Introduction
Osteosarcoma is a malignant tumor originating from
mesenchymal tissues (1). It is
one of the most common primary tumors, especially in adolescents
aged 10 to 20 years (2).
Osteosarcoma is highly malignant, with pulmonary metastasis
occurring in approximately 85–90% of patients with osteosarcoma
(3,4). In recent years, with the in-depth
research on the pathogenesis and improvement of osteosarcoma
therapies, the 5-year survival rate of localized osteosarcoma has
been increased to 60–70% (5), but
the 5-year survival rate of metastatic osteosarcoma is only 20–30%
(6). In recent years, with the
development of tissue engineering, increasing attention has been
paid to the development of bone substitutes with custom-made
microarchitecture and physicomechanical properties comparable to
native bone, such as bioactive three-dimensional (3D) porous
polymer or ceramic scaffolds or its conjugated forms, which can
mimic the host tissue to facilitate the transferring of nutrients
to ensure defective bone restoration (7–9).
Moreover, 3D-printed multifunctional polyetheretherketone bone
scaffold was widely applied in the multimodal treatment of
osteosarcoma and osteomyelitis (10). Clinical results reveal that the
efficacy of current chemotherapy drugs in patients with metastatic
osteosarcoma is insufficient (11). Thus, the research and development
of new therapeutic drugs are deemed significant for the treatment
of osteosarcoma at this stage. It has become a top priority to
clarify the mechanism of the occurrence and development of
osteosarcoma and find effective drug treatments.
Daphne genkwa Sieb. et Zucc. (Daphne genkwa), a
traditional Chinese herb, was first recorded in Shennong's Classic
Materia Medica (12). It is
widely grown in China, Japan and other countries (13). Daphne genkwa has long been used as
an anti-inflammatory, analgesic and sedative drug for edema and
asthma (14,15). Hydroxygenkwanin (HGK) is a
flavonoid compound extracted from the flower buds of Daphne genkwa,
which is considered to be one of the active ingredients in Daphne
genkwa flowers (16). The
pharmacological effect of HGK has attracted the attention of
researchers and is now widely used in the treatment of various
tumors (17,18). Unfortunately, the study of HGK in
osteosarcoma has not received much attention.
MicroRNAs (miRNAs or miRs; endogenous non-coding
small RNAs) can modify gene expression in eukaryotic cells at
post-transcriptional levels (19). Several studies have revealed that
~3% of human genes encode miRNA synthesis, and ~60% of human genes
are regulated by miRNAs (20,21). In recent years, the importance of
miRNAs in human diseases has been highlighted. miRNAs have
therefore become a hotspot in the research of diseases. Similarly,
in osteosarcoma, miRNAs also act as regulators and influence the
progression of osteosarcoma (22). Among them, upregulation of
miR-320a has been revealed to inhibit the proliferation and
migration of osteosarcoma (23,24). SRY-box transcription factor 9
(SOX9) is a key transcription factor in chondrocytes (25). Current studies have revealed that
SOX9 plays a critical role in the migration and invasion of
osteosarcoma cells (26–29). In addition, it is worth noting
that from a bioinformatics perspective, miRNA-320a appears to be
associated with SOX9. A study on liver cancer from Chou et al
revealed that HGK could inhibit the metastasis and invasion of
hepatocellular carcinoma by promoting the expression of miR-320a
(11). Therefore, it was surmised
that HGK may regulate the proliferation, invasion and migration of
osteosarcoma cells through the miR-320a/SOX9 axis.
In the present study, the effect of HGK on
osteosarcoma was detected by treating osteosarcoma cell lines with
HGK. The specific molecular mechanism of HGK in the treatment of
osteosarcoma was further elucidated by exploring the regulatory
effect of HGK on the miR-320a/SOX9 axis. The present study lays a
theoretical foundation for the treatment of osteosarcoma by HGK,
and also provides a potential targeted drug for the treatment of
osteosarcoma.
Materials and methods
Cell culture
Human osteoblasts (hFOB 1.19; cat. no. CRL-11372)
and osteosarcoma cells (MG-63; cat. no. CRL-1427; and U2OS; cat.
no. HTB-96) were obtained from American Type Culture Collection
(ATCC) and cultured in Dulbecco's modified Eagle's medium (DMEM;
cat. no. 12491-015) supplemented with 10% fetal bovine serum (FBS;
cat. no. 10099-141), 100 U/ml penicillin and 100 µg/ml streptomycin
(cat. no. 15070063; all from Gibco; Thermo Fisher Scientific, Inc.)
in an incubator at 37°C with 5% CO2.
Hydroxygenkwanin (HGK; purity >99%) was purchased
from ChemFaces. Various concentrations (0, 10, 20, 40 and 60
µmol/l) of HGK were applied to treat hFOB 1.19 cells to test the
safety of the drug. In addition, MG-63 and U2OS cells were also
treated with various concentrations of HGK (10, 20, 40 and 60
µmol/l) for 48 h, and assigned as the treatment groups. Moreover,
MG-63 and U2OS cells as well as hFOB 1.19 cells received 0 µmol/l
HGK and were assigned as the control groups.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium romide (MTT)
assay
The cells of each cell line were seeded in a 96-well
plate at a density of 1×103 cells/well. After the
indicated treatments for 48 h, 10 µl MTT solution (cat. no. ST316;
Beyotime Institute of Biotechnology) was added to the cells at 37°C
for 4 h. The medium containing MTT was then removed and 200 µl
dimethyl sulfoxide (DMSO; cat. no. ST1276; Beyotime Institute of
Biotechnology) was added into each well. Following brief slight
shaking, the optical density (OD) value of each well was measured
at a wavelength of 490 nm with a microplate reader (Molecular
Devices, LLC). Relative cell viability was calculated according to
the following formula: Cell relative viability (%)=OD (test)/OD
(control) ×100%.
Colony formation assay
The cells of MG-63 and U2OS cell lines were seeded
in a 6-well plate at a density of 100 cells/well. Next, the cells
of each well were treated with various concentrations of HGK (0,
10, 20 and 40 µmol/l), and the medium was changed every 2 days.
After 14 days, the cells were fixed with 4% paraformaldehyde (cat.
no. P0099; Beyotime Institute of Biotechnology) for 30 min at room
temperature. Subsequently, the cells were stained with 0.1% crystal
violet (cat. no. C0121; Beyotime Institute of Biotechnology) for 15
min at room temperature. Finally, cell clones were captured with a
camera (EOS 3000D; Canon, Inc.) and clones with a cell count >50
were recorded.
Wound healing assay
Treated or untreated MG-63 and U2OS cells were
seeded in a 6-well plate at a density of 2×105
cells/well. The wound healing assay was carried out when the cell
confluence reached 90%. The specific procedure of the assay was as
follows: Cells were scratched vertically with a pipette, and then
the scratched cells were washed with phosphate-buffered saline
(PBS; cat. no. C0221A; Beyotime Institute of Biotechnology).
Subsequently, the cells were cultured in a serum-free medium
containing HGK (10, 20 and 40 µmol/l). At 0 and 48 h, images of the
cells were captured with an inverted microscope at a magnification
of ×100, and the width of the scratch was measured. The relative
migration rate was calculated as follows: Relative migration
rate=(0 h scratch width-48 h scratch width)/0 h scratch width
×100%.
Transwell assay
The culture plate used in the Transwell assay was an
8-µm pore Transwell plate (product no. 3428, Corning, Inc.). Prior
to the initiation of assay, the upper chamber of the Transwell
plate was coated with 1:4 diluted BD Matrigel Matrix (cat. no.
356234; BD Biosciences) at 37°C for 4 h. The treated or untreated
MG-63 and U2OS cells (2×104) were resuspended in a
serum-free medium and then transferred to the upper chamber of the
Transwell plate while the corresponding dose of drug (10, 20 or 40
µmol/l HGK) was added. A medium containing 20% FBS was then placed
into the lower chamber of the Transwell plate. The Transwell plate
was cultured in an incubator for 48 h at 37°C. After the Transwell
upper chamber was removed, the invasive cells were fixed with 4%
paraformaldehyde for 15 min at 4°C and then stained with 0.1%
crystal violet staining solution at room temperature for 30 min.
Following staining, the invaded cells were counted under an
inverted microscope at a magnification of ×250 and the relevant
results were recorded.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from hFOB 1.19, MG-63 and U2OS cells was
isolated using TRIzol reagent (cat. no. 15596-018; Invitrogen;
Thermo Fisher Scientific, Inc.). The total RNA was subjected to
reverse transcription (PrimeScript™ RT reagent kit; cat.
no. RR037A; Takara Bio, Inc.) and the product was triply diluted
with double distilled water. RT-qPCR was then applied to detect the
expression levels of SOX9 and miR-320a using the TB
Green® Premix Ex Taq™ II kit (cat. no.
RR820A) and Mir-X miRNA qRT-PCR TB Green Kit (cat. no. 638314; both
from Takara Bio, Inc.), respectively, and an ABI
StepOnePlus™ system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primers used in this experiment were
provided by Sangon Biotech Co., Ltd. and are listed in Table I. GAPDH was the internal reference
of SOX9 and U6 was the internal reference of miR-320a. The reaction
system was as follows: 2 µl cDNA, 10 µl SYBR, 0.8 µl primers and
6.4 µl double distilled water. The thermocycling conditions were as
follows: 95°C for 30 sec, 40 cycles at 95°C for 3 sec and 60°C for
30 sec. The RT-qPCR data were analyzed using the 2−ΔΔCq
method (30).
 | Table I.Specific primer sequences for
RT-quantitative polymerase chain reaction. |
Table I.
