Introduction
Intervertebral disc (IVD) degeneration (IDD) is a
common natural aging process characterized by chronic back and low
back pain (1,2). With the increasing aging population
in the world, IDD has become the leading cause of spinal related
disability worldwide, with an increasing incidence of IDD and few
inexpensive and effective treatments (3). Therefore, there is an urgent need
for effective treatment to alleviate the progression of IDD.
Intervertebral disc is an avascular organ composed
of peripheral annulus and central nucleus pulposus (4), in which the nucleus pulposus cells
(NPCs) are responsible for regulating the synthesis and
decomposition of components of extracellular matrix (ECM) (5), including synthesis of collagen type
II (collagen II), sry-type high-mobility-group box 9 (SOX-9) and
proteoglycans (mainly aggrecan) and the decomposition of matrix
metalloproteinases (MMPs) (6). It
has previously been observed that the process of IDD is closely
associated with apoptosis and inflammatory response of NPCs and
these pro-inflammatory molecules are secreted by nucleus pulposa
(7). In the process of
degeneration, elevated levels of inflammatory factors promote ECM
degradation, leading to cell phenotypic changes and a host of
degenerative events (2).
Therefore, finding effective drugs to inhibit NPCs apoptosis, ECM
degradation and inflammation may be a feasible strategy for the
prevention and treatment of IDD.
Evodiamine (Evo), a type of indole quinazoline
alkaloid extracted from dried fruit of Evodia rutaecarpa
(8). In traditional Chinese
medicine, Evodia rutaecarpa is widely used for treating
various infection-related diseases, such as diarrhea, ulcerative
colitis and beriberi (9–11), showing strong anti-inflammatory
activity. A previous study suggested that Evo can enhance NLRP3
inflammasome activation by inducing α-tubulin acetylation, thereby
improving innate immunity to bacterial infections (11). It can also reduce the peripheral
hypersensitivity and anxiety of mice with nerve injury or
inflammation through TRPV1 (12)
and regulate the TLR4/NF-κB signaling pathway to inhibit
lipopolysaccharide (LPS)-induced HUVECs injury and promote cell
proliferation (13). In addition,
Shi et al (14)
demonstrate that Evo possesses an important protective effect on
LPS-induced acute kidney injury and cytotoxicity. However, the role
and mechanism of Evo in IDD have yet to be studied, to the best of
the authors' knowledge.
Previous studies have shown that Sirtuin 1 (SIRT1)
possesses a protective effect in IDD (15,16), while Evo regulates SIRT1 level in
colorectal cancer and inhibits the migration and invasion of
colorectal cancer (17). Another
study also reported that Evo can induce apoptosis of human melanoma
A375-S2 cells by regulating SIRT (18). Therefore, it was hypothesized that
Evo might serve a role in the progression of IDD by regulating
SIRT1. Notably, PI3K/Akt pathway also serves an important role in
the pathogenesis of IDD (19,20). The activation of PI3K/Akt pathway
can inhibit interleukin-1β (IL-1β)-induced NP cell apoptosis
(21), possibly by increasing ECM
content, preventing apoptosis and alleviating oxidative damage and
inflammatory reaction (19). In
Ren et al (22), Sirt1 as
the upstream of PI3K/Akt can directly connect with PI3K/Akt and
curcumin served a role in diabetic cardiomyopathy treatment by
modulating the Sirt1-Foxo1 and PI3K/Akt pathways. Qi et al
(23) demonstrate that tyrosol
upregulates SIRT1, inhibits apoptosis and inflammation of
IL-1β-stimulated NPCs and regulates ECM remodeling by activating
the PI3K/Akt pathway. Based on the above studies, it was
hypothesized that Evo could serve a protective role in IDD by
regulating SIRT1 and PI3K/Akt pathway. Therefore, the aim of the
present study was to investigate the effects of Evo on LPS-induced
NPCs apoptosis, ECM degradation and inflammation and to
preliminarily analyze its underlying mechanism.
Materials and methods
Cell culture
Immortalized human nucleus pulposus cells (NPCs;
iCell Bioscience Inc.; cat. no. iCell-0028a) were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 µg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.)
at 37°C in 5% CO2. The cells were passaged once after
three days. The cells cultured to logarithmic phase were used in
following experiments.
Cell Counting Kit-8 (CCK-8) assay
Cells (1×103 cells/well) were seeded into
96-well plates and incubated at 37°C with 5% CO2. Cell
proliferation was determined using CCK-8 reagent (Dojindo Molecular
Technologies, Inc.), according to the manufacturer's protocol.
Following incubation with 1 µg/ml LPS and Evo at different
concentrations (5, 10 and 20 µM) for 24 h, 10 µl CCK-8 solution was
added to each well for 4 h at 37°C. Absorbance was measured at a
wavelength of 450 nm using a microplate reader.
TUNEL assay
The effects of Evo on the apoptosis of LPS-induced
NPCs cells were detected using TUNEL according to the
manufacturer's protocol. In brief, cells were collected and washed
three times with PBS. Following fixing with 4% paraformaldehyde at
room temperature for 20 min, the cells were washed twice with PBS.
Then, 0.2% Triton-X-100 was added to the cells at room temperature
for 5 min. Subsequently, 50 µl TUNEL assay solution (Roche
Diagnostics GmbH) was added to the cells and incubated at 37°C in
the dark for 60 min. 0.5 µg/ml of DAPI solution (Beyotime
Biotechnology) was applied to stain cell nuclei for 3–5 min at room
temperature. The detection solution was discarded and cells were
washed three times with PBS. Subsequently, three fields of view
were selected at random and then cells were sealed with
anti-fluorescence quenched sealing solution for observation under a
fluorescence microscope (Zeiss GmbH; magnification, ×200).
