Bone marrow mesenchymal stem cells (BMSCs) were
first discovered in the bone marrow by Friedenstein et al
(1). Due to the multi-directional
differentiation potential of BMSCs, under specific induction
conditions, they can develop into osteoblasts, adipocytes,
chondrocytes and osteoblasts fibroblasts, and even differentiate
into myoblasts (2–4), and are therefore defined as
pluripotent cells. In addition, BMSCs can also undergo self-renewal
and generate immunomodulatory responses (5,6).
Studies have shown that BMSCs are capable of differentiating into
multiple lineages, including tissues other than their origin, such
as neurons, hepatocytes and skeletal muscle cells (7–10).
BMSCs are easy to obtain, easy to expand in vitro and still
have good differentiation potential after they are isolated from
adult bone marrow (11). BMSCs
have become extremely important seed cells in gene therapy, tissue
engineering, cell replacement therapy and regenerative medicine due
to their potential for multi-directional differentiation,
self-renewal and immune regulation.
Circular RNA (circRNA) is a large class of
non-coding RNA (ncRNA) that is ubiquitous in eukaryotic cells.
Unlike normative linear RNAs, circRNAs form covalently closed
continuous loops without 5′ or 3′ polarity (12). circRNAs are abundantly expressed
in cells and tissues, are highly conserved in evolution and are
relatively stable, and they are generally considered to be
by-products of mis-splicing or messenger RNA (mRNA) processes
(13). With the rapid development
of high-throughput RNA sequencing (RNA-Seq) technology and
bioinformatics methods, a large number of circRNAs have been
discovered and identified in a number of species; for example,
circ_28313, circ_0016624, circ_0006393, circ_0076906 and
circ_0048211. Numerous studies have shown that circRNAs play an
important role in the osteogenic differentiation of BMSCs (11,13). Further research on the role of
circRNAs in the osteogenic differentiation of BMSCs can provide a
new theoretical and experimental basis for bone tissue engineering
and clinical treatment.
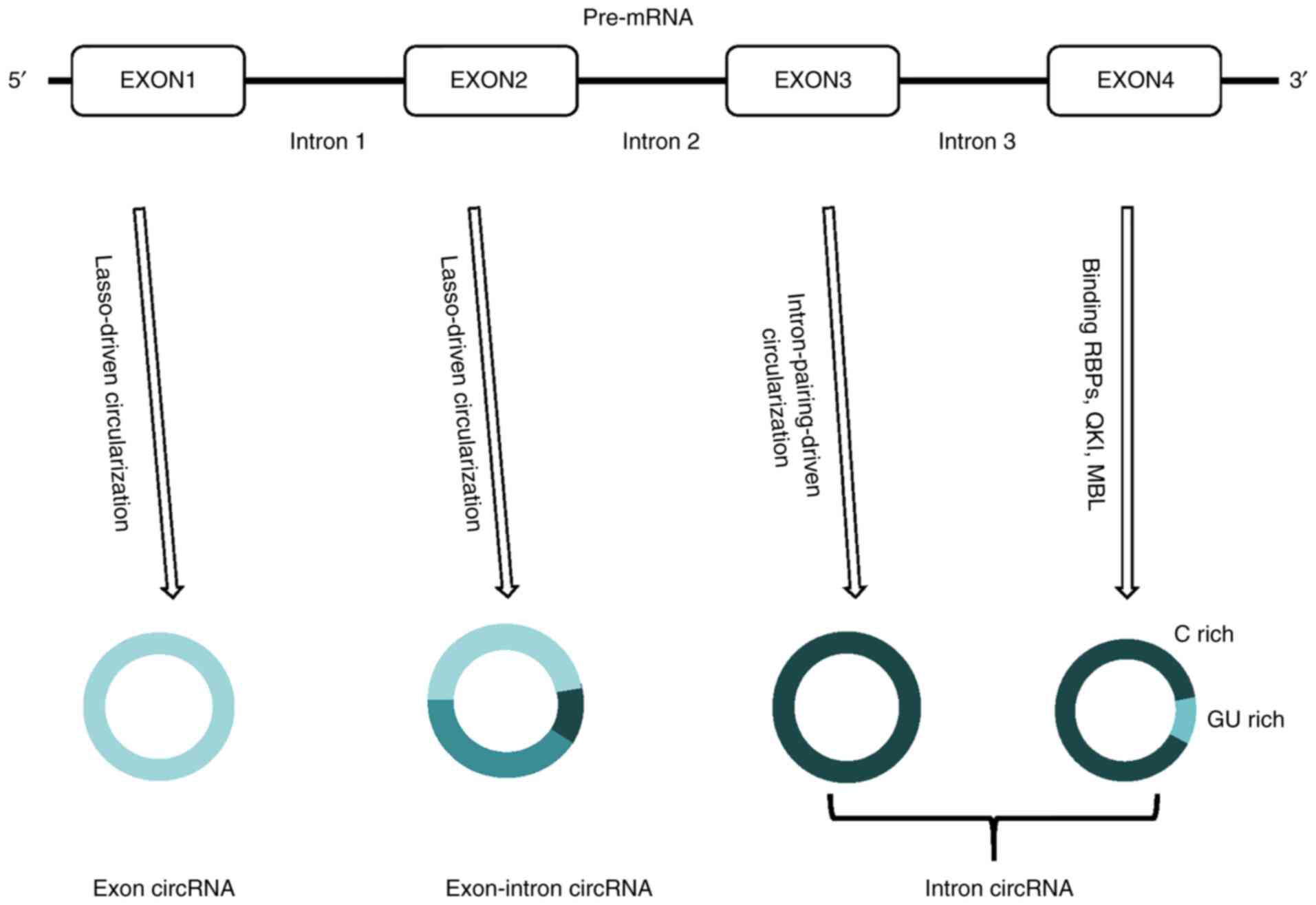
According to the genomic origin and structural
characteristics of circRNAs, they are mainly divided into three
types: Exonic circRNA, exon-intron circRNA and intronic circRNA
(18,19). The production of circRNAs is a
highly complex biological process and they are produced by
different cyclization mechanisms. Usually, eukaryotic pre-mRNA
catalyzes the removal of introns and ligates exons by a spliceosome
mechanism to form linear RNA transcripts with 5′ or 3′ polarity
