Introduction
Gastric cancer (GC) is a type of gastrointestinal
tumor and the fifth commonest type of cancer overall (1). GC also ranks second in
cancer-related mortalities due to lethal malignancy and poor
prognosis rates and therefore remains a major global public health
problem (2,3). Early stage GC can be treated by
endoscopic resection or alternative, less invasive, surgical
approaches. However, in most cases GC is asymptomatic at the early
stage and progresses to advanced stages prior to diagnosis.
Therefore, it is important to identify novel biomarkers to predict
disease relapse or distant metastasis.
Current molecular genetic analysis suggests that GC
is a heterogeneous disease that is complicated by certain
epidemiological and histopathological characteristics. The Cancer
Genome Atlas Research Network (4)
demonstrates that for 295 primary GC tissues, which divide GC into
four molecular subtypes (tumors positive for the Epstein-Barr
virus, genomically stable tumors, microsatellite unstable tumors
and tumors with chromosomal instability), dysregulated signaling
pathways and driver mutations contribute towards GC. These included
genes related to carcinogenic or tumor suppressor signaling
pathways, increased amplification rates of Janus kinase 2,
programmed cell death-ligands 1 and 2 and chromosomal aberrations,
in addition to differential gene expression and epigenetic
alterations. A global gene expression profile identifies numerous
driver mutations in 300 GC samples provided by the Asian Cancer
Research Group, which identifies that tumor protein p53,
N-α-acetyltransferase 10, phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α, Kirsten rat sarcoma virus and
phosphatase and tensin homolog mutations are present in GC tissues
(5).
As a differentially expressed regulator, acidic
nuclear phosphoprotein 32 family member E (ANP32E), has attracted
significant interest as it acts as a member of the histone
chaperones with leucine-rich repeats that removes histone variant
H2A.Z from chromatin. These proteins serve important biological
functions in multiple cellular process, including cell adhesion and
tumor progression (6,7). One study demonstrates that ANP32E
upregulates E2F transcription factor 1 (E2F1) expression to promote
tumor formation in triple-negative breast cancer (TNBC) cells
(8). Other studies report that
ANP32E is involved in DNA double-strand breakages, inducing
nucleosome recombination and DNA repair (9–11).
However, the biological role of ANP32E in GC remains to be
elucidated.
NUF2, an important component of the NDC80
kinetochore complex, is critical for chromosome segregation and is
involved in the cell cycle and proliferation of tumor cells
(12). NUF2 is significantly
dysregulated in numerous types of cancer and functions as a
valuable prognostic biomarker in predicting tumors in their early
stages (13,14). However, the precise function and
specific mechanisms of NUF2 in GC remain to be clarified.
In the present study, ANP32E was demonstrated to be
significantly highly expressed in GC tissues and three GC cell
lines. ANP32E significantly induced the proliferation and colony
formation of GC and suppressed apoptosis by upregulating the
expression of NUF2. These results suggested that ANP32E may be used
as a potential prognostic biomarker in the treatment of GC.
Materials and methods
Patient samples
Patients with GC (19 males and 11 females, aged
54-67) were enrolled from The First Affiliated Hospital of Jiamusi
University (Jiamusi, China) between May 2017 and June 2021. The
subsequent experimental protocols were approved by the Ethics
Committee of the First Affiliated Hospital of Jiamusi University
(approval no. 202078). None of the patients, who were diagnosed as
gastric cancer by three independent pathologists, received any
interventions before surgery. Written informed consent was provided
by each patient. GC and paired normal adjacent tissue samples 5 cm
away from the tumor tissues were also obtained in addition to the
cancerous tissue sample.
ANP32E and NUF2 expression analysis
using the cancer genome atlas (TCGA) database
The mRNA levels of ANP32E and NUF2 expression, and
the correlation between ANP32E and NUF2 expression in gastric
cancer were analyzed using GEPIA (http://gepia.cancer-pku.cn/), which is based on TCGA
data. A total of 408 tumors and 211 normal tissues were included in
this study.
Cell lines and culture
Human GC HGC27, AGS and MKN45 cell lines and the
human normal gastric epithelial GES-1 cell line were purchased from
the American Type Culture Collection. Cells and cultured in their
respective media according to the supplier's protocol, including
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) and DMEM (Gibco;
Thermo Fisher Scientific, Inc.), which were supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Thermo Fisher Scientific, Inc.). Cells
were cultured at 37°C with 5% CO2. Reverse
transcription-quantitative (RT-q) PCR and western blot analysis of
ANP32E was performed in all the cell lines. Other experiments were
conducted in MKN45 and HGC27 cells.
ANP32E knockdown
Lipofectamine® RNAiMAX Transfection
Reagent (4 µl; Invitrogen; Thermo Fisher Scientific, Inc.) was used
to perform cell transfection after seeding 2×105 cells
in 6-well plates. Small interfering (si)RNA (40 nM) against ANP32E
and NUF2 was used to knockdown ANP32E and NUF2 expression levels in
GC cell lines via transient transfection for 48 h at 37°C. The
siRNA constructs used were as follows: siANP32E-1,
5′-GCAGCAAATCACATACTTAGA-3′; siANP32E-2,
5′-GGATGGCGATGAAGATGATGA-3; siNUF2, 5′-CAAUAAGAUCUUAACAGGAGCU-3′;
and siControl, 5′-UUCUCCGAACGUGUCACGU-3′. The knockdown efficiency
of the aforementioned constructs was determined using western
blotting assay.
ANP32E overexpression
The pcDNA3.1 vector was used for the overexpression
of ANP32E in HGC27 and MKN45 cell. Empty vectors were used as
negative control for overexpression. The coding sequence of ANP32E
was cloned into the pcDNA3.1 vector, then the empty vectors or
ANP32E-ovexpressing vectors (1 µg) were transfected into cells
(2×105 cells/well) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) and the cultured at
37°C for 10 h. At 48 h post-transfection, ANP32E overexpression was
examined by western blotting.
Cell proliferation
Cell proliferation assay was assessed using the Cell
Counting Kit-8 (CCK-8) assay in HGC27 and MKN45 cells. Briefly,
transfected GC cells (2.5×103 cells/well) were seeded
into a 96-well plate, supplemented with 100 µl culture medium and
maintained for 5 days at 37°C. CCK-8 reagent (10%; Beyotime
Institute of Biotechnology) was subsequently added to each well on
days 1, 2, 3 or 4, following the initial culturing for 5 days.
