Introduction
Cytidine triphosphate synthase (CTPS) is a key
enzyme responsible for de novo synthesis of CTP, which is an
essential nucleotide and precursor for RNA and DNA synthesis
(1); therefore, CTPS activity
affects cell cycle progression. It has been reported that CTPS
forms filamentous structures termed cytoophidia (Greek for
‘cellular snakes’) in Drosophila (2,3),
bacteria (4), yeast (3), zebrafish (5), human and rat cells (6,7),
which suggests that the cytoophidium is an evolutionarily conserved
subcellular structure that may serve an essential role in
regulating metabolism (8).
Cytoophidia are mesoscale, intracellular,
filamentous structures that contain metabolic enzymes; they are not
membrane-bound cell organelles. They comprise a type of
intracellular compartment and are involved in cell metabolism
(2). Certain studies have
reported that cytoophidia may serve as metabolic stabilizers and a
buffer system in response to environmental changes (5,9,10).
Cytoophidia respond to nutrient stress by elongating following
nutrient deprivation in Drosophila (11) and budding yeast (12). In Schizosaccharomyces
pombe, cytoophidia formation decreases following cold or heat
shock (13). Certain studies have
reported that cytoophidium sequester the active binding sites of
enzymes, thereby inhibiting CTPS activity in Escherichia
coli and Drosophila tissue (14,15). However, Strochlic et al
(16) reported that Drosophila
CTPS within cytoophidia is catalytically active. These
aforementioned studies suggest that CTPS activity following
cytoophidia formation differs with cell type.
The changes in CTPS activity are reported to be
associated with cancer progression (17,18). Significantly higher activity of
CTPS has been reported in acute lymphocytic leukemia cells compared
with lymphocytes of healthy controls (19). CTPS also promotes malignant
progression of triple-negative breast cancer (20). Cytoophidia formed by CTPS have
been reported in human hepatocellular carcinoma cells but not in
adjacent non-cancerous hepatocytes (21). To the best of our knowledge, the
potential association between CTPS cytoophidia and cancer cell
proliferation is has not been previously elucidated.
Toosendanin (TSN) is a triterpenoid derivative
extracted from the bark of Melia toosendan Sieb et Zucc and
exerts anticancer effects on numerous types of human cancer cell,
such as colorectal cancer cells and glioma cells (22–25). Our previous studies demonstrated
that TSN induces apoptosis of human gastric cancer MKN45 cells
(26) and induces formation of
CTPS cytoophidia. To the best of our knowledge, however, the
association between formation of CTPS cytoophidia and apoptosis in
MKN45 cells remains unknown. The present study evaluated whether
the CTPS formed cytoophidia affected TSN-induced MKN45 cell
proliferation or apoptosis. The results of the present study may
facilitate further understanding of the role of CTPS cytoophidia in
cancer cell apoptosis.
Materials and methods
Cell culture
The gastric cancer MKN45 cell line was purchased
from the Beijing Beina Chuanglian Biotechnology Institute and
cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; Biological
Industries) and antibiotics (100 U/ml penicillin and 100 µg/ml
streptomycin) at 37°C in a 5% CO2 humidified
environment. All cells were cultured in 6-well plates for drug
treatment or transfection with plasmids.
Generation of constructs
Total RNA was extracted from MKN45 cells using
Trizol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. For reverse transcription
(RT), 1 µg RNA was used with ReverTra Ace™ qPCR RT Master Mix with
gDNA Remover (Toyobo Life Science) at 37°C for 15 min and
denaturation at 98°C for 5 min, cDNA was stored at −20°C. The
overexpression vector of CTPS (OE-CTPS) was constructed using the
seamless cloning technique. The linearized pcDNA3.1(+) vector was
PCR amplified with primers as follows: forward
5′-CATAAGCTTAAGTTTAAACGCTAGCCAGC-3′ and reverse
5′-TACCCATACGATGTTCCAGATTACGCTTGAGGATCCACTAGTCCAGTGTGG-3′. The
full-length coding sequences of human CTPS were amplified using PCR
with primers as follows: forward
5′-GCGTTTAAACTTAAGCTTATGAAGTACATTCTGGTTACTGGTGGT-3′ and reverse
5′-TGGAACATCGTATGGGTAGTCATGATTTATTGATGGAAACTTCAG-3′. The seamless
cloning reaction was performed using ClonExpress II One Step
Cloning kit C112 (Vazyme Biotech Co., Ltd.) according to the
manufacturer's protocol. To generate the point mutation
CTPSR294D, site-directed mutagenesis was performed on
the pcDNA3.1(+)-CTPS plasmid using primers as follows: Forward
5′-GACAGATATGATGACTTGCTGGAG-3′ and reverse
5′-AGCCATCTCTTTCCATTTCATCA-3′ (underlined section indicates the
mutated site). The PCR products were digested with Dpn I (New
England BioLabs, Inc.) at 37°C for 1 h and ligated using Quick
Ligation kit (New England BioLabs, Inc.) before transformation, as
previously described (27). All
PCR reactions were performed in a total volume of 50 µl using 2X
PrimeSTAR Max Premix (Takara Bio). Empty pcDNA3.1(+) vector served
as a negative control. The destination constructs were fully
sequenced (Sangon Biotech Co., Ltd.) before use for transfection.
The cells were seeded in 6-well plates and transfected with 4 µg of
Empty pcDNA3.1(+) vector, pcDNA3.1(+)-CTPS,
pcDNA3.1(+)-CTPSR294D at 37°C for 6 h using
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions, then cultured for 48
h.
Immunofluorescence assay
MKN45 cells were treated with 0, 60, 80 and 120 nM
TSN at 37°C for 72 h or transfected with OE-CTPS and
OE-CTPSR294D vectors. Cells were fixed using 4%
paraformaldehyde for 30 min at 4°C. After washing with PBST (1X
PBS; 0.2% Triton X-100), the cells were blocked with 5% bovine
serum albumin (BSA; 0.5% Triton X-100 in PBS) for 60 min at 37°C.
The cells were incubated with primary rabbit anti-human CTPS
antibodies (1:100; cat. no. abs138045; Absin Bioscience Inc.) at
4°C for 12 h. The cells were washed with PBST three times and
incubated with Alexa Fluor 488-conjugated goat anti-rabbit
secondary antibodies (1:500; cat. no. 111-545-003 Jackson
ImmunoResearch Laboratories, Inc.) for 2 h at room temperature. The
cells were washed with PBST and slides were counterstained with
DAPI to visualize the nuclei for 5 min at room temperature. All
samples were imaged using the 63× objective of a laser-scanning
confocal microscope (Leica TCS SP8 Confocal Microscope; Leica
Microsystems GmbH). The proportion of the cells which contained
CTPS cytoophidia was calculated from a minimum of five randomly
chosen fields from three individual experiments using ImageJ 1.43
software (National Institutes of Health).