(20). Unlike the normative
splicing of linear RNA, most circRNAs are produced by a
backsplicing process that does not follow the 5′-3′ order of the
specification (20,21). Exon cyclization between the
downstream 5′ splice site (splicing donor) and the upstream 3′
splice site (shear acceptor) in the same pre-mRNA yields a circular
product (circRNA) without a terminal structure [e.g., a 5′ cap or
polyadenylation (poly A) tail] (18,22,23). In 2013, Jeck et al
(18) proposed a model for two
exon cyclization mechanisms. One mechanism is known as
lariat-driven circularization or exon skipping. The partially
folded pre-mRNA transcript brings the original non-adjacent exons
close to other exons, causing exon skipping, creating overlapping
regions, and forming a lasso intermediate containing exons and
introns. The intron in the lasso is removed, eventually producing
an exon circRNA. In general, introns located between cyclized exons
are spliced out, and in some cases are not spliced to form
exon-intron circRNAs (24).
Another mechanism is known as intron-pairing driven circularization
or direct backsplicing. This model forms a circular structure by
linking the downstream splice donor to the upstream splice acceptor
by ALU (identified as the canonical ALU repeat) complementarity
across the flanking intron or base assignment of other RNA
secondary structures. Intron circRNAs produced by intron lasso are
resistant to degradation by de-branching enzymes (18,24). In distinguishing intron circRNAs
from exon circRNAs, intron circRNAs contain a unique 2′-5′ linkage,
which is formed by sequences near the 7 nt GU-rich 5′ splice site
and close to 11 nt-rich C-sequences at branch point sites (25). Fig.
1 shows a schematic illustration for the biogenesis of
circRNAs.
In addition, a recent study confirmed another
circRNA biogenesis model acting through RNA-binding proteins
(RBPs). In this case, the alternative splicing factor protein
quaking and muscleblind protein bring the two flanking intron
sequences together by binding to some of the circRNAs flanking the
intron and forming a bridge, promoting the circularization to form
circRNAs (26,27). This mechanism is similar to the
intron-pairing-driven circularization pathway, except that RBPs
regulate adjacent splice sites instead of the direct base pairing
between complementary motifs observed in the intron-pairing-driven
model.
New evidence suggests that circRNAs can act as
microRNA (miR/miRNA) sponges, interact with RBPs and regulate gene
transcription, and that certain circRNAs can be translated into
proteins or peptides (18,24,25,28–33).
