Introduction
Bipolar disorder (BD) is a common chronic and
cyclical psychiatric disorder characterized by unusual mood swings
between mania/hypomania and depression (1). Although the etiology of BD is not
precisely known, a distinct genetic component is considered to
participate in its pathogenesis (2). Due to the complex and multifactorial
nature of the disease, a predisposition to BD is believed to arise
from the interaction of multiple low-impact genes, and in
combination with environmental factors, may lead to a bipolar
phenotype (3). Classic genetic
epidemiological studies of family, twin, and adopted children have
yielded substantial evidence that genes might cause a
predisposition to bipolar disorder (4). In twin studies, it has been reported
that the heritability of BD varies between 37 and 69%, and the risk
of developing the disorder in first-degree biological relatives of
patients with BD is seven-fold higher, as compared to the general
population (5). However, a
monozygotic concordance of <100% suggests that genes alone do
fully explain this phenomenon (4).
Physical and mental traumas, mitochondrial
dysfunction, neuroinflammation, altered-neurogenesis and apoptosis,
oxidation, endoplasmic reticulum stress, and epigenetic changes,
including histone alterations and methylation are the biological
factors considered operative in BD (1,6).
In a previously published study, it has been suggested that the
NOD-like receptor protein 3 (NLRP3) inflammasome may mediate the
association between mitochondrial dysfunction and inflammatory
system activation (7). In
addition, it has been postulated that the nucleotide-binding
oligomerization domain-like receptor (NLR) family consists of
intracellular pattern recognition receptors that play a critical
role in triggering the host's immune and inflammatory responses
(8). A number of NLRs (>20)
have been identified in humans, with NLRP3 being the most familiar.
The NLRP3 gene is expressed primarily in peripheral blood
leukocytes (9).
The NLRP3 gene has a size of 32.9 kb, is located on
chromosome 1q44, and contains nine exon regions (10). NLRP3 gene variants have been
reported to increase the activation levels of inflammasomes and
IL-1β and IL-18 by causing changes in their functions (10,11). To date, 60 single-nucleotide
polymorphisms (SNPs) have been identified in the NLRP3 gene
(11). These genetic variants of
the NLRP3 gene appear to be a major predictor of the amplitude of
autoinflammatory response (12).
The most prevalent of these variants are rs35829419, rs10754558,
rs4612666, rs4925648 and rs10925019. It has been stated in the
literature that NLRP3 variants are associated with several
inflammatory diseases, including rheumatoid arthritis, psoriatic
arthritis, type 1 diabetes, multiple sclerosis, preeclampsia and
mood disorders (13–18). However, in a meta-analysis study,
certain variants were shown to exert a protective effect (11). Thus, the association between the
NLRP3 gene variants and autoinflammatory diseases remains largely
unknown, due to the use of a relatively small sample size,
insufficient statistical power, and/or heterogeneity of diseases
(11). To the best of our
knowledge, no genetic study has been conducted to date to establish
the role of the NLRP3 gene in the development of BD. It was
hypothesized that the NLRP3 gene may lead to a susceptibility to
bipolar I (BPI) disorder. Accordingly, the present study aimed to
determine the frequency of genetic variants in exon 2 and exon 3 of
the NLRP3 gene in BPI patients, to reveal their role in its
pathogenesis, and to determine the association between the clinical
findings.
Patients and methods
Patients
The present study was initiated following the
approval of Süleyman Demirel University Faculty of Medicine
Clinical Research Ethics Committee (date, 02.11.2020; reference no.
345). All procedures performed involving human participants
complied with the ethical standards of the institutional and/or
national research committee and the 1964 Helsinki declaration and
any subsequent amendments or comparable ethical standards. All
participants signed a written informed consent. The patient group
consisted of 123 (62 females and 61 males) patients with bipolar I
disorder and 107 (55 females and 52 males) healthy controls
(matched by age and sex) who were recruited between January and
July, 2021 from general psychiatric clinics of Süleyman Demirel
University Hospital. All participants were ≥18 years of age. The
phenotyping of tge patients was based on clinical interviews,
medical records and a family history method according to DSM-5
diagnostic criteria using The Structured Clinical Interview for
DSM-5 (SCID-5-CV). The clinical evaluation of the patients was
performed by an experienced psychiatrist. The sociodemographic and
clinical characteristics of the patients were recorded using the
Mood Disorders Patient Registration Form (SKIP-TURK) (19). In the BPI patient group, those
with any concomitant chronic/malignant disease were excluded. The
control group consisted of healthy volunteers, blood donors,
hospital staff, medical students with no family history of mood
disorders and good social functions were selected. All patients
with BPI were in the euthymic period. The Young mania rating scale
(YMRS), Hamilton depression rating scale (HAM-D), functioning
assessment short test (FAST) and global assessment of functioning
(GAF) were administered to the participants by the clinician. Those
with a HAM-D score <7 and a YMRS score <5 were defined as
being in remission.
Evaluation instruments
SCID-5-CV, a semi-structured interview guide, was
used for DSM-5 diagnoses (20).
In previous studies, Turkish validity and reliability study of this
scale have been conducted (21–25). YMRS, developed by Young et
al (22), was used to
evaluate mania symptoms. The HAM-D, developed by Hamilton was used
to evaluate depressive symptoms (24). The GAF scale and FAST were used to
measure global function (26).
The Turkish validity and reliability of the assessment tools used
were studied (27).
DNA extraction
A volume of 5 ml peripheral blood samples were
obtained from patients and controls in EDTA-containing tubes.