Specific primer sequences for
RT-quantitative polymerase chain reaction.
| Gene | Primer
sequence | Species |
|---|
| SOX9 | Forward:
5′-AGCGAACGCACATCAAGAC-3′ | Human |
|
| Reverse:
5′-CTGTAGGCGATCTGTTGGGG-3′ |
|
| GAPDH | Forward:
5′-CCACTCCTCCACCTTTGAC-3′ | Human |
|
| Reverse:
5′-ACCCTGTTGCTGTAGCCA-3′ |
|
| miR-320a |
5′-CTCAACTGGTGTCGTGGAGTCG | Human |
|
|
GCAATTCAGTTGAGCGGAAGA-3′ (RT) |
|
|
| Forward:
5′-TCGGCAGGGCCTTCTCTTCCCG-3′ |
|
|
| Reverse:
5′-CAGTGCAGGGTCCGAGGT-3′ |
|
| U6 |
5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGC | Human |
|
|
ACCAGAGCCAACAAAATATGG-3′ (RT) |
|
|
| Forward:
5′-CTCGCTTCGGCAGCACA-3′ |
|
|
| Reverse:
5′-AACGCTTCACGAATTTGCGT-3′ |
|
Western blot analysis
Total proteins from treated or untreated MG-63 and
U2OS cells were extracted with the RIPA reagent (cat. no. P0013B)
containing 1% protease inhibitor (P1030; both from Beyotime
Institute of Biotechnology). Next, the total protein concentration
was determined by a BCA protein kit (cat. no. P0012; Beyotime
Institute of Biotechnology). Subsequently, equal amounts of total
protein (30 µg of protein per lane) were separated on a 12% gel
using SDS-PAGE (cat. no. P0012A; Beyotime Institute of
Biotechnology). Following electrophoresis the samples were
transferred to a PVDF membrane (ISEQ00010/IPVH00010; Millipore;
Merck KGaA). The membrane was sealed with 5% skim milk (cat. no.
D8340; Beijing Solarbio Science & Technology Co., Ltd.) and
incubated with the primary antibodies at 4°C overnight, including
those against N-cadherin (product no. 14215; 1:1,000) and
E-cadherin (product no. 14472; 1:1,000; both from Cell Signaling
Technology, Inc.), vimentin (product code ab92547; 1:3,000), SOX9
(product code ab185966; 1:5,000) and GAPDH (product code ab8245;
1:5,000; all from Abcam). The following day, the membrane was
incubated with the secondary antibodies: Goat anti-mouse IgG
H&L (HRP) (product code ab6789; 1:5,000) and goat anti-rabbit
IgG H&L (HRP) (product code ab6721; 1:10,000; both from Abcam)
at room temperature for 1 h. The bands were visualized using
enhanced chemiluminescence (ECL) solution (cat. no. WBKLS0500;
Millipore; Merck KGaA) and BioRad Gel Imager (Bio-Rad Laboratories,
Inc.). The intensity of each band was quantified using Image J
1.50i software (National Institutes of Health).
Cell transfection
The cells were seeded in a 6-well plate at a density
of 2×105 cells/well. When the cell confluence reached
~80%, the cell transfection was started. MiR-320a mimic (product
no. miR10000510-1-5; sense, 5′-AAAAGCUGGGUUGAGAGGGCGA-3′ and
antisense, 5′-GCCCUCUCAACCCAGCUUUUUU-3′), miR-320a inhibitor
(product no. miR20000510-1-5; 5′-UCGCCCUCUCAACCCAGCUUUU-3′), mimic
control (product no. miR1N0000001-1-10; sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′) and inhibitor control (product no.
miR2N0000001-1-10; 5′-CAGUACUUUUGUGUAGUACAA-3′) were all obtained
from Guangzhou RiboBio Co., Ltd. The overexpressed plasmid of SOX9
was constructed via inserting the sequence of SOX9 into the pcDNA
3.1 vector, with the empty vector of pcDNA 3.1 as the negative
control (NC). The design and construction of the overexpressed
plasmid of SOX9 were completed by Shanghai GenePharma Co., Ltd. The
transfection reagent Lipofectamine 2000 (cat. no. 11668-027) used
in this experiment was purchased from Invitrogen; Thermo Fisher
Scientific, Inc. and cell transfection was performed according to
the manufacturer's instructions. Specifically, 10 µl transfection
reagent and 4 µg miR-320a mimic, inhibitor, overexpressed plasmid
of SOX9 or their control were diluted with 250 µl Opti-MEM (cat.
no. 31985-070; Gibco; Thermo Fisher Scientific, Inc.),
respectively. After standing for 5 min, the mixtures were remixed.
After standing again for 20 min, the corresponding cells were added
for transfection for 48 h at 37°C. At 48 h post-transfection,
RT-qPCR was used to measure the transfection efficiency.
Bioinformatic analyses and dual
luciferase reporter assay
The targeted binding sites of miR-320a and SOX9 were
predicted by starBase v2.0 (https://starbase.sysu.edu.cn/starbase2/index.php).
The 3′-untranslated region (UTR) sequence of SOX9 gene was obtained
from NCBI (https://www.ncbi.nlm.nih.gov/). The SOX9 wild-type
sequence (5′-gagaagcauuugguaAGCUUUa-3′) and the mutant sequence
(the mutant site was the miR-320a-binding region)
(5′-gagaagcauuugguaUACACUa-3′) were then constructed into the
pmirGLO plasmid (cat. no. E1330; Promega Corporation), named
SOX9-WT and SOX9-MUT. Afterwards, the SOX9-WT or SOX9-MUT plasmid
and miR-320a mimic or mimic control were co-transfected into MG63
and U2OS cells. Subsequently, cells were transfected using
Lipofectamine 2000 (cat. no. 11668-027; Invitrogen; Thermo Fisher
Scientific, Inc.) according to the method described above. After 24
h, the fluorescence intensity of cells in each group was detected
by Dual Luciferase Reporter gene detection system (cat. no. E1910;
Promega Corporation). Renilla luciferase activity was used to
normalize Firefly luciferase activity.
Xenograft assays
A total of 10 male, 6-week-old BALB/c nude mice
(Weitong Lihua Laboratory Animal Technology Co., Ltd.) were used to
study the antitumor activity of HGK against osteosarcoma. The
BALB/c nude mice were kept at 24–26°C and in 40–70% humidity, with
a 12-h light/dark cycle and free access to food and water. BALB/c
nude mice were divided into control and HGK groups (n=5, each
group), and the animals were inoculated subcutaneously in the right
flank with MG-63 cells (3×106) in 100 µl. Drug treatment
was started on day 10, where the nude mice in the HGK group were
intraperitoneally injected with 100 µl HGK (1.0 mg/kg body weight)
every two days, and the control group was treated with an equal
volume of PBS. The tumor volume was measured according to the
following formula: Tumor volume=length × width2/2. After
4 weeks, these nude mice were sacrificed by cervical dislocation
following anesthesia with an intraperitoneal injection of sodium
pentobarbital (60 mg/kg), and then the tumors were photographed,
and the weight was measured. The maximum allowed tumor size did not
exceed 1,000 mm3. The study was approved by the Animal
Ethics Committee of Nanfang Hospital (Guangzhou, China; approval
no. NFYY-2021-121).
Statistical analysis
GraphPad Prism 8.0 (GraphPad Software, Inc.) was
employed for statistical analysis. Each experiment was repeated
three times. Data are presented as the mean ± standard deviation.