Cell viability analysis
MKN45 cells were seeded in 96-well plates at a
density of 1×104 cells/well. The cells were treated with
different concentrations of TSN (0, 60, 80 and 120 nM) for 24, 48
and 72 h at 37°C, then incubated with 0.5 µg/µl MTT at 37°C for 4
h. Subsequently, the supernatant was removed and 150 µl DMSO was
added to each well. The absorbance at 570 nm was quantified using a
multi-well plate reader (Spark 10M, Tecan Group, Ltd.).
Cell cycle analysis
Following transfection with plasmids or treatment
with TSN as aforementioned, cells were washed twice with PBS and
detached from the plate surface by digestion using trypsin. Cells
were centrifuged at 300 × g at 4°C for 10 min, the pellet was
resuspended in PBS, centrifuged again at 300 × g at 4°C for 10 min
and resuspended in ice-cold 70% ethanol and stored at 4°C for 18 h.
Samples were washed once in PBS and resuspended in DNA staining
solution (propidium iodide, 5 µg/ml; RNase A, 0.5 mg/ml; PBS) and
incubated at 37°C in the dark for 30 min. All samples were assessed
using a Cytomics FC500 Flow Cytometer (Beckman Coulter, Inc.) and
analyzed using CXP Software version 2.3 (Beckman Coulter,
Inc.).
Early apoptosis assay
Following transfection with plasmids or treatment
with TSN as aforementioned, the cells were detached from the plate
surface, digested by trypsin and centrifuged at 300 × g at 4°C for
10 min, washed twice with cold PBS, then 500 µl Annexin V-FITC
binding buffer (No. C1062M, Beyotime Institute of Biotechnology)
was added to each sample. The cells were incubated with 5 µl
FITC-annexin V and 5 µl PI for 15 min at 25°C in the dark. After
washing, aliquots of 2×104 cells/sample were examined
using a Cytomics FC500 Flow Cytometer and analyzed with CXP
Software ver.2.3 (Beckman Coulter).
EdU proliferation assay
Cells were seeded in 96-well plates at
1×104 cells/well and placed in a humidified incubator at
37°C with 5% CO2 for 12 h following treatment with TSN
or transfection with plasmids for 48 h as aforementioned. Cell
proliferation was assessed using the EdU Cell Proliferation Assay
kit (Guangzhou RiboBio Co., Ltd.) as described by Wang et al
(28). The percentage of
EdU-positive cells was calculated from five random fields using
ImageJ (National Institutes of Health).
JC-1 staining for mitochondrial
membrane potential
To determine the mitochondrial membrane potential,
cells were seeded in 6-well plates at a density of 1×104
cells/well and transfection with plasmids or treatment with 80 nM
TSN for 48 h. JC-1 staining was performed as described by Sabarwal
et al (29).
Acridine orange nuclear staining
Cells were cultured on glass cover slides and
transfected with plasmids or treatment with 80 nM TSN for 48 h.
Cells were rinsed twice with PBS and fixed with 4% paraformaldehyde
in PBS for 10 min. Subsequently, the cells were stained with 0.1
mg/ml acridine orange (Solarbio Life Science Co., Ltd. Beijing) for
1–2 min. The images were captured using a Leica TCS SP8 confocal
laser-scanning microscope (Leica Microsystems GmbH) at 488 nm
excitation and 515 nm emission wavelengths (magnification,
×63).
RT-quantitative (q)PCR
Following transfection with plasmids or treatment
with 80 nM TSN for 48 h, mRNA expression levels of cyclin D1
(CCND1), Bax and Bcl-2 were assessed using RT-qPCR. The extraction
of RNA and synthesis of first-strand cDNA were performed as
described by Zhang et al (30) using ReverTra Ace qPCR RT Master
Mix with gDNA Remover (Toyobo Life Science). TB Green®
Premix Ex Taq™ (Tli RNase H Plus; cat. no. RR420L, Takara Bio Inc.
Beijing) was used for qPCR. The thermocycling conditions were as
follows: 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec,
and 60°C for 30 sec. β-actin was used as an endogenous control for
data normalization. Experiments were performed in triplicate. mRNA
expression levels were quantified using the 2−ΔΔCq
method (31). Sequences of the
primers used are presented in Table
I.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence,
5′→3′ |
|---|
| CCND1 | F:
tattgcgctgctaccgttga |
|
| R:
ccaatagcagcaaacaatgtgaaa |
| Bcl-2 | F:
atgtgtgtggagagcgtcaac |
|
| R:
agacagccaggagaaatcaaac |
| Bax | F:
aagctgagcgagtgtctcaag |
|
| R:
caaagtagaaaagggcgacaac |
| β-actin | F:
agcgagcatcccccaaagtt |
|
| R:
gggcacgaaggctcatcatt |
Western blotting
Whole-cell lysate was extracted using RIPA lysis
buffer (cat. no. P0013B, Beyotime Institute of Biotechnology). The
lysate was centrifuged at 12,470 × g for 15 min at 4°C. The
supernatant was collected and the protein concentration was
determined using the BCA Protein Assay kit (Beyotime Institute of
Biotechnology). Protein (12 µg/lane) was loaded and separated by
10% SDS-PAGE, followed by transfer to polyvinylidene fluoride
membranes (EMD Millipore). Membranes were blocked with 5% (w/v)
fat-free milk in Tris-buffered saline containing 0.05% Tween-20 at
20–25°C for 1 h and probed with primary antibodies at 4°C
overnight, primary antibodies as follows: CTPS (1:300; cat. no.
abs138045; Absin Bioscience Inc.), Bcl-2 (1:300; cat. no. ab196495;
Abcam,), Bax (1:300; cat. no. ab53154; Abcam), CCND1 (1:5,000; cat.
no. 60186-1-Ig; ProteinTech Group, Inc.) and β-actin (1:10,000;
cat. no. ab8227; Abcam,). The membranes were then incubated with
IRDye® 800CW Goat anti-Rabbit IgG Secondary Antibody
(1:15,000; code: 926-32211, LI-COR Biosciences) antibodies or IRDye
800CW Goat anti-Mouse IgG Secondary Antibody (1:15,000; code:
926-32210, LI-COR Biosciences) for 90 min at 20–25°C and washed
with PBS. The immunoblots were visualized using an Odyssey IR
Imaging System (LI-COR Biosciences). Image Studio version 4.0
(LI-COR Biosciences) was used to analyze the bands and each band
was normalized to β-actin.
Statistical analysis
All data are presented as the mean ± SEM, and data
were obtained from three replications. Representative bands of
western blotting were selected from independent experiments. All
statistical tests were performed using GraphPad Prism 5.0 software
(GraphPad Software, Inc.). One-way ANOVA was used to compare
independent groups with Dunnett's multiple comparisons test for
comparisons against a single control and Tukey's multiple
comparisons test when ≥3 groups were analyzed.
