Introduction
The imbalance in the inflammatory response and organ
injury following sepsis, cancer, trauma and shock are significantly
affected by immune system dysfunction (1). In human immunity, the spleen is a
major organ, being central to humoral and cellular immunity. The
spleen performs a crucial role in antibacterial and antiviral
immune reactivity, and is a major site of antibody production.
Functional deficiency of the spleen results in increased
susceptibility to systemic infections by bacteria or viruses
(2).
The gram-negative bacterial outer membrane component
lipopolysaccharide (LPS) can cause immune responses in the central
and peripheral nervous systems, thereby inducing sickness in mice
(3). Peripheral administration of
LPS can induce the expression of proinflammatory cytokines in both
the central and peripheral nervous systems, including inducible
nitric oxide synthase, cyclooxygenase 2, interleukin (IL)-1β and
tumor necrosis factor (TNF)-α, by activating Toll-like receptor 4
(TLR4) and regulating gene expression by initiating an
intracellular signaling pathway involving nuclear factor (NF)-κB
activation (4). LPS primarily
activates pathways that are both independent and dependent on
myeloid differentiation primary response 88 (MyD88). In both
pathways, recognition by TLR4 of the lipid A-region of LPS is
involved, suggesting that MyD88 signaling pathways have important
functions in the immune response and inflammation (5). A previous study demonstrated that LPS
could upregulate pro-inflammatory factor levels, including IL-1β
and TNF-α, thus causing spleen injury in rats (6).
The cysteine protease ubiquitin-specific protease 8
(USP8) is a member of the USP/UBP subfamily (7). USP8 mediates the stability of
ubiquitin protein ligases (E3s), including that of neuregulin
receptor degradation protein-1 (NRDP1), an E3 that is expressed
mainly in skeletal muscle, the prostate, the heart and the brain
(8). The growth-regulated enzyme
USP8 is essential and indispensable for cell survival and
proliferation (9). Conditional
knockout of USP8 in mice has been reported to result in marked loss
of expression of receptor tyrosine kinases, such as MET
proto-oncogene, receptor tyrosine kinase, Erb-B2 receptor tyrosine
kinase 3 and epidermal growth factor receptor (10). Our previous study reported that the
production of LPS-induced proinflammatory factors was reduced
following USP8 overexpression; therefore, USP8 may be regarded as a
novel candidate for the treatment of neuroinflammatory disorders
(11). In addition, our previous
study revealed that by modulating the TLR4/MyD88/NF-κB signaling
pathway, USP8 could protect against LPS-induced cognitive and motor
deficits in mice (12). However,
the role of USP8 in LPS-induced spleen injury and its effects on
the regulation of associated signaling pathways is poorly
understood. The present study hypothesized that USP8 could inhibit
the production of pro-inflammatory mediators, thereby protecting
against LPS-induced spleen injury.
Materials and methods
Ethical approval
A total of 119 healthy male C57BL/6J mice (age,
10–12 weeks; weight, 20–25 g) were obtained from the Medical
Laboratory Animal Center of Guangdong Province. All mice were
housed in a under automatically controlled temperature (21–25°C),
relative humidity (45–65%) and a 12-h light/dark cycle. The
National Institutes of Health guidelines were followed strictly
when performing all of the animal procedures (13). The Animal Care and Use Committee of
Jinan University (Guangzhou, China; approval no. IACUC-20180726-03)
approved all the animal procedures. Anesthesia was used during all
surgeries, and pain and suffering were minimized as much as
possible.
Experimental protocol
Mice (n=9/group) were randomly assigned to the
following six groups: Control group, saline group, LPS group, USP8
+ LPS group, USP8 group and negative control (NC) + LPS group. USP8
(lentiviruses encoding mouse USP8) were constructed and produced by
OBiO Technology (Shanghai) Corp., Ltd., and 1×108 TU/ml
(4 µl) was administered intracerebroventricularly (i.c.v.) on day 1
using a microsyringe with the following stereotaxic coordinates:
−0.02 cm anterior, −0.15 cm lateral and −0.26 cm dorsal from the
bregma, according to a previously described procedure (14). Age-matched NC mice were injected
with an empty lentiviral plasmid (EF-1aF/GFP&Puro) at the same
coordinates and with the same volume [OBiO Technology (Shanghai)
Corp., Ltd.; 1×108 TU/ml; 4 µl). Injection of USP8
(i.c.v.; 1×108 TU/ml; 4 µl) started 7 days before the
i.p. injection of LPS (MilliporeSigma; 750 µg/kg), which was
injected once a day for 7 days. The saline group was injected with
saline at an equal volume.
Enzyme-linked immunosorbent assay
(ELISA)
Animals were anesthetized using 1.5% isoflurane
(15,16), then sacrificed by cardiac perfusion,
and the spleen tissues were harvested and homogenized. Spleen
samples from the six groups were homogenized in
radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime
Institute of Biotechnology) containing 1 mM phenylmethylsulfonyl
fluoride (PMSF; Beyotime Institute of Biotechnology). The
bicinchoninic acid assay (Beyotime Institute of Biotechnology) was
used to quantify the protein concentrations. The levels of TNF-α
and IL-1β in serum and spleen homogenates were determined using
ELISA kits (cat. nos. MTA00B and MLB00C; R&D Systems, Inc.)
according to the manufacturer's protocols. Serum samples were
obtained from whole blood samples (~800 µl) collected from the
orbital sinus, by centrifugation at 12,000 × g for 20 min at 4°C.
Mice were immediately sacrificed following blood collection.
Staining with hematoxylin and eosin
(H&E)
Mice were anesthetized using 1.25%
2,2,2-tribromoethanol injected intraperitoneally (200 mg/kg), the
heart was exposed and paraformaldehyde (PFA; 4%) was then injected
through the right ventricle. Subsequently, 4% PFA was used to fix
the collected spleen samples for 24 h at 4°C. The spleen samples
were divided into two equal parts and immersed in distilled water
with 40% sucrose overnight at 4°C. On day 2, the spleen samples
were embedded in paraffin and were cut into 4-µm sections, before
being stained with H&E (0.5% eosin) at room temperature for 10
sec to evaluate their morphology. The sections were observed under
a light microscope.
