Introduction
Cervical cancer is attributed to human
papillomavirus (HPV) infection and is one of the most serious
malignancies in the world affecting the female reproductive tract
(1–3). Although effective early screening
and vaccination against HPV have greatly reduced cervical cancer
morbidity and mortality worldwide, the clinical signs and symptoms
of cervical cancer remain insidious until the advanced stage of
this disease, resulting in a poor therapeutic outcome (4,5).
Therefore, the identification of novel therapeutic targets is of
considerable importance for providing valuable strategies to be
used in cervical cancer treatment. It can also improve the survival
rate of these patients.
Protein tyrosine kinase 6 (PTK6) is an intracellular
non-receptor tyrosine kinase, which belongs to the SRC family of
proteins and was originally identified in metastatic breast tumors
(6). PTK6 contributes to the
differentiation of normal epithelial cells and exhibits elevated
expression in breast, ovarian and lung cancer (7–9).
It is noteworthy that PTK6 participates in the regulation of
different signaling pathways in a variety of cancer types.
Epithelial-mesenchymal transition plays a vital role in regulating
the tumorigenic activity of PTK6 by directly affecting tumor
metastasis and colonization (10). Ono et al (11) indicated that PTK6 was involved in
the progression of pancreatic cancer via the ERK signaling pathway.
An additional study demonstrated that knockdown of PTK6 expression
could inhibit proliferation, invasion, migration, and malignant
progression of hepatocellular carcinoma cells (12). In addition, Wang et al
(5) demonstrated that PTK6
expression was upregulated in cervical cancer tissues; this
phenomenon was associated with a poor disease prognosis, suggesting
that overexpression of PTK6 was closely associated with cervical
cancer prognosis (5). However,
little is known regarding the current mechanism of PTK6 in cervical
cancer. Therefore, the present study aimed to explore whether PTK6
was involved in the malignant progression of cervical cancer and
discuss its potential mechanism.
GRB2-associated binding (GAB) 1 is a multi-site
docking protein with multiple tyrosine residues (13). Following phosphorylation of the
tyrosine residues, GAB1 provides binding sites for multiple
effector proteins to induce a variety of tyrosine kinase signaling
events (14,15). A large number of studies have
shown that increased expression of GAB2 is strongly associated with
tumor proliferation and metastasis in breast, prostate and ovarian
cancers (16–18). Recent research has suggested that
GAB1, a downstream gene of c-Met, is negatively modulated by
microRNA (miR)-23b-3p in cervical cancer, indicating that GAB1 also
exhibits an essential role in cervical cancer (19). Subsequently, the Biogrid database
(https://thebiogrid.org/) predicted that PTK6
could bind to GAB1. Therefore, it is reasonable to hypothesize that
PTK6 may interact with GAB1 to participate in the malignant
progression of cervical cancer. The present study elaborated the
impact of PTK6 and GAB1 on the aggressive phenotype of cervical
cancer cells and explored its underlying mechanisms to identify
novel therapeutic strategies for cervical cancer.
Materials and methods
Cell culture
Immortalized human normal cervical epithelial cells
(Ect1/E6E7, CVCL_3679) and cervical cancer cell lines (SiHa,
CVCL_0032; HeLa, CVCL_0030; Ca-Ski, CVCL_1100; C-33A, CVCL_1094)
were purchased from American Type Culture Collection and cultured
in Dulbecco's modified Eagle's medium (DMEM) (Merck KGaA)
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin solution (both from Beijing Solarbio
Science & Technology Co., Ltd.) in a 5% CO2
incubator at 37°C.
Bioinformatics
BiogridDataBase (version 4.4; http://thebiogrid.org/) is a database that can be used
to predict the interactions between proteins. The Protein-Protein
Affinity Predictor (PPA-Pred2) database (https://www.iitm.ac.in/bioinfo/PPA_Pred/prediction.html#)
was used to predict the binding of PTK6 and GAB1.
Cell transfection
C-33A cells were harvested in the logarithmic growth
phase, transferred into 6-well plates, and subsequently transfected
when they had reached 80% confluence. Small interfering RNA (siRNA)
targeting PTK6-1 (si-PTK6-1; 5′-GCCATTAAGGTGATTTCTCGAGG-3′) and
PTK6-2 (si-PTK6-2; 5′-TTCAATTGCACCTATGTTTTTAC-3′), the
corresponding empty vector [siRNA-negative control (NC);
5′-AAGACAUUGUGUGUCCGCCTT-3′], the overexpression plasmid targeting
GAB1 (Ov-GAB1), and its empty vector (Ov-NC) were synthesized by
Geneseed Biotech Co., Ltd. According to the manufacturer's
instructions, Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc., Shanghai, China), was used to transfect
the aforementioned vectors into C-33A cells (1×105
cells/well) at a concentration of 50 ng/ml at 37°C for 24 h.
Following incubation, the medium was replaced with fresh DMEM and
the cells were routinely cultured for 48 h. Subsequently, the cells
were used to assess the transfection efficiency.
Cell counting kit (CCK)-8 assay
Following incubation of the transfected C-33A cells
(5×103 cells/ml) in 96-well plates for 24, 48, and 72 h,
10 µl CCK-8 solution (Beyotime Institute of Biotechnology) was
added and incubated with the cells for an additional 2 h. The OD
values were measured using a microplate reader (BioTek™
ELx800; BioTek Instruments, Inc.) at 450 nm and the growth curve
was plotted.
Wound healing assay
The C-33A cells were seeded at a density of
1×105 cells/well into 12-well plates. When the cell
confluence had reached 80%, serum-free DMEM was added to the cells,
which were cultured at 37°C, overnight. Subsequently, a 200-µl
pipette tip was utilized to make scratches in the cell monolayer.
Following 24 h of incubation, an inverted microscope (Olympus
Corporation) was used to monitor the wounds. The migratory rate was
calculated as the ratio of 0 h scratch width/24 h scratch
width.