Results
TSN induces CTPS cytoophidia formation
in MKN45 cells
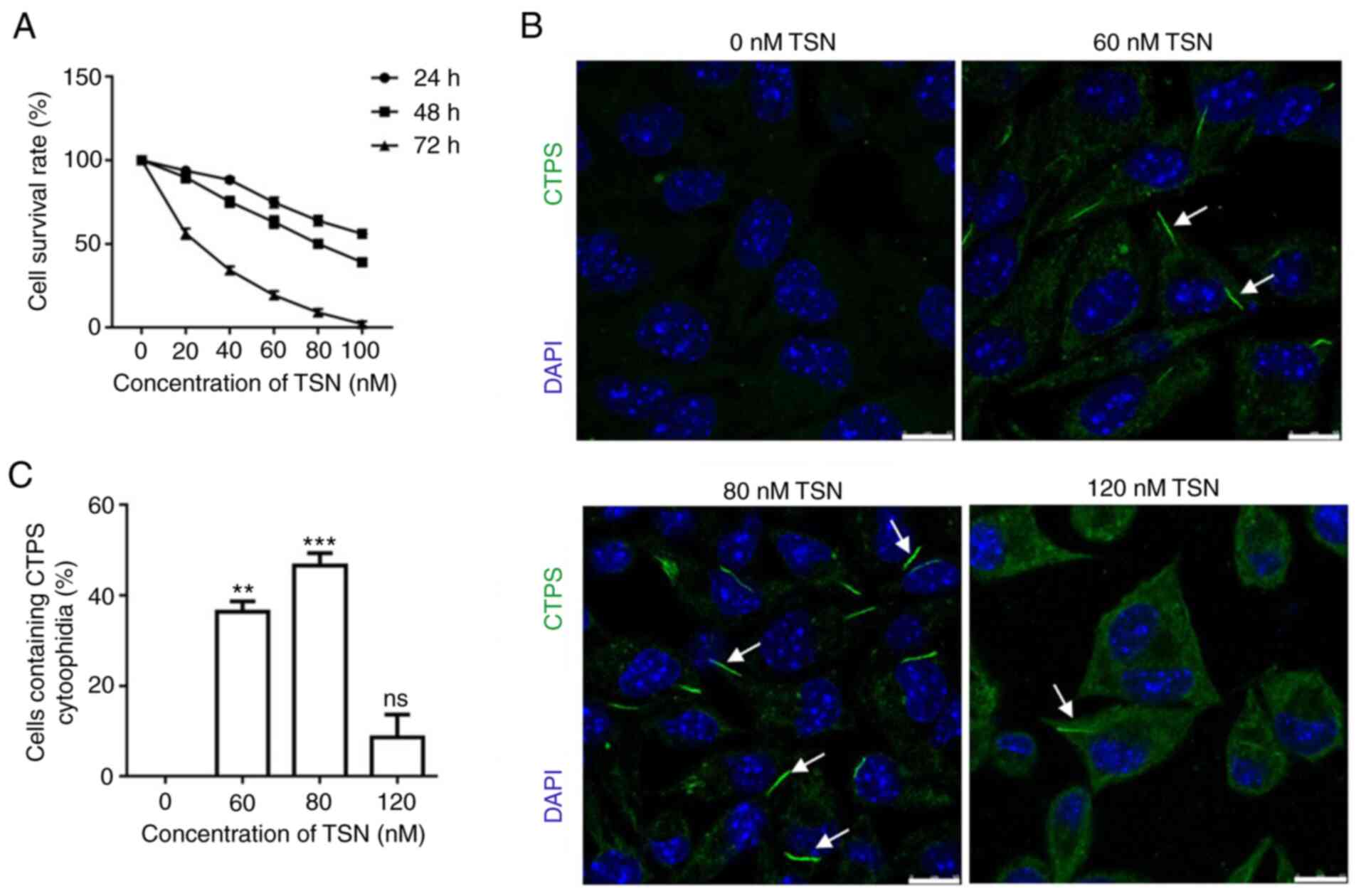
Cell survival rate markedly decreased as the TSN
concentration and treatment duration increased (Fig. 1A). CTPS cytoophidia were observed
in MKN45 cells treated with different concentrations (0, 60, 80 and
120 nM) of TSN for 72 h (Fig.
1B). Compared with the control (0 nM TSN), cytoophidia were
detected in 36.4% of MKN45 cells treated with 60 nM TSN and 46.65%
of MKN45 cells treated with 80 nM TSN; this showed that CTPS
cytoophidia formation was significantly increased compared with the
control. However, the percentage decreased to 16.01% in MKN45 cells
treated with 120 nM TSN (Fig.
1C). These results indicated that TSN decreased cell viability
while induced CTPS cytoophidia formation in MKN45 cells.
CTPS cytoophidia formation inhibits
proliferation of MKN45 cells
To determine the effect of CTPS cytoophidia on the
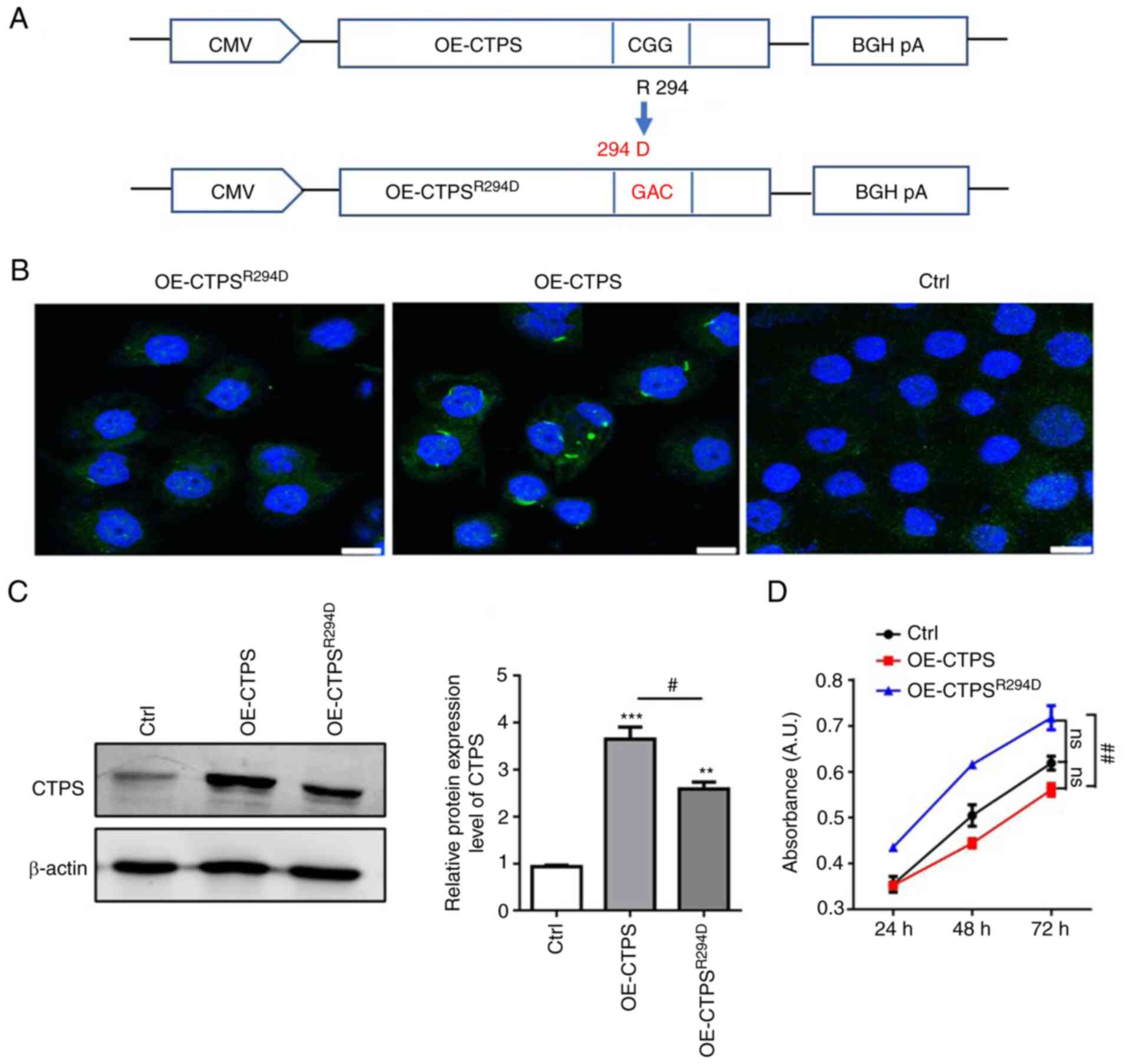
proliferation rate of gastric cancer MKN45 cells, OE-CTPS and
R294D-CTPS mutant (OE-CTPSR294D) vector were generated
(Fig. 2A). The formation of CTPS
cytoophidia and CTPS protein expression levels were assessed. CTPS
assembled into cytoophidia in OE-CTPS cells; but did not assemble
into cytoophidia in OE-CTPSR294D cells (Fig. 2B). CTPS protein expression levels
in OE-CTPSR294D cells were significantly lower compared
with those in OE-CTPS cells (Fig.
