Introduction
Malignant lymphomas are a group of malignant
hematological tumors originating in lymph nodes or other lymphoid
tissue and include Hodgkin's lymphoma and non-Hodgkin lymphoma
(NHL) (1). Diffuse large B-cell
lymphoma (DLBCL) is the most common subtype of NHL worldwide
(2). Currently, in Europe, the
incidence of DLBCL is ~3.8/100,000 individuals (3). DLBCL is an aggressive and highly
heterogeneous hematological tumor (4), which accounts for 80% of invasive
lymphomas (5) and can lead to
diffuse destruction of normal lymph node structure (4). At present, there is no effective
clinical treatment for patients with DLBCL; thus, novel treatment
methods for DLBCL are needed.
Previous studies on the mechanism of 5′-nucleotidase
domain-containing 2 (NT5DC2) in certain types of malignancy have
shown that knockout of NT5DC2 can inhibit angiogenesis and
recruitment of tumor-associated macrophages to suppress colorectal
cancer progression (6). NT5DC2
inhibition by regulating p53 signaling has also been reported to
suppress non-small cell lung cancer metastasis (7). NT5DC2 upregulation is associated
with poor overall and relapse-free survival of hepatocellular
carcinoma (HCC) and is an independent prognostic factor for overall
survival (8). Furthermore, NT5DC2
may promote liver cancer cell proliferation by stabilizing EGF
receptor (9) and causes
tumorigenicity of glioma stem cells by increasing Fyn expression
(10). However, to the best of
our knowledge, there are no studies on the value of NT5DC2 for the
treatment of DLBCL.
The IGF2BP family consists of three members, namely
IGF2BP1, 2 and 3, which bind directly to and stabilize the mRNA of
their target gene (11,12). IGF2BP2 serves an important role in
myogenesis (13,14), maintenance of glioblastoma stem
cells (15,16), and invasion and migration of
cancer cells (primarily HCC) (17). Previous studies have shown that
IGF2BP2 enhances HCC proliferation in vitro and in
vivo via an N6-methyladenosine-flap structure-specific
endonuclease 1-dependent mechanism (18). Upregulation of IGF2BP2 in
pancreatic cancer via multiple mechanisms has also been reported to
promote tumor cell proliferation through activation of the PI3K/Akt
signaling pathway (19). However,
the functions of RBP IGF2BP2, and the interactions between IGF2BP2
and NT5DC2 in DLBCL remain to be explored.
The present study aimed to investigate whether
downregulation of IGF2BP2 and NT5DC2 suppresses cell proliferation,
and induces cycle arrest and apoptosis in DLBCL cells by regulating
the p53 signaling pathway.
Materials and methods
Cell culture
Human B lymphocytes (GM12878) and DLBC cell lines
(SU-DHL-8, OCI-Ly8 and OCI-Ly7) were purchased from BeNa Culture
Collection; Beijing Beina Chunglian Institute of Biotechnology.
GM12878 cells were cultured in Iscove's modified Dulbecco's medium
(IMDM; Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (P/S). SU-DHL-8 cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing
10% FBS and 1% P/S. OCI-Ly8 and OCI-Ly7 cells were cultured in IMDM
containing 20% FBS and 1% P/S. All cells were cultured in a
humidified atmosphere of 5% CO2 at 37°C.
Cell transfection
The pGPU6/GFP/Neo vector (Shanghai GenePharma Co.,
Ltd.) was used to construct a vector containing short hairpin RNA
(shRNA) targeting NT5DC2. In addition, an IGF2BP2 genomic fragment
was cloned into a pcDNA3.1 vector (Shanghai GenePharma Co., Ltd.)
to overexpress IGF2BP2. OCI-Ly7 cells (4×105 cells/well)
were seeded in a 6-well plate and cultured until they reached
70–80% confluence. Subsequently, OCI-Ly7 cells were transfected
with 5 µg shRNA-NT5DC2#1/2, with non-targeting sequences (shRNA-NC)
as a negative control, or 4 µg overexpression (Oe)-IGF2BP2, with
empty vector (Oe-NC) as a negative control, using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 48 h. Following 48 h transfection,
the transfection efficiency was validated by reverse
transcription-quantitative PCR (RT-qPCR) and western blot analysis;
the cells were collected for subsequent experiments 48 h after
transfection.
RT-qPCR analysis
Total RNA was extracted from transfected cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and cDNA was generated using PrimeScript™ RT
Reagent kit (Takara Bio, Inc.) according to the manufacturer's
protocol. RT-qPCR was performed with SYBR Premix Ex Taq™
II kit (Takara Bio, Inc.) using a 7500 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
amplification conditions were as follows: 95°C for 10 sec, followed
by 40 cycles at 95°C for 5 sec and 60°C for 30 sec, and a final
extension step at 72°C for 10 min. The following primer sequences
were used: NT5DC2, forward 5′-GCAGCCATCTACGCCAACA-3′ and reverse
5′-TCACGGGCGGTACTGAAGA-3′; IGF2BP2, forward
5′-GTTCCCGCATCATCACTCTTAT-3′ and reverse
5′-GAATCTCGCCAGCTGTTTGA-3′; and GAPDH, forward
5′-CCATGGGGAAGGTGAAGGTC-3′ and reverse 5′-AGTGATGGCATGGACTGTGG-3′.
The relative expression levels of NT5DC2 and IGF2BP2 were
calculated using the 2−ΔΔCq method (20) and normalized to GAPDH.
Western blot analysis
Total protein was extracted from human B lymphocyte
and DLBC cells using RIPA lysis buffer (Beyotime Institute of
Biotechnology) and quantified with BCA Protein Assay kit (Beyotime
Institute of Biotechnology). Equal quantities of protein (20 µg)
were separated by SDS-PAGE (Beyotime Institute of Biotechnology) on
12% gels and transferred onto PVDF membranes (MilliporeSigma). The
membranes were blocked with 5% non-fat milk for 2 h at room
temperature and then incubated with the following primary
antibodies at 4°C overnight: Anti-NT5DC2 (1:1,000; cat. no.
orb312336, Biorbyt Ltd.), anti-CDK4 (1:1,000; cat. no. orb48321;
Biorbyt Ltd), anti-CDK6 (1:1,000; cat. no. orb538814; Biorbyt
Ltd.), anti-cyclin D1 (1:1,000; cat. no. ab134175; Abcam),
anti-Bcl-2 (1:1,000; cat. no. ab196495; Abcam), anti-Bax (1:1,000;
cat. no. ab32503; Abcam), anti-cleaved caspase 3 (1:1,000; cat. no.