Transwell assay
Matrigel (BD Biosciences) was placed in 24-well
Transwell plates with 8-µm pores at 37°C for 30 min. The C-33A
cells (5×104 cells/ml) in 200 µl serum-starved medium
were plated into the upper chamber, and the lower chamber was
filled with 600 µl DMEM supplemented with 10% FBS. Following 24 h
of cell culture, sterile cotton swabs were used to remove
non-invading cells, while the invading cells were immobilized using
4% formaldehyde for 15 min at room temperature. The invading cells
were observed in 5 random fields using an inverted microscope
following staining with 0.1% crystal violet solution at room
temperature for 30 min.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
The induction of apoptosis was detected using a
TUNEL kit (EMD Millipore). The C-33A cells were fixed using 4%
paraformaldehyde at room temperature for 15 min following washing
with PBS. A total of 0.3% Triton-X 100/PBS was used to permeabilize
the cells for 10 min, followed by rinsing with PBS. Subsequently,
3% H2O2 was added to inhibit endogenous
peroxidation, after which was the incubation of C-33A cells with
TUNEL regent for 60 min at 37°C. 3,3-Diaminobenzidine was added to
stain the cells according to the manufacturer's instructions.
Finally, nuclei were stained with DAPI at room temperature for 10
min. 10 visual fields were randomly selected and an Olympus BX51
fluorescence microscope (Olympus Corporation) was used to determine
the number of apoptotic cells.
Reverse transcription-quantitative PCR
(RT-qPCR)
C-33A cells that were in the logarithmic phase were
collected and total RNA was extracted from these cells using
TRIzol® reagent (Sigma-Aldrich; Merck KGaA) according to
the manufacturer's instructions. cDNA was synthesized using the
QuantiTect Reverse Transcription kit (Qiagen GmbH) according to the
manufacturer's instructions. An ABI 7500 Real-Time PCR instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to
conduct PCR amplification according to the operating procedures of
the SYBR Green PCR kit (Takara Bio, Inc.). The following
thermocycling conditions were used: Initial denaturation at 95°C
for 8 min; followed by 35 cycles of denaturation at 95°C for 30 sec
and annealing at 60°C for 1 min; and extension for 10 min at 72°C.
The nucleotide sequences used are listed as follows: PTK6 forward,
5′-TGCCCCATTGGGATGACTG-3′ and reverse, 5′-GTACAGCGCCAGGATGTGTTT-3′;
GAB1 forward, 5′-GATGGTTCGTGTTACGCAGTG-3′ and reverse,
5′-CGCTGTCTGCTACCAAGTAGAA-3′; GAPDH forward,
5′-TGTGGGCATCAATGGATTTGG-3′ and reverse,
5′-ACACCATGTATTCCGGGTCAAT-3′. The 2−ΔΔCq method was used
to measure the gene expression levels, which were normalized
compared with those of GAPDH (20).
Western blot analysis
RIPA lysis buffer (Beyotime Institute of
Biotechnology) was used to extract total protein from collected
C-33A cells. The protein concentration was determined using a BCA
assay and 20 µg of each protein sample was separated using 10%
SDS-PAGE gel electrophoresis. The proteins were then transferred to
a polyvinylidene fluoride membrane at room temperature (30-min
transfer), after which 5% skim milk was used for blocking of the
non-specific binding sites at room temperature for 1 h. The primary
antibodies targeting PTK6 (product code ab233392; 1:1,000
dilution), Bcl-2 (product code ab32124; 1:1,000 dilution),
baculoviral IAP repeat containing 5 (Birc5; product code ab76424;
1:5,000 dilution), Bax (product code ab32503; 1:1,000 dilution),
matrix metalloproteinase (MMP)12 (product code ab52897; 1:1,000
dilution), MMP9 (product code ab76003; 1:1,000 dilution), GAB1
(product code ab133486; 1:1,000 dilution), and GAPDH (product code
ab181602; 1:10,000 dilution) were purchased from Abcam and were
incubated with the corresponding membranes at 4°C overnight.
HRP-conjugated secondary antibodies (product no. 7074; 1:5,000;
Cell Signaling Technology, Inc.) were used to probe the membranes
at room temperature for 2 h the following day. Following the
addition of the ECL solution (Vazyme Biotech Co., Ltd.; Nanjing,
China), a gel imager (C150 model; Azure Biosystems, Inc.) was used
to achieve visualization of the protein bands. The gray values of
the protein bands were analyzed using ImageJ (v1.51; National
Institutes of Health) and the relative expression levels of each
protein were calculated.
Co-immunoprecipitation (Co-IP)
assay
For immunoprecipitation, C-33A cells were harvested
and lysed in 300 µl Pierce™ IP lysis buffer (Thermo
Fisher Scientific, Inc.). A total of 400 µg protein was incubated
with the antibody solution containing 2 µg PTK6 (product code
ab233392; 1:30 dilution), or GAB1 (product code ab133486; 1:100
dilution), and goat anti-rabbit IgG (HRP) (product code ab205718;
1:20 dilution; all from Abcam) overnight at 4°C. Subsequently, the
cell lysates were incubated for 3 h following the addition of 30 µl
Protein G/A agarose beads (Invitrogen; Thermo Fisher Scientific,
Inc.). Following 600 × g centrifugation at 4°C for 10 min, the
pellets were washed three times with 1 ml lysis buffer before
resuspension, in resuspended in 2X SDS-PAGE loading buffer.
Finally, immunoblotting was used to detect immunoprecipitation
products.
Statistical analysis
GraphPad Prism 7.0 software (GraphPad Software,
Inc.) was used to analyze the data. The experimental data are
presented as the mean ± SD. The comparison between the two groups
was performed by the unpaired Student's t-test, whereas the
comparison among multiple groups was performed by one-way ANOVA
followed by Tukey's post hoc test. Each experiment was repeated at
least three times unless otherwise specified. P<0.05 was used to
indicate a statistically significant difference.
Results
PTK6 expression is increased and PTK6
deficiency exacerbates cell apoptosis in cervical cancer
The expression levels of PTK6 were examined in human
normal cervical epithelial cells (Ect1/E6E7) and in cervical cancer
cell lines (SiHa, HeLa, Ca-Ski and C-33A) by RT-qPCR (Fig. 1A) and western blotting (Fig. 1B) analyses. PTK6 expression was
significantly increased in cervical cancer cell lines compared with
that noted in Ect1/E6E7 cells. C-33A cells were selected for
subsequent experiments since they displayed the highest expression
of PTK6 among SiHa, HeLa, Ca-Ski, and C-33A cells. Subsequently,
PTK6 expression was knocked down and the transfection efficacy was
assessed via RT-qPCR (Fig. 1C)
and western blotting (Fig. 1D)
analyses. siRNA-PTK6-1 was selected for subsequent experiments due
to the most efficient knockdown of PTK6 expression noted in the
siRNA-PTK6-1 group compared with that of the siRNA-PTK6-2 group.