Therefore, circRNAs mainly have the following functions: i) miRNA
sponges. miRNAs are a class of linear non-coding RNAs that bind
directly to target mRNAs through base-pair pairing, silencing or
degrading target mRNAs, thereby participating in the regulation of
pathological and physiological processes (34). circRNAs have miRNA sponge binding
sites that serve as competitive endogenous RNAs to inhibit miRNA
binding to targets and thereby inhibit mRNA translation. ii)
Interact with RBPs. circRNAs bind to RBPs to form RNA-protein
complexes (RPCs). These RPCs can regulate RBPs and then interact
with linear RNA to exert biological functions (35,36). iii) Regulate gene transcription.
circRNAs are abundantly present in the nucleus and can bind to
RBPs, especially transcription-related factors, including RNA
polymerase II and transcription factors, and recruit them to the
parental gene, thereby affecting the expression of the parental
gene and regulating the transcription process (37). iv) Translate into proteins or
peptides. Previous studies have found that circRNAs also have the
function of translating proteins. When synthetic circRNAs contain
an internal ribosome entry site sequence that is efficiently
translated, circRNAs bind directly to the ribosome and are
translated in eukaryotic cells (26,38). Another study confirmed that
natural eukaryotic endogenous circRNAs can drive protein
translation through methylation of adenosine N6, suggesting that
circRNAs have a function to encode proteins (39,40).
In humans, osteoblasts, which are involved in bone
formation, are inseparable from the differentiation of bone marrow
mesenchymal stem cells. Studies have shown that circRNAs play an
important role in the osteogenic differentiation of BMSCs, and
different circRNAs can either promote or inhibit the osteogenic
differentiation of BMSCs (41).
Fu et al (42) found
differentially expressed circRNAs in patients with osteoporosis
(OP), and the study identified 237 upregulated and 279
downregulated circRNAs, which also confirmed that the role of
circRNAs in the osteogenic differentiation of BMSCs is important in
the process. Another study found that circRNAs were differentially
expressed in patients with traumatic femoral head necrosis, and
identified 234 upregulated and 148 downregulated circRNAs (43). Chen et al (44) found that circRNAs, such as
circ_28313, circ_0016624, circ_0006393, circ_0076906 and
circ_0048211, were differentially expressed in patients with OP and
play an important role in the differentiation, proliferation and
apoptosis of BMSCs.
Steroid-induced osteonecrosis of the femoral head
(SONFH) is a common orthopedic disease. Chen et al (46) showed that there are differentially
expressed circRNAs in patients with SONFH. Bioinformatics analysis
found that the expression of circRNA CDR1as was upregulated, and
further experiments found that it may play a key role in the
adipogenic/osteogenic differentiation of SONFH-BMSCs through the
CDR1as-miR-7-5p-WNT5B axis. Knockdown of CDR1as promoted osteogenic
differentiation and inhibited adipogenic differentiation of BMSCs,
while overexpression of CDR1as inhibited osteogenic differentiation
and promoted adipogenic differentiation of BMSCs (46). This study provides new insights
into the molecular mechanism of the osteogenic/adipogenic
differentiation of SONFH-BMSCs and the diagnosis and treatment of
SONFH. Phosphatase and tensin homolog (PTEN) is a classic tumor
suppressor that inhibits phosphatidylinositol 3-phosphate kinase
(PI3K)/AKT signaling (47).
Another study found that the expression of circUSP45 was increased
in patients with glucocorticoid-induced osteonecrosis of the
femoral head (GIONFH) (48).
Overexpression of circUSP45 decreased the expression of osteogenic
genes and inhibited the proliferation of BMSCs, and further
experiments found that circUSP45 could directly interact with
miR-127-5p. miR-127-5p regulates osteogenesis with its target PTEN
(48). circUSP45 decreases the
osteogenic differentiation of GIONFH by sponging miR-127-5p through
the PTEN/AKT signaling pathway. Differentially expressed circRNAs
were found in elderly patients with OP, and through further
experiments, it was found that circRNA008876 can play a biological
role as an miR-150-5p sponge (49), which provides a potential
biomarker and therapeutic target for senile OP. A previous study
showed that Shh coreceptor growth arrest-specific 1 (GAS1) is
expressed in mesenchymal cells, and in a GAS1-deficient mouse
model, mice have abnormal dentition (50). Another study has also shown that
inhibiting the expression of circ_0003865 in patients with OP can
promote the osteogenic differentiation of BMSCs, and that
circ_0003865 regulates the expression of the GAS1 gene by sponging
miR-3653-3p (51). A summary of
the effect of the inhibition of circRNAs on the osteogenic
differentiation of BMSCs is shown in Table I.
Although a number of circRNAs play an inhibitory
role in the osteogenic differentiation of BMSCs, other circRNAs
that can promote the osteogenic differentiation of BMSCs have been
discovered following continuous exploration, such as circATRNL1,
circRNA-016901, hsa_circ_0000219, hsa_circ_0004588 and
hsa_circ_0005936 (52).
Runt-related transcription factor 2 (Runx2) belongs
to the Runx family, with the DNA-binding domain runt, and consists
of Runx1, Runx2 and Runx3 (53).
