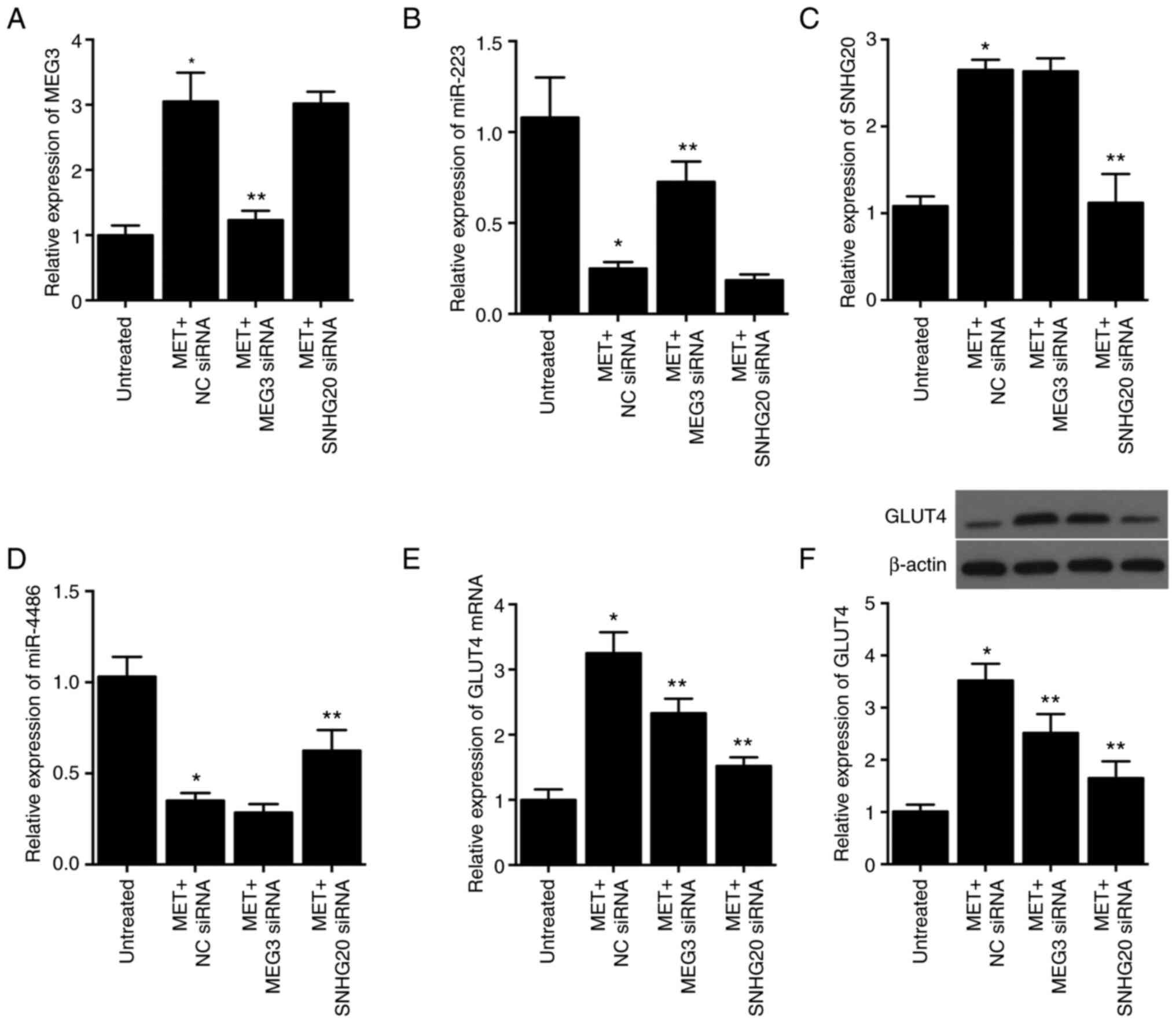 | Figure 6.MET treatment upregulates the
expression of lncRNA-MEG3, lncRNA-SNHG20 and GLUT4, and
downregulates the expression of miR-223 and miR-4486 in HCC-94
cells, while the effect of MET is reversed by siRNAs targeting
lncRNA-MEG3 and lncRNA-SNHG20 as determined via RT-qPCR and Western
blotting. (A) MET treatment upregulated the expression of
lncRNA-MEG3 in HCC-94 cells, and the successful transfection of
lncRNA-MEG3 siRNA was validated by the significantly reduced
lncRNA-MEG3 level in MET-treated HCC-94 cells. (B) MET treatment
led to the downregulation of miR-223 in HCC-94 cells, and the
suppressive effect of MET was reversed by the transfection of
lncRNA-MEG3 siRNA. (C) MET treatment upregulated the expression of
lncRNA-SNHG20 in HCC-94 cells, and successful transfection of
lncRNA-SNHG20 siRNA was validated by the significantly reduced
lncRNA-SNHG20 level in MET-treated HCC-94 cells. (D) MET treatment
significantly suppressed the expression of miR-4486 in HCC-94
cells, and the inhibitory effect of MET was partially reversed by
the transfection of lncRNA-SNHG20 siRNA. (E) RT-qPCR showed that