2C). MKN45 cell viability decreased after being transfected
with OE-CTPS compared with control; however, cell viability
increased following transfection with OE-CTPSR294D
(Fig. 2D). These results
indicated that the formation of CTPS cytoophidia decreased cell
viability in MKN45 cells.
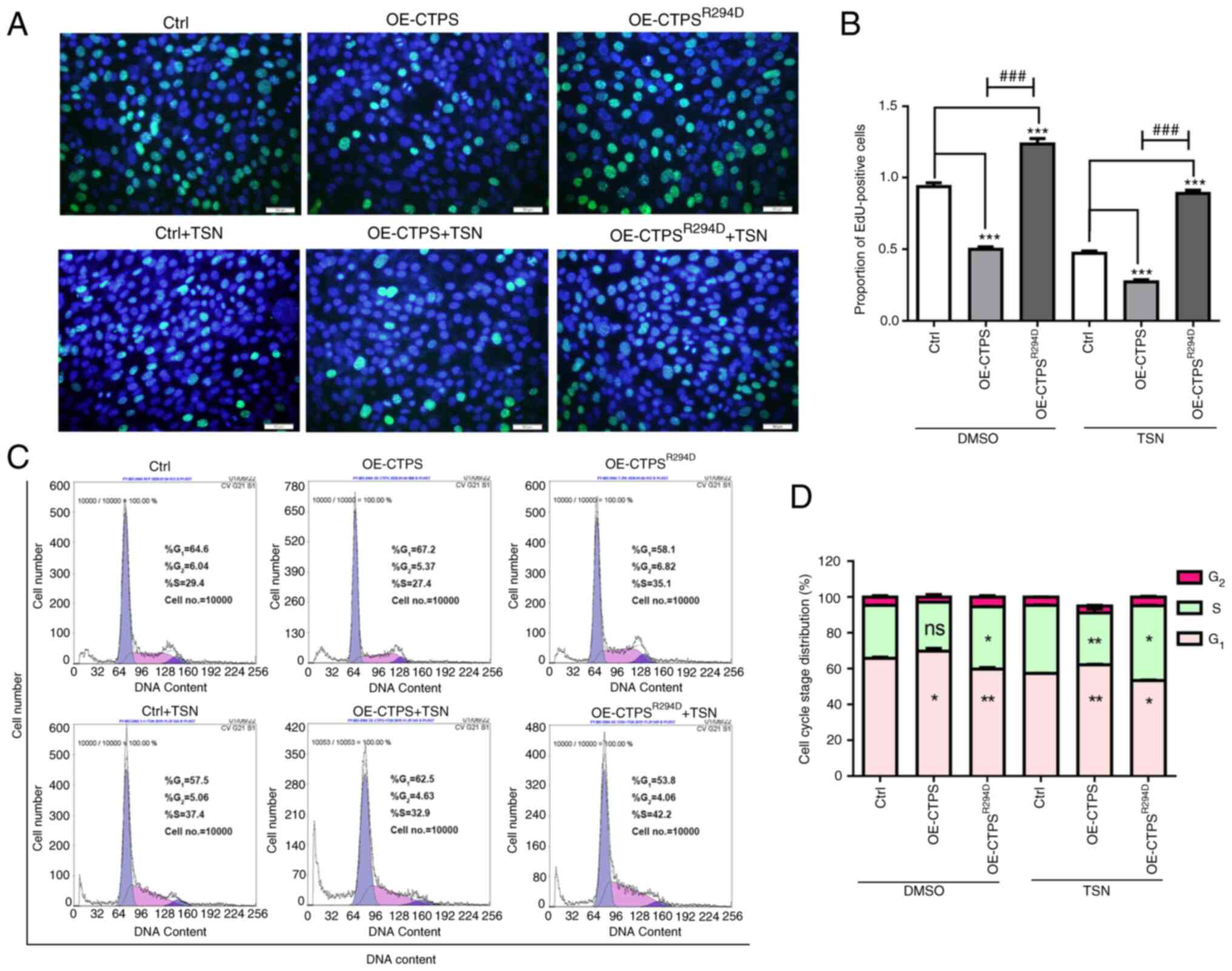
The proliferation rate of gastric cancer MKN45 cells
was also assessed. Compared with the control, the percentage of
EdU-positive cells significantly decreased in OE-CTPS cells
(Fig. 3A and B). Furthermore, the
percentage of G1/G0-phase cells significantly
increased and the percentage of S-phase cells markedly decreased in
OE-CTPS cells compared with the control (Fig. 3C and D). Following treatment with
80 nmol/l TSN, compared with group of control+TSN, the EdU-positive
rate decreased, the percentage of G1/G0-phase
cells increased and S-phase cells decreased in OE-CTPS +TSN group.
The EdU-positive rate increased, percentage of
G1/G0-phase cells decreased and the
percentage of S-phase cells increased in OE-CTPSR294D
cells compared with the control. The same changes were observed in
group of OE-CTPSR294D+TSN compared with control+TSN
group.