Studies have shown that Runx2 plays an important role in the
osteogenic differentiation of BMSCs (54). Ji et al (55) found that the expression of
hsa_circ_0006215 was decreased in the BMSCs of patients with OP.
Lentiviral experiments found that overexpression of
hsa_circ_0006215 promoted the osteogenic differentiation of BMSCs,
and hsa_circ_0006215 combined with miRNA-942-5p to regulate the
expression of RUNX2 and vascular endothelial growth factor (VEGF)
in BMSCs (55). The results of
this study suggest that hsa_circ_0006215 plays an important role in
osteogenesis and may be a new target for the treatment of elderly
OP. A recent study showed that exosome-modified circ-Rtn4 could
attenuate TNF-α-induced cytotoxicity and apoptosis in murine
MC3T3-E1 cells, and miR-146a was identified as a target of
circ-Rtn4 (56). These findings
suggest that circ-Rtn4 may serve as a new candidate for the
treatment of OP. Previous studies have found that low-level laser
irradiation (LLLI) can promote osteoblast proliferation and bone
repair (57,58), LLLI can promote BMSC proliferation
(59,60), and LLLI can increase the
expression of VEGF, thereby inducing the angiogenesis necessary for
wound healing (61). Liu et
al (62) showed that LLLI can
regulate the proliferation of BMSCs, and circRNA_0001052 can
regulate the proliferation of BMSCs through the Wnt4/β-catenin
pathway as an miR-124-3p sponge. The study also demonstrated that
circRNA_0001052 plays an important role in the proliferation of
BMSCs in response to LLLI treatment, which provides a potential
clinical application for the treatment of OP.
Articular cartilage damage is one of the main
pathological changes in osteoarthritis, and cartilage repair is the
key to solving osteoarthritis. Zheng et al (63) found that circATRNL1
(hsa_circ_0020093) was highly expressed during the chondrogenic
differentiation of BMSCs, and also found that the chondrogenic
differentiation-related factors SRY-related HMG box 9 (SOX9), type
II collagen (COL2) and aggrecan were highly expressed.
Overexpression of circATRNL1 enhanced the proliferation of BMSCs
and simultaneously enhanced the expression of SOX9, COL2 and
aggrecan, as well as the degree of chondrogenic differentiation of
BMSCs, and miR-338-3p was its target (63). This study demonstrated that
circATRNL1 promotes the cartilage differentiation of BMSCs by
regulating miR-338-3p, which provides new insights into cartilage
repair. A previous study has shown that circ-016901 promotes the
proliferation of irradiation-induced BMSCs and attenuates
irradiation-induced apoptosis by regulating the
miR-1249-5p/homeodomain interacting protein kinase 2 axis (64). Li et al (65) found that circ458420810|58485447,
circ43400193|43461320, circ183498456|183537970 and
circ106417736|106434369 acted together on miR-326-5p, and that
overexpression of miR-326-5p could promote the osteogenic
differentiation of BMSCs while inhibiting the adipogenic
differentiation. Another study showed that lentivirus-mediated
small interfering RNA has_circ_0000885 plasmid transfection into
BMSCs and an osteoclast co-culture system could promote BMSC cell
proliferation, inhibit apoptosis and promote osteogenic
differentiation (66). This
provides a new target for the treatment of patients with OP. There
are differentially expressed circRNAs in postmenopausal OP
patients, and further research found that hsa_circ_0009127,
hsa_circ_0090759, hsa_circ_0058392, hsa_circ_0090247 and
hsa_circ_0049484 were involved in the regulation of autophagy, and
the PI3K-AKT, FoxO and MAPK signaling pathways, thereby regulating
the osteogenic differentiation process of BMSCs (42). BMSCs were isolated from
ovariectomized (OVX) mice and normal mice, and further experiments
found that circRNA_0020 and circRNA_3832 were downregulated in the
OVX mice, and that overexpression of circRNA_0020 and circRNA_3832
could promote the osteogenic differentiation of BMSCs and promote
cell proliferation (67).