the MET treatment upregulated the expression of GLUT4 mRNA in
HCC-94 cells, and the effect of MET was partially reversed by
siRNAs targeting lncRNA-MEG3 and lncRNA-SNHG20. (F) The results of
western blotting and semi-quantification showed that MET treatment
upregulated the expression of GLUT4 protein in HCC-94 cells, and
the effect of MET was partially reversed by siRNAs targeting
lncRNA-MEG3 and lncRNA-SNHG20. *P<0.05 vs. untreated cells;
**P<0.05 vs. MET + NC siRNA group. MET, metformin; lnc, long
non-coding; MEG3, maternally expressed gene 3; SNHG20, small
nucleolar RNA host gene 20; miR, microRNA; GLUT4, glucose
transporter type 4 insulin-responsive; siRNA, small interfering
RNA; RT-qPCR, reverse transcription-quantitative PCR; NC, negative
control. |
Luciferase activities of lncRNAs and
GLUT4 are inhibited by their targeting miRNAs
Luciferase assays were performed to confirm the
regulatory relationship among lncRNA-SNHG-miR-4486 (Fig. 7A), miR-4486-GLUT4 (Fig. 7B), lncRNA-MEG3-miR-223 (Fig. 7C) and miR-223-GLUT4 (Fig. 7D). WT and MUT luciferase vectors
containing target sequences of the above miRNAs were constructed
and then transfected into HCC-94 cells with corresponding miRNA