Unpaired t-test was utilized for comparison between two groups,
while one-way analysis of variance (ANOVA) and Tukey's post hoc
test were used for comparison among multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
HGK suppresses the proliferation,
migration and invasion of osteosarcoma cells
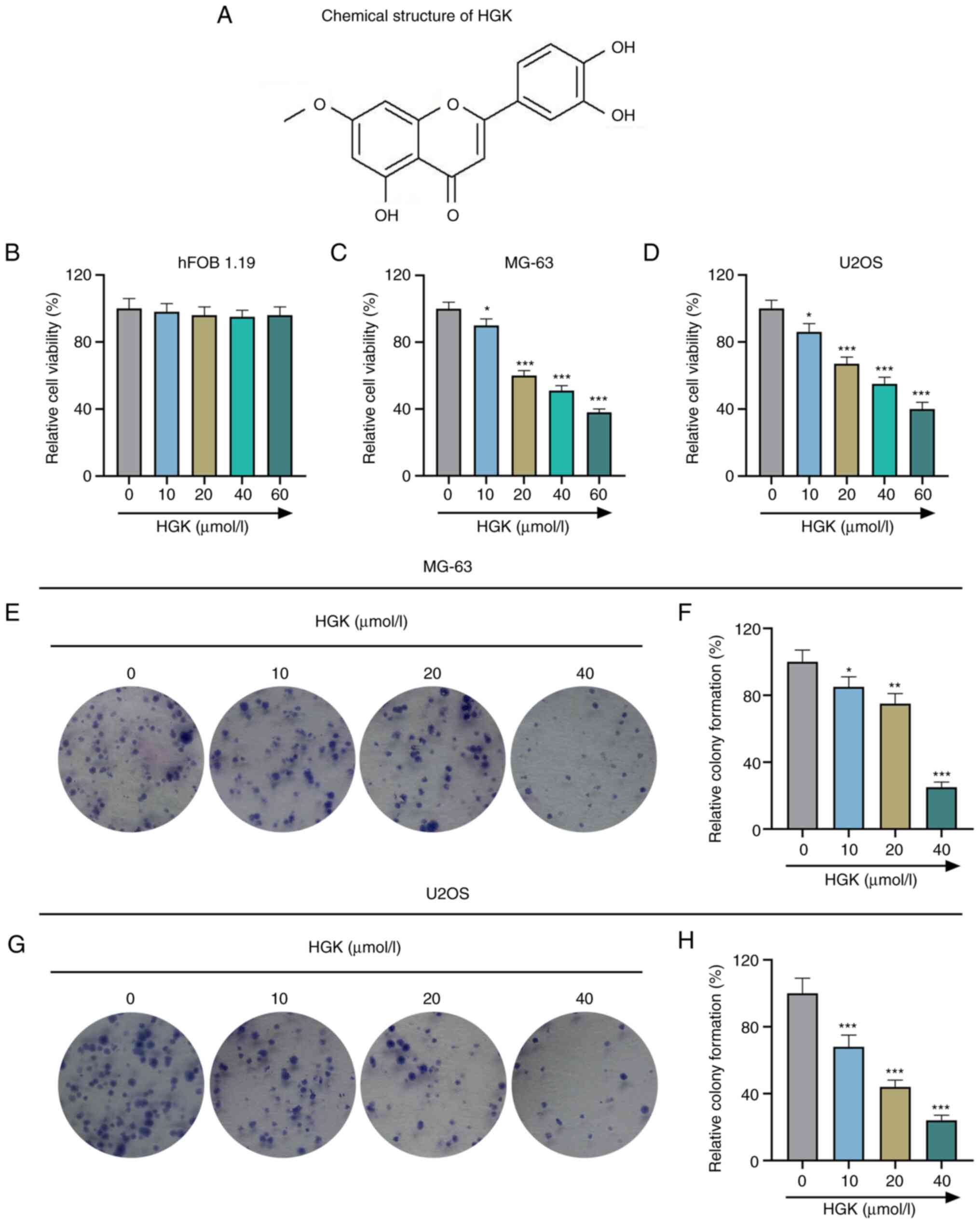
The chemical structure formula of HGK is presented
in Fig. 1A. In order to test the
safety of HGK, hFOB1.19 (an immortalized human fetal osteoblastic
cell line) was first treated with various concentrations of HGK (0,
10, 20, 40 and 60 µmol/l), and the results revealed that HGK had no
significant effect on hFOB1.19 cells (P>0.05; Fig. 1B). This suggested that HGK within
a concentration of 60 µmol/l exerted no toxic effect on normal
osteoblasts. Similarly, osteosarcoma cells MG-63 and U2OS were
treated with various concentrations of HGK as well. As demonstrated
in Fig. 1C and D, HGK decreased
the viability of MG-63 and U2OS cells in a concentration-dependent
manner as compared with the control group (P<0.05 and
P<0.001). From the results, it was determined that the
half-maximal lethal concentration of HGK was ≤40 µmol/l, thus, the
concentrations of 10, 20 and 40 µmol/l were selected for the
subsequent experiments. The effects of HGK on the proliferation,
migration and invasion of MG-63 and U2OS cells were then also
evaluated. Colony formation assay revealed that the cell
proliferation in the HGK groups was reduced compared with the
control group (Fig. 1E-H;
P<0.05, P<0.01 and P<0.001). In addition, the wound
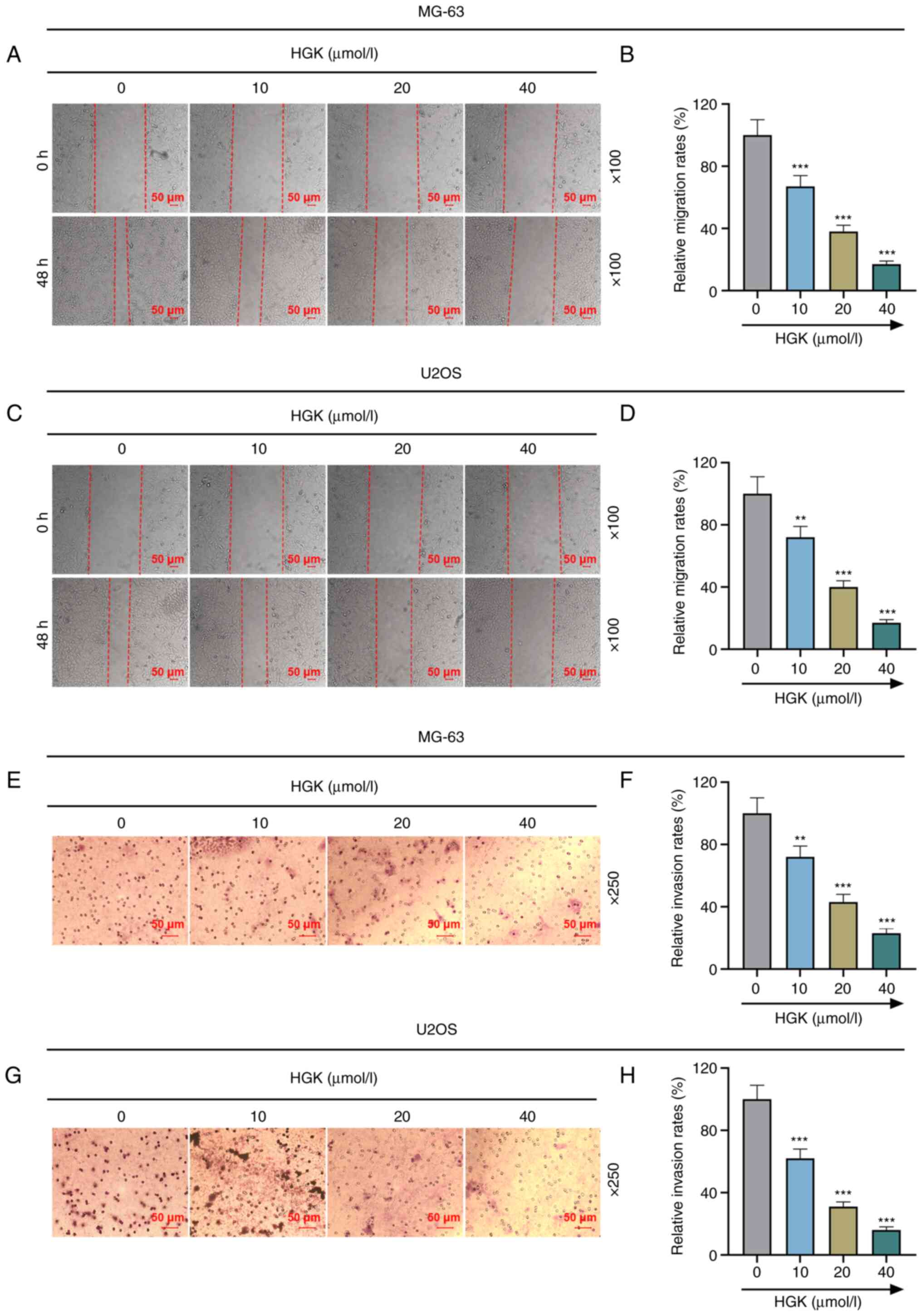
healing assay demonstrated that the migration of cells in the HGK
groups was diminished compared with the control group (Fig. 2A-D; P<0.01 and P<0.001).
Moreover, Transwell assays revealed that the invasion of cells in
the HGK groups was decreased compared with the control group
(Fig. 2E-H; P<0.01 and
P<0.001). All these results indicated that HGK inhibited cell
proliferation, migration and invasion in a concentration-dependent
manner.