Following knockdown of PTK6 expression, reduced cell viability was
observed compared with that of the siRNA-NC group (Fig. 1E). PTK6 depletion distinctly
aggravated the induction of apoptosis in C-33A cells (Fig. 1F). In addition, western blot
analysis demonstrated that the expression levels of the
apoptosis-related proteins Bcl-2 and Birc5 were downregulated,
while those of Bax were upregulated in C-33A cells following
knockdown of PTK6 expression (Fig.
1G). These findings confirmed that the proliferation of
PTK6-silenced C-33A cells was inhibited, while the induction of
their apoptosis was promoted.
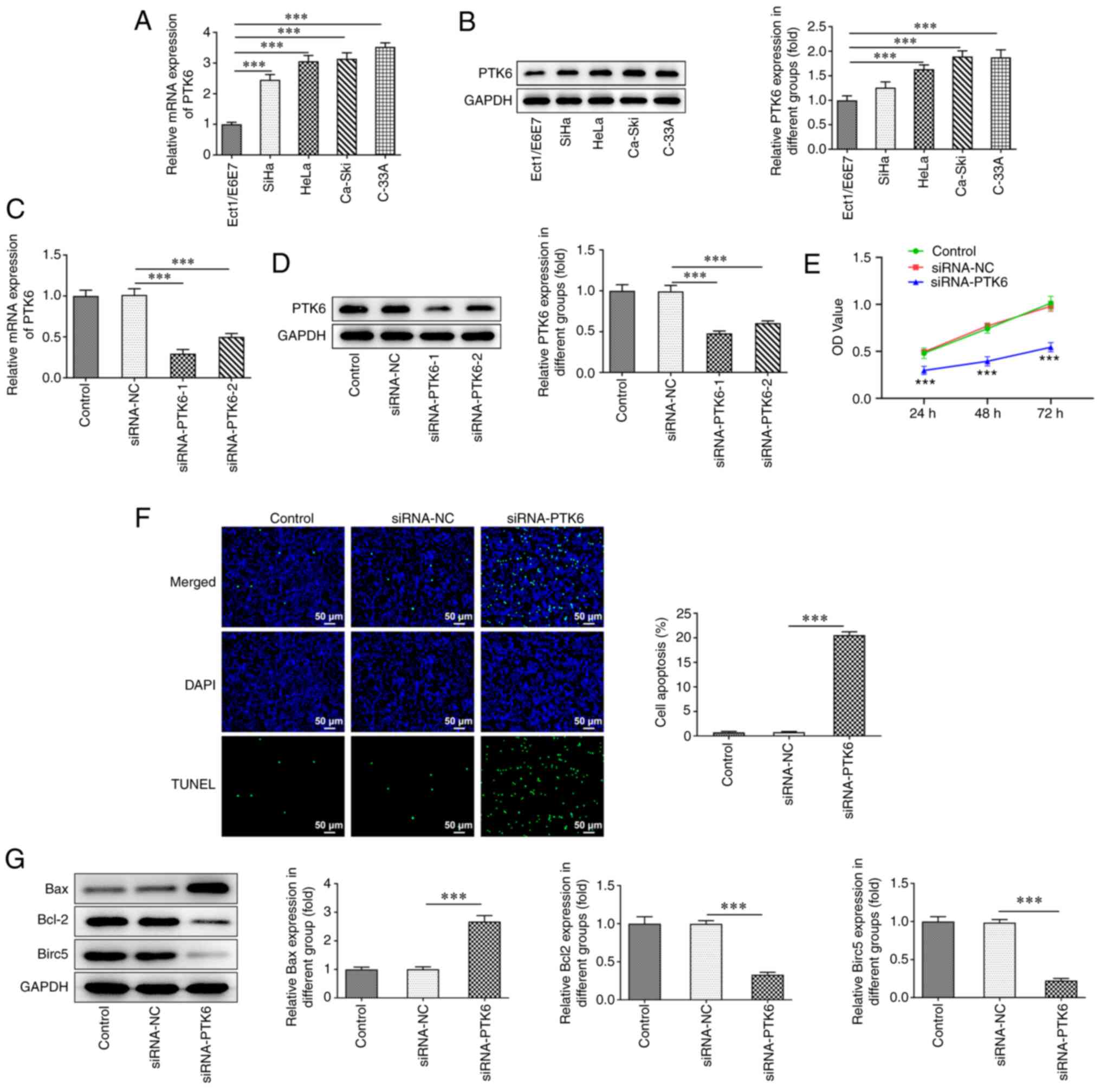 | Figure 1.PTK6 expression is increased and PTK6
deficiency exacerbates cell apoptosis in cervical cancer. (A)
RT-qPCR and (B) western blotting respectively assessed PTK6
expression in immortalized human normal cervical epithelial cells
(Ect1/E6E7) and cervical cancer cell lines (SiHa, HeLa, Ca-Ski,
C-33A). The interference effect of PTK6 was detected by (C) RT-qPCR
and (D) western blotting, and the siRNA-PTK6-1 with more marked
interference (as siRNA-PTK6) was selected for subsequent
experiments. (E) C-33A cell proliferation was detected by CCK-8
assays. (F) TUNEL staining assays detected the apoptosis of C-33A
cells. (G) Western blotting detected the protein levels of Bcl-2,
Bax and Birc5 in C-33A cells. ***P<0.001 vs. Ect1/E6E7 or
siRNA-NC. PTK6, protein tyrosine kinase 6; RT-qPCR, reverse
transcription-quantitative PCR; siRNA, small interfering RNA;
CCK-8, Cell Counting Kit-8; TUNEL, terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling;
Birc5, baculoviral IAP repeat containing 5; NC, negative
control. |
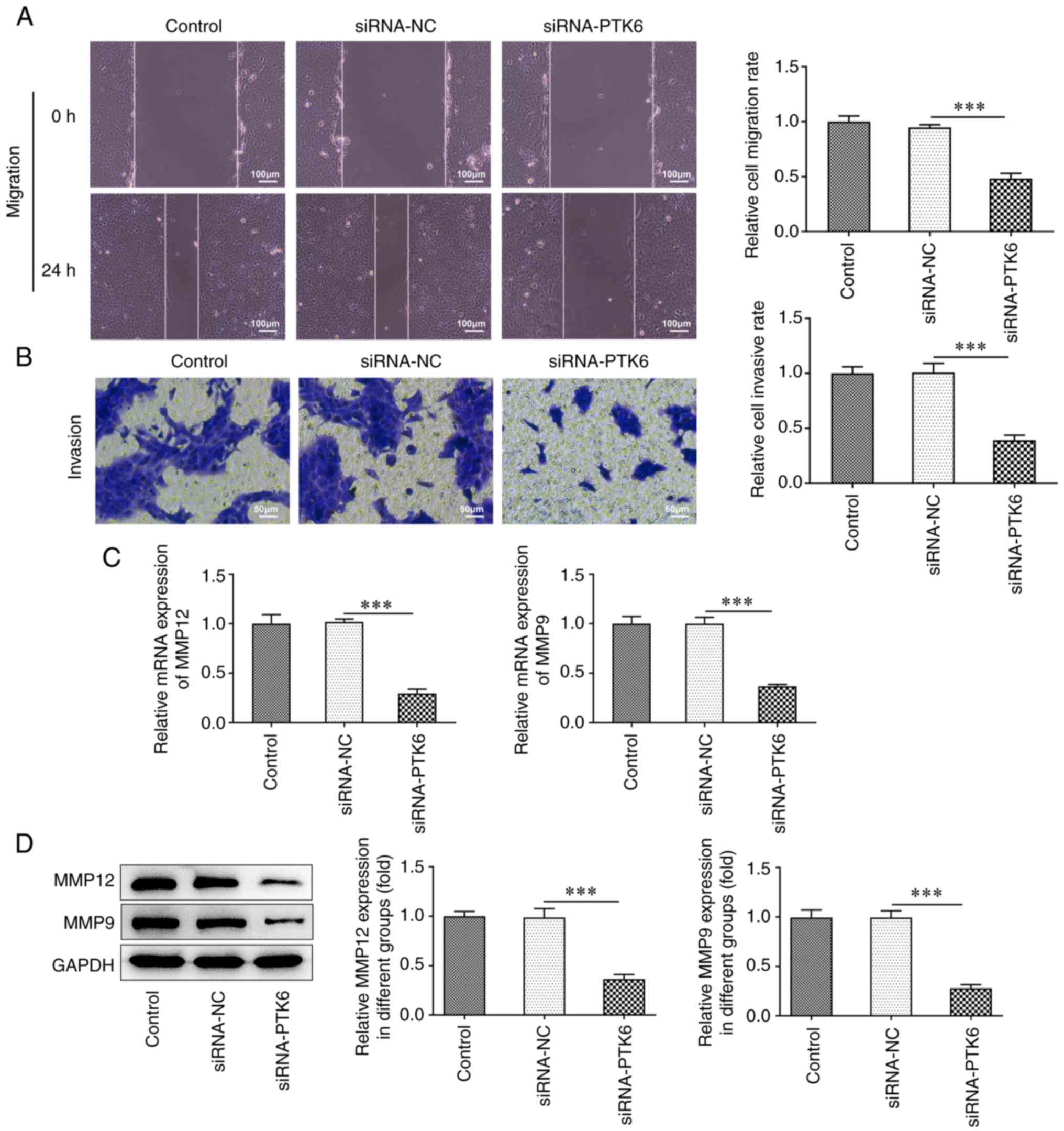
Lack of PTK6 expression mitigates cell