mimics. As shown in Fig. 7, the
luciferase activities of lncRNA-SNHG20 WT (Fig. 7A) and GLUT4 WT (Fig. 7B) were significantly inhibited by
miR-4486. The luciferase activities of lncRNA-MEG3 WT (Fig. 7C) and GLUT4 WT (Fig. 7D) were significantly repressed by
miR-223.
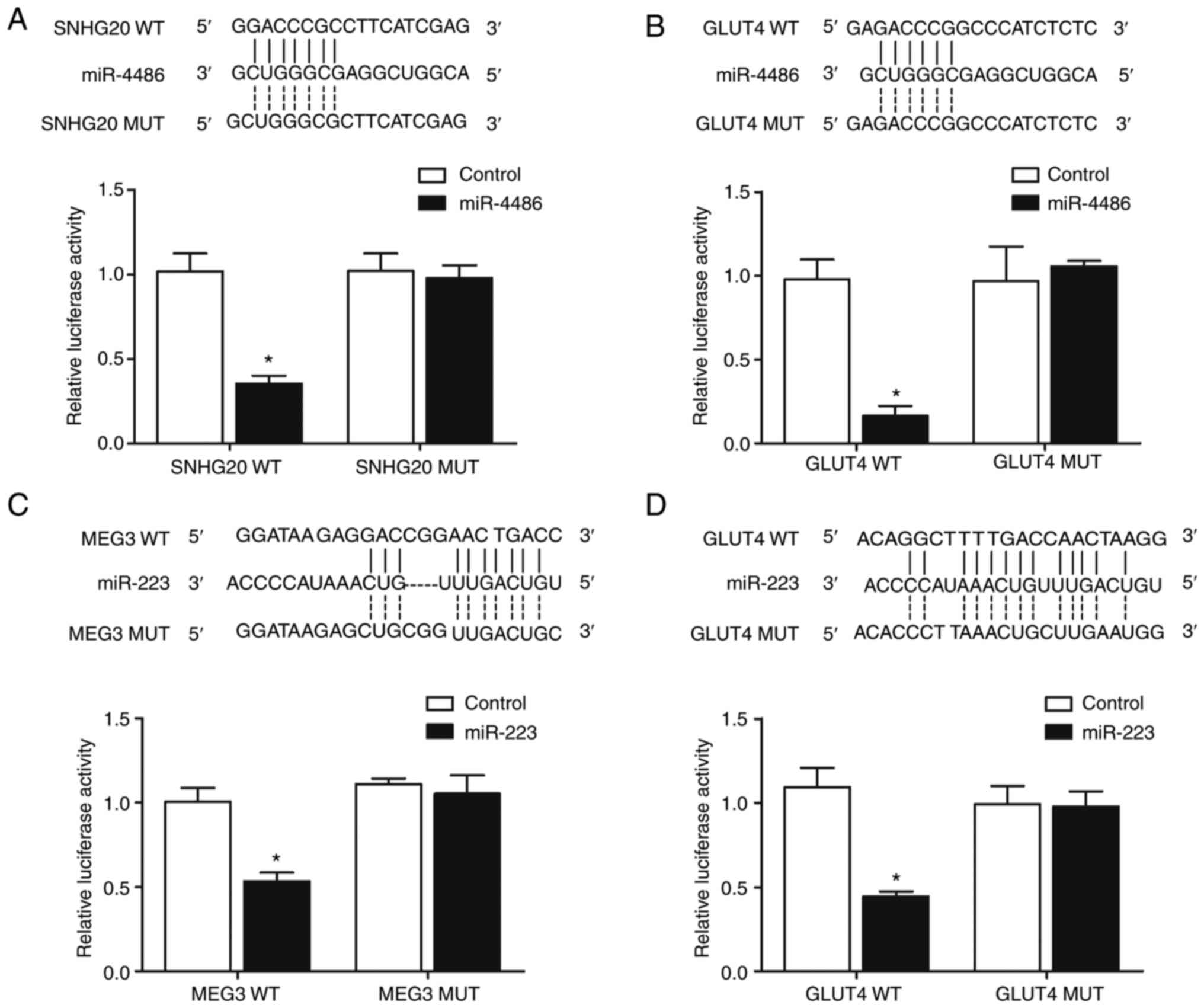 | Figure 7.Targeting relationship between
miR-4486 and lncRNA-SNHG20, miR-4486 and GLUT4, miR-223 and
lncRNA-MEG3, as well as between miR-223 and GLUT4 as determined via
a luciferase assay and sequence analysis. (A) Sequence analysis
indicated the potential binding between miR-4486 and lncRNA-SNHG20,
which was proved by the reduced luciferase activity in cells
co-transfected with lncRNA-SNHG20 WT and miR-4486. (B) Sequence
analysis indicated the potential binding between miR-4486 and
GLUT4, which was proved by the reduced luciferase activity in cells
co-transfected with GLUT4 WT and miR-4486. (C) Sequence analysis
indicated the potential binding between miR-223 and lncRNA-MEG3,
which was proved by the reduced luciferase activity in cells
co-transfected with lncRNA-MEG3 WT and miR-223. (D) Sequence
analysis indicated the potential binding between miR-223 and GLUT4,
