Introduction
Diabetic retinopathy (DR) is the most commonly
observed microvascular complication associated with diabetes and is
the major cause of blindness among working-age population (1). The disturbed energy homeostasis
following chronic hyperglycemia (2,3),
and the subsequent activation of innate immune signaling are the
most significant mechanisms contributing to microvascular
impairment found in diabetes (4,5).
In relationship to this, an enhanced level of
glucose induces oxidative phosphorylation, therefore depolarizing
the plasma membrane and leading to enhanced mitochondrial ion flux
levels (6,7). In particular, mitochondria are the
main source of reactive oxygen species (ROS) generation under
oxidative stress (8).
Translocator protein (TSPO) is a transporting protein located at
the outer mitochondrial membrane (OMM) and contributes to multiple
process including cholesterol import (9), Ca2+ signaling and
mitochondrial metabolism. TSPO forms a complex with VDAC to
facilitate mitochondrial ion flux and metabolite transport across
the OMM (10). TSPO-VDAC complex
selectively promotes the mitochondrial Ca2+ channel,
therefore precipitates the activation of the
Ca2+−dependent NADPH oxidase, further mediates ATP
production and transportation, ROS generation and mitochondrial
membrane potential (Δψm) transitions (11).
Those overly produced ATP and disturbed ROS can be
recognized as self-derived damage-associated molecular patterns by
pattern recognition receptors of the innate immune system. The
priming igniter of innate immune system, the NOD-like receptors
pyrin domain-containing 3 (NLRP3) inflammasome, is proven to be
located to cytoplasmic granular structures, in close vicinity to
mitochondria. More direct evidence is that the NLRP3 may be
activated by VDAC through its capturing function targeting ROS
(12,13). Notably, blockade of TSPO-VDAC ion
channels results in highly diminished mitochondrial ROS generation
and therefore significantly impairs NLRP3 inflammasome activation,
suggesting the crucial role of TSPO-VDAC complex in innate immune
response ignition (14).
The significance of how the TSPO-VDAC-NLRP3
signaling cascade contributes to the pathogenesis of DR is notably
apparent. However, its expression and possible function in the PDR
are still not well understood. Therefore, it is important to gain
insights into the clinical implications of TSPO-VDAC-NLRP3
signaling in the pathogenesis and development of DR.
Materials and methods
Participants
Age- and sex-paired 54 DR and 22 ARC patients were
consecutively recruited from the outpatient clinic of Eye & ENT
Hospital of Fudan University during February 2018 to July 2019
(Table I). The study followed the
guidelines of the Declaration of Helsinki, and all experimental
protocols were approved (approval no. 2020142) by the Human Ethics
Committee of Eye & ENT Hospital of Fudan University (Shanghai,
China). Written informed consent was obtained from the enrolled
participants. The detailed explanations of the study purpose were
given to all the participants and the signatures of the informed
consent form were obtained correspondingly.
 | Table I.Clinical characteristics and
laboratory results of patients. |
Table I.
Clinical characteristics and
laboratory results of patients.
| Characteristic | DR group (n=54) | Control group
(n=22) | P-value |
|---|
| Age, years | 59.11±1.188 | 61.00±1.484 | 0.2563a |
| Female sex, n
(%) | 30 (55.6) | 12 (54.5) | >0.05b |
| hypertension | 37/17 | 10/12 | >0.05b |
| Fasting blood
glucose, mmol/l | 11.09±0.4479 | 6.323±0.1091 | <0.0001 |
| IOP | 14.63±0.3608 | 14.68±0.5481 | 0.9085 |
| HbA1c | 7.748±0.2018 | 5.809±0.1369 | 0.0001 |
Patients with known systemic inflammatory,
autoimmune, immunosuppressive disease or other diabetic
complications such as nephropathy were excluded. Patients were also
excluded if they had been subjected to intraocular procedures,
intravitreal treatments, uveitis, trauma, or immunosuppressive drug
administration.
All the selected DR patients were assessed by
ultra-wide fluorescein fundus angiography (Optos 200Tx; Optos PLC).
Body mass index was determined using the weight (kg)/height
(m2) formula. Based on the DR Disease Severity Scale,
diabetics were placed into one of the following two groups: i)
non-proliferative DR (NPDR) and ii) proliferative DR (PDR)
(Table II). According to the
standard set by Aiello et al (15), patients with PDR are further
divided into active neovascularization group and inactive
neovascularization group by their presentation in fundus image and
fluorescein angiography. Patients with age-related cataract were
selected as control group. All eyes of the subjects underwent an
overall ophthalmic evaluation, including slit-lamp biomicroscopy,
IOP measurement (Goldmann applanation tonometry), fundus
examination and ultrasound B-scanning.
 | Table II.Demographic, clinical and laboratory
data of DR patient characteristics. |
Table II.
Demographic, clinical and laboratory
data of DR patient characteristics.
| Characteristic | NPDR group
(n=18) | PDR group
(n=35) | P-value |
|---|
| Age, years | 60.77±1.462 | 56.05±1.888 | 0.8446a |
| Female, n (%) | 9 (50) | 21 (60) |
>0.0500b |
| Active PDR, n
(%) | 0/18 | 18/17 | NA |
| Duration of
diabetes, years | 8.971±0.7328 | 6.947±0.8110 | 0.3604 |
| hypertension | 12/6 | 25/10 |
>0.0500b |
| Fasting blood
glucose, mmol/l | 8.92±0.3948 | 12.26±0.5682 | 0.0039 |
| IOP | 14.32±0.6625 | 14.80±0.4296 | 0.507 |
| HbA1c | 7.17±0.2440 | 8.060±0.2696 | 0.0708 |
| Vitreoretinal
condition |
|
|
|
|
Vitreous hemorrhage | 0/18 | 18/17 |
|
|
Diabetic macular edema | 0/18 | 20/15 |
|
|
Traction retinal
detachment | 0/18 | 21/14 |
|
Whole blood sample preparation
Whole blood samples (12 ml) were collected from each
participant using sterile tubes containing lithium heparin prior to
mRNA and protein extraction. The remaining blood samples were
utilized for glycated hemoglobin and fasting plasma glucose
determination. Peripheral blood mononuclear cells (PBMCs) were
isolated from heparinized blood using Hypaque density-gradient
centrifugation (Lymphoprep; Takeda Pharmaceutical Company, Ltd.).