Western blotting
Spleen tissue homogenates were harvested in RIPA
lysis buffer containing 1 mM PMSF and then centrifuged at 6,000 × g
for 15 min at 4°C. The detection of cytoplasmic and nuclear NF-κB
was carried out following protein extraction using the
NE-PER® kit (Thermo Fisher Scientific, Inc.). SDS-PAGE
on 8–10% gels was used to separate equal amounts of protein, which
were then electrotransferred onto polyvinylidene fluoride membranes
(MilliporeSigma). Subsequently, 5% skimmed milk solution was used
to block the membranes, after which they were incubated at 4°C
overnight with primary antibodies against P38 (cat. no. 9218S),
phosphorylated-(p)-P38 (cat. no. 4511S), ERK (cat. no. 4695S),
p-ERK (cat. no. 4370S), JNK (cat. no. 9252S), p-JNK (cat. no.
9255S), IκBα (cat. no. 4814T), p-IκBα (cat. no. 2859), NF-κB (cat.
no. 8242), MyD88 (cat. no. 4283S), USP8 (cat. no. 11832), Lamin-B1
(cat. no. 13435S) and GAPDH (cat. no. 2118S) (1:1,000 dilution;
Cell Signaling Technology, Inc.), as reported previously (17). Following incubation with horseradish
peroxidase-conjugated goat anti-rabbit (cat. no. 23240) or goat
anti-mouse IgG (cat. no. 91196) secondary antibodies (1:2,000
dilution; Cell Signaling Technology, Inc.) for 1 h at room
temperature, an enhanced chemiluminescence kit (MilliporeSigma) was
used to detect the immunoreactive protein bands. The blots
underwent scanning densitometry (Bio-Rad GelDoc XR imaging system;
Bio-Rad Laboratories, Inc.) and semi-quantification of signal
intensity was performed using Image J software (version 1.45;
National Institutes of Health).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. SPSS 13.0 software (IBM Corp.) was used for all
statistical analyses. Data were analyzed using one-way ANOVA
followed by post hoc pairwise comparisons using Tukey's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General appearance and alterations to
spleen morphology
All mice were observed and weighed every day
immediately before intraperitoneal injection of LPS. Following the
administration of LPS, the mice displayed classic indications of
sickness, such as reduced motion, a dispirited appearance,
insensitivity to external stimulation, and reduced drinking and
eating, which could be ameliorated by USP8 pretreatment (data not
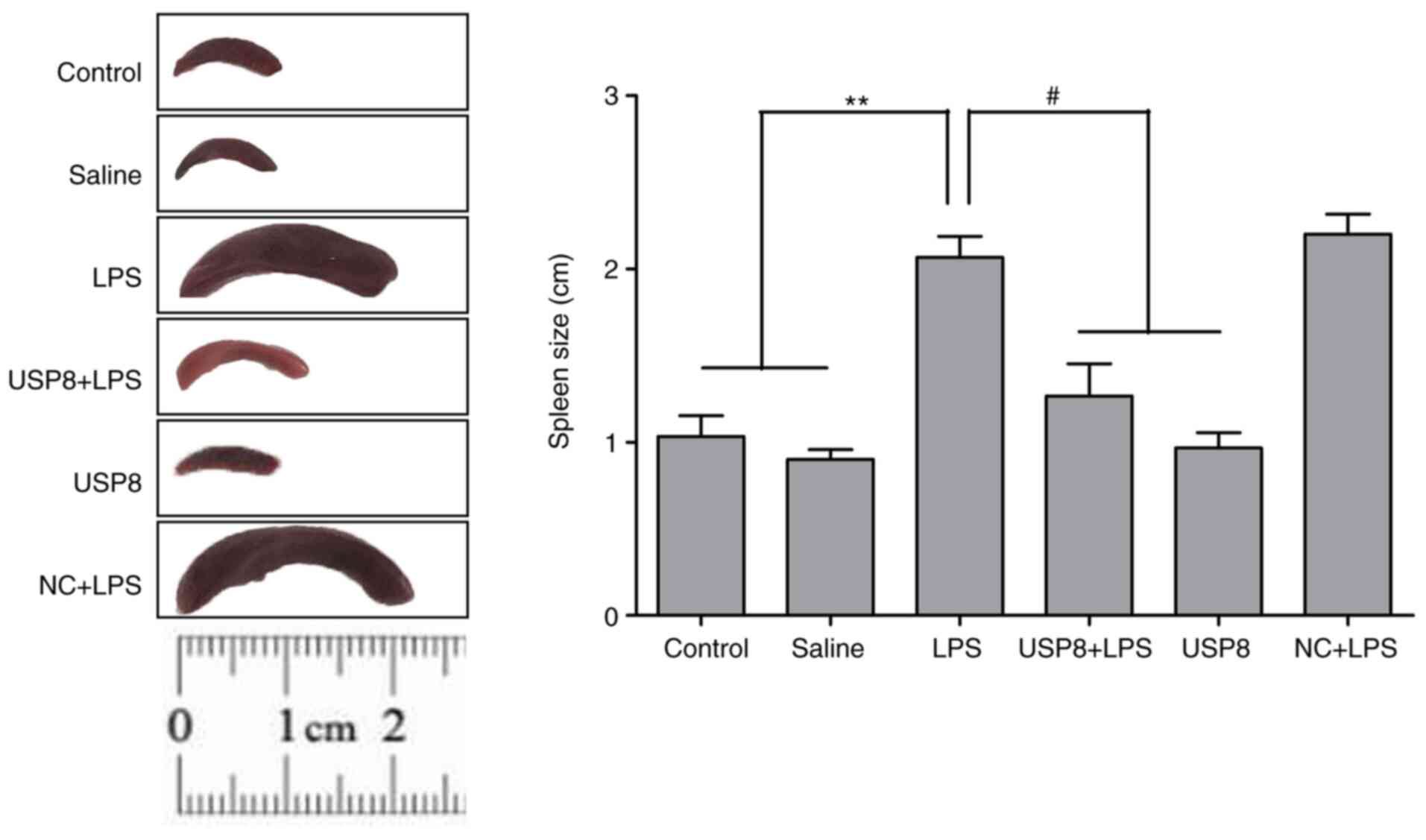
shown). Notably, the LPS-injected group exhibited splenomegaly, in
which the spleen was twice as large as that in the USP8 + LPS group
(Fig. 1). Spleen size was defined
as the largest length of the spleen in the resected specimen. As
shown in Fig. 1, spleen size was
significantly reduced in the USP8 + LPS group compared with that in
the LPS group.