which was proved by the reduced luciferase activity in cells
co-transfected with GLUT4 WT and miR-223. *P<0.05 vs. control
group. miR, microRNA; lnc, long non-coding; SNHG20, small nucleolar
RNA host gene 20; GLUT4, glucose transporter type 4
insulin-responsive; MEG3, maternally expressed gene 3; WT,
wild-type; MUT, mutant. |
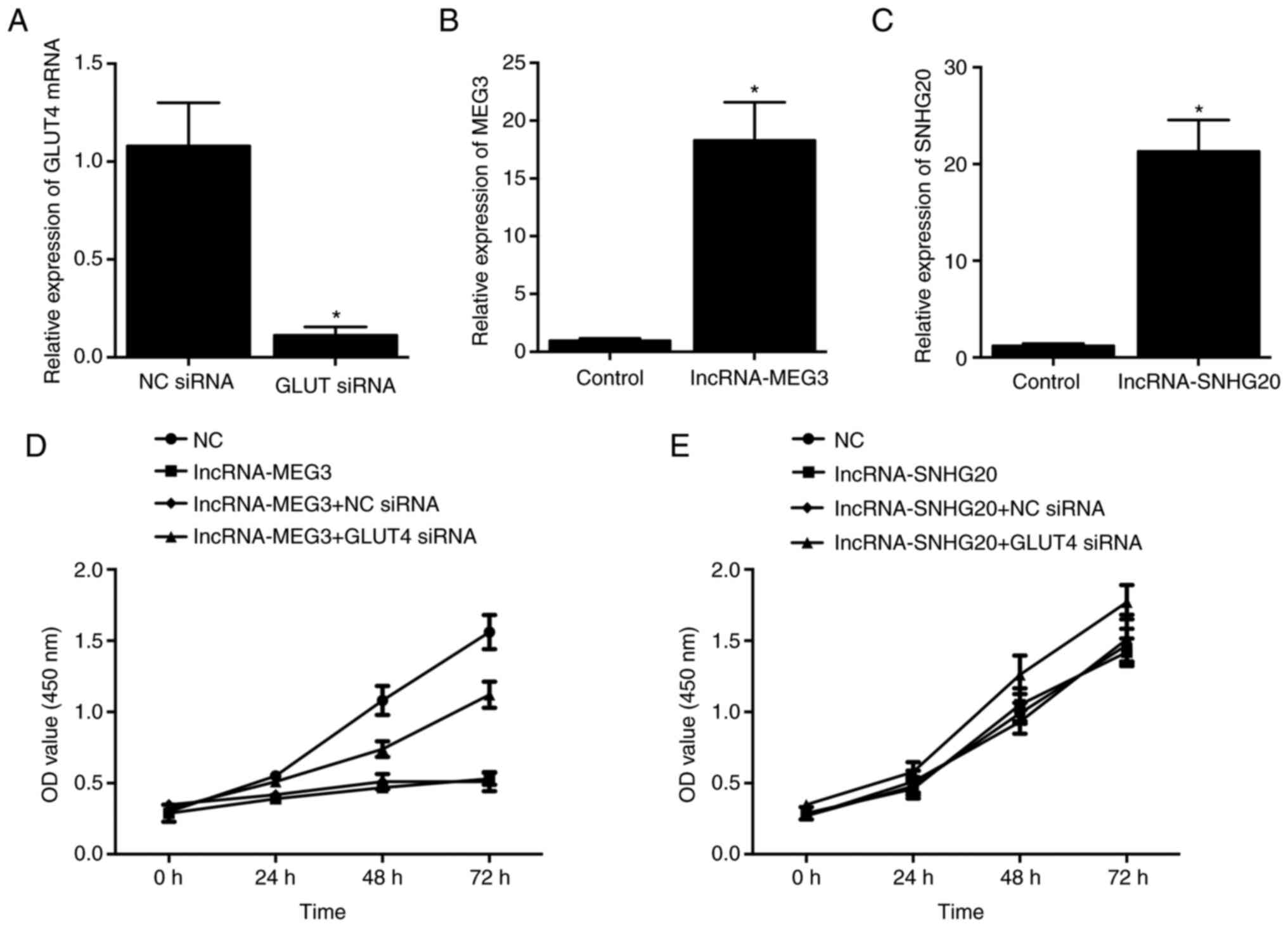
Knockdown of GLUT4 increases the
viability of HCC-94 cells
HCC-94 cells were then transfected with GLUT4 siRNA,
and successful transfection was validated by significantly reduced
expression of GLUT4 mRNA compared with the NC siRNA group (Fig. 8A). Then, lncRNA-MEG3 (Fig. 8B) and lncRNA-SNHG20 (Fig. 8C) were overexpressed in HCC-94
cells. Accordingly, it was found that the overexpression of
lncRNA-MEG3 reduced cell viability (Fig. 8D), while the overexpression of
lncRNA-SNHG20 exhibited no effect on the viability of HCC-94 cells
(Fig. 8E). Moreover, when
lncRNA-MEG3-overexpressing cells were transfected with GLUT4 siRNA,
the reduced cell viability was clearly restored (Fig. 8D). Moreover, transfection with
GLUT4 siRNA in HCC-94 cells overexpressing lncRNA-SNHG20 also
increased the viability of HCC-94 cells (Fig. 8E).