Western blotting
The extraction of total proteins from NPCs cells was
conducted by radioimmunoprecipitation (RIPA) lysing buffer (Beijing
Solarbio Science & Technology Co., Ltd.) and the protein
concentrations were quantified using a bicinchoninic acid kit
(Beyotime Institute of Biotechnology). The samples were subjected
to 12% gel with sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene
fluoride (PVDF) membranes before being blocked with 5% non-fat milk
for 2 h at room temperature. Subsequently, the membranes were
incubated overnight at 4°C with the following primary antibodies
(all purchased from Abcam): Anti-Bcl2 (1:1,000; cat. no. ab32124),
anti-Bax (1:1,000; cat. no. ab32503), anti-MMP13 (1:1,000; cat. no.
ab219620), anti-Aggrecan (1:1,000; cat. no. ab3778), anti-Collagen
II (1:1,000; cat. no. ab34712), anti-SOX9 (1:1,000; cat. no.
ab185996), anti-SIRT1 (1:1,000; cat. no. ab110304),
anti-phosphorylated (p-)P13K (1:1,000; cat. no. ab32503), anti-P13K
(1:1,000; cat. no. ab32089), anti-p-AKT (1:1,000; cat. no.
ab182651), anti-AKT (1:1,000; cat. no. ab191606) and anti-GAPDH
(1:1,000; cat. no. ab181602). Following that, the membranes were
incubated with a goat anti-rabbit horseradish peroxidase-conjugated
IgG secondary antibodies (1:5,000; cat. no. ab150077) for 2 h at
room temperature. Protein bands were visualized using enhanced
chemiluminescence reagent (Cytiva). Protein expression levels were
semi-quantified using ImageJ software (version 1.46; National
Institutes of Health) with GAPDH as the loading control.
Detection kit
The levels of TNF-α (cat. no. PT518) and IL-6 (cat.
no. PI330) in NPCs cells were quantified using ELISA kits (Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. The effect of Evo on the activity of caspase-3 in
LPS-treated NPCs cells was detected using caspase-3 activity assay
kit (cat. no. BC3830; Beijing Solarbio Science & Technology
Co., Ltd.).
Cell transfection
Cells (1×105 cells/well) were seeded into
6-well plates and cultured for 24 h at 37°C with 5% CO2.
siRNA targeting SIRT1 (si-SIRT1 forward: 5′-GGAUGAAAGUGAAAUUGAA-3′,
reverse: 5′-UUCAAUUUCACUUUCAUCC-3′) and a control non-targeting
siRNA (si-NC forward: 5′-UUCUCCGAACGUGUCACGUTT-3′, reverse:
5′-ACGUGACACGUUCGGAGAATT-3′) were designed and synthesized by
Shanghai GenePharma Co., Ltd. Subsequently, All plasmids were
transfected using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Cells in the blank control group (Control) were
untreated. At 48 h post-transfection, transfection efficiency was
assessed via RT-qPCR.
Data analysis
The data were plotted with GraphPad Prism 8.0
software (GraphPad Software, Inc.). The measurement data are
expressed as the mean ± standard deviation from ≥3 independent
experiments. One-way ANOVA followed by Tukey's post hoc test was
used for comparison between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Evo inhibits LPS-induced NPCs
apoptosis
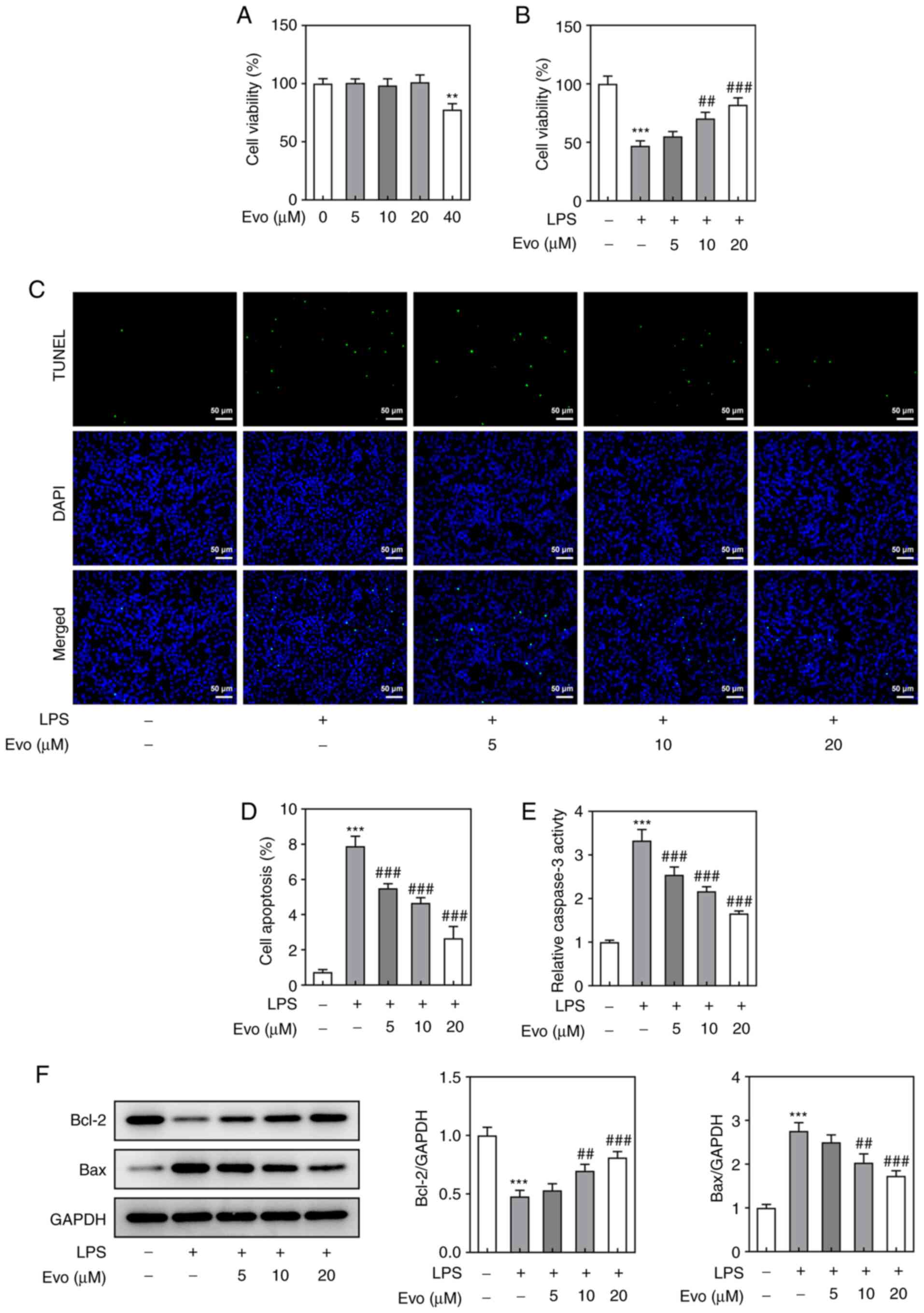
First, the effect of Evo on NPCs viability was
detected. The results showed that Evo at 5, 10 and 20 µM had no
significant effect on NPCs viability, but Evo at 40 µM caused
damage on NPCs (Fig. 1A).