Expression levels of USP8 protein in
the spleen of LPS-induced mice
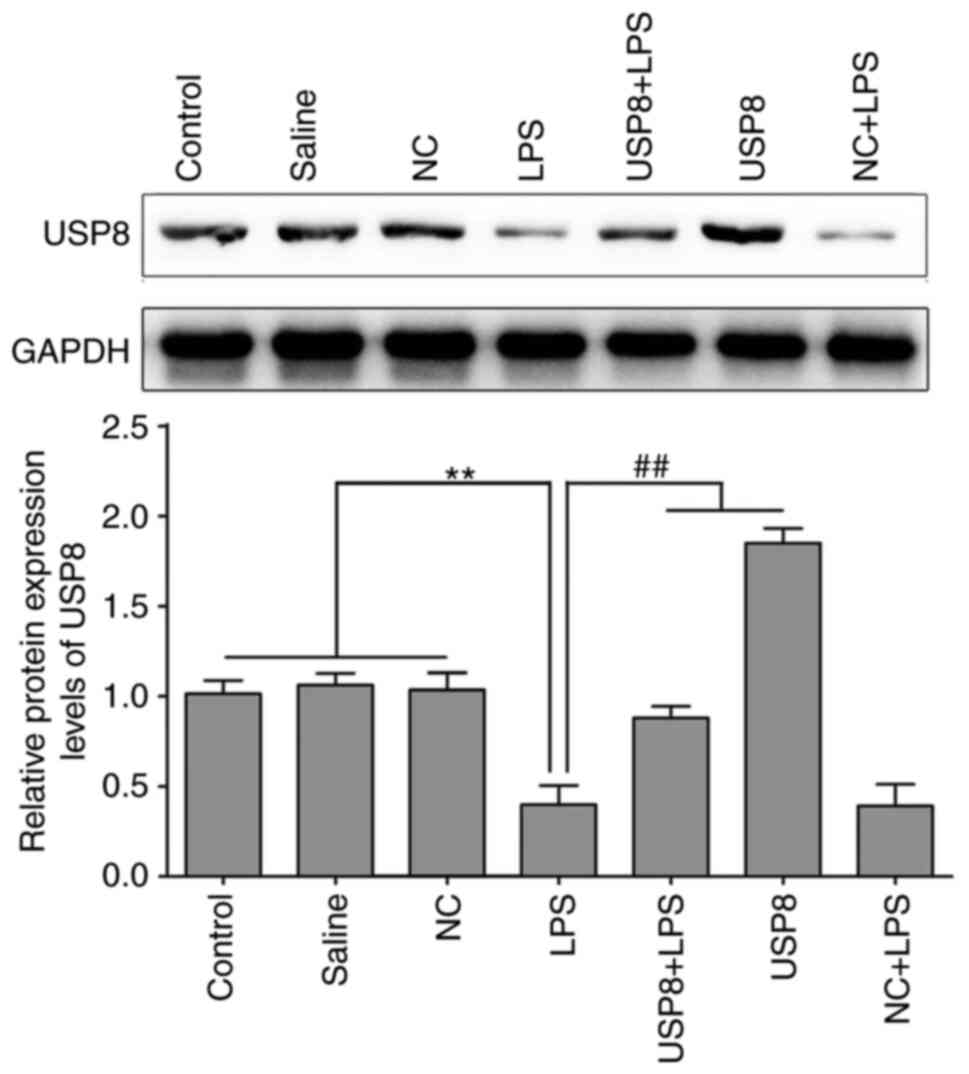
Western blotting was used to assess the protein
expression levels of USP8 in the spleen after injecting the mice
with LPS continuously for 7 days. As shown in Fig. 2, USP8 expression was significantly
increased in the USP8 group compared with in the NC group, thus
indicating successful USP8 overexpression. In the LPS group, the
expression levels of USP8 in the spleen were significantly
decreased compared with those in the control, saline and NC groups,
whereas i.c.v. injection of USP8 into the mice resulted in a
significant increase in USP8 expression levels compared with in the
LPS group (Fig. 2).