HGK inhibits the proliferation,
migration and invasion of osteosarcoma cells by promoting the
expression of miR-320a
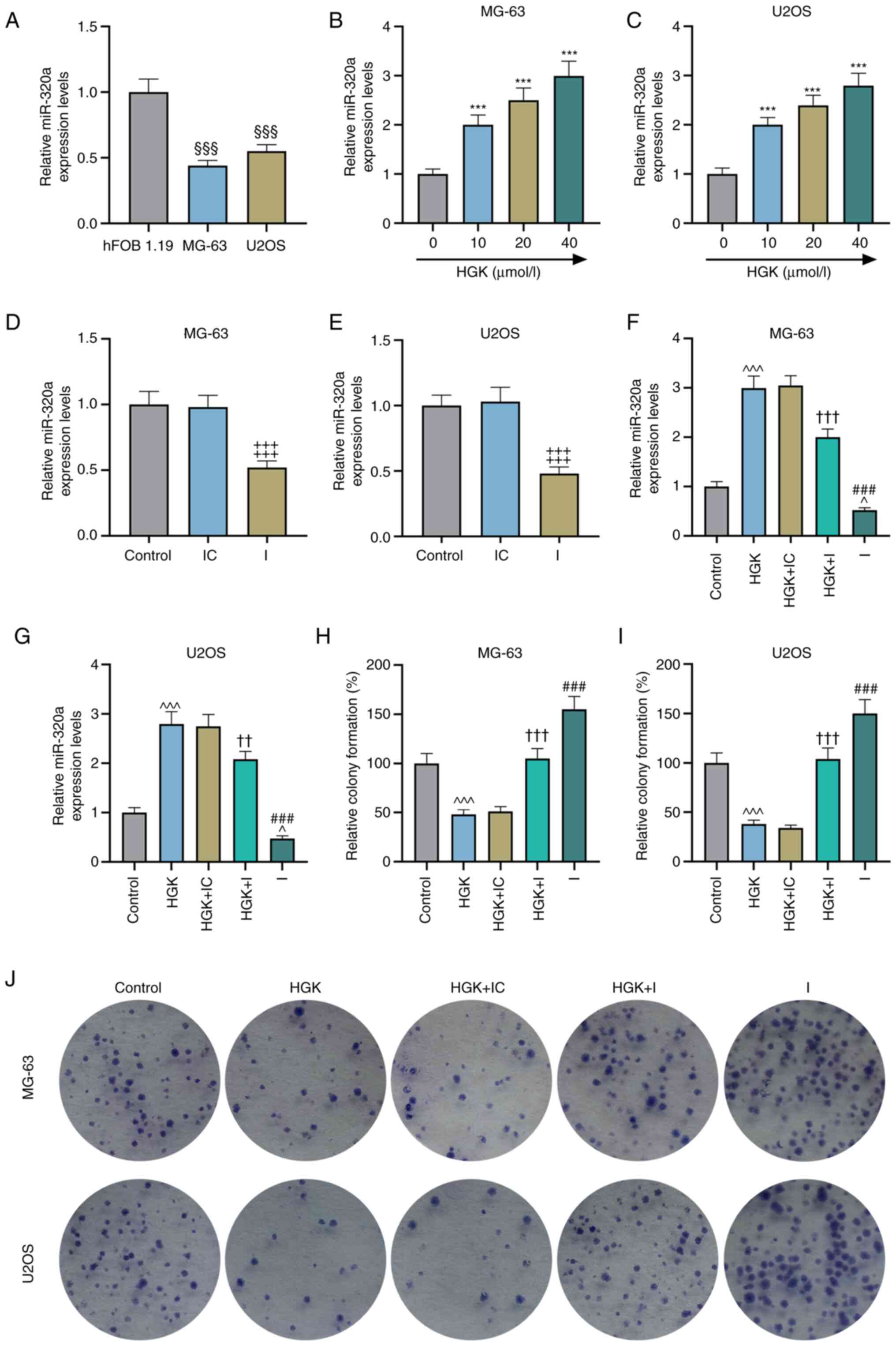
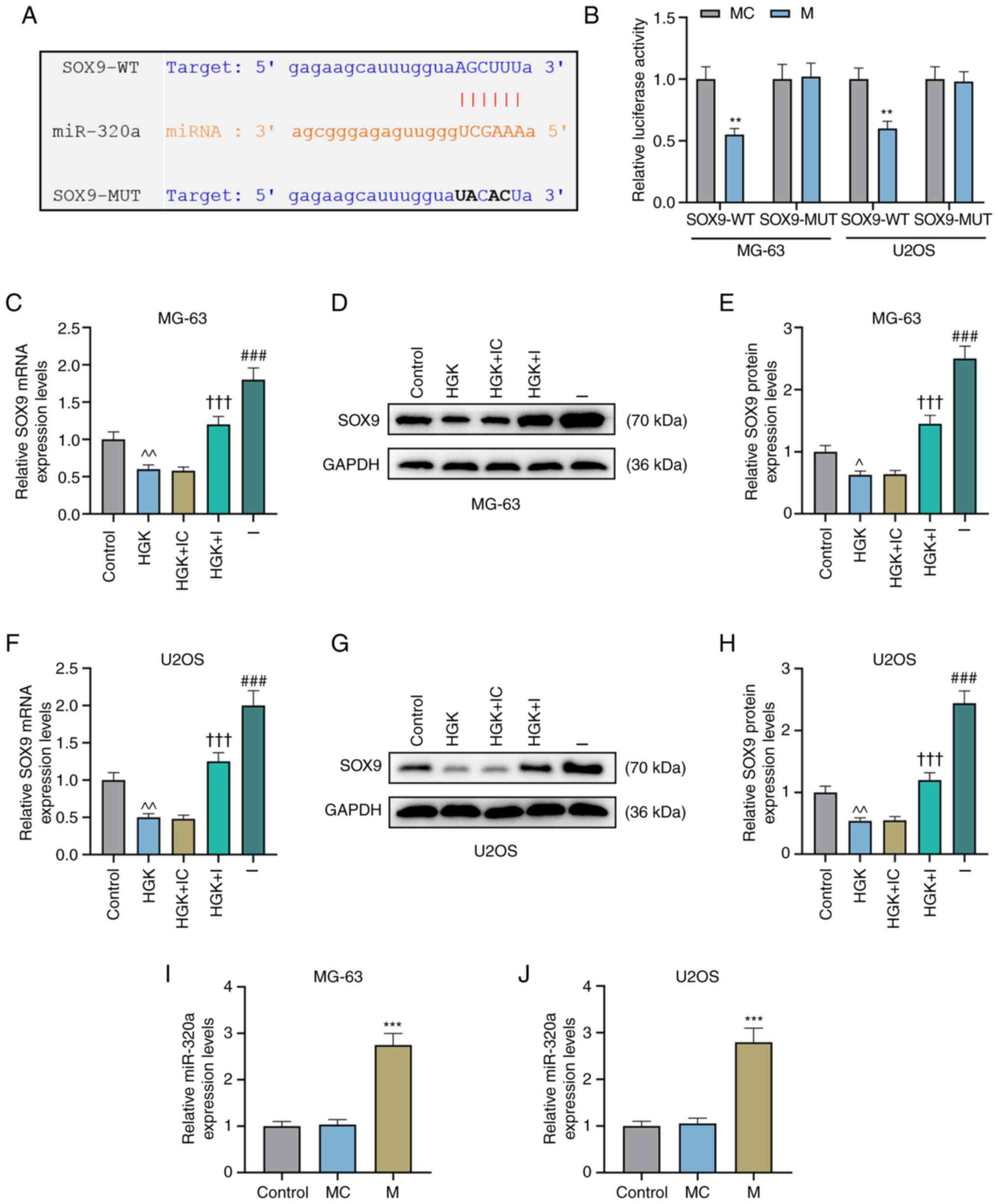
The expression of miR-320a in osteosarcoma cells was
lower than that in osteoblasts (Fig.
3A; P<0.001). Further detection revealed that HGK could
promote the expression of miR-320a in MG-63 and U2OS cells in a
concentration-dependent manner (Fig.
3B and C; P<0.001). To verify the effects of HGK and
miR-320a on osteosarcoma cells, a miR-320a inhibitor was used to
decrease the expression of miR-320a. The transfection efficiency of
miR-320a inhibitor is presented in Fig. 3D and E (P<0.001). Moreover, it
was also determined that miR-320a inhibitor could reverse the
promotive effect of HGK on miR-320a expression (Fig. 3F and G; P<0.01 and P<0.001).