Therefore, the maximum Evo concentration was 20 µM in the following
experiments. Subsequently, CCK-8 assay (Fig. 1B) and TUNEL staining (Fig. 1C and D) showed significant
apoptosis of NPCs induced by LPS. It should be noted that Evo
enhanced NPCs viability in a concentration-dependent manner. In
addition, caspase-3 activity and the expression levels of
apoptosis-related proteins in NPCs were also assessed (Fig. 1E and F). Following LPS induction,
caspase-3 activity was increased, Bax protein was significantly
upregulated and Bcl-2 protein was downregulated, indicating that
LPS induced apparent apoptosis. Similarly, this change also could
be reversed by Evo.
Evo effectively alleviates LPS-induced
extracellular matrix degradation and inflammation in NPCs
Subsequently, the effects of Evo on LPS-induced ECM
degradation and inflammation in NPCs was analyzed. The protein
expression level of ECM catabolism gene MMP-13 was upregulated
after LPS stimulation, but Evo treatment attenuated the effect of
LPS; the protein expressions of ECM synthesis genes (Aggrecan,
collagen II and SOX-9,) in NPCs were downregulated following LPS
treatment, while Evo treatment reversed these changes (Fig. 2A). ELISA was used to measure the
expression levels of pro-inflammatory cytokines (TNF-α and IL-6) in
cells. The results indicated that TNF-α and IL-6 levels were
significantly increased following LPS treatment (Fig. 2B and C). Similarly, Evo treatment