Cells were incubated at 37°C for 3 h. The optical density at 450 nm
was analyzed with an absorbance microplate reader. All the
experiments were repeated four times.
Colony formation
Transfected GC cells (2×103 cells/well)
were seeded into 6-well plates. After 14 days, colonies (>50
cells) that had formed were subsequently fixed in 4%
paraformaldehyde for 10 min, stained with crystal violet for 20 min
and dried at room temperature. Images were captured using a
camera.
Cell apoptosis
Trypsin and PBS were used to digest and wash the
cells. Subsequently, 5.0×105 cells (HGC27 or MKN45) were
seeded in six well plates. When the cell confluence reached 60-80%,
the cells were transfected with si control (Ctrl) or siANP32E1/2,
respectively. After transfection for 48 h at 37°C, the cells were
resuspended using 1X annexin-binding buffer and were stained using
an Alexa Fluor 488, annexin V and PI cell apoptosis analysis kit
(Thermo Fisher Scientific, Inc.), according to the manufacture's
protocols. PAC-1 (2 µM; Selleck Chemicals; cat. no. S2738) was used
to induce cell apoptosis, then the cells were subjected to cell
apoptosis assay. Subsequently, a cytoFLEX flow cytometer (Beckman
Coulter, Inc.) was used to detect cell apoptosis. UR plus LR
quadrants were used to calculate apoptosis. The data were analyzed
by Cytexpert (version 2.4.0.28, Beckman Coulter, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA derived from tissues (5-10 mg) or cell
lines (5×105) was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.). The tumor tissues and
normal adjacent tissues were placed on ice and cut into pieces and
then homogenized with TRIzol® reagent according to the
manufacturer's protocols. Subsequently, purified total RNA was
subjected to reverse-transcription to obtain complementary DNA
using Moloney Murine Leukemia Virus Reverse Transcriptase (Promega
Corporation) according to the manufacturer's protocols. ANP32E mRNA
expression levels were determined using SYBR green master mix
(Takara Biotechnology Co., Ltd.) on a 7500-fast machine (Thermo
Fisher Scientific, Inc.). The thermocycling conditions as follow:
Pre denaturation: 95°C, 10 min; thermal cycling: 45 cycles;
denaturation: 95°C, 20 sec; annealing: 60°C, 20 sec; elongation:
72°C, 30 sec. The qPCR primers used were: ANP32E forward (F),
5′-TGCCTGTGTGTCAATGGGG-3′ and reverse (R),
5′-GCAGAGCTTCTACTGTACTGAGA-3′; and GAPDH F,
5′-TGACTTCAACAGCGACACCCA-3′ and R, 5′-CACCCTGTTGCTGTAGCCAAA-3′.
Cyclin D forward (F), 5′-CCTCGGTGTCCTACTTCA-3′ and Reverse (R),
5′-CTCCTCGCACTTCTGTTC-3′; Cyclin E forward (F),
5′-GTTATAAGGGAGACGGGGAG-3′, Reverse (R),
5′-TGCTCTGCTTCTTACCGCTC-3′. ANP32E mRNA expression levels were
normalized to GAPDH, which was used as the internal reference gene.
The mRNA expression was quantified using the 2−∆∆Cq
method (15). The experiments
were conducted for three independent repeats.
Luciferase assay
To explore the interaction between ANP32E and NUF2,
luciferase reporter assay was performed. Briefly, the PGL3 plasmids
(Promega Corporation; cat. no. E1751) consisting of a firefly
reporter were subjected to construct the plasmids containing the
sequence of NUF2 promoter. Then, the plasmids with NUF2 promoter
were co-transfected with Ctrl or ANP32E-overexpressed vectors into
HGC27 cells using Lipofectamine 3000 (Thermo). After transfected
for 48 h, the luciferase activity was evaluated using
dual-luciferase reporter assay system (Promega Corporation).
Western blotting
RIPA lysis buffer (Beyotime Institute of
Biotechnology) was used to lyse HGC27 and MKN45 cells transfected
with siCtrl or siANP32E1/2, respectively. Protein concentration was
determined using a BCA kit (Thermo Fisher Scientific, Inc.). A
total of 30-50 µg protein was separated using SDS-PAGE on a 12%
gel. Subsequently, separated protein was transferred to a PVDF
membrane. Then, 5% non-fat milk was used to block the membrane for
1 h at room temperature. Membranes were incubated separately with
specific primary antibodies against ANP32E (1:1,000; Abcam; cat.
no. ab5993), GAPDH (1:10,000; Cell Signaling Technology, Inc.; cat.
no. 5174) and NUF2 (1:1,000; Abcam; cat. no. ab122962), overnight
at 4°C. Next, they were incubated with HRP-conjugated anti-rabbit
secondary antibodies (1:4,000; ProteinTech Group, Inc.; cat. no.
SA00001-2) at 25°C for 2 h, followed by visualization by using
SuperSignal West Pico PLUS (Thermo Fisher Scientific, Inc.; cat.
no. 34580). GAPDH was used as the loading control. Quantification
of the western blotting results was performed using Image J
(1.8.0.172; National Institutes of Health).
