Introduction
Psoriasis is an immune-mediated chronic inflammatory
skin disease, in which intralesional T lymphocytes and their
proinflammatory signals trigger keratinocytes to rapidly
proliferate and initiate the inflammatory process (1). Previous studies reported that the
IL-23/T helper (Th)17 axis played a crucial role in the
pathogenesis of psoriasis (2,3).
IL-17A is the most important effector cytokine of Th17 cells, which
is overexpressed in psoriasis, resulting in apparent epidermal
hyperplasia and inflammatory reaction. Targeted anti-IL-17
therapies have been demonstrated to be of great efficacy in
moderate and severe plaque psoriasis (4). Furthermore, in a mouse model of
psoriasis, the crucial roles of IL-17 have also been demonstrated
(5).
Notch signaling is an evolutionarily conserved
intracellular signal transduction system, which is involved in the
regulation of processes of differentiation, proliferation,
migration and apoptosis of epidermal cells, and participates in the
pathogenesis of certain human skin diseases, including psoriasis
(6). In mammals, four Notch
receptors (Notch1, 2, 3 and 4) and five ligands (jagged 1 and 2, as
well as δ-like 1, 3 and 4) have been confirmed. Although the
process of activation and transduction of Notch signaling is
complex, in essence, it involves the cleavage of cytoplasmic Notch
by γ-secretase and the release of the Notch intracellular domain
(NICD) that possesses a nuclear localization signal (NLS) that can
translocate signals to the nuclei and further regulate downstream
target genes, such as hairy and enhancer of split 1 (Hes1)
(7). Notch signaling has been
reported to be crucial for Th17 cell differentiation, which is
activated in Th17 cell polarized conditions in humans and mice
(8–12). A previous study suggested that
blocking Notch signaling with a γ-secretase inhibitor could
significantly alleviate mouse psoriasis-like skin inflammation,
which resulted in a dose-dependent decrease in the percentage of
Th17 cells and its transcription factor RAR-related orphan receptor
(RORγt), as well as a decrease in Notch1, Hes1 and IL-17A mRNA
expression and IL-17A secretion in splenic CD4+ T cells
in a 5% imiquimod (IMQ)-induced mouse psoriasis-like skin model,
indicating that Notch1/Hes1 signaling can regulate Th17 cell
differentiation and function in the disease-specific inflammation
of psoriasis (13). NF-ΚB
activator1(Act1) is an important adaptor molecule in IL-17
signaling transduction (14),
which can interact with Notch1 signaling and form an Act1-NICD1
complex to induce Notch1 activation and facilitate IL-17 crosstalk
with Notch1 signaling (15).
PI3K/AKT (also called protein kinase B) and mTOR
signaling is important in the regulation of innate and adaptive
immune responses (16), which has
been reported to be upregulated in lesions of patients with
psoriasis and in IMQ-induced mouse psoriasiform inflammation
(17,18). mTOR exists in two distinct protein
complexes, namely mTORC1 and mTORC2. PI3K/AKT/mTORC1 signaling has
been reported to positively regulate Th17 cell differentiation
through several means, including the regulation of transcription
factor hypoxia-inducible factor 1α expression, signal transducers
and activators of transcription 3 tyrosine phosphorylation,
downregulation of the Th17 cell differentiation negative regulator
growth factor independent 1 (Gfi1) and RORγt nuclear translocation
(19,20), among which, Gfi1 expression and
RORγt nuclear translocation are dependent on the signaling
molecules 40S ribosomal S6 kinase (S6K)1 and S6K2, which are
located downstream of mTORC1. Gfi1 expression is suppressed by the
transcription factor early growth response protein 2, which is
positively regulated by S6K1 (19). RORγt does not possess a NLS but is
localized in the nuclei of Th17 cells. S6K2, the nuclear
counterpart of S6K1, possesses a RORγt-associated NLS, and can bind
and transport RORγt to nuclei. Thus, mTORC1 can accelerate RORγt
translocation into nuclei during Th17 differentiation (19). PTEN is the critical negative
regulator of PI3K/AKT/mTOR signaling, which was reported to be a
target of activated Notch1 in T-cell acute lymphoblastic leukemia,
via Hes1-mediated suppression of the PTEN promoter (21,22). In addition, the regulatory
function of the Notch1/Hes1/PTEN axis has also been identified in
other human diseases, including asthma, diabetic nephropathy and
hepatocellular carcinoma (23–27). Based on these previous studies and
our previous research, it could be hypothesized that a feedback
loop between Notch1 and IL-17A exists via the
Notch1/Hes1-PTEN/AKT/mTORC1 signaling axis within the pathological
environment of psoriasis. Thus, PI3K/AKT signaling was blocked in
the present study using LY294002 to explore the possible regulatory
effect of the Notch1/Hes1-PTEN/AKT/IL-17A feedback loop on Th17
cell differentiation and IL-17A production in mouse psoriasis-like
skin inflammation.
Materials and methods
Mice and treatment
A total of 24 male BALB/c mice (age, 6–8 weeks old;
weight range, 16–20 g) were purchased from Jinan Pengyue
Experimental Animal Breeding Co., Ltd., China and bred in a
specific pathogen-free environment in the animal center of Binzhou
Medical University Hospital (Binzhou, China). They were
group-housed at up to five mice per cage in a ventilated,
temperature-controlled 23±1°C, 55% relative humidity condition with
a 12-h light/dark cycle. Autoclaved water and food were available
ad libitum to mice. The experimental mice were randomly
divided into 3 groups, namely the control group (n=8), the model
group (5% IMQ-induced group; n=8) and the intervention group (5%
IMQ-induced plus LY294002-treated group; n=8). Daily topical
application of 5% IMQ cream (62.5 mg; 3M Health Care Limited) on
the shaved back skin of the model and intervention mice were
employed to induce psoriasis-like skin inflammation in these
groups, as previously described (5), while an equivalent quantity of
Vaseline (Qingdao Hainuo Biological Engineering Co., Ltd.) was
applied to the control mice. LY294002 (cat. no. HY-10108;
MedChemExpress) dissolved in DMSO (cat. no. D2650; Sigma-Aldrich;
Merck KGaA) was intraperitoneally injected (10 mg/kg/day) in the
intervention mice since the beginning of IMQ application. Control
and model mice received an equivalent volume of DMSO
intraperitoneal injection. The experimental mice were anesthetized
by 4% isoflurane for the induction and 2% for the maintenance to
complete daily treatment. After 6 consecutive days, mice were
euthanized by inhaling 10% isoflurane for 5 min. Animal death was
confirmed by observing respiratory and cardiac arrest and the
absence of active paw reflex for >5 min. Next, the skin tissues,
inguinal lymph nodes and spleens were acquired to conduct
subsequent experiments. During the experimental process, if their
weights reduced 20%, the skins occurred infection and suppuration
or they appeared obvious fidgety, mice would be subjected to
euthanasia. All animal procedures were approved (approval no.
20190104-15) by the Laboratory Animal Ethics Committee of Binzhou
Medical University Hospital (Binzhou, China), followed the
3R-principle (replacement, reduction, refinement) and carried out
in accordance with the UK Animals (Scientific Procedures) Act,
1986, and associated guidelines, and the EU Directive 2010/63/EU
for animal experiments.
Skin structural characteristics
scoring and histopathological examination
Changes in skin structural characteristics were
recorded daily, and the severity of psoriasis-like inflammation was
estimated by the target lesion score based on the clinical
psoriasis area and severity index (5). Erythema, thickening and scaling were
scored on a scale from 0 to 4. The cumulative score of the three
indicators from 0 to 12 served as a measure of the severity of
psoriasis-like inflammation. Skin samples were fixed in 10% neutral
formalin for 24 h at 4°C, embedded with paraffin, sectioned, and
stained with hematoxylin and eosin using standard procedures
(hematoxylin staining for 8 min and eosin staining for 1 min at
room temperature) (28).
Histopathological characteristics were evaluated by well-trained
pathologists in a double-blinded manner. Image-Pro Plus 6.0 imaging
system (Media Cybernetics, Inc.) was employed to measure the
epidermis thickness from the stratum corneum to the basement
membrane.
Preparation of single cell suspension
from skin tissues and spleen
Skin samples were cut into 0.5 cm × 0.5 cm pieces
and incubated in the presence of 0.5% trypsin (cat. no. Y0002311;
Sigma-Aldrich; Merck KGaA) at 37°C for 2 h. Upon separation of the
epidermis, the dermis was digested with DNase I (cat. no. D8071;
Beijing Solarbio Science & Technology Co., Ltd.) and
collagenase IV (cat. no. C5138; Sigma-Aldrich; Merck KGaA) in DMEM
(cat. no. E600008-0500; Sangon Biotech Co., Ltd.) for 37°C, 1 h.
Cells were harvested and resuspended (1×106 cells/ml)
for use in subsequent flow cytometry analysis.
Spleen samples were triturated and filtered through
cell strainers (70 and 40 µm; cat. no. F613462 and F613461; Sangon
Biotech Co., Ltd.). Cell suspension was collected and treated
according to the protocol of mouse splenic mononuclear cell
isolation kit (cat. no. LDS1090PK; Tianjin Haoyang Biological
Products Technology Co., Ltd.). Splenic mononuclear cells were
acquired and suspended in RPMI-1640 medium (cat. no. E600028;
Sangon Biotech Co., Ltd.) with 10% fetal bovine serum (cat. no.
E510008-0500; Sangon Biotech Co., Ltd.), 1% penicillin-streptomycin
liquid, 1% nonessential amino acid and 0.01% β-mercaptoethanol
(cat. nos. P1400, N1250 and M8210, respectively; Beijing Solarbio
Science & Technology Co., Ltd.). Then cells were counted and
adjusted to 106 cells/ml for subsequent treatment and
experiments.
Splenic mononuclear cells treatment by
LY294002
Splenic mononuclear cells isolated from 5%
IMQ-induced model mice were divided into 0, 10 and 50 µM
LY294002-treated group. In LY294002-treated groups, LY294002 was
dissolved in DMSO. At the same time, splenic mononuclear cells
isolated from control mice were used as control group and treated
with DMSO only.
Flow cytometric analysis of the
proportion of Th17 cells (IL-17A+ CD4+ T
cells/CD4+ T cells)
Cells were firstly stimulated with phorbol
12-myristate 13-acetate, ionomycin, brefeldin A and monensin (Cell
Stimulation Cocktail Plus Protein Transport Inhibitors; cat. no.
4975; eBioscience; Thermo Fisher Scientific, Inc.) for 4 h at 37°C
under 5% CO2. Next, the cells were collected (630 × g
for 10 min at room temperature), washed two times with ice-cold
phosphate-buffered saline (PBS) (cat. no. E607008-0500; Sangon
Biotech Co., Ltd.) and surface-stained with an Allophycocyanin
(APC) anti-mouse CD4 Antibody (0.8 µg/ml, cat. no. 100516;
BioLegend, Inc.) for 30 min at 4°C in the dark. Next, the cells
were fixed and permeabilized using Perm/Fix (cat. no. 88-8824-00;
eBioscience) solution and intracellularly stained with a
P-phycoerythrin (PE) anti-mouse IL-17A Antibody (2.5 µg/ml, cat.
no. 506904; BioLegend, Inc.) for 30 min at 4°C in the dark.