Genomic DNA was isolated from collected peripheral blood samples of
the subjects using a DNA Isolation kit (cat. no. 11796828001; Roche
Applied Science) following the manufacturer's instructions. After
extraction, all DNA samples were stored at 20°C. The DNA
concentration in dissolved solution was evaluated through optical
density (OD) measurement at 260 nm and its 260/280 ratio using a
NanoDrop (Thermo Fisher Scientific, Inc.).
Sanger sequencing and data
analysis
Genetic analyses for NLRP3 gene (GenBank Accession
no. NM_001243133.2) exons 2 and 3 were performed using Sanger-based
DNA sequencing. Sequencing was performed from polymerase chain
reaction (PCR)-amplified DNA using conventional protocols. PCR was
performed using the following primers: for exon 2 forward,
5′-GTCTCCTCTCTCATGCCAAATA-3′ and reverse,
5′-CAAGAGCCACACAAACATGAA-3′; for exon 3 forward,
5′-CGTGACAGTCCTTCTGGAAA-3′ and reverse,
5′-CCCATTTATCCACCTACCATACA-3′. Various web tools were used for
primer synthesis [NCBI (USA gov.), Ensembl database (EMBL-EBI),
Primer3web (Whitehead Institute for Biomedical Research)
(https://www.ncbi.nlm.nih.gov/,
https://www.ensembl.org/index.html,
https://primer3.ut.ee/]. In detail, the following
protocol was applied: 25 µl Master mix, containing 2.5 µl of 10X
Buffer, 0.5 µl PCR grade nucleotide mix (10 mM), 0.5 µl forward
primer (10 µM), 0.5 µl reverse primer (10 µM), 3 µl DNA sample,
0.25 µl FastStart Taq DNA polymerase and 0.5 µl DMSO [FastStart
High Fidelity PCR System, dNTPack (Roche Diagnostic)]. The
amplification conditions were as follows: (94°C, 10 min) 1 cycle,
(94°C, 2 min, 55°C 30 sec, 72°C 1 min) 35 cycles, (72°C 7 min) 1
cycle, and 4°C permanent storage (SimpliAmp Thermal Cycler; Applied
Biosystems; Thermo Fisher Scientific, Inc.). At the end of the PCR
analysis, agarose gel electrophoresis was performed to observe the
band formation in the PCR samples. Subsequently, the gel was
visualized with a UV Transilluminator (ECX-F20; Vilber Lourmat).
The PCR product was then purified: 2 ml exosap were added to 5 ml
of the PCR product and incubated at 37°C for 30 min and at 85°C for
15 min. The amplification products were sequenced in both
directions using BigDye Terminator v3.1 (Thermo Fisher Scientific,
Inc.) and specific primers for each region, in the ABI3500 Genetic
Analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. Sequences were
analyzed with the SeqScape Software v3.0 (Thermo Fisher Scientific,
Inc.) using the GRCh37/hg19 sequence as reference. Each SNP was
assessed for deviation from Hardy-Weinberg equilibrium (HWE) using
a HWE calculator web tool (https://wpcalc.com/en/equilibrium-hardy-weinberg/).
Statistical analysis
Categorical variables were analyzed using the
Chi-squared test or Fisher's exact test, where appropriate.
Normality was tested using the Kolmogorov-Smirnov and Shapiro-Wilk
W test. An independent samples (unpaired) t-test was used for age
distribution evaluation. To evaluate the relative risk conferred by
a particular allele or genotype, the odds ratio (OR) and 95%
confidence interval (95% CI) were calculated. Deviation from HWE
was analyzed using the Chi-squared test. Statistical analysis was
performed using SPSS 18.0 software (SPSS, Inc.). Power analysis was
performed by the GPower 3.1 software (https://g-power.apponic.com/). It was determined that
it would be appropriate to analyze a total of 123 patients and 107
healthy individual controls, with a medium effect size of 0.30,
type 1 error rate of 0.05, and power of the test as 87%. P<0.05
was considered to indicate a statistically significant
difference.
Results
Description of the phenotype
The demographic and clinical characteristics of the
123 patients with BPI and the 107 healthy controls recruited for
the sequence analysis are presented in Table I. The mean age was 41.20±11.86
years for the BPI group and 42.09±8.45 years for the healthy
control group. The BPI prevalence was distributed equally between
the two sexes. The BPI patient and control groups did not differ
significantly as regards age and sex parameters (P>0.05).
According to the DSM-5 criteria, age of onset was defined as the
age at which probands first encountered symptoms, related to a
manic or severe depressive episode. There was no authorized age of
onset thresholds for the clinical and genetic study of the BPI. In
the present study, occurrence at an age <22 years was accepted
as early onset, based on the study of Hamshere et al
(28). Patients with intermediate
and late-onset occurrences were grouped as ‘late-onset’, generating
a comparable subgroup sample size and accounting for genetic
homogeneity (29). There was a
significant difference between sexes at the age of onset (P=0.024).
The age of onset in males [standard deviation (SD), 27.28±9.7]
occurred considerably earlier than the age of onset in female (SD,
31.06±8.61). The mean values of the HAM-D, YMRS, GAF and FAST
scales which were used for the classification between groups are
presented in Table I.
 | Table I.Characteristics of the controls and
patients with bipolar disorder in the present study. |
Table I.