Discussion
In the present study, a PCOS rat model was
established to analyze the effect of MET treatment on EH. It was
found that EH in PCOS rats was inhibited by MET treatment. In
addition, immunohistochemistry was performed to evaluate the
expression of GLUT4 in the endometrium of PCOS rats treated in
response to different conditions. Also, the diminished expression
of GLUT4 could be contributed to a complicated network of GLUT4
regulation, including potential functions of lncRNAs and miRNAs,
which was not been extensively studied. In the endometrium of PCOS
rats, the deregulation of GLUT4 was restored by MET treatment.
Meanwhile, qPCR was performed to measure the differential
expression of lncRNA-MEG3, lncRNA-SNHG20, miR-223 and miR-4486
under different conditions. The decreased expression of
lncRNA-MEG3/lncRNA-SNHG20 and increased expression of
miR-223/miR-4486 in the endometrium of PCOS rats were recovered by
MET treatment.
MET is a derivative of galegine, which is a natural
compound synthesized by the herb Galega officinalis
(7). MET is the most frequently
used oral antidiabetic drug and improves serum lipid profiles,
influences the process of hemostasis and exhibits anti-inflammatory
effects (32). In the 1920s,
initially discovered as derivatives of galegine, MET and phenformin
were successfully isolated, although their uses in clinical
applications were not confirmed until later on (33).
MEG3 was first discovered as a compound similar to
that encoded by the Glycosyltransferase-like protein (Gtl2) gene in
mice, and it is highly expressed in tissues in normal human organs,
especially in pituitary and other brain tissues (34). Nevertheless, the mRNA of MEG3 is
not expressed in tumors of the pituitary gland or in cancer cells
of humans and a number of other mammalian species (35). More importantly, as one of several
isoforms of MEG3 proteins, the MEG3a protein acts as a powerful
inhibitor of cell growth (35).
In addition, the colony formation assay showed that MEG3a induced
an ~60% reduction in the number of cell colonies in response to
MEG3a transfection into cancer cells, including HeLa cervical
carcinoma, H4 neuroglioma cancer and MCF7 breast adenocarcinoma
cancer cells. The MEG3 gene is located at locus 14q32.3 of the
human genome. Of note, the 14q32.3 locus contains a gene encoding a
tumor suppressor that participates in the pathogenesis and
metastasis of several tumors, including nasopharyngeal carcinoma,
meningiomas, colorectal carcinoma and leukemia (36,37). In the present study, HCC-94 cells
were transfected with lncRNA-MEG3/SNHG20 siRNAs and the expression
of lncRNAs, their competing miRNAs and GLUT4 were analyzed. It was
found that MET upregulated the expression of lncRNAs/GLUT4 and
downregulated the expression of their competing miRNAs.
LncRNA MEG3 enhances pyroptosis in HAEC cells. In
addition, MEG3 acts as a sponge of miR-223 based on the
complementarity between the sequence of MEG3 and miR-223, reducing
expression levels of miR-223, while increasing expression levels of
NACHT, LRR and PYD domains-containing protein 3 to enhance levels
of pyroptosis. Moreover, suppression of the expression level of
miR-223 also reduces the activity of melatonin in inhibiting the
pyroptosis of HAEC cells in the presence of oxidized low-density
lipoprotein (27).
SNHG20 is an lncRNA initially discovered in liver
carcinoma cells (38). Increased
levels of SNHG20 expression are considered a valuable biomarker for
the prognosis of hepatocellular carcinoma (38). In addition, an oncogenic role of
SNHG20 is also present in colorectal cancer due to high expression
levels of SNHG20 being closely associated with the metastasis of
colorectal cancer (39).
Decreased levels of SNHG20 expression were shown to
reduce the migration and proliferation of glioma cells as SNHG20
acts as a ceRNAs to reduce the levels of miR-4486 expression
(28). In the present study,
luciferase assays were performed to explore the inhibitory role of
miRNAs on their targeting lncRNAs and the GLUT4 gene. The
luciferase activities of lncRNA-SNHG20 WT and GLUT4 WT were
significantly inhibited by miR-4486, while the luciferase
activities of lncRNA-MEG3 WT and GLUT4 WT were remarkably repressed
by miR-223.