significantly downregulated the expression of TNF-α and IL-6 in
cells in a concentration-dependent manner. These results suggested
that Evo effectively alleviated LPS-induced ECM degradation and
inflammation in NPCs.
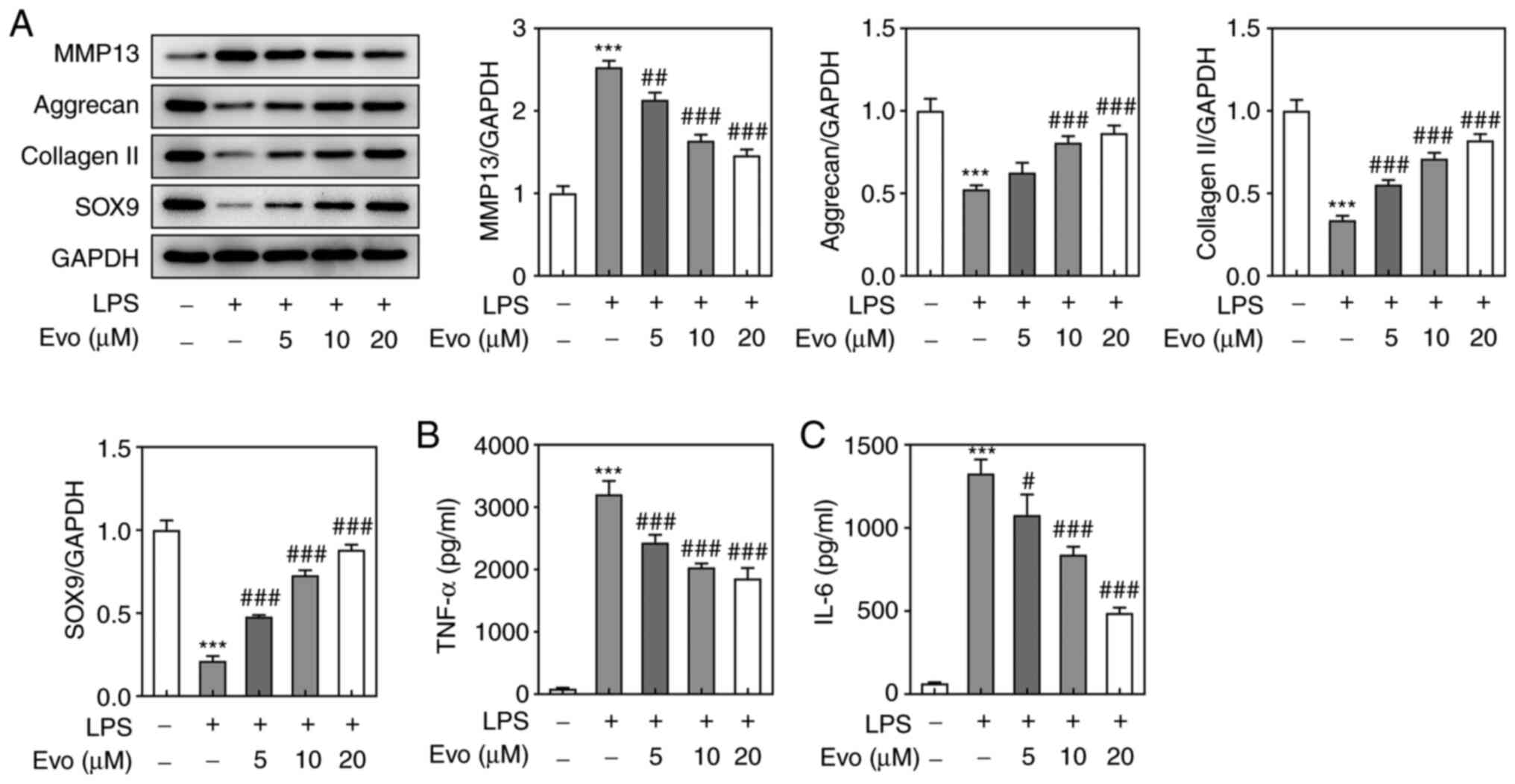 | Figure 2.Evo effectively alleviated LPS-induced
extracellular matrix degradation and inflammation in NPCs. (A)
Western blotting was applied to examine the expression of MMP-13,
Aggrecan, collagen II and SOX-9 proteins. The concentrations of (B)
TNF-α and (C) IL-6 in NPCs were determined by ELISA. ***P<0.001
vs. Control. #P<0.05, ##P<0.01 and
###P<0.001 vs. LPS. Evo, evodiamine; LPS,
lipopolysaccharide; NPCs, human nucleus pulposus cells; SOX-9,
sry-type high-mobility-group box 9; MMP, matrix metalloproteinase;
TNF, tumor necrosis factor; IL, interleukin. |
Evo activates the PI3K/AKT pathway by
upregulating SIRT1
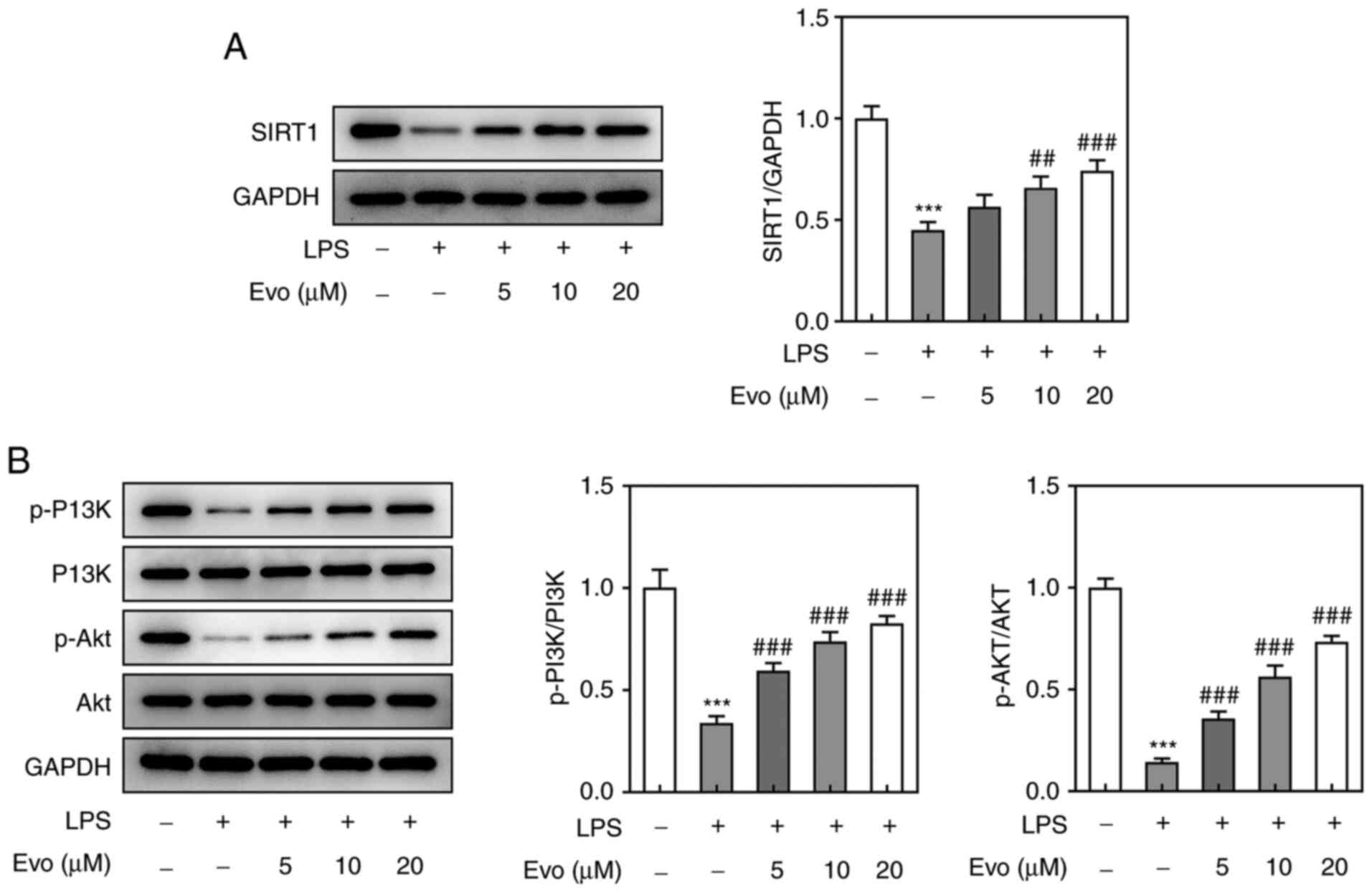
To further investigate the mechanism of Evo's role
in IDD cell model, western blotting revealed that Evo conspicuously
alleviated LPS-induced downregulation of SIRT1 (Fig. 3A). A previous study established
that SIRT1 acts as a key role in the survival of human NP degraded
cells by regulating the Akt pathway (23). Therefore, the effect of Evo on the
P13K/Akt pathway in LPS-induced NPCs was then examined. The
expression of p-Akt and p-P13K was downregulated by LPS stimulation
and this effect was also salvaged by Evo (Fig. 3B). To further verify whether Evo