Detection and analysis were conducted with a FACSCanto flow
cytometer (BD Biosciences). The characterized phenotype of Th17
cells was IL-17A+ CD4+ and the results are
expressed as a percentage of total CD4+ T cells.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
According to the manufacturer's instructions, total
RNA was extracted from each skin sample and spleen using
TRIzol® (cat. no. B511311; Sangon Biotech Co., Ltd.).
The purity and integrity of each RNA sample were confirmed,
respectively, by measuring an absorbance 260/A280 ratio of 1.8-2.0,
as detected by spectrophotometry (NV3000; Vastech Inc.), and by
observing intact 28S and 18S ribosomal RNA bands with a 2:1 ratio
in 1.5% agarose gel electrophoresis with AG-CelRed Nucleic acid gel
stain (cat. no. AG11918; Hunan Accurate Biotechnology Co., Ltd.).
cDNA was synthesized utilizing the Evo M-MLV Mix Kit with gDNA
Clean for qPCR (cat. no. AG11728; Hunan Accurate Biotechnology Co.,
Ltd.) according to the manufacturer's protocols. RT-qPCR was
performed with gene-specific primers using SYBR® Green
Premix Pro Taq HS qPCR kit (cat. no. AG11701; Hunan Accurate
Biotechnology Co., Ltd.) on a CFX96 Touch™ Real-Time PCR
Detection System (Bio-Rad Laboratories, Inc.). All primers were
designed by Hunan Accurate Biotechnology Co., Ltd. The National
Center for Biotechnology Information accession numbers are as
follows: 14433 (GAPDH), 18128 (Notch1), 15205 (Hes1), 19211 (PTEN),
11651 (AKT), 74370 (mTORC1), 72508 (S6K1), 58988 (S6K2) and 16171
(IL-17A). The primer sequences were as follows: Notch1 sense,
5′-TGCCAGTATGATGTGGATGAG-3′ and antisense,
5′-GGTCCCTGTGTAACCTTCTGT-3′; Hes1 sense,
5′-AGCCCACCTCTCTCTTCTGA-3′, and antisense,
5′-AGGCGCAATCCAATATGAAC-3′; PTEN sense,
5′-AAGGGACGGACTGGTGTAATGATTTG-3′ and antisense,
5′-CGCCTCTGACTGGGAATTGTGAC-3′; AKT sense,
5′-GACTGACACCAGGTATTTCGATGA-3′ and antisense,
5′-CTCCGCTCACTGTCCACACA-3′; mTORC1 sense,
5′-CCATCGGTGCAAACCTACAG-3′ and antisense,
5′-TCCATTCACTGTAGGCCTGG-3′; S6K1 sense, 5′-TTCTGTCGGGAGTAGCACTG-3′
and antisense, 5′-CCCCTTTACCAAGTACCCGA-3′; S6K2 sense,
5′-TCACTGCAGAGAACCGGAAGA-3′ and antisense,
5′-GGGGTTCCGCTTCAGAAACTT-3′; IL-17A sense,
5′-TTTAACTCCCTTGGCGCAAAA-3′ and antisense,
5′-CTTTCCCTCCGCATTGACAC-3′; and GAPDH sense,
5′-AAATGGTGAAGGTCGGTGTGAAC-3′ and antisense,
5′-CAACAATCTCCACTTTGCCACTG-3′. The thermocycling conditions were as
follows: 95°C for 5 min, followed by 40 cycles of denaturation for
10 sec at 95°C and annealing/elongation for 30 sec at 60°C. GAPDH
was used as the internal control, and the expression levels of
target genes were calculated based on the following formula
2−ΔΔCq (ΔΔCq=ΔCq-treated-ΔCq-control) (29).
Western blot detection and
analysis
Total protein from skin samples and treated splenic
mononuclear cells were extracted using RIPA lysis buffer (cat. no.
R0020; Beijing Solarbio Science & Technology Co., Ltd.). Equal
quantities of denatured protein (50 µg) were separated by 10%
sodium dodecyl sulphate-polyacrylamide gel electrophoresis and
transferred to polyvinylidene fluoride (cat. no. ipvh00010;
Sigma-Aldrich; Merck KGaA) membranes. After blocking in 5% skim
milk in 0.1% TBS-Tween 20 (TBST; cat. no. T1082; Beijing Solarbio
Science & Technology Co., Ltd.) for 2 h at room temperature,
the membranes were incubated overnight at 4°C with primary
antibodies (all from Affinity Biosciences) against Notch1 (1:1,000;
cat. no. AF5307), NICD1 (1:1,000; cat. no. AF5307), Hes1 (1:1,000;
cat. no. AF7575), PTEN (1:1,000; cat. no. AF6351), AKT (1:1,000;
cat. no. AF6261), phosphorylated (p)-AKT (Thr308) (1:1,000; cat.
no. AF3262), p-AKT (Ser473) (1:1,000; cat. no. AF0016), mTORC1
(1:1,000; cat. no. AF6308), p-mTOR (Ser2448) (1:1,000, cat. no.
AF3308), S6K1 (p70S6K) (1:1,000; cat. no. AF6226), S6K2 (p70S6kβ)
(1:1,000; cat. no. AF3486) and IL-17A (1:1,000; cat. no. DF6127).
The next day, each membrane was washed 3 times for 5 min with
0.1%TBST, on a shaking table, at room temperature. The membranes
were incubated with the corresponding horseradish
peroxidase-conjugated goat anti-rabbit IgG antibodies (1:1,000;
cat. no. 14708; Cell Signaling Technology, Inc.) for 1 h at room
temperature. Anti-GAPDH antibody (1:1,000; cat. no. AF7021;
Affinity Biosciences) was used to confirm equal protein loading in
each lane. The protein bands were detected using ECL kit (cat. no.
MA0186; Dalian Meilun Biology Technology Co., Ltd.) and x-ray film,
and were quantified using ImageJ software v 4.1 (National
Institutes of Health).
Statistical analysis
Shapiro-Wilk test was performed to evaluate the
normality of the data, and the data were expressed as the means ±
SD or median (interquartile range). According to Levene's test of
homogeneity of variance, one-way ANOVA was used to compare the
differences among the experimental groups for homogeneous variance,
followed by Student-Newman-Keuls (SNK) or Tukey's test performing
the multiple comparison for three groups or four groups
respectively; Welch ANOVA followed by Tamhane's T2 test was used
for comparison of heterogeneous variance. Kruskal-Wallis H test
followed by the Dunn-Bonferroni test were used to analyze the
abnormally distributed data. The difference of lesions' scores was
analyzed with the method of repeated measures ANOVA followed by
Bonferroni's adjustments. Data were analyzed using SPSS 19.0 (IBM
Corp.) and GraphPad Prism 5 (GraphPad Software, Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
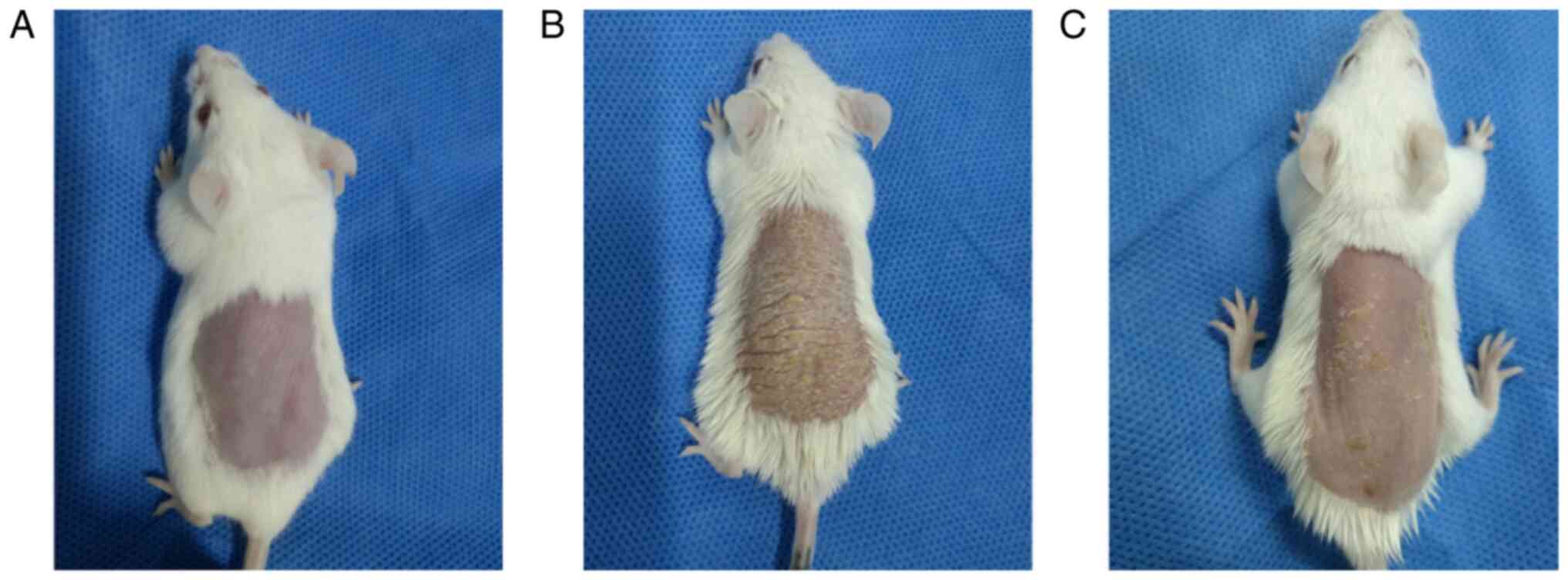
LY294002 alleviates the severity of
psoriasiform skin lesions
Control mice presented no signs of erythema,
thickening or scaling during the 6 days. By contrast, the model
mice presented signs of psoriasis-like inflammation on their shaved
back skin from the second day onwards, which exacerbated
progressively and was most severe on the 6th day. LY294002
inhibition alleviated the degree of erythema, thickening and
scaling in the intervention mice (Fig. 1). The scores of lesions of control
mice, model mice and intervention mice were listed in Table I and were significantly different
among the three experimental groups (F=1751.182, P<0.01);
moreover, the differences between every two experimental groups
were significant (all P<0.01).
 | Table I.Psoriasis area and severity index
scores of experimental mice. |
Table I.