Characteristics of the controls and
patients with bipolar disorder in the present study.
| Characteristic | BPI | Control | P-value |
|---|
| No. of
subjects | 123 | 107 | - |
| Male/female (no. of
subjects) | 61/62 | 52/55 | 0.896a |
| Age, years (mean ±
SD) | 41.20±11.86 | 42.09±8.45 | 0.509b |
| Age at onset (mean
± SD) |
|
| - |
|
Early-onset | 19.35±2.41 |
|
|
|
Late-onset | 33.83±8.61 |
|
|
| Age at onset, n
(%) |
|
| - |
|
Early-onset | 37 (30.1) |
|
|
|
Late-onset | 86 (69.9) |
|
|
| Age at onset (mean
± SD) |
|
| 0.024 |
|
Male | 27.28±9.7 |
|
|
|
Female | 31.06±8.61 |
|
|
| Illness duration,
years | 11.98±9.2 |
| - |
| Family history, n
(%) |
|
| - |
|
Positive | 58 (47.2) |
|
|
|
Negative | 65 (52.8) |
|
|
| First illness
period, n (%) |
|
| - |
|
Euphoric mania | 89 (72.4) |
|
|
| Mixed
episode | 12 (9.8) |
|
|
|
Hypomania | 5 (4.1) |
|
|
|
Depression | 17 (13.8) |
|
|
| Pharmacotherapy, n
(%) |
|
| - |
| Mood
stabilizers |
|
|
|
|
Lithium | 58 (50.9) |
|
|
|
Valproate | 54 (47.4) |
|
|
|
Lamotrigine | 7 (6.1) |
|
|
|
Carbamazepine | 2 (1.8) |
|
|
|
Antipsychotic | 8 (6.5) |
|
|
|
Combination | 77 (62.6) |
|
|
|
No treatment | 3 (2.4) |
|
|
| HAM-D (mean ±
SD) | 3.97±5.07 | 0.98±1.30 | <0.001 |
| YMRS (mean ±
SD) | 1.89±5.37 | 0.09±0.29 | <0.001 |
| GAF (mean ±
SD) | 80.69±11.29 | 91.81±4.73 | <0.001 |
| FAST (mean ±
SD) | 6.87±7.03 | 0.92±1.07 | <0.001 |
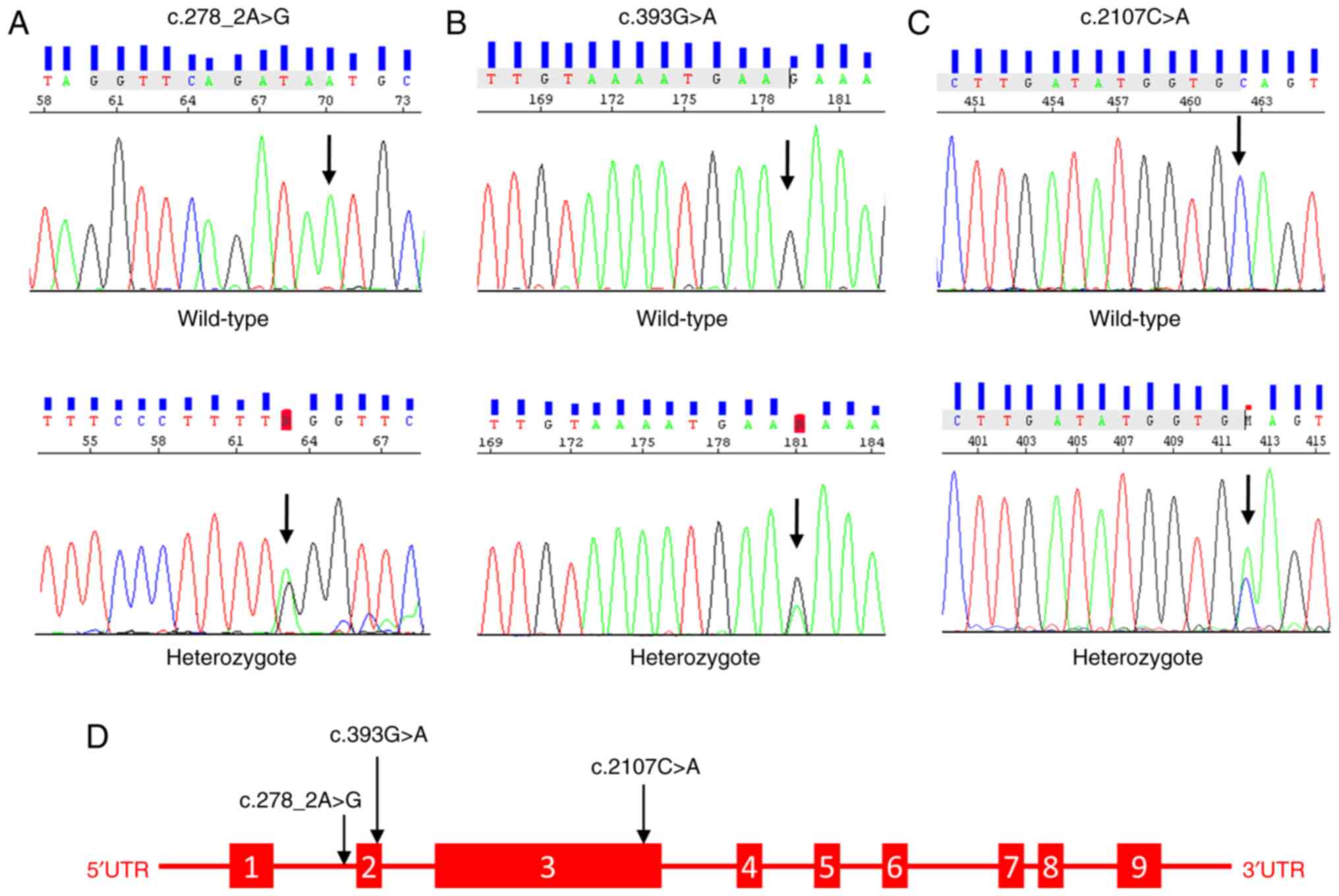
Genotype and allele distribution of
variants
In both groups, Sanger-sequencing was used to
genotype variations of the NLRP3 gene (NM_001243133.2) in exons 2
and 3. In addition to the missense-mutating variant rs35829419
(c.2107C>A, p.Q705K) identified after alignment with the
reference genome (GRCh38.p13), two previously unreported in the
database, novel variants (c.393G>A and c.278 2A>G) were
discovered in the splice region, posing a risk for the development
of BPI. These novel variants were absent in the control group. The
schematic structure of the NLRP3 gene and the Sanger chromatogram
of the detected variants are presented in Fig. 1. According to the statistical
analysis, individuals carrying the CA genotype of rs35829419 (OR,
0.202; 95% CI, 0.080-0.508), the GA genotype of c.393G>A (OR,
0.846; 95% CI, 0.784-0.912) or the AG genotype of c.278_2A>G
(OR, 0.886; 95% CI, 0.832-0.944) were associated with an increased
risk of developing BPI (P<0.001). The genotype and allele
frequencies and the estimated relative risks (OR) of the variants
are presented in Table II. In
addition to these variants, BPI patients had rs1178937944 (n=2),
rs759389274 (n=2), rs763971331 (n=2), and rs770170892 (n=1)
missense variants in exon 2.