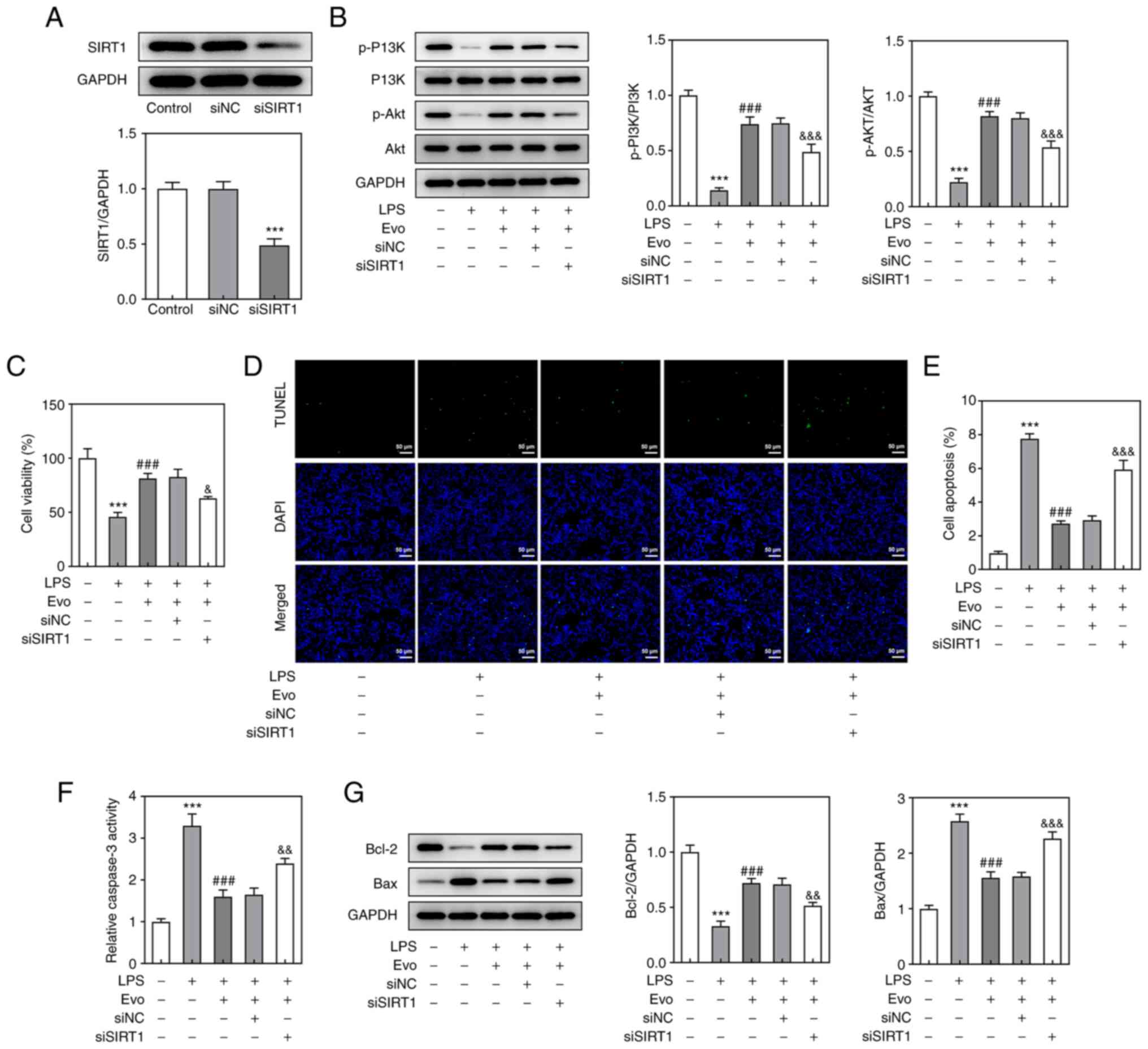
activates the P13K/AKT pathway by upregulating SIRT1, a SIRT1
interfering plasmid was constructed (Fig. 4A) for repeated experiments and it
was found that in the absence of SIRT1, Evo's activation of
P13K/AKT pathway was weakened compared with the si-NC group
(Fig. 4B). These results
demonstrated that Evo could upregulate SIRT1 and activate the
PI3K/Akt pathway, but this positive effect was significantly
weakened after SIRT1 silencing, indicating that Evo might activate
the PI3K/Akt pathway by upregulating SIRT1.
SIRT1 knockdown attenuates the
inhibitory effects of Evo on LPS-induced apoptosis, inflammation
and ECM degradation in NPCs
Considering that Evo serves a role by upregulating
SIRT1, CCK-8 assay (Fig. 4C) and
TUNEL staining (Fig. 4D and E)
were used again to detect Evo's effect on LPS-induced apoptosis in
NPCs following SIRT1 knockdown. The inhibitory effect of Evo on
LPS-induced NPCs apoptosis and increased caspase-3 activity was
weakened after SIRT1 knockdown (Fig.
4F). At the same time, the expression of apoptotic proteins
detected by western blotting also confirmed this result (Fig. 4G). As shown in Fig. 5A, si-SIRT1 transfection partially
eliminated the promotive effect of Evo on ECM synthesis.
Furthermore, si-SIRT1 also increased the concentrations of TNF-α
and IL-6 compared with Evo group (Fig. 5B and C), which indicated that
SIRT1 knockdown attenuates the inhibitory effect of Evo on
LPS-induced NPCs inflammation.