invasion and migration in cervical cancer
To confirm the effects of siRNA-PTK6-1 on the
invasive and migratory activities of cervical cancer cells, wound
healing and Transwell assays were performed. The cell migratory
(Fig. 2A) and invasive (Fig. 2B) capacities of the siRNA-PTK6
cell group prominently decreased compared with those of the
siRNA-NC group. Furthermore, the expression levels of the
migration-related proteins MMP12 and MMP9 were markedly reduced in
the siRNA-PTK6 group compared with those noted in the siRNA-NC
group (Fig. 2C and D). The
results suggested that PTK6 reduction suppressed the invasive and
migratory activities of C-33A cells.
Knockdown of PTK6 expression reduces
GAB1 expression
To examine further the specific mechanism of action
of PTK6, its potential binding to GAB1 was predicted via the
Biogrid database. The predicted binding threshold of PTK6 and GAB1
was-16.55 kcal/mol (<-5) as determined by the PPA-Pred2 database
(data not shown). RT-qPCR and western blot analyses demonstrated
upregulated GAB1 expression in the cervical cancer cell lines SiHa,
HeLa, Ca-Ski, and C-33A, which was consistent with the expression
levels of PTK6 expression in cervical cancer cells (Fig. 3A and B). Co-IP assays indicated
that in the IgG group, neither PTK6 nor GAB1 proteins were
precipitated, indicating that these two proteins could not bind to
IgG. However, in both the PTK6 and GAB1 groups, the GAB1 or PTK6
proteins were precipitated (Fig. 3C
and D). These findings indicated a strong binding affinity
between the PTK6 and GAB1 proteins. Furthermore, the protein and
mRNA expression levels of GAB1 in the siRNA-PTK6 group were
downregulated compared with those in the siRNA-NC group, indicating
a positive association between PTK6 and GAB1 (Fig. 3E and F).