They were then cultured under room temperature in RPMI-1640 medium
(cat. no. #11875093) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.; cat. #26010074) and 1%
penicillin/streptomycin and seeded at a concentration of
106 cells/ml into 24-well plates for overnight. To
determine the effect of cytokine production, PBMCs from ARC and PDR
patients were stimulated for 4 h with 100 ng/ml lipopolysaccharides
(Sigma-Aldrich; Merck KGaA) and then incubated for an additional 15
min with RPMI-1640 containing 1 mM adenosine 5-triphosphate
(lipopolysaccharide/ATP; Sigma-Aldrich; Merck KGaA).
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA was extracted from isolated PBMCs of DR
patients and ARC patients using the RNA easy Mini kit (Qiagen)
according to the manufacturer's instructions. The high-capacity
cDNA reverse transcription kit (cat. #4368813. Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used for to cDNA synthesis
according to the manufacturer's instructions. Reactions were
performed for 10 min at 25°C, 2 h at 37°C, and 5 sec at 85°C. cDNA
was subsequently kept at −20°C until it was used for qPCR
amplification. TSPO, VDAC, NLRP3, apoptosis-associated speck like
protein with a caspase recruitment domain (ASC) and caspase-1 gene
expression levels were measured by qPCR using specific TaqMan
FAM/MGB assays (Applied Biosystems; assay ID Hs00559362_m1 for
TSPO, assay ID Hs01631624_gH for VDAC1, assay ID Hs00918082_m1 for
NLRP3, assay ID Hs01547324_gH for ASC and assay ID Hs00354836_m1
for caspase-1).
Reactions were performed in an Applied Biosystems
7500 real-time PCR system. Gene expression levels were normalized
using a TaqMan VIC/MGB eukaryotic 18S rRNA endogenous control assay
(Applied Biosystems, cat. #4319413E). Reaction system consists of 6
µl 2× TaqMan Fast Advanced Master Mix (Applied Biosystems; cat. #
A44360), 0.6 µl of 20× TaqMan gene expression assay, 0.6 µl 20×
TaqMan endogenous control, 3.8 µl of water, and 1 µl of cDNA sample
solution.
The thermal cycling parameters were 95°C for 10 min,
followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec. The
following primer sequences were used: TSPO,
5′-CATGGGGTATGGCTCCTA-3′ (forward) and 5′-AGACCCAAGGGAACCATA-3′
(reverse); VDAC1, 5′-GGTGCTCTGGTGCTAGGTTA-3′ (forward) and
5′-CAGCGGTCTCCAACTTCTTG-3′ (reverse); NLRP3,
5′-GTAGGTGTGGAAGCAGGACT-3′ (forward) and 5′-CTTGCTGACTGAGGACCTGA-3′
(reverse); ASC, 5′-AACCCAAGCAAGATGCGGAAG-3′ (forward) and
5′-TTAGGGCCTGGAGGAGCAAG-3′ (reverse); caspase-1,
5′-CTCAGGCTCAGAAGGGAATG-3′ (forward) and 5′-CGCTGTACCCCAGATTTTGT-3′
(reverse). Relative expression levels were determined by the
2−ΔΔCq method (16).
All reactions were performed in triplicate.
ELISA for IL-1β and IL-18
The concentration of IL-1β and IL-18 in the culture
supernatants of PBMCs from DR and ARC patients was measured by
ELISA following the manufacturer's instructions (R&D Systems,
Inc.; cat. nos. QK201 and DL180.). The minimal detectable
concentration of IL-1β was 3.9 pg/ml and the minimal detectable
concentrations of IL-18 was 26.6 pg/ml. All the samples were
measured in replication for two times.
Statistical analysis
Statistical Package for the Social Sciences
Statistical Software (SPSS v18.0; SPSS, Inc.) was used for
statistical evaluations. Non-parametric Kruskal-Wallis test,
one-way ANOVA analysis of variance and Bonferroni correction were
used to evaluate group variations between diabetic patients and
cataract patients according to normality assumption and homogeneity
of variances. Unpaired Student's t-tests and Mann-Whitney U tests
were employed to examine variations between all groups. Spearman's
correction test was used to examine study parameters between
groups. Graphs were drawn using Prism version 5 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient clinical features
The clinical characteristics and laboratory results
of patients were summarized in Table
I. No significant deviations among groups in age and sex were
found (P>0.05). No significant difference was found in the mean
duration of diabetes between PDR and NPDR groups (Table II). HbA1c and fasting glucose
levels were also found to be significantly elevated in the PDR
group than in the cataract group (P<0.0001). Fasting glucose
levels were significantly higher in PDR group than that in NPDR
group (Table II), while no
significant difference was found regarding HbA1c between two
groups.
Expression levels of TSPO/VDAC Complex
and NLPR3 inflammasomes
The mRNA expression levels of TSPO, VDAC, NLRP3, ASC
and caspase-1 were evaluated in the PBMCs of patients with DR and
controls by using RT-qPCR. VDAC expression was found to be
associated with increasing levels of TSPO. The mRNA expression
levels of TSPO and VDAC in PDR and NPDR patients were significantly
higher than that in controls (Fig. 1A
and B). Among DR patients, the expression of TSPO and VDAC were
significantly higher in PDR group compared with that in NPDR group
(Fig. 1C and D). TSPO/VDAC
expression levels were also significantly higher in the active PDR
subgroup than that in the inactive PDR subgroup (Fig. 1E and F).
