Introduction
Reprogramming of energy metabolism is one of the
hallmarks of cancer (1).
Disordered energy metabolism is closely associated with tumor
development and the essence of tumors is the disordered
proliferation of cells, which includes not only the dysregulation
of genes but also the dysregulation of energy metabolism. The
energy metabolism associated with abnormal changes occurs in the
metabolic process of tumor development because tumor division
(rapid proliferation and DNA replication) requires a large amount
of direct energy in the form of ATP (2). Glucose, protein and lipid substances
are associated with energy metabolism. In the early days, the focus
of research on tumor metabolism was glucose metabolism (3). However, in addition to glucose
metabolism, fatty acid metabolism in cancer is dysregulated,
specifically in the expression and activity of lipid-metabolizing
enzymes (4). Abnormal fatty acid
metabolism has also been reported to be involved in tumorigenesis
and tumor progression. Lipids are another important energy source
that promotes tumor metastasis and tumor metabolism depends on the
activity of enzymes involved in fatty acid metabolism (5).
Fatty acid metabolism includes fatty acid uptake,
de novo synthesis and β-oxidation. Deciphering the complex
role of fatty acid β-oxidation in tumors contributes to a
comprehensive understanding of its pathogenesis. In fatty acid
metabolism, the role of fatty acid β-oxidation is crucial and
mitochondrial trifunctional protein (MTP) is involved in the
second, third and fourth steps of fatty acid β-oxidation (Fig. 1). MTP [the structure of the
heterotetramer based on the membrane binding domain (6)] is a protein located on the inner
mitochondrial membrane and a heterotetramer consisting of two α
subunits (HADHA) and two β subunits (HADHB). Several forms made of
α and β subunit coexist (α2β2, α4β4, α6β6), but it is generally
accepted that only the α2β2 octomer conserves the enzymatic
activities responsible of the beta oxidation (6) (Fig.
1). Therefore, HADH, which participates in and regulates fatty
acid metabolism, is a component of MTP. The α subunit of MTP is
encoded by the HADHA gene and has the activities of
enoyl-CoAhydratase (ECH) and L-β hydroxyacylCoA dehydrogenase
(HACD) and the β subunit of MTP is encoded by the HADHB gene and
has the activity of β-ketoacylCoAthiolase (KT) (6–8).
ECH, HACD and KT are key enzymes in fatty acid β-oxidation.
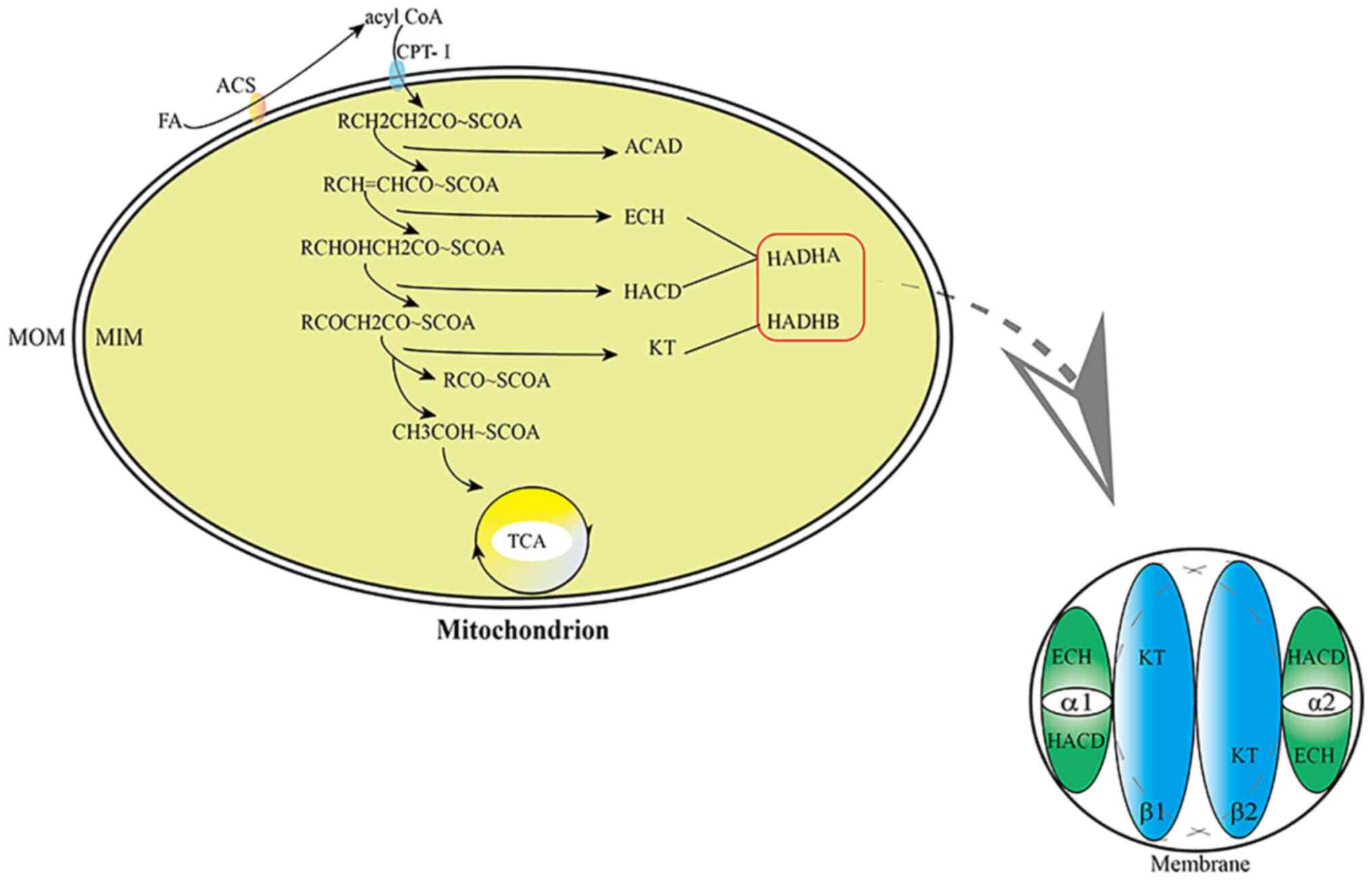 | Figure 1.Steps of fatty acid β-oxidation and
the structure of HADH. MOM, mitochondrial outer membrane; MIM,
mitochondrial inner membrane; FA, fatty acid; ACS, acetyl CoA
synthase; CPT-I, carnitine palmitoyltransferase-I; ACAD, acyl-CoA
dehydrogenase; ECH, enoyl-CoAhydratase; HACD, L-β hydroxyacylCoA
dehydrogenase; KT, β-ketoacylCoAthiolase; TCA, tricarboxylic acid
cycle. |
The HADHA and HADHB genes are located on chromosome
2 (2p23); specifically, the HADHA gene is located at 2p24.1-23.3 of
chromosome 2 (9,10). The gene of HADHA mutations may
result in LCHAD deficiency (11).