Statistical analysis
Data were analyzed using SPSS 25.0 (IBM Corp.) and
GraphPad Prism 7.0 software (GraphPad Software, Inc.). One-way
ANOVA followed by Bonferroni's test and unpaired Student's t-test
were used to assess statistical differences among groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
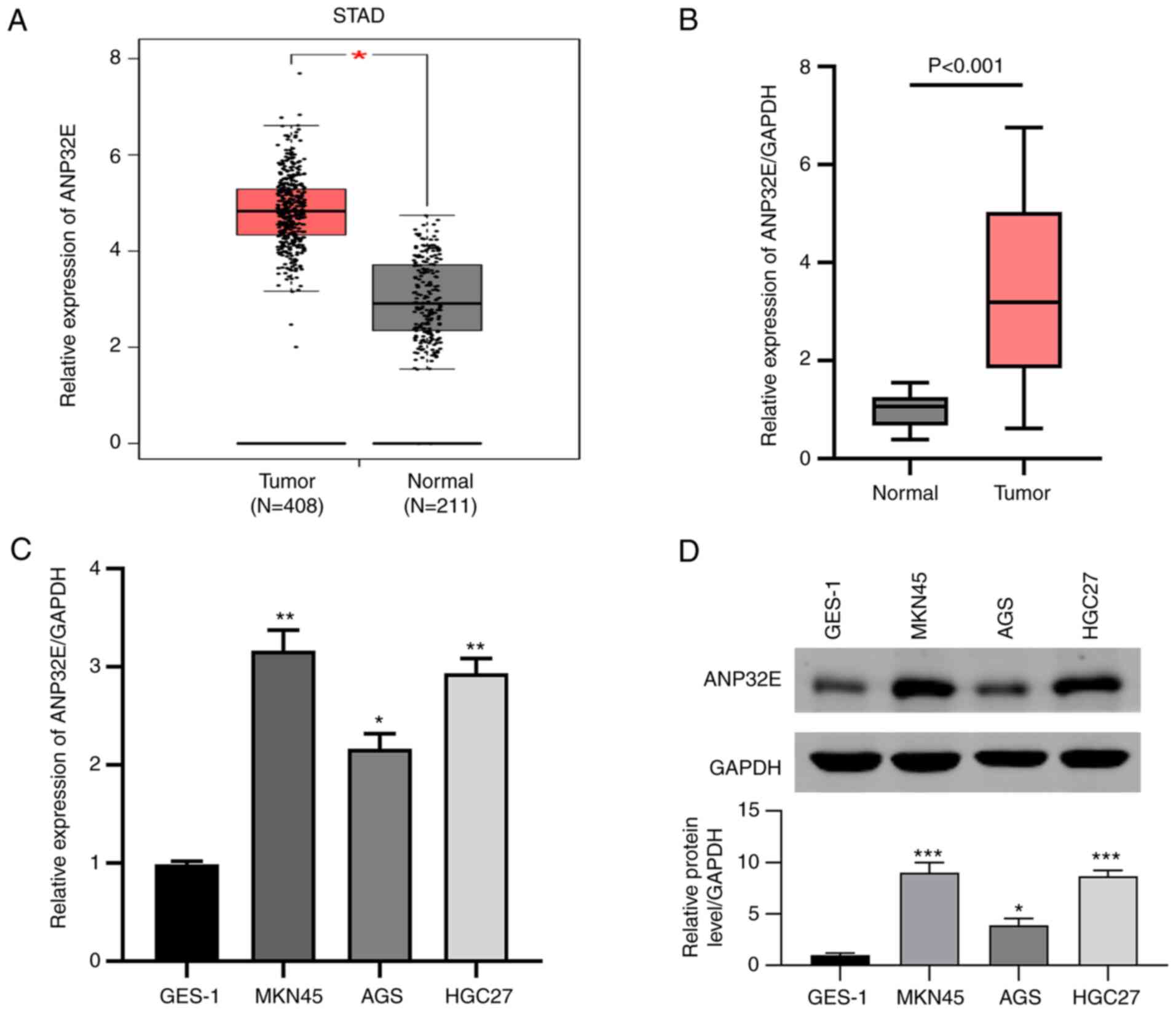
ANP32E expression levels increase in
GC tissues
The results demonstrated that ANP32E expression
levels were significantly upregulated in GC tissues compared with
normal adjacent tissues using RNA sequencing data downloaded from
TCGA (P<0.05; Fig. 1A). ANP32E
mRNA expression levels in the 30 GC tissues and paired normal
adjacent samples were determined using RT-qPCR. The results
demonstrated that ANP32E was significantly upregulated in GC
tissues compared with healthy tissues (P<0.001; Fig. 1B), consistent with TCGA analysis.
Regarding to the association between ANP32E expression and
clinicopathological characteristics in gastric cancer patients, the
results showed that ANP32E expression were significantly associated
with TNM stage (P=0.028, Table I)
and tumor size (P=0.003, Table
I). Subsequently, ANP32E mRNA and protein expression levels in
several GC cell lines were determined using RT-qPCR and western
blotting, respectively. The results demonstrated that ANP32E was
highly expressed in MKN45, AGS and HGC27 cell lines, in contrast to
GES-1, which displayed the lowest ANP32E levels among the cell
lines tested (Fig. 1C and D).
 | Table I.Correlation between ANP32E expression
and clinicopathological characteristics in 30 patients of gastric
cancer. |
Table I.
Correlation between ANP32E expression
and clinicopathological characteristics in 30 patients of gastric
cancer.
|
| ANP32E
expression |
|
|---|
|
|
|
|
|---|
| Characteristics | High | Low | P-value |
|---|
| Age |
|
|
|
|
<60 | 5 | 6 | 0.704 |
| ≥60 | 10 | 9 |
|
| Sex |
|
|
|
|
Male | 10 | 9 | 0.704 |
|
Female | 5 | 6 |
|
| TNM stage |
|
|
|
|
I–II | 4 | 10 | 0.028 |
|
III–IV | 11 | 5 |
|
| T stage |
|
|
|
|
T1-T2 | 6 | 8 | 0.464 |
|
T3-T4 | 9 | 7 |
|
| Lymph node
metastasis |
|
|
|
|
Positive | 7 | 7 | 1 |
|
Negative | 8 | 8 |
|
| Tumor size |
|
|
|
| <4
cm | 3 | 11 | 0.003 |
| ≥4
cm | 12 | 4 |
|
ANP32E silencing effectively inhibits
GC cell proliferation and induces cell apoptosis
As ANP32E mRNA and protein expression levels were
significantly increased in HGC27 and MKN45 cells, these two cell
lines were selected for assessment of the biological function of
ANP32E. The results demonstrated that ANP32E expression levels were
markedly suppressed by siANP32E-1 or siANP32E-2, in HGC27 and MKN45
cell lines, respectively (Fig.