USP8 alters the splenic structure in
the LPS-induced systemic inflammation model
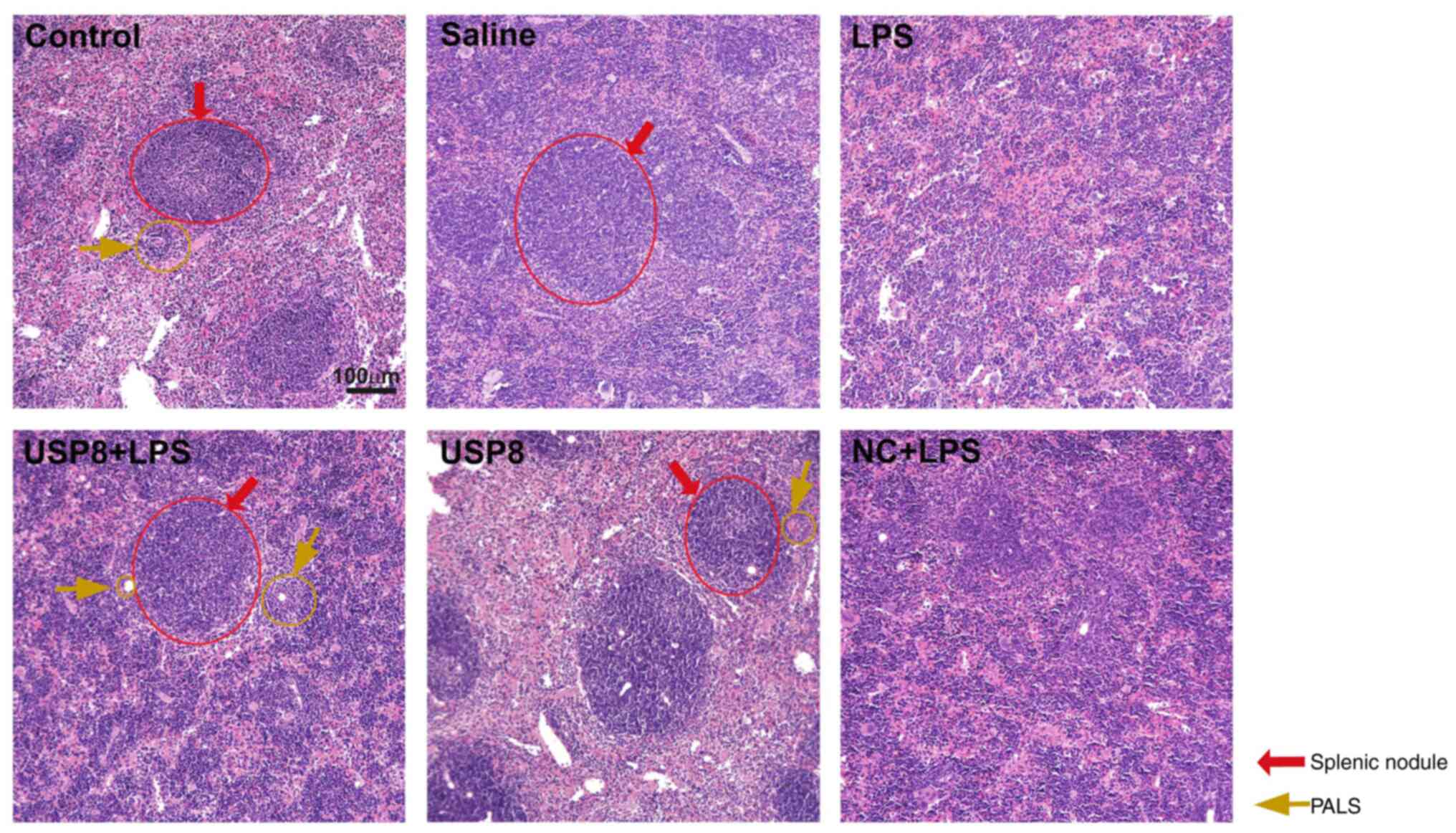
The spleens of the saline and control mouse groups
had thin capsules, with clearly visible white and red pulp after
H&E staining. The white pulp comprised splenic nodules and a
periarteriolar lymphoid sheath (PALS). In the spleen, the marginal
zone formed a unique region at the interface of both the white and
red pulps (Fig. 3). The white pulp
was disorganized in the LPS-treated group, without typical splenic
nodules and PALS, both the marginal zone and germinal center had
disappeared. The structure of the white pulp in the USP8 + LPS
group exhibited splenic nodules with an obvious germinal center and
a typical PALS; however, inflammation still existed.
USP8 decreases serum and spleen levels
of pro-inflammatory cytokines in LPS-treated mice
The TLR pathway mediates the LPS-induced
inflammatory immune response, producing expression levels of
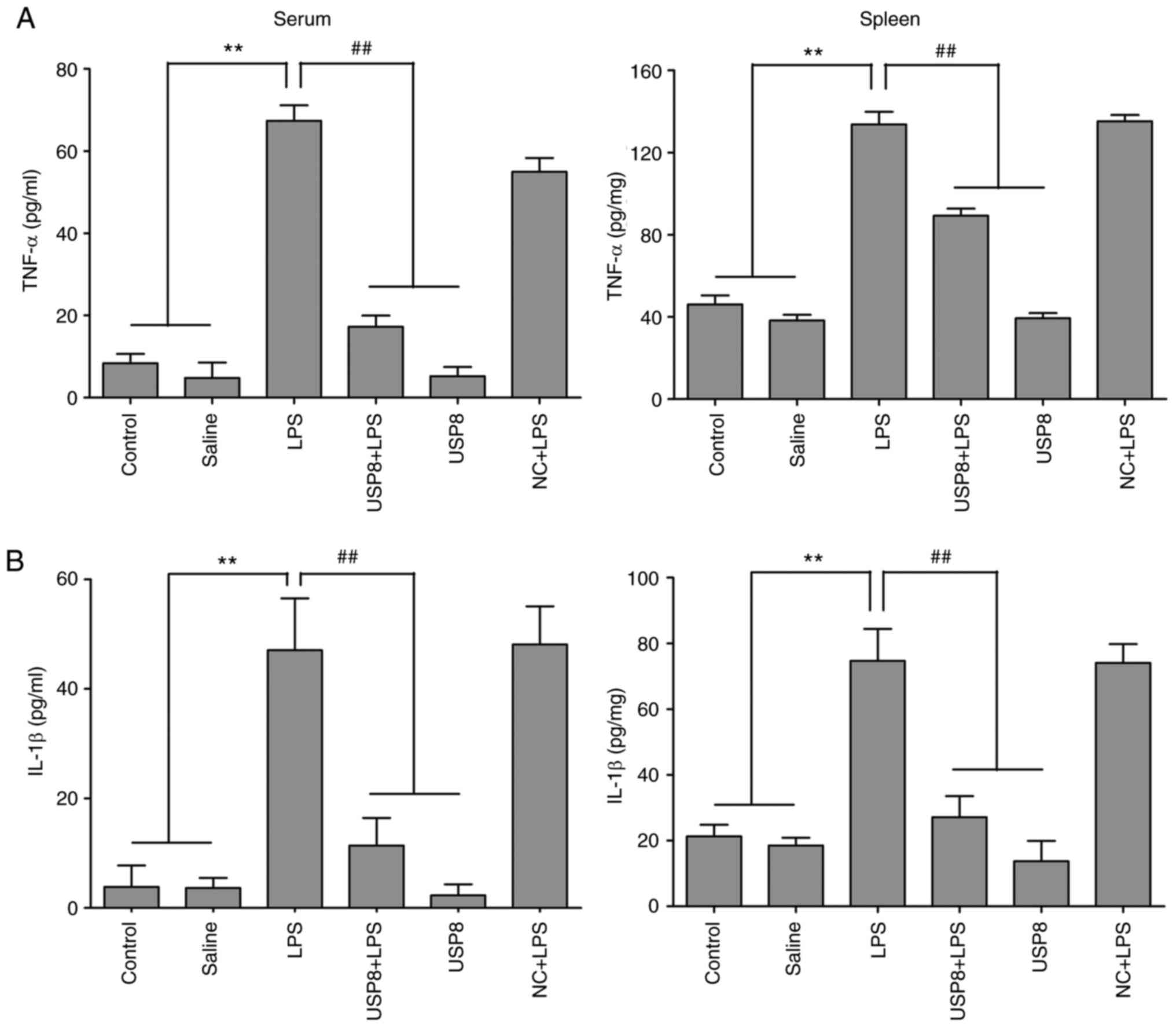
pro-inflammatory cytokines, including IL-1β and TNF-α (18). The present study determined the
concentrations of IL-1β and TNF-α in the serum and spleen
homogenates using ELISA. Following injection with LPS, IL-1β and
TNF-α levels in the spleen tissue homogenates were increased
compared with those in the control and saline groups (Fig. 4). Furthermore, the levels of IL-1β
and TNF-α in the serum samples were upregulated in the LPS-treated
mice. However, significant decreases in the levels of IL-1β and
TNF-α were observed in mice treated with USP8 + LPS compared with
those in the LPS-treated mice (Fig.