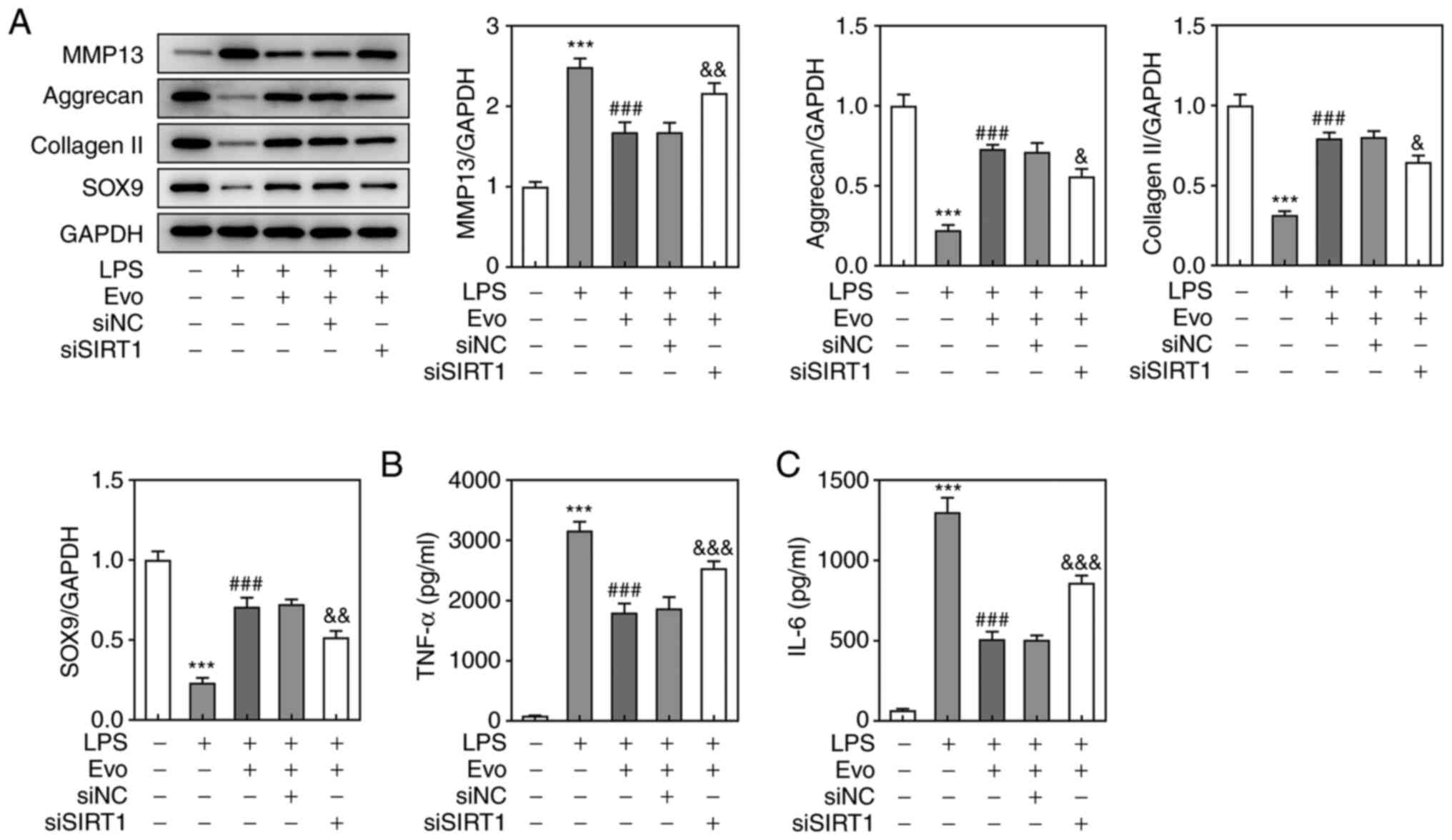 | Figure 5.SIRT1 knockdown attenuates the
inhibitory effects of Evo on LPS-induced inflammation and ECM
degradation in NPCs. (A) Western blotting was applied to examine
the expression of MMP-13, Aggrecan, collagen II and SOX-9 proteins.
The concentrations of (B) TNF-α and (C) IL-6 in NPCs were
determined by ELISA.***P<0.001 vs. Control.
###P<0.001 vs. LPS. &P<0.05,
&&P<0.01 and
&&&P<0.001 vs. si-NC. SIRT1, Sirtuin 1;
Evo, evodiamine; LPS, lipopolysaccharide; ECM, extracellular
matrix; NPCs, human nucleus pulposus cells; SOX-9, sry-type
high-mobility-group box 9; MMP, matrix metalloproteinase; TNF,
tumor necrosis factor; IL, interleukin; si, short interfering; NC,
negative control. |
Discussion
IVD degeneration is a multifactorial pathological
process associated with low back pain, which has been the leading
cause of disability worldwide (1,24).
Increasing studies have proved that the pathological changes of
intervertebral disc disease are closely associated with the
degradation of ECM, apoptosis and inflammation (25–27). The present study demonstrated for
the first time, to the best of the authors' knowledge that Evo
could effectively reduce LPS-induced NPCs apoptosis, ECM
degradation and inflammation, possibly by upregulating SIRT1 and
activating the PI3K/Akt pathway.
Evo, as an effective ingredient of Traditional
Chinese medicine, has a variety of pharmacological effects such as
antioxidant, anti-tumor, anti-ulcer and neuroprotection, especially
in anti-infection and anti-apoptosis (10,11). Evo, for example, inhibits
p2×7-dependent TNF-α expression and ERK1/2 phosphorylation, thereby
inhibiting oxidative stress and inflammatory response (28). Another study found that Evo
inhibits the secretion of interleukin (IL-10) and IL-2 by
LPS-stimulated endothelial cells, inhibiting inflammatory responses
(29). It also relieves
DSS-induced ulcerative colitis by increasing Lactobacillus
acidophilus levels and acetate production (10). In addition, Evo also regulates the
TLR4/NF-κB signaling pathway to inhibit LPS-induced HUVECs injury
and promote cell proliferation (13). In the present study, Evo was also
shown to inhibit LPS-induced apoptosis of NPCs in a
concentration-dependent manner and Evo treatment reversed the
upregulation of MMP-13, as well as the downregulation of collagen
II, SOX-9 and aggrecan in LPS-stimulated NPCs. Furthermore,
compared with LPS group, Evo markedly reduced the production of
pro-inflammatory factors TNF-α and IL-6, thus effectively
alleviating the degradation of ECM, apoptosis and inflammatory
pathological characteristics in the progression of IDD.