2A). Subsequently, the CCK-8 assay was performed to determine
GC cell proliferation, which demonstrated that ANP32E silencing
suppressed the proliferation of both the HGC27 and MKN45 cell lines
(Fig. 2B and C). The effect of
ANP32E expression on colony formation in the HGC27 and MKN45 cell
lines was investigated. The results demonstrated that colony
formation ability was significantly suppressed following ANP32E
knockdown in GC cells (P<0.01; Fig. 2D and E). Moreover, qPCR analysis
indicated that the expression of cyclin D and cyclin E were
significantly decreased after ANP32E knockdown (Fig. 2F). Taken together, these results
indicated that ANP32E knockdown may inhibit GC cell
proliferation.
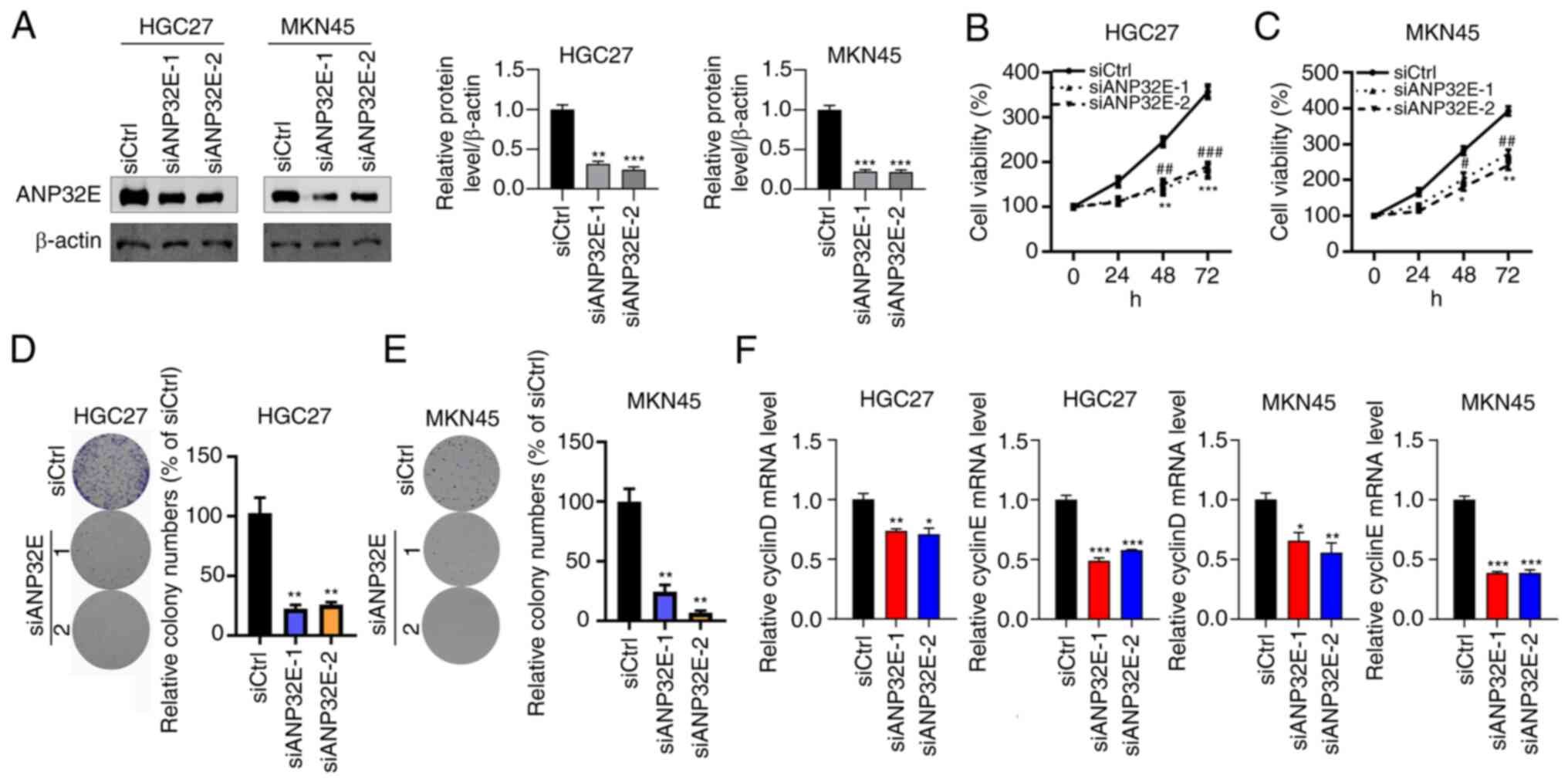 | Figure 2.ANP32E downregulation inhibits gastric
cancer cell growth. (A) Western blotting was performed to determine
the protein expression levels of ANP32E in siCtrl, siANP32E-1 and
siANP32E-2 transfected HGC27 and MKN45 cells. The protein
expression levels of ANP32E were normalized to β-actin. (B and C)
Cell proliferation was assessed in siCtrl, siANP32E-1 and
siANP32E-2 transfected (B) HGC27 and (C) MKN45 cell lines. siCtrl
vs. siANP32E-1 *P<0.05, **P<0.01 and ***P<0.001; and
siCtrl vs. siANP32E-2 #P<0.05, ##P<0.01 and ###P<0.001.
