Introduction
Hepatocellular carcinoma (HCC) is the third most
common type of malignant tumour in China (1). Even following treatment with
chemotherapy, radiotherapy and immunotherapy, its five-year
survival rate remains <20% in China (2,3).
Further research into the molecular mechanism underlying the
occurrence of HCC is therefore key for identifying novel
therapeutic targets.
Multiple studies have shown the tumorigenic function
of β-catenin in tumorigenesis and β-catenin is the core molecule of
the Wnt/β-catenin pathway (4,5).
Its stability is regulated by the intracellular β-catenin
degradation complex. Composed of scaffold proteins (APC and axin)
and kinases (glycogen synthase kinase 3β and casein kinase 1α),
this degradation complex phosphorylates β-catenin (6). E3 ubiquitin ligase binds to
phosphorylated β-catenin, causing its ubiquitination and subsequent
degradation (7). When Wnt protein
binds to LDL receptor-related protein 5 and 6 and Frizzled on the
cell membrane, Frizzled recruits dishevelled segment polarity
protein 1 (Dvl) via its C-terminus. Dvl recruits axin in the
degradation complex via its DIX domain, thereby promoting
dissociation of the complex (8).
This results in increased protein levels of β-catenin; β-catenin
enters the cell nucleus, where it activates expression of target
genes, such as Axin2, c-Myc (8).
In HCC, this cascade is abnormally activated due to an axin
mutation and a constitutively activating mutation of β-catenin
(9,10). The Wnt/β-catenin pathway is
hijacked to promote proliferation and migration of HCC cells and
remodel the tumour microenvironment, thereby reprogramming tumour
metabolism to promote progression of HCC (11–15). Therefore, it is important to
determine the regulatory mechanism of this pathway to improve HCC
treatment.
SAC3D1 is a protein that binds to spindle assembly
(16). SAC3D1 is extensively
expressed, particularly in liver and kidney tissue. SAC3D1
functions in multiple biological processes (such as cell cycle
regulation) (17). Inflammatory
signals upregulate expression of SAC3D1 (18), although its function in tumours is
poorly understood.
The present study evaluated the role of SAC3D1 in
HCC and the molecular mechanisms underlying its biological
function.
Materials and methods
Cell lines
HCC (MHCC97 and Huh7), hepatoblastoma (HepG2) and
293T cell lines were provided by The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences. All cell lines were
used following authentication by STR. Cells were incubated in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), penicillin and
streptomycin) with 5% CO2 at 37°C.
Lipofectamine® 8000 (Beyotime Institute of
Biotechnology) was used to transfect plasmids (1 µg/µl) into cells
at room temperature for 20 min.
Overexpression and knockdown of
SAC3D1
The coding sequence) of SAC3D1 was inserted into the
vector of pLVX. For knockdown of SAC3D1, the oligo was annealed and
inserted into the vector of pLKO.1. The oligo sequences for the
knockdown of SAC3D1 were: short hairpin (sh)SAC3D1 1#,
5′-aaggagtacagccgacccg-3′; shSAC3D1 2#,
5′-aagccctggcccgcttcgc-3′.
Clinical tissue
A total of 30 HCC and adjacent non-cancerous tissue
(2 cm away from the cancerous tissues) samples were obtained from
Bozhou People's Hospital (Bozhou, China) from June 2019 to May 2021
(age, 31–82 years old, 13 females and 17 males) after written
informed consent was obtained from patients. These patients did not
receive the chemo- or radiotherapy before the surgery. The present
study was approved by the Ethics Committee of Bozhou People's
Hospital (E-2019-04).
Reverse transcription-quantitative
(RT-q)PCR
TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract RNA from the tissue.
PrimeScript™ RT kit (Takara Biotechnology Co., Ltd.) was
used for RT according to the manufacturer's instructions.
SYBR-Green kit (Toyobo Life Science) was used for qPCR. The
thermocycle conditions were: 95°C for 5 min, 94°C for 30 sec, 56°C
for 15 sec, 72°C for 20 sec; 35 cycles, and 4°C forever. The mRNA
levels of SAC3D1 were calculated according to the 2−ΔΔCq
method (19). The primer
sequences were as follows: SAC3D1 forward, 5′-tctttctgctctataa-3′
and reverse, 5′-cggaaggcagcatcta-3′ and 18S forward,
5′-aggccctgtaattggaatgagtc-3′ and reverse,
5′-gctcccaagatccaactacgag-3′.
Immunohistochemistry (IHC)
Tissues were fixed with 4% formalin at 4°C
overnight, embedded with paraffin and cut into 5 µm sections. After
being dewaxed with xylene and rehydrated in descending alcohol
series, antigen retrieval was performed for 30 min in EDTA solution
at 100°C. Tissue was cooled to room temperature, treated with 3%
hydrogen peroxide to quench the endogenous peroxidase activity and
blocked with 10% BSA for 20 min at room temperature. After washing
three times, tissue sections were incubated with SAC3D1 antibody
(Abcam, ab122809, 1:200) for 8 h at 4°C. Tissue was washed and
incubated with horseradish peroxidase (HRP)-linked IgG (Cell
Signaling Technology, #7074, 1:100) for 2 h at room temperature.