 | Table II.Allele and genotype frequencies of
the NLRP3 variants. |
Table II.
Allele and genotype frequencies of
the NLRP3 variants.
| Variant | BPI (N=123) | Controls
(N=107) | Pearson's
χ2 test (df=1) | P-value | OR (95% CI) |
|---|
| rs35829419 |
|
|
|
|
|
| Genotype |
|
| 13.371 |
<0.001a | 0.202
(0.080-0.508) |
| CC | 95 (77.2%) | 101 (94.4%) |
|
|
|
| CA | 28 (22.8%) | 6 (5.6%) |
|
|
|
| AA | 0 | 0 |
|
| 4.324
(1.754-10.660) |
| Alelles |
|
| 11.746 | 0.001a |
|
| C | 218 (88.6%) | 202 (97.1%) |
|
|
|
| A | 28 (11.4%) | 6 (2.9%) |
|
|
|
|
| HWE P=0.154 | HWE P=0.765 |
|
|
|
| c.393G>A |
|
|
|
|
|
| Genotype |
|
| - |
<0.001a | 0.846
(0.784-0.912) |
| GG | 104 (84.6%) | 107 |
|
|
|
| GA | 19 (15.4%) | 0 |
|
|
|
| AA | 0 | 0 |
|
| 1.091
(1.049-1.135) |
| Alelles |
|
| 18.718 |
<0.001a |
|
| G | 208 (91.6%) | 214 |
|
|
|
| A | 19 (8.4%) | 0 |
|
|
|
|
| HWE P=0.353 | HWE - |
|
|
|
| c.278-2A>G |
|
|
|
|
|
| Genotype |
|
| - |
<0.001a | 0.886
(0.832-0.944) |
| AA | 109 (88.6%) | 107 |
|
|
|
| AG | 14 (11.4%) | 0 |
|
|
|
| GG | 0 | 0 |
|
| 1.060
(1.028-1.093) |
| Alelles |
|
| 12.561 |
<0.001a |
|
| A | 232 (94.3%) | 214 |
|
|
|
| G | 14 (5.7%) | 0 |
|
|
|
|
| HWE P=0.503 | HWE - |
|
|
|
HWE was tested in the population as it was a
mandatory quality control step for population-based genetic
association studies. The genotype distributions of the NLRP3 gene
variants were observed to be in balance in both control and BPI
populations. Therefore, it proved the absence of selection bias,
population stratification, or genotyping errors in the present
study (30). The frequencies and
HWE values of the variations identified in the control group and
patients with BPI disorder are listed in Table II.
Analysis of the genotype-phenotype
association
The association between the genotypes and several
phenotypic traits of the patients was studied. While the
association between family history and c.393G>A variant was
significant (P=0.043), no association was detected with other
variants. Patients with c.278_2A>G variant AG genotype had
significantly early onset of the disease (P=0.003). There was no
significant association between the first disease period, lithium
use, scales and variants (P>0.05). The association between
phenotypic traits and variations is depicted in Table III.
 | Table III.Association between some phenotypic
traits and variants. |
Table III.
Association between some phenotypic
traits and variants.