GLUT4 is highly expressed in tissues with a high
level of insulin expression (40). Therefore, the presence of GLUT4
enhances glucose uptake, while the inhibition of GLUT4 signaling
results in insulin resistance (41). Furthermore, celastrol regulates
the expression of some miRNAs involved in the regulation of insulin
(42,43). For example, celastrol reverses the
role of Palmitic acid in inhibiting expression levels of GLUT4
(44). More recently, it was
demonstrated that MET treatment increases expression levels of
GLUT4 in the endometrium of patients with PCOS and EH (7). Furthermore, the oral dose of MET
promotes the protein expression of myocyte-specific enhancer factor
2A and AMPK (7). These results
clearly demonstrated that MET alleviates insulin resistance in
endometrial tissues by increasing levels of GLUT4 expression, thus
improving the conditions of patients with PCOS (7).
In summary, the findings of the current study
demonstrated that the knockdown of GLUT4 was associated with the
development of EH during PCOS, while MET was effective for the
treatment of EH by upregulating the expression of GLUT4 via the
lncRNA-MEG3/miR-223/GLUT4 and lncRNA-SNHG20/miR-4486/GLUT4
signaling pathways. Specifically, MET administration upregulated
the expression of lncRNA-MEG3 and lncRNA-SNHG20, thereby
downregulating the expression of miR-223 and miR-4486,
respectively, leading to an increase in GLUT4 expression.
Acknowledgements
Not applicable.
Funding
This study was supported by the Zhejiang Chinese Medical
University Scientific Research Fund Project (ID: 2020ZG51) and
College Students Science and Technology Innovation Program and
Xinmiao Talent Program of Zhejiang Province (2015R41038).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZZ and JL conceived and designed the study. JL, YCZ
and LC collected the literature, JL, YCZ, LC, RLL, YMN and XZZ
performed the experiments, JL, YCZ and LC collected and analyzed
the data. XZZ and JL confirm the authenticity of all the raw data.
JL, XZZ and YCZ drafted the article. RLL and YMN revised the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was performed in line with the Guide for
the Care and Use of Laboratory Animal by National Research Council
(US) Committee (8th edition) and the protocols were approved by the
Animal Ethics Committee of Zhejiang Chinese Medical University
Laboratory animal research center (approval no. ZSLL-2016-42;
Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Norman RJ, Dewailly D, Legro RS and Hickey
TE: Polycystic ovary syndrome. Lancet. 370:685–697. 2007.
View Article : Google Scholar
|
|
2
|
Ehrmann DA: Polycystic ovary syndrome. N
Engl J Med. 352:1223–1236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Homburg R: Management of infertility and
prevention of ovarian hyperstimulation in women with polycystic
ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 18:773–788.
2004. View Article : Google Scholar
|
|
4
|
Shang K, Jia X, Qiao J, Kang J and Guan Y:
Endometrial abnormality in women with polycystic ovary syndrome.
Reprod Sci. 19:674–683. 2012. View Article : Google Scholar
|
|
5
|
Mioni R, Mozzanega B, Granzotto M,
Pierobon A, Zuliani L, Maffei P, Blandamura S, Grassi S, Sicolo N
and Vettor R: Insulin receptor and glucose transporters mRNA
expression throughout the menstrual cycle in human endometrium: A
physiological and cyclical condition of tissue insulin resistance.
Gynecol Endocrinol. 28:1014–1018. 2012. View Article : Google Scholar
|
|
6
|
Mozzanega B, Mioni R, Granzotto M,
Chiarelli S, Xamin N, Zuliani L, Sicolo N, Marchesoni D and Vettor
R: Obesity reduces the expression of GLUT4 in the endometrium of
normoinsulinemic women affected by the polycystic ovary syndrome.
Ann N Y Acad Sci. 1034:364–374. 2004. View Article : Google Scholar
|
|
7
|
Carvajal R, Rosas C, Kohan K, Gabler F,
Vantman D, Romero C and Vega M: Metformin augments the levels of
molecules that regulate the expression of the insulin-dependent
glucose transporter GLUT4 in the endometria of hyperinsulinemic
PCOS patients. Hum Reprod. 28:2235–2244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Emons G, Beckmann MW, Schmidt D and
Mallmann P; Uterus commission of the Gynecological Oncology Working
G, : New WHO classification of endometrial hyperplasias.
Geburtshilfe Frauenheilkd. 75:135–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Antonsen SL, Ulrich L and Høgdall C:
Patients with atypical hyperplasia of the endometrium should be
treated in oncological centers. Gynecol Oncol. 125:124–128. 2012.