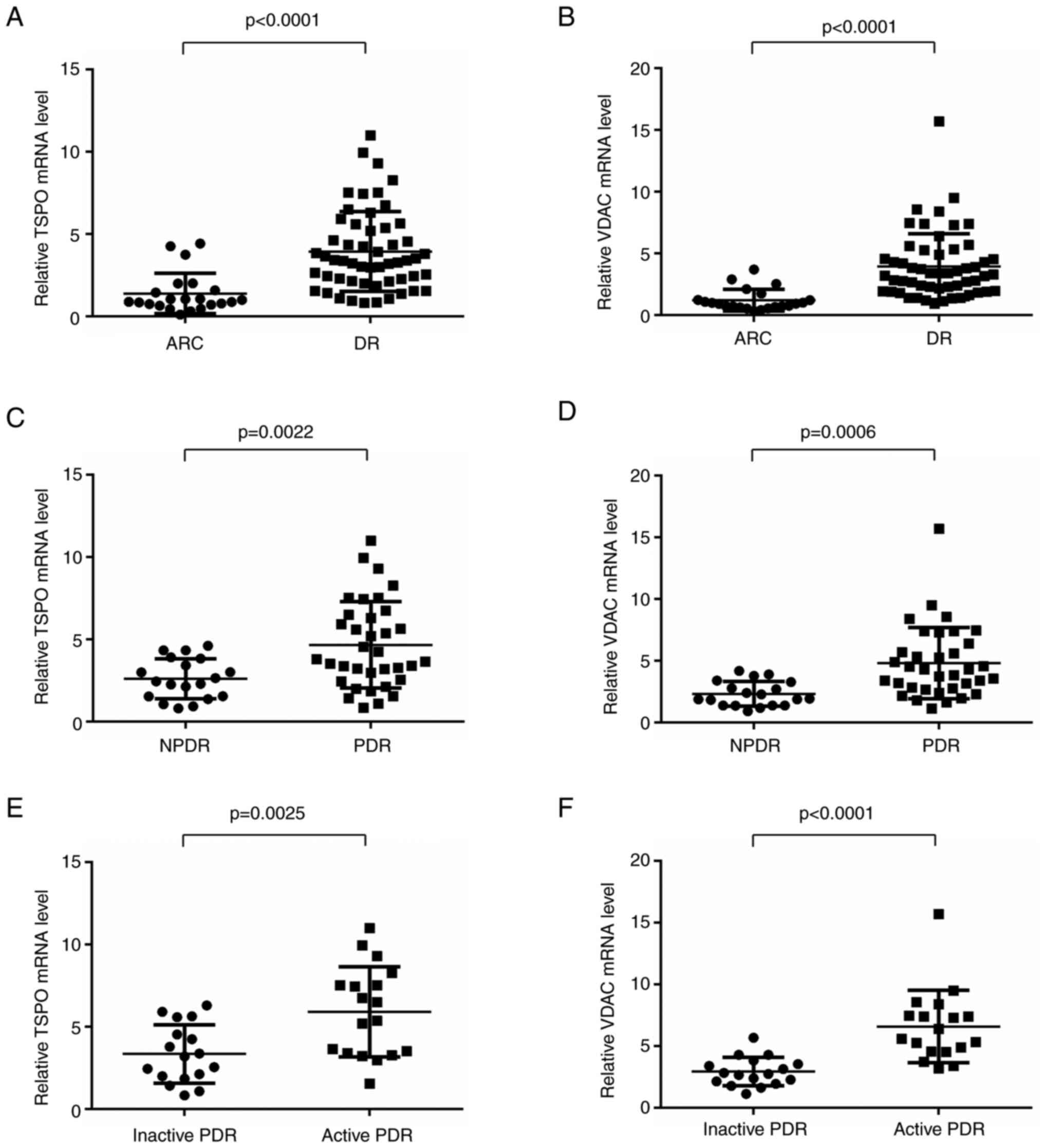 | Figure 1.Expression levels of TSPO, VDAC and
NLPR3 in PDR and NPDR patients (A and B) mRNA expression levels of
TSPO and VDAC in DR patients were significantly higher than that in
controls (DR, n=54; ARC, n=22). (C and D) The expression of TSPO
and VDAC was significantly higher in the PDR group compared with
that in the NPDR group (PDR, n=35; NPDR, n=18). (E and F) Relative
TSPO and VDAC mRNA levels in PBMCs from active PDR patients were
significantly higher than that in inactive PDR cohorts (active PDR,
n=18; inactive PDR, n=35). Data are presented as the median and
interquartile range. TSPO, translocator protein; VDAC,
voltage-dependent anion channel; DR, diabetic retinopathy; PDR,
proliferative DR; NPDR, non-proliferative DR. |