|
| Q705K | c.393G>A | c.278-2A>G |
|---|
|
|
|
|
|
|---|
| Phenotype | CC | CA | P-value | GG | GA | P-value | AA | AG | P-value |
|---|
| Family history, n
(%) |
|
| 0.930a |
|
| 0.043a |
|
| 0.426a |
|
With | 45 (77.6) | 13 (22.4) |
| 45 (77.6) | 13 (22.4) |
| 50 (86.2) | 8 (13.8) |
|
|
Without | 50 (23.1) | 15 (76.9) |
| 59 (90.8) | 6 (9.2) |
| 59 (90.8) | 6 (9.2) |
|
| Age of onset, n
(%) |
|
| 0.460a |
|
| 0.351a |
|
| 0.003a |
|
Early-onset | 27 (73) | 10 (27) |
| 33 (89.2) | 4 (10.8) |
| 28 (75.7) | 9 (24.3) |
|
|
Late-onset | 68 (79.1) | 18 (20.9) |
| 71 (82.6) | 15 (17.4) |
| 81 (94.2) | 5 (5.8) |
|
| Lithium treatment,
n (%) |
|
| 0.149a |
|
| 0.094a |
|
| 0.341a |
|
With | 48 (82.8) | 10 (17.2) |
| 45 (77.6) | 13 (22.4) |
| 53 (91.4) | 5 (8.6) |
|
|
Without | 40 (71.4) | 16 (28.6) |
| 50 (89.3) | 6 (10.7) |
| 48 (85.7) | 8 (14.3) |
|
| Scales (mean ±
SD) |
|
|
|
|
|
|
|
|
|
|
HAM-D | 3.71±4.47 | 4.81±6.73 | 0.324b | 3.93±5.19 | 4.16±4.49 | 0.858b | 4.12±5.32 | 2.86±2.18 | 0.385b |
|
YMRS | 2.11±5.99 | 1.15±2.14 | 0.416b | 2.06±5.82 | 1±1.2 | 0.432b | 1.98±5.69 | 1.21±1.48 | 0.618b |
|
GAF | 81.31±11.3 | 78.63±11.09 | 0.281b | 80.66±11.19 | 80.84±12.09 | 0.950b | 80.52±11.47 | 81.93±10.14 | 0.664b |
|
FAST | 6.63±7.18 | 7.67±6.59 | 0.505b | 7.13±7.52 | 5.53±3.36 | 0.364b | 7.01±7.37 | 5.86±3.71 | 0.567b |
Discussion
The present study comparatively analyzed the effects
of exon 2 and 3 NLRP3 variants on disease development, their
frequency, and the association between various phenotypic traits in
BPI patients and healthy individuals. To the best of our knowledge,
the present study is the first to demonstrate an association
between NLRP3 variants and BPI. The variant rs35829419 and two
novel splice-site variants c.393G>A and c.278_2A>G identified
in the NLRP3 gene demonstrated a significant association between
BPI susceptibility and some phenotypic traits.
NLRP3 is the most intensively studied receptor in
the NOD-like receptor family (31). Several studies have reported that
the NOD-like receptor is a pattern recognition receptor that
functions as a redox sensor in the inflammatory system. It has been
reported that these recognition receptors can recognize distress
signals, arising from physical and psychological stress (32,33). The variants in the NLRP3 gene can
enhance the activation of the inflammasome, by causing changes in
gene function. To date, ~60 SNPs have been identified in the NLRP3
gene (11). The gain-of-function
variant rs35829419, which has been associated with a
pro-inflammatory phenotype, has been demonstrated to promote the
disease state by triggering NLRP3 inflammation activation (34). Therefore, it has been previously
attempted to investigate the role of the rs35829419 variant in
various inflammatory diseases. The association of the rs35829419
variant with several autoinflammatory disorders has been
investigated, including celiac disease, Crohn's disease, psoriatic
arthritis, myasthenia gravis, multiple sclerosis, type 1 diabetes,
and systemic lupus erythematosus (15,16,35–38). In Parkinson's disease, in which
inflammation is effective in pathogenesis, no significant
association has been demonstrated between the frequency of the
NLRP3 rs35829419 variant and the control group (39). In another study evaluating the
effects of NLRP3 variants on the susceptibility and severity of
rheumatoid arthritis (RA), no significant association was revealed
between variants with joint damage and increased susceptibility
(13). By contrast, in a study
conducted to determine the association between inflammation-related
genetic variants and susceptibility to psoriatic arthritis, a
significant association was revealed between the
destructive/deformative subset of psoriatic arthritis and the C
allele of variant rs35829419 (14). In a previous study, a significant
association was found between pediatric celiac disease and the
NLRP3 variant rs358294199 in the Brazilian population (15). Similarly, allele and genotype
frequencies of NLRP3 rs358294199 have been observed to be similar
in case-control studies of patients with multiple sclerosis and
ulcerative colitis in the Iranian population (16,40). As regards glucose homeostasis in
patients with polycystic ovary syndrome, it has also been
previously observed that the NLRP3 rs358294199 variant is
significantly associated with the response to glycemic load
(41). Moreover, it has been
argued that the individuals with the A-risk allele of rs35829419
are more likely to develop the disease, whereas on the contrary,
other researchers suggested a protective role; therefore, the
clinical significance of this polymorphism has not been fully
elucidated (11). The findings of
the present study were consistent with other studies, suggesting
that the rs35829419 variant may induce susceptibility to
inflammatory diseases (14–16,40,41). In the present study, a substantial
increase in BPI susceptibility was found in individuals carrying
the A-risk allele of the rs35829419 variant, leading to the
assumption that patients with BPI may have a higher inflammatory
response than healthy individual controls. Therefore, it was
suggested that the NLRP3 gene may play a critical role in the
genetic etiology of BPI, and with the inflammation mediating this
association.
The variants in the exon and splice regions of genes
can alter the amino acid sequence, and thus affecting protein
functions. Novel variants that cause amino acid changes in these
regions are difficult to classify, since there are no entries in
population databases, and segregation analyses cannot be performed.
In the present study, two novel splice-site variants in the NLRP3
exon-2 region were identified. In total, 19 individuals (15.4%) in
the single BPI patient group (MAF=0.084) presented with the novel
c.393G>A variant, which resulted in synonymous alterations.