Next, the effects of HGK and miR-320a inhibitor on proliferation,
migration and invasion of MG-63 and U2OS cells were assessed. The
results of the colony formation assays suggested that the
proliferation of cells in the miR-320a inhibitor group was
increased compared with the control group (Fig. 3H-J; P<0.001). Moreover,
miR-320a inhibitor could reverse the inhibitory effect of HGK on
cell proliferation (Fig. 3H-J;
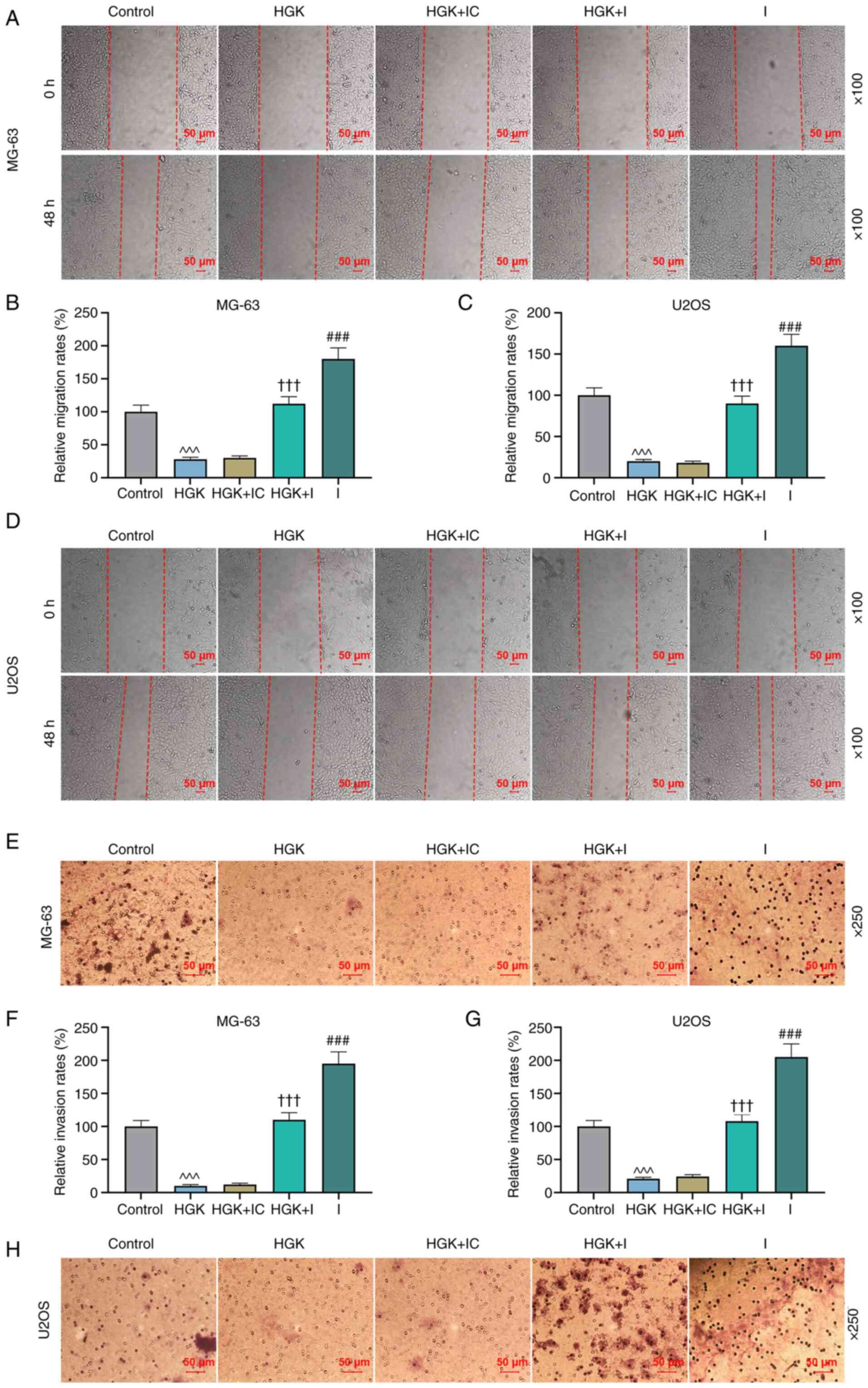
P<0.001). Wound healing assay revealed that cell migration in
the miR-320a inhibitor group was increased compared with the
control group (Fig. 4A-D;
P<0.001). Similarly, miR-320a inhibitor could reverse the
inhibitory effect of HGK on the cell migration (Fig. 4A-D; P<0.001). The Transwell
assay demonstrated that cell invasion in the miR-320a inhibitor
group was promoted compared with the control group (Fig. 4E-H; P<0.001). In addition,
miR-320a inhibitor could also reverse the effect of HGK on cell
invasion (Fig. 4E-H;
P<0.001).
HGK inhibits the expression of SOX9 by
promoting miR-320a expression
The targeted binding sites of miR-320a and SOX9 were
first predicted by starBase v2.0 (Fig. 5A). Subsequently, a dual-luciferase
reporter assay revealed that the fluorescence intensity of cells in
the SOX9-WT + miR-320a mimic group was significantly lower than
that in the mimic control group (Fig.
5B; P<0.01), while the fluorescence intensity in the
SOX9-MUT + miR-320a mimic group displayed no significant change
compared to that in the mimic control group (Fig. 5B). This indicated that miR-320a
indeed targeted SOX9. Further study demonstrated that the
expression of SOX9 was depleted in the HGK group compared with the
control group (Fig. 5C-H;
P<0.05 and P<0.01). However, miR-320a inhibitor could abolish
the inhibitory effect of HGK on SOX9 expression (Fig. 5C-H; P<0.001).
MiR-320a inhibits the migration,
invasion and epithelial-mesenchymal transition (EMT)-related
protein expression levels of osteosarcoma cells by inhibiting the
expression of SOX9
In order to further assess the effect of the
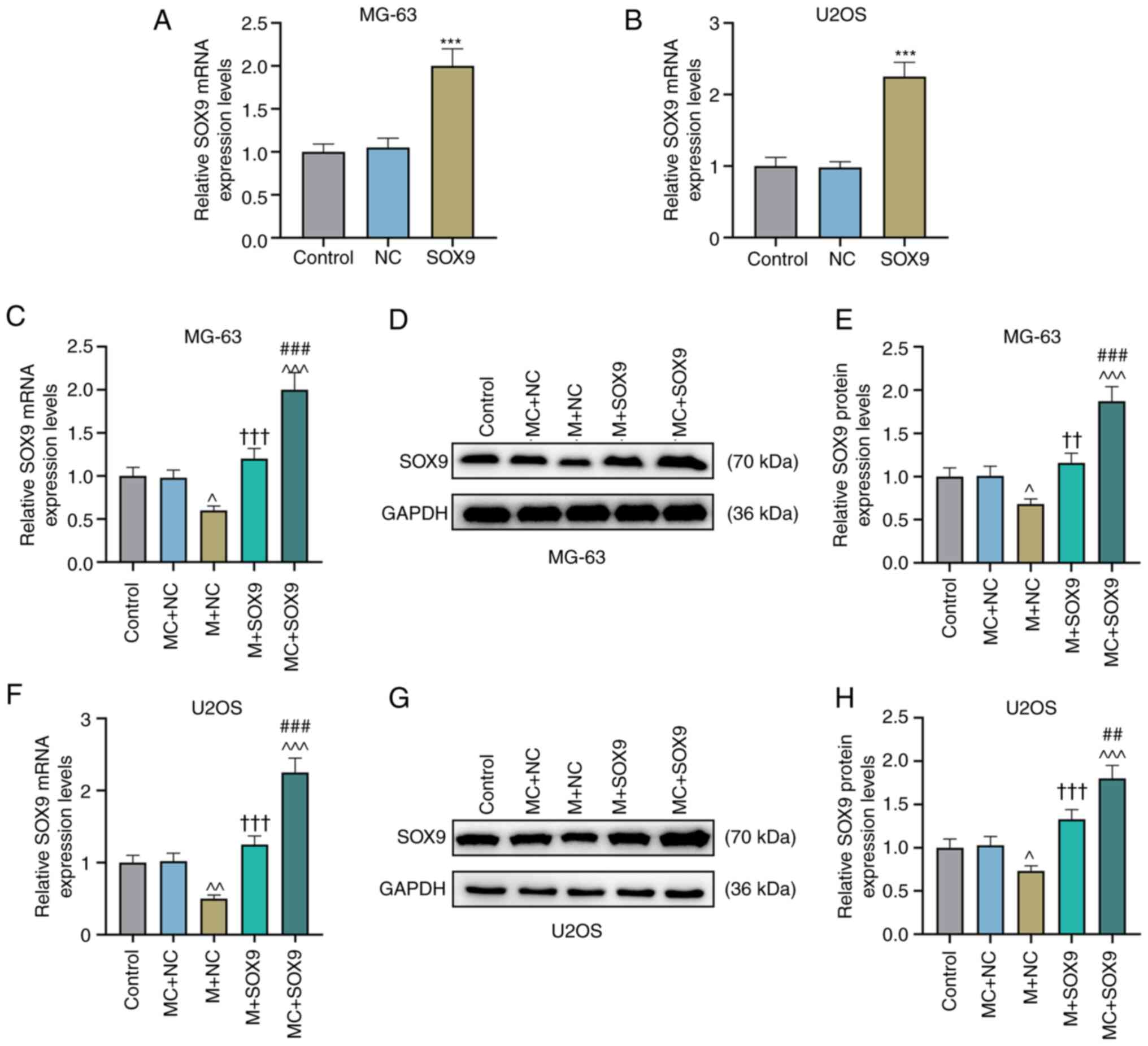
miR-320a/SOX9 axis on osteosarcoma, miR-320a mimic was used to
promote the expression of miR-320a (Fig. 5I and J; P<0.001). Concurrently,
SOX9 overexpressed plasmid was also employed to upregulate the
expression of SOX9 (Fig. 6A and
B; P<0.001). It was then determined that the expression of
SOX9 was inhibited in the miR-320a mimic + NC group as compared
with the MC + NC group (Fig.