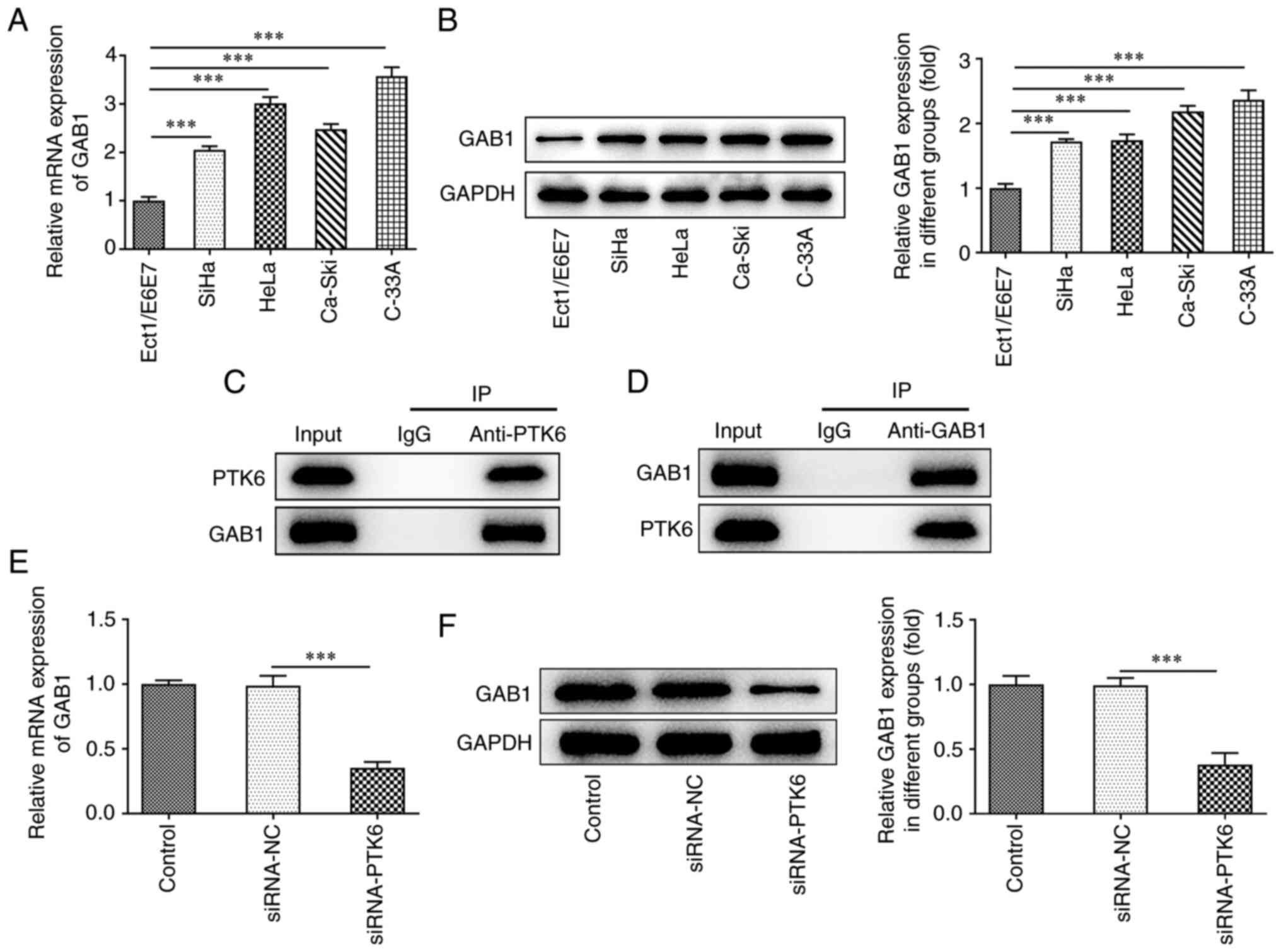 | Figure 3.Silencing of PTK6 decreases GAB1
expression. (A) RT-qPCR and (B) western blotting, respectively,
determined GAB1 expression in immortalized human normal cervical
epithelial cells (Ect1/E6E7) and cervical cancer cell lines (SiHa,
HeLa, Ca-Ski, C-33A). (C and D) Co-IP assay was performed using
control IgG beads and immunoblotted for PTK6 and GAB1. The
expression of GAB1 (E) mRNA and (F) protein levels following PTK6
interference in cervical cancer C-33A cells. ***P<0.001 vs.
Ect1/E6E7 or siRNA-NC. PTK6, protein tyrosine kinase 6; GAB1,
GRB2-associated binding 1; RT-qPCR, reverse
transcription-quantitative PCR; Co-IP, co-immunoprecipitation;
siRNA, small interfering RNA; NC, negative control. |
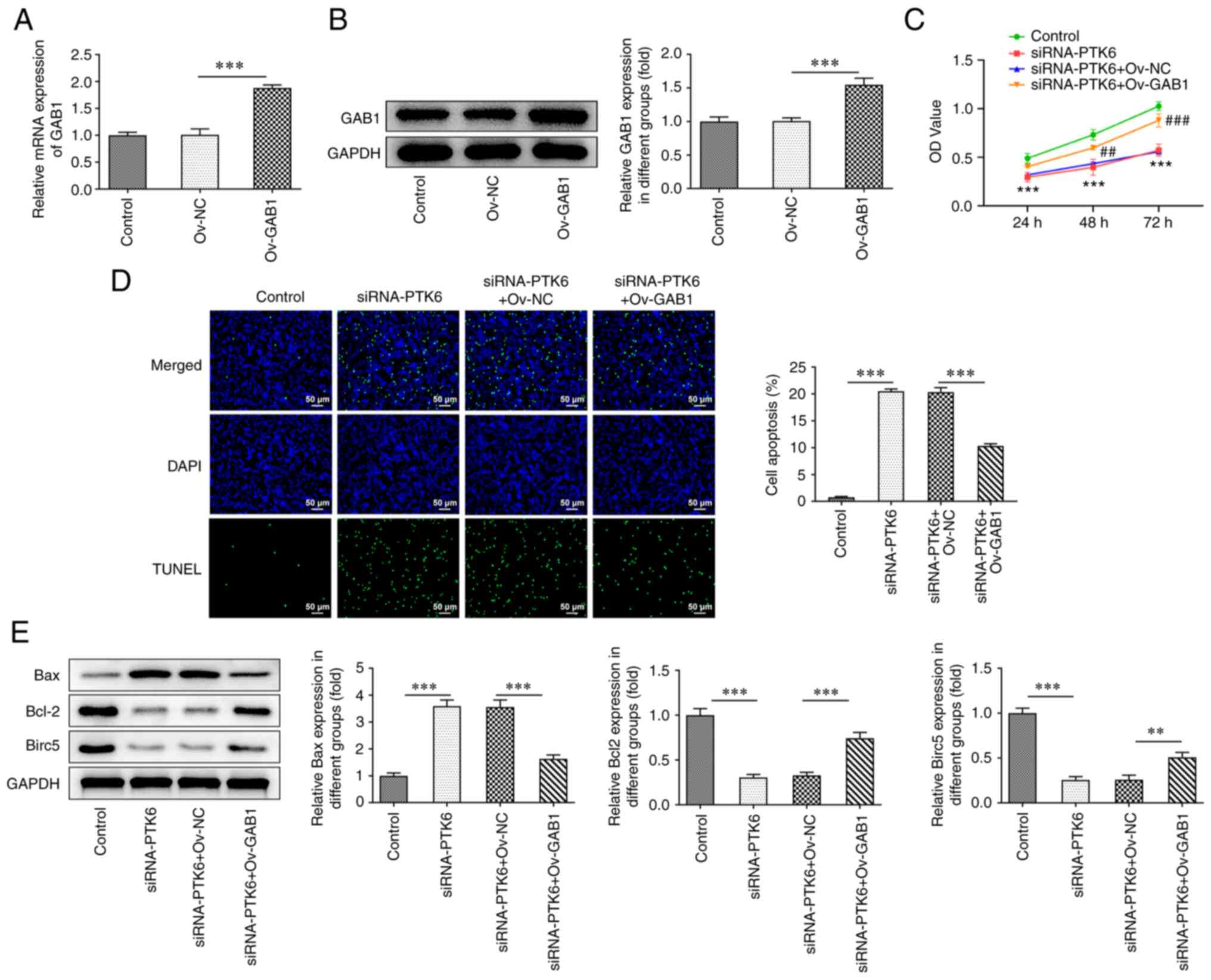
Suppressive effects of knockdown of
PTK6 expression on cervical cancer cell proliferation are reversed
by elevation of GAB1 expression
To clarify whether PTK6 binds to GAB1 and to assess
its involvement in the malignant progression of cervical cancer
cells, an Ov-GAB1 plasmid was constructed and transfected into
C-33A cells. GAB1 expression was successfully increased in the