To further investigate the mechanism of Evo
alleviating IDD pathological process, the present study focused on
Sirt1, the most prominent and widely explored member of the sirtuin
family, which has been shown to serve a role in various cellular
processes including cell survival and apoptosis in a number of
studies (30,31). It is noteworthy that SIRT1 serves
a protective role in IDD. For example, SIRT1 enhances the
proliferation of aging nucleus pulposus cells by inhibiting P16 and
serves a protective role in disc degeneration in rodents (16). CircERCC2 can regulate the
apoptosis, autophagy and ECM degradation in tert-butyl
hydroperoxide-induced NPCs by targeting miR-182-5p/SIRT1 (32). Notably, it has also been reported
that Evo exerts a role in a variety of diseases by mediating SIRT1,
Zhou et al (17) suggest
that Evo inhibits migration and invasion of colorectal cancer by
regulating SIRT1 level. The present study demonstrated that the
expression of Sirt1 was downregulated in LPS-induced NPCs and
increased in a concentration-dependent manner following Evo
treatment. These results indicated that Evo serves a therapeutic
role in IDD by upregulating sirt1.
PI3K/Akt pathway is a classic pathway involved in
regulating cell proliferation, differentiation, apoptosis and other
biological processes (33,34).
Existing research has shown that activation of PI3K/Akt pathway can
inhibit IL-1β-induced apoptosis of NPCs and enhance the
adaptability of NPCs to hypoxia microenvironment (19). Furthermore, tyrosol performs an
active role in IDD by upregulating SIRT1, which inhibits apoptosis
and inflammation of IL-1β-stimulated NPCs by activating the
PI3K/Akt pathway (23). The
present study showed that Evo rescued LPS-induced inactivation of
the PI3K/Akt pathway in NPCs. Notably, downregulation of Sirt1
reversed Evo's activation of Akt and PI3K phosphorylation, thereby
partly eliminated Evo's inhibitory effect on NPCs apoptosis and
promoting the degradation of ECM and inflammation in NPCs,
Therefore, it was hypothesized that Evo may serve a protective role
in IDD by upregulating Sirt1 and then activating PI3K/Akt.
In conclusion, the present study is, to the best of
the authors' knowledge, the first to demonstrate that Evo has a
protective effect on IDD, possibly by upregulating Sirt1 and then
activating the PI3K/Akt pathway to inhibit apoptosis, ECM
degradation and inflammation in LPS-stimulated NPCs. Although the
present study confirmed that Evo protects IDD and its possible
mechanism, the signaling pathway is complex and subject to multiple
factors, which requires further study of the interaction of
SIRT1/PI3K/Akt with other signaling mediators. In addition, further
overexpression of Sirt1 to verify the underlying mechanism of EVO
is a next research objective. In general, more research on the
mechanism of Evo's role in IDD will contribute to the development
of new biologic therapies for IDD.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JK participated in the study design, conducted the
experiments and participated in manuscript writing. NZ performed
the data analysis and manuscript writing. JK and NZ confirm the
authenticity of all raw data. Both authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vergroesen PP, Kingma I, Emanuel KS,
Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH and Smit TH:
Mechanics and biology in intervertebral disc degeneration: A
vicious circle. Osteoarthritis Cartilage. 23:1057–1070. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kos N, Gradisnik L and Velnar T: A brief
review of the degenerative intervertebral disc disease. Med Arch.
73:421–424. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
González Martínez E, García-Cosamalón J,
Cosamalón-Gan I, Esteban Blanco M, García-Suarez O and Vega JA:
Biology and mechanobiology of the intervertebral disc. Neurocirugia
(Astur). 28:135–140. 2017.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao Z, Luo R, Li G, Song Y, Zhan S, Zhao
K, Hua W, Zhang Y, Wu X and Yang C: Exosomes from mesenchymal stem
cells modulate endoplasmic reticulum stress to protect against
nucleus pulposus cell death and ameliorate intervertebral disc
degeneration in vivo. Theranostics. 9:4084–4100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng X, Zhang G, Zhang L, Hu Y, Zhang K,
Sun X, Zhao C, Li H, Li YM and Zhao J: Mesenchymal stem cells
deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus
cell apoptosis and reduce intervertebral disc degeneration. J Cell
Mol Med. 22:261–276. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molinos M, Almeida CR, Caldeira J, Cunha
C, Gonçalves RM and Barbosa MA: Inflammation in intervertebral disc
degeneration and regeneration. J R Soc Interface. 12:201504292015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Ge J, Zheng Q, Zhang J, Sun R and
Liu R: Evodiamine and rutaecarpine from Tetradium ruticarpum in the
treatment of liver diseases. Phytomedicine. 68:1531802020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu D, Qiu C, Wang Y, Qiao T and Cui YL:
Intranasal co-delivery of berberine and evodiamine by
self-assembled thermosensitive in-situ hydrogels for improving
depressive disorder. Int J Pharm. 603:1206672021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang MX, Lin L, Chen YD, Zhong YP, Lin YX,
Li P, Tian X, Han B, Xie ZY and Liao QF: Evodiamine has therapeutic
efficacy in ulcerative colitis by increasing Lactobacillus
acidophilus levels and acetate production. Pharmacol Res.
159:1049782020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li CG, Zeng QZ, Chen MY, Xu LH, Zhang CC,
Mai FY, Zeng CY, He XH and Ouyang DY: Evodiamine augments NLRP3
inflammasome activation and anti-bacterial responses through
inducing α-tubulin acetylation. Front Pharmacol. 10:2902019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang WD, Chen XY, Wu C, Lian YN, Wang YJ,
Wang JH, Yang F, Liu CH and Li XY: Evodiamine reduced peripheral
hypersensitivity on the mouse with nerve injury or inflammation.