4). These findings indicated that USP8 could effectively clear
inflammation from the spleens of mice treated with LPS.
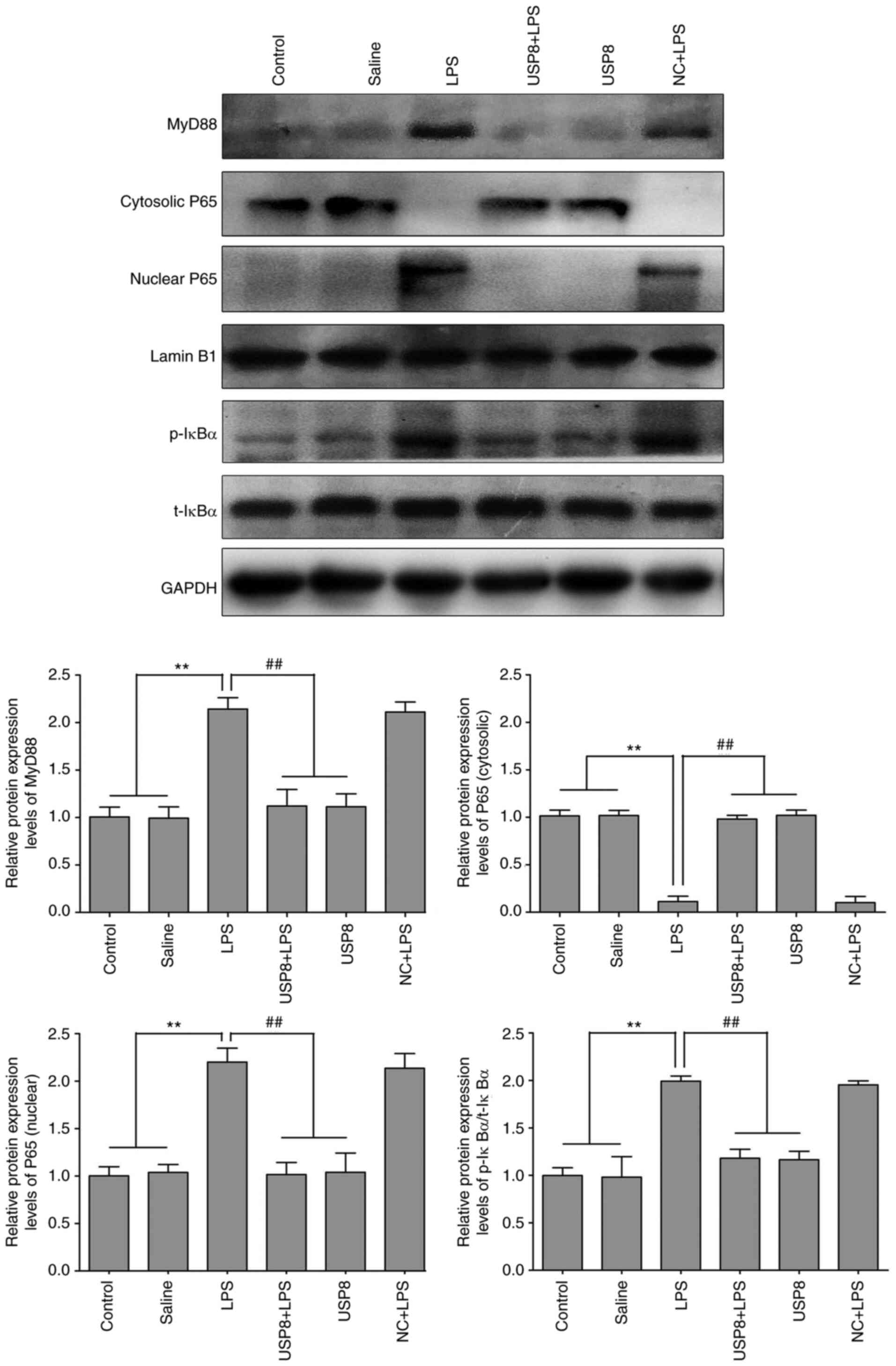
USP8 protects against LPS-induced
spleen injury by inhibiting the NF-κB pathway
NF-κB is an important transcription factor for the
secretion of pro-inflammatory mediators (19). Therefore, the present study
investigated the molecular mechanism by which USP8 exerted its
therapeutic effects on pro-inflammatory-associated splenomegaly by
determining the expression levels of NF-κB pathway-related
proteins. When treated with LPS, the NF-κB P65 subunit was shown to
move into the nucleus, which may upregulate the expression levels
of genes encoding pro-inflammatory cytokines, such as TNF-α and
IL-1β. Therefore, the protein expression levels of IκBα and MyD88
were detected. The expression levels of p-IκBα and MyD88 were
significantly increased in the mice treated with LPS compared with
those in the mice in the control and saline groups, whereas in the
USP8 + LPS group, their levels were significantly lower than those
in the LPS group (Fig. 5). These
results indicated that USP8 may attenuate splenomegaly via NF-κB
pathway inhibition.