Signals were developed with DAB and nuclei were counterstained with
hematoxylin at room temperature for 5 min. The signals were
examined under the light microscope (magnification, 20 fold) and
analyzed with Vectra 2.0 (InForm).
Western blotting
RIPA (Cell Signaling technology, #9806) buffer was
used to extract protein from cells and tissue. Then, centrifugation
(4°C, 12000g) for 2 h was performed, the supernatant was collected
in a clean tube and concentration was determined via BCA kit.
Proteins (20 µg/lane) were separated by electrophoresis using 10%
SDS-PAGE and transferred to a PVDF membrane, which was blocked
using 10% BSA for 30 min at room temperature. Then, the membrane
was incubated with primary antibody for ≥8 h at 4°C. The next day,
membranes were washed twice, incubated with the HRP-linked
secondary antibody overnight at 4°C, and signals were visualized
using chemiluminescence reagent (Millipore cat. no. WBKLS0050) and
analyzed using Image Lab software 5.0 (Bio-Rad Laboratories, Inc.).
The antibodies were as follows: Anti-SAC3D1 (1:1,000; cat. no.
25857-1-AP; ProteinTech Group, Inc.), anti-tubulin (1:4,000; cat.
no. sc-5286; Santa Cruz Biotechnology, Inc.), anti-Flag (1:3,000;
cat. no. F9291; Sigma-Aldrich; Merck KGaA), anti-ubiquitin
(1:1,000; cat. no. #3936; Cell Signaling Technology, Inc.),
anti-GAPDH (1:3,000; cat. no. 6004-1-Ig; ProteinTech Group, Inc.),
anti-β-catenin (1:1,000; cat. no. 51067-2-AP; ProteinTech Group,
Inc.), anti-histone (1:1,000; cat. no. 17168-1-AP; ProteinTech
Group, Inc.), anti-axin (1:1,000; cat. no. 16541-1-AP; ProteinTech
Group, Inc.) and anti-hemagglutinin (HA; 1:5,000; cat. no.
51064-2-AP; ProteinTech Group, Inc.).
MTT assay
Cells were incubated in fresh DMEM containing 10%
MTT reagent (5 mg/ml in PBS) for 4 h. Dimethyl sulfoxide was used
to dissolve the purple formazan and optical density at 540 nm was
evaluated.
Anchorage-independent growth
assay
A total of 500 µl gel [20% FBS, 40% 2X RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc.)and 40% agar (~1.25%)] was
added into a 24-well plate. When the gel solidified, the cell
suspension (1×104 cells/ml) in RPMI-1640 was made. Then,
gel [25% FBS (Gibco), 37.5% 2X RPMI-1640 (Gibco), 37.5% agar (1%)
and 0.8% 2 mM L-glutamine] was made, the cell suspension was added
to the gel and mixed and 500 µl mixture was added to each well.
When the gel solidified, the plate was placed in the incubator at
37°C. After 14 days, colonies (>50 cells) were photographed by a
light microscope with 10-fold magnification and counted
manually.
Ubiquitination assay
Cells were incubated with proteasome inhibitor MG132
(20 µM, Sigma-Aldrich; Merck KGaA) overnight at 37°C.
Immunoprecipitation lysis buffer [50 mM Tris-HCl (pH 8.0), 150 mM
NaCl, 1% NP-40 and protease and phosphatase inhibitors] was used to
extract protein. A total of 1 µg β-catenin antibody (cat. no.
#9562; Cell Signaling Technology, Inc.) and 500 µg total cell
lysate was used to perform immunoprecipitation at 4°C. After 8–10
h, Protein A/G beads (~40 µl; Bimake; cat. no. B23202) was added
for 4 h incubation at 4°C. After washing with TBST, 1X loading
buffer was added to beads for 5 min at 100°C. The immunoprecipitate
was examined by western blot analysis and anti-ubiquitin antibody
as aforementioned.
Immunoprecipitation assay
pLvx and pCMV-HA plasmids were obtained from Adgene.
pLvx/Flag-SAC3D1 and PCMV/HA-Axin plasmids were co-transfected into
cells using Lipofectamine 8000 (Beyotime Institute of
Biotechnology) at room temperature for 20 min. After 48 h,
immunoprecipitation lysis buffer [50 mM Tris-HCl (pH 8.0), 150 mM
NaCl, 1% NP-40 and protease and phosphatase inhibitors] was used to
extract protein from cells. After centrifugation (4°C, 12,000 g)
for 20 min, the supernatant was collected 20 µl. Beads conjugated
with anti-Flag antibody (0.25 µg) (cat. no. A2220; Sigma-Aldrich;
Merck KGaA) were incubated with the 750 µl supernatant (containing
500 µg protein) for 2 h. Then, wash buffer [50 mM Tris-HCl (pH
8.0), 150 mM NaCl, 1% NP-40] was used to wash the beads. The
immunoprecipitate was eluted by 1X loading buffer and boiled at
100°C for 5 min, then examined by western blot analysis as
aforementioned.