A previous study has shown that the expression of
osteopontin (OPN) is increased in osteoarthritis, that it
accelerates the renewal and remodeling of subchondral bone in
osteoarthritis, and that it mediates the degeneration of articular
cartilage induced by subchondral bone metabolism (68). Liu et al (69) found that circ_0005564 was highly
expressed and decreased the mRNA expression of RUNX2 and OPN during
the osteogenic differentiation of BMSCs, and that knockdown of
circ_0005564 inhibited osteoblast differentiation in BMSCs. Another
recent study (70) found that
circ-DAB1 (hsa_circ_0113689) was significantly upregulated during
the osteogenic differentiation of human BMSCs, and that
overexpression of circ-DAB1 could promote the proliferation and
osteogenic differentiation of BMSCs. miR-1270 and miR-944 are
targets of circ-DAB1, and further experiments found that circ-DAB1
promotes the cell proliferation and osteogenic differentiation of
BMSCs through the NOTCH/recombination signal binding protein for
immunoglobulin-κ-J region (RBPJ) pathway (70). Zhong et al (71) showed that circ_1983 acts as a
sponge of miR-6931 to promote the osteogenic differentiation of
BMSCs. Hao et al (72)
found that circPVT1 was decreased in the femoral head of rats with
SIONFH and glucocorticoid (GC)-treated BMSCs, whereas miR-21-5p was
upregulated, and overexpression of circPVT1 attenuated GC-induced
BMSC apoptosis and cell viability inhibition. circPVT1 acts as a
sponge for miR-21-5p (72). Bone
morphogenetic protein 2 (BMP2) plays an important role in
osteogenesis. It was found that circ_0000020 was able to regulate
the expression of BMP2 (73);
circ_0000020 is upregulated during osteogenic differentiation,
while the expression of miR-142-5p is significantly decreased
(73). Silencing circ_0000020
inhibits osteogenic differentiation and promotes apoptosis, and
inhibits the activity and mineralization of alkaline phosphatase.
circ_0000020 can directly act on miR-142-5p (73). In conclusion, circ_0000020
positively regulates the osteogenic differentiation of BMSCs by
regulating BMP2 expression via sponging miR-142-5p. Based on the
aforementioned studies, circRNAs play a key role in the osteogenic
differentiation of BMSCs. Different circRNAs can promote or inhibit
the osteogenic differentiation of BMSCs, which also provides a new
direction for OP and bone defect repair. The effect of the
promotion of circRNAs on the osteogenic differentiation of BMSCs is
shown in Table II.
BMSCs are an important source of osteogenic seed
cells in tissue engineering, and circRNAs play a key role in the
osteogenic differentiation of BMSCs. Further studies on the
functional specificity of circRNA target genes and the interactions
between circRNAs are important for elucidating their mechanism of
action. This research also provides a new theoretical and
experimental basis for bone tissue engineering and clinical
treatment.
Not applicable.
Funding: Not applicable.
Not applicable.
JW and BY drafted the manuscript and revised the
manuscript. TW, FZ, YZ, YG and XJ contributed to manuscript
conception. All authors read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Friedenstein AJ, Chailakhyan RK and
Gerasimov UV: Bone marrow osteogenic stem cells: In vitro
cultivation and transplantation in diffusion chambers. Cell Tissue
Kinet. 20:263–272. 1987.
|
|
2
|
Kim N and Cho SG: Clinical applications of
mesenchymal stem cells. Korean J Intern Med. 28:387–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alves H, Mentink A, Le B, van Blitterswijk
CA and de Boer J: Effect of antioxidant supplementation on the
total yield, oxidative stress levels, and multipotency of bone
marrow-derived human mesenchymal stromal cells. Tissue Eng Part A.
19:928–937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ren C, Gong W, Li F and Xie M: Pilose
antler aqueous extract promotes the proliferation and osteogenic
differentiation of bone marrow mesenchymal stem cells by
stimulating the BMP-2/Smad1, 5/Runx2 signaling pathway. Chin J Nat
Med. 17:756–767. 2019.PubMed/NCBI
|
|
5
|
Furuta T, Miyaki S, Ishitobi H, Ogura T,
Kato Y, Kamei N, Miyado K, Higashi Y and Ochi M: Mesenchymal stem
cell-derived exosomes promote fracture healing in a mouse model.
Stem Cells Transl Med. 5:1620–1630. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watanabe Y, Tsuchiya A, Seino S, Kawata Y,
Kojima Y, Ikarashi S, Starkey Lewis PJ, Lu WY, Kikuta J, Kawai H,
et al: Mesenchymal stem cells and induced bone marrow-derived
macrophages synergistically improve liver fibrosis in mice. Stem
Cells Transl Med. 8:271–284. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrari G, Cusella-De Angelis G, Coletta
M, Paolucci E, Stornaiuolo A, Cossu G and Mavilio F: Muscle
regeneration by bone marrow-derived myogenic progenitors. Science.
279:1528–1530. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petersen BE, Bowen WC, Patrene KD, Mars
WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS and Goff JP:
Bone marrow as a potential source of hepatic oval cells. Science.