Psoriasis area and severity index
scores of experimental mice.
| Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 |
|---|
| Control mice | 0.000±0.000 | 0.000±0.000 | 0.000±0.000 | 0.000±0.000 | 0.000±0.000 | 0.000±0.000 |
| Model mice | 0.000±0.000 | 2.875±0.354 | 5.500±0.535 | 7.00±0.535 | 8.625±0.518 | 10.125±0.835 |
| Intervention
mice | 0.000±0.000 |
2.000±0.535a |
3.625±0.518a |
4.375±0.518a |
6.500±0.535a |
6.000±0.535a |
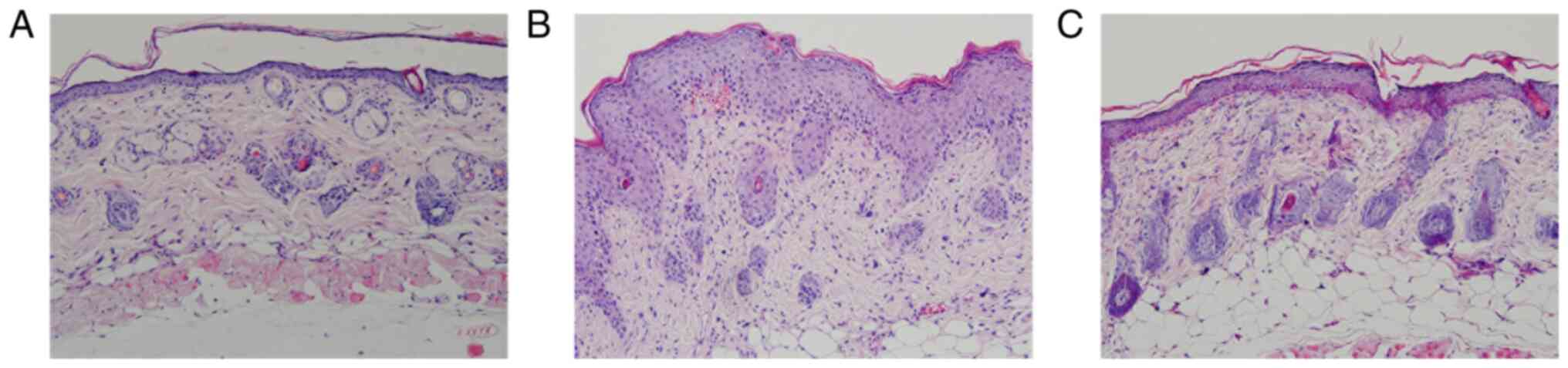
LY294002 attenuates the pathological
characteristics of psoriasiform skin lesions
Histopathological examination revealed that, in
control mice, the epidermis was thin and consisted of 1–2 cell
thick layers. Model mice presented marked epidermal hyperplasia
with hyperkeratosis, trochanterellus extension, parakeratosis with
Munro's micro-abscess, extensive inflammatory cell infiltration and
dermal telangiectasias, which were similar to previously reported
findings and matched the pathological characteristics of psoriasis
(5). Compared with the model
group, LY294002 inhibition notably ameliorated the degree of
epidermal hyperplasia and dermal inflammatory cell infiltration in
the intervention group (Fig. 2).
Further comparison of epidermal thickness showed significant
differences among the three groups (F=192.233; P<0.01) and
between two groups (model mice, 104.669±11.127 µm vs. intervention
mice, 49.983±7.656 µm and control mice, 26.480±4.301 µm; all
P<0.01).
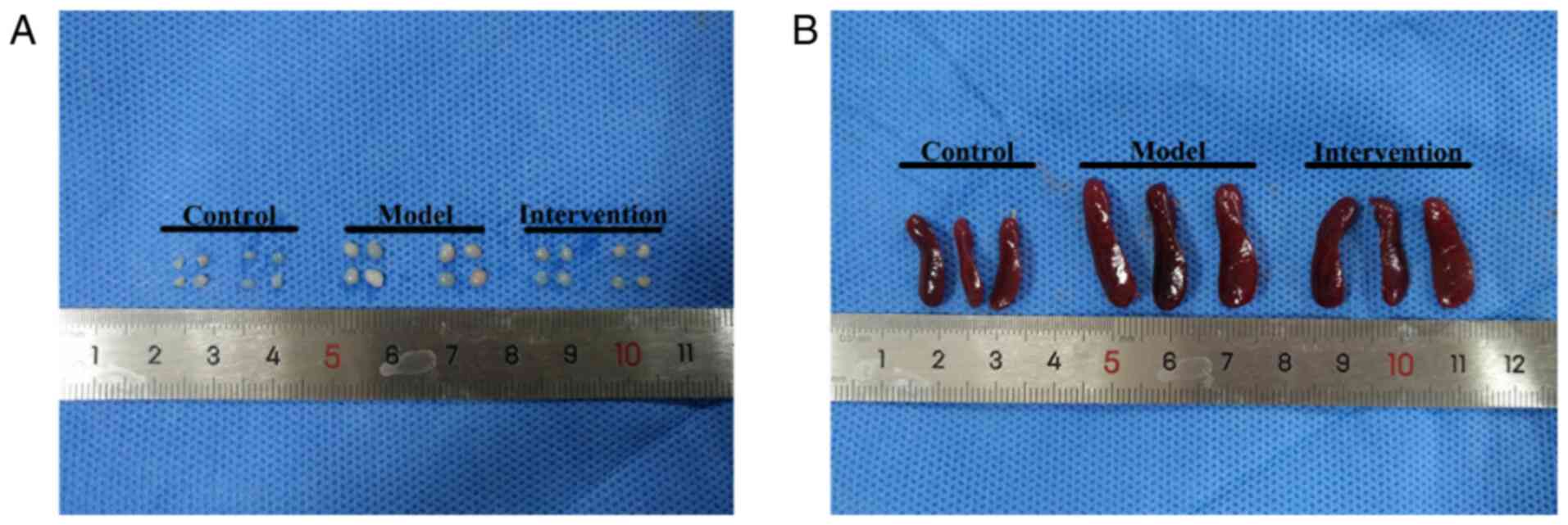
LY294002 mitigates splenomegaly and
lymphadenopathy
The volumes of the spleen and inguinal lymph nodes
in model mice were significantly larger than those of the spleen
and inguinal lymph nodes of control mice, while lessened after
intervention with LY294002 (Fig.
3). As shown in Table II,
compared with those of control mice, the spleen weight and index of
model mice were significantly increased (both P<0.01). However,
upon AKT inhibition by LY294002, they were markedly decreased in
the intervention mice (both P<0.01). In parallel with the
changes in spleen, the weight of inguinal lymph nodes notably
increased in the model mice compared with that of the control mice,
and upon LY294002 treatment, it was markedly reduced in the
intervention mice (both P<0.01; Table II).
 | Table II.Inguinal lymph node weight, spleen
mass and spleen index of experimental mice (each n=8). |
Table II.
Inguinal lymph node weight, spleen
mass and spleen index of experimental mice (each n=8).
| Group | Inguinal lymph node
weight (g) | Spleen mass
(g) | Spleen index
(mg/g) |
|---|
| Control | 4.863±0.544 | 0.253±0.024 | 0.118±0.010 |
| Model |
15.760±0.716a |
0.408±0.024a |
0.214±0.013a |
| Intervention |
9.943±0.701b |
0.348±0.009b |
0.175±0.006b |
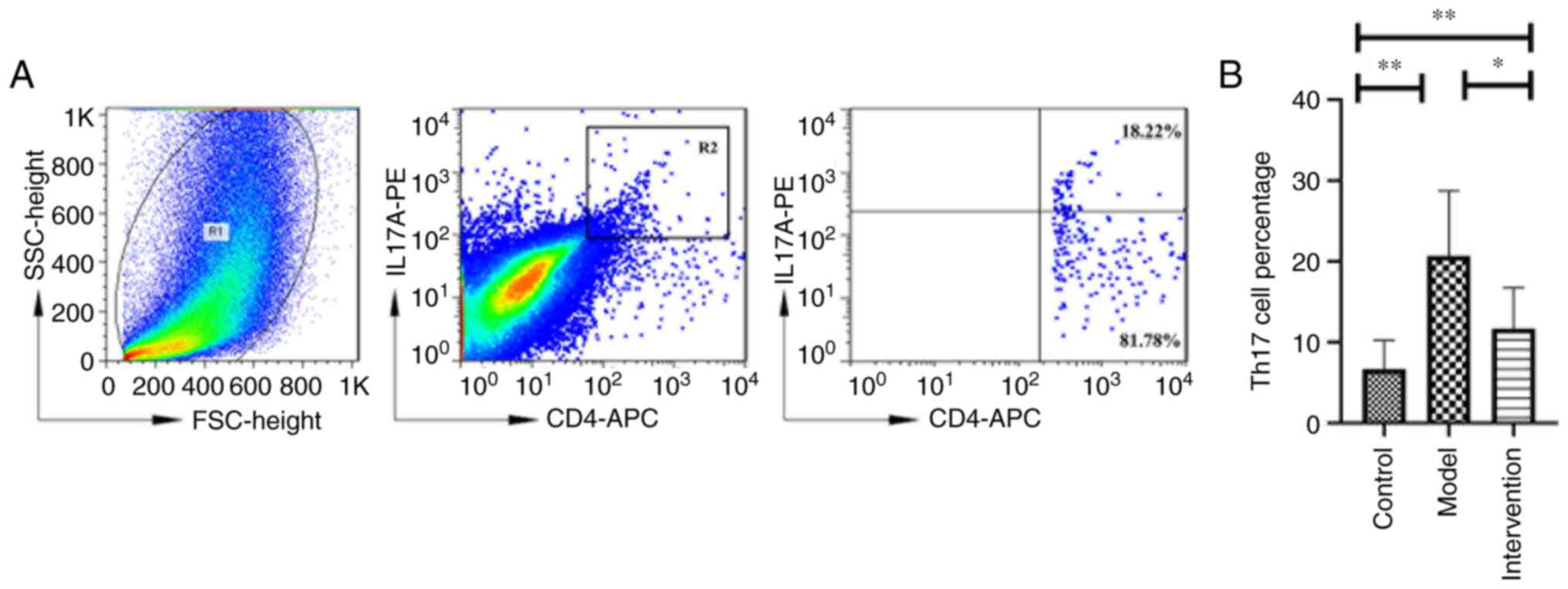
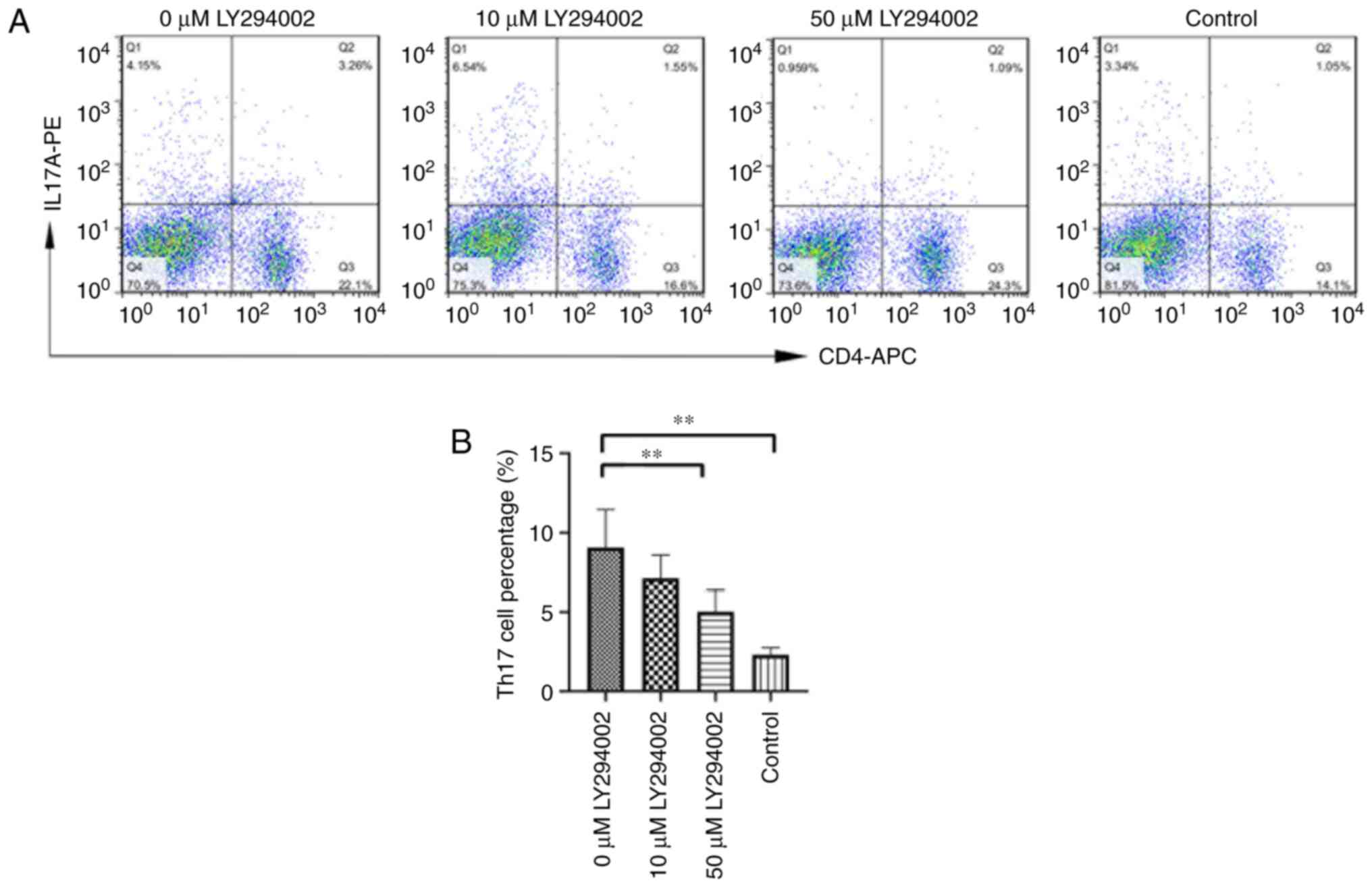
LY294002 inhibition reduces Th17 cell
percentage
Representative images of Th17 cell percentage and
IL-17A+ CD4+ cells gated on CD4+ T
cells in the three aforementioned groups are shown in Fig. 4. There were significant
differences in Th17 cell percentage in the skin tissues among the
three experimental groups (F=8.482; P<0.01), which was markedly