Human Protein Atlas (HPA)
database
To analyze the correlation between the expression of
SAC3D1 and survival, HPA database was used
(proteinatlas.org/search/SAC3D1). The Cut-off value is FPKM
5.6.
Statistical analysis
Data are presented as the mean ± standard deviation
of two or three experimental repeats. Parametric one-way analysis
of variance followed by Tukey-Kramer's post hoc test was used to
test differences between groups using SPSS 15.0 (SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
SAC3D1 expression is upregulated in
HCC
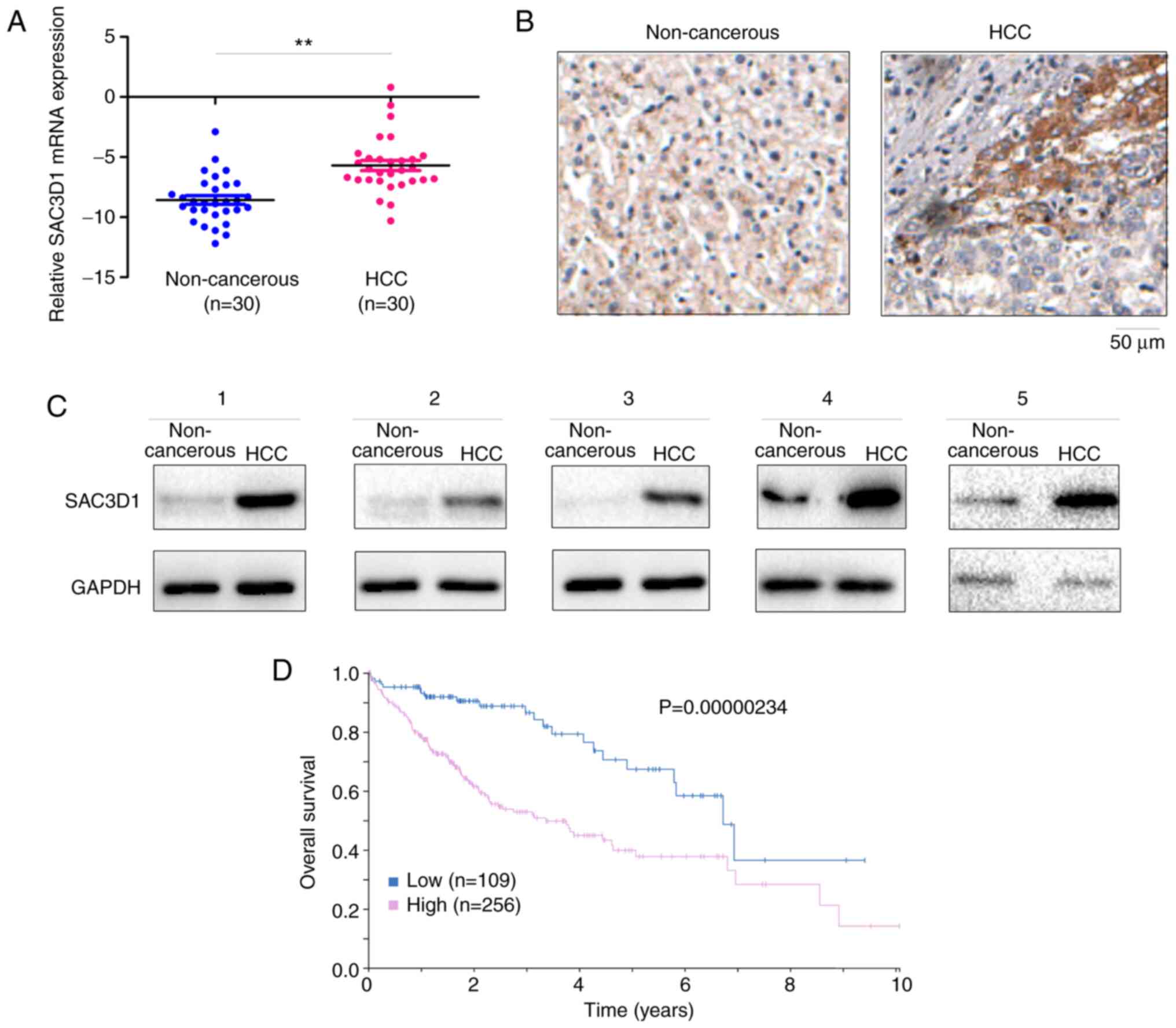
To determine expression of SAC3D1 in HCC, mRNA
transcripts of SAC3D1 in 30 HCC tissue and paired adjacent samples
were measured. The mean mRNA level of SAC3D1 in HCC tissue was
significantly elevated (Fig. 1A).
The results were verified by IHC staining (Fig. 1B). SAC3D1 protein level in 5
tumour and paired non-cancerous tissue samples was analysed. In HCC
tissue, the level of SAC3D1 protein was relatively high (Fig. 1C). From the Human Protein Atlas
database, it was determined that higher SAC3D1 expression was
associated with poorer outcome (Fig.
1D). These findings indicated that SAC3D1 expression may be
associated with HCC.