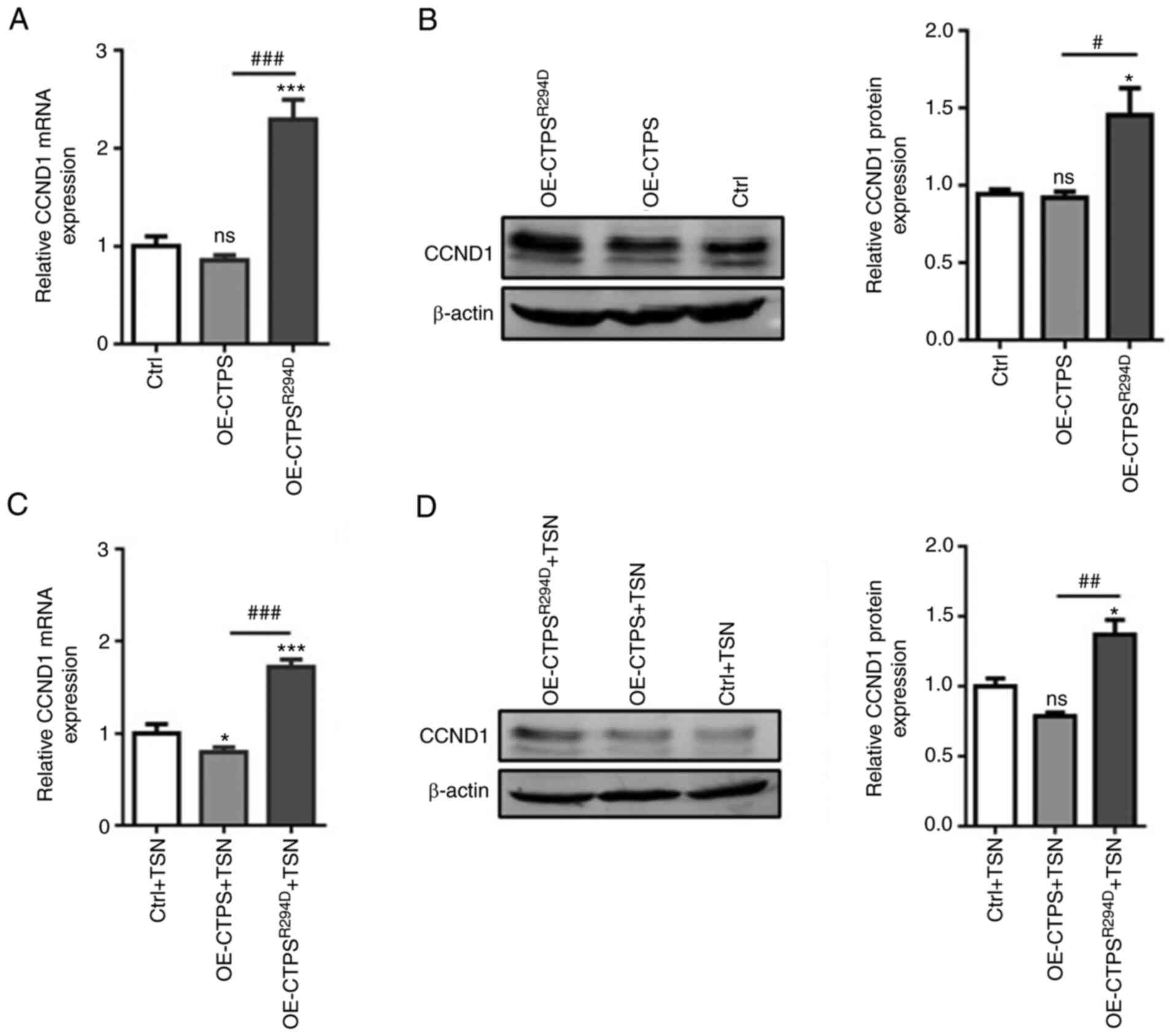
Furthermore, mRNA and protein expression levels of
CCND1 markedly decreased in OE-CTPS cells compared with the control
but significantly increased in OE-CTPSR294D cells
compared with both the control and OE-CTPS (Fig. 4A and B). The same changes of CCND1
mRNA and protein expression levels were observed following
treatment with 80 nmol/l TSN in OE-CTPS cells and
OE-CTPSR294D cells compared with control. (Fig. 4C and D). These results indicated
that CTPS cytoophidia formation could inhibit MKN45 cells
proliferation by affecting cell cycle progression.
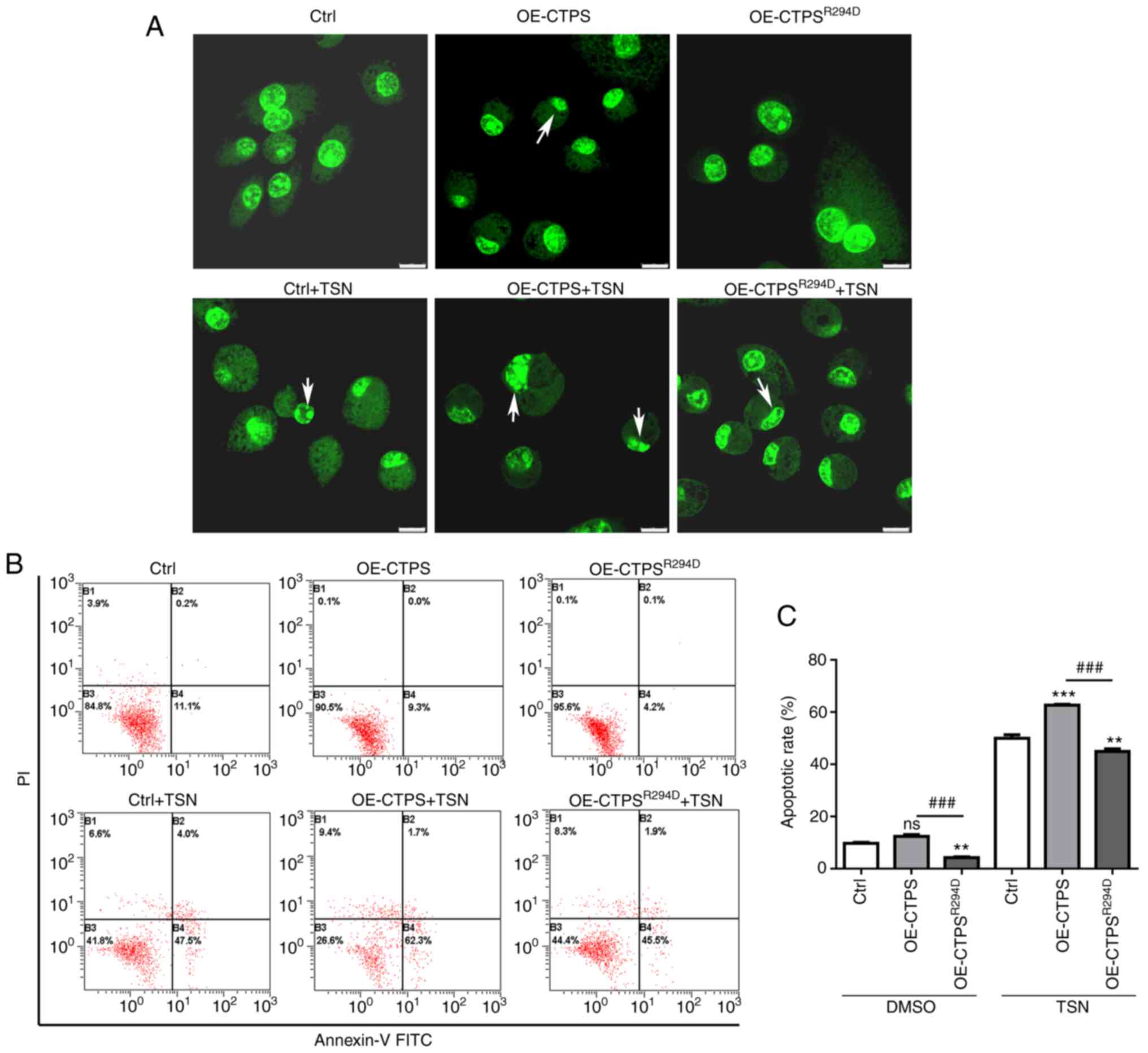
CTPS cytoophidia formation promotes
apoptosis of MKN45 cells
To assess the effect of CTPS cytoophidia on
apoptosis of gastric cancer MKN45 cells, cells were transfected
with OE-CTPS or OE-CTPSR294 vectors. Subsequently,
morphological changes and the presence of early apoptotic cells
were evaluated. Compared with the control, chromosomes were
markedly more aggregated and marginalized in OE-CTPS cells;
however, no notable morphological changes in OE-CTPSR294
cells were observed compared with the control (Fig. 5A). FITC-annexin-V/PI staining
demonstrated that apoptosis rate was higher in OE-CTPS cells
compared with the control and significantly lower in
OE-CTPSR294 cells compared with both control and OE-CTPS
(Fig. 5B and C). Following