higher in model mice (20.521±8.023%) than in the control mice
(6.670±3.545%; P<0.01). However, upon LY294002 treatment, the
Th17 cell percentage significantly decreased in the intervention
mice (11.540±5.278%) compared with that in the model mice
(P<0.05).
LY294002 inhibition downregulates
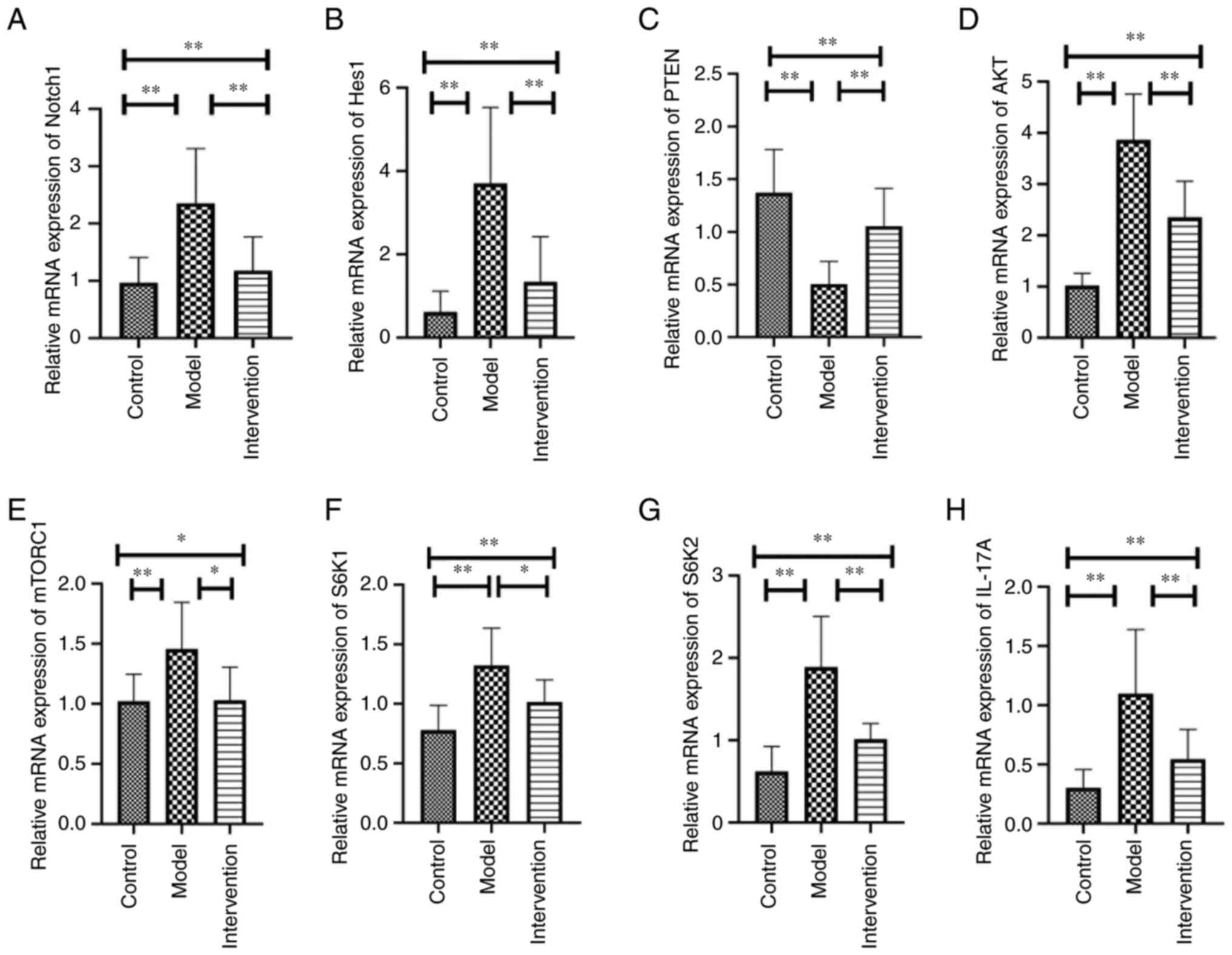
Notch1, Hes1, AKT, mTORC1, S6K1, S6K2 and IL-17A mRNA expression,
but upregulates PTEN mRNA expression
The mRNA expression levels of Notch1, Hes1, PTEN,
AKT, mTORC1, S6K1, S6K2 and IL-17A in skin samples among the three
experimental groups presented remarkable differences (all
P<0.05; Table III; Fig. 5). In addition, further comparisons
between two groups showed that the mRNA expression levels of
Notch1, Hes1, AKT, mTORC1, S6K1, S6K2 and IL-17A were significantly
higher in model mice than in control mice (all P<0.05; Table III; Fig. 5), while LY294002 inhibition
resulted in a significantly reduced mRNA expression of the
aforementioned molecules in the intervention mice (all P<0.05;
Table III; Fig. 5). PTEN mRNA expression in model
mice was significantly lower compared with that in control mice,
but was considerably reversed by LY294002 treatment in intervention
mice (both P<0.01; Table
III; Fig. 5).
 | Table III.mRNA expression of Notch1, Hes1,
PTEN, AKT, mTORC1, S6K1, S6K2 and IL-17A in skin samples of
experimental mice (each n=8). |
Table III.
mRNA expression of Notch1, Hes1,
PTEN, AKT, mTORC1, S6K1, S6K2 and IL-17A in skin samples of
experimental mice (each n=8).
| Group | Notch1 | Hes1 | PTEN | AKT | mTORC1 | S6K1 | S6K2 | IL-17A |
|---|
| Control | 0.966±0.442 | 0.617±0.501 | 1.369±0.411 | 1.024±0.238 | 1.021±0.224 | 0.789±0.189 | 0.621±0.305 | 0.302±0.156 |
| Model |
2.351±0.958a |
3.704±1.823a |
0.504±0.215a |
3.864±0.891a |
1.455±0.389a |
1.323±0.313a |
1.888±0.615a |
1.097±0.543a |
| Intervention |
1.178±0.590b |
1.341±1.085b |
1.052±0.359b |
2.352±0.706b |
1.030±0.274c |
1.016±0.185c |
1.015±0.189b |
0.545±0.252b |
| F-value | 9.146 | 13.154 | 13.361 | 35.941 | 5.339 | 11.473 | 19.893 | 10.402 |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.05 | <0.01 | <0.01 | <0.01 |
LY294002 inhibition reduces the
protein levels of Notch1, NICD1, Hes1, p-AKT (Ser473), p-AKT
(Thr308), p-mTOR (Ser2448), S6K1 (p70S6K), S6K2 (p70S6Kβ) and
IL-17A, but increases PTEN protein levels
There were marked differences in the protein levels
of Notch1, NICD1, Hes1, PTEN, p-AKT (Ser473), p-AKT (Thr308),
p-mTOR (Ser2448), S6K1 (p70S6K), S6K2 (p70S6Kβ) and IL-17A in skin
tissues among the three experimental groups (all P<0.05;
Table IV; Fig. 6). The protein levels of Notch1,
NICD1, Hes1, p-AKT (Ser473), p-AKT (Thr308), p-mTOR (Ser2448), S6K1
(p70S6K), S6K2 (p70S6Kβ) and IL-17A were higher in the model mice
than in control mice (all P<0.05; Table IV; Fig. 6). However, upon LY294002
treatment, the expression of all of these proteins were markedly
decreased in the intervention mice (all P<0.05). By contrast,
the PTEN protein level was significantly reduced in model mice
compared with that in control mice (P<0.01), whereas following
LY294002 treatment, it was elevated in the intervention mice
(P<0.01; Table IV; Fig. 6).