Mitochondrial trifunctional protein deficiency (MTPD) is a
metabolic disease of fatty acid β-oxidation caused by HADHB gene
mutations. It can also be seen that HADH is closely associated with
fatty acid β-oxidation (12).
Overall, HADH serves an important role in fatty acid
β-oxidation and alterations in fatty acid metabolism are associated
with tumorigenesis. However, there is no review summarizing the
role of HADH in cancer. The present review summarized the altered
fatty acid metabolism of HADHA and HADHB in several tumors and
explored their potential clinical value.
The roles of HADH in different organs
Cerebrum
Lipid droplets (LDs) are significantly enriched in
glioblastoma multiforme (GBM) (13). Studies have shown that fatty acid
β-oxidation and immunity mediate radioresistance in GBM (14) and fatty acid β-oxidation is
critical for tumor survival (15).
Wang et al (16) showed that telmisartan can inhibit
the proliferation of GBM by inducing fatty acid β-oxidation. The
increased fatty acid β-oxidation is associated with the high
expression level of HADHA. The second and third steps of
HADHA-catalyzed fatty acid β-oxidation contribute to the
upregulation of fatty acid β-oxidation. This demonstrates that
telmisartan can not only affect fatty acid metabolism to treat
hypertension but also act on HADHA to interfere with the
proliferation of GBM through fatty acid metabolism, which provides
a therapeutic strategy for tumor treatment.
Oral cavity
Oral squamous cell carcinoma (OSCC) accounts for
>90% of head and neck squamous cell carcinomas (17). Some studies have shown that fatty
acid β-oxidation produces a large number of byproducts and some
byproducts can also be used as early diagnostic indicators of OSCC
(18–20). This suggests that there is an
association between oral cancer and fatty acid β-oxidation.
Huang et al (21) constructed a prognostic risk
scoring model for OSCC (containing the survival-related metabolic
gene HADHB). The HADHB gene was significantly downregulated in this
model. This study demonstrated that the risk score is an
independent prognostic factor for OSCC and provides a more accurate
and personalized prediction of OSCC prognosis. Furthermore,
tumor-infiltrating immune cells were negatively correlated with the
risk score.
Esophagus
Abnormal fatty acid β-oxidation serves a role in
esophageal cancer (EC) (22).
Moreover, studies have shown that adipose tissue and adipocytes are
closely associated with the occurrence of EC and affect the
occurrence and metastasis of EC, which is associated with abnormal
fatty acid metabolism (22,23). In addition, esophageal squamous
cell carcinoma (ESCC) is particularly prominent in China,
accounting for ~88% of EC cases (24). The 5-year survival rate of
esophageal adenocarcinoma (EA) is only 20% (25), reflecting the importance of
identifying genes or proteins associated with abnormal fatty acid
metabolism.
Wang et al (26) found that the HADHA gene was one of
10 hub genes and was identified between EC and normal samples and
between EA and ESCC. However, in patients with ESCC and EA, HADHA
was not associated with overall survival. In EC, the expression of
HADHA is reduced, which suggests that HADHA is a tumor suppressor
gene. Compared with EA, the HADHA gene is suppressed in ESCC.
Liver
The liver is one of the most important organs in the
body. Liver cancer has a specific metabolic pattern (27) and metabolism in liver cancer is
diverse and heterogeneous. It has been reported in the literature
that the prognosis and development of liver cancer are closely
associated with fatty acid metabolism (27). Accordingly, the present study
reviewed and summarized the relationship between enzymes associated
with fatty acid metabolism and liver cancer.
Tanaka et al (28) analyzed the interaction of tumor
metabolism, differentiation and malignant potential in 41 patients
with completely resected liver cancer. They found that
dedifferentiation will speed up when the level of HADHA is
decreased. HADHA was downregulated in HCC. These results suggest
that HADHA is associated with tumor differentiation and altered
fatty acid β-oxidation. To verify that fatty acid β-oxidation
disorder is associated with the development of hepatocellular
carcinoma (HCC) without cirrhosis, Khare et al (29) established a new mouse model. The
study revealed that a mouse model deficient in long-chain
3-hydroxyacyl-CoA dehydrogenase (LCHAD) developed HCC at an early
age and demonstrated altered expression of early cancer markers.
The HADHA mRNA transcript was significantly downregulated.
Impairment in the oxidation of long-chain fatty acids due to a
reduction in HADHA transcript may serve a cancer-promoting role in
HCC. This suggests that mitochondrial dysfunction serves an
important role in HCC. Micro RNA (miR)-612 inhibits
epithelial-mesenchymal transition (EMT) in HCC. Liu et al
(30) investigated its biological
role in HCC expression and found that miR-612 reduction leads to
HADHA upregulation and that miR-612 can inhibit pseudopodia (mainly
responsible for extracellular matrix degradation, local cell
migration and invasion, extravasation of blood itself and
spatiotemporal spread to distant organs), EMT and HCC metastasis
through HADHA-mediated fatty acid programming. Shi et al
(31) constructed a computational
framework to predict HCC prognosis. Its risk score [Metabolic genes
derived from Pathways (MGP) score] consists of the HADHA gene. The
metabolic pathway involved in HADHA is downregulated in liver
cancer. The HADHA gene is a risk factor for HCC. Shi et al
(31) propose that the MGP score
can predict the prognosis of liver cancer and provide a basis for
precise treatment.
Pancreas
Hypoxia is a common phenomenon in pancreatic cancer
and is a key determinant of, and an important therapeutic target
for, pancreatic cancer. To adapt to hypoxic conditions, pancreatic
cancer cells proliferate by altering their own metabolism and
increasing the uptake of fatty acids to ensure tumor progression
(32). Signaling pathways
regulating pancreatic cancer stem cell (PCSC) growth, survival and
metabolomic plasticity are poorly understood (33).
Di Carlo et al (34) showed that PCSCs have specific and
common proteome and lipidome regulation. Their study revealed that
HADHA protein was upregulated in PCSCs. This may be a new target
for pancreatic cancer treatment.
The published research about the gene of HADH on the
site of pancreas is poor and further experimental or meta-analysis
investigations will yield some significant results. Perhaps these
new findings will bring good prospects in treating this tumor.