Colony formation was analyzed in siCtrl, siANP32E-1 and siANP32E-2
transfected (D) HGC27 and (E) MKN45 cells. **P<0.01. (F) RT-qPCR
assay was performed to examine the expression level of
proliferation markers, including Cyclin D and Cyclin E in HGC27 and
MKN45 cells. *P<0.05, **P<0.01 and ***P<0.001. ANP32E,
acidic nuclear phosphoprotein 32 family member E; si, small
interfering RNA; Ctrl, control; RT-qPCR, reverse
transcription-quantitative PCR. |
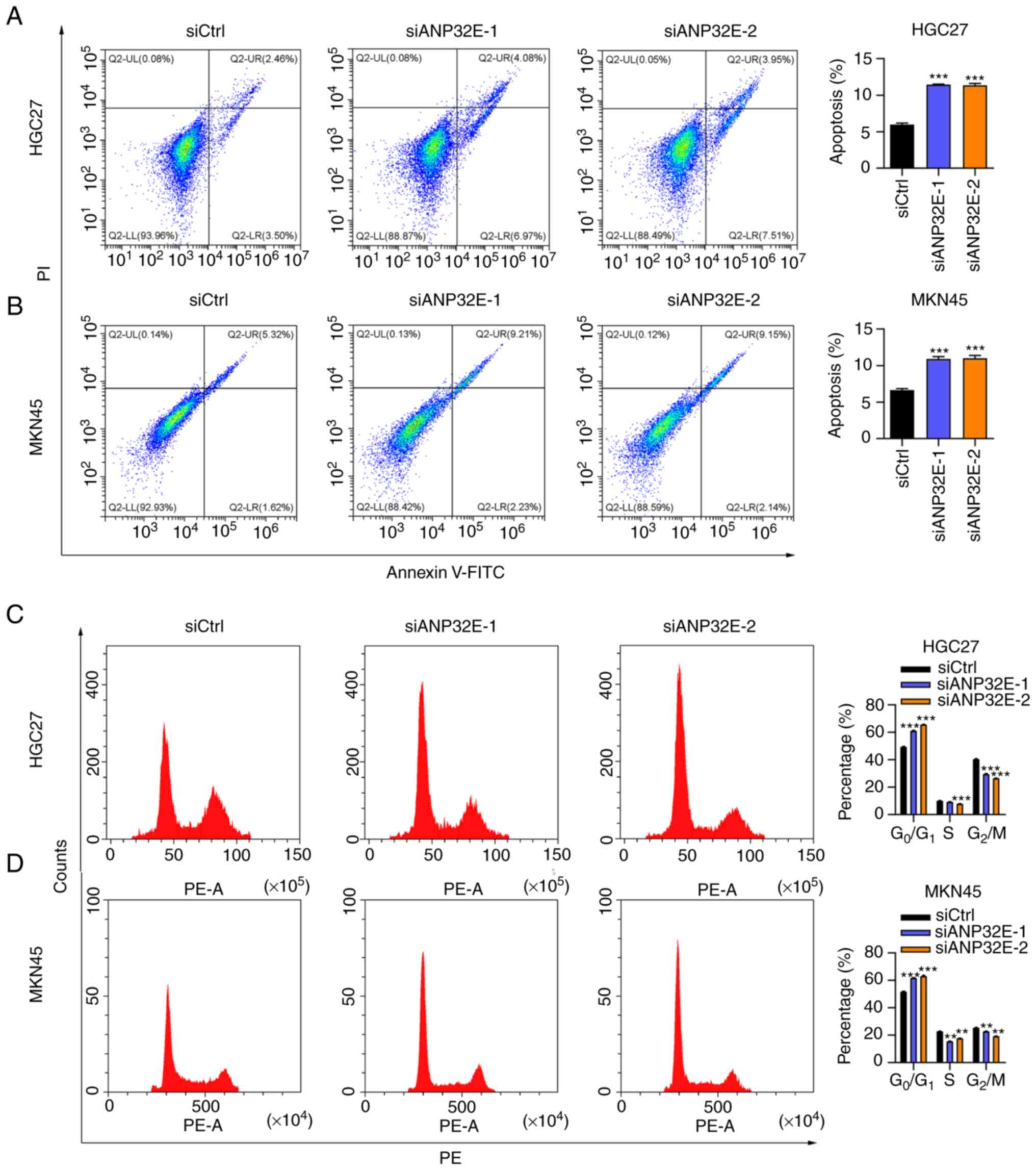
ANP32E silencing regulates cell
apoptosis and cell cycle progression
The downregulation of ANP32E significantly induced
cell apoptosis in the HGC27 and MKN45 cell lines as indicated by
the flow cytometry assay (P<0.05; Fig. 3A and B). The proportion of cells
in the S and G2/M phases were decreased and the
proportion of cells in the G0/G1 phase were
increased following ANP32E knockdown in HGC27 and MKN45 cells
(Fig. 3C and D). Collectively,
these data suggested that ANP32E downregulation may promote cell
apoptosis and cell cycle arrest.
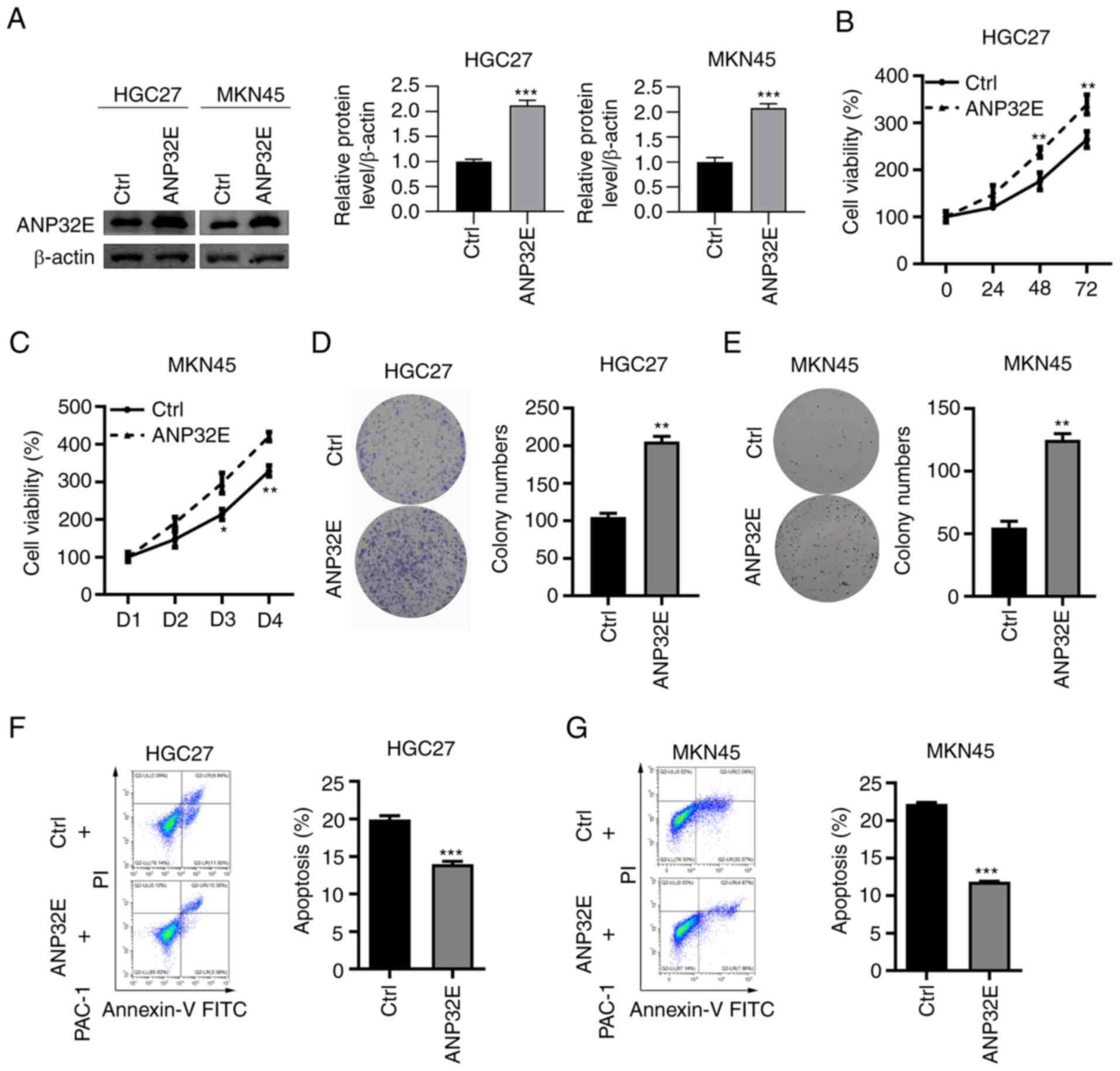
ANP32E overexpression promotes GC cell
proliferation
Based on the above results, ANP32E may act as an
oncogene in GC development. In turn, ANP32E was overexpressed in GC
cells to analyze its function. The results demonstrated that ANP32E
protein expression levels were markedly upregulated in HGC27 and
MKN45 cell lines (Fig. 4A).
Overexpression of ANP32E significantly increased cell proliferation
and colony formation in both HGC27 and MKN45 cell lines (P<0.01;
Fig. 4B-E). Furthermore, cell
apoptosis of GC cells following ANP32E overexpression was
investigated. The results demonstrated that ANP32E overexpression
significantly decreased the percentage of apoptosis induced by PAC1
in HGC27 and MKN45 cell lines (P<0.001; Fig. 4F and G).
ANP32E induces GC cell progression via
upregulating NUF2 expression
To investigate whether ANP32E regulated GC cell
growth by regulating another molecule, the correlation between
ANP32E and NUF2 expression levels was investigated. The results
demonstrated a strong positive relationship in GC tissues (Fig. 5A). Compared with healthy tissues,
NUF2 was upregulated in GC tissues (Fig. 5B). Furthermore, the mRNA and
protein expression levels of NUF2 were upregulated in
ANP32E-overexpressing HGC27 cells, which was rescued after treated
with siNUF2 (Fig. 5C and E). The
luciferase assay results of the luciferase activity in
NUF2-transfected HGC27 cells were significantly higher than that in
cells transfected with pGL3-basic vectors (Fig. 5D). To explore whether NUF7
upregulation contributes to the oncogenic role of ANP32E in GC