#9661; Cell Signaling Technology, Inc.), anti-cytochrome c
(cyto-c; 1:1,000; cat. no. #4272; Cell Signaling Technology, Inc.),
anti-cleaved poly-ADP ribose polymerase (PARP; 1:1,000; cat. no.
ab32561; Abcam), anti-IGF2BP2 (1:2,000; cat. no. ab124930; Abcam),
anti-p53 (1:1,000; cat. no. orb99409; Biorbyt Ltd.), anti-p21
(1:1,000; cat. no. orb38089; Biorbyt Ltd.), anti-PCNA (1:1,000;
cat. no. ab92552; Abcam) and anti-GAPDH (1:2,500; cat. no. ab9485;
Abcam). Subsequently, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibody (1:2,000; cat. no. ab6721;
Abcam) at room temperature for 1 h. Finally, protein bands were
visualized using Pierce™ ECL Western Blotting Substrate
(Pierce; Thermo Fisher Scientific, Inc.) and the expression levels
of each protein were semi-quantified using AlphaImager™
2000 Imaging System (ProteinSimple).
Cell counting kit-8 (CCK-8) assay
Transfected OCI-Ly7 cells (1×103
cells/well) were seeded into a 96-well plate, which was placed in a
humidified incubator at 37°C for ≥24 h. At 24, 48 and 72 h, 10 µl
CCK-8 reagent was added to each well and the 96-well plate was then
incubated at 37°C for 2 h according to the manufacturer's protocol
(Beyotime Institute of Biotechnology). Finally, the OD value at 450
nm was determined using a microplate reader.
5-Bromo-2-deoxyuridine (BrdU)
staining
Transfected OCI-Ly7 cells (5×103
cells/well) were seeded on a pre-treated glass coverslip in a
24-well plate. Subsequently, 200 µl BrdU (Beyotime Institute of
Biotechnology) was added to the cells and incubated at 37°C in the
presence of 5% CO2 for 24 h. The transfected OCI-Ly7
cells were then fixed in 4% paraformaldehyde for 15 min at room
temperature, permeabilized with PBS containing 0.1% Tween-20 and
blocked with 2% BSA (Sigma Aldrich; Merck KGaA) at room temperature
for 2 h. The transfected OCI-Ly7 cells were then incubated with
primary antibody against BrdU (1:500; cat. no. ab6326; Abcam) for 1
h at 4°C and with a secondary antibody (cyanine 3-conjugated goat
anti-mouse IgG; cat. no. A0521; 1:500; Beyotime Institute of
Biotechnology) for 30 min at room temperature, then stained with 20
mg/ml DAPI at 37°C for 30 min. After washing with PBS,
BrdU/DAPI-positive cells were observed under a fluorescence
microscope (magnification, ×200).
Flow cytometry
Transfected OCI-Ly7 cells (1×103
cells/well) were seeded into a 96-well plate and cultured for 48 h
at 37°C. For cell cycle analysis, transfected OCI-Ly7 cells were
collected and fixed with 70% ethanol at 4°C overnight. After
washing with PBS, the transfected OCI-Ly7 cells were incubated with
1 mg/ml RNase A for 20 min at 37°C, followed by staining with
propidium iodide (PI) and 1% Triton X-100 for 20 min at 4°C. The
experimental data were collected using a flow cytometer (BD
FACScan; Becton, Dickinson and Company). For apoptosis analysis,
cells were washed with PBS and resuspended with 100 µl binding
buffer, followed by addition of 10 µl PI/FITC-Annexin V (Roche
Diagnostics) and incubation at room temperature for 1 h in the
dark. Flow cytometry (BD FACScan; Becton, Dickinson and Company)
was used to detect apoptosis within 1 h. The cell cycle
distribution and apoptosis of transfected OCI-Ly7 cells were
analyzed by Cell Quest acquisition and analysis software (V6.0; BD
Biosciences).
RNA immunoprecipitation (RIP)
assay
An EZ-Magna RIP™ RNA-Binding Protein
Immunoprecipitation Kit (cat. no. 17-704; MilliporeSigma) was
employed to perform RIP assay following the manufacturer's
instructions. A total of 2×106 OCI-Ly7 cells were
incubated with 100 µl RIP Lysis Buffer (MilliporeSigma) for 5 min
at 4°C and centrifuged at 40,000 × g at 4°C for 10 min to obtain
the cell lysate. Subsequently, 50 µl protein A/G magnetic beads
conjugated with 5 µg anti-IGF2BP2 (cat. no. ab117809; 2 µg/mg;
Abcam) or IgG antibody (cat. no. ab172730; 2 µg/mg; Abcam) were
added to 800 µl cell lysate and incubated overnight at 4°C. After
being washed with washing buffer six times, proteinase K was then
used to isolate the immunoprecipitated RNA at 55°C for 30 min. The
expression levels of NT5DC2 in the immunoprecipitated RNA were
detected by RT-qPCR analysis as aforementioned.
RNA pull-down assay
An RNA pull down kit (cat. no. P0202: Geenseed used
to perform RNA pull down assay according to the manufacturer's
instructions. NT5DC2 (3 µg) was synthesized in vitro by
Shanghai GenePharma Co., Ltd., labeled with biotin using the RNA 3′
End Desthiobiotinylation kit (Thermo Fisher Scientific, Inc.) and
incubated with streptavidin magnetic beads (Thermo Fisher
Scientific, Inc.). Cellular lysates (50 µl) were incubated with
NT5DC2 or control probes overnight at 4°C. The magnetic beads (30
µl) were mixed with RIPA lysis buffer (Invitrogen; Thermo Fisher
Scientific, Inc.), incubated overnight at 4°C and centrifuged at
1,000 × g for 1 min at 4°C. After washing with RIP wash buffer (50
mM Tris; pH 7.5; 300 mM NaCl and 0.1% NP-40) three times, the
magnetic beads were boiled in SDS buffer to denature the protein
bound to RNA. The protein expression levels of IGF2BP2 were
determined by western blot analysis as aforementioned. The
sequences of NT5DC2 and non-targeting control were as follows:
NT5DC2, forward 5′-TAATACGACTCACTATAGGGGCAGCCATCTACGCCAACA-3′ and
reverse 5′-TCACGGGCGGTACTGAAGA-3′; non-targeting control, forward
5′-TAATACGACTCACTATAGGGCCATGGGGAAGGTGAAGGTC-3′ and reverse
5′-AGTGATGGCATGGACTGTGG-3′.