There was a significant association with positive family history
concerning the novel variant c.393G>A, as compared with
phenotypic traits. There are only a few studies in the literature
having addressed the family history of BPI patients. As previously
reported, a family history of bipolar disorder could influence the
course of the disorder and its prevalence rates (42). It has been revealed that patients
with bipolar disorder who have a family history of depression have
an early onset of the disease, experience more severe
deterioration, experience multiple episodes during their lifetime,
and are hospitalized more frequently (43). In their study involving data from
patient families, Post et al (44) reported that the illness occurred
early in adult patients with a family history of bipolar disorder
and was associated with the occurrence of multiple psychiatric
disorders in their children. In another study in patient family
data, 22.5% of children with BD were observed to present with a
higher incidence of depression, anxiety, conduct disorder,
attention-deficit/hyperactivity disorder, and substance use
disorders in comparison with their parents (45). In total, 14 individuals (11.4%)
belonging to the BPI patient group were heterozygous for novel
splice-site variant, c.278_2A>G. This variant was classified as
‘likely pathogenic’ in the VarSome and Franklin databases. The
comparison of the novel c.278_2A>G variant with phenotypic
features, revealed that there was an early onset of the disease in
patients with the AG genotype. The early-onset BP has been
generally associated with a worse prognosis than late-onset BP,
including more psychotic features, substance addiction, comorbidity
with panic and obsessive-compulsive disorders, a lower lithium
response, and further suicide attempts (46–48). In a previous study, it has been
suggested that early-onset BD has a high degree of homogeneity and
a unique genetic etiology that has not yet been established in
practice (49). In a study
investigating the role of age at onset and family history in the
clinic for BD, a significantly higher prevalence of family history
and a higher number of attacks per year were observed in the
early-onset group (50). Although
a number of interesting preliminary studies have been conducted for
the investigation of the basis of the different phenotypic
characteristics of BD disease among individuals, the results
obtained in genome-wide association studies (GWAS) with large
samples need to be replicated to prove their actual validity. GWAS
can identify genetic variations between early and late-onset BD,
and the proportion of common SNPs that constitute each subtype can
be estimated (49–51).
To the best of our knowledge, no genotype-phenotype
correlation of NLRP3 gene variants has been described in BPI
patients to date. The present study proved that NLRP3 gene
variants, which may play an important role in modulating
inflammatory responses, might genetically predispose to a chronic
neuroinflammatory disease, including BPI. Furthermore, it was
suggested that the rs35829419, c.393G>A, and c.278_2A>G NLRP3
variants may change the degree of systemic inflammation, and thus
contribute to adverse inflammatory outcomes. However, there were
some significant limitations to this study. For instance, there was
a relatively small number of patients that comprised the study
population. Another limitation was the inability to perform
segregation analyses and functional studies, which were critical
for demonstrating the effect of the identified variants on the
disease.
In conclusion, the present study revealed that NLRP3
gene variants may be associated with an elevated risk of BPI. These
findings need to be confirmed by family-based studies and
replicated in different studies and large population samples. In
addition, large-scale genetic studies, including GWAS, whole-exome
sequencing, and whole-genome sequencing seem to be the most
promising perspectives for future research.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific Research
Projects Coordination Unit of Süleyman Demirel University with the
project no. TSG-2020-8134.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KHÖ and GÖÜ contributed to the conception and design
of the study. KHÖ and GÖÜ performed experiments and samples
collection. KHÖ performed data analysis and interpretation. KHÖ and
GÖÜ drafted the manuscript. KHÖ and GÖÜ confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by Süleyman Demirel
University Faculty of Medicine Clinical Research Ethics Committee
(date, 02.11.2020, no. 345). All patients provided written informed
consent prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pereira AC, Oliveira J, Silva S, Madeira
N, Pereira CM and Cruz MT: Inflammation in bipolar disorder (BD):
Identification of new therapeutic targets. Pharmacol Res.
163:1053252021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gubert C, Andrejew R, Moritz CE, Dietrich
F, Vasconcelos-Moreno MP, Dos Santos BT, Fijtman A, Kauer-Sant'Anna
M, Kapczinski F, da Silva Magalhães PV and Battastini AM: Bipolar
disorder and 1513A>C P2RX7 polymorphism frequency. Neurosci
Lett. 16:143–147. 2019. View Article : Google Scholar
|
|
3
|
Prata DP, Costa-Neves B, Cosme G and
Vassos E: Unravelling the genetic basis of schizophrenia and
bipolar disorder with GWAS: A systematic review. J Psychiatr Res.
114:178–207. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gordovez FJ and McMahon FJ: The genetics
of bipolar disorder. Mol Psychiatry. 25:544–559. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gunderson JG, Zanarini MC, Choi-Kain LW,
Mitchell KS, Jang KL and Hudson JI: Family study of borderline
personality disorder and its sectors of psychopathology. Arch Gen
Psychiatry. 68:753–762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berk M, Post R, Ratheesh A, Gliddon E,
Singh A, Vieta E, Carvalho AF, Ashton MM, Berk L, Cotton SM, et al:
Staging in bipolar disorder: From theoretical framework to clinical
utility. World Psychiatry. 16:236–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sigitova E, Fišar Z, Hroudová J, Cikánková
T and Raboch J: Biological hypotheses and biomarkers of bipolar
disorder. Psychiatry Clin Neurosci. 71:77–103. 2017. View Article : Google Scholar
|
|
8
|
Mangan MSJ, Olhava EJ, Roush WR, Seidel
HM, Glick GD and Latz E: Targeting the NLRP3 inflammasome in
inflammatory diseases. Nat Rev Drug Discov. 17:6882018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang AQ, Zeng L, Gu W, Zhang LY, Zhou J,
Jiang DP, Du DY, Hu P, Yang C, Yan J, et al: Clinical relevance of
single nucleotide polymorphisms within the entire NLRP3 gene in
patients with major blunt trauma. Crit Care. 15:R2802011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Villani AC, Lemire M, Fortin G, Louis E,
Silverberg MS, Collette C, Baba N, Libioulle C, Belaiche J, Bitton
A, et al: Common variants in the NLRP3 region contribute to Crohn's
disease susceptibility. Nat Genet. 41:71–76. 2009. View Article : Google Scholar
|
|
11
|
Wu Z, Wu S and Liang T: Association of
NLRP3 rs35829419 and rs10754558 polymorphisms with risks of
autoimmune diseases: A systematic review and meta-analysis. Front
Genet. 12:6908602021. View Article : Google Scholar
|
|
12
|
Verma D, Lerm M, Julinder RB, Eriksson P,
Söderkvist P and Särndahl E: Gene polymorphisms in the NALP3
inflammasome are associated with interleukin-1 production and
severe inflammation: Relation to common inflammatory diseases?