treatment of transfected cells with 80 nmol/l TSN, apoptotic bodies
were prominent in OE-CTPS+TSN cells compared with in groups of
control+TSN; however, in OE-CTPSR294+TSN cells no
apoptotic bodies was observed, chromosome aggregation or
marginalization occurred only in some cells. Apoptosis rate was
more pronouncedly increased in group of OE-CTPS +TSN cells compared
with both control+TSN and OE-CTPSR294+TSN groups.
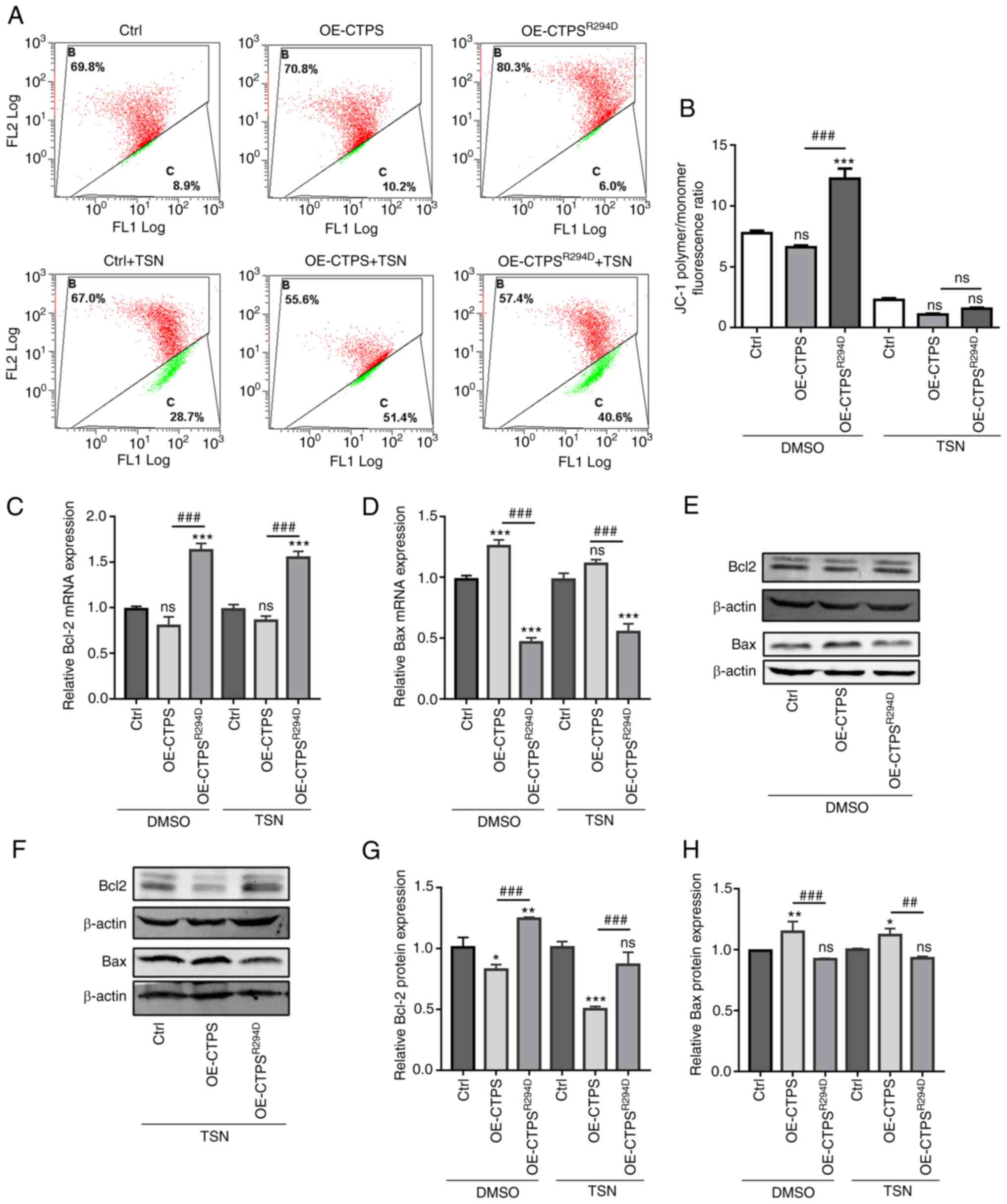
Mitochondrial membrane potential in OE-CTPS cells
was markedly lower compared with control cells; however, the
potential in OE-CTPSR294 cells was significantly higher
compared with OE-CTPS cells without TSN treatment and markedly
higher compared with OE-CTPS cells with TSN treatment (Fig. 6A and B). Furthermore, mRNA and
protein expression levels of Bax and Bcl-2 were assessed by RT-qPCR
and western blotting. The mRNA and protein expression levels of Bax
increased significantly, whereas Bcl-2 mRNA expression levels
markedly decreased and protein expression levels significantly
decreased, in OE-CTPS cells compared with control cells.
Furthermore, mRNA and protein expression levels of Bax
significantly decreased, whereas mRNA and protein expression levels
of Bcl-2 significantly increased in OE-CTPSR294 cells
compared with OE-CTPS cells in the presence or absence of TSN
treatment (Fig. 6C-H).