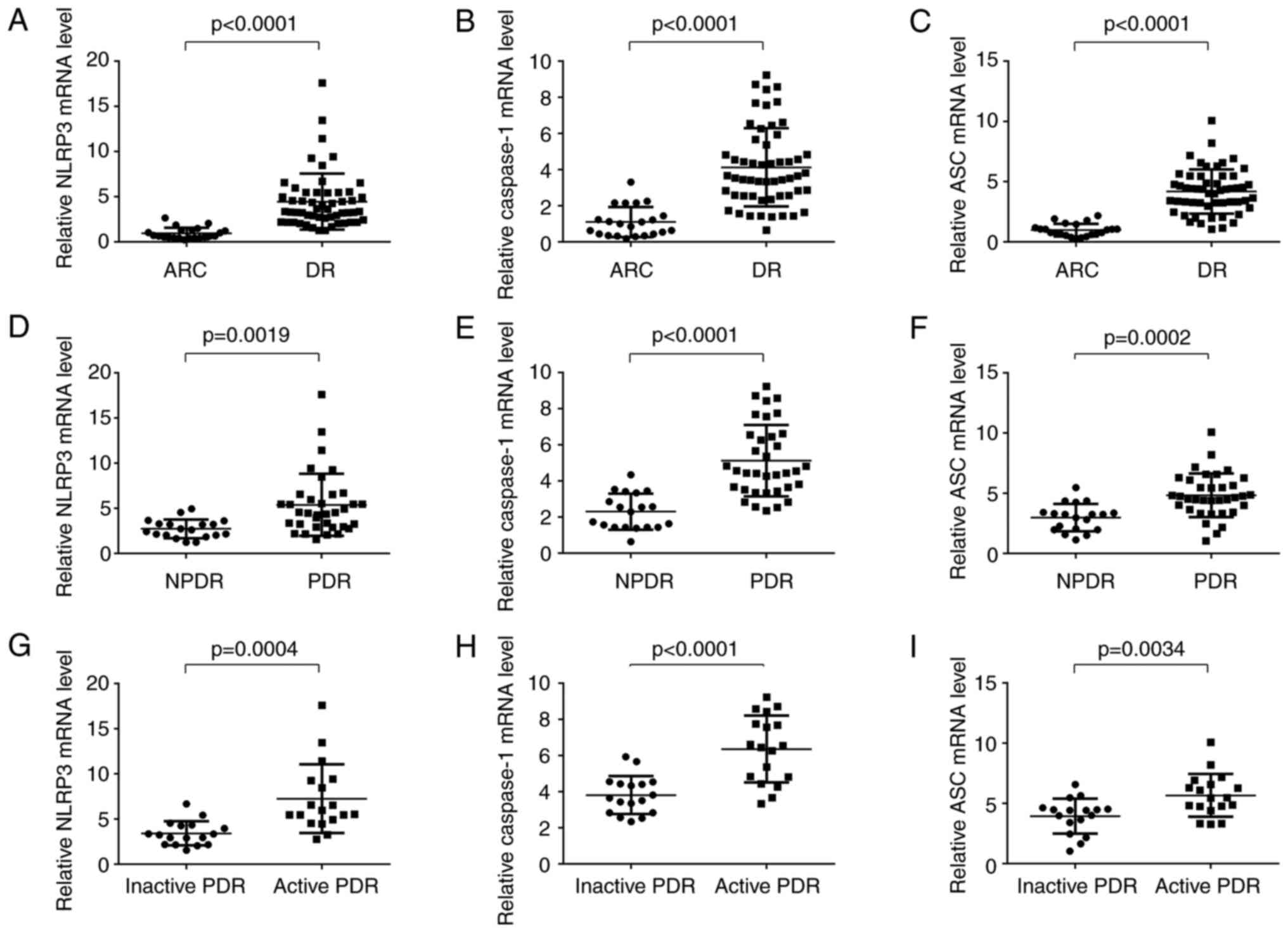
The expression levels of NLRP3 and its key molecules
ASC and caspase-1 were significantly upregulated in patients with
DR compared with ARC patients (Fig.
2A-C). The expression levels of NLRP3, ASC and caspase-1 were
significantly higher in PDR patients compared with NPDR patients
(Fig. 2D-F). Meanwhile, higher
levels of NLRP3, ASC and caspase-1 were detected in patients with
active PDR than that of those with inactive PDR (Fig. 2G-I). As expected, there was a
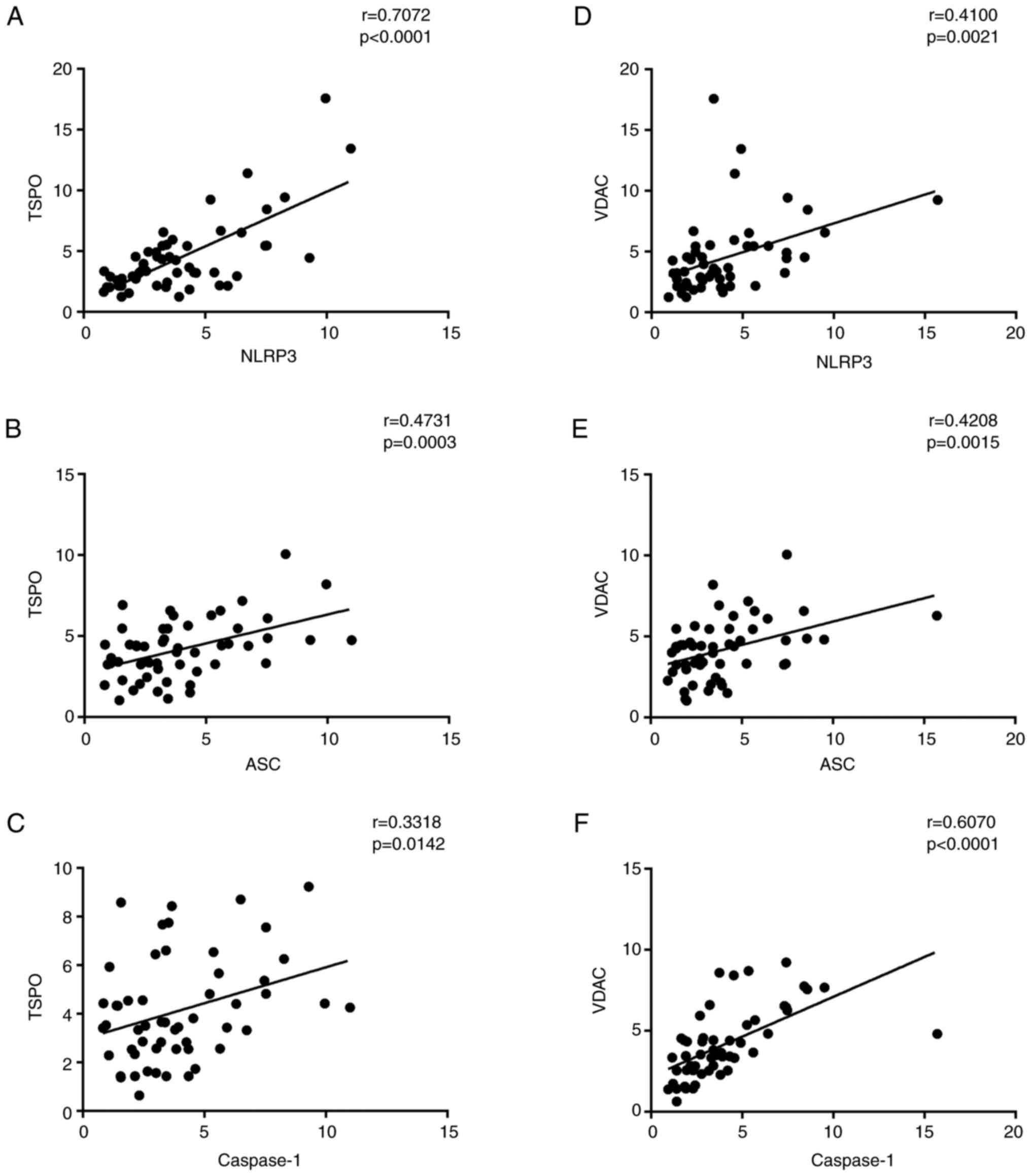
strong positive correlation between NLRP3-related inflammasome
activation proteins (NLRP3, ASC, caspase-1, IL-1β, and IL-18) and
TSPO-related proteins (TSPO and VDAC), respectively (Fig. 3).