284:1168–1170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sanchez-Ramos J, Song S, Cardozo-Pelaez F,
Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W,
Patel N, et al: Adult bone marrow stromal cells differentiate into
neural cells in vitro. Exp Neurol. 164:247–256. 2000. View Article : Google Scholar
|
|
10
|
Owen M and Friedenstein AJ: Stromal stem
cells: Marrow-derived osteogenic precursors. Ciba Found Symp.
136:42–60. 1988.PubMed/NCBI
|
|
11
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lasda E and Parker R: Circular RNAs:
Diversity of form and function. RNA. 20:1829–1842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu CX and Sun S: An emerging role for
circular RNAs in osteoarthritis. Yonsei Med J. 59:349–355. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsu MT and Coca-Prados M: Electron
microscopic evidence for the circular form of RNA in the cytoplasm
of eukaryotic cells. Nature. 280:339–340. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kos A, Dijkema R, Arnberg AC, van der
Meide PH and Schellekens H: The hepatitis delta (delta) virus
possesses a circular RNA. Nature. 323:558–560. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salzman J, Gawad C, Wang PL, Lacayo N and
Brown PO: Circular RNAs are the predominant transcript isoform from
hundreds of human genes in diverse cell types. PLoS One.
7:e307332012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou LD and Zhang J: Circular RNAs: An
emerging type of RNA in cancer. Int J Immunopathol Pharmacol.
30:1–6. 2017. View Article : Google Scholar
|
|
20
|
Chen LL and Yang L: Regulation of circRNA
biogenesis. RNA Biol. 12:381–388. 2015. View Article : Google Scholar
|
|
21
|
Vicens Q and Westhof E: Biogenesis of
circular RNAs. Cell. 159:13–14. 2014. View Article : Google Scholar
|
|
22
|
Wang Y and Wang Z: Efficient backsplicing
produces translatable circular mRNAs. RNA. 21:172–179. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen I, Chen CY and Chuang TJ: Biogenesis,
identification, and function of exonic circular RNAs. Wiley
Interdiscip Rev RNA. 6:563–579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar
|
|
25
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar
|
|
26
|
Conn SJ, Pillman KA, Toubia J, Conn VM,
Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA and
Goodall GJ: The RNA binding protein quaking regulates formation of
circRNAs. Cell. 160:1125–1134. 2015. View Article : Google Scholar
|
|
27
|
Ashwal-Fluss R, Meyer M, Pamudurti NR,
Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and
Kadener S: circRNA biogenesis competes with pre-mRNA splicing. Mol
Cell. 56:55–66. 2014. View Article : Google Scholar
|
|
28
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
You X, Vlatkovic I, Babic A, Will T,
Epstein I, Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, et al:
Neural circular RNAs are derived from synaptic genes and regulated
by development and plasticity. Nat Neurosci. 18:603–610. 2015.
View Article : Google Scholar
|
|
31
|
Huang S, Yang B, Chen BJ, Bliim N,
Ueberham U, Arendt T and Janitz M: The emerging role of circular
RNAs in transcriptome regulation. Genomics. 109:401–407. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Han P, Zhou T, Guo X, Song X and
Li Y: circRNADb: A comprehensive database for human circular RNAs
with protein-coding annotations. Sci Rep. 6:349852016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Granados-Riveron JT and Aquino-Jarquin G:
The complexity of the translation ability of circRNAs. Biochim
Biophys Acta. 1859:1245–1251. 2016. View Article : Google Scholar
|
|
34
|
Ebert MS and Sharp PA: MicroRNA sponges:
Progress and possibilities. RNA. 16:2043–2050. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abdelmohsen K, Kuwano Y, Kim HH and
Gorospe M: Posttranscriptional gene regulation by RNA-binding
proteins during oxidative stress: Implications for cellular
senescence. Biol Chem. 389:243–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yin QF, Yang L, Zhang Y, Xiang JF, Wu YW,
Carmichael GG and Chen LL: Long noncoding RNAs with snoRNA ends.
Mol Cell. 48:219–230. 2012. View Article : Google Scholar
|
|
37
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015. View Article : Google Scholar
|
|
38
|
Chen CY and Sarnow P: Initiation of
protein synthesis by the eukaryotic translational apparatus on
circular RNAs. Science. 268:415–417. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang
Y, Jin Y, Yang Y, Chen LL, Wang Y, et al: Extensive translation of
circular RNAs driven by N6-methyladenosine. Cell Res.
27:626–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pamudurti NR, Bartok O, Jens M,
Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E,
Perez-Hernandez D, Ramberger E, et al: Translation of CircRNAs. Mol
Cell. 66:9–21.e7. 2017. View Article : Google Scholar
|
|
41
|
Lin Z, Tang X, Wan J, Zhang X, Liu C and
Liu T: Functions and mechanisms of circular RNAs in regulating stem
cell differentiation. RNA Biol. 18:2136–2149. 2021. View Article : Google Scholar
|
|
42
|
Fu M, Fang L, Xiang X, Fan X, Wu J and
Wang J: Microarray analysis of circRNAs sequencing profile in
exosomes derived from bone marrow mesenchymal stem cells in
postmenopausal osteoporosis patients. J Clin Lab Anal.