Arthritis Rheum. 58:888–894. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kastbom A, Johansson M, Verma D,
Söderkvist P and Rantapää-Dahlqvist S: CARD8 p.C10X polymorphism is
associated with inflammatory activity in early rheumatoid
arthritis. Ann Rheum Dis. 69:723–726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Juneblad K, Kastbom A, Johansson L,
Rantapää-Dahlqvist S, Söderkvist P and Alenius GM: Association
between inflammasome-related polymorphisms and psoriatic arthritis.
Scand J Rheumatol. 50:206–212. 2021. View Article : Google Scholar
|
|
15
|
Pontillo A, Brandao L, Guimaraes R, Segat
L, Araujo J and Crovella S: Two SNPs in NLRP3 gene are involved in
the predisposition to type-1 diabetes and celiac disease in a
pediatric population from northeast Brazil. Autoimmunity.
43:583–589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imani D, Azimi A, Salehi Z, Rezaei N,
Emamnejad R, Sadr M and Izad M: Association of nod-like receptor
protein-3 single nucleotide gene polymorphisms and expression with
the susceptibility to relapsing-remitting multiple sclerosis. Int J
Immunogenet. 45:329–336. 2018. View Article : Google Scholar
|
|
17
|
Xu L, Li S, Liu Z, Jiang S, Wang J, Guo M,
Zhao X, Song W and Liu S: The NLRP3 rs10754558 polymorphism is a
risk factor for preeclampsia in a Chinese Han population. J Matern
Neonatal Med. 32:1792–1799. 2019. View Article : Google Scholar
|
|
18
|
Zhou XY, Fernando SM, Pan AY, Laposa R,
Cullen KR, Klimes-Dougan B and Andreazza AC: Characterizing the
NLRP3 inflammasome in mood disorders: Overview, technical
development, and measures of peripheral activation in adolescent
patients. Int J Mol Sci. 22:125132021. View Article : Google Scholar
|
|
19
|
Özerdem A, Yazıcı O, Tunca Z and Tırpan K:
Establishment of computerized registry program for bipolar illnes
in turkey: SKIP-TURK. J Affect Disord. 84:82–86. 2004.
|
|
20
|
First MB, Williams JB, Karg RS and Spitzer
RL: User's guide for the SCID-5-CV Structured Clinical Interview
for DSM-5® disorders: Clinical version: American
Psychiatric Publishing, Inc. 2016.
|
|
21
|
Elbir M, Topbaş ÖA, Bayad S, Kocabaş T,
Topak OZ, Çetin Ş, Özdel O, Ateşçi F and Aydemir Ö: Adaptation and
reliability of the structured clinical interview for
DSM-5-disorders-clinician version (SCID-5/CV) to the Turkish
language. Turk Psikiyatri Derg. 30:51–56. 2019.(In Turkish).
PubMed/NCBI
|
|
22
|
Young RC, Biggs JT, Ziegler VE and Meyer
DA: A rating scale for mania: Reliability, validity and
sensitivity. Br J Psychiatry. 133:429–435. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karadag F, Oral T, Yalcin FA and Erten E:
Reliability and validity of Turkish translation of young mania
rating scale. Turk Psikiyatri Derg. 13:107–114. 2002.(In Turkish).
PubMed/NCBI
|
|
24
|
Hamilton M: A rating scale for depression.
J Neurol Neurosurg Psychiatry. 23:56–62. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akdemir A, Türkçapar MH, Örsel SD,
Demirergi N, Dag I and Özbay MH: Reliability and validity of the
Turkish version of the hamilton depression rating scale. Compr
Psychiatry. 42:161–165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rosa AR, Sánchez-Moreno J, Martínez-Aran
A, Salamero M, Torrent C, Reinares M, Comes M, Colom F, Van Riel W,
Ayuso-Mateos JL, et al: Validity and reliability of the functioning
assessment short test (FAST) in bipolar disorder. Clin Pract
Epidemiol Ment Health. 3:52007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aydemir O and Uykur B: Reliability and
validity study of the Turkish version of functioning assessment
short test in bipolar disorder. Turk Psikiyatri Derg. 23:193–200.
2012.(In Turkish). PubMed/NCBI
|
|
28
|
Hamshere ML, Gordon-Smith K, Forty L,
Jones L, Caesar S, Fraser C, Hyde S, Tredget J, Kirov G, Jones I,
et al: Age-at-onset in bipolar-I disorder: Mixture analysis of 1369
cases identifies three distinct clinical sub-groups. J Affect
Disord. 116:23–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grigoroiu-Serbanescu M, Martinez M, Nöthen
MM, Grinberg M, Sima D, Propping P, Marinescu E and Hrestic M:
Different familial transmission patterns in bipolar I disorder with
onset before and after age 25. Am J Med Genet. 105:765–773. 2001.
View Article : Google Scholar
|
|
30
|
Namipashaki A, Razaghi-Moghadam Z and
Ansari-Pour N: The essentiality of reporting hardy-weinberg
equilibrium calculations in population-based genetic association
studies. Cell J. 17:187–192. 2015.PubMed/NCBI
|
|
31
|
Bryant C and Fitzgerald KA: Molecular
mechanisms involved in inflammasome activation. Trends Cell Biol.