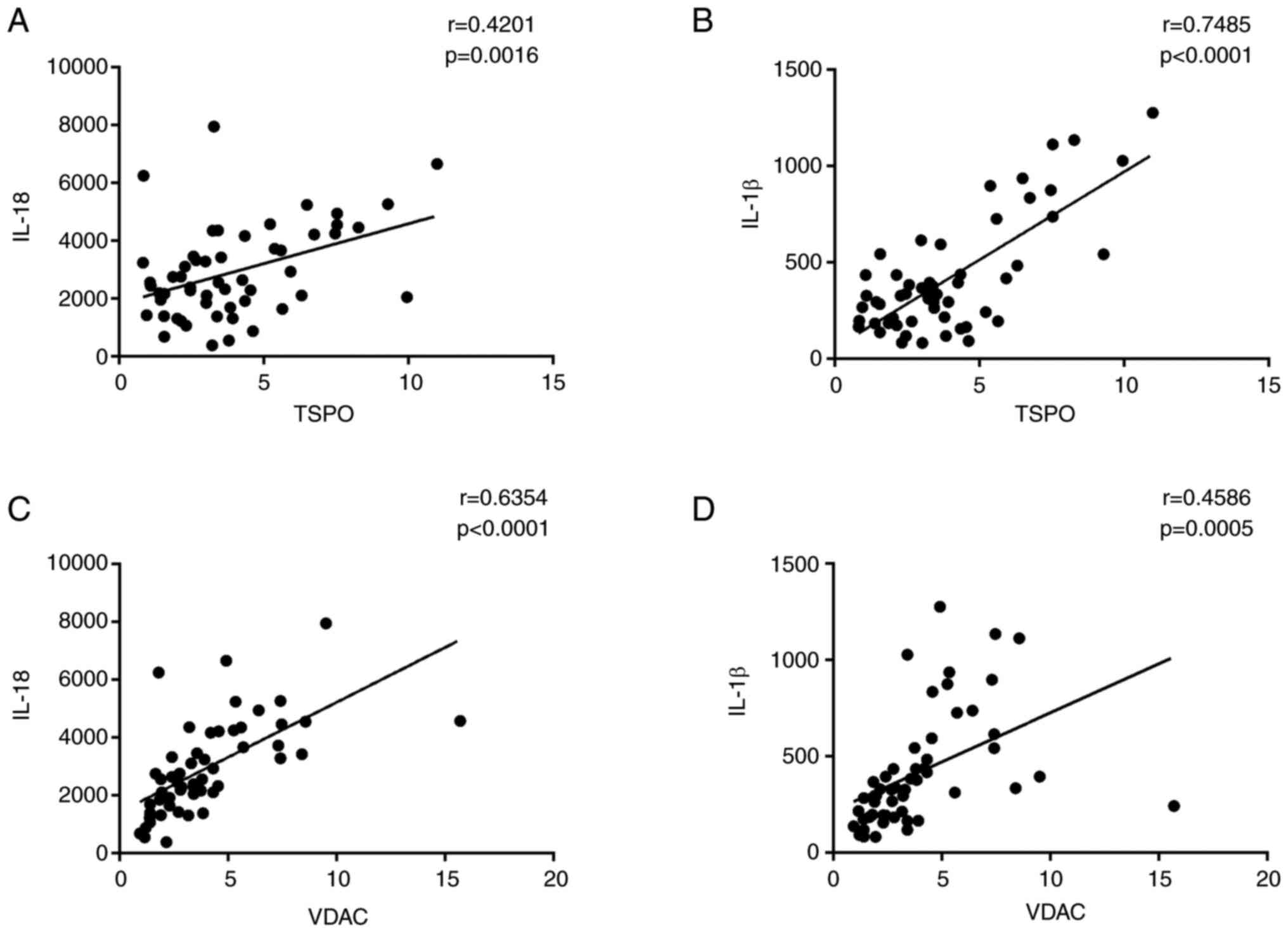
Concentration levels of IL-1β and
IL-18 in PBMC of patients with DR and ARC
One of the features of activated inflammasomes is to
promote IL-1β and IL-18 production (17). Therefore, the protein levels of
IL-1β and IL-18 were investigated by using ELISA. The expression
levels of IL-1β and IL-18 were significantly higher in DR patients
compared with those in the ARC group (Fig. 4A and B). In PDR patients, the
expression levels of IL-1β and IL-18 were significantly elevated
compared with those in NPDR patients (Fig. 4C and D). Meanwhile, DR patients
with active neovascularization showed significantly higher levels
of IL-1β and IL-18 expression in comparison with active DR patients
(Fig. 4E and F).