Bioinformatics analysis
StarBase v2.0 (https://starbase.sysu.edu.cn/) was used to predict the
potential binding of NT5DC2 with IGF2BP2.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0 software (GraphPad Software, Inc.) and data are presented
as the mean ± standard deviation of ≥3 repeats. Statistical
differences between two or multiple groups were determined using
unpaired Student's t-test or one-way ANOVA followed by Tukey's post
hoc test, respectively. P<0.05 was considered to indicate a
statistically significant difference.
Results
NT5DC2 is upregulated in DLBC cells
and knockdown of NT5DC2 inhibits proliferation of DLBC cells
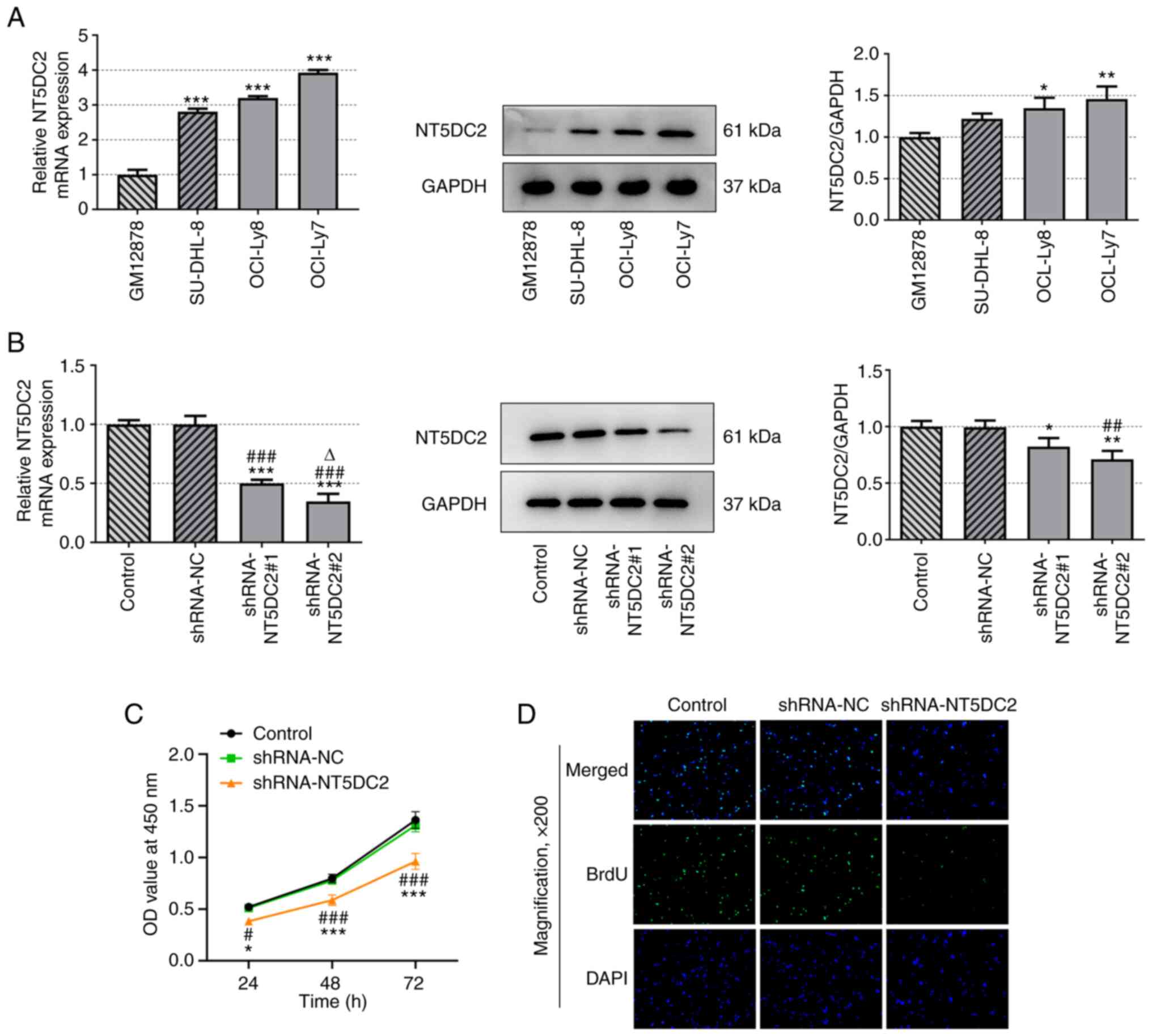
The mRNA and protein expression levels of NT5DC2 in
DLBC cells were increased compared with those in GM12878 cells.
mRNA and protein expression of NT5DC2 was highest in OCI-Ly7 cells
compared with in the other DLBC cell lines (Fig. 1A).
The mRNA and protein expression levels of NT5DC2
were decreased in DLBC cells transfected with shRNA-NT5DC2#1/2
compared with in the untransfected group, and were lower in the
shRNA-NT5DC2#2 group compared with in the shRNA-NT5DC2#1 group
(Fig. 1B). Therefore,
shRNA-NT5DC2#2 was used in the subsequent experiments. Knockdown of
NT5DC2 suppressed the viability (Fig.
1C) and proliferation (Fig.
1D) of OCI-Ly7 cells. These findings indicated that NT5DC2
silencing may have a suppressive role in DLBC cell
proliferation.