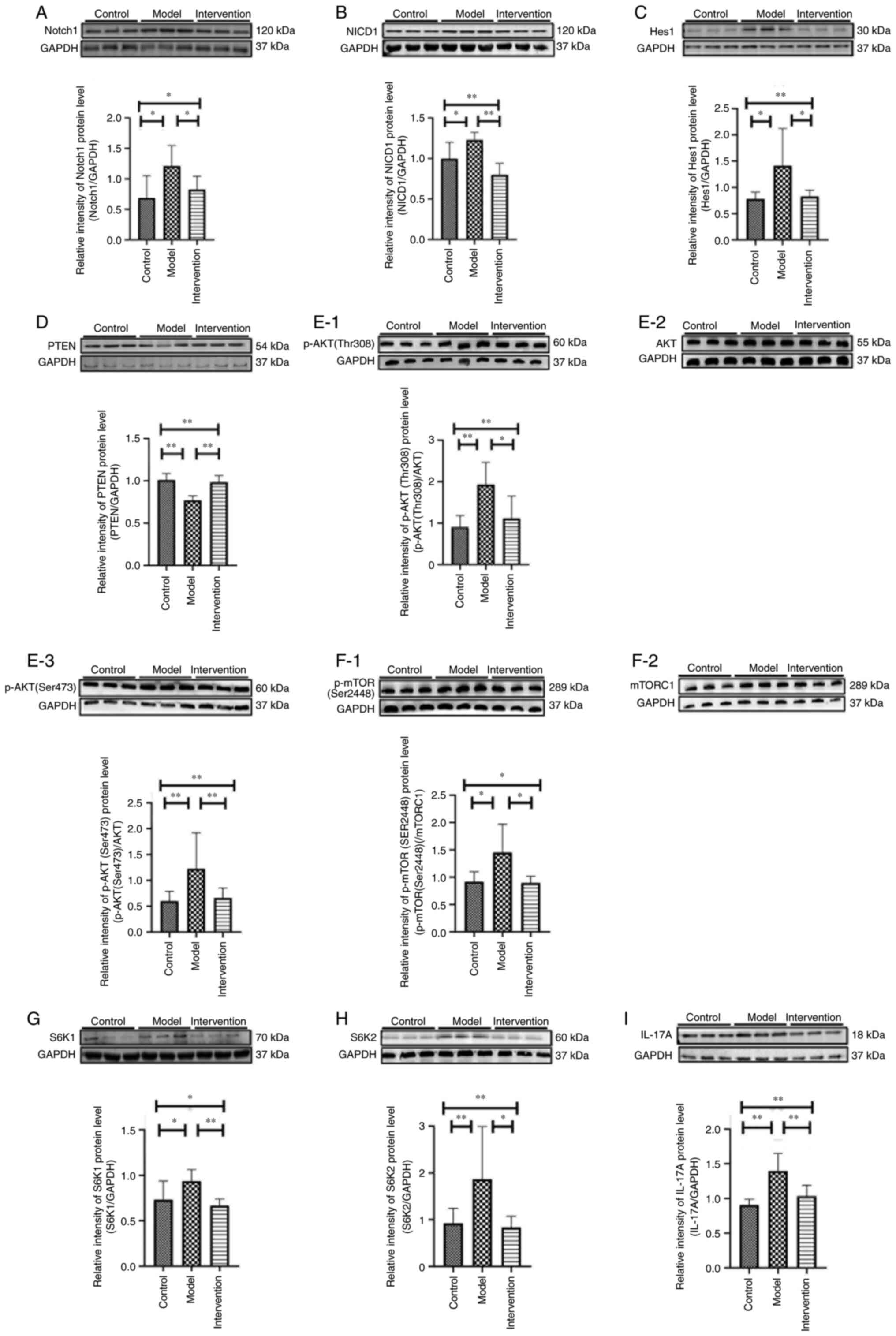 | Figure 6.Protein levels of (A) Notch1, (B)
NICD1, (C) hairy and enhancer of split 1, (D) PTEN, (E-1) p-AKT
(Thr308), (E-2) AKT, (E-3) p-AKT (Ser473), (F-1) p-mTOR (Ser2448),
(F-2) mTORC1, (G) S6K1 (p70S6Kα), (H) S6K2 (p70S6Kβ) and (I) IL-17A
in skin samples of experimental mice. *P<0.05 and **P<0.01.
NICD1, Notch intracellular domain 1; p, phosphorylated; S6K, S6
kinase. Since protein bands of p-AKT (Thr308), p-AKT (Ser473) and
AKT are not from the same western blot membrane, they are evaluated
with each corresponding loading control, then the protein levels of
p-AKT (Thr308) and p-AKT (Ser473) are calculated by comparing with
that of AKT. Similarly, protein bands of p-mTOR (Ser2448) and
mTORC1 are evaluated with each corresponding loading control, then
the protein level of p-mTOR (Ser2448) is calculated by comparing
with that of mTORC1. |
 | Table IV.Protein levels of Notch1, NICD1,
Hes1, PTEN, p-AKT (Thr308), p-AKT (Ser473), p-mTOR (Ser2448), S6K1
(p70s6kα), S6K2 (p70s6kβ) and IL-17A in skin samples of
experimental mice (each n=8). |
Table IV.
Protein levels of Notch1, NICD1,
Hes1, PTEN, p-AKT (Thr308), p-AKT (Ser473), p-mTOR (Ser2448), S6K1
(p70s6kα), S6K2 (p70s6kβ) and IL-17A in skin samples of
experimental mice (each n=8).
| Group | Notch1 | NICD1 | Hes1 | PTEN | p-AKT(Thr308) |
|---|
| Control | 0.688±0.365 | 0.996±0.205 | 0.735 (0.130) | 1.011±0.077 | 0.908±0.273 |
| Model |
1.211±0.339b |
1.229±0.095b | 1.188
(0.680)b |
0.770±0.053a |
1.928±0.536a |
| Intervention |
0.824±0.220d |
0.798±0.141c | 0.819
(0.210)d |
0.984±0.079c |
1.111±0.540d |
|
F-value/χ2 | 4.476 | 11.800 | 9.696 | 21.027 | 8.037 |
| P-value | <0.05 | <0.01 | <0.01 | <0.01 | <0.01 |
| Group | p-AKT (Ser473) | p-mTOR
(Ser2448) | S6K1 | S6K2 | IL-17A |
| Control | 0.601±0.190 | 0.843 (0.190) | 0.731±0.205 | 0.824 (0.63) | 0.905±0.084 |
| Model |
1.226±0.696b | 1.184
(0.900)b |
0.936±0.128b | 1.419
(0.990)a |
1.396±0.250a |
| Intervention |
0.659±0.197d | 0.920
(0.200)d |
0.667±0.073c | 0.839
(0.440)d |
1.033±0.159c |
|
F-value/χ2 | 3.833 | 8.983 | 5.577 | 10.433 | 12.310 |
| P-value | <0.05 | <0.05 | <0.05 | <0.01 | <0.01 |
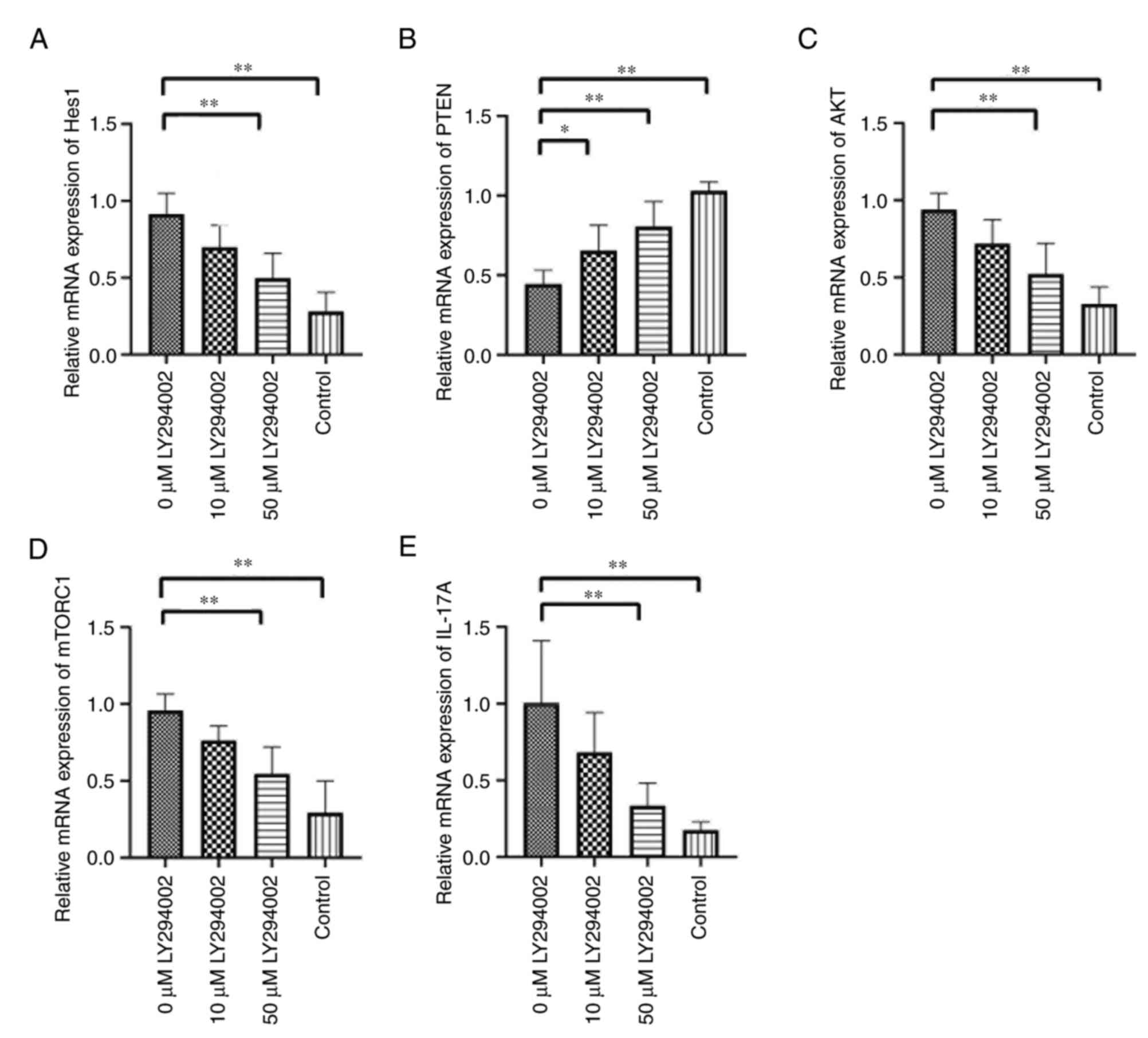
High dose of LY294002 presents even
more obviously regulatory effect on the percentage of Th17 cells as
well as mRNA expression and protein levels of Hes1, PTEN, AKT,
mTORC1 and IL-17A
The percentage of Th17 cells, mRNA expression of
Hes1, AKT, mTORC1 and IL-17A as well as protein levels of Hes1,
p-AKT (Ser473), p-AKT (Thr308), p-mTOR (Ser2448) and IL-17A were
significantly increased, while the expression levels of PTEN were
obviously decreased in splenic mononuclear cells from 5%
IMQ-induced mice compared with control mice (P<0.01; Tables V and VI; Fig.