Mol Pain. 16:17448069209025632020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Yang W, Peng F, Chen T, Fu Y and
Tian M: Evodiamine inhibits injury of HUVECs induced by
lipopolysaccharide through TLR4/NF-κB signaling pathway. Xi Bao Yu
Fen Zi Mian Yi Xue Za Zhi. 35:1088–1093. 2019.(In Chinese).
PubMed/NCBI
|
|
14
|
Shi Y, Hua Q, Li N, Zhao M and Cui Y:
Protective effects of evodiamine against LPS-induced acute kidney
injury through regulation of ROS-NF-κB-mediated inflammation. Evid
Based Complement Alternat Med. 2019:21908472019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo J, Shao M, Lu F, Jiang J and Xia X:
Role of Sirt1 plays in nucleus pulposus cells and intervertebral
disc degeneration. Spine (Phila Pa 1976). 42:E757–E766. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia X, Guo J, Lu F and Jiang J: SIRT1
plays a protective role in intervertebral disc degeneration in a
puncture-induced rodent model. Spine (Phila Pa 1976). 40:E515–E524.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou P, Li XP, Jiang R, Chen Y, Lv XT, Guo
XX, Tian K, Yuan DZ, Lv YW, Ran JH, et al: Evodiamine inhibits
migration and invasion by Sirt1-mediated post-translational
modulations in colorectal cancer. Anticancer Drugs. 30:611–617.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang C, Wang MW, Tashiro S, Onodera S and
Ikejima T: Roles of SIRT1 and phosphoinositide 3-OH kinase/protein
kinase C pathways in evodiamine-induced human melanoma A375-S2 cell
death. J Pharmacol Sci. 97:494–500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ouyang ZH, Wang WJ, Yan YG, Wang B and Lv
GH: The PI3K/Akt pathway: A critical player in intervertebral disc
degeneration. Oncotarget. 8:57870–57881. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan Y, Yao X, Dai Z, Wang Y and Lv G: Bone
morphogenetic protein 2 alleviated intervertebral disc degeneration
through mediating the degradation of ECM and apoptosis of nucleus
pulposus cells via the PI3K/Akt pathway. Int J Mol Med. 43:583–592.
2019.PubMed/NCBI
|
|
21
|
Guo HT, Yang SD, Zhang F, Liu S, Yang DL,
Ma L, Wang H and Ding WY: 17β-Estradiol protects against
interleukin-1β-induced apoptosis in rat nucleus pulposus cells via
the mTOR/caspase-3 pathway. Mol Med Rep. 20:1523–1530.
2019.PubMed/NCBI
|
|
22
|
Ren BC, Zhang YF, Liu SS, Cheng XJ, Yang
X, Cui XG, Zhao XR, Zhao H, Hao MF, Li MD, et al: Curcumin
alleviates oxidative stress and inhibits apoptosis in diabetic
cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J
Cell Mol Med. 24:12355–12367. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qi W, Ren D, Wang P, Song Z, Wu H, Yao S,
Geng L, Su Y and Bai X: Upregulation of Sirt1 by tyrosol suppresses
apoptosis and inflammation and modulates extracellular matrix
remodeling in interleukin-1β-stimulated human nucleus pulposus
cells through activation of PI3K/Akt pathway. Int Immunopharmacol.
88:1069042020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brinjikji W, Diehn FE, Jarvik JG, Carr CM,
Kallmes DF, Murad MH and Luetmer PH: MRI findings of disc
degeneration are more prevalent in adults with low back pain than
in asymptomatic controls: A systematic review and meta-analysis.
AJNR Am J Neuroradiol. 36:2394–2399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cazzanelli P and Wuertz-Kozak K: MicroRNAs
in intervertebral disc degeneration, apoptosis, inflammation, and
mechanobiology. Int J Mol Sci. 21:36012020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiang Q, Kang L, Wang J, Liao Z, Song Y,
Zhao K, Wang K, Yang C and Zhang Y: CircRNA-CIDN mitigated
compression loading-induced damage in human nucleus pulposus cells
via miR-34a-5p/SIRT1 axis. EBioMedicine. 53:1026792020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo HY, Guo MK, Wan ZY, Song F and Wang
HQ: Emerging evidence on noncoding-RNA regulatory machinery in
intervertebral disc degeneration: A narrative review. Arthritis Res
Ther. 22:2702020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xue Y, Guo T, Zou L, Gong Y, Wu B, Yi Z,
Jia T, Zhao S, Shi L, Li L, et al: Evodiamine attenuates
P2X7-mediated inflammatory injury of human umbilical
vein endothelial cells exposed to high free fatty acids. Oxid Med
Cell Longev. 2018:50828172018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu YY, He KW and Zhu HD: Chinese herbal
medicinal ingredients affect secretion of NO, IL-10, ICAM-1 and
IL-2 by endothelial cells. Immunopharmacol Immunotoxicol.
37:324–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang BL: Sirt1 and the mitochondria. Mol
Cells. 39:87–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiao F and Gong Z: The beneficial roles of
SIRT1 in neuroinflammation-related diseases. Oxid Med Cell Longev.
2020:67828722020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie L, Huang W, Fang Z, Ding F, Zou F, Ma
X, Tao J, Guo J, Xia X, Wang H, et al: CircERCC2 ameliorated
intervertebral disc degeneration by regulating mitophagy and
apoptosis through miR-182-5p/SIRT1 axis. Cell Death Dis.
10:7512019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie Y, Shi X, Sheng K, Han G, Li W, Zhao
Q, Jiang B, Feng J, Li J and Gu Y: PI3K/Akt signaling transduction
pathway, erythropoiesis and glycolysis in hypoxia (review). Mol Med
Rep. 19:783–791. 2019.PubMed/NCBI
|
|
34
|
Pompura SL and Dominguez-Villar M: The
PI3K/AKT signaling pathway in regulatory T-cell development,
stability, and function. J Leukoc Biol. Jan 22–2018.(Epub ahead of
print). doi: 10.1002/JLB.2MIR0817-349R. View Article : Google Scholar : PubMed/NCBI
|