Ov-GAB1 group compared with that noted in the Ov-NC groups
(Fig. 4A and B). In addition,
following overexpression of GAB1, it was observed that C-33A cell
viability (Fig. 4C) was increased
in the siRNA-PTK6 group, while the induction of apoptosis was
reduced (Fig. 4D). Moreover,
western blot analysis indicated an increase in the expression
levels of Bcl-2 and Birc5 and a decrease in the expression levels
of Bax in C-33A cells following GAB1 overexpression (compared with
siRNA-PTK6 + Ov-NC; Fig. 4E).
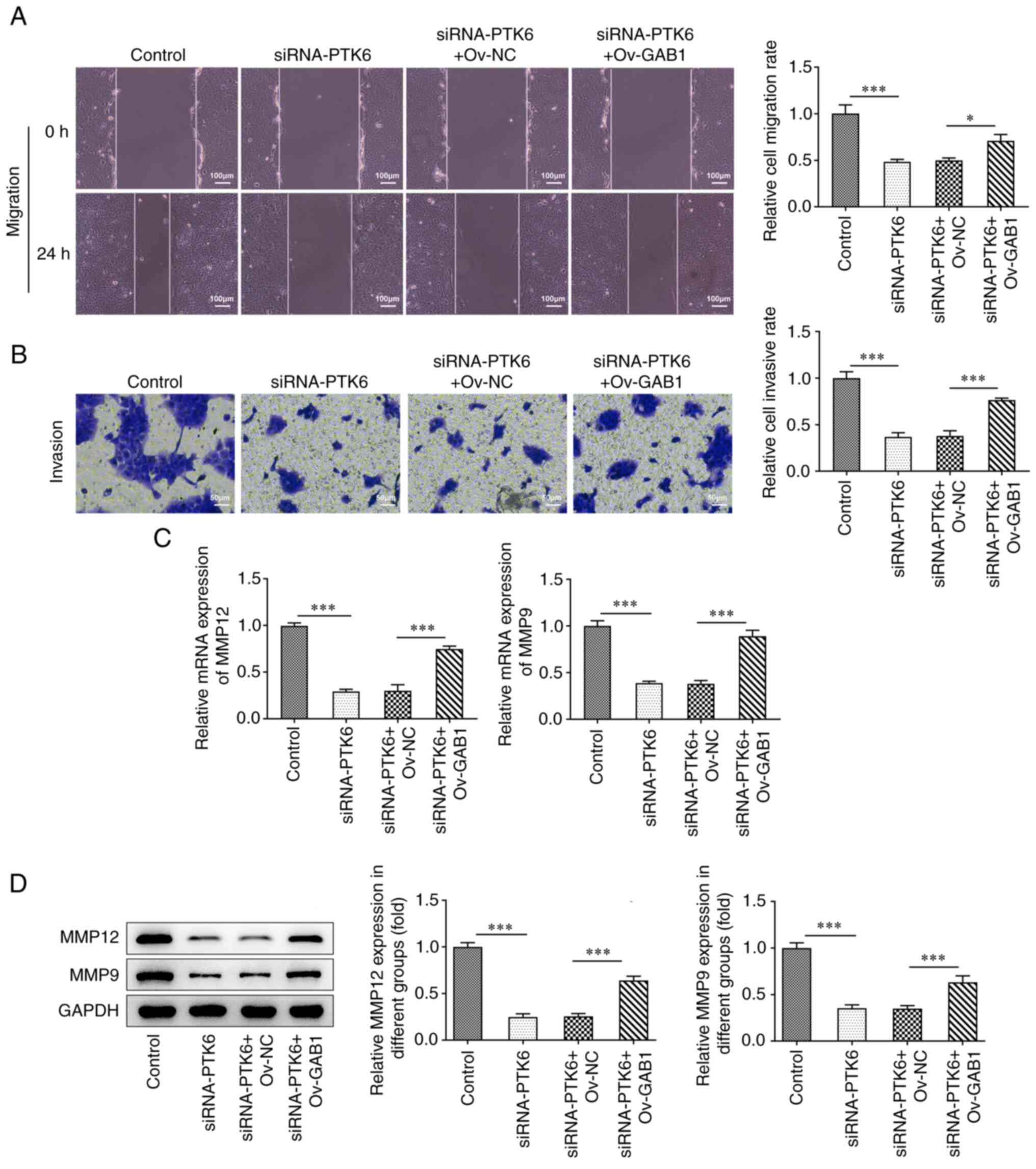
Suppressive effects of the knockdown
of PTK6 expression on cervical cancer cell migration and invasion
are reversed following overexpression of GAB1 expression
Overexpression of GAB1 caused a partial reversal of
the suppressive effects of PTK6 knockdown on the migration and
invasion of C-33A cells (Fig. 5A and
B). Moreover, the expression levels of MMP12 and MMP9 were
increased in C-33A cells co-transfected with Ov-GAB1 and siRNA-PTK6
compared with those noted in the siRNA-PTK6 + Ov-NC group (Fig. 5C and D). In general, the
overexpression of GAB1 could counteract the reduced migratory and
invasive activities observed following knockdown of PTK6 expression
in C-33A cells.