cells, the present study used siRNAs to silence NUF2 in
ANP32E-overexpressing HGC27 cells. Firstly, it was shown that HGC27
cells transfected with siNUF2 had decreased mRNA compared the cells
transfected with siCtrl (Fig.
S1). In addition, NUF2 was also markedly downregulated in
ANP32E-overexpressing HGC27 cells transfected with siNUF2 (Fig. 5E). While overexpression of ANP32E
promoted cell proliferation and colony formation, suppression of
NUF2 combined with ANP32E overexpression abolished the oncogene
roles mediated by ANP32E overexpression, including promoting cell
growth as well as inhibiting cell apoptosis (Fig. 5F-H). Overall, these results
suggested that ANP32E may promote GC cell proliferation and
suppress cell apoptosis via increasing NUF2 expression levels.
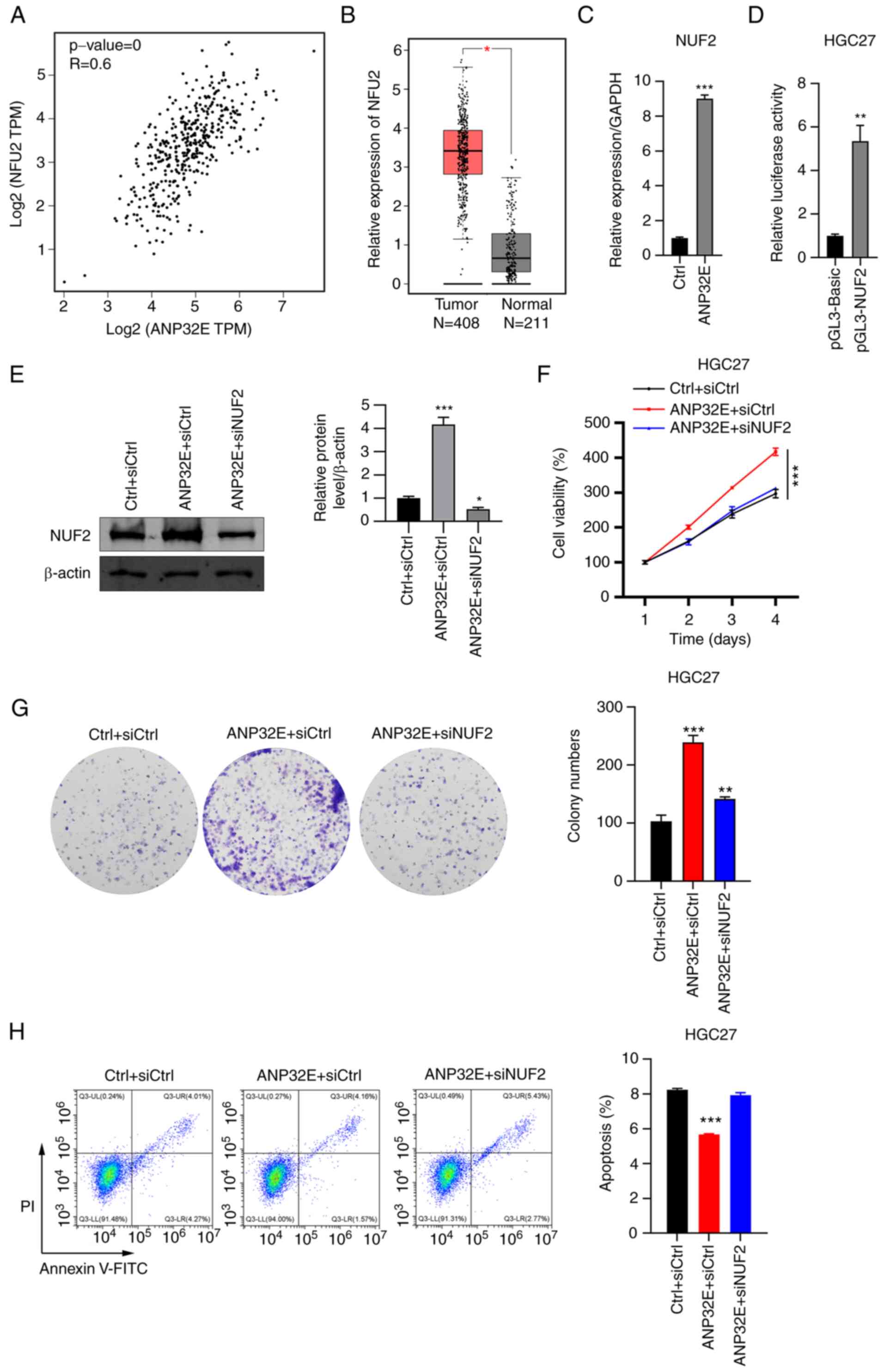 | Figure 5.ANP32E promotes GC cell proliferation
via NUF2 upregulation. (A) Spearman's correlation between ANP32E
and NUF2 expression levels was analyzed in tumor tissues using
TCGA. r=0.6, P<0.05. (B) NUF2 expression levels in tumor (n=408)
and adjacent normal (n=211) tissues were analyzed using TCGA. (C)
Reverse transcription-quantitative PCR was used to determine NUF2
mRNA expression levels in Ctrl and ANP32E-overexpressing HGC27
cells. The NUF2 mRNA expression levels were normalized to GAPDH.
(D) Luciferase reporter assay was performed to determine the
interaction between ANP32E and NUF2. (E) Western blotting was used
to determine NUF2 protein expression levels of NUF2 in Ctrl +
siCtrl, ANP32E-overexpressing + siCtrl and ANP32E-overexpressing +
siNUF2 HGC27 cells. . (F) Cell proliferation and (G) colony
formation was assessed in Ctrl + siCtrl, ANP32E-overexpressing +
siCtrl and ANP32E-overexpressing + siNUF2 HGC27 cells. (H)
PI/Annexin V-FITC staining and flow cytometry were performed to
analyze cell apoptosis in Ctrl + siCtrl, ANP32E-overexpressing +
siCtrl and ANP32E-overexpressing + siNUF2-treated HGC27 cells.
*P<0.05, **P<0.01 and ***P<0.001. ANP32E, acidic nuclear
phosphoprotein 32 family member E; GC, gastric cancer; NUF2, NUF2
component of NDC80 kinetochore complex; TCGA, The Cancer Genome
Atlas; si, small interfering RNA; Ctrl, control. |
Discussion
Surgery was previously considered as the only
radical treatment for GC (16).
Diagnostic and other therapeutic approaches have markedly improved
in recent years. However, targeted therapy is especially effective
in patients with GC (17).
Therefore, an improved understanding of the molecular pathogenesis
of GC development and progression is important to improve GC
therapeutics. In the present study, an in vitro assay was
used to determine the biological functions of ANP32E via its