6C-H; P<0.05 and P<0.01). Subsequently, the effect of
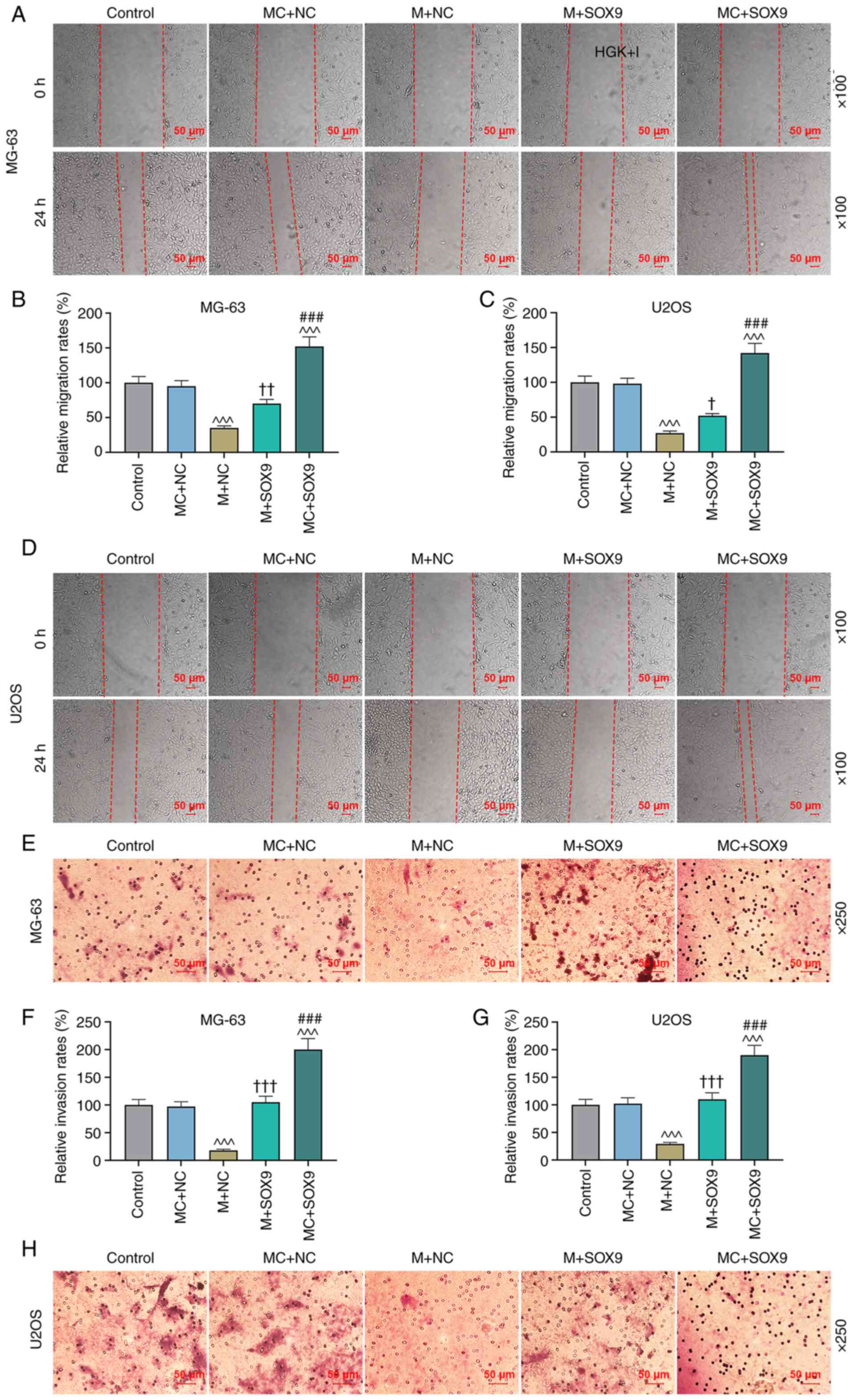
miR-320a/SOX9 on the migration and invasion of osteosarcoma cells
was examined. As demonstrated in Fig.
7A-D, the migration of MG-63 and U2OS cells was enhanced in the
MC + SOX9 group compared with the MC + NC group (P<0.05,
P<0.01 and P<0.001). In addition, SOX9 could reverse the
effect of miR-320a mimic on the migration of MG-63 and U2OS cells
(Fig. 7A-D; P<0.05 and
P<0.01). According to Fig.
7E-H, the invasion of MG-63 and U2OS cells was promoted in MC +
SOX9 group, compared with the MC + NC group (P<0.001). In
addition, SOX9 could reverse the effect of miR-320a mimic on the
invasion of MG-63 and U2OS cells (Fig. 7E-H; P<0.001). In order to
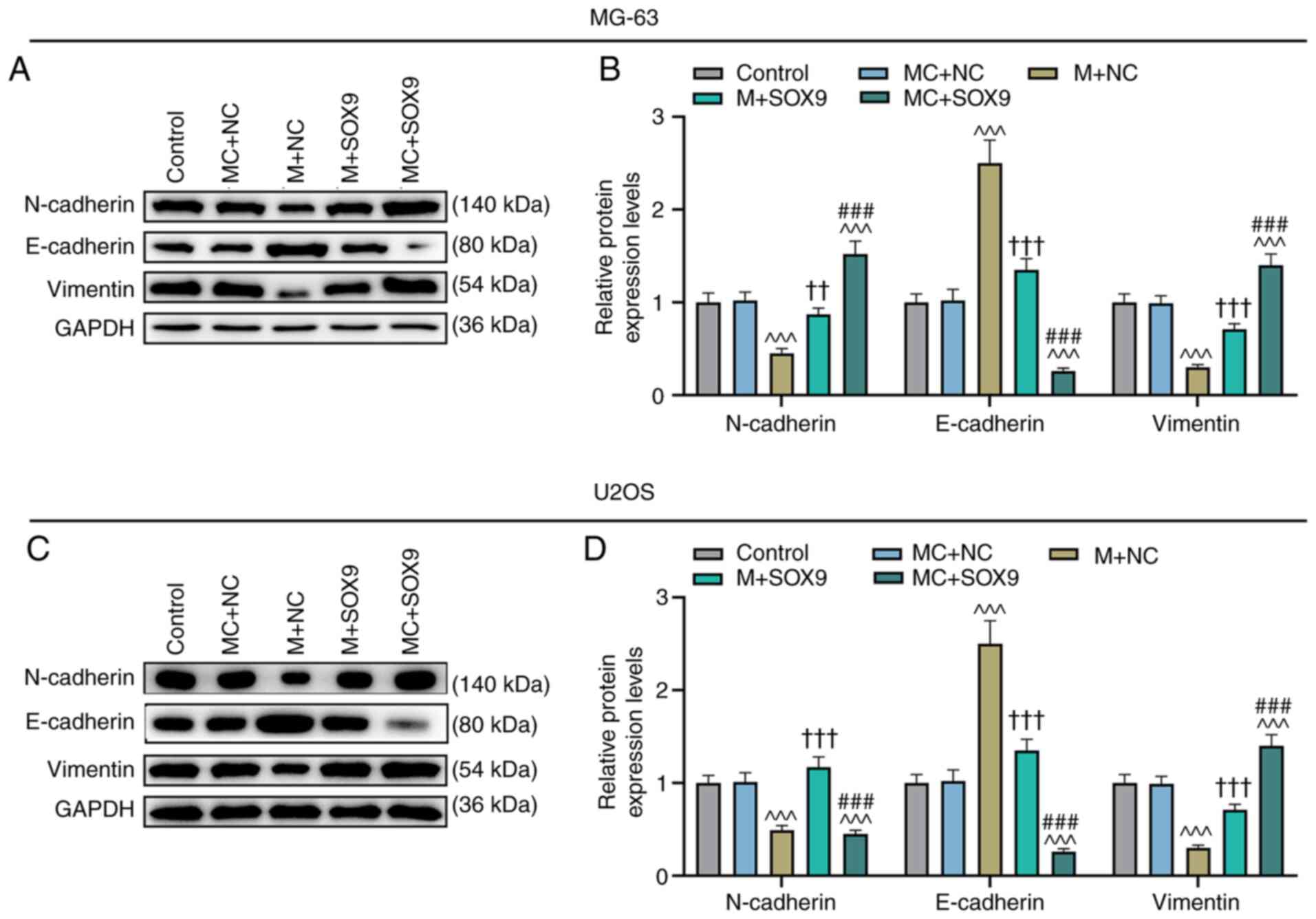
further confirm the effects of the miR-320a/SOX9 axis on the
migration and invasion of cells, EMT-related proteins were also
detected. The results revealed that miR-320a mimic could increase
the expression of E-cadherin while diminishing the expression
levels of N-cadherin and vimentin, compared with those in the MC +
NC group (Fig. 8A-D; P<0.001).
The effects of SOX9 were the opposite of those obtained with the
miR-320a mimic (Fig. 8A-D;
P<0.001). Similarly, upregulation of SOX9 could also reverse the
effects of miR-320a mimic on the expression levels of E-cadherin,
N-cadherin and vimentin (Fig.
8A-D; P<0.01 and P<0.001).