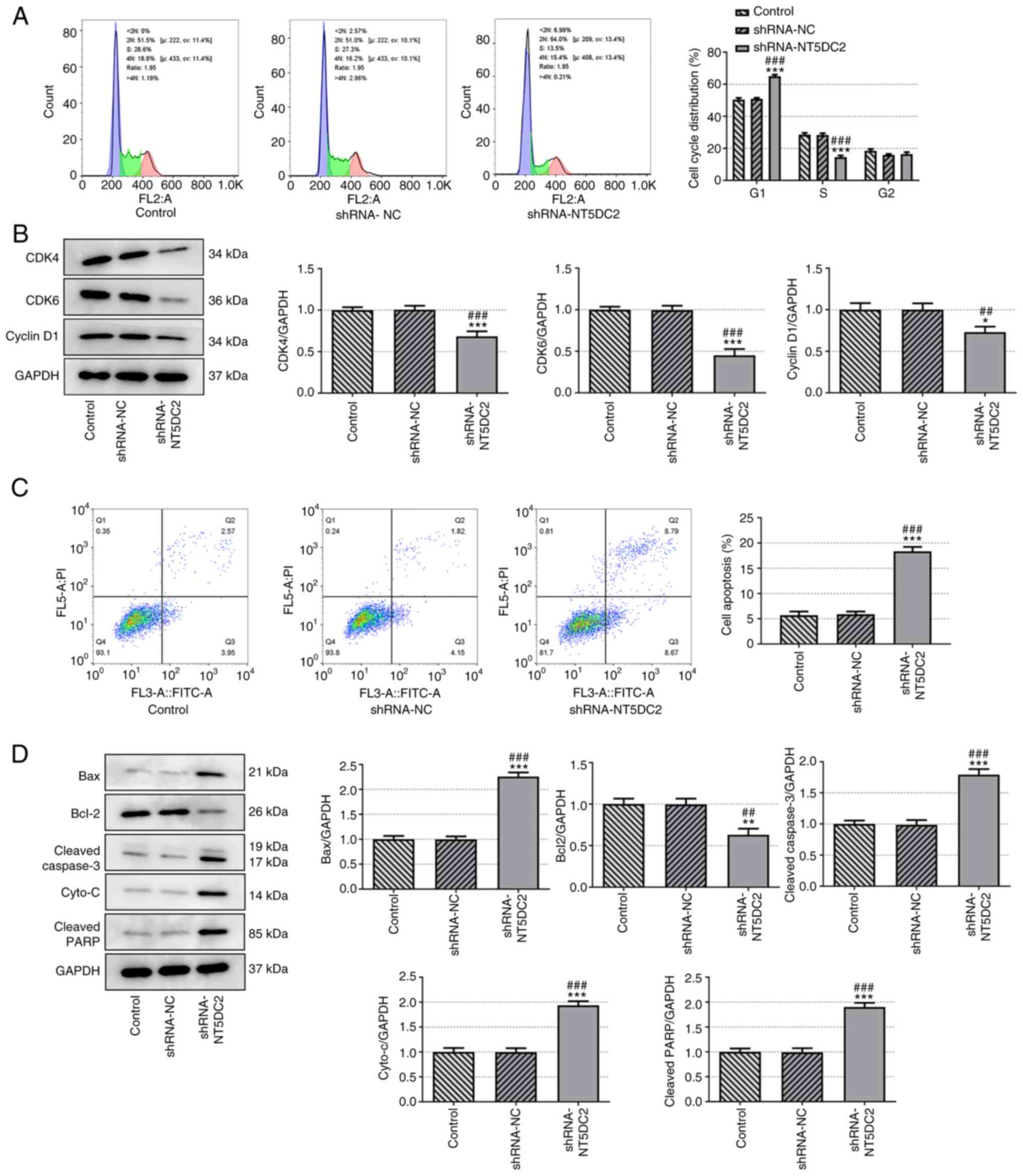
Knockdown of NT5DC2 induces DLBC cell
cycle arrest and apoptosis
Knockdown of NT5DC2 induced DLBC cell cycle arrest,
as indicated by an increased population of cells in
G0/G1 phase and a decreased population of
cells in S phase (Fig. 2A).
The expression levels of cell cycle-associated
proteins (CDK4, CDK6 and cyclin D1) were significantly decreased in
the shRNA-NT5DC2 group (Fig. 2B).
In addition, knockdown of NT5DC2 promoted OCI-Ly7 cell apoptosis
(Fig. 2C), decreased Bcl-2
expression, and increased expression of Bax, cleaved caspase 3,
cyto-c and cleaved PARP (Fig.
2D). These findings suggested that NT5DC2 depletion stimulated
cell cycle arrest and apoptosis in DLBC.
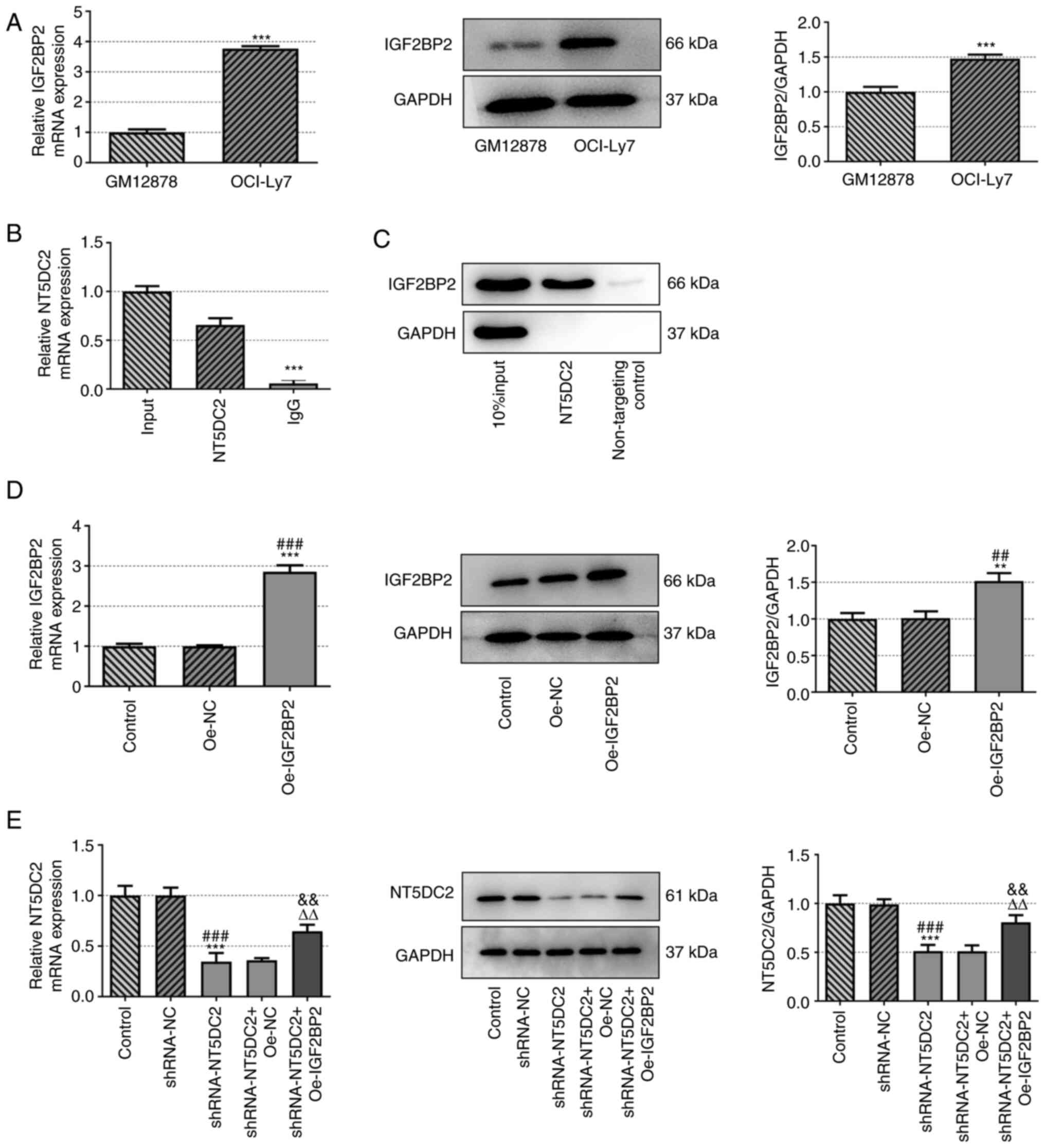
IGF2BP2 is upregulated in DLBC cells
and is the RBP of NT5DC2
Based on starBase, the potential interaction between
IGF2BP2 and NT5DC2 was predicted. The mRNA and protein expression
levels of IGF2BP2 were increased in OCI-Ly7 cells compared with in
GM12878 cells (Fig. 3A). The
interaction between IGF2BP2 and NT5DC2 was confirmed by RIP and RNA
pull-down assays (Fig. 3B and
C).