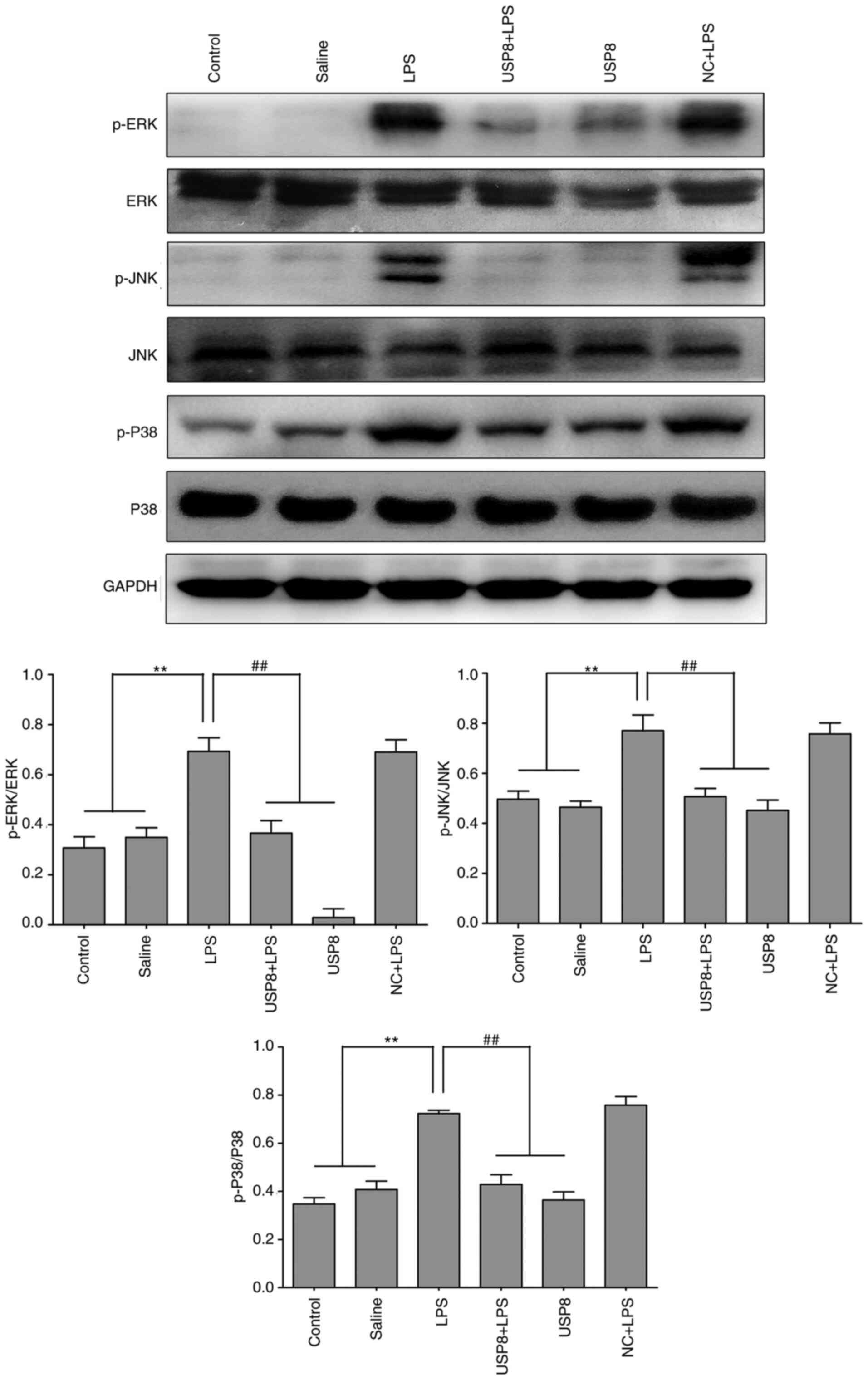
USP8 protects against LPS-induced
spleen injury by inhibiting the MAPK pathway
Growing evidence has suggested that MAPK has a vital
function in regulating the synthesis and release of
pro-inflammatory factors (20). The
present study investigated how USP8 functions in MAPK signaling.
LPS increased the levels of p-ERK, p-P38 (also known as MAPK14) and
p-JNK (also known as MAPK8) compared with those in the control and
saline groups, as indicated by western blotting, whereas in the
USP8 + LPS group, their expression levels were decreased (Fig. 6). These results suggested that USP8
may mediate attenuation of the inflammatory response via the MAPK
pathway.
Discussion
The cellular ubiquitin proteasome system (UPS)
efficiently degrades proteins; in particular, it regulates
structurally abnormal, misfolded or damaged proteins, and short
half-life regulatory proteins (21). The UPS comprises ubiquitin and
various other enzymes, such as USP8, which belong to the
deubiquitinating enzyme family (8).
USP8 regulates growth-related proteins and the stability of its
effector protein, NRDP1, in response to various stimuli (22). NRDP1 regulates TLR signaling via
inhibition of activator protein 1 and NF-κB, which attenuates
pro-inflammatory cytokine production (23). In the brains of cecal ligation and
puncture (CLP) model mice, our previous study revealed that
neuroinflammation occurred and that USP8 had protective functions
against CLP-induced neuroinflammation, and cognitive and motor
impairments. This previous observation may facilitate the
development of novel therapeutic strategies to treat
sepsis-associated encephalopathy (24). The spleen is the largest immune
organ in the body and contains numerous lymphocytes, which have a
vital function in the immune response, and exhibit antitumor,
anti-inflammatory and hematopoietic properties (25). Our previous studies reported that
USP8 could regulate inflammation in vitro and in vivo
(11,12,22);
however, to the best of our knowledge, the impact of USP8 on spleen
injury remains unknown. The aim of the present study was to
investigate the protective effects of USP8 against spleen injury in
LPS-induced mice via attenuation of inflammation.