7,Fig. 8,9). LY294002 treatment on 5% IMQ-induced
mouse splenic mononuclear cells dramatically reduced Th17 cell
percentage, Hes1, AKT, mTORC1 and IL-17A mRNA expression and their
corresponding protein levels, while increased PTEN expression
levels were confirmed in 50 µM LY294002-treated group (P<0.01;
Tables V and VI; Fig.
7,Fig. 8,9).
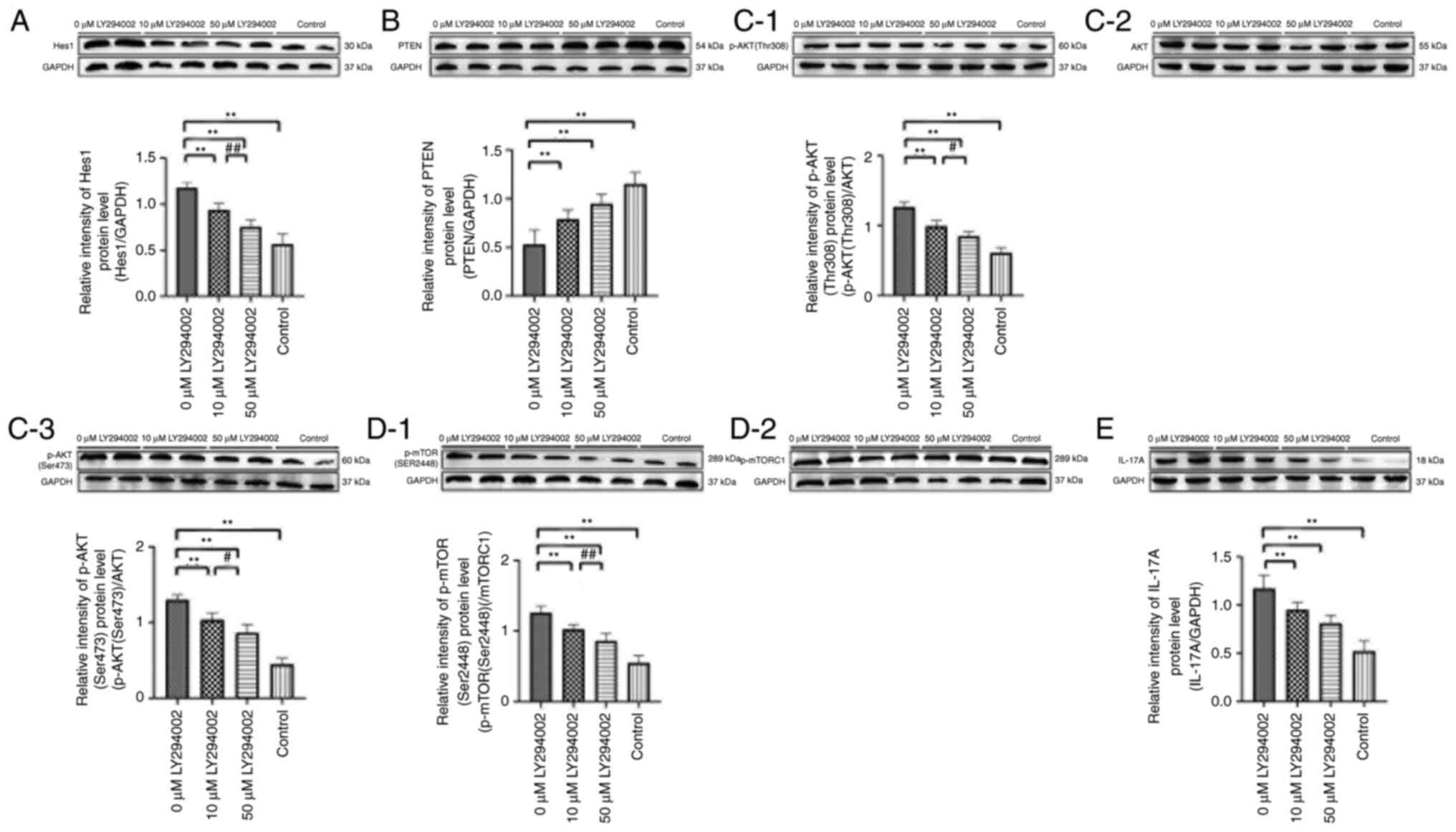 | Figure 9.Protein levels of (A) hairy and
enhancer of split 1, (B) PTEN, (C-1) p-AKT (Thr308), (C-2) AKT,
(C-3) p-AKT (Ser473), (D-1) mTORC1, (D-2) p-mTOR (Ser2448), (E)
IL-17A in mouse splenic mononuclear cells of control group, 0 µM
LY294002 group, 10 µM LY294002 group and 50 µM LY294002 group.
**P<0.01, #P<0.05 and ##P<0.01. p-,
phosphorylated. Since protein bands of p-AKT (Thr308), p-AKT
(Ser473) and AKT are not from the same western blot membrane, they
are evaluated with each corresponding loading control, then the
protein levels of p-AKT (Thr308) and p-AKT (Ser473) are calculated
by comparing with that of AKT. Similarly, protein bands of p-mTOR
(Ser2448) and mTORC1 are evaluated with each corresponding loading
control, then the protein level of p-mTOR (Ser2448) is calculated
by comparing with that of mTORC1. |
 | Table V.mRNA expression of Hes1, PTEN, AKT,
mTORC1 and IL-17A and Th17 cell percentage in splenic mononuclear
cells of experimental mice (each n=6). |
Table V.
mRNA expression of Hes1, PTEN, AKT,
mTORC1 and IL-17A and Th17 cell percentage in splenic mononuclear
cells of experimental mice (each n=6).
| Group | Hes1 | PTEN | AKT | mTORC1 | IL-17A | Th17 cell percent
(%) |
|---|
| 0 µM LY294002 | 0.914±0.135 | 0.444±0.087 | 0.939±0.107 | 0.959±0.105 | 1.005±0.406 | 9.074±2.381 |
| 10 µM LY294002 | 0.698±0.144 |
0.654±0.160b | 0.720±0.156 | 0.764±0.095 | 0.682±0.260 | 7.130±1.449 |
| 50 µM LY294002 |
0.497±0.163a |
0.806±0.157a |
0.522±0.200a |
0.546±0.175a |
0.334±0.149a |
5.024±1.410a |
| Control |
0.281±0.125a,c |
1.031±0.055a,c |
0.329±0.110a,c |
0.293±0.207a,c |
0.175±0.054a,d |
2.305±0.469a,c |
| F-value | 21.728 | 24.183 | 18.815 | 21.005 | 12.772 | 20.261 |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
 | Table VI.Protein levels of Hes1, PTEN,
p-AKT(Thr308), p-AKT(Ser473), p-mTOR (Ser2448) and IL-17A in
splenic mononuclear cells of experimental mice (each n=6). |
Table VI.
Protein levels of Hes1, PTEN,
p-AKT(Thr308), p-AKT(Ser473), p-mTOR (Ser2448) and IL-17A in
splenic mononuclear cells of experimental mice (each n=6).
| Group | Hes1 | PTEN | p-AKT (Thr308) | p-AKT (Ser473) | p-mTOR
(Ser2448) | IL-17A |
|---|
| 0 µM LY294002 | 1.179±0.060 | 0.530±0.149 | 1.264±0.072 | 1.304±0.065 | 1.256±0.095 | 1.173±0.134 |
| 10 µM LY294002 |
0.939±0.070a |
0.788±0.097a |
0.992±0.077a |
1.039±0.088a |
1.026±0.061a |
0.951±0.074a |
| 50 µM LY294002 |
0.756±0.073a,b |
0.948±0.100a |
0.845±0.062a,c |
0.866±0.108a,c |
0.857±0.109a,c |
0.810±0.085a |
| Control |
0.569±0.111a,b |
1.151±0.119a,b |
0.608±0.073a,b |
0.450±0.084a,b |
0.550±0.107a,b |
0.522±0.107a,b |
| F-value | 62.235 | 29.712 | 88.796 | 100.548 | 58.847 | 42.321 |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Discussion
Psoriasis is an immune-driven inflammatory disease,
in which dendritic cells, T cells and keratinocytes serve critical
roles (30). Originally, Th1
cells were considered to be the major mediators of psoriasis.
Increasing evidence derived from animal and human studies suggested
that Th17 cells and IL-17 may be major mediators of psoriasis
(31,32). IL-17A is an important member of
the IL-17 family (IL-17A-F). IL-17A is generally referred to as
IL-17. IL-17 blockade, either via cytokine IL-17A or its receptor
IL-17RA, can markedly reverse the clinical disease severity and
molecular features of patients with psoriasis (4,33).
Our previous research demonstrated increased IL-17A serum levels in
patients with psoriasis, as well as elevated IL-17A levels both in
the sera and skin tissues of mice with psoriasis-like skin
inflammation (34–36). In the present study, elevated
IL-17A mRNA and protein levels, as well as increased Th17 cell
percentage were observed in mouse psoriasis-like skin lesions.
Psoriasis is a systemic disease, associated with increased risk for
comorbidities (37). It has been
reported that IMQ can induce spleen enlargement through systemic
effects and alter the immune cell composition of IMQ-induced
psoriatic model mice (38,39).
Our previous study also demonstrated an increased percentage in
Th17 cells in splenic CD4+ T cells in this type of
psoriatic mouse model (33).
Additionally, the present study detected enlarged spleen and
inguinal lymph nodes, further indicating its enhanced systemic
inflammatory response in mouse psoriasis-like inflammation. Upon
effective interruption by LY294002, splenomegaly and
lymphadenopathy were fully mitigated (Table II; Fig. 3).
Th17 cell differentiation is regulated by a complex
network of transcription factors and intracellular signaling
cascades, including PI3K/AKT/mTOR complexes, which are tightly
regulated by feedback loops, in part through mTOR linkage with AKT
(40). Two major pathways have
been reported to intersect at AKT, namely the PI3K/p-AKT
(Thr308)/mTORC1 and mTORC2/p-AKT (Ser473)/FOXO1/3a signaling
pathways (41). The roles of
secondary messengers in Th17 differentiation remain contested,
although several distinct signaling pathways have been confirmed to
regulate Th17 differentiation through mTORC1 (20). S6K is an important downstream
target of mTORC1, and presents high homology with the kinases S6K1
and S6K2. PI3K/AKT/mTORC1/S6K1/2 signaling has been reported to
control Th17 cell differentiation by regulating RORγt nuclear
translocation and the expression of the Th17 cell differentiation
negative regulator Gfi1 (19).