Stomach and colorectum
Fatty acid metabolism is an important pathway of
cellular energy metabolism. Fatty acid β-oxidation supports
intestinal stem cell renewal, which is associated with the HADH
gene (35). A study revealed that
HADH is a noncanonical target gene in colon cancer (36). However, the exact role of fatty
acid metabolism in gastric cancer (GC) and colorectal cancer (CRC)
is poorly understood and there is no fatty acid metabolism therapy
for CRC (37). GC and CRC are
difficult to diagnose early, and treat in the advanced stage, and
they have a poor prognosis (38,39). The 5-year survival rate of
patients with GC is low, although great progress has been made in
the treatment of GC (40).
Therefore, it is urgent to explore the relationship among fatty
acid metabolism and GC and CRC.
Du et al (39) found that HADH has prognostic value
in GC and that HADH is significantly associated with disease-free
survival. The analysis of the HADH gene shows that low expression
levels lead to improved chances of survival. This finding reveals
that the HADH gene may act as a tumor suppressor. Shen et al
(41) studied the mechanism of GC
and found that HADH was decreased in GC samples compared with
normal gastric tissue, which significantly promoted the development
of GC. The mechanism may be associated with increased expression of
phosphorylated (p-)Akt and reduced expression of PTEN. Gao et
al (42) reported that the
phosphorylation of sirtuin 6 (SIRT6) is significantly increased
following palmitic acid treatment in colon cancer cells. This is
the result of increased binding of SIRT6 to the promoter of
HADHB.
There are no predictors for routine neoadjuvant
chemotherapy (nCRT) in patients with rectal cancer. Croner et
al (43) successfully
verified the expression of the regulatory protein HADHA following
nCRT-II (5-fluorouracil ± oxaliplatin). HADHA protein is
upregulated in good responders (patients who are sensitive to
nCRT-II) following nCRT-II, which serves as the basis for nCRT-II.
The difference in HADHA protein expression between nCRT-I
(5-fluorouracil) and nCRT-II may provide new research directions
for imbalances in genomic methylation leading to carcinogenesis.
Imbalances in genomic methylation lead to carcinogenesis. Zhu et
al (44) found that
hypermethylation of the HADHB gene in CRC correlates with its
transcriptional downregulation. HADHB reduces the migration and
invasiveness of cancer cells, suggesting that HADHB may be a tumor
suppressor gene (TSG). Peng et al (45) found significant changes in the
expression of the HADHB gene in a differentially expressed gene
study of metformin-treated type-2 diabetes and colorectal cancer.
The expression level of HADHB is upregulated following metformin
treatment in CRC cells. The authors suggest that the HADHB gene may
regulate the functions of ATPase, basal transcription factor and
the mitochondrion by mutations and/or structural changes. Hu et
al (46) were the first to
explore the genes associated with cetuximab (CTX) sensitivity in
colorectal cancer through clustered regularly interspaced short
palindromic repeats Cas9. It was also confirmed that HADHB is
associated with the CTX sensitivity of colorectal cancer, which
provides a theoretical basis for further research on the drug
sensitivity mechanism of colorectal cancer. In addition, Ren et
al (47) also confirm that
the expression of HADH is a relevant prognostic indicator in
intestinal cancer.
Lymph
Malignant lymphoma is a malignant tumor of lymph
nodes and lymphoid tissue (48).
Oncogene mutations and cancer metabolic programming provide
distinctive perspectives on tumor initiation and progression
(49). Reprogramming of energy
metabolism also exists in lymphoma and its association with fatty
acid β-oxidation is gradually becoming known.
A study on malignant lymphoma by Yamamoto et
al (49) demonstrated that
HADHA tends to be overexpressed in its high-grade subtype. This was
associated with significantly lower overall survival and was an
independent prognostic predictor for diffuse large B-cell lymphoma.
This result suggests that the HADHA target may provide a new
therapeutic strategy for malignant lymphoma. Sekine et al
(50) studied the antitumor
effect of HADHB in malignant lymphoma. HADHB is overexpressed in
high-grade lymphoma subtypes. This is an independent predictor of
poor prognosis. These studies provide new directions for the
treatment of malignant lymphoma.
Lung
Metabolic differences persist even within the same
tumor (51). For example,
compared with nonoxidative lung cancer, oxidative lung cancer is
characterized by different carbon sources involved in the
tricarboxylic acid cycle. The carbon source of oxidative lung
cancer is derived from substances other than glucose, and fatty
acid β-oxidation can provide it with ATP. This reveals whether
there is a potential mechanism between fatty acid β-oxidation and
the occurrence and development of lung cancer (51).
Amoedo et al (52) analyzed lung adenocarcinoma with a
high-resolution respiration method and found that [18F]
fluorodeoxyglucose binding was poor in tumors with high
mitochondrial respiration, the expression of mitochondrial
trifunctional fatty acid oxidase (MTP; HADHA) increased and the
genetic inhibition of MTP changed the growth of tumors with high
mitochondrial respiration in the body. These findings provide
proof-of-concept data for preclinical, precise, bioenergetic
medicine in oxidative lung cancer. A study by Madhusudhan et
al (3) revealed that
mitochondrial complex I is a target of non-small cell lung cancer
(NSCLC), while QDC (selective toxin for NSCLC cell lines) interacts
with mitochondrial complex I of the electron transport chain and
catalytic long chain HADHA binding of fatty acid β-oxidation.
Breast
Breast cancer (BC) is a systemic metabolic disease
for which no significant improvement in morbidity and mortality has
occurred and in which metastasis, relapse and drug resistance are
common (53). Fatty acids may
affect the progression of BC (54).
A study by Zhou et al (55) showed that estrogen has two
receptors: ERα and ERβ. ERβ is located in mitochondria and binds to
ERα. A previous study (56)
demonstrated that ERα interacts with the mitochondrial protein
HADHB (affecting thiolytic cleavage activity in β-oxidation). Thus,
it was demonstrated that HADHB is associated with ERβ and
colocalized in the mitochondria of BC cells. The enzymatic activity
of HADHB is enhanced when ERβ expression is specifically inhibited,
suggesting that ERβ serves an inhibitory role in HADHB enzymatic
activity. Orogen affects the primary role of mitochondria,
producing most of the cellular energy and reactive oxygen species
(ROS). Compared with ERα, ERβ affects the enzymatic activity of
HADHB in an opposite manner; specifically, ERα activates the
enzymatic activity of HADHB and ERβ inhibits the enzymatic activity
of HADHB. The authors speculate that the increase in ROS production
may be due to the stimulation of HADHB enzymatic activity by ERα
when ERα positivity is present and that when ERα negativity is
present or the ERα/ERβ ratio is low, ERβ inhibits HADHB enzymatic
activity, thereby affecting fatty acid β-oxidation. There is no
difference in the expression of HADHA in ER (+) and ER (−) and
HADHA is unlikely to have been influenced by ER status (57). A meta-analysis by Mamtani et
al (57) assessing the risk
of BC occurrence, metastasis and recurrence showed that HADHA is
expressed at low levels in BC (especially ER-negative BC). In
addition, HADHA is also underexpressed in metastatic and relapsed
patients. This study supports the possibility that HADHA is
involved in the occurrence of BC and suggests that alteration of
the metabolism of long-chain fatty acids in breast tissue should be
an indicator of possible carcinogenesis. This provides the basis
for the primary prevention of BC. Ji et al (58) found that 5–7 exons of the HADHA
gene (located at chr2:26453059-2645723) produced hsa-circ-0053063
(with a unique closed-loop structure). The authors confirmed that
it functions as a tumor suppressor gene.