Viral or bacterial infections can cause splenomegaly
resulting from the infiltration of a large number of inflammatory
cells, accompanied by congestion of the spleen (2). results of the present study indicated
that systemic inflammation, induced by LPS, may cause splenomegaly,
which could be ameliorated by USP8 pretreatment. In addition, the
spleen was shown to be damaged by LPS administration, with marked
congestion in the white pulp, a reduction in typical splenic
nodules and PALS, and disappearance of the marginal zone and
germinal center; notably, all of these findings were ameliorated by
USP8 treatment. Thus, USP8 might exert a protective effect on
LPS-induced spleen injury.
It has previously been indicated that the expression
of the transcription factor NF-κB is increased in various tissues,
including the spleen, following LPS injection (26). Under LPS stimulation in mice,
phosphorylated IκBα has been reported to be degraded via
proteasome-mediated activation, resulting in active NF-κB nuclear
translocation (27). Intranuclear
blockage of NF-κB has been demonstrated to suppress
pro-inflammatory cytokines, such as IL-1β and TNF-α (28,29).
Furthermore, in a previous study, USP8 increased NRDP1 levels,
potently downregulated MyD88 and TLR4 levels, and blocked inhibitor
of NF-κB kinase subunit β and IκBα phosphorylation, thus decreasing
p65 nuclear translocation, resulting in and inhibition of NF-κB
signaling pathway activation in LPS-induced mice (12). In the present study, USP8 reduced
the protein levels of MyD88, NF-κB and IκBα, which suppressed the
release of pro-inflammatory cytokines, including TNF-α and IL-1β.
Therefore, it was hypothesized that treatment with USP8 could
ameliorate pro-inflammatory cytokine release, thereby attenuating
spleen injury following LPS injection.
The MAPK serine/threonine protein kinases are common
in mammals. MAPKs include ERK, JNK and P38 (30). The levels of p-P38 have been shown
to be significantly increased by LPS treatment in spleen
homogenates from rats (31).
Mormède et al (32) observed
that splenic MAPK signaling pathways were activated by i.p.
injection of LPS. Increased expression of USP8 has been reported to
induce increased surface localization of LepRb, which in turn can
enhance leptin-mediated activation of the MAPK/ERK pathway and CREB
activation (33). The present
results demonstrated that USP8 treatment reduced the expression
levels of p-JNK, p-ERK and p-P38 levels in the spleen of
LPS-induced mice, which is beneficial to reducing inflammation. The
results suggested that the attenuation of inflammatory responses by
USP8 acts via the MAPK pathway.
In conclusion, USP8 contributed to suppressing the
release of pro-inflammatory cytokines and prevented spleen injury
following LPS injection in mice. These benefits of USP8 were
associated with inhibition of inflammation-related signaling
pathways, particularly the MAPK and NF-κB pathways. These data
provided strong evidence that USP8 could be applied therapeutically
to treat inflammation-mediated spleen injury.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Natural Science
Foundation of China (grant nos. 81200930 and 82071568), the
Training Program for Outstanding Young Teachers in Higher Education
Institutions of Guangdong Province (grant no. YQ2015024), the
Fundamental Research Funds for the Central Universities (grant no.
21617482) and the Flagship Specialty Construction Project of the
First Affiliated Hospital of Jinan University-Department of
Neurology (grant no. 11001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WB and LHZ designed the study. LHZ, WB and JWZ
participated in designing the present study and reviewing the data,
and confirmed the authenticity of all the raw data. ZHZ, RYZ and
JYZ performed the majority of experiments. LW and XTL assisted in
carrying out the H&E experiment. WY assisted with data analysis
and interpretation, and critically read the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The Animal Care and Use Committee of Jinan
University (Guangzhou, China; approval no. IACUC-20180726-03)
approved all the animal procedures.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang LN, Yao YM and Sheng ZY: The role of
regulatory T cells in the pathogenesis of sepsis and its clinical
implication. J Interferon Cytokine Res. 32:341–349. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altamura M, Caradonna L, Amati L,
Pellegrino NM, Urgesi G and Miniello S: Splenectomy and sepsis: The
role of the spleen in the immune-mediated bacterial clearance.
Immunopharmacol Immunotoxicol. 23:153–161. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noh H, Jeon J and Seo H: Systemic
injection of LPS induces region-specific neuroinflammation and
mitochondrial dysfunction in normal mouse brain. Neurochem Int.
69:35–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Badshah H, Ali T and Kim MO: Osmotin
attenuates LPS-induced neuroinflammation and memory impairments via
the TLR4/NFκB signaling pathway. Sci Rep. 6:244932016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rahimifard M, Maqbool F, Moeini-Nodeh S,
Niaz K, Abdollahi M, Braidy N, Nabavi SM and Nabavi SF: Targeting
the TLR4 signaling pathway by polyphenols: A novel therapeutic
strategy for neuroinflammation. Ageing Res Rev. 36:11–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiao K, Zou WH, Yang Z, Rehman ZU, Ansari
AR, Yuan HR, Zhou Y, Cui L, Peng KM and Song H: The role of
visfatin on the regulation of inflammation and apoptosis in the
spleen of LPS-treated rats. Cell Tissue Res. 359:605–618. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nijman SM, Luna-Vargas MP, Velds A,
Brummelkamp TR, Dirac AM, Sixma TK and Bernards R: A genomic and
functional inventory of deubiquitinating enzymes. Cell.
