Introduction
Acute pancreatitis is an acute inflammatory process
of the pancreas caused by the intracellular activation of digestive
enzymes and autodigestion of the pancreas (1). Destruction of the pancreatic
parenchyma induces a rapid inflammatory process at the injury site
(2). During the early phase of
acute pancreatitis, pancreatic acinar cells produce tumor necrosis
factor-a (TNF-α), interleukin (IL)-6, and chemokine monocyte
chemoattractant protein-1 (MCP-1). These findings have been
observed in pancreatic acinar cells of patients with acute
pancreatitis (3). IL-6, a member
of the inflammatory cytokine family, is the primary inducer of
acute-phase protein responses during all types of injuries, and its
level is directly correlated with the severity of the injury.
Therefore, the severity of pancreatitis is indicated by the degree
and duration of IL-6 elevation (4,5).
In an experimental pancreatitis model, a
cholecystokinin (CCK) analog, cerulein, induced pancreatic
inflammation similar to human acute pancreatitis. Treatment with
high doses of cerulein increases the levels of serum digestive
enzymes (amylase and lipase) and cytokines in the pancreas
(6,7). Previously, we demonstrated that
cerulein induces the activation of Janus kinase (JAK) 2, signal
transducer and activator of transcription (STAT) 3, and IL-1β
expression, which were suppressed by treatment with the JAK
inhibitor AG490 in pancreatic acinar cells. In cerulein-stimulated
pancreatitis in rats, AG490 treatment inhibited pancreatic changes,
such as edema and inflammation, and reduced serum IL-6 levels
(8,9). Therefore, the Jak2/Stat3 pathway may
be the upstream signaling pathway for cytokine expression in the
pathogenesis of pancreatitis.
The JAK/STAT pathway mediates cytokine signal
transduction in the immune system and in other tissues (10). In pancreatic acinar cells,
cerulein binds to the G protein-coupled receptor CCK2 and activates
the JAK2/STAT3 pathway for cell proliferation (11). Subsequently, JAK/STAT activation
leads to the induction of suppressors of cytokine signaling (SOCS)
proteins, which suppress cytokine signaling (12). SOCS inhibits JAK activity and
negatively regulates immune cell response (13). Our previous study revealed that
ligands of peroxisome proliferator activated receptor-γ (PPAR-γ),
such as 15-deoxy-Δ12, 14-prostaglandin J2 (15d-PGJ2) and
troglitazone, and suppressor of cytokine signaling (SOCS) 3 inhibit
IL-6 expression by suppressing JAK2/STAT3 in cerulein-stimulated
pancreatic acinar cells (9).
Berlato et al reported that transfection of SOCS3 into mouse
macrophages decreased nitric oxide, TNF-α, and IL-6 levels
(14). Therefore, a relationship
exists between JAK2/STAT3, PPAR-γ, and SOCS3 in cells exposed to
cytokines or inflammatory stimuli.
PPAR-γ, a nuclear hormone receptor, regulates the
metabolism of fatty acids and glucose (15). It also contributes to the
inactivation of genes involved in inflammation (16). When PPAR-γ is activated by
ligands, dimerization with the retinoic acid X receptor (RXR) binds
to the peroxisome proliferator response element (PPRE) to induce
various target genes (17).
Rollins et al reported that PPAR-γ agonists (15d-PGJ2 or
troglitazone) significantly reduced the severity of pancreatitis in
mice by reducing serum amylase activity and the levels of
pro-inflammatory cytokines, such as IL-6 and TNF-α (18). Furthermore, PPAR-γ induces
inflammation regulation by SOCS3. Berger et al showed that
docosahexaenoic acid (DHA)-mediated PPAR-γ activation limits Th17
cell differentiation by inducing SOCS3 expression (19). DHA may bind to PPAR-γ and
transactivate the SOCS3 promoter to prevent the phosphorylation of
STAT3 (20). Therefore, SOCS3
might prevent JAK/STAT3 activation and decrease the expression of
inflammatory genes.
Lutein is a lipophilic oxygenated carotenoid present
in green leafy vegetables, fruits, and egg yolk. Since lutein
exerts antioxidant and anti-inflammatory effects, it suppresses
oxidative stress-mediated inflammatory diseases such as
neurodegenerative disorders, diabetic retinopathy, and colon
diseases (21). Dietary lutein
supplementation (50 mg/kg body weight) inhibits LPS-induced
elevation of splenic levels of IL-1 and PPAR-γ in chickens
(22). Additionally, lutein
protects against vancomycin-induced renal injury by upregulating
PPAR-γ (23).
In the present study, we investigated whether lutein
activates PPAR-γ and induces SOCS3 expression, thereby inhibiting
cerulein-stimulated activation of JAK2/STAT3 and IL-6 expression in
pancreatic acinar AR42J cells. To investigate the involvement of
PPAR-γ activation in IL-6 expression, cerulein-stimulated cells
were treated with the PPAR-γ antagonist GW9662 in the presence of
lutein or the PPAR-γ agonist troglitazone.
Materials and methods
Materials
Lutein, troglitazone, the PPAR-γ antagonist GW9662,
and cerulein were obtained from Sigma-Aldrich; Merck KGaA. Lutein,
troglitazone, and GW9662 were dissolved in DMSO. Cerulein was
dissolved in PBS containing 0.1% BSA (10−4 M). For each
experiment, the amount of vehicle DMSO was <0.1%.
Cell line and culture conditions
Rat pancreatic acinar AR42J cells (pancreatoma, ATCC
CRL 1492) were purchased from the American Type Culture Collection
(ATCC) and cultured as previously described (9).