Discussion
PTK6, a protein kinase, may act as an oncogene based
on its high expression levels noted in a variety of cancer types,
and has thus attracted considerable attention (21–23). Notably, strategies for
pharmaceutical kinase inhibition have been clinically demonstrated
and pursued in programs targeting some tumor types (24). Thus, in-depth studies of PTK6
signaling function would be critical for the future production or
improvement of its inhibitors with clinical relevance. Increased
expression of PTK6 has been reported to promote the development of
prostate cancer through the activation of carcinogenic signaling
pathways, which are involved in regulating cell growth, migration,
and apoptosis (25). Dwyer et
al (26) demonstrated that
PTK6 was overexpressed in 86% of patients with breast cancer and
correlated with tumor grade, while it was weakly expressed or
undetectable in normal breast tissues (27). Wang et al (5) indicated that PTK6 was also
overexpressed in cervical squamous cell carcinoma and was related
to the short-term survival of these patients, implying that it may
be associated with the prognosis of patients with cervical cancer.
However, the specific mechanism by which PTK6 contributes to the
development of cervical cancer has not yet been addressed. The
present study revealed that PTK6 expression was increased in
cervical cancer cells compared with that observed in human normal
cervical epithelial cells (Ect1/E6E7). It should be noted that the
study was selected in C-33A cells owing to the relatively high
expression of PTK6 in C-33A cells. This was one of the limitations
of the present study, and future studies will add cell lines for
further validation. Moreover, in line with the results of Wang
et al (5), the
insufficient expression of PTK6 hindered C-33A cell proliferation,
migration, and invasion. Therefore, it was tentatively hypothesized
that targeting PTK6 may serve as a potential therapeutic strategy
in the management of cervical cancer.
GAB1 is a multi-site docking protein containing
multiple tyrosine residues (28);
it can achieve binding to the non-receptor tyrosine kinase PTK6 and
participate in PTK6 signal transduction events (14,15). Previous research on cervical
cancer revealed that GAB1 expression was inhibited by the
overexpression of the tumor suppressor gene miR-23b-3p, suggesting
that this protein plays a similar role to PTK6 and that it is
associated with poor prognosis of cervical cancer (19). Therefore, it was hypothesized that
PTK6 may participate in the development of cervical cancer by
binding to GAB1. In the present study, the Biogrid database
(https://thebiogrid.org/) predicted that PTK6
could bind to GAB1. Subsequently, in vitro cell experiments,
demonstrated that depletion of PTK6 expression markedly ameliorated
the proliferation, invasion, and migration of C-33A cells, while
overexpression of GAB1 counteracted the PTK6-mediated inhibition of
the aggressive phenotypes of C-33A cells, implying an indivisible
association between them. Therefore, it was surmised that
chemotherapeutic drugs would be an effective strategy to improve
the treatment of cervical cancer by inhibiting the activation of
PTK6 or GAB1 pathways. The present study proposed for the first
time, to the best our knowledge, that the knockdown of PTK6
expression could promote the malignant phenotype of cervical cancer
due to the activation of GAB1, providing a possible target for the
clinical diagnosis and treatment of cervical cancer.
A previous study concerning hepatocellular carcinoma
proposed that PSPC1 is responsible for the subcellular localization
of PTK6/β-catenin tumor switch (29), and another study indicated that
the ectopic expression of PTK6 facilitated the migration and
metastasis of cancer cells via AKT activation (30). A main disadvantage of the present
study is the lack of in vivo experiments that could verify
the findings presented by the in vitro studies. Therefore, a
mouse tumor-bearing model needs to be constructed to further
investigate the effects of PTK6 on cervical cancer. This will be
preceded by further clarification of the association between PTK6
expression and human papillomavirus.
In summary, PTK6 activated GAB1 to increase the
proliferation, invasion, and migration of cervical cancer cells,
which in turn promoted the malignant progression of cervical
cancer. This suggests that PTK6 and GAB1 may be considered as
prognostic or therapeutic markers for patients with cervical
cancer. The identification of effective small molecule inhibitors
for PTK6 and GAB1 is expected to improve the therapeutic efficacy
of this treatment strategy against cervical cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shenzhen Nanshan District
Technology Research and Development and Creative Design Project
(grant no. 2020030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL, NY, XT, LO, MJ and SZ conceived and designed the
study, as well as acquired and interpreted the data. JL was a major
contributor in writing the manuscript. JL, NY and SZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen R, Sheng C, Ma R, Zhang L, Yang L and
Chen Y: PLAC1 is an independent predictor of poor survival, and
promotes cell proliferation and invasion in cervical cancer. Mol
Med Rep. 24:8002021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Auguste P: Cervical cancer screening:
Updated guidelines from the American cancer society. Am Fam
Physician. 104:314–315. 2021.PubMed/NCBI
|
|
3
|
Olusola P, Banerjee HN, Philley JV and
Dasgupta S: Human papilloma virus-associated cervical cancer and
health disparities. Cells. 8:6222019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Z and Ma D: The precision prevention
and therapy of HPV-related cervical cancer: New concepts and
clinical implications. Cancer Med. 7:5217–5236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang XJ, Xiong Y, Ma ZB, Xia JC and Li YF:
The expression and prognostic value of protein tyrosine kinase 6 in
early-stage cervical squamous cell cancer. Chin J Cancer.
35:542016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shin WS, Shim HJ, Lee YH, Pyo M, Park JS,
Ahn SY and Lee ST: PTK6 localized at the plasma membrane promotes
cell proliferation and migration through phosphorylation of Eps8. J
Cell Biochem. 118:2887–2895. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao C, Chen Y, Zhang W, Zhang J, Xu Y, Li
W, Chen S and Deng A: Expression of protein tyrosine kinase 6
(PTK6) in nonsmall cell lung cancer and their clinical and
prognostic significance. Onco Targets Ther. 6:183–188.