SAC3D1 promotes growth of HCC cells
both in liquid media and on soft agar
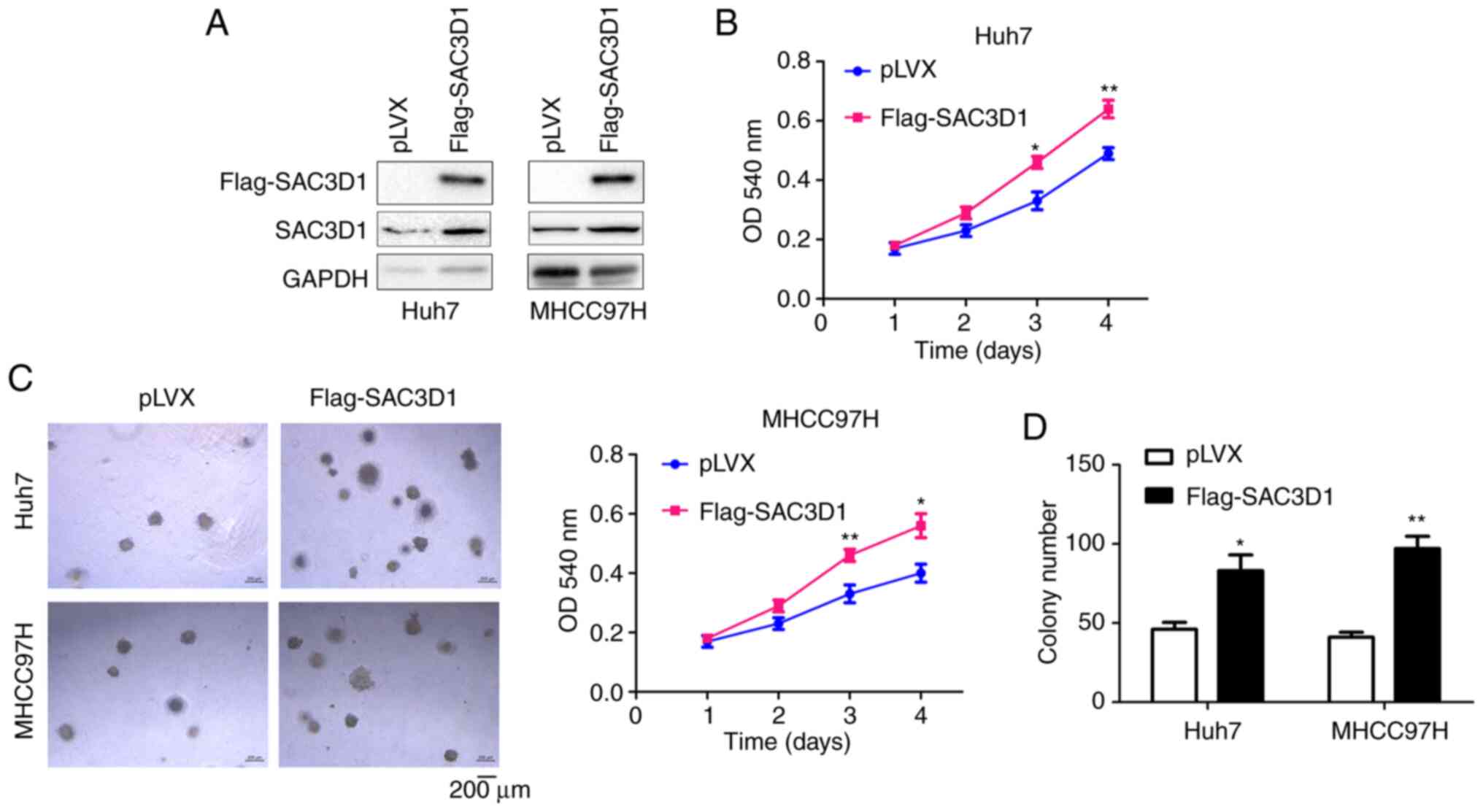
To investigate the role of SAC3D1 in HCC cells,
exogenous SAC3D1 was first stably expressed in Huh7 and MHCC97H
cells (Fig. 2A). The effect of
SAC3D1 expression on cell proliferation were investigated. MTT
assay showed that SAC3D1 accelerated cell proliferation (Fig. 2B). A growth assay on soft agar was
performed to determine the effect of SAC3D1 expression on
anchorage-independent growth (Fig. 2C
and D). The results showed that overexpression of SAC3D1
promoted cell growth.