Experimental protocol
To investigate the effect of lutein on PPAR-γ
activation and SOCS3 expression in unstimulated cells, AR42J cells
(8×105 cells/ml/well) were treated with 5 µM lutein for
1, 2, or 3 h. Activation of PPAR-γ was increased by lutein
treatment (peak at 1 h and still elevated at 2 h) in unstimulated
cells. The levels of SOCS3 were increased by lutein (elevated at 2
h and peaked at 3 h) in unstimulated cells. Therefore, to determine
the effective dose of lutein, the cells were treated with 1, 2, and
5 µM lutein for 1 h to assess its effect on PPAR-γ expression and
for 3 h to measure SOCS3 expression levels.
To determine the effect of lutein on
cerulein-stimulated cells, AR42J cells were treated with 5 µM
lutein for 2 h and then stimulated with cerulein (10−8
M) for 1 h (JAK2/STAT3 activation), 6 h (IL-6 mRNA expression), and
24 h (IL-6 protein expression) based on our previous studies
(8,21,24). A 1 h incubation time for
cerulein-induced JAK2/STAT3 activation was adapted from our
previous study (8,24), while incubation times for IL-6
mRNA and protein levels were adapted from the study by Ahn and Kim
(21).
To assess whether PPAR-γ and SOCS3 contribute to the
inhibitory effect of lutein on cerulein-stimulated IL-6 expression,
cells were pretreated with 10 µM GW9662 in the presence of 5 µM
lutein or 40 µM troglitazone for 2 h and stimulated with cerulein
for 1 h (JAK2/STAT3 activation), 6 h (IL-6 mRNA), and 24 h (IL-6
protein expression).
Real-time polymerase chain reaction
(PCR) analysis
IL-6 mRNA expression levels were determined using a
method described previously (25). The IL-6 (accession number M26745)
primers 5′-GCCCTTCAGGAACAGCTATGA-3′ (forward primer) and
5′-TGTCAACAACATCAGTCCCAAGA-3′ (reverse primer) were used to
generate a 242 bp PCR product. For β-actin (accession number
XM_032887061.1), the forward primer used was
5′-ACCAACTGGGACGATATGGAG-3′ and the reverse primer was
5′-GTCAGGATCTTCATGAGGTAGTC-3′, which gives a 353 bp PCR product.
β-actin, a reference gene, was amplified in the same reaction.
Enzyme-linked immunosorbent assay
IL-6 level in the culture medium was measured using
an enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen;
Thermo Fisher Scientific, Inc.) (25).
Western blot analysis
Preparation of whole-cell, cytosolic, and nuclear
extracts, as well as western blot analysis were performed as
previously described (26).
Briefly, the extracts (6–40 µg protein/per lane) were loaded onto
8–10% SDS polyacrylamide gels and separated by electrophoresis. The
following antibodies were used: p-JAK2 (#3771), p-STAT3 (#9131),
JAK2 (#3230) (Cell Signaling Technology), STAT3 (sc-483), PPAR-γ
(sc-7273), aldolase A (sc-12059), histone H3 (sc-10809), SOCS3
(sc-518020), and actin (sc-1615; Santa Cruz Biotechnology). Primary
antibodies were detected with horseradish peroxidase-conjugated
secondary antibodies and visualized using the enhanced
chemiluminescence detection system (Santa Cruz Biotechnology) and
BioMax MR film (Kodak) using the enhanced chemiluminescence
detection system (Santa Cruz Biotechnology). Actin, aldolase A, and
histone H3 served as controls in whole-cell, cytosolic, and nuclear
extracts, respectively. The levels of PPAR-γ and SOCS3 in the
whole-cell extracts were compared to those of actin. The levels of
PPAR-γ in the cytosolic and nuclear extracts were compared to those
of aldolase A and histone H3, respectively. The phospho-specific
forms of JAK2 and STAT3 were compared with those of total JAK2 and
STAT3. The intensity of each protein band was quantified using
ImageJ software (National Institutes of Health). The data represent
the mean ± standard error (SE) from three immunoblots. For the
relative protein expression, the relative density of the protein
band was normalized to actin, aldolase A, histone 3, total JAK2, or
total STAT3.
Statistical analysis
Values are represented as mean ± SE (n=12 per
group). Statistical analysis was performed using analysis of
variance (ANOVA), followed by individual comparisons using Tukey's
post-hoc test. Differences were considered significant at a P-value
of ≤0.05.