123:773–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soares L, Seroogy C, Skrenta H,
Anandasabapathy N, Lovelace P, Chung CD, Engleman E and Fathman CG:
Two isoforms of otubain 1 regulate T cell anergy via GRAIL. Nat
Immunol. 5:45–54. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naviglio S, Mattecucci C, Matoskova B,
Nagase T, Nomura N, Di Fiore PP and Draetta GF: UBPY: A
growth-regulated human ubiquitin isopeptidase. EMBO J.
17:3241–3250. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niendorf S, Oksche A, Kisser A, Löhler J,
Prinz M, Schorle H, Feller S, Lewitzky M, Horak I and Knobeloch KP:
Essential role of ubiquitin-specific protease 8 for receptor
tyrosine kinase stability and endocytic trafficking in vivo. Mol
Cell Biol. 27:5029–5039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu L, Bi W and Lu D, Zhang C, Shu X, Wang
H, Qi R, Shi Q and Lu D: Regulation of ubiquitin-specific
processing protease 8 suppresses neuroinflammation. Mol Cell
Neurosci. 64:74–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J, Bi W, Zhang J, Xiao S, Zhou R,
Tsang CK, Lu D and Zhu L: USP8 protects against
lipopolysaccharide-induced cognitive and motor deficits by
modulating microglia phenotypes through TLR4/MyD88/NF-κB signaling
pathway in mice. Brain Behav Immun. 88:582–596. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory Animals. National Academies Press (US); Washington DC,
USA: pp. 21–48. 1996
|
|
14
|
Haley TJ and Mccormick WG: Pharmacological
effects produced by intracerebral injection of drugs in the
conscious mouse. Br J Pharmacol Chemother. 12:12–15. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tunstall ME and Sheikh A: Comparison of
1.5% enflurane with 1.25% isoflurane in oxygen for caesarean
section: Avoidance of awareness without nitrous oxide. Br J
Anaesth. 62:138–143. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rao Z, Pan X, Zhang H, Sun J, Li J, Lu T,
Gao M, Liu S, Yu D and Ding Z: Isoflurane preconditioning
alleviated murine liver ischemia and reperfusion injury by
restoring AMPK/mTOR-Mediated Autophagy. Anesth Analg.
125:1355–1363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin S, Yin Q, Zhong Q, Lv FL, Zhou Y, Li
JQ, Wang JZ, Su BY and Yang QW: Heme activates TLR4-mediated
inflammatory injury via MyD88/TRIF signaling pathway in
intracerebral hemorrhage. J Neuroinflamm. 9:462012. View Article : Google Scholar
|
|
18
|
Hernández-Romero MC, Delgado-Cortés MJ,
Sarmiento Ml, de Pablos RM, Espinosa-Oliva AM, Argüelles S, Bández
MJ, Villarán RF, Mauriño R, Santiago M, et al: Peripheral
inflammation increases the deleterious effect of CNS inflammation
on the nigrostriatal dopaminergic system. Neurotoxicology.
33:347–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ozato K, Tsujimura H and Tamura T:
Toll-like receptor signaling and regulation of cytokine gene
expression in the immune system. Biotechniques. (Suppl):66–68. 7072
passim. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koistinaho M and Koistinaho J: Role of p38
and p44/42 mitogen-activated protein kinases in microglia. Glia.
40:175–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gallastegui N and Groll M: The 26S
proteasome: Assembly and function of a destructive machine. Trends
Biochem Sci. 35:634–642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu X, Yen L, Irwin L, Sweeney C and
Carraway KL III: Stabilization of the E3 ubiquitin ligase Nrdp1 by
the deubiquitinating enzyme USP8. Mol Cell Biol. 24:7748–7757.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Chen T, Zhang J, Yang M, Li N, Xu
X and Cao X: The E3 ubiquitin ligase Nrdp1 ‘preferentially’
promotes TLR-mediated production of type I interferon. Nat Immunol.
10:744–752. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bi W, Lan X, Zhang J, Xiao S, Cheng X,
Wang H, Lu D and Zhu L: USP8 ameliorates cognitive and motor
impairments via microglial inhibition in a mouse model of
sepsis-associated encephalopathy. Brain Res. 1719:40–48. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang WH, Yao YM, Shi ZG, Yu Y, Wu Y, Lu LR
and Sheng ZY: Effect of recombinant
bactericidal/permeability-increasing protein on endotoxin
translocation and lipopolysaccharide-binding protein/CD14
expression in rats after thermal injury. Crit Care Med.
29:1452–1459. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mallard C: Innate immune regulation by
toll-like receptors in the brain. ISRN Neurol. 2012:7019502012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nair AR, Masson GS, Ebenezer PJ, Del Piero
F and Francis J: Role of TLR4 in lipopolysaccharide-induced acute
kidney injury: Protection by blueberry. Free Radic Biol Med.
71:16–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Petrova TV, Akama KT and Van Eldik LJ:
Cyclopentenone prostaglandins suppress activation of microglia:
Down-regulation of inducible nitric-oxide 12,14-synthase by
15-deoxy-prostaglandin J2. Proc Natl Acad Sci USA. 96:4668–4673.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye SM and Johnson RW: Regulation of
interleukin-6 gene expression in brain of aged mice by nuclear
factor kappaB. J Neuroimmunol. 117:87–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nithianandarajah-Jones GN, Wilm B,
Goldring CE, Müller J and Cross MJ: ERK5: Structure, regulation and
function. Cell Signal. 24:2187–2196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meng AH, Ling YL, Zhang XP, Zhao XY and
Zhang JL: CCK-8 inhibits expression of TNF-alpha in the spleen of
endotoxic shock rats and signal transduction mechanism of p38 MAPK.
World J Gastroenterol. 8:139–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mormède C, Palin K, Kelley KW, Castanon N
and Dantzer R: Conditioned taste aversion with lipopolysaccharide
and peptidoglycan does not activate cytokine gene expression in the
spleen and hypothalamus of mice. Brain Behav Immun. 18:186–200.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bland T, Sahin GS, Zhu M, Dillon C, Impey
S, Appleyard SM and Wayman GA: USP8 deubiquitinates the leptin
receptor and is necessary for leptin-mediated synapse formation.
Endocrinology. 160:1982–1998. 2019. View Article : Google Scholar : PubMed/NCBI
|