Discussion
TSN exhibits anticancer effects on numerous types of
human cancer cell (32), such as
suppresses hepatocellular carcinoma proliferative and metastasis
(33), induces the apoptosis of
human Ewing's sarcoma (34). In
the present study, TSN significantly inhibited proliferation of
MKN45 cells in a time- and dose-dependent manner. CTPS formed
cytoophidia following TSN-induced inhibition of MKN45 cell
proliferation; moreover, the number of CTPS cytoophidia increased
with TSN dosage. However, high concentrations of TSN led to cell
death and affected the formation of CTPS cytoophidia; fewer CTPS
cytoophidia were observed when cells were treated with 120 nM TSN.
These data suggested that cytoophidia formation may affect
proliferation rate and apoptosis of cancer cells.
Cytoophidia are a type of intracellular compartment
conserved across prokaryotes and eukaryotes and are involved in
cell metabolism (35). The first
reported component of the cytoophidia was CTPS (2–4).
CTPS is a cytosol-associated glutamine amidotransferase enzyme that
catalyzes de novo biosynthesis of CTP, a key nucleotide.
Polymerization of CTPS into filamentous structures (cytoophidia)
regulates its enzymatic activity (8,35).
The formation of cytoophidia is reported to inhibit CTPS activity
in E. coli and Drosophila (14,15). In the present study, R294D-CTPS
mutants were generated and used to evaluate the effect of CTPS
cytoophidia on TSN-induced proliferation and apoptosis. Although
the R294D-CTPS mutant did not form cytoophidia, CTPS activity was
not affected (10,36). As expected, significantly fewer
EdU-positive cells were observed in the OE-CTPS group compared with
the control in the present study. However, a significantly higher
percentage of EdU-positive cells was observed in
OE-CTPSR294D compared with OE-CTPS cells. The decrease
in EdU-positive cells demonstrated that formation of CTPS
cytoophidia affected the proliferation rate of MKN45 cells.
Proliferating cells have been reported to
demonstrate higher RNA and DNA synthesis rates during
G1- and S-phase (37).
Therefore, proliferating cells synthesize increased amounts of
ribonucleotides and deoxyribonucleotides. As CTPS is key for de
novo synthesis of CTP, a precursor for RNA and DNA synthesis,
it can be hypothesized that CTPS activity increases in
G1-phase of the cell cycle to support increased
synthesis of nucleic acids. The present study demonstrated that the
percentage of G1/G0-phase cells significantly
increased and the percentage of S-phase cells markedly decreased in
OE-CTPS cells compared with the control. Moreover, the mRNA and
protein expression levels of CCND1 markedly decreased in OE-CTPS
cells compared with the control. OE-CTPSR294D cells
which demonstrated significantly decreased percentage of
G1/G0-phase cells and increased the percentage of S-phase cells,
meanwhile increased CCND1 mRNA and protein expression levels
compared with both OE-CTPS and control cells. The aforementioned
effects of OE-CTPS and R294D-CTPS mutation were greater following
treatment with 80 nmol/l TSN, and subG1 peak was observed
simultaneously, but the proportion of subG1 values were not
calculated as the software cannot analyze it. The aforementioned
results suggested that formation of CTPS cytoophidia affected RNA
synthesis during the cell cycle, thereby inhibiting MKN45 cell
proliferation induced by TSN.
TSN has been reported to suppress proliferation and
induce apoptosis in numerous types of human cancer cell, such as
hepatocellular carcinoma (26,33). The present study assessed the
effect of CTPS cytoophidia on TSN-induced apoptosis in MKN45 cells.
Following formation of TSN-induced CTPS cytoophidia, the number of
apoptotic bodies and apoptotic rate increased markedly in OE-CTPS
cells compared with the control. A decrease in mitochondrial
membrane potential occurs during early cell apoptosis; the present
study demonstrated that mitochondrial membrane potential markedly
decreased following formation of TSN-induced CTPS cytoophidia in
OE-CTPS cells. However, the mitochondrial membrane potential
increased significantly when the formation of CTPS cytoophidia was
prevented in OE-CTPSR294D cells. Furthermore, mRNA and
protein expression levels of Bcl-2 markedly decreased whereas those
of proapoptotic Bax markedly increased in OE-CTPS compared with
control; however, when formation of CTPS cytoophidia was prevented
in OE-CTPSR294D cells, increased Bcl-2 mRNA and protein
expression levels and decreased Bax mRNA and protein expression
levels were observed compared with both OE-CTPS and control
cells.
In conclusion, the results of the present study
suggested that CTPS promoted cell proliferation and inhibited
apoptosis in MKN-45 cells. However, when CTPS formed cytoophidia
after MKN45 cells were treated with TSN, CTPS activity was
inhibited, which arrested the cell cycle in G1 phase,
inhibiting cell proliferation and promoting apoptosis. However, the
mechanism by which TSN induces CTPS to form cytoophidia is still
unclear and requires further study.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 31801148), Natural Science
Foundation of Heilongjiang Province (grant no. LH2021C099), the
Scientific Research Fund of Heilongjiang Provincial Education
Department (grant no. 135209260) and the Basic Scientific Research
Fund of Heilongjiang Provincial Institutions of University (grant
no. YSTSXK201874).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XPF analyzed data for the work and drafted the
manuscript. WC and YP performed the experiments. CL performed data
analysis. ZZZ performed the photography using the confocal laser
microscope. SLS and WWZ designed the experiments and revised the
manuscript. WWZ obtained funding. All authors have read and
approved the final manuscript. YP and WWZ confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Levitzki A and Koshland DE Jr: Role of an
allosteric effector. Guanosine triphosphate activation in cytosine
triphosphate synthetase. Biochemistry. 11:241–246. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu JL: Intracellular compartmentation of
CTP synthase in Drosophila. J Genet Genomics. 37:281–296. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noree C, Sato BK, Broyer RM and Wilhelm
JE: Identification of novel filament-forming proteins in
Saccharomyces cerevisiae and Drosophila melanogaster. J Cell Biol.
190:541–551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ingerson-Mahar M, Briegel A, Werner JN,
Jensen GJ and Gitai Z: The metabolic enzyme CTP synthase forms
cytoskeletal filaments. Nat Cell Biol. 12:739–746. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang CC, Keppeke GD, Antos CL, Peng M,
Andrade LEC, Sung LY and Liu JL: CTPS forms the cytoophidium in
zebrafish. Exp Cell Res. 405:1126842021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carcamo WC, Satoh M, Kasahara H, Terada N,
Hamazaki T, Chan JYF, Yao B, Tamayo S, Covini G, von Mühlen CA and
Chan EKL: Induction of cytoplasmic rods and rings structures by
inhibition of the CTP and GTP synthetic pathway in mammalian cells.
PLoS One. 6:e296902011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen K, Zhang J, Tastan ÖY, Deussen ZA,
Siswick MY and Liu JL: Glutamine analogs promote cytoophidium
assembly in human and Drosophila cells. J Genet Genomics.