Results
Lutein promotes expression and nuclear
translocation of PPAR-γ and expression of SOCS3 in unstimulated
AR42J cells
First, we determined the effect of lutein on PPAR-γ
activation and SOCS expression in the unstimulated cells. Cells
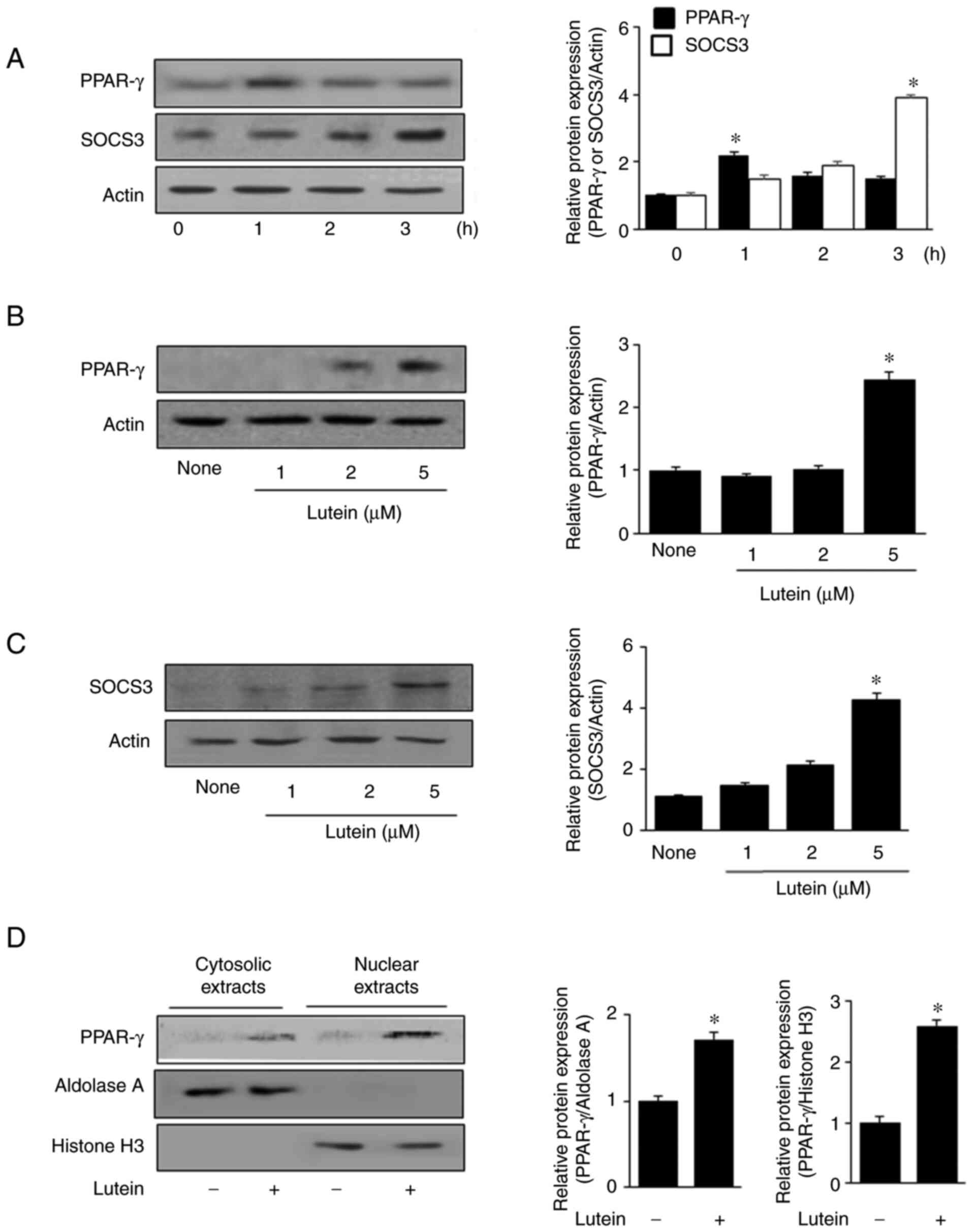
were treated with 5 µM lutein for 1, 2, and 3 h. Fig. 1A shows that the PPAR-γ and SOCS3
levels increased at 1 and 3 h, respectively. Therefore, for the
dose experiment, lutein (1, 2, and 5 µM) treatment was conducted
for 1 h to assess PPAR-γ expression levels, and for 3 h to measure
SOCS3 expression levels (Fig. 1B and
C). Treatment with 5 µM lutein resulted in a maximum increase
in the expression levels of both PPAR-γ and SOCS3 in unstimulated
cells. Thus, for the next experiment on PPAR-γ activation, cells
were treated with 5 µM lutein.
To observe the nuclear translocation of PPAR-γ, the
cells were treated with 5 µM lutein for 1 h, and PPAR-γ protein
levels were determined by western blot analysis (Fig. 1D). PPAR-γ expression was observed
at low levels in both cytosolic and nuclear extracts of the
untreated cells (lutein, -) and lutein treatment increased PPAR-γ
expression in both cytosolic and nuclear extracts. Aldolase A and
histone H3, the indices of cytosolic and nuclear extracts, were not
altered by lutein treatment.
Lutein suppresses activation of
JAK2/STAT3 and expression of IL-6 but increases SOCS3 expression
level in AR42J cells stimulated with cerulein
Fig. 2A and B show
that cerulein increased IL-6 mRNA and protein levels, which were
reversed by lutein. Without cerulein stimulation, both mRNA and
protein levels of IL-6 were reduced by lutein treatment.