The mRNA and protein expression levels of IGF2BP2
were increased in OCI-Ly7 cells transfected with Oe-IGF2BP2
(Fig. 3D). Furthermore, the mRNA
and protein expression levels of NT5DC2 were decreased in OCI-Ly7
cells transfected with shRNA-NT5DC2; this was reversed by IGF2BP2
overexpression (Fig. 3E). These
findings indicated that IGF2BP2 was the RBP of NT5DC2.
Upregulation of IGF2BP2 reverses the
regulatory effect of NT5DC2 knockdown on the proliferation and
apoptosis of DLBC cells
Upregulation of IGF2BP2 enhanced the viability
(Fig. 4A) and proliferation
(Fig. 4B) of OCI-Ly7 cells
transfected with shRNA-NT5DC2. Upregulation of IGF2BP2 also
promoted cell cycle progression of OCI-Ly7 cells transfected with
shRNA-NT5DC2, as indicated by a decreased number of cells in the
G0/G1 phase and an increased number of cells
in the S phase (Fig. 4C).
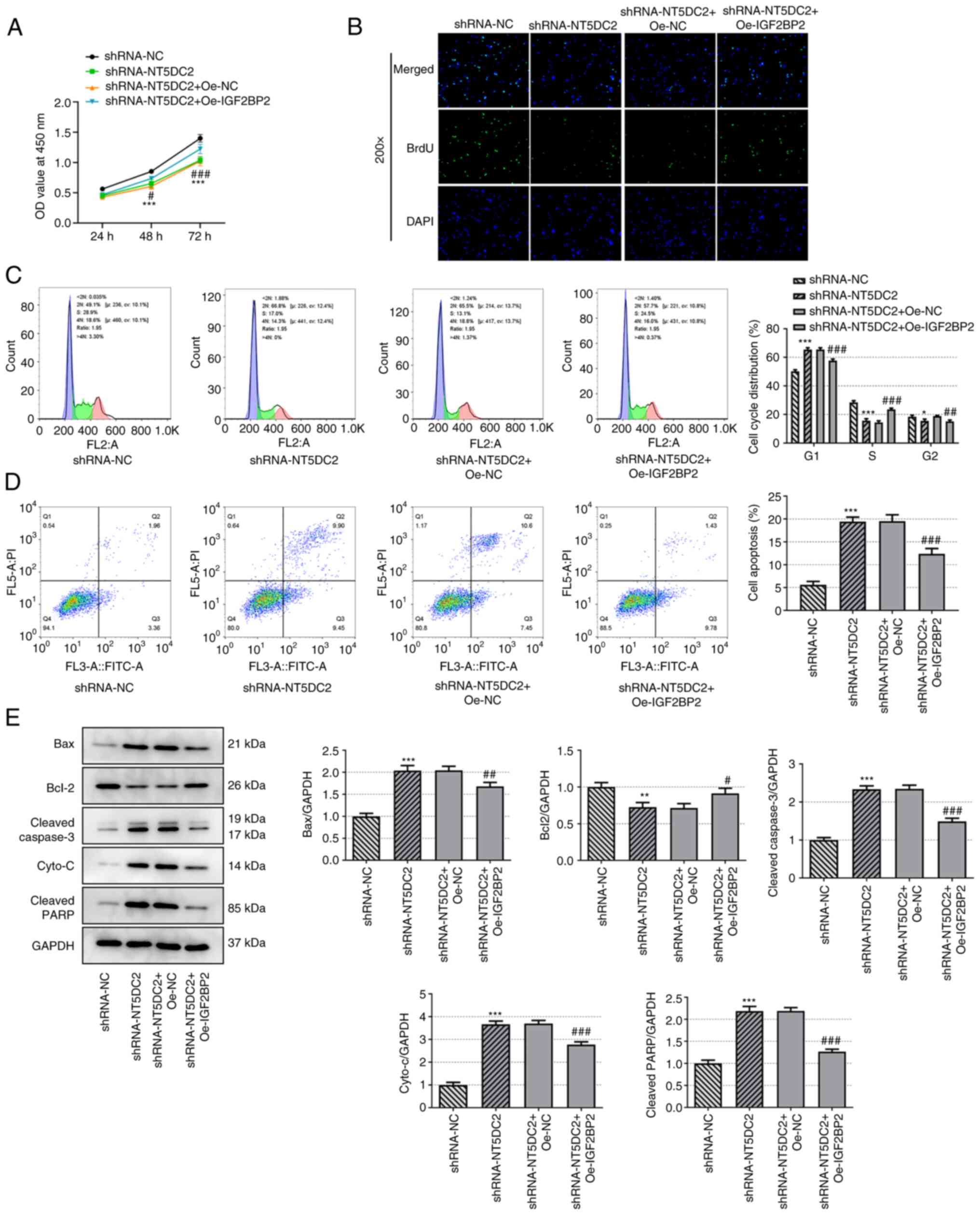 | Figure 4.Upregulation of IGF2BP2 reverses the
regulatory effect of knockdown of NT5DC2 on the proliferation and
apoptosis of diffuse large B-cell carcinoma cells. (A) Viability of
OCI-Ly7 cells transfected with shRNA-NT5DC2 and Oe-IGF2BP2 was
analyzed by Cell Counting Kit-8 assay. (B) Proliferation of OCI-Ly7
cells transfected with shRNA-NT5DC2 and Oe-IGF2BP2 was observed by
BrdU staining. (C) Cell cycle analysis and (D) apoptosis of OCI-Ly7
cells transfected with shRNA-NT5DC2 and Oe-IGF2BP2 was detected by
flow cytometry. (E) Expression levels of apoptosis-associated
proteins were analyzed by western blotting. *P<0.05, **P<0.01
and ***P<0.001 vs. shRNA-NC; #P<0.05, ##P<0.01 and
###P<0.001 vs. shRNA-NT5DC2 + Oe-NC. IGF2BP2, insulin-like
growth factor 2 mRNA-binding protein 2; NT5DC2, 5′-nucleotidase
domain-containing 2; shRNA, short hairpin RNA; oe, overexpression;
BrdU, 5-bromo-2-deoxyuridine; NC, negative control; cyto-c,
cytochrome c; PARP, poly-ADP ribose polymerase; OD, optical
density. |
The apoptosis of OCI-Ly7 cells transfected with
shRNA-NT5DC2 was decreased by upregulation of IGF2BP2 (Fig. 4D). In addition, upregulation of
IGF2BP2 promoted Bcl-2 expression, and suppressed the expression
levels of Bax, cleaved caspase 3, cyto-c and cleaved PARP in
OCI-Ly7 cells transfected with shRNA-NT5DC2 (Fig. 4E). Collectively, IGF2BP2 elevation
may reverse the effects of NT5DC2 knockdown on DLBC cell
proliferation and apoptosis.