The present study demonstrated a significant increase in Th17 cell
percentage, as well as in the mRNA and protein expression levels of
AKT, mTORC1, S6K1, S6K2 and IL-17A in skin lesions of psoriatic
model mice (Fig. 4,Fig. 5,6), which confirmed that PI3K/AKT
signaling was involved in the pathogenesis of psoriasis. To further
evaluate the possible effect of PI3K/AKT on Th17 cell
differentiation and IL-17A production in the disease-specific
inflammation process of psoriasis, PI3K/AKT signaling was blocked
by LY294002, which resulted in a coordinated decrease in the
aforementioned increased levels of PI3K/AKT signaling molecules
AKT, mTORC1, S6K1 and S6K2 as well as Th17 cell percentage with its
effector cytokine IL-17A in intervention mice (Fig. 4,Fig.
5,6). Importantly, marked
alleviation of psoriasiform inflammation, thickened epidermis and
inflammatory cell-infiltrated dermis were also observed in
intervention mice (Figs. 1 and
2). Therefore, our previous study
together with the current findings suggested that the
PI3K/AKT/mTORC1/S6K1/2 axis can exert its function in the
development of psoriasis by regulating Th17 cells.
Notch signaling plays a critical role in the lineage
commitment of cells and fates of lymphocytes, and is also well
known for its pivotal role in Th17 cell differentiation (10,11,41). Notch signaling is initiated
through the binding of the Notch receptor with a Notch ligand;
subsequently, a series of enzymatic reactions result in the
cleavage of NICD by γ-secretase. NICD is then translocated to the
nucleus, where it activates the transcription of downstream genes
(42). Thus, Notch signaling
activation is controlled by γ-secretase. The effects of γ-secretase
inhibitors on murine and human Th17 cell differentiation have been
reported, leading to a marked reduction in IL-17A production
(11). Our previous research
verified increased expression of Notch1 and its target gene Hes1 in
patients with psoriasis and in a mouse model of psoriasis, and
demonstrated that the γ-secretase inhibitor DAPT could notably
decrease IL-17A production by CD4+ T cells in Th17
polarizing situations, both in psoriasiform mice and in patients
with psoriasis (13,35). Signal transduction is a complex
network involving numerous intercellular signaling molecules,
interactions, crosstalk and feedback loops. PTEN is a crucial
negative regulator of PI3K/AKT/mTOR signaling, which was
demonstrated to be markedly decreased in psoriasiform skin of model
mice in the present study (Figs.
5 and 6). Notch1 plays an
important role in regulating the expression of PTEN and the
activity of PI3K/AKT signaling through Hes1 (21,43,44). The Notch1/Hes1/PTEN signaling axis
has been reported to be involved in certain human diseases
(21–27). In hepatocellular carcinoma cells,
knockdown of Notch1 was correlated with the reduction of p-AKT
(26). Downregulation of Notch1
resulted in PTEN activation and AKT dephosphorylation, and
inhibited the invasion and metastasis of the human gastric cancer
cell lines SGC7901 and MKN74 (27). In addition, promoter dual
luciferase reporter assays demonstrated that the PTEN DNA sequence
has promoter activity. Hes1 binds to the PTEN promoter region and
can directly regulate the promoter of PTEN, leading to the
activation of PI3K/AKT signaling (24). Thus, the Hes1-mediated negative
regulation of PTEN to activate the PI3K/AKT/mTORC1/S6K1/2 axis may
be an important mechanism of Notch1/Hes1 signaling driving Th17
cell differentiation and IL-17A production.
In addition, Notch1 activation induced by IL-17 has
been confirmed to occur via the signaling molecule Act1, which is a
critical adaptor molecule in IL-17 signaling transduction (14,15). The crosstalk of IL-17 with Notch1
was first demonstrated in oligodendrocyte progenitor cells
(14). IL-17R interacts with
Notch1 through its extracellular domain, thus forming the
Act1-NICD1 complex and subsequently translocating into nuclei
(14). As a result, Act1 and the
transcription factor recombination signal binding protein for
immunoglobulin kJ region, the core element of Notch signaling, are
recruited to the promoters of Th17-induced Notch1 target genes
(15). Based on the
aforementioned studies, it can be proposed that a feedback loop of
Notch1/Hes1-PTEN/PI3K/AKT/mTORC1/S6K1/2 may exist to mediate the
effects of IL-17A. The present study confirmed increased mRNA and
protein expression levels of Notch1, NICD1 and Hes1; decreased
PTEN; elevated p-AKT, p-mTORC1, S6K1 and S6K2; and increased Th17
cell percentage and IL-17A levels in mouse psoriasis-like skin
inflammation, which further demonstrated that both the PI3K/AKT and
Notch1 signaling pathways are hyperactivated in psoriasis and play
important roles in the pathogenesis of psoriasis. To corroborate
the crosstalk and feedback loop of
Notch1/Hes1-PTEN/PI3K/AKT/mTORC1/S6K1/2 around Th17 cells and
IL-17A, PI3K/AKT signaling was inhibited by LY294002 while
establishing a model of psoriasiform inflammation. Following
LY294002 inhibition, with the exception of reduced expression of
AKT, mTORC1, S6K1, S6K2 and IL-17A as well as Th17 cell percentage,
decreased Notch1, NICD1 and Hes1 were observed as well as increased
PTEN, which suggested the action of IL-17A crosstalk with Notch1
and the continuous feedback loop of
Notch1/Hes1-PTEN/PI3K/AKT/mTORC1/S6K1/2 around IL-17A. Our study
group have focused on the studies of Th17 cells and IL-17A for more
than ten years, especially in the field of atopic dermatitis and
psoriasis (13,34-36,45,46). In recent five years, close attention
was paid to the regulatory effect of Notch1/Hes1 signaling on Th17
cell and γδT17 cell differentiation in psoriasis, and related in
vitro/in vivo studies were performed through blocking
Notch1/Hes1 signaling by γ-secretase inhibitor and it was reported
that Notch1 inhibition by DAPT could significantly suppress Th17
cell differentiation and IL-17A production (13,35,36). In the present study, for the first
time, Notch1/Hes1-PTEN/AKT/IL-17A feedback loop was proposed, the
crosstalk between Notch1/Hes1 signaling and PI3K/AKT/mTORC1
signaling and their coordinate regulatory effect on Th17 cell
differentiation in psoriatic inflammation. A study on metastasis of
gastric cancer revealed that the PI3K/AKT and Notch1 signaling
blockers LY294002 and DAPT coordinately inhibited the metastasis of
gastric cancer through mutual enhancement (47). Thus, based on our previous
findings and the present results, the Notch1/Hes1-PTEN/AKT/IL-17A
feedback loop may be an important mechanism in the regulation of
Th17 differentiation in psoriasis. Blocking the
Notch1/Hes1-PTEN/AKT/IL-17A feedback loop may be a potential
therapeutic method for the treatment of psoriatic inflammation.
IMQ-induced mouse psoriasis-like skin inflammation
reached peak severity at day 7–8, then diminished gradually
(48), thus in most of mouse
psoriatic inflammation studies established by 5% IMQ, mice were
administered a daily topical application for 6–7 consecutive days.
Due to the short duration of classic psoriatic features and
character of gradual diminishment, LY294002 intervention was
conducted at the same time of experimental model establishment. The
limitation of the present study is that it did not explore the
intervention effects of different dose of LY294002 on experimental
mice and their intervention effects after the establishment of
psoriatic lesions. In the in vitro experiment of the present
study, 10 and 50 µM LY294002 were exerted on splenic mononuclear
cells from 5% IMQ-induced mice; notably, the even more obvious
intervention effects of 50 µM LY294002 were confirmed. Related
in vitro/in vivo studies shall be performed in the future to
more deeply evaluate the potential therapeutic effect of LY294002
in psoriatic inflammation.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science
Foundation of China (grant no. 81803145).
Availability of data and materials
All data generated or analyzed during this study are
included in this manuscript or are available from the corresponding
author on reasonable request.
Authors' contributions
YWL and XXL carried out the experiments, performed
the statistical analysis and wrote the first draft of the
manuscript. FHF participated in the design of the study and carried
out the experiments, particularly for the development of the
experimental model. BL and RQQ participated in the design of the
study and analyzed the data. XYX helped to carry out the
experiments. LM was responsible for the design of the study,
reviewed and edited this article. All authors read and approved the
final manuscript and agree to be accountable for all aspects of the
work in ensuring that questions related to the accuracy or
integrity of the work are appropriately investigated and resolved.
LM and YWL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no.
20190104-15) by the Laboratory Animal Ethics Committee of Binzhou
Medical University Hospital (Binzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yamanaka K, Yamamoto O and Honda T:
Pathophysiology of psoriasis: A review. J Dermatol. 48:722–731.
2021. View Article : Google Scholar
|
|
2
|
Furue K, Ito T, Tsuji G, Kadono T and
Furue M: Psoriasis and the TNF/IL23/IL17 axis. G Ital Dermatol
Venereol. 154:418–424. 2019. View Article : Google Scholar
|
|
3
|
Molinelli E, Campanati A, Brisigotti V and
Offidani A: Biologic therapy in psoriasis (part II): Efficacy and
safety of new treatment targeting IL23/IL-17 pathways. Curr Pharm
Biotechnol. 18:964–978. 2017. View Article : Google Scholar
|
|
4
|
Ly K, Smith MP, Thibodeaux Q, Reddy V,
Liao W and Bhutani T: Anti IL-17 in psoriasis. Expert Rev Clin
Immunol. 15:1185–1194. 2019. View Article : Google Scholar
|
|
5
|
van der Fits L, Mourits S, Voerman JS,
Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens
EP and Lubberts E: Imiquimod-induced psoriasis-like skin
inflammation in mice is mediated via the IL-23/IL-17 axis. J
Immunol. 182:5836–5845. 2009. View Article : Google Scholar
|
|
6
|
Gratton R, Tricarico PM, Moltrasio C, Lima
Estevão de Oliveira AS, Brandão L, Marzano AV, Zupin L and Crovella
S: Pleiotropic role of Notch signaling in human skin diseases. Int
J Mol Sci. 21:42142020. View Article : Google Scholar
|
|
7
|
Gratton R, Tricarico PM, d'Adamo AP,
Bianco AM, Moura R, Agrelli A, Brandão L, Zupin L and Crovella S:
Notch signaling regulation in autoinflammatory diseases. Int J Mol
Sci. 21:88472020. View Article : Google Scholar
|
|
8
|
Fiúza UM and Arias AM: Cell and molecular
biology of Notch. J Endocrinol. 194:459–474. 2007. View Article : Google Scholar
|
|
9
|
Auderset F, Coutaz M and Tacchini-Cottier
F: The role of Notch in the differentiation of CD4+ T helper cells.