Kidney
Clear cell renal cell carcinoma (ccRCC) is
histologically characterized by the presence of numerous LDs in the
cytoplasm (59,60). There is evidence of a link between
obesity and ccRCC and ccRCC is a metabolic disease (61). Abnormal fatty acid metabolism
occurs in ccRCC (62) and the
β-oxidase of ccRCC is changed (63).
Zhao et al (64) showed that HADHA is a prognostic
indicator of ccRCC. In tumor tissues, the expression of HADHA is
downregulated; the expression of HADHA is also significantly
correlated with tumor grade, stage, size, metastasis and
tumor-specific survival. Downregulation of HADHA expression is
associated with poor tumor prognosis and HADHA is an independent
prognostic factor. Liu et al (65) further studied the tumor suppressor
effect of HADHA overexpression in ccRCC. HADHA overexpression
inhibits the formation of cytoplasmic LDs and tumor cells take up
fatty acids and store them as LDs, leading to proliferation.
Overall, overexpression of HADHA disrupts fatty acid metabolism and
inhibits tumor growth. A study by Zhao et al (66) also suggests that abnormal fatty
acid metabolism may be involved in the occurrence and development
of ccRCC, with downregulation of fatty acid oxidation. HADHA and
HADHB are involved in fatty acid oxidation and HADHA and HADHB are
protective factors. In studying the relationship between enzymes
and prognosis, both HADHA and HADHB were associated with good
overall survival rates. Taken together, these results suggest that
HADHA is a potential prognostic marker for ccRCC.
Although the survival rate of children with Wilms
tumor (WT) is high, the mortality rate of recurrent WT is increased
(67). Chemotherapy is usually
required to treat WT and enhancement of fatty acid metabolism can
alleviate the hypoxic state caused by chemotherapy (68).
In a study by Wang et al (69), the expression levels of HADHA and
HADHB were low in tumor tissue. The reason for this finding may be
that the majority of the tissues did not undergo chemoradiotherapy;
therefore, the tumor was not under hypoxic conditions. Wu et
al (68) found that the
expression level of HADHA in tumors was lower than in adjacent
normal tissues and correlated with histopathological type. In a
prognostic analysis, it was found that high HADHA expression was
closely associated with poor prognosis. The reason for the
differential expression of HADHA in ccRCC and WT may be associated
with the different tumor tissue origins. Specifically, ccRCC
originates from mutated kidney cells, whereas WT does not.
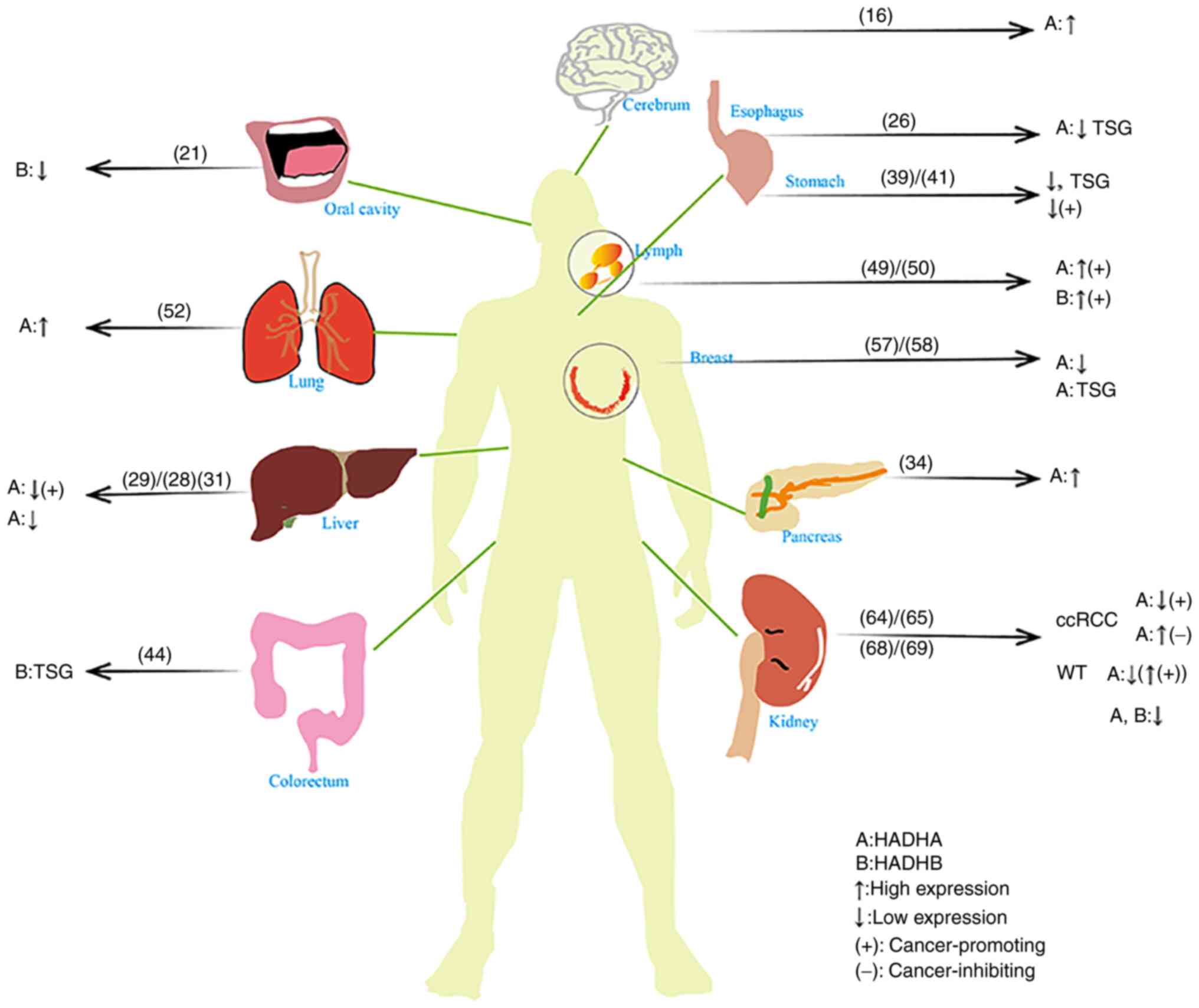
Conclusions
With the increase in research, the role of energy
metabolism reprogramming in tumors is gradually becoming known and
fatty acid β-oxidation serves an important role. The present review
summarized the expression of HADH in some tumors of 11 organs to
explore its role in tumor intervention and prognosis. Compared with
HADHB, there are more studies on HADHA.