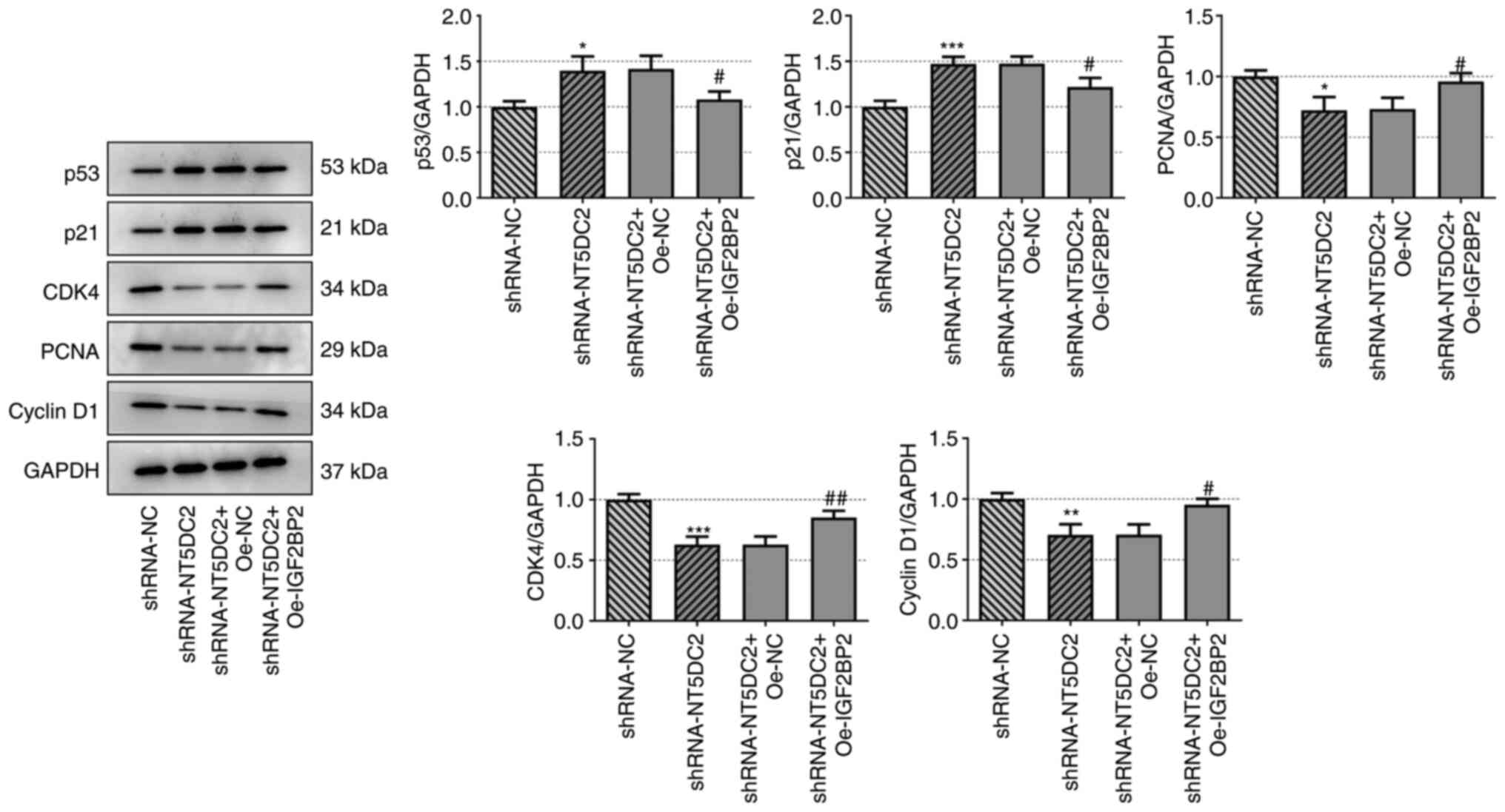
NT5DC2 regulates the p53 signaling
pathway
Knockdown of NT5DC2 increased the expression levels
of p53 and p21, but the decreased expression levels of PCNA, CDK4
and cyclin D1. These effects were reversed by upregulation of
IGF2BP2 (Fig. 5). Taken together,
NT5DC2 may have a regulatory effect on p53 signaling.
Discussion
The incidence of DLBCL in China has been increasing
at a rate of ≥25,000 cases/year (21), and the mortality rate of malignant
lymphoma was estimated at 7.07/100,000 in Spain in 2018 (22). Although rituximab and
anthracycline-based combination chemotherapy regimens have the
potential to cure DLBCL, 30–40% of patients still have poor
prognosis due to refractory or recurrent disease, as well as severe
side effects and development of drug resistance due to long-term
chemotherapy (23). Therefore, it
is necessary to identify novel effective treatments for patients
with DLBCL.
NT5DC2 is a member of the NT5DC family, which has a
highly similar sequence to 5′-nucleotidase, cytosolic II (NT5C2).