19:455–464. 2009. View Article : Google Scholar
|
|
32
|
Zhou R, Yazdi AS, Menu P and Tschopp J: A
role for mitochondria in NLRP3 inflammasome activation. Nature.
469:221–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei P, Yang F, Zheng Q, Tang W and Li J:
The potential role of the NLRP3 inflammasome activation as a link
between mitochondria ROS generation and neuroinflammation in
postoperative cognitive dysfunction. Front Cell Neurosci.
13:732019. View Article : Google Scholar
|
|
34
|
Zhang Q, Fan HW, Zhang JZ, Wang YM and
Xing HJ: NLRP3 rs35829419 polymorphism is associated with increased
susceptibility to multiple diseases in humans. Genet Mol Res.
14:13968–13980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pontillo A, Vendramin A, Catamo E, Fabris
A and Crovella S: The missense variation Q705K in CIAS1/NALP3/NLRP3
gene and an NLRP1 haplotype are associated with celiac disease. Am
J Gastroenterol. 106:539–544. 2011. View Article : Google Scholar
|
|
36
|
Roberts RL, Topless RKG, Phipps-Green AJ,
Gearry RB, Barclay ML and Merriman TR: Evidence of interaction of
CARD8 rs2043211 with NALP3 rs35829419 in Crohn's disease. Genes
Immun. 11:351–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Agah E, Nafissi S, Saleh F, Sarraf P,
Tafakhori A, Mousavi SV, Saghazadeh A, Sadr M, Sinaei F, Mohebbi B,
et al: Investigating the possible association between NLRP3 gene
polymorphisms and myasthenia gravis. Muscle Nerve. 63:730–736.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pontillo A, Girardelli M, Kamada AJ,
Pancotto JAT, Donadi EA, Crovella S and Sandrin-Garcia P:
Polimorphisms in inflammasome genes are involved in the
predisposition to systemic lupus erythematosus. Autoimmunity.
45:271–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Redenšek S, Flisar D, Kojović M,
Kramberger MG, Georgiev D, Pirtošek Z, Trošt M and Dolžan V:
Genetic variability of inflammation and oxidative stress genes does
not play a major role in the occurrence of adverse events of
dopaminergic treatment in Parkinson's disease. J Neuroinflammation.
16:502019. View Article : Google Scholar
|
|
40
|
Hanaei S, Sadr M, Rezaei A, Shahkarami S,
Daryani NE, Bidoki AZ and Rezaei N: Association of NLRP3 single
nucleotide polymorphisms with ulcerative colitis: A case-control
study. Clin Res Hepatol Gastroenterol. 42:269–275. 2018. View Article : Google Scholar
|
|
41
|
Herman R, Jensterle M, Janež A, Goričar K
and Dolžan V: Genetic variability in antioxidative and inflammatory
pathways modifies the risk for PCOS and influences metabolic
profile of the syndrome. Metabolites. 10:4392020. View Article : Google Scholar
|
|
42
|
Antypa N and Serretti A: Family history of
a mood disorder indicates a more severe bipolar disorder. J Affect
Disord. 156:178–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zalar B, Blatnik A, Maver A, Klemenc-Ketiš
Z and Peterlin B: Family history as an important factor for
stratifying participants in genetic studies of major depression.
Balkan J Med Genet. 21:5–12. 2018. View Article : Google Scholar
|
|
44
|
Post RM, Altshuler LL, Kupka R, McElroy
SL, Frye MA, Rowe M, Grunze H, Suppes T, Keck PE Jr, Leverich GS
and Nolen WA: Multigenerational transmission of liability to
psychiatric illness in offspring of parents with bipolar disorder.
Bipolar Disord. 21:doi: 10.1111/bdi.12668. 2018.(Epub ahead of
print). PubMed/NCBI
|
|
45
|
Axelson D, Goldstein B, Goldstein T, Monk
K, Yu H, Hickey MB, Sakolsky D, Diler R, Hafeman D, Merranko J, et
al: Diagnostic precursors to bipolar disorder in offspring of
parents with bipolar disorder: A longitudinal study. Am J
Psychiatry. 172:638–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Studart-Bottó P, Bezerra-Filho S, Sarmento
S and Miranda-Scippa Â: Social support in patients with bipolar
disorder and differing ages at onset. Clin Psychol Psychother.
29:351–359. 2022. View
Article : Google Scholar
|
|
47
|
Geoffroy PA, Etain B, Scott J, Henry C,
Jamain S, Leboyer M and Bellivier F: Reconsideration of bipolar
disorder as a developmental disorder: Importance of the time of
onset. J Physiol. 107:278–285. 2013.
|
|
48
|
Grigoroiu-Serbanescu M, Rietschel M,
Hauser J, Czerski PM, Herms S, Sun X, Wickramaratne P and Elston
RC: Commingling analysis of age-of-onset in bipolar I disorder and
the morbid risk for major psychoses in first degree relatives of
bipolar I probands. J Affect Disord. 168:197–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kennedy KP, Cullen KR, Deyoung CG and
Klimes-Dougan B: The genetics of early-onset bipolar disorder: A
systematic review. J Affect Disord. 184:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Soni A, Singh P, Kumar S, Shah R, Batra L
and Verma M: Role of age at onset in the clinical presentation of
bipolar disorder in Indian population. Ind Psychiatry J. 30:41–46.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu LS, Huang MC, Fann CS, Lane HY, Kuo CJ,
Chiu WC, Kwok PY and Cheng AT: Genome-wide association study of
early-onset bipolar I disorder in the Han Taiwanese population.
Transl Psychiatry. 11:3012021. View Article : Google Scholar : PubMed/NCBI
|