Different tumors yield different HADHA expression
results. For example, tumors with high HADHA expression inhibiting
tumorigenesis or low HADHA expression promoting tumorigenesis
include HCC, GC and ccRCC (Fig.
2). In contrast, tumors with high HADHA expression that promote
tumorigenesis is lymphomas. In GBM, pancreas and lung, the
expression of HADHA is high. In OSCC, esophagus, HCC, GC, BC and
WT, the expression of HADHA is low.
Relatively few studies have been performed on HADHB.
A tumor with high HADHB expression that promote tumorigenesis is
lymphoma. The low expression of HADHB is included in OSCC and WT.
(Fig. 2).
In addition, a number of scholars have constructed
predictive models for tumors or screened out related genes. In a
model of oral cancer, HADHB is expressed at low levels (21); HADHA is a TSG in esophageal cancer
(26) and BC (58) and HADHB is a TSG in CRC (44). HADHA is one of the liver cancer
models and HADHA is expressed at low levels (29).
In 11 organs, the HADH is involved in fatty acid
oxidation and located in 2p23 (70). However, the dissimilarities of
organs are unclear in cancers. The gene of HADH is not only
expressed in mitochondria but also in cytoplasm (71). Research about the mechanisms of
HADH is not plentiful in various types of cancer. There are some
relevant articles about the mechanisms of HADH, including PPARγ
(72), p-Akt and PTEN (41), RoR2, Dvl2, ATF2 and ATF4 (36), LCL-K and MD901 (50) and TNFα, IL6-JAK-STAT3 and
interferon-γ (73).
HADHA, a key enzyme in fatty acid oxidation, serves
a significant role in tumors by influencing fatty acid metabolism.
For example, in HCC, reduction in the levels of HADHA is
significantly correlated with the progression of de-differentiation
which influence fatty acid oxidation and the progression of HCC
(28). It has been found that
HADHA can promote invadopodium formation, Wnt/β-catenin
signaling-mediated EMT and HCC metastasis via lipid programming
(30). In addition, in lymph,
downregulation of HADHA can cause G0/G1
arrest which is similar to treatment with the inhibitor of fatty
acid oxidation (FAO) (49). HADHA
is association with mitochondrial complex I to influence fatty acid
oxidation (3). HADHA is regulated
by the way of VHL/HIF-2α-independent and high expression HADHA
decrease the formation of cytoplasmic lipid droplets in ccRCC.
The majority of articles describe the expression of
HADH as an essential enzyme in cancer, but the precise mechanism
remains to be elucidated. Under the background of tumor energy
metabolism, acidic and hypoxic conditions are the usual environment
in tumor metabolism (74). In
addition, resistance to radiotherapy and chemotherapy is
association with hypoxia (75).
With the response to hypoxia, a number of tumor cells will regulate
special ways of energy, such as glucose and fatty acid metabolism
(76). Under hypoxia, although
some cells initiate programmed apoptosis, other tumor cells adapt
to the hypoxic environment, which is closely associated with tumor
recurrence (77). Energy
metabolism is associated with hypoxia. Hypoxia inducible factor-1
(HIF-1) can promote the occurrence of tumors (78) which serves a significant role in
adapting hypoxic environment (75). HIF-1 regulates glycolysis and
pyruvate metabolism (75). At the
same time, fatty acid metabolism also can be influenced by HIF-1
and HIF-2 (79). Fatty acid
oxidation can be affected by HIF-1a; the high expression of HIF-1a
and the low expression of the fatty acid oxidative gene is
association with reduced PPARγ-mediated fatty acid oxidation
(80). The gene of HADH is an
essential gene of fatty acid oxidation, which seems to be a
survival strategy for tumor cells in an anoxic environment
(80). It is hypothesized that
the interaction between the gene of HADH and HIF might be the
potential mechanism regulating fatty acid oxidation. Further basic
experiments are required.
Overall, the abovementioned studies have shown that
the expression of HADH is different in tumors occurring in
different organs. However, HADH is closely associated with tumors
and can be used as a prognostic indicator and therapeutic target
for tumors. Exploring its specific mechanism in tumors is the next
undertaking and one which could eventually aid in clinical
decision-making.
Acknowledgements
Not applicable.
Funding
The present review was supported by grants from Jinan Science
and Technology Bureau (grant no. 202019134) and the Shandong
Provincial Natural Science Foundation (grant nos. ZR2017MH091,
ZR2020QH263 and ZR2021QH142).
Availability of data and materials
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
Authors' contributions
XW and HS performed the literature search and wrote
the manuscript. JL, YJ and YZ supervised and revised the
manuscript. Data authentication is not applicable. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tennant DA, Durán RV and Gottlieb E:
Targeting metabolic transformation for cancer therapy. Nat Rev
Cancer. 10:267–277. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Madhusudhan N, Hu B, Mishra P,
Calva-Moreno JF, Patel K, Boriack R, Ready JM and Nijhawan D:
Target discovery of selective non-small-cell lung cancer toxins
reveals inhibitors of mitochondrial complex I. ACS Chem Biol.
15:158–170. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang M, Han J, Xing H, Zhang H, Li Z,
Liang L, Li C, Dai S, Wu M, Shen F and Yang T: Dysregulated fatty
acid metabolism in hepatocellular carcinoma. Hepat Oncol.
3:241–251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mancini R, Noto A, Pisanu ME, De Vitis C,
Maugeri-Saccà M and Ciliberto G: Metabolic features of cancer stem
cells: The emerging role of lipid metabolism. Oncogene.
37:2367–2378. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia C, Fu Z, Battaile KP and Kim JP:
Crystal structure of human mitochondrial trifunctional protein, a
fatty acid β-oxidation metabolon. Proc Natl Acad Sci USA.