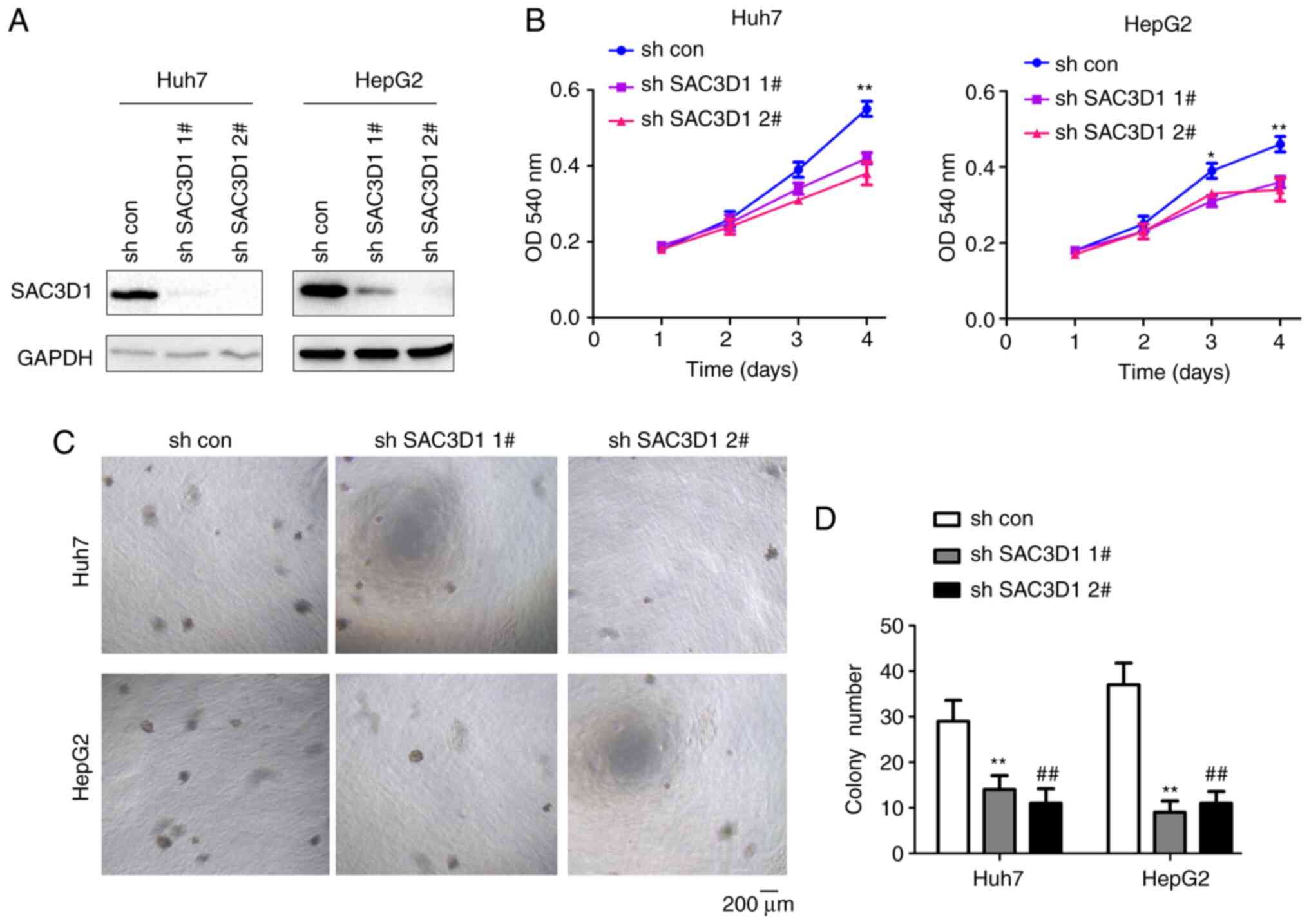
SAC3D1 was then knocked down in two HCC cell lines
using shRNA (Fig. 3A).
Downregulation of SAC3D1 inhibited the proliferation HepG2 and Huh7
cells (Fig. 3B), as well as their
growth on agar (Fig. 3C and
D).
SAC3D1 promotes β-catenin
accumulation
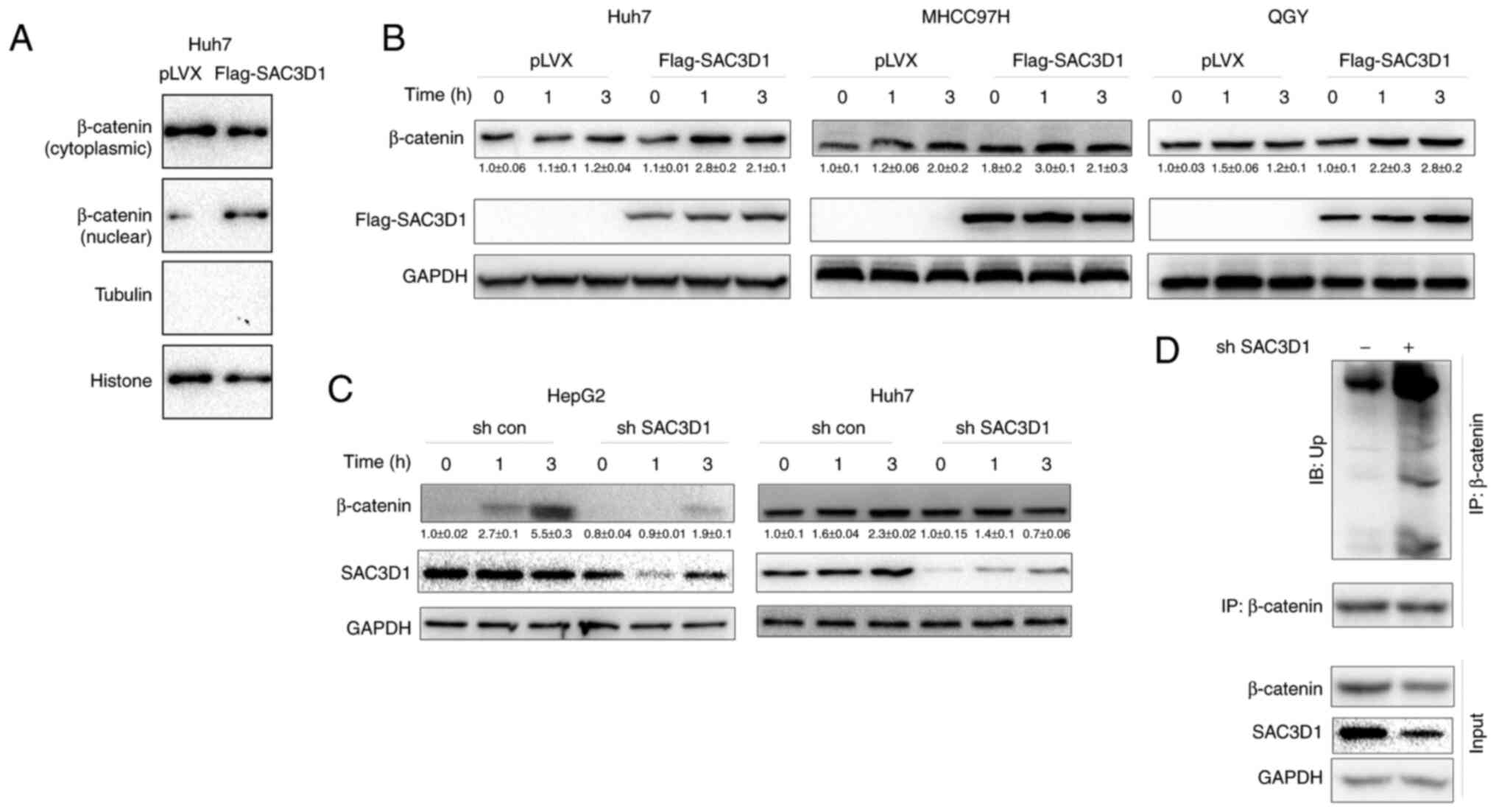
To test whether SAC3D1 regulates the Wnt/β-catenin
signalling pathway, the effect of SAC3D1 on levels of nuclear
β-catenin in control Huh7 cells and Huh7 cells overexpressing
SAC3D1 was evaluated. SAC3D1 promoted nuclear localization of
β-catenin (Fig. 4A), suggesting
that SAC3D1 elevated levels of nuclear β-catenin protein.
Consistently, Wnt3a promoted accumulation of β-catenin when SAC3D1
was overexpressed (Fig. 4B) and
knockdown of SAC3D1 inhibited elevation of nuclear protein levels
induced by Wnt3a (Fig. 4C).
Moreover, knockdown of SAC3D1 elevated levels of ubiquitinated
β-catenin (Fig. 4D).
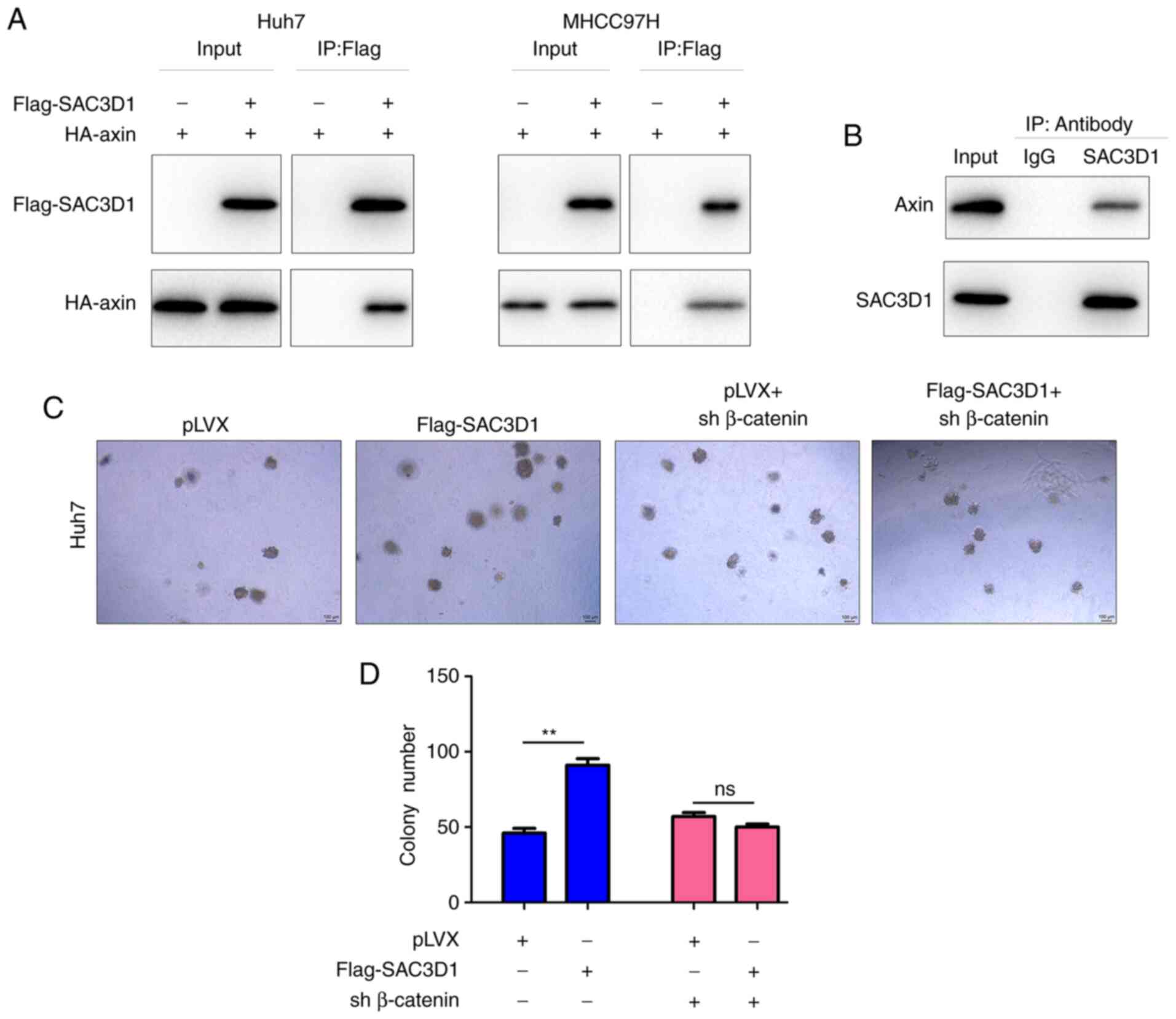
SAC3D1 forms a complex with axin
To study the molecular mechanism underlying