36:e239162022. View Article : Google Scholar
|
|
43
|
Zhang Y, Jia S, Wei Q, Zhuang Z, Li J, Fan
Y, Zhang L, Hong Z, Ma X, Sun R, et al: CircRNA_25487 inhibits bone
repair in trauma-induced osteonecrosis of femoral head by sponging
miR-134-3p through p21. Regen Ther. 16:23–31. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen W, Zhang B and Chang X: Emerging
roles of circular RNAs in osteoporosis. J Cell Mol Med.
25:9089–9101. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang M, Jia L and Zheng Y: circRNA
expression profiles in human bone marrow stem cells undergoing
osteoblast differentiation. Stem Cell Rev Rep. 15:126–138. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen G, Wang Q, Li Z, Yang Q, Liu Y, Du Z,
Zhang G and Song Y: Circular RNA CDR1as promotes adipogenic and
suppresses osteogenic differentiation of BMSCs in steroid-induced
osteonecrosis of the femoral head. Bone. 133:1152582020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rademacher S and Eickholt BJ: PTEN in
Autism and neurodevelopmental disorders. Cold Spring Harb Perspect
Med. 9:a0367802019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kuang MJ, Xing F, Wang D, Sun L, Ma JX and
Ma XL: CircUSP45 inhibited osteogenesis in glucocorticoid-induced
osteonecrosis of femoral head by sponging miR-127-5p through
PTEN/AKT signal pathway: Experimental studies. Biochem Biophys Res
Commun. 509:255–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Geng Y, Chen J, Chang C, Zhang Y, Duan L,
Zhu W, Mou L, Xiong J and Wang D: Systematic analysis of mRNAs and
ncRNAs in BMSCs of senile osteoporosis patients. Front Genet.
12:7769842021. View Article : Google Scholar
|
|
50
|
Seppala M, Thivichon-Prince B, Xavier GM,
Shaffie N, Sangani I, Birjandi AA, Rooney J, Lau JNS, Dhaliwal R,
Rossi O, et al: Gas1 regulates patterning of the murine and human
dentitions through sonic hedgehog. J Dent Res. 101:473–482. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang X, Chen T, Deng Z, Gao W, Liang T,
Qiu X, Gao B, Wu Z, Qiu J, Zhu Y, et al: Melatonin promotes bone
marrow mesenchymal stem cell osteogenic differentiation and
prevents osteoporosis development through modulating circ_0003865
that sponges miR-3653-3p. Stem Cell Res Ther. 12:1502021.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xiang S, Li Z and Weng X: Changed cellular
functions and aberrantly expressed miRNAs and circRNAs in bone
marrow stem cells in osteonecrosis of the femoral head. Int J Mol
Med. 45:805–815. 2020.PubMed/NCBI
|
|
53
|
Komori T: Roles of Runx2 in skeletal
development. Adv Exp Med Biol. 962:83–93. 2017. View Article : Google Scholar
|
|
54
|
Komori T: Regulation of proliferation,
differentiation and functions of osteoblasts by Runx2. Int J Mol
Sci. 20:16942019. View Article : Google Scholar
|
|
55
|
Ji H, Cui X, Yang Y and Zhou X: CircRNA
hsa_circ_0006215 promotes osteogenic differentiation of BMSCs and
enhances osteogenesis-angiogenesis coupling by competitively
binding to miR-942-5p and regulating RUNX2 and VEGF. Aging (Albany
NY). 13:10275–10288. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cao G, Meng X, Han X and Li J: Exosomes
derived from circRNA Rtn4-modified BMSCs attenuate TNF-α-induced
cytotoxicity and apoptosis in murine MC3T3-E1 cells by sponging
miR-146a. Biosci Rep. 40:BSR201934362020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mikami R, Mizutani K, Aoki A, Tamura Y,
Aoki K and Izumi Y: Low-level ultrahigh-frequency and
ultrashort-pulse blue laser irradiation enhances osteoblast
extracellular calcification by upregulating proliferation and
differentiation via transient receptor potential vanilloid 1.
Lasers Surg Med. 50:340–352. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rosa AP, de Sousa LG, Regalo SC, Issa JP,
Barbosa AP, Pitol DL, de Oliveira RH, de Vasconcelos PB, Dias FJ,
Chimello DT and Siéssere S: Effects of the combination of low-level
laser irradiation and recombinant human bone morphogenetic
protein-2 in bone repair. Lasers Med Sci. 27:971–977. 2012.