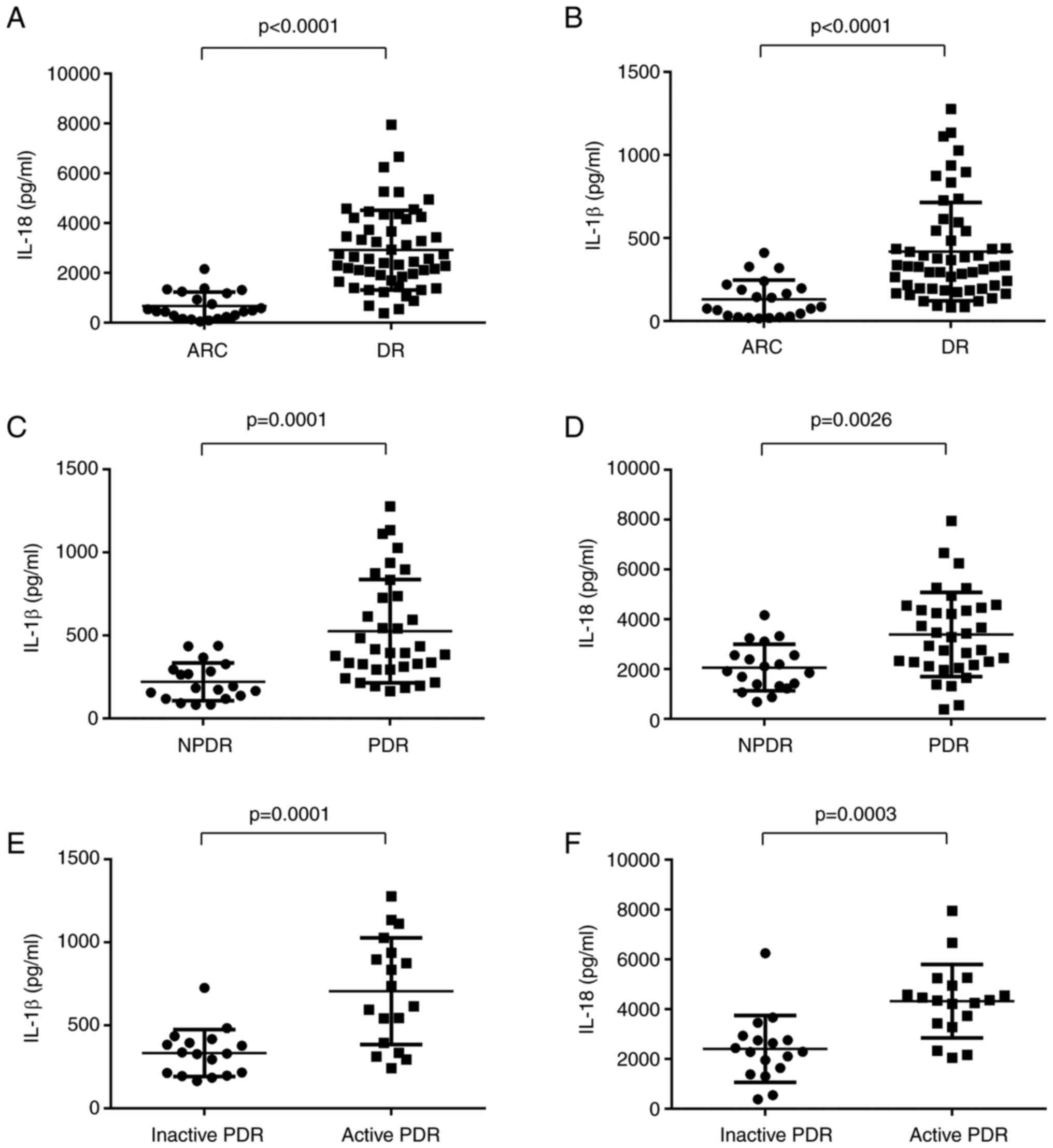 | Figure 4.Expression levels of IL-1β and IL-18
in ARC, DR and NPDR. (A and B) Expression levels of IL-1β and IL-18
were significantly higher in DR patients compared with those in ARC
group (DR, n=54; ARC, n=22). (C and D) Relative IL-18 and IL-1β
protein levels in PBMCs from PDR patients were significantly
elevated compared with that in NPDR cohorts (PDR, n=35; NPDR,
n=18). (E and F) Relative IL-18 and IL-1β protein levels in PBMCs
from active PDR patients were higher than that in inactive PDR
cohorts (active PDR, n=18; inactive PDR, n=35). Data are presented
as the median and interquartile range. DR, diabetic retinopathy;
PBMCs, peripheral blood mononuclear cells; PDR, proliferative DR;
NPDR, non-proliferative DR. |
Positive correlations between TPSO,
VDAC and NLRP3 cascade levels and IL-1β and IL-18 expression
levels
Accumulating evidence has linked the role of TSPO
and VDAC with the NLRP3 activation in numerous pathologic
conditions (18–20). In accordance with those studies,
positive correlations were found among NLRP3, ASC and caspase-1
levels and TSPO (Fig. 3A-C) and
VDAC expression levels (Fig.
3D-F). As expected, positive correlations were also found
between IL-1β and IL-18 protein levels and TSPO (Fig. 5A and B) and VDAC expression levels
(Fig. 5C and D).
Discussion
DR is considered a para-inflammation entity
triggered by metabolic and biochemical disorder. Investigating the
interface between metabolic dysregulation and inflammatory
activities is essential for understanding the pathogenesis of DR.