116:6069–6074. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang K, Li N, Wang X, Dai J, Liu P, Wang
C, Chen XW, Gao N and Xiao J: Cryo-EM structure of human
mitochondrial trifunctional protein. Proc Natl Acad Sci USA.
115:7039–7044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Fakhri M and Middleton B: The existence
of an inner-membrane-bound, long acyl-chain-specific
3-hydroxyacyl-CoA dehydrogenase in mammalian mitochondria. Biochim
Biophys Acta. 713:270–279. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
L IJ, Ruiter JP, Hoovers JM, Jakobs ME and
Wanders RJ: Common missense mutation G1528C in long-chain
3-hydroxyacyl-CoA dehydrogenase deficiency. Characterization and
expression of the mutant protein, mutation analysis on genomic DNA
and chromosomal localization of the mitochondrial trifunctional
protein alpha subunit gene. J Clin Invest. 98:1028–1033. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ushikubo S, Aoyama T, Kamijo T, Wanders
RJ, Rinaldo P, Vockley J and Hashimoto T: Molecular
characterization of mitochondrial trifunctional protein deficiency:
Formation of the enzyme complex is important for stabilization of
both alpha- and beta-subunits. Am J Hum Genet. 58:979–988.
1996.PubMed/NCBI
|
|
11
|
Schwab KO, Ensenauer R, Matern D, Uyanik
G, Schnieders B, Wanders RA and Lehnert W: Complete deficiency of
mitochondrial trifunctional protein due to a novel mutation within
the beta-subunit of the mitochondrial trifunctional protein gene
leads to failure of long-chain fatty acid beta-oxidation with fatal
outcome. Eur J Pediatr. 162:90–95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang J, Yuan D, Tan X, Zeng Y, Tang N,
Chen D, Tan J, Cai R, Huang J and Yan T: Analysis of a family with
mitochondrial trifunctional protein deficiency caused by HADHA gene
mutations. Mol Med Rep. 25:472022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taïb B, Aboussalah AM, Moniruzzaman M,
Chen S, Haughey NJ, Kim SF and Ahima RS: Lipid accumulation and
oxidation in glioblastoma multiforme. Sci Rep. 9:195932019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang N, Xie B, Xiao W, Fan M, Xu S, Duan
Y, Hamsafar Y, Evans AC, Huang J, Zhou W, et al: Fatty acid
oxidation fuels glioblastoma radioresistance with CD47-mediated
immune evasion. Nat Commun. 13:15112022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang K and Rich JN: A delicate initiation:
Lipolysis of lipid droplets fuels glioblastoma. Mol Cell.
81:2686–2687. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Zhang T, Li C, Guo J, Xu B and Xue
L: Telmisartan attenuates human glioblastoma cells proliferation
and oncogenicity by inducing the lipid oxidation. Asia Pac J Clin
Oncol. 18:217–223. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marur S and Forastiere AA: Head and neck
squamous cell carcinoma: Update on epidemiology, diagnosis, and
treatment. Mayo Clin Proc. 91:386–396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shetty SR, Babu S, Kumari S, Shetty P,
Hegde S and Castelino R: Status of salivary lipid peroxidation in
oral cancer and precancer. Indian J Med Paediatr Oncol. 35:156–158.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malik UU, Siddiqui IA, Hashim Z and Zarina
S: Measurement of serum paraoxonase activity and MDA concentrations
in patients suffering with oral squamous cell carcinoma. Clin Chim
Acta. 430:38–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaur J, Politis C and Jacobs R: Salivary
8-hydroxy-2-deoxyguanosine, malondialdehyde, vitamin C, and vitamin
E in oral pre-cancer and cancer: Diagnostic value and free radical
mechanism of action. Clin Oral Investig. 20:315–319. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang ZD, Yao YY, Chen TY, Zhao YF, Zhang
C and Niu YM: Construction of prognostic risk prediction model of
oral squamous cell carcinoma based on nine survival-associated
metabolic genes. Front Physiol. 12:6097702021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zemanova M, Vecka M, Petruželka L,
Staňková B, Žák A and Zeman M: Plasma phosphatidylcholines fatty
acids in men with squamous cell esophageal cancer:
Chemoradiotherapy improves abnormal profile. Med Sci Monit.
22:4092–4099. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zuijdgeest-van Leeuwen SD, van der Heijden
MS, Rietveld T, van den Berg JW, Tilanus HW, Burgers JA, Wilson JH
and Dagnelie PC: Fatty acid composition of plasma lipids in
patients with pancreatic, lung and oesophageal cancer in comparison
with healthy subjects. Clin Nutr. 21:225–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang AH, Liu Y, Wang B, He YX, Fang YX and
Yan YP: Epidemiological studies of esophageal cancer in the era of
genome-wide association studies. World J Gastrointest Pathophysiol.
5:335–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abbas G and Krasna M: Overview of
esophageal cancer. Ann Cardiothorac Surg. 6:131–136. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang F, Zhang L, Xu Y, Xie Y and Li S:
Comprehensive analysis and identification of key driver genes for
distinguishing between esophageal adenocarcinoma and squamous cell
carcinoma. Front Cell Dev Biol. 9:6761562021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen D, Zhang Y, Wang W, Chen H, Ling T,
Yang R, Wang Y, Duan C, Liu Y, Guo X, et al: Identification and
characterization of robust hepatocellular carcinoma prognostic
subtypes based on an integrative metabolite-protein interaction
network. Adv Sci (Weinh). 8:e21003112021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanaka M, Masaki Y, Tanaka K, Miyazaki M,
Kato M, Sugimoto R, Nakamura K, Aishima S, Shirabe K, Nakamuta M,
et al: Reduction of fatty acid oxidation and responses to hypoxia
correlate with the progression of de-differentiation in HCC. Mol
Med Rep. 7:365–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khare T, Khare S, Angdisen JJ, Zhang Q,
Stuckel A, Mooney BP, Ridenhour SE, Gitan RS, Hammoud GM and Ibdah
JA: Defects in long-chain 3-hydroxy acyl-CoA dehydrogenase lead to
hepatocellular carcinoma: A novel etiology of hepatocellular
carcinoma. Int J Cancer. 147:1461–1473. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Lu LL, Wen D, Liu DL, Dong LL, Gao
DM, Bian XY, Zhou J, Fan J and Wu WZ: MiR-612 regulates invadopodia
of hepatocellular carcinoma by HADHA-mediated lipid reprogramming.