Curr Top Microbiol Immunol. 360:115–134. 2012.
|
|
10
|
Eagar TN, Tang Q, Wolfe M, He Y, Pear WS
and Bluestone JA: Notch 1 signaling regulates peripheral T cell
activation. Immunity. 20:407–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keerthivasan S, Suleiman R, Lawlor R,
Roderick J, Bates T, Minter L, Anguita J, Juncadella I, Nickoloff
BJ, Le Poole IC, et al: Notch signaling regulates mouse and human
Th17 differentiation. J Immunol. 187:692–701. 2011. View Article : Google Scholar
|
|
12
|
Radtke F, Fasnacht N and MacDonald HR:
Notch signaling in the immune system. Immunity. 32:14–27. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma L, Xue H, Qi R, Wang Y and Yuan L:
Effect of γ-secretase inhibitor on Th17 cell differentiation and
function of mouse psoriasis-like skin inflammation. J Transl Med.
16:592018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian Y, Liu C, Hartupee J, Altuntas CZ,
Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, et al: The
adaptor Act1 is required for interleukin 17-dependent signaling
associated with autoimmune and inflammatory disease. Nat Immunol.
8:247–256. 2007. View
Article : Google Scholar
|
|
15
|
Wang C, Zhang CJ, Martin BN, Bulek K, Kang
Z, Zhao J, Bian G, Carman JA, Gao J, Dongre A, et al: IL-17 induced
NOTCH1 activation in oligodendrocyte progenitor cells enhances
proliferation and inflammatory gene expression. Nat Commun.
8:155082017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weichhart T and Säemann MD: The
PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic
applications. Ann Rheum Dis. 67 (Suppl 3):iii70–iii74. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mercurio L, Albanesi C and Madonna S:
Recent updates on the involvement of PI3K/AKT/mTOR molecular
cascade in the pathogenesis of hyperproliferative skin disorders.
Front Med (Lausanne). 8:6656472021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chamcheu JC, Chaves-Rodriquez MI, Adhami
VM, Siddiqui IA, Wood GS, Longley BJ and Mukhtar H: Upregulation of
PI3K/AKT/mTOR, FABP5 and PPARβ/δ in human psoriasis and
imiquimod-induced murine psoriasiform dermatitis model. Acta Derm
Venereol. 96:854–856. 2016.
|
|
19
|
Kurebayashi Y, Nagai S, Ikejiri A, Ohtani
M, Ichiyama K, Baba Y, Yamada T, Egami S, Hoshii T, Hirao A, et al:
PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by
regulating Gfi1 expression and nuclear translocation of RORγ. Cell
Rep. 1:360–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagai S, Kurebayashi Y and Koyasu S: Role
of PI3K/Akt and mTOR complexes in Th17 cell differentiation. Ann N
Y Acad Sci. 1280:30–34. 2013. View Article : Google Scholar
|
|
21
|
Palomero T, Sulis ML, Cortina M, Real PJ,
Barnes K, Ciofani M, Caparros E, Buteau J, Brown K, Perkins SL, et
al: Mutational loss of PTEN induces resistance to NOTCH1 inhibition
in T-cell leukemia. Nat Med. 13:1203–1210. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hales EC, Taub JW and Matherly LH: New
insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling
axis: Targeted therapy of γ-secretase inhibitor resistant T-cell
acute lymphoblastic leukemia. Cell Signal. 26:149–161. 2014.
View Article : Google Scholar
|
|
23
|
Li X, Zou F, Lu Y, Fan X, Wu Y, Feng X,
Sun X and Liu Y: Notch1 contributes to TNF-α-induced proliferation
and migration of airway smooth muscle cells through regulation of
the Hes1/PTEN axis. Int Immunopharmacol. 88:1069112020. View Article : Google Scholar
|
|
24
|
Liu X, Zhang Y, Shi M, Wang Y, Zhang F,
Yan R, Liu L, Xiao Y and Guo B: Notch1 regulates PTEN expression to
exacerbate renal tubulointerstitial fibrosis in diabetic
nephropathy by inhibiting autophagy via interactions with Hes1.
Biochem Biophys Res Commun. 497:1110–1116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian T, Fu X, Lu J, Ruan Z, Nan K, Yao Y
and Yang Y: MicroRNA-760 inhibits doxorubicin resistance in
hepatocellular carcinoma through regulating Notch1/Hes1-PTEN/Akt
signaling pathway. J Biochem Mol Toxicol. 32:e221672018. View Article : Google Scholar
|
|
26
|
Zhang X, Hu Y, Gao H, Lan X and Xue Y:
Downregulation of Notch1 inhibits the invasion and metastasis of
human gastric cancer cells SGC7901 and MKN74 in vitro
through PTEN activation and dephosphorylation of AKT and FAK. Mol
Med Rep. 16:2318–2324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sokolowski KM, Balamurugan M,
Kunnimalaiyaan S, Wilson J, Gamblin TC and Kunnimalaiyaan M: Role
of Akt inhibition on Notch1 expression in hepatocellular carcinoma:
Potential role for dual targeted therapy. Am J Surg. 211:755–760.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lan XO, Wang HX, Qi RQ, Xu YY, Yu YJ, Yang
Y, Guo H, Gao XH and Geng L: Shikonin inhibits CEBPD downregulation
in IL-17-treated HaCaT cells and in an imiquimod-induced psoriasis
model. Mol Med Rep. 22:2263–2272. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Greb JE, Goldminz AM, Elder JT, Lebwohl
MG, Gladman DD, Wu JJ, Mehta NN, Finlay AY and Gottlieb AB:
Psoriasis. Nat Rev Dis Primers. 2:160822016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Cesare A, Di Meglio P and Nestle FO:
The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J
Invest Dermatol. 129:1339–1350. 2009. View Article : Google Scholar
|
|
32
|
Li B, Huang L, Lv P, Li X, Liu G, Chen Y,
Wang Z, Qian X, Shen Y, Li Y and Fang W: The role of Th17 cells in
psoriasis. Immunol Res. 68:296–309. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hawkes JE, Chan TC and Krueger JG:
Psoriasis pathogenesis and the development of novel targeted immune
therapies. J Allergy Clin Immunol. 140:645–653. 2017. View Article : Google Scholar
|
|
34
|
Ma L, Xue HB, Guan XH, Shu CM, Wang F,
Zhang JH and An RZ: The imbalance of Th17 cells and CD4(+)
CD25(high) Foxp3(+) Treg cells in patients with atopic dermatitis.
J Eur Acad Dermatol Venereol. 28:1079–1086. 2014. View Article : Google Scholar
|
|
35
|
Ma L, Xue H, Gao T, Gao M and Zhang Y:
Notch1 signaling regulates the Th17/Treg immune imbalance in
patients with psoriasis vulgaris. Mediators Inflamm.
2018:30695212018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Li X, Xing X, Xue H, Qi R, Ji H
and Ma L: Notch-Hes1 signaling regulates IL-17A+ γδ
+T cell expression and IL-17A secretion of mouse
psoriasis-like skin inflammation. Mediators Inflamm.
2020:82971342020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Korman NJ: Management of psoriasis as a
systemic disease: What is the evidence? Br J Dermatol. 182:840–848.
2020. View Article : Google Scholar
|
|
38
|
Kim CH, Yoo JK, Jeon SH, Lim CY, Lee JH,
Koo DB and Park MY: Anti-psoriatic effect of myeloid-derived
suppressor cells on imiquimod-induced skin inflammation in mice.
Scand J Immunol. 89:e127422019.
|
|
39
|
Frenzel DF, Borkner L, Scheurmann J, Singh
K, Scharffetter-Kochanek K and Weiss JM: Osteopontin deficiency
affects imiquimod-induced psoriasis-like murine skin inflammation
and lymphocyte distribution in skin, draining lymph nodes and
spleen. Exp Dermatol. 24:305–307. 2015. View Article : Google Scholar
|
|
40
|
Chamcheu JC, Adhami VM, Esnault S, Sechi
M, Siddiqui IA, Satyshur KA, Syed DN, Dodwad SJ, Chaves-Rodriquez
MI, Longley BJ, et al: Dual inhibition of PI3K/Akt and mTOR by the
dietary antioxidant, delphinidin, ameliorates psoriatic features in
vitro and in an imiquimod-induced psoriasis-like disease in mice.
Antioxid Redox Signal. 26:49–69. 2017. View Article : Google Scholar
|
|
41
|
Coutaz M, Hurrell BP, Auderset F, Wang H,
Siegert S, Eberl G, Ho PC, Radtke F and Tacchini-Cottier F: Notch
regulates Th17 differentiation and controls trafficking of IL-17
and metabolic regulators within Th17 cells in a context-dependent
manner. Sci Rep. 6:391172016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nagase H and Nakayama K:
γ-Secretase-regulated signaling typified by Notch signaling in the
immune system. Curr Stem Cell Res Ther. 8:341–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Palomero T, Dominguez M and Ferrando AA:
The role of the PTEN/AKT pathway in NOTCH1-induced leukemia. Cell
Cycle. 7:965–970. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu S, Ma X, Ai Q, Huang Q, Shi T, Zhu M,
Wang B and Zhang X: NOTCH1 functions as an oncogene by regulating
the PTEN/PI3K/AKT pathway in clear cell renal cell carcinoma. Urol
Oncol. 31:938–948. 2013. View Article : Google Scholar
|
|
45
|
Ma L, Xue HB, Guan XH, Qi RQ, An RZ, Shu
CM, Zhang YJ, Wei YH and Zhang JH: Possible role of Th17 cells and
IL-17 in the pathogenesis of atopic dermatitis in northern China. J
Dermatol Sci. 68:66–68. 2012. View Article : Google Scholar
|
|
46
|
Ma L, Xue HB, Wang F, Shu CM and Zhang JH:
MicroRNA-155 may be involved in the pathogenesis of atopic
dermatitis by modulating the differentiation and function of T
helper type 17 (Th17) cells. Clin Exp Immunol. 181:142–149. 2015.
View Article : Google Scholar
|
|
47
|
Peng X, Zhou J, Li B, Zhang T, Zuo Y and
Gu X: Notch1 and PI3K/Akt signaling blockers DAPT and LY294002
coordinately inhibit metastasis of gastric cancer through mutual
enhancement. Cancer Chemother Pharmacol. 85:309–320. 2020.
View Article : Google Scholar
|
|
48
|
Zhao J, Di T, Wang Y, Liu X, Liang D,
Zhang G and Li P: Multi-glycoside of tripterygium wilfordii Hook.
f. ameliorates imiquimod-induced skin lesions through a
STAT3-dependent mechanism involving the inhibition of Th17-mediated
inflammatory responses. Int J Mol Med. 38:747–757. 2016. View Article : Google Scholar : PubMed/NCBI
|