HGK inhibits the osteosarcoma tumor
growth of nude mice in vivo
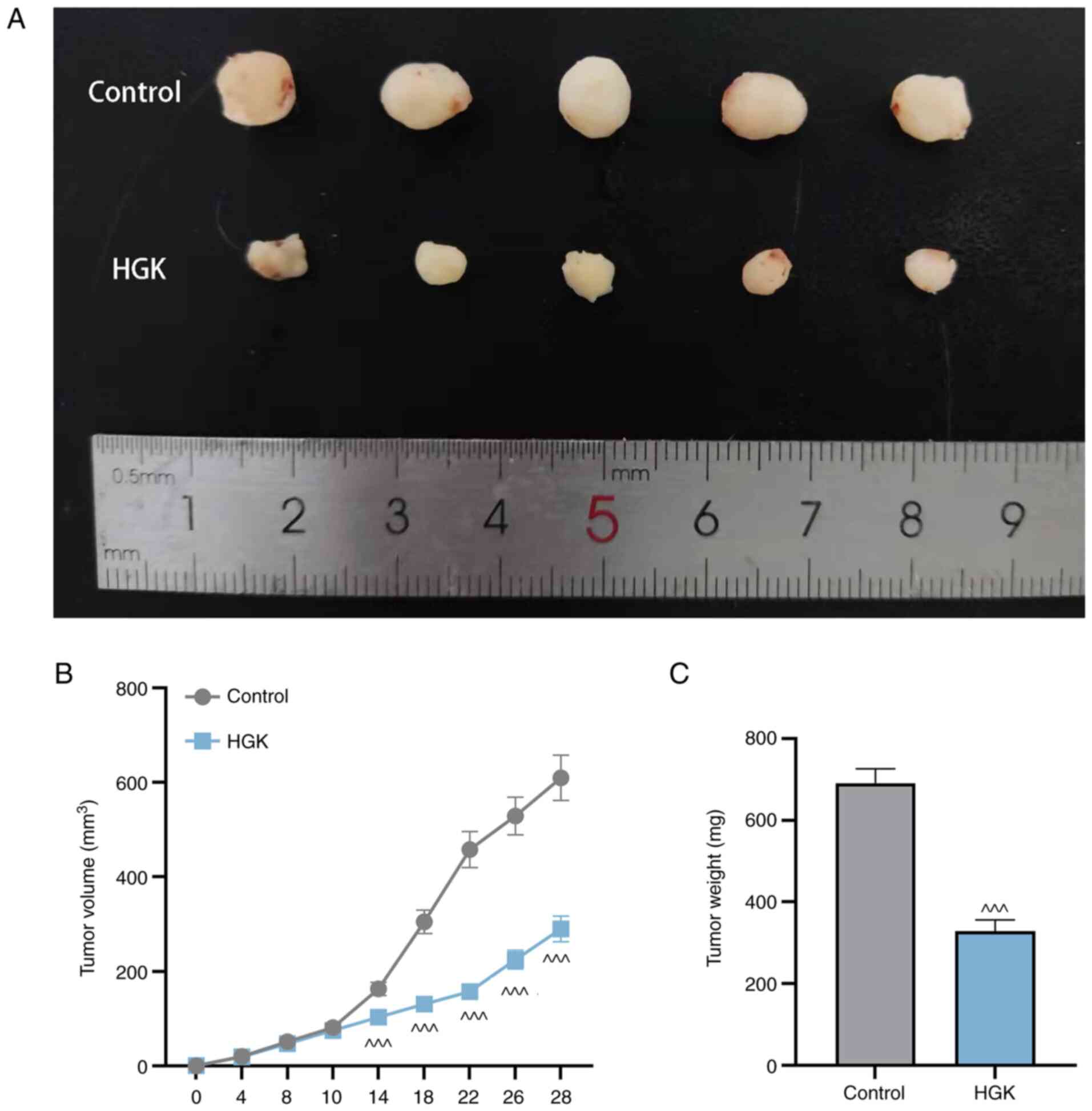
A xenograft mouse model was constructed in the
present study to evaluate the antitumor activity of HGK in vivo. As
revealed in Fig. 9A-C, HGK
inhibited the tumor volume and weight in the HGK group compared
with the control group.
Discussion
The medicinal value of Daphne genkwa dated back to
ancient times (31). Daphne
genkwa is often used to treat edema and make expectoration easy
(32). As one of the active
ingredients of Daphne genkwa, HGK has also been identified to
possess powerful biological functions in the treatment of assorted
diseases, especially its antitumor effect. For example, Huang et al
indicated that HGK inhibits the invasion and migration of oral
squamous cell cancer cells by downregulating the expression level
of vimentin (33). Chen et al
(17) reported that HGK can
inhibit the expression of HDAC to induce the expression of tumor
suppressor p21, and promote the acetylation and activation of p53
and p65, thus inhibiting the growth, migration and invasion of
liver cancer cells and increasing the cell apoptosis. Notably, this
aforementioned study, revealed that the antitumor effect of HGK was
mainly demonstrated through the inhibition of the migration and
invasion of tumor cells. It is important to note that the primary
cause of mortality for osteosarcoma is pulmonary metastasis, and
current chemotherapy regimens appear to be ineffective against
metastasis of osteosarcoma (34).
The outstanding ability of HGK to suppress migration and invasion
makes it a noteworthy potential drug for our research, and
indicates that it may be a potential drug for the treatment of
osteosarcoma. In the present study, it was revealed that HGK did
not affect the viability of normal human osteoblasts, indicating
that the safety of HGK was reliable. In addition, further study
revealed that HGK could reduce the proliferation, migration and
invasion of osteosarcoma cells.
In our subsequent study, it was determined that HGK
could promote the expression of miR-320a in osteosarcoma cells. In
fact, the role of miR-320a in osteosarcoma has been extensively
studied, involving doxorubicin resistance, and its effect on
proliferation, migration, and invasion of osteosarcoma cells
(23,24,35). In addition, it has been reported
that overexpression of miR-320a promotes stress oxidation levels,
while reducing the viability, proliferation and mineralization
capacities of osteosarcoma cells (36). A previous study even proposed
miR-320a as a possible biomarker for osteosarcoma (37). Therefore, it can be theorized that
the role of HGK in osteosarcoma may be realized by regulating
miR-320a. Notably, a recent study by Chou et al revealed that HGK
can inhibit tumor progression by promoting the expression of
miR-320a in lung cancer (11).
Their research adds to the credibility of our theory. In order to
determine the association between miR-320a and HGK, osteosarcoma
cells were treated with HGK and an increased expression of miR-320a
was detected. This suggested that HGK had a regulatory effect on
miR-320a, but whether it further affected the migration and
invasion of osteosarcoma needed to be further explored. MiR-320a
inhibitor was used to decrease miR-320a expression, and the
aforementioned conjecture was verified by miR-320a inhibitor. The
results clearly revealed that HGK-inhibited migration and invasion
of osteosarcoma cells were partially counteracted by miR-320a
inhibitor.
The starBase database is commonly used to predict
the downstream target molecules and targeted binding sites of
miRNAs (38). In the present
study, the targeted bindings of miR-320a and SOX9 were predicted
through starBase database. This result was also confirmed by dual
luciferase reporter assay. Notably, SOX9 has been reported to be an
essential transcription factor for normal differentiation of
osteoblasts and also plays an important role in the progression of
osteosarcoma (27,29). In fact, the results of the present
study demonstrated that HGK could decrease the expression of SOX9.
In addition, miR-320a inhibitor could partially offset the effects
of HGK on SOX9 expression. This indicated that HGK can inhibit the
expression of SOX9 by promoting miR-320a expression. Further
exploration revealed that miR-320a alleviated the migration and
invasion abilities of osteosarcoma cells by inhibiting SOX9
expression. These results were further confirmed using western blot
analysis. Additionally, EMT has been demonstrated to be an
important process of migration and invasion, during which the
connexins (such as E-cadherin) are gradually decreased and the
expression levels of mesenchymal marker proteins (N-cadherin and
vimentin) are promoted in cells (39). The results of the present study
indicated that upregulation of miR-320a could promote the
expression of E-cadherin while inhibiting the expression levels of
N-cadherin and vimentin. However, overexpression of SOX9 could
reverse the regulatory effects of miR-320a on the expression of
E-cadherin, N-cadherin and vimentin. Moreover, our results also
confirmed that miR-320a decreased the cell migration and invasion
of osteosarcoma cells by inhibiting the expression of SOX9. In
addition, similar to a previous study (11), the present study revealed that HGK
had no significant effect on hFOB1.19 cells, while inhibiting the
viability of osteosarcoma cells. This suggested that HGK
selectively killed tumor cells without significant toxicity to
normal cells. The mechanism revealing how HGK could selectively
kill tumor cells remains unclear and needs to be further
explored.
Collectively, the present experiments demonstrated
that HGK could attenuate the proliferation, migration and invasion
of osteosarcoma cells by regulating the miR-320a/SOX9 axis.
Unfortunately, our study was only conducted in vitro, and further
investigation in vivo and clinical trials are required. HGK is a
potential drug for the treatment of osteosarcoma and is expected to
provide a new direction for the clinical research on
osteosarcoma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
XD made substantial contributions to conception and
design of the study. YW, HZ and GA acquired, analyzed and
interpreted the data. XD drafted the manuscript and critically
revised it for important intellectual content. XD and GA confirm
the authenticity of all the raw data. All authors read and approved
the final version for the manuscript to be published and agree to
be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Animal Ethics
Committee of Nanfang Hospital (Guangzhou, China; approval no.
NFYY-2021-121). Animal experiments were performed in Nanfang
Hospital, which has project co-operation and is linked with the
Traditional Chinese Medicine Hospital of Binzhou (Binzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mannerström B, Kornilov R, Abu-Shahba AG,
Chowdhury IM, Sinha S, Seppänen-Kaijansinkko R and Kaur S:
Epigenetic alterations in mesenchymal stem cells by
osteosarcoma-derived extracellular vesicles. Epigenetics.
14:352–364. 2019. View Article : Google Scholar
|
|
2
|
Brown HK, Tellez-Gabriel M and Heymann D:
Cancer stem cells in osteosarcoma. Cancer Lett. 386:189–195. 2017.