J Hematol Oncol. 13:122020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi Q, Liu Y, Lu M, Lei QY, Chen Z, Wang L
and He X: A pathway-guided strategy identifies a metabolic
signature for prognosis prediction and precision therapy for
hepatocellular carcinoma. Comput Biol Med. 144:1053762022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hao X, Ren Y, Feng M, Wang Q and Wang Y:
Metabolic reprogramming due to hypoxia in pancreatic cancer:
Implications for tumor formation, immunity, and more. Biomed
Pharmacother. 141:1117982021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Carlo C, Brandi J and Cecconi D:
Pancreatic cancer stem cells: Perspectives on potential therapeutic
approaches of pancreatic ductal adenocarcinoma. World J Stem Cells.
10:172–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Di Carlo C, Sousa BC, Manfredi M, Brandi
J, Dalla Pozza E, Marengo E, Palmieri M, Dando I, Wakelam MJO,
Lopez-Clavijo AF and Cecconi D: Integrated lipidomics and
proteomics reveal cardiolipin alterations, upregulation of HADHA
and long chain fatty acids in pancreatic cancer stem cells. Sci
Rep. 11:132972021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen L, Vasoya RP, Toke NH, Parthasarathy
A, Luo S, Chiles E, Flores J, Gao N, Bonder EM, Su X and Verzi MP:
HNF4 regulates fatty acid oxidation and is required for renewal of
intestinal stem cells in mice. Gastroenterology. 158:985–999.e9.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Voloshanenko O, Schwartz U, Kranz D,
Rauscher B, Linnebacher M, Augustin I and Boutros M:
β-catenin-independent regulation of Wnt target genes by RoR2 and
ATF2/ATF4 in colon cancer cells. Sci Rep. 8:31782018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou Y, Li X, Guan A, Zhou H, Zhu Y, Wang
R and Li R: EPHX2 inhibits colon cancer progression by promoting
fatty acid degradation. Front Oncol. 12:8707212022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nassar D and Blanpain C: Cancer stem
cells: Basic concepts and therapeutic implications. Annu Rev
Pathol. 11:47–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Du Z, Zhang X, Gao W and Yang J:
Differentially expressed genes PCCA, ECHS1, and HADH are potential
prognostic biomarkers for gastric cancer. Sci Prog.
104:3685042110113442021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moehler M, Baltin CT, Ebert M, Fischbach
W, Gockel I, Grenacher L, Hölscher AH, Lordick F, Malfertheiner P,
Messmann H, et al: International comparison of the German
evidence-based S3-guidelines on the diagnosis and multimodal
treatment of early and locally advanced gastric cancer, including
adenocarcinoma of the lower esophagus. Gastric Cancer. 18:550–563.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shen C, Song YH, Xie Y, Wang X, Wang Y,
Wang C, Liu S, Xue SL, Li Y, Liu B, et al: Downregulation of HADH
promotes gastric cancer progression via Akt signaling pathway.
Oncotarget. 8:76279–76289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gao T, Li M, Mu G, Hou T, Zhu WG and Yang
Y: PKCζ phosphorylates SIRT6 to mediate fatty acid β-oxidation in
colon cancer cells. Neoplasia. 21:61–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Croner RS, Sevim M, Metodiev MV, Jo P,
Ghadimi M, Schellerer V, Brunner M, Geppert C, Rau T, Stürzl M, et
al: Identification of predictive markers for response to
neoadjuvant chemoradiation in rectal carcinomas by proteomic
isotope coded protein label (ICPL) analysis. Int J Mol Sci.
17:2092016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu Y, Lu H, Zhang D, Li M, Sun X, Wan L,
Yu D, Tian Y, Jin H, Lin A, et al: Integrated analyses of
multi-omics reveal global patterns of methylation and
hydroxymethylation and screen the tumor suppressive roles of HADHB
in colorectal cancer. Clin Epigenetics. 10:302018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Peng WF, Bai F, Shao K, Shen LS, Li HH and
Huang S: The key genes underlying pathophysiology association
between the type 2-diabetic and colorectal cancer. J Cell Physiol.
233:8551–8557. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu TT, Yang JW, Yan Y, Chen YY, Xue HB,
Xiang YQ and Ye LC: Detection of genes responsible for cetuximab
sensitization in colorectal cancer cells using CRISPR-Cas9. Biosci
Rep. 40:BSR202011252020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ren J, Feng J, Song W, Wang C, Ge Y and Fu
T: Development and validation of a metabolic gene signature for
predicting overall survival in patients with colon cancer. Clin Exp
Med. 20:535–544. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Krause JR: WHO classification of tumours
of haematopoietic and lymphoid tissues: An overview. Crit Values.
2:30–32. 2009. View Article : Google Scholar
|
|
49
|
Yamamoto K, Abe S, Honda A, Hashimoto J,
Aizawa Y, Ishibashi S, Takemura T, Hanagata N, Yamamoto M, Miura O,
et al: Fatty acid beta oxidation enzyme HADHA is a novel potential
therapeutic target in malignant lymphoma. Lab Invest. 100:353–362.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sekine Y, Yamamoto K, Kurata M, Honda A,
Onishi I, Kinowaki Y, Kawade G, Watabe S, Nomura S, Fukuda S, et
al: HADHB, a fatty acid beta-oxidation enzyme, is a potential
prognostic predictor in malignant lymphoma. Pathology. 54:286–293.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hensley CT, Faubert B, Yuan Q, Lev-Cohain
N, Jin E, Kim J, Jiang L, Ko B, Skelton R, Loudat L, et al:
Metabolic heterogeneity in human lung tumors. Cell. 164:681–694.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Amoedo ND, Sarlak S, Obre E, Esteves P,
Bégueret H, Kieffer Y, Rousseau B, Dupis A, Izotte J, Bellance N,
et al: Targeting the mitochondrial trifunctional protein restrains
tumor growth in oxidative lung carcinomas. J Clin Invest.
131:e1330812021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ginsburg O, Bray F, Coleman MP, Vanderpuye
V, Eniu A, Kotha SR, Sarker M, Huong TT, Allemani C, Dvaladze A, et
al: The global burden of women's cancers: A grand challenge in
global health. Lancet. 389:847–860. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pauwels EK and Kairemo K: Fatty acid
facts, part II: Role in the prevention of carcinogenesis, or, more
fish on the dish? Drug News Perspect. 21:504–510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhou Z, Zhou J and Du Y: Estrogen receptor
beta interacts and colocalizes with HADHB in mitochondria. Biochem
Biophys Res Commun. 427:305–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou Z, Zhou J and Du Y: Estrogen receptor
alpha interacts with mitochondrial protein HADHB and affects
beta-oxidation activity. Mol Cell Proteomics. 11:M111.011056. 2012.