knockdown and overexpression in GC cell lines.
ANP32E is an oncogene in numerous types of solid
tumors (18), including thyroid
carcinoma, breast cancer (8) and
lung cancer (19). ANP32E
functions as an important gene that promotes cell proliferation and
invasion of thyroid carcinoma via the activation of
AKT/mTOR/hexokinase 2-mediated glycolysis (18). Other studies report that ANP32E
induces TNBC cell proliferation and metastasis via the upregulation
of E2F1 expression (8,20). In the present study, ANP32E was
significantly upregulated in GC tissues, compared with adjacent
normal tissues, which implied that ANP32E may serve a role in tumor
progression. However, the present study could not obtain the
expression of ANP32E at protein levels owing the lack of suitable
antibody and the protein expression of ANP32E in gastric cancer
tissues should be explored in the future. It was also observed that
ANP32E expression markedly affected the proliferation, apoptosis
and cell cycle in GC cells in vitro. The results indicated
that ANP32E is an oncogene in GC, which was consistent with
previous studies (20). Among the
gastric cancer lines, GES-1 is a normal human gastric epithelial
cell (21), AGS (poorly
differentiated) and HGC27 (undifferentiated) are two
well-characterized gastric cancer cell lines (22,23), whereas MKN45 (poorly
differentiated) was established from the tumor of a 62-year-old
patient with gastric cancer (24). The present study observed higher
expression of ANP32E in the gastric cancer cell lines compared with
normal gastric epithelial cell line. To the best of the authors'
knowledge, the mechanism by which ANP32E acts as a GC oncogene was
examined for the first time in the present study. The public
microarray data also demonstrated a strong positive association
between ANP32E and NUF2 expression. It can therefore be
hypothesized that the upregulation of NUF2 may be an important
mechanism by which ANP32E regulates GC progression. NUF2 is known
to modulate tumor cell proliferation, invasion and apoptosis
(25). NUF2 functions as a
prognostic marker in breast cancer (26). Furthermore, NUF2 expression can be
used to predict the early recurrence of hepatocellular carcinoma
following surgical resection, serving as a promising prognostic
biomarker (13). Other studies
report that NUF2 knockdown suppresses proliferation and induces
apoptosis of human osteosarcoma Saos-2 cells and hepatocellular
carcinoma (14,27). As in a previous study (25), the present study indicated that
ANP32E overexpression may be one of the causes of abnormal NUF2
expression in GC, which promotes GC progression.