2013.PubMed/NCBI
|
|
8
|
Ito K, Park SH, Katsyv I, Zhang W, De
Angelis C, Schiff R and Irie HY: PTK6 regulates growth and survival
of endocrine therapy-resistant ER+ breast cancer cells. NPJ Breast
Cancer. 3:452017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan G, Lin G, Lucito R and Tonks NK:
Protein-tyrosine phosphatase 1B antagonized signaling by
insulin-like growth factor-1 receptor and kinase BRK/PTK6 in
ovarian cancer cells. J Biol Chem. 288:24923–24934. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ito K, Park SH, Nayak A, Byerly JH and
Irie HY: PTK6 inhibition suppresses metastases of triple-negative
breast cancer via SNAIL-dependent E-cadherin regulation. Cancer
Res. 76:4406–4417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ono H, Basson MD and Ito H: PTK6 promotes
cancer migration and invasion in pancreatic cancer cells dependent
on ERK signaling. PLoS One. 9:e960602014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Song B, Lin Y, Cao L, Feng S,
Zhang L and Wang F: PTK6 promotes hepatocellular carcinoma cell
proliferation and invasion. Am J Transl Res. 8:4354–4361.
2016.PubMed/NCBI
|
|
13
|
Bongartz H, Gille K, Hessenkemper W,
Mandel K, Lewitzky M, Feller SM and Schaper F: The multi-site
docking protein Grb2-associated binder 1 (Gab1) enhances
interleukin-6-induced MAPK-pathway activation in an SHP2-, Grb2-,
and time-dependent manner. Cell Commun Signal. 17:1352019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simister PC and Feller SM: Order and
disorder in large multi-site docking proteins of the Gab
family-implications for signalling complex formation and inhibitor
design strategies. Mol Biosyst. 8:33–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaughan TY, Verma S and Bunting KD:
Grb2-associated binding (Gab) proteins in hematopoietic and immune
cell biology. Am J Blood Res. 1:130–134. 2011.PubMed/NCBI
|
|
16
|
Shi XY, Wang H, Wang W and Gu YH:
MiR-98-5p regulates proliferation and metastasis of MCF-7 breast
cancer cells by targeting Gab2. Eur Rev Med Pharmacol Sci.
24:109142020.PubMed/NCBI
|
|
17
|
Tian J, Zhang H, Mu L, Wang M, Li X, Zhang
X, Xie E, Ma M, Wu D and Du Y: The miR-218/GAB2 axis regulates
proliferation, invasion and EMT via the PI3K/AKT/GSK-3β pathway in
prostate cancer. Exp Cell Res. 394:1121282020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang Z, Li T, Chen W, Wu D, Qin Y, Liu M,
Wu G, He L, Li H and Gu H: Gab2 promotes cancer stem cell like
properties and metastatic growth of ovarian cancer via
downregulation of miR-200c. Exp Cell Res. 382:1114622019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Campos-Viguri GE, Peralta-Zaragoza O,
Jimenez-Wences H, Longinos-González AE, Castañón-Sánchez CA,
Ramírez-Carrillo M, Camarillo CL, Castañeda-Saucedo E,
Jiménez-López MA, Martínez-Carrillo DN and Fernández-Tilapa G:
MiR-23b-3p reduces the proliferation, migration and invasion of
cervical cancer cell lines via the reduction of c-Met expression.
Sci Rep. 10:32562020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sahu R and Pattanayak SP: Strategic
developments & future perspective on gene therapy for breast
cancer: Role of mTOR and Brk/PTK6 as molecular targets. Curr Gene
Ther. 20:237–258. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li T, Wan Y, Su Z, Li J, Han M and Zhou C:
SRF potentiates colon cancer metastasis and progression in a
microRNA-214/PTK6-dependent manner. Cancer Manag Res. 12:6477–6491.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu XL, Ye YL, Wu ZM, He QM, Tan L, Xiao
KH, Wu RY, Yu Y, Mai J, Li ZL, et al: Overexpression of PTK6
predicts poor prognosis in bladder cancer patients. J Cancer.
8:3464–3473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harvey AJ and Crompton MR: The Brk protein
tyrosine kinase as a therapeutic target in cancer: Opportunities
and challenges. Anticancer Drugs. 15:107–111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng Y and Tyner AL: Context-specific
protein tyrosine kinase 6 (PTK6) signalling in prostate cancer. Eur
J Clin Invest. 43:397–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dwyer AR, Kerkvliet CP, Krutilina RI,
Playa HC, Parke DN, Thomas WA, Smeester BA, Moriarity BS, Seagroves
TN and Lange CA: Breast tumor kinase (Brk/PTK6) mediates advanced
cancer phenotypes via SH2-domain dependent activation of RhoA and
Aryl hydrocarbon receptor (AhR) signaling. Mol Cancer Res.
19:329–345. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ostrander JH, Daniel AR, Lofgren K, Kleer
CG and Lange CA: Breast tumor kinase (protein tyrosine kinase 6)
regulates heregulin-induced activation of ERK5 and p38 MAP kinases
in breast cancer cells. Cancer Res. 67:4199–4209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fan Y, Yang F, Cao X, Chen C, Zhang X,
Zhang X, Lin W, Wang X and Liang C: Gab1 regulates SDF-1-induced
progression via inhibition of apoptosis pathway induced by
PI3K/AKT/Bcl-2/BAX pathway in human chondrosarcoma. Tumour Biol.
37:1141–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lang YD, Chen HY, Ho CM, Shih JH, Hsu EC,
Shen R, Lee YC, Chen JW, Wu CY, Yeh HW, et al: PSPC1-interchanged
interactions with PTK6 and β-catenin synergize oncogenic
subcellular translocations and tumor progression. Nat Commun.
10:57162019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng Y, Wang Z, Bie W, Brauer PM, Perez
White BE, Li J, Nogueira V, Raychaudhuri P, Hay N, Tonetti DA, et
al: PTK6 activation at the membrane regulates
epithelial-mesenchymal transition in prostate cancer. Cancer Res.
73:5426–5437. 2013. View Article : Google Scholar : PubMed/NCBI
|