In the present study, it was found that the elevated expression
levels of TSPO-VDAC complex are associated with the activation of
NLRP3 inflammasome, demonstrating the inflammatory crosstalk in the
development of DR.
Hyperglycemia induces various stress conditions
including disorderly metabolic rates, mitochondrial respiratory
chain overreaction and accumulation of cytosolic damage signals
such as NADPH oxidase and ROS (21). TSPO is the most abundant channel
protein in the OMM to exchange metabolites between cellular
compartments including mitochondria. It is proven that TSPO and its
coordinating protein VDAC are crucial components in the process of
mitochondrial metabolism activity and are essential for cytosolic
ROS production (11), therefore
initiates innate immune responses and pathological angiogenesis in
retinal microglia (22). In this
aspect, the mRNA expression of TSPO and VDAC were examined in PBMCs
of patients with DR and controls. The present results revealed that
the mRNA expression levels of TSPO and VDAC were significantly
greater in DR patients in comparison with those in controls.
Moreover, the levels of TSPO and VDAC were significantly higher in
PDR patients compared with those in DR patients in earlier stage,
indicating that TSPO and VDAC are positively correlated with the
severity of DR. Notably, DR patients with active neovascularization
showed higher expression levels of TSPO and VDAC compared with
those in inactive DR patients. This phenomenon is in parallel with
a recent study conducted by Wolf et al (22), which demonstrated that TSPO plays
important role in promoting the secretion of IL-1β, and
subsequently mediates neovascular formation in retinal microglia.
Those results suggested that TSPO and VDAC may participate in the
pathogenic angiogenesis in DR through activating the innate immune
responses.
A previous study conducted by Chen et al
(23) demonstrated that NLRP3
inflammasome contributes to the inflammatory responses in different
stages of DR, but how TSPO-VDAC exert their roles in DR and whether
they are connected with NLRP3 inflammasomes in DR, and whether
their expression changes upon the severity of DR remain unclear.
The NLRP3 inflammasome is activated upon ‘cellular damage signal’
and works as a molecular platform to integrate and ripen
pro-inflammatory cytokines, but the underlying mechanism of NLRP3
activation remains unclear. One of the models proposes that NLRP3
is activated by a common pathway of ROS (18). NLRP3 was proven to be localized in
close vicinity to mitochondria for efficient sensing of the
presence of the fast-fading ROS (17). Zhou et al provided more
direct evidence by demonstrating that knocking down VDAC
significantly diminishes mitochondrial ROS generation and therefore
considerably impairs NLRP3 inflammasome activation (12). In the present study, the mRNA
expression of NLRP3 inflammasome adaption proteins (NLRP3, ASC and
caspase-1) and protein levels of IL-1β and IL-18 were examined. Our
data revealed that the expression levels of NLRP3 inflammasome
activation proteins (NLRP3, ASC, caspase-1, IL-1β and IL-18) were
significantly greater in DR patients than those in ARC patients.
Furthermore, the TSPO and VDAC levels were found to be positively
correlated with NLRP3, ASC, caspase-1, IL-1β and IL-18 expression.
The present results were in consistency with a previous study
conducted by Nakahira et al (17), who found that TSPO ligands
inhibited NLRP3 inflammasome activation through mitochondrial
disturbance in BMDM cells.
In addition, it was revealed that the levels of
NLRP3, ASC, caspase-1, IL-1β and IL-18 expression were
significantly higher in PDR patients compared with those in NPDR
patients. Meanwhile, active DR patients showed significantly more
elevated expression levels of NLRP3, ASC, caspase-1, IL-1β and
IL-18 compared with those in inactive DR patients. ASC is the
adaptor protein of NLRP3 and plays critical role in the formation
of inflammasome (24). After
being recruited by NLRP3, ASC interacts with cleaved caspase-1, and
in turn permits the maturation and secretion of IL-1β and IL-18
(25); the latter ones are potent
proinflammatory cytokines that can drive downstream molecular
cascades such as IL-6 and VEGF (26).
The aforementioned evidence is in consistency with
the present findings that the expression levels of TSPO-VDAC and
NLRP3 activation proteins (NLRP3, ASC, caspase-1, IL-1β and IL-18)
develop in parallel with the severity of DR and are related to the
clinical complications of DR such as neovascularization.
There was a limitation to the present study; the
cytokines were only partially examined in protein level due to
inadequate blood sample volumes as the count of PBMCs was not
enough for sufficient protein extraction for western blotting.
Therefore, RT-qPCR and ELISA were used to evaluate the expression
of related cytokines.
In summary, the present study demonstrated for the
first time that metabolic and inflammatory markers measured in
peripheral blood are associated with DR. The present findings
emphasized the critical need for further characterization of the
TSPO and VDAC pathways in DR patients, as changes in their
expression may result in activation of NLRP3 pathways, resulting in
inflammation and neovascularization. Future longitudinal studies
examining the relationship between mitochondrial markers and the
progression of DR, as well as the assessment of inflammatory
markers in other tissues relevant to the pathophysiology of DR, are
necessary.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Youth Project of the
National Natural Science Fund (grant nos. 82101150 and 82101149),
the Shanghai clinical three-year action plan-major clinical
research project (grant no. SHDC2020CR2041B) and the Shanghai
Municipal Health Committee (grant no. 20204Y0056).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YG collected samples and performed the experiments
and data analyses. ZS contributed significantly to analysis and
manuscript preparation. QS contributed to the conception of the
study, performed the data analyses and wrote the manuscript. YG, ZS
and QS confirm the authenticity of all the raw data. LW, RJ and GX
helped in performing the analysis with constructive discussions.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study followed the guidelines of the Declaration
of Helsinki, and all experimental protocols were approved (approval
no. 2020142) by the Human Ethics Committee of Eye & ENT
Hospital of Fudan University (Shanghai, China). Written informed
consent was obtained from the enrolled participants. The detailed
explanations of the study purpose have been given to all the
participants, and the signatures of the informed consent form have
been obtained correspondingly.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Semeraro F, Morescalchi F, Cancarini A,
Russo A, Rezzola S and Costagliola C: Diabetic retinopathy, a
vascular and inflammatory disease: Therapeutic implications.