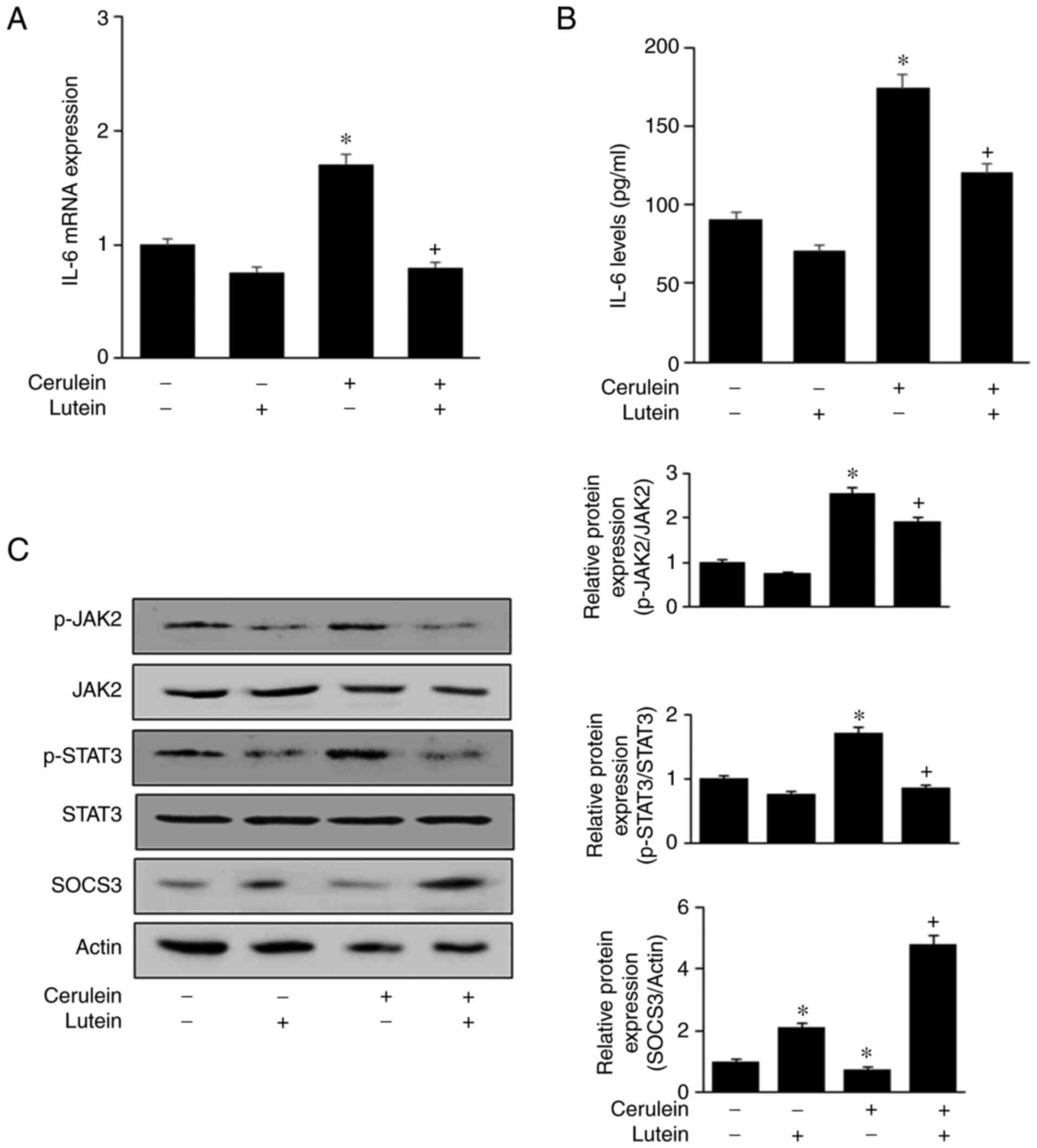 | Figure 2.Lutein suppresses activation of
JAK2/STAT3 and expression of IL-6, but induces SOCS3 expression in
AR42J cells stimulated with cerulein. Cells were cultured with 5 µM
lutein for 2 h and stimulated in the presence or absence of
cerulein (10–8 M) for (A) 6 h or (B) 24 h. (A) mRNA level of IL-6
was determined using reverse transcription-quantitative PCR and
normalized to the level of β-actin. (B) IL-6 levels in the medium
were determined by ELISA (n=12 per each group). (C) Cells were
cultured with 5 µM lutein for 2 h and then treated with cerulein
(10–8 M) for 1 h. Total and phospho-specific forms of JAK2 and
STAT3, and level of SOCS3 in whole-cell extracts were determined by
western blot analysis (left panel). For the relative protein
expression, the densitometry analysis for the ratio of p-JAK2/JAK2,
p-STAT3/STAT3 and SOCS3/actin represent the mean ± SE from three
immunoblots (right panel). *P<0.05 vs. the unstimulated and
untreated cells (Cerulein-, Lutein-); +P<0.05 vs.
cerulein-treated cells without lutein treatment (Cerulein+,
Lutein-). JAK2, Janus kinase; STAT, signal transducer and activator
of transcription; IL, interleukin; SOCS3, suppressor of cytokine
signaling; p-, phosphorylated. |
To investigate the underlying mechanism, the
activation of JAK2/STAT3 was assessed in cells treated with or
without lutein. Cerulein increased phospho-specific forms of
JAK2/STAT3, which were decreased by lutein treatment. The total
levels of JAK2/STAT3 were unchanged in cells stimulated with
cerulein (Fig. 2C). The
expression level of SOCS3, a negative feedback regulator of
JAK2/STAT3, was reduced by cerulein, which was inhibited by lutein
in AR42J cells. Lutein significantly increased SOCS3 expression
level in the unstimulated cells. These results demonstrated that
lutein inhibits JAK2/STAT3 activation and IL-6 expression, which
may be mediated by SOCS3 expression in cerulein-stimulated AR42J
cells.
PPAR-γ antagonist GW9662 abolishes the
effect of lutein on induction of SOCS3 expression and inhibition of
IL-6 expression in AR42J cells treated with cerulein
GW9662 reversed the inhibitory effects of lutein on
cerulein-induced expression of IL-6 (Fig. 3A and B). These results demonstrate
that lutein inhibits cerulein-induced IL-6 expression by activating
PPAR-γ. In addition, GW9662 suppressed the effect of lutein on
SOCS3 expression (Fig. 3C). Thus,
lutein may act as a PPAR-γ agonist to activate PPAR-γ and increase
SOCS3 levels in cerulein-stimulated cells.