View Article : Google Scholar
|
|
10
|
Kurman RJ, Kaminski PF and Norris HJ: The
behavior of endometrial hyperplasia. A long-term study of
‘untreated’ hyperplasia in 170 patients. Cancer. 56:403–412. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheung AP: Ultrasound and menstrual
history in predicting endometrial hyperplasia in polycystic ovary
syndrome. Obstet Gynecol. 98:325–331. 2001. View Article : Google Scholar
|
|
12
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
mTOR regulation of autophagy. FEBS Lett. 584:1287–1295. 2010.
View Article : Google Scholar
|
|
13
|
Brosius J: Waste not, want not-transcript
excess in multicellular eukaryotes. Trends Genet. 21:287–288. 2005.
View Article : Google Scholar
|
|
14
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar
|
|
15
|
Rapicavoli NA, Qu K, Zhang J, Mikhail M,
Laberge RM and Chang HY: A mammalian pseudogene lncRNA at the
interface of inflammation and anti-inflammatory therapeutics.
Elife. 2:e007622013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu G, Gong AY, Wang Y, Ma S, Chen X, Chen
J, Su CJ, Shibata A, Strauss-Soukup JK, Drescher KM and Chen XM:
LincRNA-Cox2 promotes late inflammatory gene transcription in
macrophages through modulating SWI/SNF-mediated chromatin
remodeling. J Immunol. 196:2799–2808. 2016. View Article : Google Scholar
|
|
17
|
Paladini L, Fabris L, Bottai G, Raschioni
C, Calin GA and Santarpia L: Targeting microRNAs as key modulators
of tumor immune response. J Exp Clin Cancer Res. 35:1032016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shao R, Li X, Feng Y, Lin JF and Billig H:
Direct effects of metformin in the endometrium: A hypothetical
mechanism for the treatment of women with PCOS and endometrial
carcinoma. J Exp Clin Cancer Res. 33:412014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen ZQ, Zhu HT and Lin JF: Reverse of
progestin-resistant atypical endometrial hyperplasia by metformin
and oral contraceptives. Obstet Gynecol. 112:465–467. 2008.
View Article : Google Scholar
|
|
20
|
Pal N, Broaddus RR, Urbauer DL,
Balakrishnan N, Milbourne A, Schmeler KM, Meyer LA, Soliman PT, Lu
KH, Ramirez PT, et al: Treatment of low-risk endometrial cancer and
complex atypical hyperplasia with the levonorgestrel-releasing
intrauterine device. Obstet Gynecol. 131:109–116. View Article : Google Scholar
|
|
21
|
Li X, Guo YR, Lin JF, Feng Y, Billig H and
Shao R: Combination of Diane-35 and metformin to treat early
endometrial carcinoma in PCOS women with insulin resistance. J
Cancer. 5:173–181. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stanosz S: An attempt at conservative
treatment in selected cases of type I endometrial carcinoma (stage
I a/G1) in young women. Eur J Gynaecol Oncol. 30:365–369. 2009.
|
|
23
|
Li X, Feng Y, Lin JF, Billig H and Shao R:
Endometrial progesterone resistance and PCOS. J Biomed Sci.
21:22014. View Article : Google Scholar
|
|
24
|
Cui P, Li X, Wang X, Feng Y, Lin JF,
Billig H and Shao R: Lack of cyclical fluctuations of endometrial
GLUT4 expression in women with polycystic ovary syndrome: Evidence
for direct regulation of GLUT4 by steroid hormones. BBA Clin.
4:85–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Cui P, Jiang HY, Guo YR, Pishdari B,
Hu M, Feng Y, Billig H and Shao R: Reversing the reduced level of
endometrial GLUT4 expression in polycystic ovary syndrome: A
mechanistic study of metformin action. Am J Transl Res. 7:574–586.
2015.PubMed/NCBI
|
|
26
|
Zhang Y, Hu M, Meng F, Sun X, Xu H, Zhang
J, Cui P, Morina N, Li X, Li W, et al: Metformin ameliorates
uterine defects in a rat model of polycystic ovary syndrome.