View Article : Google Scholar
|
|
3
|
Gross AC, Cam H, Phelps DA, Saraf AJ, Bid
HK, Cam M, London CA, Winget SA, Arnold MA, Brandolini L, et al:
IL-6 and CXCL8 mediate osteosarcoma-lung interactions critical to
metastasis. JCI Insight. 3:e997912018. View Article : Google Scholar
|
|
4
|
Meazza C and Scanagatta P: Metastatic
osteosarcoma: A challenging multidisciplinary treatment. Expert Rev
Anticancer Ther. 16:543–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haghiralsadat F, Amoabediny G,
Naderinezhad S, Zandieh-Doulabi B, Forouzanfar T and Helder MN:
Codelivery of doxorubicin and JIP1 siRNA with novel EphA2-targeted
PEGylated cationic nanoliposomes to overcome osteosarcoma multidrug
resistance. Int J Nanomedicine. 13:3853–3866. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sreeja S, Parameshwar R, Varma PRH and
Sailaja GS: Hierarchically porous osteoinductive Poly(hydroxyethyl
methacrylate-co-methyl methacrylate) scaffold with sustained
doxorubicin delivery for consolidated osteosarcoma treatment and
bone defect repair. ACS Biomater Sci Eng. 7:701–717. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu M, Li H, Zhai D, Chang J, Chen S and Wu
C: Hierarchically porous nagelschmidtite bioceramic-silk scaffolds
for bone tissue engineering. J Mater Chem B. 3:3799–3809. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Torres AL, Gaspar VM, Serra IR, Diogo GS,
Fradique R, Silva AP and Correia IJ: Bioactive polymeric-ceramic
hybrid 3D scaffold for application in bone tissue regeneration.
Mater Sci Eng C Mater Biol Appl. 33:4460–4469. 2013. View Article : Google Scholar
|
|
10
|
Zhu C, He M, Sun D, Huang Y, Huang L, Du
M, Wang J, Wang J, Li Z, Hu B, et al: 3D-Printed multifunctional
polyetheretherketone bone scaffold for multimodal treatment of
osteosarcoma and osteomyelitis. ACS Appl Mater Interfaces.
13:47327–47340. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chou LF, Chen CY, Yang WH, Chen CC, Chang
JL, Leu YL, Liou MJ and Wang TH: Suppression of hepatocellular
carcinoma progression through FOXM1 and EMT inhibition via
hydroxygenkwanin-induced miR-320a expression. Biomolecules.
10:202019. View Article : Google Scholar
|
|
12
|
Chen YY, Tang YP, Shang EX, Zhu ZH, Tao
WW, Yu JG, Feng LM, Yang J, Wang J, Su SL, et al: Incompatibility
assessment of genkwa flos and glycyrrhizae radix et Rhizoma with
biochemical, histopathological and metabonomic approach. J
Ethnopharmacol. 229:222–232. 2019. View Article : Google Scholar
|
|
13
|
Li Y, Geng L, Liu Y, Chen M, Mu Q, Zhang
X, Zhang Z, Ren G and Liu C: Identification of three Daphne species
by DNA barcoding and HPLC fingerprint analysis. PLoS One.
13:e02017112018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Lan XY, Ji J, Zhang CF, Li F, Wang
CZ and Yuan CS: Anti-inflammatory and anti-angiogenic activities in
vitro of eight diterpenes from Daphne genkwa based on hierarchical
cluster and principal component analysis. J Nat Med. 72:675–685.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Chou G, Hseu Y, Yang H, Kwan H and
Yu Z: Isolation of anticancer constituents from flos genkwa (Daphne
genkwa Sieb.et Zucc.) through bioassay-guided procedures. Chem Cent
J. 7:1592013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li YN, Yin LH, Xu LN and Peng JY: A simple
and efficient protocol for large-scale preparation of three
flavonoids from the flower of Daphne genkwa by combination of
macroporous resin and counter-current chromatography. J Sep Sci.
33:2168–2175. 2010. View Article : Google Scholar
|
|
17
|
Chen CY, Chen CC, Chuang WY, Leu YL, Ueng
SH, Hsueh C, Yeh CT and Wang TH: Hydroxygenkwanin inhibits class I
HDAC expression and synergistically enhances the antitumor activity
of sorafenib in liver cancer cells. Front Oncol. 10:2162020.
View Article : Google Scholar
|
|
18
|
Wang Y, Xu YS, Yin LH, Xu LN, Peng JY,
Zhou H and Kang W: Synergistic anti-glioma effect of
hydroxygenkwanin and apigenin in vitro. Chem Biol Interact.
206:346–355. 2013. View Article : Google Scholar
|
|
19
|
Correia de Sousa M, Gjorgjieva M, Dolicka
D, Sobolewski C and Foti M: Deciphering miRNAs' action through
miRNA editing. Int J Mol Sci. 20:62492019. View Article : Google Scholar
|
|
20
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar
|
|
21
|
Wang S, Lv S, Guan Y, Gao G, Li J, Hei F
and Long C: Cardiopulmonary bypass techniques and clinical outcomes
in Beijing Fuwai Hospital: A brief clinical review. ASAIO J.
57:414–420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ram Kumar RM, Boro A and Fuchs B:
Involvement and clinical aspects of MicroRNA in osteosarcoma. Int J
Mol Sci. 17:8772016. View Article : Google Scholar
|
|
23
|
Zhou B, Li L, Li Y, Sun H and Zeng C: Long
noncoding RNA SNHG12 mediates doxorubicin resistance of
osteosarcoma via miR-320a/MCL1 axis. Biomed Pharmacother.
106:850–857. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang S, Zhu X, Ke Y, Xiao D, Liang C,
Chen J and Chang Y: LncRNA FTX inhibition restrains osteosarcoma
proliferation and migration via modulating miR-320a/TXNRD1. Cancer
Biol Ther. 21:379–387. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lefebvre V, Angelozzi M and Haseeb A: SOX9
in cartilage development and disease. Curr Opin Cell Biol.
61:39–47. 2019. View Article : Google Scholar
|
|
26
|
Zhang XD, Wang YN, Feng XY, Yang JY, Ge YY
and Kong WQ: Biological function of microRNA-30c/SOX9 in pediatric
osteosarcoma cell growth and metastasis. Eur Rev Med Pharmacol Sci.
22:70–78. 2018.
|
|
27
|
Zhang W, Wei L, Sheng W, Kang B, Wang D
and Zeng H: miR-1225-5p functions as a tumor suppressor in
osteosarcoma by targeting Sox9. DNA Cell Biol. 39:78–91. 2020.
View Article : Google Scholar
|
|
28
|
Ma L, Zhang L, Guo A, Liu LC, Yu F, Diao
N, Xu C and Wang D: Overexpression of FER1L4 promotes the apoptosis
and suppresses epithelial-mesenchymal transition and stemness
markers via activating PI3K/AKT signaling pathway in osteosarcoma
cells. Pathol Res Pract. 215:1524122019. View Article : Google Scholar
|
|
29
|
Qu H, Xue Y, Lian W, Wang C, He J, Fu Q,
Zhong L, Lin N, Lai L, Ye Z and Wang Q: Melatonin inhibits
osteosarcoma stem cells by suppressing SOX9-mediated signaling.
Life Sci. 207:253–264. 2018. View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kai H, Koine T, Baba M and Okuyama T:
Pharmacological effects of Daphne genkwa and Chinese medical
prescription, ‘Jyu-So-To’. Yakugaku Zasshi. 124:349–354. 2004.(In
Japanese). View Article : Google Scholar
|
|
32
|
Chen Y, Guo J, Tang Y, Wu L, Tao W, Qian Y
and Duan JA: Pharmacokinetic profile and metabolite identification
of yuanhuapine, a bioactive component in Daphne genkwa by
ultra-high performance liquid chromatography coupled with tandem
mass spectrometry. J Pharm Biomed Anal. 112:60–69. 2015. View Article : Google Scholar
|
|
33
|
Huang YC, Lee PC, Wang JJ and Hsu YC:
Anticancer effect and mechanism of hydroxygenkwanin in oral
squamous cell carcinoma. Front Oncol. 9:9112019. View Article : Google Scholar
|
|
34
|
Villanueva F, Araya H, Briceño P, Varela
N, Stevenson A, Jerez S, Tempio F, Chnaiderman J, Perez C,
Villarroel M, et al: The cancer-related transcription factor RUNX2
modulates expression and secretion of the matricellular protein
osteopontin in osteosarcoma cells to promote adhesion to
endothelial pulmonary cells and lung metastasis. J Cell Physiol.
234:13659–13679. 2019. View Article : Google Scholar
|
|
35
|
Li C, Zhang S, Qiu T, Wang Y, Ricketts DM
and Qi C: Upregulation of long non-coding RNA NNT-AS1 promotes
osteosarcoma progression by inhibiting the tumor suppressive
miR-320a. Cancer Biol Ther. 20:413–422. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De-Ugarte L, Balcells S, Guerri-Fernandez
R, Grinberg D, Diez-Perez A, Nogues X and Garcia-Giralt N: Effect
of the tumor suppressor miR-320a on viability and functionality of
human osteosarcoma cell lines compared to primary osteoblasts. Appl
Sci. 10:28522020. View Article : Google Scholar
|
|
37
|
Lian F, Cui Y, Zhou C, Gao K and Wu L:
Identification of a plasma four-microRNA panel as potential
noninvasive biomarker for osteosarcoma. PLoS One. 10:e01214992015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:(Database Issue). D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|