 | Figure 3.GW9662, a PPAR-γ antagonist, reverses
the effect of lutein on induction of SOCS3 expression and
inhibition of IL-6 expression in AR42J cells stimulated with
cerulein. Cells were co-treated with lutein (5 µM) and GW9662 (1
µM) for 2 h, and then cultured with cerulein for (A) 6 h, (B) 24 h
or (C) 1 h. (A) mRNA expression level of IL-6 was determined using
reverse transcription PCR and normalized to the expression level of
β-actin. (B) IL-6 levels in the cell culture media were determined
by ELISA (n=12 per each group). (C) Total and phospho-specific
forms of JAK2 and STAT3, and level of SOCS3 in whole-cell extracts
were determined using western blot analysis (left panel). For the
relative protein expression, the densitometry analysis for the
ratio of p-JAK2/JAK2, p-STAT3/STAT3 and SOCS3/actin represent the
mean ± SE from three immunoblots (right panel). *P<0.05 vs. the
unstimulated and untreated cells (Cerulein-, Lutein-, GW9662-);
+P<0.05 vs. Cerulein-treated cells without any treatment
(Cerulein+, Lutein-, GW9662-). ++P<0.05 vs. Cerulein and
lutein-treated cells without GW9662 treatment (Cerulein+, Lutein+,
GW9662-). PPAR-γ, peroxisome proliferator activated receptor-γ;
SOCS3, suppressor of cytokine signaling; IL, interleukin; JAK2,
Janus kinase; STAT, signal transducer and activator of
transcription; p-, phosphorylated. |
PPAR-γ agonist troglitazone inhibits
IL-6 expression, but increases SOCS3 expression level in AR42J
cells stimulated with cerulein
Troglitazone reduced mRNA and protein levels of IL-6
in AR42J cells stimulated with cerulein (Fig. 4A and B). The cerulein-induced
increase in JAK2 and STAT3 phosphorylation was inhibited by
troglitazone (Fig. 4C). In
addition, troglitazone markedly increased SOCS3 expression level in
the unstimulated cells. The cerulein-induced decrease in SOCS3
levels was inhibited by troglitazone treatment. These results
demonstrate that PPAR-γ activation by troglitazone inhibits
cerulein-induced IL-6 expression and JAK2/STAT3 activation by
upregulating SOCS3 expression.
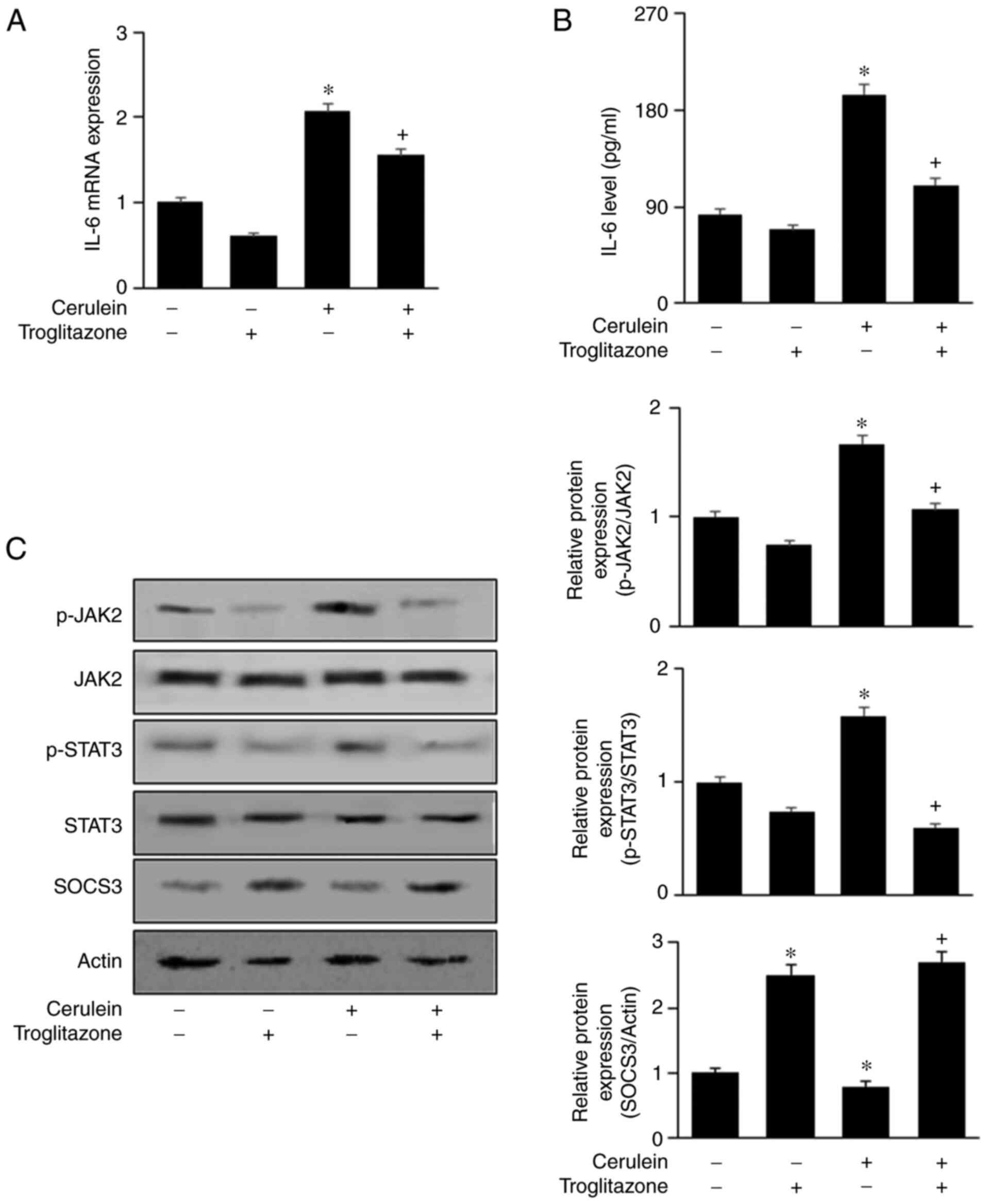 | Figure 4.PPAR-γ agonist troglitazone inhibits
IL-6 expression but induces SOCS3 expression in AR42J cells
stimulated with cerulein. Cells were treated with 40 µM
troglitazone for 2 h and cultured with cerulein for (A) 6 h, (B) 24
h or (C) 1 h. (A) IL-6 mRNA level was determined by reverse
transcription-quantitative PCR and normalized to the level of
β-actin. (B) IL-6 levels in the medium were measured by ELISA (n=12
per each group). (C) Levels of total and phospho-specific forms of
JAK2, STAT3, and SOCS3 in whole-cell extracts were determined using
western blot analysis (left panel). For the relative protein
expression, the densitometry analysis for the ratio of p-JAK2/JAK2,
p-STAT3/STAT3 and SOCS3/actin represent mean ± SE from three
immunoblots (right panel). *P<0.05 vs. the unstimulated and
untreated cells (cerulein-, Troglitazone-); +P<0.05 vs.