Mutant NT5C2 has been reported to be associated with proliferation,
metastasis and chemotherapy resistance of acute lymphoblastic
leukemia cells (24,25). The present results indicated that
NT5DC2 was increased in DLBCL, cells and knockdown of NT5DC2
markedly suppressed the viability and proliferation of OCI-Ly7
cells in vitro. These findings were consistent with previous
studies, which showed that NT5DC2 is oncogenic in numerous types of
tumor, such as HCC, glioblastoma, leiomyosarcoma and colorectal
cancer (6,9,10,26). The occurrence and development of
tumors are associated with dysregulation of the cell cycle and cell
cycle arrest is one of the primary causes of the apoptosis of tumor
cells (27). In the present
study, knockdown of NT5DC2 increased the
G0/G1 phase ratio and decreased the S phase
ratio in OCI-Ly7 cells, which suppressed the viability and
proliferation of OCI-Ly7 cells. The cell cycle is regulated by
multiple proteins, including cell cycle-promoting (cyclin D1,
cyclin D3, CDK4 and CDK6) and -arresting proteins (p18, p21 and
p27) (9). The present data showed
that the expression levels of cyclin D1, CDK4 and CDK6 was
downregulated in NT5DC2-knockdown OCI-Ly7 cells, which led to cell
cycle arrest.
Apoptosis, also known as type-I programmed cell
death, is an active process of programmed cell death regulated by
genes, including Bcl-2 and Bax, under physiological or pathological
conditions (28). Apoptosis in
different tissues shows common characteristics, such as
morphological (formation of apoptotic bodies) and biochemical
changes (ectropion of phosphatidylserine in the membrane and DNA
fragmentation) (29). The present
study revealed that knockdown of NT5DC2 induced the apoptosis of
OCI-Ly7 cells, which was accompanied by decreased expression levels
of anti-apoptotic proteins (Bcl-2) and increased expression levels
of pro-apoptotic proteins (Bax, cleaved caspase 3, cyto-c and
cleaved PARP); these effects were reversed by IGF2BP2
overexpression.
The signaling pathway regulated by the tumor
suppressor gene p53 is inhibited in numerous types of cancer, such
as colon, breast, lung and esophageal cancer (30). In vitro and in vivo
studies have revealed that reactivation of p53 may eliminate tumors
initiated by transforming events independent of p53 (31,32). The tumor suppressor protein p53
serves a key role in tumors, and its dysfunction is associated with
the pathogenesis of B-cell malignancy, including DLBCL (33,34). Drakos et al (35) found that the activated p53
signaling pathway led to cell cycle arrest and apoptosis in DLBCL
cells. Evasion of apoptosis of DLBCL cells occurs via p53
inactivation, and an effective and precise therapeutic strategy for
DLBCL has been observed using the mouse double minute 2 homolog-p53
inhibitor APG-115 (36). The
present study reported that p53 expression levels were markedly
upregulated in NT5DC2-knockdown OCI-Ly7 cells, whereas the opposite
results were detected in cells transfected with Oe-IGF2BP2, which
was consistent with the findings reported in a previous study
(7).
There are certain limitations in the present study.
Firstly, the present study only investigated the role of NT5DC2 in
DLBCL in vitro; in vivo data to verify these findings
should be collected in future work. Secondly, further clarification
is required to identify the detailed and specific regulatory
mechanism underlying NT5DC2/p53 signaling-mediated DLBCL. To the
best of our knowledge, there have been no studies on drugs
targeting NT5DC2 for clinical application. Further investigation of
the molecular role of NT5DC2 is required for the clinical
application of NT5DC2 as a therapeutic target.
In conclusion, the present study demonstrated that
knockdown of NT5DC2 suppressed proliferation, and induced cell
cycle arrest and apoptosis in DLBCL cells by regulating the p53
signaling pathway, which was reversed by IGF2BP2 overexpression.
Therefore, IGF2BP2 and NT5DC2 may be promising targets to improve
treatment efficacy in DLBCL.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific Research Fund
of Heilongjiang Provincial Education Department (grant no.
2018-KYYWF-0932); the North Medicine and Functional Food
Characteristic Subject Project in Heilongjiang Province (grant no.
2018-TSXK-02) and the Innovation and Entrepreneurship Training
Program Project of College Students in Heilongjiang Province (grant
no. 201910222086).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HQ and CW were involved in designing the
experiments. YC, YW, CL, DZ and YY performed the experiments,
analyzed the data and interpreted the results. YC and YW drafted
the manuscript, and HQ and CW revised the manuscript. HQ and CW
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Li L and Fu R: Advances in targeted drug
therapy for relapse/refractory diffuse large B-cell lymphoma. Chin
J Clin Oncol. 46:581–585. 2019.
|
|
2
|
Dunleavy K, Erdmann T and Lenz G:
Targeting the B-cell receptor pathway in diffuse large B-cell
lymphoma. Cancer Treat Rev. 65:41–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiappella A, Santambrogio E, Castellino
A, Nicolosi M and Vitolo U: Integrating novel drugs to
chemoimmunotherapy in diffuse large B-cell lymphoma. Expert Rev
Hematol. 10:697–705. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu P and Zhao W: The interpretation of the
Chinese society of clinical oncology clinical guidelines for the
diagnosis and treatment of diffuse large B-cell lymphoma. West Chin
Med J. 34:351–354. 2019.
|
|
5
|
Vaqué JP, Martínez N, Batlle-López A,
Pérez C, Montes-Moreno S, Sánchez-Beato M and Piris MA: B-cell
lymphoma mutations: Improving diagnostics and enabling targeted
therapies. Haematologica. 99:222–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Z, Hou Q and Guo H: NT5DC2 knockdown
inhibits colorectal carcinoma progression by repressing metastasis,
angiogenesis and tumor-associated macrophage recruitment: A
mechanism involving VEGF signaling. Exp Cell Res. 397:1123112020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin X, Liu X, Zhang Z and Xu L: NT5DC2
suppression restrains progression towards metastasis of
non-small-cell lung cancer through regulation p53 signaling.
Biochem Biophys Res Commun. 533:354–361. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Cao J, Wang P and He X: NT5DC2 is
a novel prognostic marker in human hepatocellular carcinoma. Oncol
Lett. 20:702020.PubMed/NCBI
|
|
9
|
Li KS, Zhu XD, Liu HD, Zhang SZ, Li XL,
Xiao N, Liu XF, Xu B, Lei M, Zhang YY, et al: NT5DC2 promotes tumor
cell proliferation by stabilizing EGFR in hepatocellular carcinoma.