38:391–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aughey GN and Liu JL: Metabolic regulation
via enzyme filamentation. Crit Rev Biochem Mol Biol. 51:282–293.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Narvaez-Ortiz HY, Lopez AJ, Gupta N and
Zimmermann BH: A CTP synthase undergoing stage-specific spatial
expression is essential for the survival of the intracellular
parasite toxoplasma gondii. Front Cell Infect Microbiol. 8:832018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lynch EM, Hicks DR, Shepherd M, Endrizzi
JA, Maker A, Hansen JM, Barry RM, Gitai Z, Baldwin EP and Kollman
JM: Human CTP synthase filament structure reveals the active enzyme
conformation. Nat Struct Mol Biol. 24:507–514. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Z and Liu JL: Cytoophidia respond to
nutrient stress in Drosophila. Exp Cell Res. 376:159–167. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petrovska I, Nüske E, Munder MC,
Kulasegaran G, Malinovska L, Kroschwald S, Richter D, Fahmy K,
Gibson K, Verbavatz JM and Alberti S: Filament formation by
metabolic enzymes is a specific adaptation to an advanced state of
cellular starvation. Elife. 25:e024092014. View Article : Google Scholar
|
|
13
|
Zhang J and Liu JL: Temperature-sensitive
cytoophidium assembly in Schizosaccharomyces pombe. J Genet
Genomics. 46:423–432. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aughey GN, Grice SJ, Shen QJ, Xu Y, Chang
CC, Azzam G, Wang PY, Freeman-Mills L, Pai LM, Sung LY, et al:
Nucleotide synthesis is regulated by cytoophidium formation during
neurodevelopment and adaptive metabolism. Biol Open. 3:1045–1056.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barry RM, Bitbol AF, Lorestani A, Charles
EJ, Habrian CH, Hansen JM, Li HJ, Baldwin EP, Wingreen NS, Kollman
JM and Gitai Z: Large-scale filament formation inhibits the
activity of CTP synthetase. Elife. 16:e036382014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strochlic TI, Stavrides KP, Thomas SV,
Nicolas E, O'Reilly AM and Peterson JR: Ack kinase regulates CTP
synthase filaments during Drosophila oogenesis. EMBO Rep.
15:1184–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kizaki H, Williams JC, Morris HP and Weber
G: Increased cytidine 5′-triphosphate synthetase activity in rat
and human tumors. Cancer Res. 40:3921–3927. 1980.PubMed/NCBI
|
|
18
|
Williams JC, Kizaki H, Weber G and Morris
HP: Increased CTP synthetase activity in cancer cells. Nature.
271:71–73. 1978. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verschuur AC, Van Gennip AH, Leen R,
Meinsma R, Voute PA and van Kuilenburg AB: In vitro inhibition of
cytidine triphosphate synthetase activity by cyclopentenyl cytosine
in paediatric acute lymphocytic leukaemia. Br J Haematol.
110:161–169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin Y, Zhang J, Li Y, Guo W, Chen L, Chen
M, Chen X, Zhang W, Jin X, Jiang M, et al: CTPS1 promotes malignant
progression of triple-negative breast cancer with transcriptional
activation by YBX1. J Transl Med. 20:172022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang CC, Jeng YM, Peng M, Keppeke GD,
Sung LY and Liu JL: CTP synthase forms the cytoophidium in human
hepatocellular carcinoma. Exp Cell Res. 361:292–299. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ong ES and Ong CN: Qualitative and
quantitative analysis of toosendanin in Melia toosendan Sieb. Et
Zucc (Meliaceae) with liquid chromatography/tandem mass
spectrometry. Rapid Commun Mass Spectrom. 21:589–598. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang G, Feng CC, Chu SJ, Zhang R, Lu YM,
Zhu JS and Zhang J: Toosendanin inhibits growth and induces
apoptosis in colorectal cancer cells through suppression of
AKT/GSK-3β/β-catenin pathway. Int J Oncol. 47:1767–1774. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang C, Gao H, Liu Z, Lai J, Zhan Z, Chen
Y and Huang H: Mechanisms involved in the anti-tumor effects of
Toosendanin in glioma cells. Cancer Cell Int. 21:4922021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruan H, Song Z, Cao Q, Ni D, Xu T, Wang K,
Bao L, Tong J, Xiao H, Xiao W, et al: IMPDH1/YB-1 positive feedback
loop assembles cytoophidia and represents a therapeutic target in
metastatic tumors. Mol Ther. 28:1299–1313. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shao S, Li S, Liu C, Zhang W, Zhang Z, Zhu
S, Feng Y and Pan Y: Toosendanin induces apoptosis of MKN-45 human
gastric cancer cells partly through miR-23a-3p-mediated
downregulation of BCL2. Mol Med Rep. 22:1793–1802. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Y, Wang JJ, Ghosh S and Liu JL:
Critical roles of CTP synthase N-terminal in cytoophidium assembly.
Exp Cell Res. 354:122–133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang YM, Wu FJ, Du L, Li GY, Takahashi K,
Xue Y and Xue CH: Effects of polysaccharides from abalone (Haliotis
discus hannai Ino) on HepG2 cell proliferation. Int J Biol
Macromol. 66:354–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sabarwal A, Agarwal R and Singh RP:
Fisetin inhibits cellular proliferation and induces
mitochondria-dependent apoptosis in human gastric cancer cells. Mol
Carcinog. 56:499–514. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Tong H, Zhang Z, Shao S, Liu D,
Li S and Yan Y: Transcription factor EGR1 promotes differentiation
of bovine skeletal muscle satellite cells by regulating MyoG gene
expression. J Cell Physiol. 233:350–362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang T, Li J, Yin F, Lin B, Wang Z, Xu J,
Wang H, Zuo D, Wang G, Hua Y and Cai Z: Toosendanin demonstrates
promising antitumor efficacy in osteosarcoma by targeting STAT3.
Oncogene. 36:6627–6639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang T, Xu R, Huo J, Wang B, Du X, Dai B,
Zhu M, Zhan Y, Zhang D and Zhang Y: WWOX activation by toosendanin
suppresses hepatocellular carcinoma metastasis through JAK2/Stat3
and Wnt/β-catenin signaling. Cancer Lett. 513:50–62. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao T, Xie A, Liu X, Zhan H, Zeng J, Dai M
and Zhang B: Toosendanin induces the apoptosis of human Ewing's
sarcoma cells via the mitochondrial apoptotic pathway. Mol Med Rep.
20:135–140. 2019.PubMed/NCBI
|
|
35
|
Liu JL: The cytoophidium and its kind:
Filamentation and compartmentation of metabolic enzymes. Annu Rev
Cell Dev Biol. 32:349–372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin WC, Chakraborty A, Huang SC, Wang PY,
Hsieh YJ, Chien KY, Lee YH, Chang CC, Tang HY, Lin YT, et al:
Histidine-dependent protein methylation is required for
compartmentalization of CTP synthase. Cell Rep. 24:2733–2745. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van den Berg AA, van Lenthe H, Kipp JB, de
Korte D, van Kuilenburg AB and van Gennip AH: Cytidine triphosphate
(CTP) synthetase activity during cell cycle progression in normal
and malignant T-lymphocytic cells. Eur J Cancer. 1:108–112. 1995.
View Article : Google Scholar : PubMed/NCBI
|