cerulein-treated cells without troglitazone treatment (cerulein+,
Troglitazone-). SOCS3, suppressor of cytokine signaling; IL,
interleukin; JAK2, Janus kinase; STAT, signal transducer and
activator of transcription; p-, phosphorylated. |
Discussion
In the present study, lutein attenuated IL-6
expression in cerulein-treated pancreatic acinar cells by inducing
PPAR-γ activation and SOCS3 expression. We also investigated the
relationship between the PPAR-γ ligand and SOCS3 expression using
the PPAR-γ antagonist GW9662. Our previous studies demonstrated
that PPAR-γ ligands, such as 15d-PGJ2 and troglitazone, decreased
cerulein-induced expression of IL-6 and TGF-β by upregulating SOCS3
in pancreatic acinar cells (8,9).
GW9662 reversed the inhibitory effect of DHA on IL-6 expression in
cerulein-treated AR42J cells (27). Rollins et al demonstrated
that the PPAR-γ agonists 15d-PGJ2 and troglitazone reduced the
severity of pancreatitis by reducing serum amylase activity and
histological damage (leukocyte infiltration and vacuolization) in
the pancreas (18). 15d-PGJ2 and
ciglitazone prevented inflammation by increasing PPAR-γ target
genes, such as antioxidant enzymes (copper/zinc superoxide
dismutase and catalase), and showed neuroprotective effects
(28).
Activation of STAT proteins is tightly regulated by
SOCS proteins. SOCS proteins are direct targets of STATs that
inhibit JAK/STAT activation, and SOCS3 regulates cellular
processes, including growth, apoptosis, and inflammatory gene
expression (29). SOCS3
deficiency induces embryonic lethality through the activation of
STAT3 and mitogen-activated protein kinases (30). Cerulein activates NADPH oxidase;
consequently, large amounts of ROS activate the JAK2/STAT3 pathway
and induce IL-6 expression in pancreatic acinar cells (9,24).
Carballo et al (31)
demonstrated that hydrogen peroxide promotes the nuclear
translocation of STAT3 in human lymphocytes. This study revealed
oxidative stress as a transcription factor of STAT3. Therefore,
cerulein-induced ROS production may activate JAK/STAT, which may
induce SOCS3 expression as a negative feedback loop. Our previous
study showed that cerulein increased SOCS3 expression, but this
induction of SOCS3 was not sufficient to prevent IL-6 expression in
pancreatic acinar cells (9).
PPAR-γ agonists 15d-PGJ2 and troglitazone increased SOCS3 levels
and prevented IL-6 expression in cerulein-stimulated cells
(9). Therefore, nutrients that
act as PPAR-γ agonists to induce SOCS3 expression, may be
beneficial in preventing acute pancreatitis.
Gallmeier et al showed that acinar cells are
the main source of STATs in the pancreas (32). In rat pancreatic acinar cells,
increased STAT3 and SOCS3 mRNA levels are observed in response to
the inflammatory mediator TNF-α (33). STAT3 regulates the expression of
IL-6 during starvation-induced autophagy in cancer cells (34). In cultured vascular cells, SOCS
overexpression prevents the proliferation of vascular smooth muscle
cells and monocytes, whereas SOCS3 inhibition leads to an increase
in the magnitude of cytokine response (35). These studies support the present
finding that JAK2/STAT3 activation mediates IL-6 production in
cerulein-stimulated AR42J cells.
Lutein modulates proinflammatory mediators, such as
PPAR, which affects inflammatory signaling pathways (36). PPAR-γ has been implicated in the
regulation of glucose, lipid homeostasis, cell differentiation, and
apoptosis (37). It is a sensor
of lipophilic molecules that causes structural changes in the
PPAR-γ receptor, which activates this receptor. In its active
conformation, PPAR-γ induces regulatory actions in inflammation
(38). Dietary lutein induces
PPAR expression and inhibits inflammation in chicken immune tissues
(22). These studies support our
present findings that lutein inhibits cerulein-induced IL-6
expression through PPAR-γ activation and SOCS3 induction in
pancreatic acinar cells.
For the first time, we show that the expression of
PPAR-γ is stimulated by lutein, which increases the expression of
SOCS3 in pancreatic acinar cells. Since this antioxidant mechanism
of lutein has not been reported in any cells or tissues, the
results of this study may increase the use of lutein for preventing
and treating oxidative stress-associated diseases in various
tissues. Further studies are needed to determine whether lutein
increases SOCS3 expression via PPAR-γ activation in pancreatic
tissues of animals with acute pancreatitis.