In conclusion, the results of the present study
demonstrated that ANP32E expression levels were significantly
increased in GC and markedly promoted cell proliferation and
inhibited cell apoptosis by upregulating NUF2 expression, thereby
contributing to GC tumorigenesis. These findings have provided a
novel biomarker for potential use in GC clinical practices.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Heilongjiang Province (grant no. LH2020H004) and the
Scientific Research Project of Heilongjiang Health Commission
(grant no. 2019-314).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
MZ and QW designed this study. XZ and YZ performed
the experiments. TW, JN and QT aided with the experiments and
performed the data analysis. MZ, XZ, YZ and QW confirm the
authenticity of all the raw data. XZ, MZ and QW wrote and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Jiamusi University
(approval no. 202078). Written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qin Y, Tong X, Fan J, Liu Z, Zhao R, Zhang
T, Suo C, Chen X and Zhao G: Global burden and trends in incidence,
mortality, and disability of stomach cancer from 1990 to 2017. Clin
Transl Gastroenterol. 12:e004062021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cristescu R, Lee J, Nebozhyn M, Kim KM,
Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al: Molecular
analysis of gastric cancer identifies subtypes associated with
distinct clinical outcomes. Nat Med. 21:449–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mao Z, Pan L, Wang W, Sun J, Shan S, Dong
Q, Liang X, Dai L, Ding X, Chen S, et al: Anp32e, a higher
eukaryotic histone chaperone directs preferential recognition for
H2A.Z. Cell Res. 24:389–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorén V, Garcia-Jaraquemada A, Naves JE,
Carmona X, Mañosa M, Aransay AM, Lavin JL, Sánchez I, Cabré E,
Manyé J and Domènech E: ANP32E, a protein involved in
steroid-refractoriness in ulcerative colitis, identified by a
systems biology approach. J Crohns Colitis. 13:351–361. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiong Z, Ye L, Zhenyu H, Li F, Xiong Y,
Lin C, Wu X, Deng G, Shi W, Song L, et al: ANP32E induces
tumorigenesis of triple-negative breast cancer cells by
upregulating E2F1. Mol Oncol. 12:896–912. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gursoy-Yuzugullu O, Ayrapetov MK and Price
BD: Histone chaperone Anp32e removes H2A.Z from DNA double-strand
breaks and promotes nucleosome reorganization and DNA repair. Proc
Natl Acad Sci USA. 112:7507–7512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alatwi HE and Downs JA: Removal of H2A.Z
by INO80 promotes homologous recombination. EMBO Rep. 16:986–994.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murphy KE, Meng FW, Makowski CE and Murphy
PJ: Genome-wide chromatin accessibility is restricted by ANP32E.
Nat Commun. 11:50632020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mikami Y, Hori T, Kimura H and Fukagawa T:
The functional region of CENP-H interacts with the Nuf2 complex
that localizes to centromere during mitosis. Mol Cell Biol.
25:1958–1970. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Tan PY, Handoko YA, Sekar K, Shi
M, Xie C, Jiang XD, Dong QZ, Goh BKP, Ooi LL, et al: NUF2 is a
valuable prognostic biomarker to predict early recurrence of
hepatocellular carcinoma after surgical resection. Int J Cancer.
145:662–670. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Dai SJ, Li H, Dong L and Peng YP:
Silencing of NUF2 inhibits tumor growth and induces apoptosis in
human hepatocellular carcinomas. Asian Pac J Cancer Prev.
15:8623–8629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song Z, Wu Y, Yang J, Yang D and Fang X:
Progress in the treatment of advanced gastric cancer. Tumour Biol.
39:10104283177146262017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pellino A, Riello E, Nappo F, Brignola S,
Murgioni S, Djaballah SA, Lonardi S, Zagonel V, Rugge M, Loupakis F
and Fassan M: Targeted therapies in metastatic gastric cancer:
Current knowledge and future perspectives. World J Gastroenterol.
25:5773–5788. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang J, Gao W, Liu H, Yin G, Duan H,
Huang Z and Zhang Y: Up-regulated ANP32E promotes the thyroid
carcinoma cell proliferation and migration via activating
AKT/mTOR/HK2-mediated glycolysis. Gene. 750:1446812020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Li J, Li Y and Pang LB: Hsa-let-7c
exerts an anti-tumor function by negatively regulating ANP32E in
lung adenocarcinoma. Tissue Cell. 65:1013722020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Lan Z, Qiu G, Ren H, Zhao Y, Gu
Z, Li Z, Feng L, He J and Wang C: Over-expression of ANP32E is
associated with poor prognosis of pancreatic cancer and promotes
cell proliferation and migration through regulating β-catenin. BMC
Cancer. 20:10652020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen L, Gao Y, Zhu L, Song H, Zhao L, Liu
A, Zhang G and Shi G: Establishment and characterization of a GES-1
human gastric epithelial cell line stably expressing miR-23a. Oncol
Lett. 16:977–983. 2018.PubMed/NCBI
|
|
22
|
Keates S, Sougioultzis S, Keates AC, Zhao
D, Peek RM Jr, Shaw LM and Kelly CP: cag+ Helicobacter pylori
induce transactivation of the epidermal growth factor receptor in
AGS gastric epithelial cells. J Biol Chem. 276:48127–48134. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akagi T and Kimoto T: Human cell line
(HGC-27) derived from the metastatic lymph node of gastric cancer.
Acta Med Okayama. 30:215–219. 1976.PubMed/NCBI
|
|
24
|
Motoyama T, Hojo H and Watanabe H:
Comparison of seven cell lines derived from human gastric
carcinomas. Acta Pathol Jpn. 36:65–83. 1986.PubMed/NCBI
|
|
25
|
Xie X, Jiang S and Li X: Nuf2 is a
prognostic-related biomarker and correlated with immune infiltrates
in hepatocellular carcinoma. Front Oncol. 11:6213732021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhai X, Yang Z, Liu X, Dong Z and Zhou D:
Identification of NUF2 and FAM83D as potential biomarkers in
triple-negative breast cancer. PeerJ. 8:e99752020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu HL and Shao L: Silencing of NUF2
inhibits proliferation of human osteosarcoma Saos-2 cells. Eur Rev
Med Pharmacol Sci. 20:1071–1079. 2016.PubMed/NCBI
|