EBioMedicine. 18:157–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Liu X, Bai X, Lin Y, Li Z, Fu J,
Li M, Zhao T, Yang H, Xu R, et al: Melatonin prevents endothelial
cell pyroptosis via regulation of long noncoding RNA
MEG3/miR-223/NLRP3 axis. J Pineal Res. 64:2018. View Article : Google Scholar
|
|
28
|
Liu J, Cheng LG and Li HG: LncRNA SNHG20
promoted the proliferation of glioma cells via sponging miR-4486 to
regulate the MDM2-p53 pathway. Eur Rev Med Pharmacol Sci.
23:5323–5331. 2019.
|
|
29
|
Zhang Y, Sun X, Sun X, Meng F, Hu M, Li X,
Li W, Wu XK, Brännström M, Shao R and Billig H: Molecular
characterization of insulin resistance and glycolytic metabolism in
the rat uterus. Sci Rep. 6:306792016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Markowicz-Piasecka M, Sikora J, Szydlowska
A, Skupien A, Mikiciuk-Olasik E and Huttunen KM: Metformin-a future
therapy for neurodegenerative diseases: Theme: Drug discovery,
development and delivery in Alzheimer's disease guest editor:
Davide Brambilla. Pharm Res. 34:2614–2627. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pernicova I and Korbonits M:
Metformin-mode of action and clinical implications for diabetes and
cancer. Nat Rev Endocrinol. 10:143–156. 2014. View Article : Google Scholar
|
|
34
|
Miyoshi N, Wagatsuma H, Wakana S,
Shiroishi T, Nomura M, Aisaka K, Kohda T, Surani MA, Kaneko-Ishino
T and Ishino F: Identification of an imprinted gene, Meg3/Gtl2 and
its human homologue MEG3, first mapped on mouse distal chromosome
12 and human chromosome 14q. Genes Cells. 5:211–220. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, Zhou Y, Mehta KR, Danila DC,
Scolavino S, Johnson SR and Klibanski A: A pituitary-derived MEG3
isoform functions as a growth suppressor in tumor cells. J Clin
Endocrinol Metab. 88:5119–5126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Menon AG, Rutter JL, von Sattel JP, Synder
H, Murdoch C, Blumenfeld A, Martuza RL, von Deimling A, Gusella JF
and Houseal TW: Frequent loss of chromosome 14 in atypical and
malignant meningioma: Identification of a putative ‘tumor
progression’ locus. Oncogene. 14:611–616. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Itoyama T, Chaganti RS, Yamada Y,
Tsukasaki K, Atogami S, Nakamura H, Tomonaga M, Ohshima K, Kikuchi
M and Sadamori N: Cytogenetic analysis and clinical significance in
adult T-cell leukemia/lymphoma: A study of 50 cases from the human
T-cell leukemia virus type-1 endemic area, Nagasaki. Blood.
97:3612–3620. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang D, Cao C, Liu L and Wu D:
Up-regulation of LncRNA SNHG20 predicts poor prognosis in
hepatocellular carcinoma. J Cancer. 7:608–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li C, Zhou L, He J, Fang XQ, Zhu SW and
Xiong MM: Increased long noncoding RNA SNHG20 predicts poor
prognosis in colorectal cancer. BMC Cancer. 16:6552016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aravinthan A, Challis B, Shannon N, Hoare
M, Heaney J and Alexander GJM: Selective insulin resistance in
hepatocyte senescence. Exp Cell Res. 331:38–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, Li C, Yang M, Shi L, Tao W, Shen K,
Li X, Wang X, Yang Y and Yao Y: Association of single nucleotide
polymorphisms of miRNAs involved in the GLUT4 pathway in T2DM in a
Chinese population. Mol Genet Genomic Med. 7:e9072019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li H, Li Y, Liu D, Sun H and Liu J:
miR-224 is critical for celastrol-induced inhibition of migration
and invasion of hepatocellular carcinoma cells. Cell Physiol
Biochem. 32:448–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sha M, Ye J, Zhang LX, Luan ZY, Chen YB
and Huang JX: Celastrol induces apoptosis of gastric cancer cells
by miR-21 inhibiting PI3K/Akt-NF-κB signaling pathway.
Pharmacology. 93:39–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang X, Xue XC, Wang Y, Cao FF, You J,
Uzan G, Peng B and Zhang DH: Celastrol reverses palmitic
acid-induced insulin resistance in HepG2 cells via restoring the
miR-223 and GLUT4 pathway. Can J Diabetes. 43:165–172. 2019.
View Article : Google Scholar : PubMed/NCBI
|