The limitation of the present study is that only one
cell line was used to determine the antioxidant mechanism of lutein
and its efficacy in preventing acute pancreatitis progression.
Since acute pancreatitis is a serious state of pancreatitis, the
demonstrated inhibitory effect of lutein on cerulein-stimulated
IL-6 expression in pancreatic acinar cells does not confirm the
protective effect of lutein on the development and progression of
acute pancreatitis. Further studies on the effects of lutein on
systematic and local events in acute pancreatitis development and
progression should be performed using in vivo experiments.
Thus, it cannot be concluded that lutein reduces the inflammatory
response in acute pancreatitis.
The novel finding of the present study was that
lutein exhibits antioxidant properties via PPAR-γ activation and
SOCS3 induction in pancreatic acinar cells. Therefore, lutein
inhibited cerulein-mediated JAK2/STAT3 activation and IL-6
expression in pancreatic acinar cells. In conclusion,
lutein-induced PPAR-γ activation and SOCS3 expression may underlie
the inhibitory effect of lutein on cerulein-induced IL-6 expression
in pancreatic acinar AR42J cells.
Acknowledgements
The abstract was presented at Nutrition 2021 live
online (Annual Meeting of the American Society for Nutrition), June
7–10 2021 and published as abstract in Curr Dev Nutr 5 (Suppl 2),
293, 2021.
Funding
This study was supported in part by the BK21 FOUR project,
Yonsei University, Republic of Korea.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HK conceived and designed the experiments. JWL
assisted in the experimental design. YJA performed the experiments.
YJA and JWL analyzed the data. YJA and JWL confirmed the
authenticity of all raw data. YJA wrote the manuscript. HK reviewed
and edited the manuscript. All the authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Authors' information
Yu Jin Ahn, https://orcid.org/0000-0002-3683-4213; Joo Weon Lim,
https://orcid.org/0000-0001-7483-3820; Hyeyoung Kim,
https://orcid.org/0000-0002-7019-917X.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leach SD, Gorelick FS and Modlin IM: New
perspectives on acute pancreatitis. Scand J Gastroenterol. 27
(Suppl 192):S29–S38. 1992. View Article : Google Scholar
|
|
2
|
Banks P: Predictors of severity in acute
pancreatitis. Pancreas. 6 (Suppl 1):S7–S12. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gu H, Werner J, Bergmann F, Whitcomb DC,
Büchler MW and Fortunato F: Necro-inflammatory response of
pancreatic acinar cells in the pathogenesis of acute alcoholic
pancreatitis. Cell Death Dis. 4:e8162013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Biffl WL, Moore EE, Moore FA and Peterson
VM: Interleukin-6 in the injured patients: Marker of injury or
mediator of inflammation. Ann Surg. 224:647–664. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kishimoto T, Akira S, Narazaki M and Taga
T: Interleukin-6 family of cytokines and gp130. Blood.
86:1243–1254. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hyun JJ and Lee HS: Experimental models of
pancreatitis. Clin Endosc. 47:212–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim H: Cerulein pancreatitis: Oxidative
stress inflammation and apoptosis. Gut Liver. 2:74–80. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu JH, Kim KH and Kim H: Suppression of
IL-1beta expression by the Jak 2 inhibitor AG490 in
cerulein-stimulated pancreatic acinar cells. Biochem Pharmacol.
72:1555–1562. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu JH, Kim KH and Kim H: SOCS 3 and
PPAR-gamma ligands inhibit the expression of IL-6 and TGF-beta1 by
regulating JAK2/STAT3 signaling in pancreas. Int J Biochem Cell
Biol. 40:677–688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watowich SS, Wu H, Socolovsky M,
Klingmuller U, Constantinescu SN and Lodish HF: Cytokine receptor
signal transduction and the control of hematopoietic cell
development. Annu Rev Cell Dev Biol. 12:91–128. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrand A, Kowalski-Chauvel A, Bertrand C,
Escrieut C, Mathieu A, Portolan G, Pradayrol L, Fourmy D, Dufresne
M and Seva C: A novel mechanism for JAK2 activation by a G
protein-coupled receptor, the CCK2R: Implication of this signaling
pathway in pancreatic tumor models. J Biol Chem. 280:10710–10715.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fenner JE, Starr R, Cornish AL, Zhang JG,
Metcalf D, Schreiber RD, Sheehan K, Hilton DJ, Alexander WS and
Hertzog PJ: Suppressor of cytokine signaling 1 regulates the immune
response to infection by a unique inhibition of type I interferon
activity. Nature Immunol. 7:33–39. 2005. View Article : Google Scholar
|
|
13
|
Hilton DJ, Richardson RT, Alexander WS,
Viney EM, Willson TA, Sprigg NS, Starr R, Nicholson SE, Metcalf D
and Nicola NA: Twenty proteins containing a C-terminal SOCS box
form five structural classes. Proc Nat Acad Sci USA. 95:114–119.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berlato C, Cassatella MA, Kinjyo I, Gatto
L, Yoshimura A and Bazzoni F: Involvement of suppressor of cytokine
signaling-3 as a mediator of the inhibitory effects of IL-10 on
lipopolysaccharide-induced macrophage activation. J Immunol.
168:6404–6411. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lazar MA: PPAR gamma, 10 years later.