View Article : Google Scholar
|
|
59
|
Hou JF, Zhang H, Yuan X, Li J, Wei YJ and
Hu SS: In vitro effects of low-level laser irradiation for bone
marrow mesenchymal stem cells: Proliferation, growth factors
secretion and myogenic differentiation. Lasers Surg Med.
40:726–733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Abramovitch-Gottlib L, Gross T, Naveh D,
Geresh S, Rosenwaks S, Bar I and Vago R: Low level laser
irradiation stimulates osteogenic phenotype of mesenchymal stem
cells seeded on a three-dimensional biomatrix. Lasers Med Sci.
20:138–146. 2005. View Article : Google Scholar
|
|
61
|
Kipshidze N, Nikolaychik V, Keelan MH,
Shankar LR, Khanna A, Kornowski R, Leon M and Moses J: Low-power
helium: Neon laser irradiation enhances production of vascular
endothelial growth factor and promotes growth of endothelial cells
in vitro. Lasers Surg Med. 28:355–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu N, Lu W, Qu X and Zhu C: LLLI promotes
BMSC proliferation through circRNA_0001052/miR-124-3p. Lasers Med
Sci. 37:849–856. 2022. View Article : Google Scholar
|
|
63
|
Zheng J, Lin Y, Tang F, Guo H, Yan L, Hu S
and Wu H: Promotive role of CircATRNL1 on chondrogenic
differentiation of BMSCs mediated by miR-338-3p. Arch Med Res.
52:514–522. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wen X, Zhang J, Yang W, Nie X, Gui R, Shan
D, Huang R and Deng H: CircRNA-016901 silencing attenuates
irradiation-induced injury in bone mesenchymal stem cells via
regulating the miR-1249-5p/HIPK2 axis. Exp Ther Med. 21:3552021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li X, Chen R, Lei X, Wang P, Zhu X, Zhang
R and Yang L: Quercetin regulates ERα mediated differentiation of
BMSCs through circular RNA. Gene. 769:1451722021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhao YS, Lin P, Tu YC, An T, Wu YP and Li
XF: Lentivirus mediated siRNA hsa-circ-0000885 transfection of
BMSCs and osteoclast co-culture system on cell differentiation,
proliferation and apoptosis. Zhongguo Gu Shang. 34:978–984.
2021.(In Chinese). PubMed/NCBI
|
|
67
|
Wang H, Zhou K, Xiao F, Huang Z, Xu J,
Chen G, Liu Y and Gu H: Identification of circRNA-associated ceRNA
network in BMSCs of OVX models for postmenopausal osteoporosis. Sci
Rep. 10:108962020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lin C, Chen Z, Guo D, Zhou L, Lin S, Li
C..Li S, Wang X, Lin B and Ding Y: Increased expression of
osteopontin in subchondral bone promotes bone turnover and
remodeling, and accelerates the progression of OA in a mouse model.
Aging (Albany NY). 14:253–271. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu Z, Liu Q, Chen S, Su H and Jing T:
Circular RNA Circ_0005564 promotes osteogenic differentiation of
bone marrow mesenchymal cells in osteoporosis. Bioengineered.
12:4911–4923. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chia W, Liu J, Huang YG and Zhang C: A
circular RNA derived from DAB1 promotes cell proliferation and
osteogenic differentiation of BMSCs via RBPJ/DAB1 axis. Cell Death
Dis. 11:3722020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhong W, Li X, Pathak JL, Chen L, Cao W,
Zhu M, Luo Q, Wu A, Chen Y, Yi L, et al: Dicalcium silicate
microparticles modulate the differential expression of circRNAs and
mRNAs in BMSCs and promote osteogenesis via circ_1983-miR-6931-Gas7
interaction. Biomater Sci. 8:3664–3677. 2020. View Article : Google Scholar
|
|
72
|
Hao Y, Lu C, Zhang B, Xu Z, Guo H and
Zhang G: CircPVT1 up-regulation attenuates steroid-induced
osteonecrosis of the femoral head through regulating
miR-21-5p-mediated Smad7/TGFβ signalling pathway. J Cell Mol Med.
25:4608–4622. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhou R, Miao S, Xu J, Sun L and Chen Y:
Circular RNA circ_0000020 promotes osteogenic differentiation to
reduce osteoporosis via sponging microRNA miR-142-5p to up-regulate
bone morphogenetic protein BMP2. Bioengineered. 12:3824–3836. 2021.
View Article : Google Scholar : PubMed/NCBI
|