stabilization of β-catenin by SAC3D1, the interaction between
SAC3D1 and each protein in the β-catenin degradation complex was
analysed. In Huh7 and MHCC97H cells, exogenously expressed SAC3D1
(Flag-SAC3D1) interacted with axin (HA-Axin) in the degradation
complex (Fig. 5A). Consistent
with this observation, endogenously expressed SAC3D1 and axin
formed a complex in Huh7 cells (Fig.
5B). Moreover, knockdown of β-catenin abolished the promoting
effect of SAC3D1 on anchorage-independent growth of Huh7 cells,
suggesting that the oncogenic role of SAC3D1 was dependent on
Wnt/β-catenin signalling (Fig. 5C and
D).
Discussion
Previous study has shown that expression of SAC3D1
is upregulated in HCC (16).
Previous analysis of The Cancer Genome Atlas database showed that
high expression of SAC3D1 is associated with poor outcome
(GEPIA.cancer-pku.cn /detail.php?gene=SAC3D1). However, the
function of SAC3D1 in the progression of HCC, as well as the
molecular mechanism involved, remain unknown. Here, levels of
SAC3D1 mRNA and protein were increased in HCC tissue and the
proliferation and colony formation of HCC cells were enhanced by
SAC3D1. SAC3D1 interacted with axin, activating the Wnt/β-catenin
cascade. These findings reveal the promotive role of SAC3D1 in the
progression of HCC.
In HCC tissue, the Wnt/β-catenin cascade is
overactive. The reasons for its abnormal activation include loss of
function mutation of Axin, constitutively activating mutation of
β-catenin (10,20–22), upregulation of Wnt ligand, and
downregulation of secreted frizzled-related protein (23–25). These abnormal changes are directly
associated with the function of β-catenin degradation complex.
Therefore, the regulatory mechanism of the β-catenin degradation
complex in HCC may be valuable for identifying novel therapeutic
targets. Here, SAC3D1 interacted with axin in the degradation
complex, indicating the mechanism by which SAC3D1 activates the
Wnt/β-catenin cascade. Therefore, a promising treatment approach
may be to develop a small molecule drug to block the interaction
between SAC3D1 and axin in the degradation complex.