Cell Death Dis. 11:3352020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo S, Ran H, Xiao D, Huang H, Mi L, Wang
X, Chen L, Li D, Zhang S, Han Q, et al: NT5DC2 promotes
tumorigenicity of glioma stem-like cells by upregulating fyn.
Cancer Lett. 454:98–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bell JL, Wächter K, Mühleck B, Pazaitis N,
Köhn M, Lederer M and Hüttelmaier S: Insulin-like growth factor 2
mRNA-binding proteins (IGF2BPs): Post-transcriptional drivers of
cancer progression? Cell Mol Life Sci. 70:2657–2675. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nielsen J, Christiansen J, Lykke-Andersen
J, Johnsen AH, Wewer UM and Nielsen FC: A family of insulin-like
growth factor II mRNA-binding proteins represses translation in
late development. Mol Cell Biol. 19:1262–1270. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong C, Li Z, Ramanujan K, Clay I, Zhang
Y, Lemire-Brachat S and Glass DJ: A long non-coding RNA, LncMyoD,
regulates skeletal muscle differentiation by blocking IMP2-mediated
mRNA translation. Dev Cell. 34:181–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Gilbert JA, Zhang Y, Zhang M, Qiu Q,
Ramanujan K, Shavlakadze T, Eash JK, Scaramozza A, Goddeeris MM, et
al: An HMGA2-IGF2BP2 axis regulates myoblast proliferation and
myogenesis. Dev Cell. 23:1176–1188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Degrauwe N, Schlumpf TB, Janiszewska M,
Martin P, Cauderay A, Provero P, Riggi N, Suvà ML, Paro R and
Stamenkovic I: The RNA binding protein IMP2 preserves glioblastoma
stem cells by preventing let-7 target gene silencing. Cell Rep.
15:1634–1647. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Janiszewska M, Suvà ML, Riggi N,
Houtkooper RH, Auwerx J, Clément-Schatlo V, Radovanovic I, Rheinbay
E, Provero P and Stamenkovic I: Imp2 controls oxidative
phosphorylation and is crucial for preserving glioblastoma cancer
stem cells. Genes Dev. 26:1926–1944. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simon Y, Kessler SM, Bohle RM, Haybaeck J
and Kiemer AK: The insulin-like growth factor 2 (IGF2) mRNA-binding
protein p62/IGF2BP2-2 as a promoter of NAFLD and HCC? Gut.
63:861–863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pu J, Wang J, Qin Z, Wang A, Zhang Y, Wu
X, Wu Y, Li W, Xu Z, Lu Y, et al: IGF2BP2 promotes liver cancer
growth through an m6A-FEN1-dependent mechanism. Front Oncol.
10:5788162020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu X, Yu Y, Zong K, Lv P and Gu Y:
Up-regulation of IGF2BP2 by multiple mechanisms in pancreatic
cancer promotes cancer proliferation by activating the PI3K/Akt
signaling pathway. J Exp Clin Cancer Res. 38:4972019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lenz G and Staudt LM: Aggressive
lymphomas. N Engl J Med. 362:1417–1429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Darbà J and Marsà A: Burden of Hodgkin and
non-Hodgkin lymphoma in Spain over a 10-year period: productivity
losses due to premature mortality. Expert Rev Pharmacoecon Outcomes
Res. 21:87–92. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shiozawa T, Tadokoro J, Fujiki T, Fujino
K, Kakihata K, Masatani S, Morita S, Gemma A and Boku N: Risk
factors for severe adverse effects and treatment-related deaths in
Japanese patients treated with irinotecan-based chemotherapy: A
postmarketing survey. Jpn J Clin Oncol. 43:483–491. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barz MJ, Hof J, Groeneveld-Krentz S, Loh
JW, Szymansky A, Astrahantseff K, von Stackelberg A, Khiabanian H,
Ferrando AA, Eckert C and Kirschner-Schwabe R: Subclonal NT5C2
mutations are associated with poor outcomes after relapse of
pediatric acute lymphoblastic leukemia. Blood. 135:921–933. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dieck CL and Ferrando A: Genetics and
mechanisms of NT5C2-driven chemotherapy resistance in relapsed ALL.
Blood. 133:2263–2268. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu B, Zhou S, Hu X, Zhang H, Lan X, Li M,
Wang Y and Hu Q: NT5DC2 promotes leiomyosarcoma tumour cell growth
via stabilizing unpalmitoylated TEAD4 and generating a positive
feedback loop. J Cell Mol Med. 25:5976–5987. 2021.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ogrodnik M, Salmonowicz H, Jurk D and
Passos JF: Expansion and cell-cycle arrest: Common denominators of
cellular senescence. Trends Biochem Sci. 44:996–1008. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Voss AK and Strasser A: The essentials of
developmental apoptosis. F1000Res. 9:F1000 Faculty Rev. –148. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burgess DJ: Apoptosis: Refined and lethal.
Nat Rev Cancer. 13:792013. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ventura A, Kirsch DG, McLaughlin ME,
Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R
and Jacks T: Restoration of p53 function leads to tumour regression
in vivo. Nature. 445:661–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xue W, Zender L, Miething C, Dickins RA,
Hernando E, Krizhanovsky V, Cordon-Cardo C and Lowe SW: Senescence
and tumour clearance is triggered by p53 restoration in murine
liver carcinomas. Nature. 445:656–660. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tessoulin B, Eveillard M, Lok A, Chiron D,
Moreau P, Amiot M, Moreau-Aubry A, Le Gouill S and
Pellat-Deceunynck C: p53 dysregulation in B-cell malignancies: More
than a single gene in the pathway to hell. Blood Rev. 31:251–259.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu TX, Young KH, Xu W and Li JY: TP53
dysfunction in diffuse large B-cell lymphoma. Crit Rev Oncol
Hematol. 97:47–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Drakos E, Singh RR, Rassidakis GZ,
Schlette E, Li J, Claret FX, Ford RJ Jr, Vega F and Medeiros LJ:
Activation of the p53 pathway by the MDM2 inhibitor nutlin-3a
overcomes BCL2 overexpression in a preclinical model of diffuse
large B-cell lymphoma associated with t(14;18)(q32;q21). Leukemia.
25:856–867. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo Q, Pan W, Zhou S, Wang G, Yi H, Zhang
L, Yan X, Yuan L, Liu Z, Wang J, et al: A novel BCL-2 inhibitor
APG-2575 exerts synthetic lethality with BTK or MDM2-p53 inhibitor
in diffuse large B-cell lymphoma. Oncol Res. 28:331–344. 2020.
View Article : Google Scholar : PubMed/NCBI
|