View Article : Google Scholar
|
|
57
|
Mamtani M and Kulkarni H: Association of
HADHA expression with the risk of breast cancer: Targeted subset
analysis and meta-analysis of microarray data. BMC Res Notes.
5:252012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ji C, Hu J, Wang X, Zheng W, Deng X, Song
H, Yu Y, Luo Q, Hua K, Zhou X and Fang L: Hsa_circ_0053063 inhibits
breast cancer cell proliferation via
hsa_circ_0053063/hsa-miR-330-3p/PDCD4 axis. Aging (Albany NY).
13:9627–9645. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Massari F, Ciccarese C, Santoni M,
Brunelli M, Piva F, Modena A, Bimbatti D, Fantinel E, Santini D,
Cheng L, et al: Metabolic alterations in renal cell carcinoma.
Cancer Treat Rev. 41:767–776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Du W, Zhang L, Brett-Morris A, Aguila B,
Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI, et
al: HIF drives lipid deposition and cancer in ccRCC via repression
of fatty acid metabolism. Nat Commun. 8:17692017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhu Y, Wang HK, Zhang HL, Yao XD, Zhang
SL, Dai B, Shen YJ, Liu XH, Zhou LP and Ye DW: Visceral obesity and
risk of high grade disease in clinical t1a renal cell carcinoma. J
Urol. 189:447–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gebhard RL, Clayman RV, Prigge WF,
Figenshau R, Staley NA, Reesey C and Bear A: Abnormal cholesterol
metabolism in renal clear cell carcinoma. J Lipid Res.
28:1177–1184. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wettersten HI, Hakimi AA, Morin D, Bianchi
C, Johnstone ME, Donohoe DR, Trott JF, Aboud OA, Stirdivant S, Neri
B, et al: Grade-dependent metabolic reprogramming in kidney cancer
revealed by combined proteomics and metabolomics analysis. Cancer
Res. 75:2541–2552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhao Z, Lu J, Han L, Wang X, Man Q and Liu
S: Prognostic significance of two lipid metabolism enzymes, HADHA
and ACAT2, in clear cell renal cell carcinoma. Tumour Biol.
37:8121–8130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu S, Liu X, Wu F, Zhang X, Zhang H, Gao
D, Bi D, Qu H, Ge J, Xu Y and Zhao Z: HADHA overexpression disrupts
lipid metabolism and inhibits tumor growth in clear cell renal cell
carcinoma. Exp Cell Res. 384:1115582019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhao Z, Liu Y, Liu Q, Wu F, Liu X, Qu H,
Yuan Y, Ge J, Xu Y and Wang H: The mRNA expression signature and
prognostic analysis of multiple fatty acid metabolic enzymes in
clear cell renal cell carcinoma. J Cancer. 10:6599–6607. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ramburan A, Chetty R, Hadley GP, Naidoo R
and Govender D: Microsatellite analysis of the DCC gene in
nephroblastomas: Pathologic correlations and prognostic
implications. Mod Pathol. 17:89–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wu X, Feng R, Wang X, Guo F and Liu W:
Roles of hydroxyacyl-CoA dehydrogenase trifunctional multienzyme
complex subunit alpha, a lipid metabolism enzyme, in Wilms tumor
patients. J Cancer Res Ther. 17:1281–1285. 2021.PubMed/NCBI
|
|
69
|
Wang X, Du G, Wu Y, Zhang Y, Guo F, Liu W
and Wu R: Association between different levels of lipid
metabolism-related enzymes and fatty acid synthase in Wilms' tumor.
Int J Oncol. 56:568–580. 2020.PubMed/NCBI
|
|
70
|
Aoyama T, Wakui K, Orii KE, Hashimoto T
and Fukushima Y: Fluorescence in situ hybridization mapping of the
alpha and beta subunits (HADHA and HADHB) of human mitochondrial
fatty acid beta-oxidation multienzyme complex to 2p23 and their
evolution. Cytogenet Cell Genet. 79:221–224. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Maeyashiki C, Oshima S, Otsubo K,
Kobayashi M, Nibe Y, Matsuzawa Y, Onizawa M, Nemoto Y, Nagaishi T,
Okamoto R, et al: HADHA, the alpha subunit of the mitochondrial
trifunctional protein, is involved in long-chain fatty acid-induced
autophagy in intestinal epithelial cells. Biochem Biophys Res
Commun. 484:636–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Soliman E, Elhassanny AEM, Malur A, McPeek
M, Bell A, Leffler N, Van Dross R, Jones JL, Malur AG and Thomassen
MJ: Impaired mitochondrial function of alveolar macrophages in
carbon nanotube-induced chronic pulmonary granulomatous disease.
Toxicology. 445:1525982020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jiang H, Chen H, Wan P and Chen N:
Decreased expression of HADH is related to poor prognosis and
immune infiltration in kidney renal clear cell carcinoma. Genomics.
113:3556–3564. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhelev Z, Aoki I, Lazarova D, Vlaykova T,
Higashi T and Bakalova R: A ‘weird’ mitochondrial fatty acid
oxidation as a metabolic ‘secret’ of cancer. Oxid Med Cell Longev.
2022:23395842022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zeng W, Liu P, Pan W, Singh SR and Wei Y:
Hypoxia and hypoxia inducible factors in tumor metabolism. Cancer
Lett. 356:263–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Denko NC: Hypoxia, HIF1 and glucose
metabolism in the solid tumour. Nat Rev Cancer. 8:705–713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Milane L, Duan Z and Amiji M: Role of
hypoxia and glycolysis in the development of multi-drug resistance
in human tumor cells and the establishment of an orthotopic
multi-drug resistant tumor model in nude mice using hypoxic
pre-conditioning. Cancer Cell Int. 11:32011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang K, Han ES, Dellinger TH, Lu J, Nam
S, Anderson RA, Yim JH and Wen W: Cinnamon extract reduces VEGF
expression via suppressing HIF-1α gene expression and inhibits
tumor growth in mice. Mol Carcinog. 56:436–446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yin J, Miyazaki K, Shaner RL, Merrill AH
Jr and Kannagi R: Altered sphingolipid metabolism induced by tumor
hypoxia-new vistas in glycolipid tumor markers. FEBS Lett.
584:1872–1878. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ezzeddini R, Taghikhani M, Salek Farrokhi
A, Somi MH, Samadi N, Esfahani A and Rasaee MJ: Downregulation of
fatty acid oxidation by involvement of HIF-1α and PPARγ in human
gastric adenocarcinoma and related clinical significance. J Physiol
Biochem. 77:249–260. 2021. View Article : Google Scholar : PubMed/NCBI
|