The effect of SAC3D1 on the progression of HCC was
studied in an HCC cell model, revealing that SAC3D1 promoted cell
growth. In future, clinical specimens of HCC should be collected to
confirm the association between levels of SAC3D1 and HCC clinical
features. An animal model should be used to study the role of
SAC3D1 in the occurrence and progression of HCC. In summary, SAC3D1
was upregulated in HCC and promoted progression of HCC by
activating Wnt/β-catenin signalling.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW designed the study and wrote the manuscript. XS
performed the experiments. Both authors have read and approved the
final manuscript. HW and XS confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Bozhou People's Hospital (approval no. E-2019-04).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers. 7:62021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altomonte J. Liver cancer, . Sensitizing
hepatocellular carcinoma to oncolytic virus therapy. Nat Rev
Gastroenterol Hepatol. 15:8–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J, da Fonseca LG and Reig M:
Insights into the success and failure of systemic therapy for
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
16:617–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schunk SJ, Floege J, Fliser D and Speer T:
WNT-β-catenin signalling-a versatile player in kidney injury and
repair. Nat Rev Nephrol. 17:172–184. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and β-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kypta RM and Waxman J: Wnt/β-catenin
signalling in prostate cancer. Nat Rev Urol. 9:418–428. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leavy O. Mucosal immunology, . β-catenin
calms the gut. Nat Rev Immunol. 10:6822010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mosimann C, Hausmann G and Basler K:
Beta-catenin hits chromatin: Regulation of Wnt target gene
activation. Nat Rev Mol Cell Biol. 10:276–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clevers H. Axin and hepatocellular
carcinomas. Nat Genet. 24:206–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishizaki Y, Ikeda S, Fujimori M, Shimizu
Y, Kurihara T, Itamoto T, Kikuchi A, Okajima M and Asahara T:
Immunohistochemical analysis and mutational analyses of
beta-catenin, Axin family and APC genes in hepatocellular
carcinomas. Int J Oncol. 24:1077–1083. 2004.PubMed/NCBI
|
|
11
|
Tian W, Li J, Wang Z, Zhang T, Han Y, Liu
Y, Chu W, Liu Y and Yang B: HYD-PEP06 suppresses hepatocellular
carcinoma metastasis, epithelial-mesenchymal transition and cancer
stem cell-like properties by inhibiting PI3K/AKT and WNT/β-catenin
signaling activation. Acta Pharm Sin B. 11:1592–1606. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Y, Sheng H, Xiao Y, Hu W, Zhang Z,
Chen Y, Zhu Z, Wu D, Cao C and Sun J: Wnt/β-catenin inhibitor
ICG-001 enhances the antitumor efficacy of radiotherapy by
increasing radiation-induced DNA damage and improving tumor immune
microenvironment in hepatocellular carcinoma. Radiother Oncol.
162:34–44. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu Y, Zhang L and Xu J: LncRNA BANCR
promotes proliferation of hepatocellular carcinoma Huh-7 cells by
activating Wnt/beta-catenin signaling pathway. Minerva
Gastroenterol (Torino). Jun 11–2021.(Epub ahead of print).
View Article : Google Scholar
|
|
14
|
Yang T, Xu R, Huo J, Wang B, Du X, Dai B,
Zhu M, Zhan Y, Zhang D and Zhang Y: WWOX activation by toosendanin
suppresses hepatocellular carcinoma metastasis through JAK2/Stat3
and Wnt/β-catenin signaling. Cancer Lett. 513:50–62. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adebayo Michael AO, Ko S, Tao J, Moghe A,
Yang H, Xu M, Russell JO, Pradhan-Sundd T, Liu S, Singh S, et al:
Inhibiting glutamine-dependent mTORC1 activation ameliorates liver
cancers driven by β-catenin mutations. Cell Metab. 29:1135–1150.e6.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han ME, Kim JY, Kim GH, Park SY, Kim YH
and Oh SO: SAC3D1: A novel prognostic marker in hepatocellular
carcinoma. Sci Rep. 8:156082018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu AG, Zhong JC, Chen G, He RQ, He YQ, Ma
J, Yang LH, Wu XJ, Huang JT, Li JJ, et al: Upregulated expression
of SAC3D1 is associated with progression in gastric cancer. Int J
Oncol. 57:122–138. 2020.PubMed/NCBI
|
|
18
|
Nakajima H, Tamura T, Ito M, Shibata F,
Kuroda K, Fukuchi Y, Watanabe N, Kitamura T, Ikeda Y and Handa M:
SHD1 is a novel cytokine-inducible, negative feedback regulator of
STAT5-dependent transcription. Blood. 113:1027–1036. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guan CN, Chen XM, Lou HQ, Liao XH, Chen BY
and Zhang PW: Clinical significance of axin and β-catenin protein
expression in primary hepatocellular carcinomas. Asian Pac J Cancer
Prev. 13:677–681. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JY, Park WS, Nam SW, Kim SY, Lee SH,
Yoo NJ, Lee JY and Park CK: Mutations of beta-catenin and AXIN I
genes are a late event in human hepatocellular carcinogenesis.
Liver Int. 25:70–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kitao A, Matsui O, Yoneda N, Kozaka K,
Kobayashi S, Sanada J, Koda W, Minami T, Inoue D, Yoshida K, et al:
Hepatocellular carcinoma with β-catenin mutation: Imaging and
pathologic characteristics. Radiology. 275:708–717. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu J, Xie Y, Li M, Zhou F, Zhong Z, Liu Y,
Wang F and Qi J: Association between SFRP promoter hypermethylation
and different types of cancer: A systematic review and
meta-analysis. Oncol Lett. 18:3481–3492. 2019.PubMed/NCBI
|
|
24
|
Xu C, Zeng XH, Wang L, Tao SQ, Wu QX, Zhu
P, Deng GH and Wang YM: sFRP-4, a potential novel serum marker for
chronic hepatitis B-related hepatocellular carcinoma. Hepatobiliary
Pancreat Dis Int. 14:164–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bengochea A, de Souza MM, Lefrançois L, Le
Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P, et
al: Common dysregulation of Wnt/Frizzled receptor elements in human
hepatocellular carcinoma. Br J Cancer. 99:143–150. 2008. View Article : Google Scholar : PubMed/NCBI
|