Diabetes Metab. 45:517–527. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kowluru RA, Kowluru A, Mishra M and Kumar
B: Oxidative stress and epigenetic modifications in the
pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 48:40–61.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simó-Servat O, Simó R and Hernández C:
Circulating biomarkers of diabetic retinopathy: An overview based
on physiopathology. J Diabetes Res. 2016:52637982016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Figueras-Roca M, Molins B,
Sala-Puigdollers A, Matas J, Vinagre I, Ríos J and Adán A:
Peripheral blood metabolic and inflammatory factors as biomarkers
to ocular findings in diabetic macular edema. PLoS One.
12:e01738652017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang J and Kern TS: Inflammation in
diabetic retinopathy. Prog Retin Eye Res. 30:343–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeiner J, Loukovaara S, Losenkova K,
Zuccarini M, Korhonen AM, Lehti K, Kauppinen A, Kaarniranta K,
Müller CE, Jalkanen S and Yegutkin GG: Soluble and membrane-bound
adenylate kinase and nucleotidases augment ATP-mediated
inflammation in diabetic retinopathy eyes with vitreous hemorrhage.
J Mol Med (Berl). 97:341–354. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mishra M, Lillvis J, Seyoum B and Kowluru
RA: Peripheral blood mitochondrial DNA damage as a potential
noninvasive biomarker of diabetic retinopathy. Invest Ophthalmol
Vis Sci. 57:4035–4044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blake R and Trounce IA: Mitochondrial
dysfunction and complications associated with diabetes. Biochim
Biophys Acta. 1840:1404–1412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ilkan Z and Akar FG: The mitochondrial
translocator protein and the emerging link between oxidative stress
and arrhythmias in the diabetic heart. Front Physiol. 9:15182018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shoshan-Barmatz V, Pittala S and Mizrachi
D: VDAC1 and the TSPO: Expression, interactions, and associated
functions in health and disease states. Int J Mol Sci. 20:33482019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gatliff J, East D, Crosby J, Abeti R,
Harvey R, Craigen W, Parker P and Campanella M: TSPO interacts with
VDAC1 and triggers a ROS-mediated inhibition of mitochondrial
quality control. Autophagy. 10:2279–2296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou R, Yazdi AS, Menu P and Tschopp J: A
role for mitochondria in NLRP3 inflammasome activation. Nature.
469:221–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Afonina IS, Zhong Z, Karin M and Beyaert
R: Limiting inflammation-the negative regulation of NF-κB and the
NLRP3 inflammasome. Nat Immunol. 18:861–869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Swanson KV, Deng M and Ting JPY: The NLRP3
inflammasome: Molecular activation and regulation to therapeutics.
Nat Rev Immunol. 19:477–489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aiello LP, Avery RL, Arrigg PG, Keyt BA,
Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et
al: Vascular endothelial growth factor in ocular fluid of patients
with diabetic retinopathy and other retinal disorders. N Engl J
Med. 331:1480–1487. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakahira K, Haspel JA, Rathinam VA, Lee
SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim
HP, et al: Autophagy proteins regulate innate immune responses by
inhibiting the release of mitochondrial DNA mediated by the NALP3
inflammasome. Nat Immunol. 12:222–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimada K, Crother TR, Karlin J, Dagvadorj
J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et
al: Oxidized mitochondrial DNA activates the NLRP3 inflammasome
during apoptosis. Immunity. 36:401–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lv Q, Xu D, Ma J, Wang Y, Yang X, Zhao P,
Ma L, Li Z, Yang W, Liu X, et al: Uric acid drives intestinal
barrier dysfunction through TSPO-mediated NLRP3 inflammasome
activation. Inflamm Res. 70:127–137. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng H, Liu Y, Zhang R, Liang Y, Sun L,
Lan N and Ma B: TSPO ligands PK11195 and midazolam reduce NLRP3
inflammasome activation and proinflammatory cytokine release in
BV-2 cells. Front Cell Neurosci. 14:5444312020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Franceschi C, Garagnani P, Parini P,
Giuliani C and Santoro A: Inflammaging: A new immune-metabolic
viewpoint for age-related diseases. Nat Rev Endocrinol. 14:576–590.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wolf A, Herb M, Schramm M and Langmann T:
The TSPO-NOX1 axis controls phagocyte-triggered pathological
angiogenesis in the eye. Nat Commun. 11:27092020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen H, Zhang X, Liao N, Mi L, Peng Y, Liu
B, Zhang S and Wen F: Enhanced expression of NLRP3
inflammasome-related inflammation in diabetic retinopathy. Invest
Ophthalmol Vis Sci. 59:978–985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wan Z, Fan Y, Liu X, Xue J, Han Z, Zhu C
and Wang X: NLRP3 inflammasome promotes diabetes-induced
endothelial inflammation and atherosclerosis. Diabetes Metab Syndr
Obes. 12:1931–1942. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong Z, Liang S, Sanchez-Lopez E, He F,
Shalapour S, Lin XJ, Wong J, Ding S, Seki E, Schnabl B, et al: New
mitochondrial DNA synthesis enables NLRP3 inflammasome activation.
Nature. 560:198–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song Z, Sun M, Zhou F, Huang F, Qu J and
Chen D: Increased intravitreous interleukin-18 correlated to
vascular endothelial growth factor in patients with active
proliferative diabetic retinopathy. Graefes Arch Clin Exp
Ophthalmol. 252:1229–1234. 2014. View Article : Google Scholar : PubMed/NCBI
|