Biochimie. 87:9–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barish GD, Downes M, Alaynick WA, Yu RT,
Ocampo CB, Bookout AL, Mangelsdorf DJ and Evans RM: Nuclear
receptor atlas: Macrophage activation. Mol Endocrinol.
19:2466–2477. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao W, Shi G, Gu H and Nguyen BN: Role of
PPARγ in the nutritional and pharmacological actions of
carotenoids. Res Report Biochem. 6:13–24. 2016. View Article : Google Scholar
|
|
18
|
Rollins MD, Sudarshan S, Firpo MA,
Etherington BH, Hart BJ, Jackson HH, Jackson JD, Emerson LL, Yang
DT, Mulvihill SJ and Glasgow RE: Anti-inflammatory effects of
PPAR-gamma agonists directly correlate with PPAR-gamma expression
during acute pancreatitis. J Gastrointest Surg. 10:1120–1130. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berger H, Végran F, Chikh M, Gilardi F,
Ladoire S, Bugaut H, Mignot G, Chalmin F, Bruchard M, Derangère V,
et al: SOCS3 transactivation by PPARγ prevents IL-17-driven cancer
growth. Cancer Res. 73:3578–3590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Végran F, Berger H, Ghiringhelli F and
Apetoh L: Socs3 induction by PPARγ restrains cancer-promoting
inflammation. Cell Cycle. 12:2157–2158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahn YJ and Kim H: Lutein as a modulator of
oxidative stress-mediated inflammatory diseases. Antioxidants
(Basel). 10:14482021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Selvaraj RK, Shanmugasundaram R and
Klasing KC: Effects of dietary lutein and PUFA on PPAR and RXR
isomer expression in chickens during an inflammatory response. Comp
Biochem Physiol A Mol Integr Physiol. 157:198–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Emeka PM, Rasool ST, Morsy MA, Islam MIH
and Chohan MS: Protective effects of lutein against
vancomycin-induced acute renal injury in mice via upregulation of
peroxisome proliferator-activated receptor gamma/nuclear factor
erythroid 2-related factor 2 and inhibition nuclear
factor-kappaB/caspase 3. Kor J Physiol Pharmacol. 25:321–331. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu JH, Kim KH and Kim H: Role of NADPH
oxidase and calcium in cerulein-induced apoptosis: Involvement of
apoptosis-inducing factor. Ann NY Acad Sci. 1090:292–297. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwak MS, Lim JW and Kim H: Astaxanthin
inhibits interleukin-6 expression in cerulein/resistin-stimulated
pancreatic acinar cells. Med Inflamm. 2021:55872972021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lim JW, Kim KH and Kim H: NF-kappaB p65
regulates nuclear translocation of Ku70 via degradation of heat
shock cognate protein 70 in pancreatic acinar AR42J cells. Int J
Biochem Cell Biol. 40:2065–2077. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song EA, Lim JW and Kim H: Docosahexaenoic
acid inhibits IL-6 expression via PPARγ-mediated expression of
catalase in cerulein-stimulated pancreatic acinar cells. Int J
Biochem Cell Biol. 88:60–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kapadia R, Yi JH and Vemuganti R:
Mechanisms of anti-inflammatory and neuroprotective actions of
PPAR-gamma agonists. Front Biosci. 13:1813–1826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carow B and Rottenberg ME: SOCS3, a major
regulator of infection and inflammation. Front Immunol. 5:582014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roberts AW, Robb L, Rakar S, Hartley L,
Cluse L, Nicola NA, Metcalf D, Hilton DJ and Alexander WS:
Placental defects and embryonic lethality in mice lacking
suppressor of cytokine signaling 3. Proc Nat Acad Sci USA.
98:9324–9329. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carballo M, Conde M, El Bekay R,
Martín-Nieto J, Camacho MJ, Monteseirín J, Conde J, Bedoya FJ and
Sobrino F: Oxidative stress triggers STAT3 tyrosine phosphorylation
and nuclear translocation in human lymphocytes. J Biol Chem.
274:17580–17586. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gallmeier E, Schäfer C, Moubarak P, Tietz
A, Plössl I, Huss R, Göke B and Wagner ACC: JAK and STAT proteins
are expressed and activated by IFN-gamma in rat pancreatic acinar
cells. J Cell Physiol. 203:209–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vona-Davis LC, Frankenberry KA, Waheed U,
Peterson E and McFadden DW: Expression of STAT3 and SOCS3 in
pancreatic acinar cells. J Surg Res. 127:14–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoon S, Woo SU, Kang JH, Kim K, Kwon MH,
Park S, Shin HJ, Gwak HS and Chwae YJ: STAT3 transcriptional factor
activated by reactive oxygen species induces IL6 in
starvation-induced autophagy of cancer cells. Autophagy.
6:1125–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ortiz-Muñoz G, Martin-Ventura JL,
Hernandez-Vargas P, Mallavia B, Lopez-Parra V, Lopez-Franco O,
Muñoz-Garcia B, Fernandez-Vizarra P, Ortega L, Egido J and
Gomez-Guerrero C: Suppressors of cytokine signaling modulate
JAK/STAT-mediated cell responses during atherosclerosis.
Arterioscler Thromb Vasc Biol. 29:525–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim JE, Clark RM, Park Y, Lee J and
Fernandez ML: Lutein decreases oxidative stress and inflammation in
liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr
Res Pract. 6:113–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma Y, Shi M, Wang Y and Liu J: PPARγ and
its agonists in chronic kidney disease. Int J Nephrol.
2020:29174742020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Desvergne B and Wahli W: Peroxisome
proliferator-activated receptors: Nuclear control of metabolism.
Endocr Rev. 20:649–688. 1999. View Article : Google Scholar : PubMed/NCBI
|